Nuclear import receptor inhibits phase separation of FUS through binding to multiple sites (original) (raw)
. Author manuscript; available in PMC: 2019 Apr 19.
Summary
Liquid-liquid phase separation (LLPS) is believed to underlie formation of biomolecular condensates, cellular compartments that concentrate macromolecules without surrounding membranes. Physical mechanisms that control condensate formation/dissolution are poorly understood. The RNA-binding protein Fused in Sarcoma (FUS) undergoes LLPS in vitro and associates with condensates in cells. We show that the Importin Karyopherin-β2/Transportin-1 inhibits LLPS of FUS. This activity depends on tight binding of Karyopherin-β2 to the C-terminal proline-tyrosine nuclear localization signal (PY-NLS) of FUS. NMR analyses reveal weak interactions of Karyopherin-β2 with sequence elements and structural domains distributed throughout the entirety of FUS. Biochemical analyses demonstrate that most of these same regions also contribute to LLPS of FUS. The data lead to a model where high-affinity binding of Karyopherin-β2 to the FUS PY-NLS tethers the proteins together, allowing multiple, distributed weak intermolecular contacts to disrupt FUS self-association, blocking LLPS. Karyopherin-β2 may act analogously to control condensates in diverse cellular contexts.
Keywords: Karyopherin-β2, Transportin-1, PY-NLS, FUS, phase separation, liquid-liquid phase separation, biomolecular condensates, low-complexity sequences, Ran GTPase, M9M, RRM, RGG, zinc finger
Graphical abstract

Introduction
The RNA-binding protein Fused in Sarcoma (FUS) plays roles in transcription, RNA processing and DNA repair (Ederle and Dormann, 2017). FUS is localized primarily to the nucleus but is also found in cytoplasmic RNP granules (Crozat et al., 1993; Ryu et al., 2014). Heat shock and DNA damage promote localization of the protein to cytoplasmic and nuclear puncta (Patel et al., 2015). FUS is involved in diverse diseases including cancer and the neurodegenerative diseases amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD) (Harrison and Shorter, 2017; Schwartz et al., 2015). In ALS, FUS is often mutated in its proline-tyrosine nuclear localization sequence (PY-NLS). These alterations decrease affinity for the nuclear import factor Karyopherin-β2 (Kapβ2; also known as Transportin-1) leading to aberrant cytoplasmic localization and enrichment in RNP granules (Dormann and Haass, 2011; Zhang and Chook, 2012). Proper compartmentalization of FUS is important in maintaining cellular homeostasis, as the degree of FUS mislocalization correlates with ALS onset and severity (Dormann and Haass, 2011).
FUS is composed of multiple structural and functional elements. It has an N-terminal disordered region with low amino acid sequence complexity that is enriched in Gly, Ser, Tyr and Gln residues (LC region), followed by a segment with Arg-Gly-Gly motifs (RGG1), a folded RNA Recognition Motif (RRM) domain, two additional RGG regions (RGG2 and RGG3) flanking a zinc-finger (ZnF) domain, and a C-terminal 26-residue PY-NLS (Figure 1A) (Ederle and Dormann, 2017). FUS is highly prone to self-association, a process that can lead to different material states including phase separated liquids, amyloid fiber containing hydrogels and aggregated solids (Burke et al., 2015; Kato et al., 2012; Murakami et al., 2015; Sun et al., 2011). The LC region undergoes liquid-liquid phase separation (LLPS) at high concentrations, through weak and transient homotypic interactions (Burke et al., 2015; Lin et al., 2015). Full-length FUS also undergoes LLPS, but at much lower concentrations, consistent with previous reports that the RGG regions can contribute to self-association of the protein (Patel et al., 2015; Sun et al., 2011). RNA enhances these processes (Burke et al., 2015; Schwartz et al., 2013). Phase separation of FUS and other disordered proteins is driven by a variety of interaction types including charge-charge, cation-π, π-π stacking and hydrogen bonds, involving side chains and backbone (Banani et al., 2017; Brangwynne et al., 2015). Over time, phase separated FUS droplets mature to more solid hydrogels that contain amyloid-like fibers (Burke et al., 2015; Kato et al., 2012; Lin et al., 2015; Murakami et al., 2015; Patel et al., 2015). Disease-causing mutations accelerate maturation of FUS droplets in vitro (Murakami et al., 2015; Patel et al., 2015). A recent solid state NMR analysis of fibers formed by the FUS LC region revealed a β-strand-containing structured core spanning residues 39–95, whose formation also appears to contribute to LLPS (Murray et al., 2017). Similar LLPS and maturation behaviors have been observed for other RNA binding proteins containing disordered or LC regions (Lin et al., 2015; Molliex et al., 2015; Xiang et al., 2015). The progression from phase separated liquid to a more static solid is likely controlled in cells to produce structures of different material properties, according to specific cellular needs. However the biological factors that can control self-association, LLPS and fiber formation of FUS are not known.
Figure 1. Kapβ2 inhibits FUS turbidity and phase separation in a PY-NLS- and RanGTP-dependent manner.
A) Domain organization of FUS. B) Turbidity of 8 μM MBP-FUS ± 8 μM Kapβ2, measured for 60 min at room temperature after addition of Tev protease to remove MBP from MBP-FUS. C) Turbidity of 8 μM MBP-FUS in the presence of buffer, 8 μM Kapβ2 ± RanGTP or inhibitor M9M, or Kapβ2Δloop ± RanGTP (60 min after Tev). D) Either 8 μM Kapβ2 or buffer was added at time=60 min to turbid FUS (8 μM MBP-FUS pre-treated with Tev for 60 min) and OD395nm measured for the next 20 min. B)-D), OD395nm normalized to measurements of MBP-FU+buffer+Tev at time=60 min. C)-D), mean of 3 technical replicates, ± S.D. E) Mixtures containing 5 μM MBP-FUS, 0.5 μM MBP-FUS-SNAPSNAP-Surface 649 and either buffer or 10 μM Kapβ2 were treated with Tev and imaged 1 hr later. (Supplementary Movie 1 also shows FUS droplets at time=1 hr.) F) Mixtures of 5 μM MBP-FUS and 0.5 μM MBP-FUS-SNAPSNAP-Surface 649 were treated with Tev for 1 hr prior to addition of 10 μM of Kapβ2, which cleared FUS droplets in less than 5 min (see also Supplementary Movie 2). Kapβ2 added to phase separated FUS 48 hr after Tev treatment cleared most of the phase separated material in 120 min (Supplementary Movie 3 also shows the first 30 min after Kapβ2 addition). Images in E)-F) were obtained with spinning disk confocal microscopy (561 nm laser illumination; 60x 1.4na oil immersion objective lenses) and 20 μm length scale bars are shown. See also Figure S1.
The FUS PY-NLS and its ALS-associated mutations seem to play no direct role in FUS self-association (Ju et al., 2011; Sun et al., 2011). However, FUS PY-NLS binding to Kapβ2 controls nuclear-cytoplasmic localization of FUS and cytoplasmic concentrations of FUS likely controls self-association and disease onset (Dormann and Haass, 2011). Kapβ2 is also the only high-affinity binding partner of FUS that has been characterized to date (Zhang and Chook, 2012). Although it is well established that Kapβ2 imports FUS into the nucleus, it is not known if Kapβ2 binding directly affects FUS self-association and/or its ability to undergo LLPS.
Here, we show that Kapβ2 inhibits LLPS of FUS, in a manner dependent on interactions with the FUS PY-NLS. The Importin-α•Importin-β (Impα/β) heterodimer and the yeast Kap121, can also inhibit FUS LLPS when the FUS PY-NLS is replaced with the appropriate cognate NLSs. Thus, Importins may generally be able to control LLPS of self-associating RNA-binding proteins through high-affinity binding to their NLSs. NMR analyses reveal multiple weak interactions of Kapβ2 with both folded and disordered regions across FUS. Deletion or mutation of some of these elements (LC, RGG2 and RGG3) also decreases phase separation of FUS. Together, the data suggest that high-affinity interactions between Kapβ2 and the PY-NLS of FUS anchor the two proteins together, facilitating multiple weak interactions with FUS regions that mediate self-association, thus blocking phase separation. These effects may enable Kapβ2, and perhaps other Importin family members, to control the stability and dynamics of RNA-containing biomolecular condensates.
Results
Kapβ2 prevents and reverses turbidity of FUS solutions
Purified bacterially expressed Maltose Binding Protein-FUS fusion protein (MBP-FUS) is soluble and monomeric by gel filtration chromatography. The protein is polydisperse in dynamic light scattering experiments, however, suggesting the presence of minor high molecular weight oligomers (Figure S1A). The PY-NLS of FUS binds the 100 kDa Kapβ2 with dissociation constant (KD) of 70 nM (Figure S1B). MBP-FUS also binds Kapβ2 stably, but the affinity is difficult to quantify because of the polydispersity of MBP-FUS. An approximate KD determined by isothermal titration calorimetry (ITC) is 160 nM (Figure S1B). Addition of Kapβ2 to MBP-FUS drastically reduced polydispersity. The majority of MBP-FUS•Kapβ2 behaves as a single species, most likely the heterodimer (Figure S1C), suggesting that Kapβ2 can disrupt self-association of FUS.
Removal of MBP from MBP-FUS with the Tev protease causes FUS to self-associate, producing a turbid solution (Figure 1B and S1D, E). Addition of equimolar Kapβ2 prior to Tev cleavage prevents turbidity, consistent with formation of soluble Kapβ2•FUS heterodimer (Figure 1B, C and S1C). The ability of Kapβ2 to prevent turbidity is abolished by the M9M peptide inhibitor or the Ran GTPase (Figure 1C), which both displace cargos from Kapβ2 (Cansizoglu et al., 2007; Chook and Blobel, 1999). Kapβ2Δloop mutant, which can bind both RanGTP and PY-NLS simultaneously (Chook et al., 2002), retains the ability to block turbidity even in the presence of RanGTP (Figure 1C). Together, these data show that the ability of Kapβ2 to inhibit turbidity of FUS solutions is dependent on binding to the C-terminal PY-NLS of FUS, the same interaction that mediates nuclear import of FUS. Analogous behavior is also observed when Kapβ2 is added 60 minutes after turbidity is induced by Tev addition (Figure 1D and S1F). Thus, Kapβ2 can both inhibit and reverse turbidity caused by FUS self-association.
Kapβ2 inhibits liquid-liquid phase separation of FUS
We examined turbid solutions of fluorescently labeled FUS (5 μM MBP-FUS doped with 0.5 μM fluorescent MBP-FUS-SNAPSNAP-Surface 649) in the presence of Kapβ2 and its regulators using spinning disc confocal microscopy (Figure 1E, F and S1G). As previously reported (Burke et al., 2015; Monahan et al., 2017; Patel et al., 2015), after removal of MBP, FUS concentrates into phase separated liquid droplets. When analyzed by polarized light microscopy, the interiors of FUS droplets show no molecular order on 350 nm length scale, consistent with them being a homogeneous liquid phase (Figure S1H). FUS droplets fuse with each other and by 24 hours accumulate into large mats of phase-separated liquid (Figure 1E, S1G and Supplementary movie 1). As in the turbidity assays above, Kapβ2 can block phase separation of FUS and this activity is inhibited by both RanGTP and the M9M inhibitor (Figure 1E and S1G). Further, Kapβ2 can disrupt phase separated FUS droplets when added at either one hour or 48 hours after Tev addition, although clearance of droplets takes longer in the latter case (Figure 1F, Supplementary movies 2 and 3).
Other Importins can also prevent FUS LLPS if their cognate NLS is present
We next examined whether two other Importins with distinct cargo recognition sequences, the Impα/β heterodimer and the S. cerevisiae Importin Kap121, can also bind FUS and block its LLPS. Cognate cargo recognition sequences for Impα/β and Kap121 are the classical NLS (cNLS) and the isoleucine-lysine NLS (IK-NLS), respectively (Soniat and Chook, 2016). Initially we examined interactions of immobilized GST-Kapβ2, GST-Impα•Impβ and GST-Kap121 with MBP-FUS in pull-down binding and turbidity assays. We found that GST-Kapβ2 binds well to MBP-FUS but GST-Impα•Impβ does not (Figure S2A), consistent with Impα/β not affecting FUS turbidity (Figure 2A). In contrast, GST-Kap121 binds weakly to MBP-FUS (MBP-FUS band sub-stoichiometric to GST-Kap121 band; Figure S2A), and Kap121 partially prevents FUS turbidity (Figure 2A). When bound to IK-NLS, Kap121 no longer affects FUS turbidity suggesting that Kap121 likely uses its cargo-binding site to bind FUS weakly (Figure 2A).
Figure 2. Imp-α/β and Kap121 inhibit FUS phase separation when their NLS is introduced into FUS, but Kapβ2 does not act non-specifically to inhibit FUS phase separation.
A) Turbidity of wildtype FUS in the presence of buffer, Kapβ2, Impα/β, cNLS-bound Impα/β, Kap121 or IK-NLS-bound Kap121. B) Turbidity of FUS(cNLS) chimera (FUS PY-NLS replaced with the SV40 T antigen cNLS) in the presence of buffer, Impα/β, Impα/β•RanGTP or Impα. C) Turbidity of FUS(IK-NLS) chimera (PY-NLS replaced with IK-NLS from Pho4) in the presence of buffer, Kap121 or Kap121•RanGTP. D) Turbidity of FUS(NES) chimera (PY-NLS replaced with the NES from the NS2 protein of MVM virus) in the presence of buffer or CRM1. 8 μM proteins were used in A)-D), and OD395nm were normalized to those of MBP-FUS+buffer+Tev at time=60 min. E) Diffusion coefficients of Kapβ2 were measured at different concentrations by dynamic light scattering. Error bars represent S.D. from 3 technical replicates. See also Figure S2 and Table S1.
To learn whether the lack of activity of Impα/β could be due simply to low affinity for FUS, we replaced the PY-NLS in FUS (residues 501–526) with a high affinity cNLS to give FUS(cNLS). Pull-down binding assays showed that MBP-FUS(cNLS) binds both Impα alone and Impα/β, consistent with direct binding to Impα, as observed for all known Impα/β cargos (Figure S2A). In turbidity assays, Impα alone had no effect on FUS(cNLS) turbidity, but Impα/β blocked turbidity in a RanGTP-sensitive manner (Figure 2B). We also replaced the PY-NLS of FUS with an IK-NLS to give FUS(IK-NLS) (Kobayashi and Matsuura, 2013). Analogous to the results above, LLPS of this chimera is strongly inhibited by Kap121 in a RanGTP-sensitive manner (Figure 2C). In summary, when FUS has an appropriate high-affinity recognition signal, an Importin family member that is distinct from Kapβ2 can block its phase-separation. This inhibition requires a β-Importin family member as Impα alone has no effect.
Kapβ2 is unlikely to act non-specifically to disrupt LLPS
Previous studies reported that FUS PY-NLS does not participate directly in FUS self-association (Ju et al., 2011; Sun et al., 2011). It is therefore unclear how Kapβ2 binding to this element could block LLPS by FUS. One limiting possibility is that simply tethering any large molecule to the C-terminus of FUS may act non-specifically to alter the balance between FUS-FUS and FUS-solvent interactions, disfavoring the former and thus inhibiting LLPS. Alternatively, Kapβ2 may be acting specifically, through binding competitively to regions of FUS that mediate self-association.
To examine the former possibility, we replaced the FUS PY-NLS with a high-affinity nuclear export signal (NES) to generate a FUS(NES) chimera (Ohshima et al., 1999). In contrast to the FUS(cNLS) and FUS(IK-NLS) chimeras, phase separation of FUS(NES) was not inhibited by its cognate Kapβ protein, the 127 kDa Exportin CRM1/XPO1 (Figure 2D) even though the two proteins bind each other tightly (Figure S2A). Similarly as described above, the 60 kDa Impα does not disrupt LLPS of FUS(cNLS) (Figure 2B and S2A). Thus merely tethering large proteins to the FUS C-terminus is insufficient to inhibit LLPS.
We recently showed that tethering self-attractive proteins to a phase-separating system increases the drive to phase separate and tethering self-repulsive proteins has an opposite effect (Lin et al., 2017). To investigate whether Kapβ2 has attractive or repulsive self-interactions, we measured its diffusion interaction parameter, κD; positive κD suggests net repulsive interactions and negative κD indicates net attractive interactions (Connolly et al., 2012). As shown in Figure 2E, Kapβ2 has κD = −173 ml/g, indicating attractive self-interactions. Thus, the protein is unlikely to act non-specifically to generate repulsion between FUS molecules.
Kapβ2 interacts weakly and non-uniformly with residues in FUS LC
FUS is believed to phase separate due to weak homotypic interactions involving the LC region and C-terminal elements (Burke et al., 2015; Monahan et al., 2017; Patel et al., 2015). Kapβ2 could block phase separation by competitively binding these elements, which are outside of the PY-NLS. The affinities of Kapβ2 for full-length FUS and the FUS PY-NLS are similar (KD ~160 nM vs. ~70 nM) suggesting that such additional interactions are likely weak (Figure S1B). The lack of stable Kapβ2•FUS contacts outside the PY-NLS is consistent with observations that only the PY-NLS is observed in crystal structures of Kapβ2 bound to FUS (full-length), FUS(371–526) and FUS(456–526) (crystallographic statistics in Table S1; ITC analysis, structures and electron density maps shown in Figure S2B–H).
