Fibroblast growth factor 21 (FGF21) and bone: is there a relationship in humans? (original) (raw)
. Author manuscript; available in PMC: 2019 Jan 2.
Published in final edited form as: Osteoporos Int. 2013 Aug 3;24(12):3053–3057. doi: 10.1007/s00198-013-2464-9
Abstract
Summary
In animals, high fibroblast growth factor 21 (FGF21) states improve insulin resistance but induce bone loss. Whether FGF21 relates to bone mineral density (BMD) is unknown in humans. Contrary to prediction from animal findings, we found higher FGF21 levels associating with greater BMD in women, independent of age and body composition.
Introduction
Recent laboratory studies suggest that FGF21 is involved in reciprocal regulation of bone and energy homeostasis. Systemic administration of FGF21 protects animals from obesity and diabetes but causes severe bone loss, smothering the enthusiasm over FGF21 as a potential antiobesity therapeutic. To date, there is no information on whether FGF21 relates to BMD in humans. We thus studied the relationship between plasma FGF21 levels and BMD in healthy adults.
Methods
Fasting plasma FGF21 levels were measured by enzyme-linked immunosorbent assay and body composition by dual-energy X-ray absorptiometry.
Results
Among 40 healthy volunteers (age 32±10 year, 16 women), men had significantly higher lean body mass (_p<_0.01) and total BMD (_p<_0.05), and lower percent body fat than women (_p<_0.01). Median plasma FGF21 levels were not different between the sexes. While there was no association between FGF21 concentrations and body composition in men, FGF21 levels correlated positively with fat mass (_p<_0.01) in women. In men, no significant correlation between FGF21 with BMD was observed. However, in women, FGF21 correlated positively with total BMD (_R_2=0.69, _p=_0.003) and spine BMD (_R_2=0.76, _p=_0.001); the correlation remained significant after adjusting for age, ethnicity, and body composition. Conclusions This study reveals for the first time a strong positive association between plasma FGF21 levels and BMD in healthy women, suggesting the association between bone loss and high FGF21 states in animals may not be directly translated to humans in physiologic states. We hypothesize that FGF21 may increase bone mass particularly in women through paracrine mechanisms in the bone–adipose interface.
Keywords: Beige adipose tissue, Bone mineral density, Brown adipose tissue, FGF21, Fibroblast growth factor-21
Introduction
Fibroblast growth factor 21 (FGF21) is a newly identified member of the FGF family that plays key roles in energy balance [1]. It is expressed in liver and adipose tissue, acting locally in the regulation of ketogenesis and lipolysis. Aside the autocrine/paracrine action common to most FGFs, FGF21 is also secreted into the circulation, thus functioning as a hormone capable of modulating systemic glucose and lipid metabolism [2]. These properties define FGF21 as a factor with dual endocrine and paracrine actions, orchestrating the interplay between nutrient fluxes and fuel demands.
The effects of FGF21 manipulation in animals with respect to energy balance and bone biology are provocative and dramatic: pharmacological administration of FGF21 imparts profound metabolic benefits, reversing diabetes, hepatic steatosis, and obesity [1, 3, 4]. However, genetic FGF21 over-expression decreases bone mass and systemic FGF21 treatment leads to severe bone loss in mice [5]. Thus FGF21 appears to exert reciprocal regulation of bone and energy homeostasis in animals. The adverse skeletal effects have cast shadows over the promise of FGF21 as a potential therapeutic for obesity-related disorders [6].
To date, there is no information on whether FGF21 relates to bone mass in humans. This is clinically important in light of bone loss induced by high FGF21 states in animals. To investigate this, we determine the interrelationship between circulating FGF21 levels, bone mineral density (BMD), and body composition in healthy men and women.
Materials and methods
Subjects
Study participants consisted of 40 volunteers (16 women) recruited through local advertisement to participate in protocols related to energy expenditure/metabolism at the National Institutes of Health (ClinicalTrials.gov identifier numbers NCT00521729 and NCT01730105). Volunteers with full body compositional assessment and sufficient stored blood samples for FGF21 measurement were included in the study. Energy expenditure results of 12 subjects included in the current study have been published previously [7, 8]. All subjects were healthy and on no medications. Women were studied during the follicular phase of the menstrual cycle, except one female who was postmenopausal. Volunteers were admitted to the Metabolic Clinical Research Unit of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) in Bethesda, Maryland, for fasting blood sampling and assessment of body composition and BMD. The protocol was approved by the NIDDK-NIAMS institutional review board and informed consent was obtained from all subjects.
BMD and body composition
BMD and body composition were measured by dual-energy x-ray absorptiometry (Lunar iDXA; GE Healthcare, Madison, WI). Scan analysis was performed using GE Encore 11.10 software. The following parameters were measured in each volunteer: BMD, bone mineral content (BMC), and bone area of total body, spine, pelvis, legs, and arms; fat mass (FM) and lean body mass (LBM). The coefficient of variation of BMD, FM, and LBM were 0.5, 1.0, and 0.5 %, respectively.