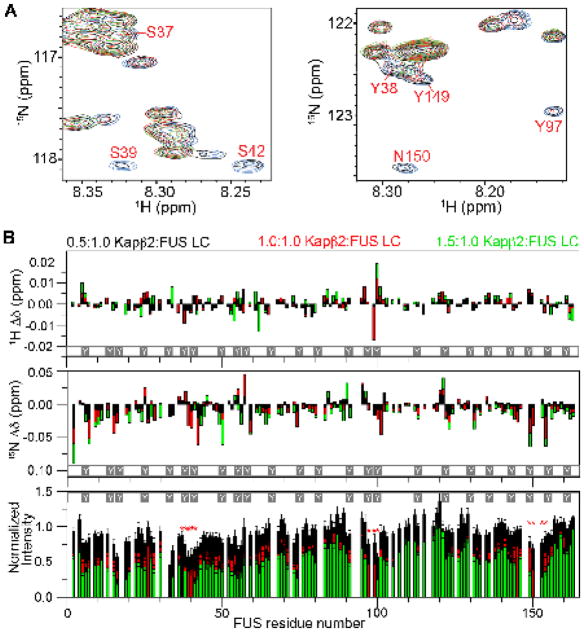
We used NMR spectroscopy to identify regions of FUS outside its C-terminal PY-NLS that contact Kapβ2 (Figure 3A–B, 4A–E and 5A–E). β-Importin proteins make many weak and highly dynamic interactions with phenylalanine-glycine (FG) repeats in various nucleoporins to traverse the nuclear pore complex (Hough et al., 2015; Milles et al., 2015). The FUS LC (residues 1–163) contains 24 motifs with the sequence [S/G]Y[S/G] (Figure 3A–B and S3A–B), potentially analogous to the FG repeats in the nuclear pore complex. Thus, we initially analyzed 1H/15N HSQC spectra of 15N-FUS LC in the absence and presence of Kapβ2 to identify such contacts (Figure 3A and S3A). At conditions where FUS LC is not phase separated (75 μM, 10 °C), many resonances progressively shift and decrease in intensity as Kapβ2 is increased from 0 to 112.5 μM (Figure 3B; chemical shift assignments from (Burke et al., 2015)). These behaviors are consistent with weak binding that is not saturated at these conditions. The attenuation/shifting of FUS LC resonances is distributed non-uniformly across the protein. The largest perturbations are observed for resonances from the segments 37SYSGY41, 97YPGY100 and 149YSPPSG154, suggesting relatively stronger binding to these elements (Figure 3B and Figure S3B). The 37SYSGY41 is part of the β-strand-containing structured core observed in solid state NMR analysis of LC fibers (Murray et al., 2017), suggesting that disruption of the core may contribute to the effects of Kapβ2. Amide resonances change similarly upon addition of Kapβ2 alone or Kapβ2•PY-NLS, indicating contacts outside the PY-NLS binding site of the karyopherin (Figure S3B).
Figure 3. Kapβ2 interacts weakly and non-uniformly with residues in FUS LC.
A) Overlay of 2D 1H-15N spectra of 75 μM 15N-FUS LC alone (blue) or with increasing concentrations of Kapβ2: 37.5 μM (0.5:1, black), 75 μM (1:1, red), 112.5 μM (1.5:1, green), showing three of the FUS LC regions (residues 37–41, 97–100, 149–154) most affected by Kapβ2 resulting in chemical shifts and intensity attenuations. B) Titrations at 10 °C of 75 μM FUS LC with increasing concentrations of Kapβ2 compared to FUS LC alone. NMR chemical shift deviations, 1H (top) and 15N (middle), and resonance intensity attenuation (bottom) are plotted. Increasing extent of chemical shift differences of 1H and 15N resonance position, as well as resonance intensity attenuation, support Kapβ2 binding weakly to across the entire FUS LC domain. Resonance intensity attenuation and chemical shifts are non-uniformly distributed as segments 37SYSGY41, 97YPGY100 and 149YSPPSG154 (white Ys mark the 24 tyrosines in FUS LC) show the largest perturbations in amide resonance intensity (red asterisks, bottom panel) in the presence of Kapβ2. These segments also show large 15N and/or 1H chemical shift deviations. See also Figure S3.
Kapβ2 interacts weakly with folded and disordered regions within FUS(164–500)
Beyond the LC region, C-terminal elements also contribute to LLPS of FUS (Burke et al., 2015; Monahan et al., 2017). Thus, we next examined interactions of FUS(164–500) with Kapβ2. Because of the complex nature of this fragment, containing two folded domains surrounded by three intrinsically disordered elements, we used NMR cross saturation transfer experiments to look for regions that directly contact Kapβ2. In these experiments, 15N/2H labeled FUS(164–500) (protonated at amide positions, deuterated at all aliphatic sites) is mixed with fully protonated Kapβ2. Irradiation of such samples in the aliphatic region of the spectrum saturates resonances of Kapβ2 and this saturation is transferred to amides of FUS that are in direct contact with the Karyopherin. Saturation is manifest as decreases in intensity of selected amide resonances in FUS, which are observed in HSQC-type 1H/15N correlation spectra. The experiment can be complicated by the dynamics of the interactions, such that decreases at bona fide interfaces may not be observed if the bound populations are low or interaction kinetics are in the wrong rate regime (Jayalakshmi and Krishna, 2002; Ueda et al., 2014). The data can be particularly complicated in interactions of disordered proteins, where different parts of the chain may contact a partner with different local dynamics.
Addition of Kapβ2 to 15N-FUS(164–526) harboring the PY-NLS causes severe line-broadening of most resonances in 1H/15N HSQC spectra, including all of those representing the folded domains and much of the disordered regions thus precluding analysis (Figure S4A). This broadening probably occurs because of the large size of the FUS•Kapβ2 complex (~160 kDa) and slow exchange between the bound and free states arising from the high-affinity interaction. Thus, to weaken the interactions and identify direct Kapβ2 contact sites we recorded spectra on 15N/2H-FUS(164–500) lacking the PY-NLS, in the presence of Kapβ2 bound to the pM affinity M9M peptide inhibitor (to exclude artifactual contacts to the PY-NLS binding site, which would be occluded in the native FUS•Kapβ2 complex).
The 1H/15N HSQC spectrum of 17 μM 15N-FUS(164–500) shows many strong resonances with 1H chemical shifts between ~7.5 ppm and 8.5 ppm, mostly representing residues in unstructured regions of the protein, as well as numerous resonances outside of this window, which represent the folded RRM (residues 285–370) and Cys4-type ZnF (residues 421–455) domains (Figure S4B and Tables S2, S3). Correspondence between the dispersed resonances and reported chemical shift assignments of the two domains (Iko et al., 2004; Liu et al., 2013) enabled us to assign most resonances from the RRM and ZnF to specific residues (Figure S4B, Figure 4A, C).
Figure 4. Kapβ2 interacts weakly with the folded RRM and ZnF domains within FUS(164–500).
A) Attenuations in intensity (I/I0) of assigned RRM domain non-proline resonances in FUS(164–500) in the cross saturation transfer experiment. Deuterated 15N-FUS(164–500) was cross saturated from protonated Kapβ2-M9M (1.5-fold molar excess) and intensities of assigned RRM resonances were measured with (I) and without (I0) irradiation in aliphatic region. B) Ribbon (left) and surface (middle and right panels) representations of the RRM (PDBID 1LCW; green), showing binding sites for Kapβ2 (magenta, residues with I/I0<0.4 in cross saturation experiment) and RNA (yellow). C) Same as (A), but shown here are I/I0 of assigned ZnF domain non-proline resonances in FUS(164–500) in the cross saturation transfer experiment. D) Selected resonances of RRM (green) and ZnF (orange) domains from TROSY 1H-15N HSQC/1H-15N HSQC NMR spectra of 15N-FUS(164–500) showing change in intensity in cross saturation and line broadening experiments upon addition of 3-fold molar excess Kapβ2-M9M. E) Homology model of the FUS ZnF domain (orange; from ZnF in ZNF265, PDBID 2K1P). Ribbon (left) and surface (middle and right) representations showing binding sites for Kapβ2 (magenta, residues with I/I0<0.55 in cross saturation experiment) and RNA (yellow). Residues with unassigned/missing/proline resonances are in white. See also Figure S4.
In cross saturation transfer experiments, minimal changes were observed in amide resonances of 15N/2H-labeled FUS(164–500) alone (not shown). In the presence of 1.5-fold excess Kapβ2•M9M, a subset of amide resonances in the RRM domain showed particularly large decreases in intensity (> 60%; Figure 4A, D). These resonances mapped to a contiguous patch on one face of the FUS RRM domain (PDBID 2LCW; (Liu et al., 2013)) defined by its two α-helices (Figure 4B). Similarly we observed greater decreases in intensity (> 45%) of certain resonances in the ZnF domain (Figure 4C, D). These resonances mapped to one face of a homology model of the FUS ZnF (PDBID 2K1P; (Iko et al., 2004; Loughlin et al., 2009)), defined by its C-terminal β-strand (Figure 4E). Although the uncertainties in intensity ratio (saturated vs unsaturated) for any individual peak is relatively large due to the broad lines induced by Kapβ2, the convergence of the affected residues to contiguous patches affords confidence that they map contact sites on the RRM and ZnF domains.
As in our analysis of the LC region, we also examined line broadening of FUS resonances upon addition of 3-fold excess Kapβ2•M9M. As detailed in Figures S4C-G, similar, although more extensive, regions of the RRM and ZnF domains were also perturbed by Kapβ2 addition in these experiments. Thus, the cross saturation transfer and line broadening data indicate that in the absence of high-affinity Kapβ2-PY-NLS binding, the RRM and ZnF domains can make weak direct contacts to regions of the Karyopherin outside of its PY-NLS binding site.
Analysis of the unfolded RGG regions of FUS(164–500) was complicated by the low sequence complexity of these elements, which produces severe overlap in 1H/15N correlation spectra. Of the 146 glycine residues in the three regions, only 30 distinct peaks could be observed in the glycine region of the spectra (105–111 15N ppm) (Figure 5A). The glycine resonances appear to be present but overlapped, rather than absent due to line broadening, based on spectra of fragments containing individual RGG elements (RGG1 alone, RGG2-ZnF and ZnF-RGG3). That is, there are many instances of peaks with identical chemical shifts appearing in spectra of different fragments, and the fragment spectra are largely subsets of the FUS(164–500) spectrum (Figure S5A–D and 5A-F). In cross saturation transfer experiments of 15N/2H-FUS(164–500) plus excess Kapβ2•M9M, only one of the 30 distinct glycine peaks, peak 8, decreased (Figure 5F). This peak could be assigned to RGG3 based on comparison to spectra of the fragments (Figure S5D).
Figure 5. Kapβ2 interacts with disordered RGG regions.
Attenuation of glycine resonances in 1H/15N HSQC NMR spectra of 15N-FUS(164–500) (A), RGG1 (B), RGG2-ZnF (C) and ZnF-RGG3 (D) upon addition of 2-fold molar excess of Kapβ2•M9M. E–F) Selected glycine amide resonances of RGG2 (E) and RGG3 (F, left) in 1H-15N HSQC spectra ± 2-fold molar excess Kapβ2•M9M. F) Right, selected glycine amide resonances of RGG3 in 1H-15N TROSY-based cross saturation transfer experiments in the presence of a 1.5-fold molar excess of Kapβ2•M9M with off- or on-resonance saturation. Cross saturation experiment was performed on 2H/15N-FUS(164–500) complexed with unlabeled Kapβ2•M9M in 1:1.5 molar ratio. See also Figure S5, Tables S2 and S3.
In line broadening experiments, several glycine peaks in FUS(164–500) decreased in intensity upon addition of excess Kapβ2 (Figure 5A). Comparison to the RGG fragments allowed some of these to be assigned to RGG2 and others to RGG3 (Figure 5A and S5C, D). In analogous experiments involving the RGG2-ZnF and ZnF-RGG3 fragments, these same peaks plus others broadened upon Kapβ2 addition (Figure 5C, D). In spectra of these fragments and of FUS(164–500), the chemical shifts of ZnF resonances are identical within experimental error to those of the isolated domain (Figure S5F), suggesting that there are no intramolecular contacts of the RGG regions with the ZnF domain (see Figure S5F legend for a more detailed discussion). Thus, the line broadening most likely represents direct Kapβ2 binding to RGG2 and RGG3. RGG1 may also make direct contacts, based on line broadening observed in spectra of isolated RGG1 plus Kapβ2•M9M (Figure 5B), although this is less certain since RGG1 resonances are severely overlapped in spectra of FUS(164–500). Consistent with binding of RGG regions, a number of unassigned resonances representing non-glycine residues in the unfolded region of the spectra of FUS(164–500) also broadened upon Kapβ2 addition (Figure S5G). We note that the most severely broadened glycine peak (#8) was the same as that affected in the cross saturation transfer experiment, showing consistency between the experiments (Figure 5F). Inefficient cross saturation transfer between Kapβ2 and the RGG2/3 regions probably results from a combination of low bound populations of individual Arg-(Gly)n or (Gly)n motifs and binding dynamics that are unfavorable to the experiment.
The combined NMR data show that Kapβ2 binds to the N-terminal LC region (Figure 3B) as well as large portions of the FUS C-terminal segment (Figure 4A–E and 5A–F), including the RGG2 and RGG3 regions, the RRM and ZnF domains.
Implications of Kapβ2 binding across FUS: SAXS analysis and RNA-binding
Small angle X-ray scattering (SAXS) analysis shows substantial compaction of full-length FUS upon binding Kapβ2. Five SAXS profiles (MBP, MBP-FUS, Kapβ2, Kapβ2•FUS and Kapβ2•MBP-FUS) were analyzed to calculate radius of gyration (_Rg_SAXS), maximum particle size (Dmax), and pair distribution function (P(r)) (Figure 6A) (Franke et al., 2017). According to the molecular weight estimation from SAXS analysis (Table S4), the polydispersity of MBP-FUS is highly concentration dependent. Thus, the parameters in Figure 6A were calculated from the merged SAXS profiles, where polydispersity of MBP-FUS (and other samples tested) is negligible (Kikhney and Svergun, 2015). To assess compactness of the SAXS samples, _Rg_Globular was estimated using a formula of 6.6*MW0.333 Å (for MW in kDa; (Erickson, 2009). MBP-FUS presents the largest values of _Rg_SAXS/_Rg_Globular, Dmax, and Dmax/_Rg_SAXS, suggesting that FUS is significantly expanded/extended in solution compared to globular proteins. In contrast, Kapβ2•FUS presents smaller values of _Rg_SAXS/_Rg_Globular, Dmax, and Dmax/_Rg_SAXS, suggesting that FUS becomes more compact upon binding Kapβ2. Consistently, the ab initio shapes computed from the experimental SAXS profiles (Figures 6A and S6A-E), as well as the pair distribution functions (Figure S6F) further support the compactness of FUS upon binding Kapβ2 (similar in buffers with 5% or 20% glycerol; Figure S6G).
Figure 6. Implications of Kapβ2 binding to multiple sites across FUS: SAXS analysis and RNA-binding.
A) SAXS profiles of MBP, MBP-FUS, Kapβ2, Kapβ2•FUS, and Kapβ2•MBP-FUS produced radius of gyration _Rg_SAXS, maximum particle size Dmax, and pair distribution function P(r). _Rg_Globular was estimated using the formula of 6.6*MW0.333 Å. Right, ab initio shapes of MBP-FUS and Kapβ2•FUS with the structures of MBP (PDBID 1Y4C), Kapβ2 (PDBID 2QMR), and the FUS PY-NLS (Figure S2F, G) coarsely fitted to the SAXS envelopes. See also Figure S6 and Table S4. B) Size exclusion chromatography (monitored by Abs280 nm, Abs260 nm and fluorescence emission at 520 nm (Em520 nm)) of 1 μM prD RNA alone and 1 μM prD + 3 μM MBP-FUS (left), and of 1 μM prD + 3 μM MBP-FUS+3.2 μM Kapβ2 (right). C) Size exclusion chromatography as in B) of 2 μM TERRA RNA alone and 2 μM TERRA + 3 μM MBP-FUS (left), and 2 μM TERRA + 3 μM MBP-FUS + 3.2 μM Kapβ2 (right). 5′ of the RNAs were labeled with 6-FAM fluorophore and proteins were visualized by Coomassie blue stained SDS/PAGE.
The RGG regions and ZnF and RRM domains were previously shown to bind RNA. The ZnF and RRM domains bind weakly to GGUG-containing RNA, with KD values in the micromolar range (Iko et al., 2004; Ozdilek et al., 2017). In contrast, the RGG regions bind RNA with KD values in the nanomolar range. We examined the effects of Kapβ2 on FUS binding to two RNAs, the 48-nucleotide prD (DNMT) RNA (KD ~0.7 μM; binds RGG1 and RGG3) and the 24-nucleotide telomeric repeat TERRA RNA (KD ~12 nM; binds RGG3) (Ozdilek et al., 2017; Takahama and Oyoshi, 2013). Figures 6B and C show MBP-FUS binding to fluorophore-labeled prD and TERRA, respectively. Addition of Kapβ2 to the MBP-FUS•RNA complexes caused efficient release of prD, but only partial release of the higher affinity TERRA, consistent with overlapping binding sites in the FUS RGG regions. Since RNA promotes aggregation and LLPS of FUS (Burke et al., 2015; Schwartz et al., 2013), our data also suggest that Kapβ2 may inhibit biological phase separation of FUS through blocking interactions with RNA.
Regions of FUS that bind Kapβ2 contribute to LLPS
We examined the temperature dependence of LLPS of full-length FUS and a series of deletion mutants to identify functionally important regions. At 8 μM, FUS phase separates at temperatures below 33°C (cloud point temperature, T cloud), as assessed by a sharp increase in turbidity when temperature is decreased slowly from 45°C (Figure 7A). Removal of the PY-NLS did not affect LLPS (FUS(1–500) Tcloud 33°C; Figure 7A), consistent with observations that the PY-NLS does not affect FUS aggregation (Ju et al., 2011; Sun et al., 2011).
Figure 7. Regions of FUS that bind Kapβ2 contribute to phase separation.
A) Temperature dependence of FUS phase separation. Turbidity (OD395nm) of 8 μM MBP-FUS proteins (wildtype (wt) and FUS mutants) after 3 h treatment with Tev protease was monitored as temperatures were decreased from 40°C or 45°C to 0°C or 5°C. Optical densities were normalized to values measured at 0°C or 5°C. T Cloud is the x-intercept of tangent at inflection point of the curve (mean of 3 technical replicates, ± S.D.). B) Left, turbidity of 8 μM MBP-FUS(1–500), in the presence of buffer or 4–64 μM Kapβ2•M9M, measured for 60 min at room temperature after treatment with Tev protease. Right, turbidity at time=60 min of experiments in the left panel, normalized to FUS turbidity in the presence of buffer (mean of 3 technical replicates, ± S.D.). See also Figure S7.
Our NMR data suggest that Kapβ2 contacts three segments of the FUS LC (Figure 3B and Figure S3B). Alanine mutation of the five tyrosines in these segments (Tyr38, Tyr 41, Tyr97, Tyr100 and Tyr149) in full-length FUS (FUS(Y5A)) substantially decreased Tcloud to 25°C (Figure 7A), consistent with the importance of tyrosine side chains in self-assembly of the FUS LC region (Kato et al., 2012; Lin et al., 2017), and in LLPS of disordered proteins in general (Banani et al., 2017; Brangwynne et al., 2015).
Deletion of the RRM or ZnF domain (FUS(ΔRRM) or FUS(ΔZnF)) does not decrease the ability of FUS to phase-separate. The Tcloud of 33.6°C for FUS(ΔZnF) is similar to that of wildtype FUS, while the Tcloud of 38°C for FUS(ΔRRM) suggests an enhancement in phase separation (Figure 7A). As described in Figures S7A-B, S5A, B, E and 5A, enhancement of LLPS by RRM deletion appears to derive from loss of inhibitory intramolecular interactions between the domain and RGG regions (see below).
We made several mutants to perturb the FUS RGG regions. Mutating all arginine residues in RGG2 and RGG3 of full-length FUS to lysines (FUS(RtoK) decreased Tcloud to 23.5°C (Figure 7A) suggesting stereospecific roles of arginine side chains in promoting LLPS. The FUS(1–452) truncation mutant has a similarly low Tcloud of 22.5°C indicating the importance of RGG3 in LLPS (Figure 7A). The last mutant, FUS(1–370), lacks both RGG2 and RGG3 and shows a drastic decrease in its ability to phase separate (Tcloud of 8°C).
At 2 μM, wildtype FUS and the mutants showed the same trends in LLPS as at 8 μM, but Tcloud was uniformly decreased as expected from theory (Figure S7C). We also observed the same patterns in temperature-dependent analyses of LLPS by fluorescence microscopy, with FUS(ΔRRM) > FUS wildtype > FUS(1–452) in their propensity to phase separate (Figure S7D). Together, these studies show that the LC and RGGs regions are the main determinants of FUS LLPS.
As detailed below, our combined data lead to a model in which FUS is anchored to Kapβ2 through high-affinity interactions of the PY-NLS. This enables distributed weak interactions to disrupt FUS self-association and phase separation. A prediction of this model is that even in the absence of the Kapβ2-PY-NLS interactions, high concentrations of the Karyopherin should disrupt FUS phase separation. Consistent with this prediction, we found that very high concentrations (64 μM) of Kapβ2•M9M are able to inhibit phase separation of FUS(1–500), which lacks the PY-NLS (Figure 7B).
Discussion
Kapβ2 is the dominant nuclear transport factor that traffics FUS into the nucleus (Dormann and Haass, 2011). This activity is based on high-affinity, RanGTP-sensitive binding of Kapβ2 to the PY-NLS of FUS (Lee et al., 2006; Zhang and Chook, 2012). Here we describe an additional biochemical consequence of this interaction - disruption of LLPS of FUS. Mechanistic studies show that in addition to the established high affinity binding of Kapβ2 to the FUS PY-NLS, regions outside of the PY-NLS binding pocket of the karyopherin make weak, distributed interactions with multiple regions of FUS. These regions include tyrosine-repeats in the LC region, the RGG elements and the folded RRM and zinc finger domains. Of these FUS elements, the LC and RGG regions contribute to LLPS. Thus, heterotypic Kapβ2-FUS interactions should compete with homotypic FUS-FUS interactions. Since the drive for phase separation is distributed across the FUS sequence, it seems logical that Kapβ2 binds in distributed fashion to block phase separation. Our data suggest a model where high-affinity and stable tethering of Kapβ2 to the FUS PY-NLS enables weak and dynamic interactions involving other regions of the two proteins, which block formation of higher-order FUS assemblies and phase separation.
Within the complex, it is possible that Kapβ2 engages all sites on FUS simultaneously. Alternatively the complex may be dynamic in nature, sampling different collection of contacts that rapidly interconvert, as observed in other IDP interactions, for example the binding of disordered cyclin-dependent kinase (CDK) inhibitor Sic1 to its receptor Cdc4 (Mittag et al., 2008). Based on structural and energetic considerations, we favor the latter model of a dynamic complex. The regions of Kapβ2 that bind the different FUS elements remain unknown. However, features of the karyopherin that are conserved among other β-Importin proteins suggest potential modes of interaction. First, Kapβ2 possesses a series of hydrophobic patches on the convex spine of its superhelical structure (Chook and Suel, 2011; Conti and Izaurralde, 2001) (Figure S7E). During nuclear import, these patches bind in dynamic fashion to the large arrays of FG repeats in the nuclear pore complex. These same regions could be repurposed to make analogous interactions with tyrosine repeats in the FUS LC region. In addition, Kapβ2 possess highly acidic surfaces and adjacent long acidic loops on the concave side of its superhelix. Portions of these acidic elements bind to the FUS PY-NLS, but parts remain solvent-exposed in the complex (Figure S7E), and could interact with the basic RGG regions of FUS. Interactions with these spatially distributed surfaces on Kapβ2 significantly constrain the FUS chain, as evidenced by our SAXS data showing compaction of the extended FUS upon binding the karyopherin.
In addition to FUS, Kapβ2 binds and imports many PY-NLS containing, RNA-binding proteins including EWS, TAF15, hnRNP A1 and hnRNP A2. Like FUS, these proteins contain folded RNA binding domains as well as LC and RGG regions, and are found in RNA granules (King et al., 2012). As shown in the companion paper by Shorter and colleagues, high-affinity binding of Kapβ2 to the PY-NLSs of these proteins prevents their self-association and likely phase separation (Guo et al, in press). Although the Kapβ2 cargos have different domain arrangements, in all cases individual elements are either disordered or connected by flexible linkers. Thus, Kapβ2 can probably contact the RGG and LC regions of the cargos when anchored to their PY-NLSs, disrupting self-association through a mechanism analogous to that of FUS.
In addition to Kapβ2, other β-Importin family members may also act to modulate phase separation of LC-containing RNA-binding proteins. We have shown here that two other Importins can inhibit LLPS by FUS when the protein is equipped with high-affinity recognition peptides. β-Importin family members share both the hydrophobic patches on the convex spine and the acid surfaces and loops on their concave side, which are likely important in disruption of LLPS by FUS (Chook and Suel, 2011; Conti and Izaurralde, 2001) (Figure S7E). These same elements could be used to disrupt LLPS by other RNA-binding proteins. While conceptually similar, this molecular mechanism is distinct from that proposed previously to account for the chaperone activity of Importins toward positively charged cargo proteins (Jakel et al., 2002). In contrast to Importins, Exportins such as CRM1 have very different charge distributions and spatial relationships between NES- and FG-binding sites (Dong et al., 2009; Fung and Chook, 2014; Port et al., 2015). Unlike the large contiguous negatively charged surfaces on the concave side of Importins, analogous surfaces of Exportins are basic (Figure S7E, F). Further, in contrast to NLSs, which bind the concave acidic surfaces of Importins, the NES binds in a hydrophobic groove that is located on the FG-repeats-binding convex surface of CRM1 (Figure S7E, F). Thus, conserved features enable Importins to disrupt LLPS of RNA binding proteins that possess appropriate NLSs, an activity that is likely not shared by Exportins resembling CRM1.
We can envision several potential mechanisms by which the ability of Kapβ2 to control FUS LLPS could be important in cell physiology. First, Kapβ2 may bind newly translated FUS and prevent it from phase separating in the cytoplasm while escorting it into the nucleus. Kapβ2-FUS interactions may also modulate cytoplasmic RNA granules, where FUS is localized upon heat shock or other cellular stresses (Dormann et al., 2010; Li et al., 2013; Patel et al., 2015). In this role, Kapβ2 may facilitate dissociation of FUS from RNA, controlling dynamics of the protein and/or its stoichiometry in the condensates. If FUS is important to granule stability, Kapβ2 could control granule formation and/or disassembly, as we observed here. Finally, by weakening intermolecular contacts, substoichiometric amounts of Kapβ2 could modulate the material properties of granules, likely affecting the chemistry that occurs within them (Banani et al., 2017); Guo et al and Hofweber et al in press). In conclusion, our data suggest an expanded role for β-Importins. Not only do they traffic proteins into the nucleus, they also may control the formation, composition and dynamics of biomolecular condensates.
STAR * METHODS
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Yuh Min Chook (yuhmin.chook@utsouthwestern.edu).
Experimental Method and Subject Details
All recombinant proteins were expressed in BL21 (DE3) E. coli cells growing in LB medium or M9 medium.
Method Details
Constructs, protein expression and purification
Kapβ2, Kapβ2Δloop (residues 337–367 replaced with a GGSGGSG linker), Impα, Impβ, Kap121 and CRM1 were expressed as GST-fusions, which were generated by inserting PCR fragments of the gene of interest (all Karyopherins, except the S. cerevisiae Kap121, are human proteins) into the pGEX-TEV plasmid, which is a pGEX4T3 vector (GE Healthcare, UK) modified to include a TEV cleavage site (Chook and Blobel, 1999). All FUS proteins were expressed from MBP-fusion constructs using the pMAL-TEV vector, which is a pMAL (New England BioLabs, Ipswich, MA) modified to contain a TEV cleavage site (Chook et al., 2002) or a pMAL-TEV vector modified to express His6-MBP instead of MBP (p6xHisMal-TEV). FUS mutations were made by site-directed mutagenesis using a Quik-Change Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA), and all constructs were sequenced. MBP-FUS(cNLS), MBP-FUS(IK-NLS) and MBP-FUS(NES) chimeras were made by inverse PCR method. FUS residues 501–526 were replaced with either SV40NLS (PKKKRKV), Pho4 residues 140–166 (140SANKVTKNKSNSSPYLNKRRGKPGPDS166) or the NES from the NS2 protein of the MVM virus (YSTVDEMTKKFGTLTIH), respectively. A MBP-FUS-SNAP construct was generated by cloning in a SNAP tag (New England BioLabs), preceded by a TGGGS linker, at the C-terminus of MBP-FUS (full-length).
All recombinant proteins were expressed individually in BL21 (DE3) E. coli cells (induced with 0.5 mM isopropyl-β-d-1-thiogalactoside (IPTG) for 12 hours at 25°C for Importins and at 18°C for FUS). Bacteria expressing Importins were lysed with the EmulsiFlex-C5 cell homogenizer (Avestin, Ottawa, Canada) in buffer containing 50 mM Tris pH 7.5, 200 mM NaCl, 20% (v/v) glycerol, 2 mM DTT, 1 mM EDTA, and protease inhibitors. To purify untagged Importins, GST-Importins were first purified by affinity chromatography using GSH sepharose beads (GE Healthcare, UK), eluted, cleaved with TEV protease, and further purified by ion-exchange and gel filtration chromatography in TB buffer (20 mM HEPES pH 7.4, 200 mM NaCl, 2 mM DTT, 2 mM Mg(OAC)2, 10% glycerol, 1 mM EGTA). For pull-down binding assays, affinity purified GST-Importins were eluted and then dialyzed against buffer containing 20 mM HEPES pH 7.4, 150 mM NaCl, 2 mM DTT and 10% glycerol.
Bacteria expressing MBP-FUS proteins for crystallization, turbidity, imaging and pull-down binding assays were lysed in 50 mM HEPES pH 7.4, 1.5 M NaCl, 10% glycerol, 2 mM DTT (high salt to disrupt association with nucleic acid). MBP-FUS proteins were purified by affinity chromatography using amylose resin (New England BioLabs, Ipswich, MA), eluted with buffer containing 20 mM HEPES pH 7.4, 150 mM NaCl, 2 mM DTT, 10% glycerol, and 20 mM maltose and either dialyzed extensively in final maltose-free buffers to remove maltose or further purified by ion-exchange chromatography. Purification of the MBP-FUS proteins always included either high salt or RNAse A treatment to eliminate RNA (purified proteins have A260/A280 ratios of 0.50–0.71), and the absence of EDTA to maintain the fold of its zinc finger domain. MBP-FUS proteins are also free of maltose since they are able to be immobilized on amylose resin.
RanGTP (GSP1 residues 1–179, Q71L) and M9M was purified as previously described (Cansizoglu et al., 2007; Fung et al., 2015). E. coli expressed His6-RanGTP was purified using affinity and cation exchange chromatography. Purified protein was concentrated and exchanged buffer into 20 mM HEPES pH 7.4, 100 mM sodium chloride, 4 mM magnesium acetate, 1 mM DTT, 10% Glycerol. cNLS, M9M and IK-NLS peptides were expressed as a GST-fusions, purified using GSH sepharose followed by cleavage of GST tag and further purified by gel filtration in buffer containing 20 mM HEPES pH 7.4, 150 mM NaCl, 2 mM DTT and 10% glycerol.
Turbidity Assays
FUS turbidity analysis at room temperature
Importins and its NLSs, M9M or buffer are mixed at room temperature for 30 minutes prior to turbidity assays. 8 μM MBP-FUS and buffer, 8 μM Importins (Importins, Importin•NLS or Kapβ2•M9M) ± 8 μM RanGTP were mixed in buffer containing 20 mM HEPES pH 7.4, 150 mM NaCl, 2 mM DTT, 2 mM Mg(OAC)2, 10% glycerol to reaction volumes of 100 μL. TEV was added at time= 0 min to final concentration of 25 μg/mL. Absorbance at 395 nm (OD395nm) was monitored at room temperature using Variskan plate reader (Thermo Fisher Scientific, Inc.).
Temperature dependent FUS turbidity analysis
Prior to tracking turbidity, 8 μM MBP-FUS proteins (in buffer containing 20 mM HEPES pH 7.4, 150 mM NaCl, 2 mM DTT, 2 mM magnesium acetate, 10% glycerol) were treated with Tev protease (final concentration 25 ug/mL Tev) in reaction volumes of 500 uL for 3 hours at room temperature, and then placed in a cuvette. OD395nm was measured using a Cary 100 UV-Visible spectrophotometer equipped with a Peltier thermal controller (Agilent Technologies, Australia). FUS reaction mixtures in cuvettes were held at 45°C or 40°C for 10 min then cooled gradually at a rate of −0.5°C/min. OD 395nm of FUS proteins were monitored every 0.5°C. T Cloud is the x-intercept of tangent at inflection point of the curve (mean of 3 technical replicates, ± S.D.).