FGF21 measurement
Blood samples were collected in EDTA-coated chilled tubes after an overnight fast, immediately separated, and stored at −80 °C for later analyses. Plasma FGF21 concentration was measured by ELISA (BioVendor, Oxford, U.K.) according to the manufacturer’s protocol, with intra-assay and inter-assay coefficients of variation of 3.6 and 3.8 %, respectively. No cross-reactivity with other human endocrine FGFs (FGF19 and FGF23) has been observed.
Statistical analysis
Statistical analysis was performed using SPSS 20.0 (SPSS, Inc., Chicago, IL). Data are expressed as mean±standard deviation for normally distributed variables. FGF21 levels were skewed, and were therefore log-transformed to normalize distribution in regression analyses. Median (interquartile range) values of FGF21 are presented in descriptive statistics. Men and women were analyzed separately. Comparisons between results were performed using the un-paired t test and the χ2 test for continuous and categorical baseline characteristics, respectively. Pearson correlation coefficients were used to examine linear relations between variables. Multivariate linear regression models using analysis of variance were constructed to determine the predictors of BMD; the model included age, ethnicity, body compositional parameters, and log FGF21 levels. Association between FGF21 and BMD was further examined by partial correlation analyses controlled for body compositional parameters. An α error of 0.05 was considered the threshold for statistical significance.
Results
Sex-specific cohort characteristics of participating volunteers are shown in Table 1. As expected, men had significantly higher LBM (_p<_0.01) and total BMD (_p<_0.05), and lower percent body fat than women (_p<_0.01). There was a strong trend for higher FM in women (_p=_0.06). Median plasma FGF21 levels were not different between the sexes.
Table 1.
Clinical characteristics
| All | Men | Women | |
|---|---|---|---|
| N | 40 | 24 | 16 |
| Age (years) | 32±10 | 32±10 | 34±10 |
| Ethnicity (N) | |||
| White | 22 | 15 | 7 |
| African American | 14 | 6 | 8 |
| Other/mixed ethnicity | 4 | 3 | 1 |
| Weight (kg) | 70.2±ll.l | 74.8±2.8 | 64.4±2.8 |
| Height (m) | 1.7±0.1 | 1.8±0.1 | 1.7±0.1* |
| Body mass index (kg/m2) | 26.7±4.9 | 26.7±4.6 | 26.8±5.5 |
| Fat mass (kg) | 24.7±12.2 | 21.8±10.6 | 29.1±13.3 |
| % Fat mass (%) | 30.0±10.9 | 25.3±8.9 | 37.0±10.2* |
| Lean body mass (kg) | 53.9±10.5 | 59.7±9.3 | 45.2±4.5* |
| Total BMD (g/cm2) | 1.31±0.12 | 1.33±0.12 | 1.27±0.11* |
| Total BMC (g) | 3,084±513 | 3,306±520 | 2,752±273* |
| Total bone area (cm2) | 2,349±227 | 2,467±210 | 2,172±103* |
| Spine BMD (g/cm2) | 1.13±0.16 | 1.12±0.16 | 1.13±0.16 |
| Pelvis BMD (g/cm2) | 1.15±0.15 | 1.18±0.16 | l.11±0.11 |
| Leg BMD (g/cm2) | 1.41±0.15 | 1.47±0.13 | 1.31±0.12* |
| Arm BMD (g/cm2) | 1.903±0.14 | 1.08±0.14 | 0.94±0.09* |
| Plasma FGF21 (pg/ml)a | 138 (108–181) | 141 (108–180) | 137 (113–184) |
BMD and body composition
Electronic supplementary material (ESM) Supplemental Table 1 shows simple correlations between age, body compositional measurements, FGF21 concentration, and bone parameters. Total and regional BMDs at multiple bone sites correlated positively with LBM in men (_p<_0.01) but not with FM or percent body fat. In women, only spine BMD correlated positively with LBM (_p<_0.05), FM (_p<_0.01), and percent body fat (_p<_0.01). A positive correlation approached significance between total BMD and percent body fat (_p=_0.06).
FGF21 and body composition
In men, there was no association between FGF21 concentrations, LBM, FM, or percent body fat. In women, FGF21 levels correlated positively with FM (_p<_0.01) and percent body fat (_p<_0.01). There was no relationship between FGF21 and LBM in women.