Monitoring interactions between Importins and FUS
In vitro pull-down binding assays were performed using GST-Kapβ2, GST-Impα, GST-Impα/β or GST-Kap121 immobilized on GSH Sepharose beads. ~ 4 μM GST-Importins were immobilized on beads. 30 μL of GST-Importins beads are incubated with 8 μM MBP-FUS proteins (total 80 μg) for 30 min at room temperature and washed three times with buffer containing 20 mM HEPES pH7.4, 150 mM NaCl, 10% glycerol, 2 mM Mg(OAC)2 and 2 mM DTT. Bound proteins were separated by SDS-PAGE and stained with Coomassie blue.
Gel filtration chromatography to assess complex formation was performed using a Superdex 200 10/300 GL column (GE Healthcare). 500 μL of protein samples were loaded onto the column and eluted with buffer containing 20 mM HEPES pH7.4, 150 mM NaCl, 10% glycerol, 2 mM Mg(OAC)2 and 2 mM DTT. Eluted fractions were visualized by SDS-PAGE/Coomassie Blue.
Binding affinities of MBP-FUS proteins to Kapβ2 were measured using isothermal titration calorimetry (ITC). ITC experiments were performed with a Malvern iTC200 calorimeter (Malvern Instruments, Worcestershire, UK). Proteins were dialyzed overnight against buffer containing 20 mM HEPES pH 7.4, 150 mM NaCl, 10% glycerol, and 2 mM β-mercaptoethanol. 50–100 μM MBP-FUS proteins were titrated into the sample cell containing 5–10 μM recombinant Kapβ2. ITC experiments were performed at 20°C with 19 rounds of 2 μL injections. Data were integrated using NITPIC (Scheuermann and Brautigam, 2015), globally fitted using SEDPHAT (Brautigam et al., 2016; Zhao et al., 2015), and plotted with GUSSI (Brautigam, 2015). Confidence intervals for reported KDs were calculated with projection method at 68.3% confidence level in SEDPHAT.
Monitoring the effects of Kapβ2 on FUS-RNA interactions
prD (5′-AUUGAGGAGCAGCAGAGAAGUUGGAGUGAAGGCAGAGAGGGGUUAAGG-3′, 48-mer) and TERRA (5′-UUAGGGUUAGGGUUAGGGUUAGGG-3′, 24-mer) were chemically synthesized (Integrated DNA Technologies, Coralville, IA). Both RNAs were 5′-end labeled with 6-FAM (Fluorescein). prD (in buffer containing 20 mM Hepes pH 7.4, 150 mM KCl, 2 mM DTT, 2 mM magnesium acetate, 10% glycerol) was heated at 95°C for 5 min and snap-cooled on ice for 10 min. TERRA in the same buffer was heated at 95°C for 5 min and cooled down to 4°C at a rate of 1°C/min for annealing on T100 thermal cycler (Bio-Rad, Hercules, CA). RNA, MBP-FUS and Kapβ2 were mixed at room temperature for at least 10 min prior to gel filtration chromatography. 1 μM prD or 2 μM TERRA ± 3 μM MBP-FUS ± 3.2 μM Kapβ2 were mixed in buffer containing 20 mM HEPES pH 7.4, 150 mM KCl, 2 mM magnesium acetate, 10% glycerol and 2 mM DTT. Gel filtration chromatography with a Superdex 200 10/300 GL column (GE Healthcare) was used to assess complex formation. 100 μL of proteins ± RNA samples were loaded onto the column and eluted with buffer containing 20 mM HEPES pH7.4, 150 mM KCl, 10% glycerol, 2 mM magnesium acetate and 2 mM DTT. Protein(s) in eluted fractions (500 μL each) were visualized by SDS-PAGE/Coomassie Blue. The RNA in each fraction was tracked by monitoring fluorescence emission at 520 nm from the 6-FAM tag (excited at 495 nm) using Varioskan plate reader (Thermo Fisher Scientific, Inc.).
Imaging of turbid FUS solution
For imaging experiments, purified MBP-FUS-SNAP was labeled with SNAP-Surface 649 fluorophore (New England BioLabs) by incubating with 5-fold excess fluorophore for 2 hours at room temperature. Unreacted fluorophore was removed by dialysis. 5 μM MBP-FUS, 0.5 μM MBP-FUS-SNAPSNAP-Surface 649 and either buffer or 10 μM Kapβ2 ± 15 μM M9M or 15 μM RanGTP were mixed at room temperature in total volumes of 100 μL in individual wells of CultureWell non-removable chamber cover glass (Grace Bio-Labs). All wells were made up to 100 μL with buffer containing 10 mM HEPES pH7.4, 150 mM NaCl, 2 mM Mg(OAC)2, 2 mM DTT and 10% glycerol. Tev protease was added to final concentration of 1.5 μM at time= 0 hr. Wells containing protein mixtures were imaged by spinning disk confocal microscopy beginning at time = 1 hr. Spinning disk confocal microscopy was executed using a Yokogawa CSU-X scanhead (Solamere Technology Group) mounted on an ASI Rapid Automated Modular Microscope system equipped with motorized XT stage and piezo z-motor in the stage (ASI), an Andor iXon Ultra 897 camera (Andor), and laser illumination using a VersaLase laser system equipped with 405, 488, 561, and 640 nm laser (Vortran Laser Technology). A multi-bandpass dichroic mirror in the Yokogawa scanhead was combined with dye specific emission filters (Chroma Technology Corp.) in a Finger Lakes Instrument filter wheel (Finger Lakes Instrument). Nikon 60x 1.4na oil immersion objective lens (Nikon) was used. The microscope system was operated using the Micro-Manager software package (http://micro-manager.org).
Polarized light microscopy was performed with the LC-PolScope, employing a liquid crystal based universal compensator to generate retardance maps that are independent of the orientation of the slow axis of birefringence (Oldenbourg, 1991; Oldenbourg and Mei, 1995). The instrument was implemented on an inverted microscope stand (Nikon Eclipse Ti-E), equipped with 60x/1.4 NA objective and condenser lens of matching NA, 546/12 nm interference filter, liquid crystal device, polarization components, and processing software as described and available from OpenPolScope.org.
To perform temperature-dependent FUS studies by fluorescence microscopy, mixtures of 2 μM MBP-FUS wt (MBP-FUS(1–452), or MBP-FUS(ΔRRM)), 20 nM FUS-GFP (gift from Avinash Patel and Tony Hyman) and Tev protease were loaded onto a CherryTemp™ heater/cooler stage (Cherry BioTech). The FUS mixtures were treated with Tev protease at room temperature for 50 min to form phase-separated FUS droplets. The phase separated mixtures were cooled using the CherryTemp™ temperature controller to either 10°C or 15°C, held at those temperatures for 2 min, and then increased by 2°C increments to a maximum temperature of 43°C or 44°C. The sample was held at each temperature for 2–3 min prior to acquisition of a 50 μm Z-stack (1 μm increments) using spinning disk confocal microscopy. Maximum projection images from the Z-stack 10–50 μm above the slide surface were generated. To calculate Tcloud, images in this same portion of the Z-stack were segmented using the Triangle algorithm in ImageJ and the total number of FUS droplets was determined after a filter for circularity (>0.5) and size (>0.5 μm2). Tcloud was determined from the x-intercept of a line fit to the first six (wt and FUS(1–452)) or eight (FUS(ΔRRM)) points in the (number of puncta) vs. temperature curve.
Dynamic light scattering analysis
Dynamic light scattering (DLS) was performed to examine polydispersity of MBP-FUS alone and the MBP-FUS•Kapβ2 complex. We used a DynPro DLS instrument (Wyatt Technology). Samples of 12 μM MBP-FUS ± 12 μM Kapβ2 in buffer containing 10 mM HEPES pH7.4, 150 mM NaCl, 10% glycerol and 2 mM 2-mercaptoethanol were loaded into the cuvette. Scattered light intensity at 25°C was analyzed using the software SEDPHAT.
To investigate whether Kapβ2 has attractive or repulsive self-interactions, we determined its diffusion coefficient at different protein concentrations, also using DLS. Prior to the experiment, Kapβ2 (in buffer containing 150 mM NaCl, 20 mM HEPES pH 7.5, 2 mM Mg(OAc)2, 2 mM DTT, 10% glycerol) was centrifuged at 16,000 × g for 10 min and filtered through an ultrafree-MC GV centrifugal filter with a 0.22 μm pore size (EMD Millipore). Measurements were performed at 25 degree on a DynaPro DLS instrument (Wyatt Technology). The scattering intensities were averaged over twenty runs, each with a 20-second acquisition time. The diffusion coefficients were analyzed using Dynamics software (Wyatt Technology). Molecules with attractive interactions form larger species that diffuse more slowly as concentration increases; conversely, molecules with repulsive interactions do not self-associate, and diffuse more rapidly at higher concentrations interactions. The concentration dependence of diffusion coefficient (D) can be described approximately by D= D0 (1_+κDc_), where D0 is the diffusion coefficient at infinite dilution, c is the protein concentration and κD is the diffusion interaction parameter. A positive κD suggests net repulsive interactions and a negative κD indicates net attractive interactions (Connolly et al., 2012).
NMR analysis of FUS LC with Kapβ2
15N-FUS LC (residues 1–163) was expressed by growing E. coli BL21 Star (DE3) in M9 minimal medium with 15NH4Cl. 15N-FUS LC was purified from inclusion bodies by resolubilizing in buffer containing 8M urea followed by HisTrap affinity chromatography and cleavage with TEV protease. The eluted protein was exchanged and concentrated into 20 mM CAPS pH 11.0 (no denaturant). To make samples for NMR, concentrated FUS LC was diluted into 20 mM MES/Bis-Tris (pH 6.6), 150 mM NaCl, 2 mM DTT, 10% glycerol and 0.01% NaN3, followed by addition of 10% v/v D2O. Samples with FUS LC and Kapβ2 variants were made identically, with Kapβ2 present in the MES/Bis-Tris buffer before addition of FUS LC. Independent samples were made for each Kapβ2 concentration.
NMR data for 15N-labeled FUS LC were acquired at 10°C and 25°C on a Bruker 850 MHz spectrometer equipped with 5 mm cryogenically cooled triple- resonance pulsed field gradient (TCI) probe. Two-dimensional (2D) 1H-15N HSQC spectra were collected with spectral widths of 8928.6 Hz and 1723.5 Hz with 1536 and 256 complex data pairs in the 1H and 15N dimensions, respectively. An inter-scan delay of 1s was employed between successive transients. States-TPPI was employed for frequency discrimination in the indirectly detected dimension. Reference HSQC spectrum (without Kapβ2) was acquired on 15N-labeled FUS LC (75 μM). To probe interactions between FUS LC and Kapβ2 or Kapβ2-FUS PY-NLS, 100 μM of unlabeled Kapβ2 or Kapβ2 bound to FUS PY-NLS (purified by size exclusion in presence of excess FUS PY-NLS and then exchanged by centrifugal filtration into NMR buffer) was added to the 15N-labeled FUS LC in the stated ratios. Importantly, titrations were performed by generating a series of independent matched samples. All spectra were recorded in 5 mm NMR tubes and sample volume was always maintained to 500 μl in 90%H2O/10%D2O.
NMR analysis of FUS(164–526), FUS(164–500) and smaller FUS fragments with Kapβ2
Preparation of 15N-labeled FUS(164–526), FUS(164–500) and RGG2-ZnF
15N-labeled His6-MBP-FUS(164–526), MBP-FUS(164–500) and MBP-RGG2-ZnF (FUS residues 371–452) were expressed by growing BL21(DE3) cells harboring the respective plasmids in M9 minimal medium (Muchmore et al., 1989) with 15NH4Cl as a sole source of nitrogen. Protein expression was induced with 1 mM IPTG, at 16 °C for 18 hours. Harvested cells were lyzed in buffer containing 20 mM Na-Phosphate buffer (pH 6.5), 1.5 M NaCl, 1 mM PMSF, 5 mM 2-mercaptoethanol (BME), 1μg/ml Leupeptin, 1 mM Benzamidine 1 μg/ml Antipain, and 10% Glycerol using EmulsiFlex-C5 (Avestin, Ottawa, Canada). 15N-MBP-FUS(164–500) was purified by affinity chromatography using Amylose beads (washed extensively, first with buffer containing 20 mM Na-Phosphate pH 6.5, 1.5 M NaCl, 5 mM BME and then with the same buffer containing 1 M NaCl). On-bead TEV reaction was performed to remove MBP tag, and cleaved 15N-FUS(164–500) was collected as flow-through and was dialyzed against buffer containing 20 mM HEPES (pH 7.4), 150 mM NaCl, 2 mM BME. The protein was further purified by cation exchange chromatography (Source 15S, GE healthcare life sciences). Fractions of clean FUS(164–500) were pooled and concentrated to 10 ml. NaCl concentration in the FUS(164–500) solution was then raised to 1M followed by gel filtration chromatography (Superdex 200 HiLoad 26/600) in buffer containing 20 mM HEPES (pH 7.4), 1 M NaCl, 2 mM BME. 15N-labeled FUS(164–500) elutes as a peak corresponding to monomeric molecular weight. Finally, 15N-labeled FUS(164–500) was dialyzed against NMR buffer containing 20 mM Bis-Tris/MES (pH 6.5), 150 mM NaCl, 2 mM DTT, 10% glycerol, 1 mM NaN3. 15N-MBP-RGG2-ZnF was purified similarly, except the gel filtration step was omitted. 15N-FUS(164–526) was purified as 15N-FUS(164–500) except the former was purified using Ni-NTA instead of amylose affinity chromatography.
Preparation of 2H/15N/12C-labeled FUS(164–500)
Deuterated 15N-labeled MBP-FUS(164–500) was expressed by growing BL21(DE3) cells harboring plasmids for MBP-FUS(164–500) in modified M9+ medium (Cai et al 2016) in 100 % D2O (99% 2H2O, Cambridge isotope limited, inc) with 15NH4Cl and 12C glucose-d6 (Cambridge isotope limited, inc) as sole source of nitrogen and carbon, respectively. The starter culture was prepared by inoculating 1 ml of LB with a single colony of freshly transformed BL21 cells. Cells were allowed to grow at 37 °C till OD600 reached 0.8 (~3 hrs). 400 μl of the previous culture was used to inoculate 10 ml of LB prepared in 100 % D2O and subsequently 10 ml of LB/D2O (OD600 0.8) culture was used to inoculate 100 ml of medium M9+ medium. Cells were grown at 37 °C until OD600 reached ~4, and the whole culture was used to inoculate 1000 ml of M9+/D2O and grown at 37 °C until OD600 reached 3.0. Protein expression was then induced with 0.8 mM IPTG, at 25°C for 30 hours. Harvested cells were lyzed and the protein purified as described for 15N-labeled FUS(164–500) preparation except that final NMR buffer was 20 mM phosphate buffer (pH 6.5), 150 mM NaCl 2mM DTT, 10% glycerol and 1 mM NaN3.
Preparation of 15N-labeled RGG1, RGG1-RRM, RRM, ZnF and ZnF-RGG3
15N-labeled His6-MBP-RGG1 (FUS residues 164–267), His6-MBP-RGG1-RRM (FUS residues 164–370), His6-MBP-RRM (FUS residues 285–371), His6-MBP-ZnF (FUS residues 415–460) and His6-MBP-ZnF-RGG3 (FUS residues 421–500) proteins were expressed using the same protocol as protonated 15N-labeled MBP-FUS(164–500). Cells were lysed in buffer containing 50 mM Tris pH 8.0, 10 mM Imidazole pH 7.5, 5 mM BME, 1 M NaCl, 1mM PMSF, 10% Glycerol, 1μg/ml Leupeptin, 1 mM Benzamidine, 1μg/ml Antipain. 15N-labeled His6-MBP-RGG1 and His6-MBP-ZnF-RGG3 proteins were first purified on Ni-NTA beads (washed extensively buffer containing 50 mM Tris pH 8.0, 10 mM Imidazole pH7.5, 1 mM BME, 1.5 M NaCl, followed by second wash with buffer containing 50 mM TRIS pH 8.0, 25 mM Imidazole pH 7.5, 1 mM BME and 200 mM NaCl). Bound material was eluted with buffer containing 50 mM Tris pH 8.0, 500 mM Imidazole pH 7.5) 1 mM BME, 200 mM NaCl, and TEV was added to eluted materials for cleavage at 4 °C. Cleaved 15N-labeled FUS fragments were further purified by cation exchange chromatography (Source 15S, GE healthcare life sciences), and pure proteins were then dialyzed against NMR buffer 20 mM Bis-Tris/MES pH 6.5, 150 mM NaCl, 2 mM DTT, 10% glycerol and 1 mM NaN3. 15N-labeled RGG1-RRM was purified similarly except an additional final gel filtration (SD 75) step. His6-MBP-RRM and His6-MBP-ZnF were purified similarly except gel filtration (SD 75 or SD peptide 10/300, respectively) was substituted for cation exchange chromatography.
Cross saturation transfer experiment
In order to identify the interfaces between FUS(164–500) and Kapβ2•M9M, cross-saturation experiment (Takahashi et al., 2000) was performed on an Agilent 800 MHz spectrometer equipped with a 5 mm cryogenically cooled triple-resonance pulsed field gradient (TRPFG) probe. Perdeuterated 2H/15N-labeled FUS(164–500) (28 μM) was mixed with 42 μM of unlabeled Kapβ2•M9M in 90% H2O/D2O. Cross saturation of 2H/15N FUS was achieved by saturating aliphatic protons of Kapβ2•M9M for 1.5 s. A train of CHIRP adiabatic pulses with RF amplitude of 125 Hz, excitation centered at 2 ppm, which provided a 2400 Hz irradiation bandwidth, was used for saturation, followed by acquisition of 1H-15N TROSY (Pervushin et al., 1997) HSQC with an intertransient delay 2 s. A reference spectrum was acquired with the same experimental setup, except that center of CHIRP pulse train was shifted to ~50000 Hz off resonance (no saturation). Intensity of cross-peaks in irridiated (I, with saturation) and reference (I0, no saturation) spectra were calculated by using nmrPipe (Delaglio et al., 1995) and Analysis module in CCPN (Vranken et al., 2005).
Line broadening experiments
NMR data for 15N-labeled FUS(164–500) were acquired at 25°C on an Agilent 600 MHz spectrometer equipped with a 5 mm cryogenically cooled triple- resonance pulsed field gradient (TRPFG) probe. The sample temperature was maintained at 25 °C during all experiments. Two-dimensional (2D) 1H-15N HSQC spectra were collected with spectral widths of 8000 Hz and 1920 Hz and acquisition times of 64 ms and 67 ms in the 1H and 15N dimensions, respectively. An inter-scan delay of 1s was employed between successive transients. Rance-Kay mode of quadrature detection was employed for frequency discrimination in the indirectly detected dimension. Reference HSQC spectrum (without Kapβ2•FUS PY-NLS or Kapβ2•M9M) was acquired for 17 μM 15N-labeled FUS(164–500). To probe interactions between FUS(164–500) and Kapβ2, 17 μM 15N-FUS(164–500) was titrated with varying concentrations of unlabeled Kapβ2•FUS PY-NYLS (FUS(164–500):Kapβ2•FUS PY-NLS in molar ratios of 1:0.5, 1:1 and 1:2) and with unlabeled Kapβ2•M9M (FUS(164–500):Kapβ2•M9M molar ratio of 1:3). All spectra were recorded in a 5 mm NMR tubes and samples always maintained to 300 μl in 90%H2O/10%D2O. Each titration experiment was performed on a freshly prepared sample to avoid a dilution effect upon addition of the Kapβ2 complex.
NMR data for 15N-FUS(164–526) were acquired similarly. To probe the interaction between FUS(164–526) and Kapβ2, 1H-15N spectra of 15 μM 15N-FUS(164–526) were acquired in presence of 15 μM Kapβ2. A reference spectrum was acquired with 15 μM 15N FUS (164–526) alone.
Interaction between FUS fragments and Kapβ2•M9M
In order to probe interaction between FUS fragments (RGG1, RGG1-RRM, RGG2-ZnF, ZnF-RGG3) and Kapβ2•M9M, different fragments (50 μM of each fragment) were titrated with Kapβ2•M9M (100 μM) in a 5 mm shigemi NMR tube. 2D 1H-15N HSQC spectra were acquired on an Agilent 600 MHz spectrometer equipped with a 5 mm cryogenically cooled triple- resonance pulsed field gradient (TRPFG) probe. All data were acquired at 25 °C in 90% H2O/10% D2O. Spectra were collected with sweep widths 8000 Hz and 1920 Hz and acquisition times of 64 ms and 67 ms in the 1H and 15N dimensions, respectively. An inter-transient delay of 1s was employed between successive transients. Rance-Kay mode of quadrature detection was employed for frequency discrimination in the indirectly detected dimension. A reference experiment was acquired for each fragment (at 50 uM concentration) without Kapβ2•M9M, with the same experimental setup. Cross-peak intensities in absence (I0) and presence (I) of Kapβ2•M9M were measured by using nmrPipe and Analysis module in CCPN.
All NMR data were processed using NMRPipe/NMRDraw processing software. Directly and indirectly detected time domain data were processed by applying a 90° phase-shifted squared sine bell or sine bell, respectively. Zero-filling was employed prior to Fourier transformation. Processed data were analyzed using the ipap.com script distributed with nmrPipe. The intensity of a resonance in the control experiment was taken as the reference intensity (I0). The decrease in intensity (I) due to the line broadening and/or chemical exchange contribution to the relaxation, arising from interaction between 15N-labeled FUS and Kapβ2 complex was determined by measuring peak volume/height. A ratio of I/I0 as a function of residue number/peak number was plotted to assess the interacting residues on FUS.
Homology modeling was used to construct a model of the FUS zinc finger. A sequence similarity search of FUS zinc finger against sequences in the PDB performed on using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins) identified PDBID 2K1P as the closest homolog (50% sequence identity). The homology model of the FUS zinc finger was built using SWISS-MODEL(Guex et al., 2009).
Small-angle X-ray scattering (SAXS)
SAXS experiments of MBP, MBP-FUS, Kapβ2, Kapβ2•FUS, and Kapβ2•MBP-FUS were carried out at Beamline 4–2 of the Stanford Synchrotron Radiation Lightsource (SSRL) in the SLAC National Accelerator Laboratory. At SSRL, the beam energy and current were 11 keV and 500 mA, respectively. A silver behenate sample was used to calibrate the q-range and detector distance. Data collection was controlled with Blu-Ice (Kim et al., 2014). We used an automatic sample delivery system equipped with a 1.5 mm-diameter thin-wall quartz capillary within which a sample aliquot was oscillated in the X-ray beam to minimize radiation damage (Kim et al., 2014). The sample was placed at 1.7 m from a MX225-HE (Rayonix, USA) CCD detector with a binned pixel size of 292 by 292 μm.
The SAXS profiles were collected at concentrations ranging from 0.5 either to 19.2 (Kapβ2) or up to 5.0 (all others) mg/mL. All protein samples were expressed and purified (as described above in Methods) in the protein storage buffers (10 mM HEPES pH 7.4, 150 mM NaCl, 20% glycerol, 2 mM Mg(OAc)2, and 2 mM DTT for Kapβ2, 10 mM HEPES pH 7.4, 150 mM NaCl, 20% glycerol, 2 mM DTT, and protease inhibitors for Kapβ2-FUS, and 50 mM HEPES pH 7.4, 500 mM NaCl, 20% glycerol, 2 mM DTT, and protease inhibitors for all others). The 20% glycerol in the protein storage buffer protects the protein samples from radiation damage during X-ray exposure (Kim et al., 2014). To assess the potential effects of high glycerol concentration on the solution behavior of the proteins, we also collected SAXS profiles for all samples in buffer with 5% glycerol. No significant change in behavior was observed (Figure S7G); thus glycerol does not affect protein compaction in its low concentration ranging 5 to 20%. All solutions were filtered through 0.1 μm membranes (Millipore) to remove any aggregates. Up to 20 one-second exposures were used for each sample and buffer maintained at 15°C. Each of the resulting diffraction images was scaled using the transmitted beam intensity, azimuthally integrated by SASTool (http://ssrl.slac.stanford.edu/~saxs/analysis/sastool.htm) and averaged to obtain fully processed data in the form of intensity versus q [q=4πsin(θ)/λ, θ=one-half of the scattering angle; λ=X-ray wavelength]. The buffer SAXS profile was subtracted from a protein SAXS profile. Subsequently, the mean of the lower concentration (0.5 – 1.0 mg/mL) profiles in the smaller scattering angle region (q < 0.15 Å−1) and the mean of the higher concentration (1.5 – 5.0 or higher mg/mL) profiles in the wider scattering angle region (q > 0.12 Å−1) were merged to obtain the final experimental SAXS profiles that are free of the concentration-dependent aggregation or polydispersity effect (Kikhney and Svergun, 2015).
The merged SAXS profiles were initially analyzed using the ATSAS package (Franke et al., 2017) to calculate radius of gyration (_Rg_SAXS), maximum particle size (Dmax), and pair distribution function (P(r)) (Figure 6E, Figure S6, and Table S4). The molecular weight (MWSAXS) of each SAXS sample was estimated using SAXS MOW (Fischer et al., 2010) with a threshold of qmax = 0.2 – 0.3 Å−1 (Table S4). The ab initio shape of the corresponding protein (Figure S7; transparent envelope) was computed from the experimental SAXS profile by running DAMMIF 20 times, and then refined through additional 50 DAMMIN runs followed by superposition and averaging with DAMAVER (Franke et al., 2017).
X-ray crystallography of Kapβ2•FUS complexes
To assemble and purify Kapβ2•FUS complexes for crystallization, bacteria expressing GST-Kapβ2Δloop and MBP-FUS were mixed and lysed together. Kapβ2•FUS complex was purified by tandem affinity chromatography using GSH sepharose beads and amylose resin, cleaved with TEV protease, and purified by gel filtration chromatography in buffer containing 20 mM HEPES, pH 7.4, 110 mM potassium acetate, 2 mM DTT, 2 mM magnesium acetate, 1 mM EGTA and 20% glycerol. Kapβ2•FUS complexes were concentrated to 10 mg/mL for crystallization.
All Kapβ2•FUS crystals were obtained by hanging drop vapor diffusion at 20°C (1.0 μL protein + 1.0 μL reservoir solution) with reservoir solution of 0.8 M Succinic acid pH 7.0. Crystals were cryo-protected by addition of ~25% glycerol, and flash-cooled by immersion in liquid nitrogen. 0.9795Å wavelength X-ray diffraction data were collected at the Advance Photon Source 19ID beamline in the Structural Biology Center at Argonne National Laboratory. Diffraction data was indexed, integrated, and scaled using HKL3000 (Minor et al., 2006). The structure was determined by molecular replacement using PHASER with a search model of human Kapβ2 (Chain A from PDB ID 4FDD) (Cansizoglu and Chook, 2007). Several rounds of refinement using PHENIX and manual model building with Coot were performed (Adams et al., 2010; Emsley et al., 2010). X-triage analysis of the dataset for KapB2•full-length FUS indicated pseudo-merohedral twinning (Adams et al., 2010). Therefore the data was refined in phenix.refine with twin law I,-k,h, and twin fraction was refined to 36% (Afonine et al., 2012). FUS residues were built into the electron density maps at the last stages of the refinement. Final models of Kapβ2•FUS complexes show excellent stereochemical parameters based on Molprobity suite in PHENIX (Chen et al., 2010). Illustrations were prepared with PyMOL (Schrodinger, 2015). Kicked OMIT maps are calculated with PHENIX by omitting FUS.
Quantification and Statistical Analysis
Turbidity analyses in Figures 1B–D, 2A–C, 5A–C and Supplemental Figure S1E were each repeated three times. Standard deviation error bars were obtained from three technical replicates.
X-ray diffraction data was indexed, integrated and scaled using software HKL3000. Completeness, _R_merge, I/σI and CC1/2 values were used to evaluate data. _R_work and _R_free were used to evaluate PHENIX-refined models, which were validated using the Molprobity suite in PHENIX.
ITC data in Figures S1B, S2B and S2C, collected in triplicates using the MicroCal iTC200 software. Individual thermograms were integrated and processed into binding isotherms using the NITPIC software. Analysis by NITPIC produces error of each titration, the incompentency of Kapβ2 and the binding isotherms. Triplicate isotherms are then populated into the SEDPHAT software for global fitting. A rigorous statistical analysis of the best fit is carried out using F-statistics. The triplicate datasets are then presented using GUSSI.
NMR analysis
Error in resonance intensity measurements reported in Figures 3, 4, 5, S3 and S5 were calculated by measuring signal-to-nosie ratio.
Data and Software Availability
The crystal structures of Kapβ2•FUS(full-length), Kapβ2•FUS(371–526) and Kapβ2•FUS(456–526) have been deposited in the Protein Data Bank under codes 5YVG, 5YVH and 5YVI.
Supplementary Material
1. Figure S1, related to Figure 1. Solution behavior of FUS complexes and microscopy of FUS LLPS.
(A) MBP-FUS is monomeric in size exclusion chromatography (left) but dynamic light scattering (DLS) analysis of 12 μM MBP-FUS shows polydispersity and presence of possibly very large MBP-FUS oligomers (right). (B) Dissociation constants (KDs) of Kapβ2 binding to MBP-FUS PY-NLS (upper) and MBP-full length FUS (lower) by isothermal titration calorimetry at 20°C. In the upper panel, 50 μM Kapβ2 was titrated into 4.5 or 6.5 μM MBP-FUS PYNLS (residues 475–526). In the lower panel, 50 μM Kapβ2 was titrated into 4.5 or 5 μM MBP-full length FUS. Thermograms, binding isotherms and data fit residuals are plotted, and KDs obtained from data integration using NITPIC (1) and global fitting of two independent ITC experiments using SEDPHAT (2), and are reported with 68.3% confidence interval in brackets. In the experiment of Kapβ2 binding to MBP-FUS PY-NLS, 2.3% of the MBP-FUS PY-NLS was incompetent. In the experiment of Kapβ2 binding to MBP-FUS, 8.6% of the MBP-FUS was incompetent. (C) A purified Kapβ2•FUS complex is heterodimeric in size exclusion chromatography (left). DLS (right) shows Kapβ2 drastically reducing the MBP-FUS polydispersity and the majority of the Kapβ2•MBP-FUS complex behaves as a single species. (D) Time course of Tev protease (added at time=0 min) cleaving MBP from MBP-FUS at room temperature. Proteins were visualized by Coomassie blue stained SDS/PAGE. (E) Turbidity (OD395nm) of 8 μM MBP-FUS proteins with either a TEV protease ceavage site or a PreScission protease cleavage site between MBP and FUS. Turbidity was measured for 60 min at room temperature after Tev or PreScission treatment. Cleavage MBP-FUS by the proteases was visualized by Coomassie blue stained SDS/PAGE. (F) Already turbid FUS (8 μM MBP-FUS treated with Tev for 60 min) were treated with either buffer, 8 μM Kapβ2 ± RanGTP or inhibitor M9M, or Kapβ2Δloop ± RanGTP at time=60 min. Turbidity measurements 20 min later, at time=80 min are shown (mean of 3 technical replicates, ± S.D.). (G) Mixtures containing 5 μM MBP-FUS, 0.5 μM MBP-FUS-SNAPSNAP-Surface 649 and either buffer or 10 μM Kapβ2 were treated with Tev protease (time=0 hr) and imaged with spinning disk confocal microscopy 1 hr after later. By time=1 hr, FUS has phase separated into liquid droplets that coalesce into large mats of phase separated protein by time=24 hr. Kapβ2 prevents FUS phase separation, but this effect is reversed if either 15 μM RanGTP or 15 μM Kapβ2 inhibitor M9M is added. *RanGTP or M9M was added to the FUS+Kapβ2 mixture at time= 1 hr and imaged either 1 hr later (time=2 hr) or at time=24 hr. Images were obtained with spinning disk confocal microscopy (561 nm laser illumination; 60x 1.4na oil immersion objective lenses). 20 μm length scale bars are shown on the bottom right of all images. (H) Polarized light microscopy of droplets (5 μM MBP-FUS+0.5 μM MBP-FUS-SNAPSNAP-Surface 649, 1–2 hr of Tev treatment). Retardance images were recorded with the LC-PolScope - white corresponds to 2.5 nm retardance. The edge of each droplet is decorated with a birefringent double layer, an optical effect that stems from the refractive index difference between droplet and surrounding medium. The droplets display higher refraction than the medium, probably due to a higher protein/nucleotide concentration. The right panel, an enlarged droplet shows the lack of detectable birefringence in the interior of the droplet. The inside of each droplet has the same level of anisotropy as the surrounding medium, both of which are caused by the shot noise (photon statistics noise) inherent in the recorded image intensities. There is no order that we can detect in the interior of the droplets.
10
Supplementary movie 1.avi., related to Figure 1. FUS droplets, 1 hour after Tev is added to 5 μM MBP-FUS.
11
12
13
14
2. Figure S2, related to Figure 2. Interactions of MBP-FUS chimeras with Importins, ITC and crystal structures of Kapβ2 bound to FUS.
A) Pull-down binding assays showing interactions between MBP-FUS wt or chimeras with GST-Kapβ2, GST-Impα/β, GST-Kap121 or GST-Crm1. B) Dissociation constants (KDs) measured at 20°C by ITC of Kap β2 binding to MBP-FUS(453–526) and MBP-FUS(371–526) (C). D) Overall structure of Kapβ2 (pink) in complex with FUS(456–526) (green). Kicked omit map (cyan mesh) within 10 Å of all Kapβ2 residues, contoured at 3.0σ, is overlaid on the structure. E) Overall structure of Kapβ2 (pink) in complex with FUS(371–526) (green). Kicked omit map (cyan mesh) within 10 Å of all Kapβ2 residues, contoured at 3.0σ, is overlaid on the structure. F) Crystals of the Kapβ2•full length FUS complex were harvested, washed in crystallization reservoir solution, dissolved in buffer and visualized by Coomassie Blue-stained SDS/PAGE. G) Stereo-images of the 4Å resolution structure of Kapβ2 (pink cartoon) bound to full length FUS where only residues 508–526 of FUS are modeled (blue ribbon with side chain sticks). Kicked omit map (light grey mesh) within 10 Å of all Kapβ2 residues, contoured at 2.5σ and 2.0σ levels are shown. There are no obvious densities for extensive FUS interaction with Kapβ2 in addition to the NLS. H) Superimposed structures of FUS from four complexes: Kapβ2-FUS full-length (blue), Kapβ2•FUS(371–526) (yellow), Kapβ2•FUS(456–526) (green) and Kapβ2•FUS(498–526) (red; PDBID 4FDD).