FGF21 and BMD
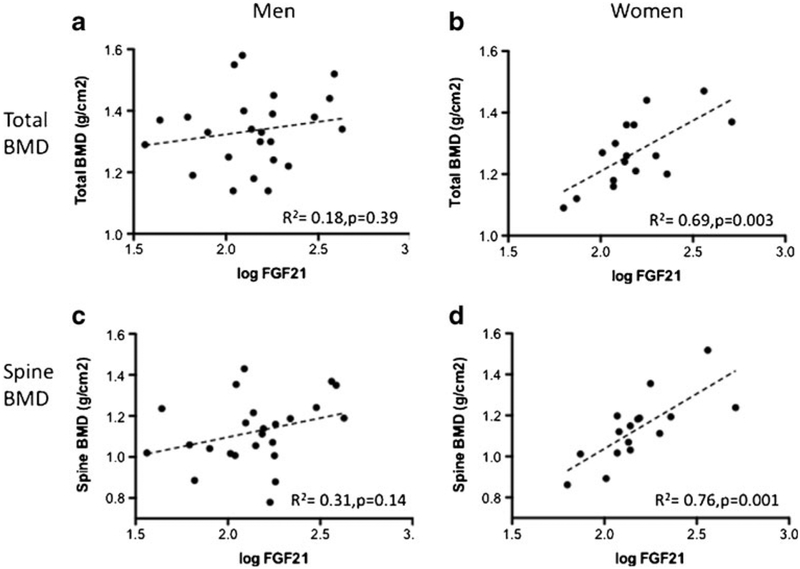
Figure 1 shows the relationship between FGF21 and BMD. There were no significant correlation between FGF21 with total [panel a] and spine BMD [panel c] in men. However, in women, FGF21 correlated positively with total [panel b] and spine BMD [panel d]. In a forward step-wise multiple regression analysis controlling for age, ethnicity, height, body weight, body mass index, LBM, FM, and percent body fat, FGF21 was the single independent predictor of total BMD (_β=_0.47, _p=_0.003) and spine BMD (_β=_0.58, _p=_0.001) in women. The positive relationship between FGF21, total BMD, and spine BMD observed in women remained significant in partial correlation analyses adjusting for body compositional parameters [ESM Supplemental Table 2]. Exclusion of the postmenopausal subject did not alter conclusions of study analyses. There was no evidence of significant col-linearity; the variance inflation factors for all models were between 1.1 and 1.9.
Fig 1.
Gender-specific correlations between bone mineral density (BMD) and plasma fibroblast growth factor-21 (FGF21) levels. No relationships were present between FGF21 and a total or c spinal BMD in men while FGF21 levels correlated positively with both b total BMD and d spine BMD in women
Discussion
To the best of our knowledge, this is the first study investigating the relationship between FGF21 levels and BMD in humans. We revealed a robust, positive correlation between plasma FGF21 concentrations with BMD in women but not men, independent of age, ethnicity, FM, and LBM. These findings provide a human perspective on FGF21-bone interaction, contrasting with the recently reported bone loss pheno-type in various high FGF21 states in rodents [5, 9].
The bone–adipose axis is emerging as a novel endocrine domain critical in reciprocal regulation of energy and skeletal homeostasis [10]. This is exemplified by FGF21, a factor that induces far-reaching effects in bone and adipose tissue. FGF21 augments substrate utilization via PPARα- and PPARγ-dependent pathways in liver and adipose, respectively [2]. High FGF21 states in animals enhance insulin sensitivity and protection against obesity [1]. This is at the expense of severe bone loss secondary to PPARγ-induced osteoblastogenesis inhibition and increased bone resorption [5]. Contrary to a predicted negative association between FGF21 and BMD based on animal findings, we observed a robust positive relationship between FGF21 and BMD at multiple sites in women. Positive correlations were present in men between FGF21 and all BMD sites, but did not reach statistical significance (Fig. 1).
Our results shed novel insight on FGF21–bone interaction in the context of known animal biology. Bone loss occurs in animals following pharmacological FGF21 administration or overexpression, while the current study indicates a positive association between FGF21 and BMD in human under physiologic conditions. It is possible the effects of FGF21 on skeleton metabolism are dependent on circulating concentration and therefore tissue exposure. We hereof attempt to decipher potential underlying mechanisms based on our findings.
A significant positive correlation between FGF21 and BMD was observed only in women. Such gender dimorphism is reminiscent of our recently reported female-specific association between higher brown adipose tissue (BAT) mass and BMD [11]. Because BAT secretes FGF21 [12] and women have more BAT than men [13], it is possible that BAT-secreted FGF21 mechanistically links higher FGF21 levels and greater BAT mass with higher BMD. Furthermore, both FGF21 and BAT mass [11] were more strongly associated with spine than total and other regional BMDs; this finding is supportive of region-specific interactions. As vertebral bone marrow fat harbors brown adipocyte-like cells [14, 15], it is biologically plausible that local BAT-derived FGF21 contributes to the stronger FGF21-BMD association observed at the spine than at other cortical bone sites.