3. Figure S3, related to Figure 3. NMR analyses Kapβ2 binding to FUS LC.
A) Overlay of 2D 1H-15N spectra of 75 μM 15N-labeled FUS LC alone (blue) or with increasing concentrations of Kapβ2: 37.5 μM (0.5:1, black), 75 μM (1:1, red), 112.5 μM (1.5:1, green). Residues in several regions of FUS LC show chemical shifts and intensity attenuations (see Figure 5A) and three of the most affected regions are 37–41, 97–100, 149–154. B) NMR chemical shift deviations at 10 °C of 75 μM FUS LC resonances in the presence of 100 μM Kapβ2 (dark blue open bars) or 100 μM Kapβ2•FUS PY-NLS complex (light blue) compared to 75 μM FUS LC alone. NMR chemical shift deviations, 1H (top) and 15N (middle), and resonance intensity attenuation (bottom) are plotted. 1H and 15N resonances show very similar changes upon addition of Kapβ2 alone or FUS PY-NLS-bound Kapβ2. Chemical shift differences of 1H (top) and 15N (middle) resonance position as well as resonance intensity attenuation (bottom) support FUS LC binding weakly to Kapβ2 across the entire FUS LC domain. Three segments 37SYSGY41, 97YPGY100 and 149YSPPSG154 (grey bars mark positions of the 24 tyrosines) show larger resonance intensity attenuation (red asterisks) and large 15N and/or 1H chemical shift differences, suggesting stronger Kapβ2-binding to those elements.
4. Figure S4, related to Figure 4. NMR analyses of Kapβ2 binding to RRM and ZnF domains within FUS(164–500).
A) Overlay of 1H/15N HSQC NMR spectrum of 15N-FUS(164–526) alone (black) or with equimolar Kapβ2. B) Overlay of 1H/15N HSQC NMR spectrum of 15N-FUS(164–500) with simulated spectra of the FUS RRM and zinc finger domains based on reported chemical shifts of the isolated domains (Liu et al, 2013; Iko et al, 2004). C) Attenuation of assigned RRM domain resonances in 1H/15N HSQC NMR spectra of 15N-FUS(164–500) upon addition of 3-fold molar excess of Kapβ2•M9M (I=resonance intensity in the presence of Kapβ2•M9M; I0=resonance intensity of FUS alone). D) Ribbon (top) and surface representations (middle and bottom panels) of the FUS RRM (PDBID 1LCW, green), showing residues with I/I0 <0.5 upon binding Kapβ2 (magenta). Unassigned residues are white. E) Attenuation in intensity of assigned ZnF domain resonances in 1H/15N HSQC NMR spectra of 15N-FUS(164–500) upon addition of 3-fold molar excess of Kapβ2•FUS PY-NLS. F) Ribbon and surface representations of the homology model of the FUS ZnF domain generated from structure of zinc finger in ZNF265, PDBID 2K1P, orange. Residues with I/I0 <0.3 upon Kapβ2 binding are in magenta and unassigned residues are white. G) Selected resonances from ZnF (orange) and RRM (green). Asterisk indicate Kapβ2•M9M complex. All other columns are Kapβ2•FUS PY-NLS.
5. Figure S5, related to Figure 5. NMR analyses of Kapβ2 binding RGG1, RGG2 and RGG3 regions of FUS(164–500).
A) Glycine residues in FUS(164–500) were assigned to RGG1, RGG2 and RGG3 regions by overlaying 1H/15N spectra of FUS(164–500) and A) RGG1 containing FUS residues 164–267, B) RGG1-RRM containing FUS residues 164–370, C) RGG2-ZnF containing FUS residues 371–452, D) ZnF-RGG3 containing FUS residues 421–500. Glycine crosspeaks observed in spectra of only RGG1/RGG1-RRM or only RGG2 or only RGG3 could be assigned to that fragment, and are indicated in the spectra. E) Attenuation in intensity of glycine peaks in 1H/15N HSQC NMR spectra of 15N-FUS(RGG1-RRM) upon addition of 2-fold molar excess of Kapβ2•M9M. F) Chemical shift differences, Δ = [(Δ1H)2 + ((10*Δ15N))2]0.5, in ZnF resonances between the isolated domain (FUS residues 422–453), FUS(164–500) (grey bars), RGG2-ZnF (yellow bars) and ZnF-RGG3 (magenta bars). The ZnF chemical shifts were identical within experimental error in all constructs. Additionally, with the exception of only a few cross peaks likely at the termini of the constructs, the glycine chemical shifts of the RGG1, RGG2-ZnF and ZnF-RGG3 fragments were virtually exact subsets of those in FUS(164–500) (panels A-D). These data strongly suggest that the ZnF domain behaves independently in FUS(164–500) and does not make significant intramolecular contacts with surrounding regions. The similarity of chemical shifts across four different constructs indicates that if such intramolecular interactions are present, the self-interacting states must be populated to a small degree. Moreover, even in the case of multi-site exchange (involving for example all three RGG regions competing for a common site on the ZnF domain), where divergent chemical shifts might be averaged out, these differences should be revealed in spectra of the smaller fragments where competition for those sites would be eliminated. Yet the chemical shifts of all the fragments are identical within experimental error to each other and to those in FUS(164–500). Because of these data, we conclude that the ZnF makes few or no intramolecular contacts in FUS. G) Attenuation of unassigned non-glycine resonances (arbitrarily numbered; Table S2) in 1H/15N HSQC NMR spectra of 15N-FUS(164–500) upon addition of 3-fold molar excess of Kapβ2•M9M. Asterisks indicate resonances not in RRM or zinc finger domains based on reported chemical shift assignments as in Figure S4A.
6. Figure S6, related to Figure 6A. SAXS analysis of MBP, MBP-FUS, Kapβ2, Kapβ2•FUS, and Kapβ2•MBP-FUS.
A-E) SAXS profiles of MBP (A), MBP-FUS (B), Kapβ2 (C), Kapβ2•FUS (D), and Kapβ2•MBP-FUS (E) were used to compute radius of gyration (Rg), maximum particle size (Dmax), pair distribution function (P(r)), and ab initio shapes. (A-E, left panels) The experimental SAXS profile (blue dots with black error bars) is shown along with the extrapolation curve (red). The corresponding Kratky plot (used to depict the level of flexibility) is also shown in blue dots along with the extrapolation curve (red). A high plateau in the Kratky plot (q=0.15 – 0.2 Å−1) suggests some flexibility in MBP-FUS (B). (A-E, middle panels) The top plot shows the pair distribution function, P(r). The maximum particle size (Dmax) was determined as the maximum pair distance in the plot. The bottom plot shows the corresponding Guinier plot with the calculated Rg fit value in Å. The linearity of the Guinier plots confirms a high degree of homogeneity for each of the five SAXS samples. (A-E, right panels) A view of the ab initio shape (represented as a transparent envelope) computed from the experimental SAXS profile. Structures of MBP (PDBID 1Y4C), Kapβ2 (PDBID 2QMR), and FUS PY-NLS of full length FUS (Figure S2F and S2G) were coarsely fitted to the SAXS envelopes. F) Comparison of the pair distribution functions, P(r) between MBP (green curves) and MBP-FUS (orange dots), and among Kapβ2 (blue curves), Kapβ2•FUS (red dots), and Kapβ2•MBP-FUS (purple dots). MBP-FUS is significantly extended compared to MBP. In contrast, FUS becomes more compact upon binding Kapβ2. G) Comparison of the SAXS profiles (at ~1.0 mg/mL) of Kapβ2, Kapβ2•FUS, Kapβ2•MBP-FUS, and MBP-FUS collected in 5% (red curves) and 20% (blue curves) glycerol buffers, respectively. SAXS profiles of the same proteins are nearly identical within their uncertainties, regardless of the glycerol concentration. Therefore, glycerol concentrations from 5 to 20% do not affect protein compaction. Note that Kapβ2•FUS in 5% glycerol was prepared at a low concentration of ~0.5 mg/mL, leading to the high standard deviation in its SAXS profile.
7. Figure S7, related to Figure 7 and Discussion. RRM participates in intramolecular interactions within FUS(164–500), temperature dependence of FUS phase separation, electrostatic surfaces of Karyopherin proteins.
A) Chemical shift differences, Δ = [(Δ1H)2 + ((10*Δ15N))2]0.5, between the isolated RRM (FUS residues 285–371) and the RRM within FUS(164–500). B) Ribbon and surface representations of the FUS RRM (PDBID 1LCW, green), with residues Δ > 0.2 colored blue. Residues that directly contact Kapβ2 based on cross saturation transfer data are colored magenta (Figure 4B). At 2 μM and 8 μM concentration, FUS(ΔRRM) has a higher Tcloud than wildtype FUS, indicating that deletion of the RRM favors phase separation. To gain insight into this behavior, we compared the chemical shifts of the isolated RRM domain to those of the RRM within FUS(164–500). A number of amide resonances, mostly on the face of the domain opposite the Kapβ2 binding site, differ between the two constructs. This observation suggests that in FUS(164–500), RRM participates in intramolecular contacts with the RGG regions, which could sequester these elements. Given that the RGG regions play important roles in phase separation, their sequestration by RRM may suppress phase separation. C) Temperature dependence of FUS phase separation. Turbidity (OD395nm) of 2 μM MBP-FUS proteins (wildtype (WT) and mutants) after 3 h treatment with Tev protease was monitored as temperature was decreased from 40°C or 45°C to 5°C. Optical densities were normalized to values measured at 5°C. TCloud is the x-intercept of the tangent at the inflection point of the curve (mean of 3 technical replicates, ± S.D.). D) Mixtures containing 2 μM MBP-FUS WT, MBP-FUS(1–452), or MBP-FUS(ΔRRM), 20 nM FUS-GFP were treated with Tev protease at room temperature for 50 min to form FUS droplets. The phase separated mixtures were cooled to either 10°C or 15°C, held a t those temperatures for 2 min, and then increased by 2°C increments to a maximum temperature of 43°C or 44°C. The sample was held at each temperature for 2–3 min prior to acquisition of a 50 μm Z-stack (1 μm increments) using spinning disk confocal microscopy. Panels show maximum projection images derived from the Z-stack 10–50 μm above the slide surface. To calculate Tcloud, images in this same portion of the Z-stack were segmented using the Triangle algorithm in ImageJ and the total number of FUS droplets was determined after a filter for circularity (>0.5) and size (>0.5 μm2). Tcloud was determined from the x-intercept of a line fit to the first six (WT and FUS(1–452)) or eight (FUS(ΔRRM)) points in the (number of puncta) vs. temperature curve. E) Electrostatic surface potential (scale of −12 kTe−1 to +12 kTe−1) of Importins Kapβ2 (PDBID 4FDD), Impβ (1QGK) and Kap121 (3W3W). PY-NLS bound to Kapβ2, IBB (NLS) bound to Impβ and IK-NLS bound to Kap121 are shown as cyan cartoons. Arrows point to acidic patches on the concave surface of the Importins. F) Electrostatic surface potential (scale of −12 kTe−1 to +12 kTe−1) of Exportins CRM1 (PDBID 3GB8), CSE1 (1WA5) and XPO5 (3A6P). The NES (residues 1–15) of cargo SNUPN bound to CRM1 is shown as a cyan cartoon. Cargos bound to CSE1 (Impα) and XPO5 (pre-miRNA), along with bound RanGTP, were removed to allow viewing of the concave surfaces of the Exportin. Arrows point to basic patches on the concave surface of the Exportins.
8
Supplementary movie 2.avi., related to Figure 1. FUS droplets (from 5 μM MBP-FUS + Tev), disappearing within 5 minutes of addition of 10 μM of Kapβ2.
9
Supplementary movie 3.avi., related to Figure 1. Phase separated FUS (48 hours after Tev was added to 5 μM MBP-FUS) imaged for 20 minutes after addition of 10 μM of Kapβ2.
- Karyopherin-β2 inhibits liquid-liquid phase separation of FUS
- Kapβ2 binds tightly to the PY-NLS and weakly to multiple regions across FUS
- Many of these regions also promote FUS phase separation
- Kapβ2-FUS interactions compete with FUS-FUS interactions to disrupt phase separation
Acknowledgments
We thank D. Dormann and J. Shorter for critical reading of the manuscript. We thank A. Patel and A. A. Hyman for their gift of FUS-GFP, T. Cagatay and A. Gonzalez for help subcloning, J. Hu and D. Corey for their help with temperature-dependent turbidity analysis, N. Conrad and K. Lynch for advice on FUS-RNA analysis, T. Matsui and T.M. Weiss at SSRL, SLAC National Accelerator Laboratory, for assistance with collecting SAXS data, and B. Raveh for discussions on SAXS analysis. We thank D. Tomchick for advice on X-ray structure determination, and the Structural Biology Laboratory and Macromolecular Biophysics Resource at UTSW for their assistance with crystallographic and biophysical data collection. Crystallographic results are derived from work performed at Argonne National Laboratory, Structural Biology Center at the Advanced Photon Source. Argonne is operated by UChicago Argonne, LLC, for the U.S. Department of Energy (DOE), Office of Biological and Environmental Research (BER) under contract DE-AC02-06CH11357. NMR data were obtained in part at the Brown University Structural Biology Core Facility supported by the Division of Biology and Medicine, Brown University. NMR spectroscopy at UTSW is supported by NIH instrumentation grants 1S10RR26461-1 and 1S10OD018027-01. SAXS experiments were performed at the SSRL, SLAC National Accelerator Laboratory operated for DOE by Stanford University. The SSRL SMBP is supported by the DOE BER, by the National Institutes of Health (NIH), NCRR, Biomedical Technology Program (P41RR001209), and by NIGMS, NIH (P41GM103393). This work was funded by NIGMS of NIH under Awards R01GM069909 (Y.M.C.), U01GM98256-01 (Y.M.C and A.S.), R01GM56322 (M.K.R.), R01GM118530 (N.L.F), R01GM083960 (A.S), P41GM109824 (A.S), R01GM112108 (A.S), and R01GM114274 (R.O.), T32GM008203 (J.J.), T32GM008297 (J.M.B.), the Howard Hughes Medical Institute HCIA grant (M.K.R.), the Welch Foundation Grants I-1532 (Y.M.C.) and I-1544 (M.K.R.), Leukemia and Lymphoma Society Scholar Award (Y.M.C.), the University of Texas Southwestern Endowed Scholars Program (Y.M.C.). Work in M.K.R’s lab was supported by the Howard Hughes Medical Institute. R.A. was an American Heart Association Postdoctoral Fellow and H.Y.J.F was a Howard Hughes Medical Institute International Student Research fellow.
Footnotes
Declaration of Interests
The authors declare no conflict of interest.
Author Contributions
Conceptualization, Y.M.C. and M.K.R.; Methodology, Y.M.C, M.K.R., N.L.F., A.S., R.O., T.Y., R.A.; Investigation, T.Y., R.A., J.J., H.Y.J.F., Y.M.C., M.K.R., K.A.B., Y.L., W.B.P., S.J.K., D.S., M.S., J.M.B., R.O. and N.L.F.; Writing – Original Draft, Y.M.C., M.K.R., Y.L., S.J.K. and N.L.F.; Writing – Review & Editing, Y.M.C., M.K.R., T.Y., R.A.; Funding Acquisition, Y.M.C., M.K.R., R.O., A.S. and N.L.F.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afonine PV, Grosse-Kunstleve RW, Echols N, Headd JJ, Moriarty NW, Mustyakimov M, Terwilliger TC, Urzhumtsev A, Zwart PH, Adams PD. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr D Biol Crystallogr. 2012;68:352–367. doi: 10.1107/S0907444912001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banani SF, Lee HO, Hyman AA, Rosen MK. Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol. 2017 doi: 10.1038/nrm.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne CP, Tompa P, Pappu RV. Polymer physics of intracellular phase transitions. Nat Phys. 2015;11:899–904. [Google Scholar]
- Brautigam CA. Calculations and Publication-Quality Illustrations for Analytical Ultracentrifugation Data. Methods Enzymol. 2015;562:109–133. doi: 10.1016/bs.mie.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Brautigam CA, Zhao H, Vargas C, Keller S, Schuck P. Integration and global analysis of isothermal titration calorimetry data for studying macromolecular interactions. Nat Protoc. 2016;11:882–894. doi: 10.1038/nprot.2016.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke KA, Janke AM, Rhine CL, Fawzi NL. Residue-by-Residue View of In Vitro FUS Granules that Bind the C-Terminal Domain of RNA Polymerase II. Mol Cell. 2015;60:231–241. doi: 10.1016/j.molcel.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cansizoglu AE, Chook YM. Conformational heterogeneity of karyopherin beta2 is segmental. Structure. 2007;15:1431–1441. doi: 10.1016/j.str.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cansizoglu AE, Lee BJ, Zhang ZC, Fontoura BM, Chook YM. Structure-based design of a pathway-specific nuclear import inhibitor. Nat Struct Mol Biol. 2007;14:452–454. doi: 10.1038/nsmb1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen VB, Arendall WB, 3rd, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC. MolProbity: all-atom structure validation for macromolecular crystallography. Acta crystallographica Section D, Biological crystallography. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chook YM, Blobel G. Structure of the nuclear transport complex karyopherin-beta2-Ran x GppNHp. Nature. 1999;399:230–237. doi: 10.1038/20375. [DOI] [PubMed] [Google Scholar]
- Chook YM, Jung A, Rosen MK, Blobel G. Uncoupling Kapbeta2 substrate dissociation and ran binding. Biochemistry. 2002;41:6955–6966. doi: 10.1021/bi012122p. [DOI] [PubMed] [Google Scholar]
- Chook YM, Suel KE. Nuclear import by karyopherin-betas: recognition and inhibition. Biochim Biophys Acta. 2011;1813:1593–1606. doi: 10.1016/j.bbamcr.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly BD, Petry C, Yadav S, Demeule B, Ciaccio N, Moore JM, Shire SJ, Gokarn YR. Weak interactions govern the viscosity of concentrated antibody solutions: high-throughput analysis using the diffusion interaction parameter. Biophys J. 2012;103:69–78. doi: 10.1016/j.bpj.2012.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti E, Izaurralde E. Nucleocytoplasmic transport enters the atomic age. Curr Opin Cell Biol. 2001;13:310–319. doi: 10.1016/s0955-0674(00)00213-1. [DOI] [PubMed] [Google Scholar]
- Crozat A, Aman P, Mandahl N, Ron D. Fusion of CHOP to a novel RNA-binding protein in human myxoid liposarcoma. Nature. 1993;363:640–644. doi: 10.1038/363640a0. [DOI] [PubMed] [Google Scholar]
- Dong X, Biswas A, Suel KE, Jackson LK, Martinez R, Gu H, Chook YM. Structural basis for leucine-rich nuclear export signal recognition by CRM1. Nature. 2009;458:1136–1141. doi: 10.1038/nature07975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormann D, Haass C. TDP-43 and FUS: a nuclear affair. Trends Neurosci. 2011;34:339–348. doi: 10.1016/j.tins.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Dormann D, Rodde R, Edbauer D, Bentmann E, Fischer I, Hruscha A, Than ME, Mackenzie IR, Capell A, Schmid B, et al. ALS-associated fused in sarcoma (FUS) mutations disrupt Transportin-mediated nuclear import. Embo J. 2010;29:2841–2857. doi: 10.1038/emboj.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ederle H, Dormann D. TDP-43 and FUS en route from the nucleus to the cytoplasm. FEBS Lett. 2017;591:1489–1507. doi: 10.1002/1873-3468.12646. [DOI] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta crystallographica Section D, Biological crystallography. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson HP. Size and Shape of Protein Molecules at the Nanometer Level Determined by Sedimentation, Gel Filtration, and Electron Microscopy. Biol Proced Online. 2009;11:32–51. doi: 10.1007/s12575-009-9008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H, Neto MD, Napolitano HB, Polikarpov I, Craievich AF. Determination of the molecular weight of proteins in solution from a single small-angle X-ray scattering measurement on a relative scale. J Appl Crystallogr. 2010;43:101–109. [Google Scholar]
- Franke D, Petoukhov MV, Konarev PV, Panjkovich A, Tuukkanen A, Mertens HDT, Kikhney AG, Hajizadeh NR, Franklin JM, Jeffries CM, et al. ATSAS 2.8: a comprehensive data analysis suite for small-angle scattering from macromolecular solutions. J Appl Crystallogr. 2017;50:1212–1225. doi: 10.1107/S1600576717007786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung HY, Chook YM. Atomic basis of CRM1-cargo recognition, release and inhibition. Semin Cancer Biol. 2014;27:52–61. doi: 10.1016/j.semcancer.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung HY, Fu SC, Brautigam CA, Chook YM. Structural determinants of nuclear export signal orientation in binding to exportin CRM1. Elife. 2015;4 doi: 10.7554/eLife.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guex N, Peitsch MC, Schwede T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: A historical perspective. Electrophoresis. 2009;30:S162–S173. doi: 10.1002/elps.200900140. [DOI] [PubMed] [Google Scholar]
- Harrison AF, Shorter J. RNA-binding proteins with prion-like domains in health and disease. Biochem J. 2017;474:1417–1438. doi: 10.1042/BCJ20160499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough LE, Dutta K, Sparks S, Temel DB, Kamal A, Tetenbaum-Novatt J, Rout MP, Cowburn D. The molecular mechanism of nuclear transport revealed by atomic-scale measurements. Elife. 2015;4 doi: 10.7554/eLife.10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iko Y, Kodama TS, Kasai N, Oyama T, Morita EH, Muto T, Okumura M, Fujii R, Takumi T, Tate S, et al. Domain Architectures and characterization of an RNA-binding protein, TLS. Journal of Biological Chemistry. 2004;279:44834–44840. doi: 10.1074/jbc.M408552200. [DOI] [PubMed] [Google Scholar]
- Jakel S, Mingot JM, Schwarzmaier P, Hartmann E, Gorlich D. Importins fulfil a dual function as nuclear import receptors and cytoplasmic chaperones for exposed basic domains. Embo Journal. 2002;21:377–386. doi: 10.1093/emboj/21.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayalakshmi V, Krishna NR. Complete relaxation and conformational exchange matrix (CORCEMA) analysis of intermolecular saturation transfer effects in reversibly forming ligand-receptor complexes. J Magn Reson. 2002;155:106–118. doi: 10.1006/jmre.2001.2499. [DOI] [PubMed] [Google Scholar]
- Ju S, Tardiff DF, Han H, Divya K, Zhong Q, Maquat LE, Bosco DA, Hayward LJ, Brown RH, Jr, Lindquist S, et al. A yeast model of FUS/TLS-dependent cytotoxicity. PLoS Biol. 2011;9:e1001052. doi: 10.1371/journal.pbio.1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Han TW, Xie S, Shi K, Du X, Wu LC, Mirzaei H, Goldsmith EJ, Longgood J, Pei J, et al. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell. 2012;149:753–767. doi: 10.1016/j.cell.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikhney AG, Svergun DI. A practical guide to small angle X-ray scattering (SAXS) of flexible and intrinsically disordered proteins. FEBS Lett. 2015;589:2570–2577. doi: 10.1016/j.febslet.2015.08.027. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Fernandez-Martinez J, Sampathkumar P, Martel A, Matsui T, Tsuruta H, Weiss TM, Shi Y, Markina-Inarrairaegui A, Bonanno JB, et al. Integrative structure-function mapping of the nucleoporin Nup133 suggests a conserved mechanism for membrane anchoring of the nuclear pore complex. Mol Cell Proteomics. 2014;13:2911–2926. doi: 10.1074/mcp.M114.040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King OD, Gitler AD, Shorter J. The tip of the iceberg: RNA-binding proteins with prion-like domains in neurodegenerative disease. Brain Res. 2012;1462:61–80. doi: 10.1016/j.brainres.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi J, Matsuura Y. Structural basis for cell-cycle-dependent nuclear import mediated by the karyopherin Kap121p. J Mol Biol. 2013;425:1852–1868. doi: 10.1016/j.jmb.2013.02.035. [DOI] [PubMed] [Google Scholar]
- Lee BJ, Cansizoglu AE, Suel KE, Louis TH, Zhang Z, Chook YM. Rules for nuclear localization sequence recognition by karyopherin beta 2. Cell. 2006;126:543–558. doi: 10.1016/j.cell.2006.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YR, King OD, Shorter J, Gitler AD. Stress granules as crucibles of ALS pathogenesis. Journal of Cell Biology. 2013;201:361–372. doi: 10.1083/jcb.201302044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Currie SL, Rosen MK. Intrinsically disordered sequences enable modulation of protein phase separation through distributed tyrosine motifs. J Biol Chem. 2017 doi: 10.1074/jbc.M117.800466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Protter DS, Rosen MK, Parker R. Formation and Maturation of Phase-Separated Liquid Droplets by RNA-Binding Proteins. Mol Cell. 2015;60:208–219. doi: 10.1016/j.molcel.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XH, Niu CY, Ren JT, Zhang JY, Xie XD, Zhu HN, Feng W, Gong WM. The RRM domain of human fused in sarcoma protein reveals a non-canonical nucleic acid binding site. Bba-Mol Basis Dis. 2013;1832:375–385. doi: 10.1016/j.bbadis.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughlin FE, Mansfield RE, Vaz PM, McGrath AP, Setiyaputra S, Gamsjaeger R, Chen ES, Morris BJ, Guss JM, Mackay JP. The zinc fingers of the SR-like protein ZRANB2 are single-stranded RNA-binding domains that recognize 5′ splice site-like sequences. Proc Natl Acad Sci U S A. 2009;106:5581–5586. doi: 10.1073/pnas.0802466106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milles S, Mercadante D, Aramburu IV, Jensen MR, Banterle N, Koehler C, Tyagi S, Clarke J, Shammas SL, Blackledge M, et al. Plasticity of an ultrafast interaction between nucleoporins and nuclear transport receptors. Cell. 2015;163:734–745. doi: 10.1016/j.cell.2015.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor W, Cymborowski M, Otwinowski Z, Chruszcz M. HKL-3000: the integration of data reduction and structure solution--from diffraction images to an initial model in minutes. Acta crystallographica Section D, Biological crystallography. 2006;62:859–866. doi: 10.1107/S0907444906019949. [DOI] [PubMed] [Google Scholar]
- Mittag T, Orlicky S, Choy WY, Tang X, Lin H, Sicheri F, Kay LE, Tyers M, Forman-Kay JD. Dynamic equilibrium engagement of a polyvalent ligand with a single-site receptor. Proc Natl Acad Sci U S A. 2008;105:17772–17777. doi: 10.1073/pnas.0809222105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliex A, Temirov J, Lee J, Coughlin M, Kanagaraj AP, Kim HJ, Mittag T, Taylor JP. Phase Separation by Low Complexity Domains Promotes Stress Granule Assembly and Drives Pathological Fibrillization. Cell. 2015;163:123–133. doi: 10.1016/j.cell.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan Z, Ryan VH, Janke AM, Burke KA, Rhoads SN, Zerze GH, O’Meally R, Dignon GL, Conicella AE, Zheng W, et al. Phosphorylation of the FUS low-complexity domain disrupts phase separation, aggregation, and toxicity. EMBO J. 2017;36:2951–2967. doi: 10.15252/embj.201696394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Qamar S, Lin JQ, Schierle GS, Rees E, Miyashita A, Costa AR, Dodd RB, Chan FT, Michel CH, et al. ALS/FTD Mutation-Induced Phase Transition of FUS Liquid Droplets and Reversible Hydrogels into Irreversible Hydrogels Impairs RNP Granule Function. Neuron. 2015;88:678–690. doi: 10.1016/j.neuron.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray DT, Kato M, Lin Y, Thurber KR, Hung I, McKnight SL, Tycko R. Structure of FUS Protein Fibrils and Its Relevance to Self-Assembly and Phase Separation of Low-Complexity Domains. Cell. 2017;171:615–627. e616. doi: 10.1016/j.cell.2017.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima T, Nakajima T, Oishi T, Imamoto N, Yoneda Y, Fukamizu A, Yagami K. CRM1 mediates nuclear export of nonstructural protein 2 from parvovirus minute virus of mice. Biochem Biophys Res Commun. 1999;264:144–150. doi: 10.1006/bbrc.1999.1478. [DOI] [PubMed] [Google Scholar]
- Oldenbourg R. Analysis of edge birefringence. Biophys J. 1991;60:629–641. doi: 10.1016/S0006-3495(91)82092-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenbourg R, Mei G. New polarized light microscope with precision universal compensator. J Microsc. 1995;180:140–147. doi: 10.1111/j.1365-2818.1995.tb03669.x. [DOI] [PubMed] [Google Scholar]
- Ozdilek BA, Thompson VF, Ahmed NS, White CI, Batey RT, Schwartz JC. Intrinsically disordered RGG/RG domains mediate degenerate specificity in RNA binding. Nucleic Acids Res. 2017;45:7984–7996. doi: 10.1093/nar/gkx460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Lee HO, Jawerth L, Maharana S, Jahnel M, Hein MY, Stoynov S, Mahamid J, Saha S, Franzmann TM, et al. A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell. 2015;162:1066–1077. doi: 10.1016/j.cell.2015.07.047. [DOI] [PubMed] [Google Scholar]
- Port SA, Monecke T, Dickmanns A, Spillner C, Hofele R, Urlaub H, Ficner R, Kehlenbach RH. Structural and Functional Characterization of CRM1-Nup214 Interactions Reveals Multiple FG-Binding Sites Involved in Nuclear Export. Cell Rep. 2015;13:690–702. doi: 10.1016/j.celrep.2015.09.042. [DOI] [PubMed] [Google Scholar]
- Ryu HH, Jun MH, Min KJ, Jang DJ, Lee YS, Kim HK, Lee JA. Autophagy regulates amyotrophic lateral sclerosis-linked fused in sarcoma-positive stress granules in neurons. Neurobiol Aging. 2014;35:2822–2831. doi: 10.1016/j.neurobiolaging.2014.07.026. [DOI] [PubMed] [Google Scholar]
- Scheuermann TH, Brautigam CA. High-precision, automated integration of multiple isothermal titration calorimetric thermograms: new features of NITPIC. Methods. 2015;76:87–98. doi: 10.1016/j.ymeth.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrodinger, LLC. The PyMOL Molecular Graphics System, Version 1.8. 2015. [Google Scholar]
- Schwartz JC, Cech TR, Parker RR. Biochemical Properties and Biological Functions of FET Proteins. Annu Rev Biochem. 2015;84:355–379. doi: 10.1146/annurev-biochem-060614-034325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JC, Wang X, Podell ER, Cech TR. RNA seeds higher-order assembly of FUS protein. Cell Rep. 2013;5:918–925. doi: 10.1016/j.celrep.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soniat M, Chook YM. Karyopherin-beta2 Recognition of a PY-NLS Variant that Lacks the Proline-Tyrosine Motif. Structure. 2016;24:1802–1809. doi: 10.1016/j.str.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Diaz Z, Fang X, Hart MP, Chesi A, Shorter J, Gitler AD. Molecular determinants and genetic modifiers of aggregation and toxicity for the ALS disease protein FUS/TLS. PLoS Biol. 2011;9:e1000614. doi: 10.1371/journal.pbio.1000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahama K, Oyoshi T. Specific binding of modified RGG domain in TLS/FUS to G-quadruplex RNA: tyrosines in RGG domain recognize 2′-OH of the riboses of loops in G-quadruplex. J Am Chem Soc. 2013;135:18016–18019. doi: 10.1021/ja4086929. [DOI] [PubMed] [Google Scholar]
- Ueda T, Takeuchi K, Nishida N, Stampoulis P, Kofuku Y, Osawa M, Shimada I. Cross-saturation and transferred cross-saturation experiments. Q Rev Biophys. 2014;47:143–187. doi: 10.1017/S0033583514000043. [DOI] [PubMed] [Google Scholar]
- Xiang SH, Kato M, Wu LC, Lin Y, Ding M, Zhang YJ, Yu YH, McKnight SL. The LC Domain of hnRNPA2 Adopts Similar Conformations in Hydrogel Polymers, Liquid-like Droplets, and Nuclei. Cell. 2015;163:829–839. doi: 10.1016/j.cell.2015.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZC, Chook YM. Structural and energetic basis of ALS-causing mutations in the atypical proline-tyrosine nuclear localization signal of the Fused in Sarcoma protein (FUS) Proc Natl Acad Sci U S A. 2012;109:12017–12021. doi: 10.1073/pnas.1207247109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Piszczek G, Schuck P. SEDPHAT--a platform for global ITC analysis and global multi-method analysis of molecular interactions. Methods. 2015;76:137–148. doi: 10.1016/j.ymeth.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1. Figure S1, related to Figure 1. Solution behavior of FUS complexes and microscopy of FUS LLPS.
(A) MBP-FUS is monomeric in size exclusion chromatography (left) but dynamic light scattering (DLS) analysis of 12 μM MBP-FUS shows polydispersity and presence of possibly very large MBP-FUS oligomers (right). (B) Dissociation constants (KDs) of Kapβ2 binding to MBP-FUS PY-NLS (upper) and MBP-full length FUS (lower) by isothermal titration calorimetry at 20°C. In the upper panel, 50 μM Kapβ2 was titrated into 4.5 or 6.5 μM MBP-FUS PYNLS (residues 475–526). In the lower panel, 50 μM Kapβ2 was titrated into 4.5 or 5 μM MBP-full length FUS. Thermograms, binding isotherms and data fit residuals are plotted, and KDs obtained from data integration using NITPIC (1) and global fitting of two independent ITC experiments using SEDPHAT (2), and are reported with 68.3% confidence interval in brackets. In the experiment of Kapβ2 binding to MBP-FUS PY-NLS, 2.3% of the MBP-FUS PY-NLS was incompetent. In the experiment of Kapβ2 binding to MBP-FUS, 8.6% of the MBP-FUS was incompetent. (C) A purified Kapβ2•FUS complex is heterodimeric in size exclusion chromatography (left). DLS (right) shows Kapβ2 drastically reducing the MBP-FUS polydispersity and the majority of the Kapβ2•MBP-FUS complex behaves as a single species. (D) Time course of Tev protease (added at time=0 min) cleaving MBP from MBP-FUS at room temperature. Proteins were visualized by Coomassie blue stained SDS/PAGE. (E) Turbidity (OD395nm) of 8 μM MBP-FUS proteins with either a TEV protease ceavage site or a PreScission protease cleavage site between MBP and FUS. Turbidity was measured for 60 min at room temperature after Tev or PreScission treatment. Cleavage MBP-FUS by the proteases was visualized by Coomassie blue stained SDS/PAGE. (F) Already turbid FUS (8 μM MBP-FUS treated with Tev for 60 min) were treated with either buffer, 8 μM Kapβ2 ± RanGTP or inhibitor M9M, or Kapβ2Δloop ± RanGTP at time=60 min. Turbidity measurements 20 min later, at time=80 min are shown (mean of 3 technical replicates, ± S.D.). (G) Mixtures containing 5 μM MBP-FUS, 0.5 μM MBP-FUS-SNAPSNAP-Surface 649 and either buffer or 10 μM Kapβ2 were treated with Tev protease (time=0 hr) and imaged with spinning disk confocal microscopy 1 hr after later. By time=1 hr, FUS has phase separated into liquid droplets that coalesce into large mats of phase separated protein by time=24 hr. Kapβ2 prevents FUS phase separation, but this effect is reversed if either 15 μM RanGTP or 15 μM Kapβ2 inhibitor M9M is added. *RanGTP or M9M was added to the FUS+Kapβ2 mixture at time= 1 hr and imaged either 1 hr later (time=2 hr) or at time=24 hr. Images were obtained with spinning disk confocal microscopy (561 nm laser illumination; 60x 1.4na oil immersion objective lenses). 20 μm length scale bars are shown on the bottom right of all images. (H) Polarized light microscopy of droplets (5 μM MBP-FUS+0.5 μM MBP-FUS-SNAPSNAP-Surface 649, 1–2 hr of Tev treatment). Retardance images were recorded with the LC-PolScope - white corresponds to 2.5 nm retardance. The edge of each droplet is decorated with a birefringent double layer, an optical effect that stems from the refractive index difference between droplet and surrounding medium. The droplets display higher refraction than the medium, probably due to a higher protein/nucleotide concentration. The right panel, an enlarged droplet shows the lack of detectable birefringence in the interior of the droplet. The inside of each droplet has the same level of anisotropy as the surrounding medium, both of which are caused by the shot noise (photon statistics noise) inherent in the recorded image intensities. There is no order that we can detect in the interior of the droplets.
10
Supplementary movie 1.avi., related to Figure 1. FUS droplets, 1 hour after Tev is added to 5 μM MBP-FUS.
11
12
13
14
2. Figure S2, related to Figure 2. Interactions of MBP-FUS chimeras with Importins, ITC and crystal structures of Kapβ2 bound to FUS.
A) Pull-down binding assays showing interactions between MBP-FUS wt or chimeras with GST-Kapβ2, GST-Impα/β, GST-Kap121 or GST-Crm1. B) Dissociation constants (KDs) measured at 20°C by ITC of Kap β2 binding to MBP-FUS(453–526) and MBP-FUS(371–526) (C). D) Overall structure of Kapβ2 (pink) in complex with FUS(456–526) (green). Kicked omit map (cyan mesh) within 10 Å of all Kapβ2 residues, contoured at 3.0σ, is overlaid on the structure. E) Overall structure of Kapβ2 (pink) in complex with FUS(371–526) (green). Kicked omit map (cyan mesh) within 10 Å of all Kapβ2 residues, contoured at 3.0σ, is overlaid on the structure. F) Crystals of the Kapβ2•full length FUS complex were harvested, washed in crystallization reservoir solution, dissolved in buffer and visualized by Coomassie Blue-stained SDS/PAGE. G) Stereo-images of the 4Å resolution structure of Kapβ2 (pink cartoon) bound to full length FUS where only residues 508–526 of FUS are modeled (blue ribbon with side chain sticks). Kicked omit map (light grey mesh) within 10 Å of all Kapβ2 residues, contoured at 2.5σ and 2.0σ levels are shown. There are no obvious densities for extensive FUS interaction with Kapβ2 in addition to the NLS. H) Superimposed structures of FUS from four complexes: Kapβ2-FUS full-length (blue), Kapβ2•FUS(371–526) (yellow), Kapβ2•FUS(456–526) (green) and Kapβ2•FUS(498–526) (red; PDBID 4FDD).
3. Figure S3, related to Figure 3. NMR analyses Kapβ2 binding to FUS LC.
A) Overlay of 2D 1H-15N spectra of 75 μM 15N-labeled FUS LC alone (blue) or with increasing concentrations of Kapβ2: 37.5 μM (0.5:1, black), 75 μM (1:1, red), 112.5 μM (1.5:1, green). Residues in several regions of FUS LC show chemical shifts and intensity attenuations (see Figure 5A) and three of the most affected regions are 37–41, 97–100, 149–154. B) NMR chemical shift deviations at 10 °C of 75 μM FUS LC resonances in the presence of 100 μM Kapβ2 (dark blue open bars) or 100 μM Kapβ2•FUS PY-NLS complex (light blue) compared to 75 μM FUS LC alone. NMR chemical shift deviations, 1H (top) and 15N (middle), and resonance intensity attenuation (bottom) are plotted. 1H and 15N resonances show very similar changes upon addition of Kapβ2 alone or FUS PY-NLS-bound Kapβ2. Chemical shift differences of 1H (top) and 15N (middle) resonance position as well as resonance intensity attenuation (bottom) support FUS LC binding weakly to Kapβ2 across the entire FUS LC domain. Three segments 37SYSGY41, 97YPGY100 and 149YSPPSG154 (grey bars mark positions of the 24 tyrosines) show larger resonance intensity attenuation (red asterisks) and large 15N and/or 1H chemical shift differences, suggesting stronger Kapβ2-binding to those elements.
4. Figure S4, related to Figure 4. NMR analyses of Kapβ2 binding to RRM and ZnF domains within FUS(164–500).
A) Overlay of 1H/15N HSQC NMR spectrum of 15N-FUS(164–526) alone (black) or with equimolar Kapβ2. B) Overlay of 1H/15N HSQC NMR spectrum of 15N-FUS(164–500) with simulated spectra of the FUS RRM and zinc finger domains based on reported chemical shifts of the isolated domains (Liu et al, 2013; Iko et al, 2004). C) Attenuation of assigned RRM domain resonances in 1H/15N HSQC NMR spectra of 15N-FUS(164–500) upon addition of 3-fold molar excess of Kapβ2•M9M (I=resonance intensity in the presence of Kapβ2•M9M; I0=resonance intensity of FUS alone). D) Ribbon (top) and surface representations (middle and bottom panels) of the FUS RRM (PDBID 1LCW, green), showing residues with I/I0 <0.5 upon binding Kapβ2 (magenta). Unassigned residues are white. E) Attenuation in intensity of assigned ZnF domain resonances in 1H/15N HSQC NMR spectra of 15N-FUS(164–500) upon addition of 3-fold molar excess of Kapβ2•FUS PY-NLS. F) Ribbon and surface representations of the homology model of the FUS ZnF domain generated from structure of zinc finger in ZNF265, PDBID 2K1P, orange. Residues with I/I0 <0.3 upon Kapβ2 binding are in magenta and unassigned residues are white. G) Selected resonances from ZnF (orange) and RRM (green). Asterisk indicate Kapβ2•M9M complex. All other columns are Kapβ2•FUS PY-NLS.
5. Figure S5, related to Figure 5. NMR analyses of Kapβ2 binding RGG1, RGG2 and RGG3 regions of FUS(164–500).
A) Glycine residues in FUS(164–500) were assigned to RGG1, RGG2 and RGG3 regions by overlaying 1H/15N spectra of FUS(164–500) and A) RGG1 containing FUS residues 164–267, B) RGG1-RRM containing FUS residues 164–370, C) RGG2-ZnF containing FUS residues 371–452, D) ZnF-RGG3 containing FUS residues 421–500. Glycine crosspeaks observed in spectra of only RGG1/RGG1-RRM or only RGG2 or only RGG3 could be assigned to that fragment, and are indicated in the spectra. E) Attenuation in intensity of glycine peaks in 1H/15N HSQC NMR spectra of 15N-FUS(RGG1-RRM) upon addition of 2-fold molar excess of Kapβ2•M9M. F) Chemical shift differences, Δ = [(Δ1H)2 + ((10*Δ15N))2]0.5, in ZnF resonances between the isolated domain (FUS residues 422–453), FUS(164–500) (grey bars), RGG2-ZnF (yellow bars) and ZnF-RGG3 (magenta bars). The ZnF chemical shifts were identical within experimental error in all constructs. Additionally, with the exception of only a few cross peaks likely at the termini of the constructs, the glycine chemical shifts of the RGG1, RGG2-ZnF and ZnF-RGG3 fragments were virtually exact subsets of those in FUS(164–500) (panels A-D). These data strongly suggest that the ZnF domain behaves independently in FUS(164–500) and does not make significant intramolecular contacts with surrounding regions. The similarity of chemical shifts across four different constructs indicates that if such intramolecular interactions are present, the self-interacting states must be populated to a small degree. Moreover, even in the case of multi-site exchange (involving for example all three RGG regions competing for a common site on the ZnF domain), where divergent chemical shifts might be averaged out, these differences should be revealed in spectra of the smaller fragments where competition for those sites would be eliminated. Yet the chemical shifts of all the fragments are identical within experimental error to each other and to those in FUS(164–500). Because of these data, we conclude that the ZnF makes few or no intramolecular contacts in FUS. G) Attenuation of unassigned non-glycine resonances (arbitrarily numbered; Table S2) in 1H/15N HSQC NMR spectra of 15N-FUS(164–500) upon addition of 3-fold molar excess of Kapβ2•M9M. Asterisks indicate resonances not in RRM or zinc finger domains based on reported chemical shift assignments as in Figure S4A.
6. Figure S6, related to Figure 6A. SAXS analysis of MBP, MBP-FUS, Kapβ2, Kapβ2•FUS, and Kapβ2•MBP-FUS.
A-E) SAXS profiles of MBP (A), MBP-FUS (B), Kapβ2 (C), Kapβ2•FUS (D), and Kapβ2•MBP-FUS (E) were used to compute radius of gyration (Rg), maximum particle size (Dmax), pair distribution function (P(r)), and ab initio shapes. (A-E, left panels) The experimental SAXS profile (blue dots with black error bars) is shown along with the extrapolation curve (red). The corresponding Kratky plot (used to depict the level of flexibility) is also shown in blue dots along with the extrapolation curve (red). A high plateau in the Kratky plot (q=0.15 – 0.2 Å−1) suggests some flexibility in MBP-FUS (B). (A-E, middle panels) The top plot shows the pair distribution function, P(r). The maximum particle size (Dmax) was determined as the maximum pair distance in the plot. The bottom plot shows the corresponding Guinier plot with the calculated Rg fit value in Å. The linearity of the Guinier plots confirms a high degree of homogeneity for each of the five SAXS samples. (A-E, right panels) A view of the ab initio shape (represented as a transparent envelope) computed from the experimental SAXS profile. Structures of MBP (PDBID 1Y4C), Kapβ2 (PDBID 2QMR), and FUS PY-NLS of full length FUS (Figure S2F and S2G) were coarsely fitted to the SAXS envelopes. F) Comparison of the pair distribution functions, P(r) between MBP (green curves) and MBP-FUS (orange dots), and among Kapβ2 (blue curves), Kapβ2•FUS (red dots), and Kapβ2•MBP-FUS (purple dots). MBP-FUS is significantly extended compared to MBP. In contrast, FUS becomes more compact upon binding Kapβ2. G) Comparison of the SAXS profiles (at ~1.0 mg/mL) of Kapβ2, Kapβ2•FUS, Kapβ2•MBP-FUS, and MBP-FUS collected in 5% (red curves) and 20% (blue curves) glycerol buffers, respectively. SAXS profiles of the same proteins are nearly identical within their uncertainties, regardless of the glycerol concentration. Therefore, glycerol concentrations from 5 to 20% do not affect protein compaction. Note that Kapβ2•FUS in 5% glycerol was prepared at a low concentration of ~0.5 mg/mL, leading to the high standard deviation in its SAXS profile.
7. Figure S7, related to Figure 7 and Discussion. RRM participates in intramolecular interactions within FUS(164–500), temperature dependence of FUS phase separation, electrostatic surfaces of Karyopherin proteins.
A) Chemical shift differences, Δ = [(Δ1H)2 + ((10*Δ15N))2]0.5, between the isolated RRM (FUS residues 285–371) and the RRM within FUS(164–500). B) Ribbon and surface representations of the FUS RRM (PDBID 1LCW, green), with residues Δ > 0.2 colored blue. Residues that directly contact Kapβ2 based on cross saturation transfer data are colored magenta (Figure 4B). At 2 μM and 8 μM concentration, FUS(ΔRRM) has a higher Tcloud than wildtype FUS, indicating that deletion of the RRM favors phase separation. To gain insight into this behavior, we compared the chemical shifts of the isolated RRM domain to those of the RRM within FUS(164–500). A number of amide resonances, mostly on the face of the domain opposite the Kapβ2 binding site, differ between the two constructs. This observation suggests that in FUS(164–500), RRM participates in intramolecular contacts with the RGG regions, which could sequester these elements. Given that the RGG regions play important roles in phase separation, their sequestration by RRM may suppress phase separation. C) Temperature dependence of FUS phase separation. Turbidity (OD395nm) of 2 μM MBP-FUS proteins (wildtype (WT) and mutants) after 3 h treatment with Tev protease was monitored as temperature was decreased from 40°C or 45°C to 5°C. Optical densities were normalized to values measured at 5°C. TCloud is the x-intercept of the tangent at the inflection point of the curve (mean of 3 technical replicates, ± S.D.). D) Mixtures containing 2 μM MBP-FUS WT, MBP-FUS(1–452), or MBP-FUS(ΔRRM), 20 nM FUS-GFP were treated with Tev protease at room temperature for 50 min to form FUS droplets. The phase separated mixtures were cooled to either 10°C or 15°C, held a t those temperatures for 2 min, and then increased by 2°C increments to a maximum temperature of 43°C or 44°C. The sample was held at each temperature for 2–3 min prior to acquisition of a 50 μm Z-stack (1 μm increments) using spinning disk confocal microscopy. Panels show maximum projection images derived from the Z-stack 10–50 μm above the slide surface. To calculate Tcloud, images in this same portion of the Z-stack were segmented using the Triangle algorithm in ImageJ and the total number of FUS droplets was determined after a filter for circularity (>0.5) and size (>0.5 μm2). Tcloud was determined from the x-intercept of a line fit to the first six (WT and FUS(1–452)) or eight (FUS(ΔRRM)) points in the (number of puncta) vs. temperature curve. E) Electrostatic surface potential (scale of −12 kTe−1 to +12 kTe−1) of Importins Kapβ2 (PDBID 4FDD), Impβ (1QGK) and Kap121 (3W3W). PY-NLS bound to Kapβ2, IBB (NLS) bound to Impβ and IK-NLS bound to Kap121 are shown as cyan cartoons. Arrows point to acidic patches on the concave surface of the Importins. F) Electrostatic surface potential (scale of −12 kTe−1 to +12 kTe−1) of Exportins CRM1 (PDBID 3GB8), CSE1 (1WA5) and XPO5 (3A6P). The NES (residues 1–15) of cargo SNUPN bound to CRM1 is shown as a cyan cartoon. Cargos bound to CSE1 (Impα) and XPO5 (pre-miRNA), along with bound RanGTP, were removed to allow viewing of the concave surfaces of the Exportin. Arrows point to basic patches on the concave surface of the Exportins.
8
Supplementary movie 2.avi., related to Figure 1. FUS droplets (from 5 μM MBP-FUS + Tev), disappearing within 5 minutes of addition of 10 μM of Kapβ2.
9
Supplementary movie 3.avi., related to Figure 1. Phase separated FUS (48 hours after Tev was added to 5 μM MBP-FUS) imaged for 20 minutes after addition of 10 μM of Kapβ2.