Taken together, we hypothesize bone–adipose paracrine interaction to be the principal mechanism that underlies the positive association between FGF21 and BMD in physiologic states. This is supported by recent in vitro studies revealing an enhancing effect of FGF21 on the osteogenic activity of bone morphogenic protein 2 [16], a factor also known to stimulate brown adipogenesis [17]. In contrast, bone loss arising from systemic FGF21 administration could be a consequence of the activation of catabolic endocrine bone remodeling pathways by supraphysiologic FGF21 concentrations. Future studies should incorporate bone turnover marker measurements and BAT quantification to further the understanding on the interaction between FGF21, BAT, and bone. This may uncover adipose–bone crosstalk mechanisms directly relevant to humans, given the recent rediscovery of significant BAT depots in adults [18] and the identification of FGF21 as a human brown adipokine [8, 19].
Small sample size and cross-sectional design are limitations of the current study. Despite careful adjustment for potential confounders, larger confirmatory studies are required to exclude a type 1 error in the observed relationship between FGF21 and BMD, and to assess if this relationship is maintained in elderly individuals. While a type 2 error could explain the absence of these relationships in men, it is also possible that higher LBM in men overshadows the significance of fat-derived FGF21 on bone. We emphasize that a causal relationship between FGF21 and higher BMD cannot be established in the current study. However, our study provides the first human perspective on FGF21 bone biology, which is clinically relevant at a time when FGF21 therapeutic trials are poised to begin. The sequelae of FGF21 treatment in humans on bone and adipose may be more complex than what animal studies have suggested and could ultimately depend on dosing, as well as the balance of its paracrine/endocrine actions and individual BAT abundance.
In summary, the current study reveals a strong positive association between plasma FGF21 levels and BMD in healthy women, independent of age, ethnicity, and other body compositional parameters. No significant correlations were present in men. These findings indicate the recently reported association between bone loss and high FGF21 states in animals may not be directly translated to humans in physiologic states.
Supplementary Material
suppl
Acknowledgments
Paul Lee was supported by an Australian National Health Medical Research Council (NHMRC) Early Career Fellowship, the Royal Australasian College of Physicians (RACP) Foundations Diabetes Australia Fellowship, and Bushell Travelling Fellowship. This study was supported by the Intramural Research Program of NIDDK: programs Z01-DK047057–02 and Z01-DK071044.
Footnotes
Conflicts of interest None.
References
- 1.Kharitonenkov A, Shiyanova TL, Koester A et al. (2005) FGF-21 as a novel metabolic regulator. J Clin Invest 115:1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams AC, Kharitonenkov A (2012) FGF21: the center of a transcriptional nexus in metabolic regulation. Curr Diabetes Rev 8:285–293 [DOI] [PubMed] [Google Scholar]
- 3.Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A (2008) Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149:6018–6027 [DOI] [PubMed] [Google Scholar]
- 4.Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ (2007) The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 148:774–781 [DOI] [PubMed] [Google Scholar]
- 5.Wei W, Dutchak PA, Wang X et al. (2012) Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor gamma. Proc Natl Acad Sci USA 109:3143–3148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J, Cohen P, Spiegelman BM (2013) Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev 27:234–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celi FS, Brychta RJ, Linderman JD et al. (2010) Minimal changes in environmental temperature result in a significant increase in energy expenditure and changes in the hormonal homeostasis in healthy adults. Eur J Endocrinol 163:863–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee P, Brychta RJ, Linderman J, Smith S, Chen KY, Celi FS (2013) Mild cold exposure modulates fibroblast growth factor 21 (FGF21) diurnal rhythm in humans: relationship between FGF21 levels, lipolysis, and cold-induced thermogenesis. J Clin Endocrinol Metab 98:E98–E102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ables GP, Perrone CE, Orentreich D, Orentreich N (2012) Methionine-restricted C57BL/6J mice are resistant to diet-induced obesity and insulin resistance but have low bone density. PLoS One 7:e51357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guntur AR, Rosen CJ (2012) Bone as an endocrine organ. Endocr Pract 18:758–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee P, Brychta RJ, Collins MT, Linderman J, Smith S, Herscovitch P, Millo C, Chen KY, Celi FS (2012) Cold-activated brown adipose tissue is an independent predictor of higher bone mineral density in women. Osteoporos Int 24(4):1513–1518. doi: 10.1007/s00198-012-2110-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher FM, Kleiner S, Douris N et al. (2012) FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermo-genesis. Genes Dev 26:271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee P, Greenfield JR, Ho KK, Fulham MJ (2010) A critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 299:E601–E606 [DOI] [PubMed] [Google Scholar]
- 14.Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B (2012) Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone 50:546–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishio M, Yoneshiro T, Nakahara M et al. (2012) Production of functional classical brown adipocytes from human pluripotent stem cells using specific hemopoietin cocktail without gene transfer. Cell Metab 16:394–406 [DOI] [PubMed] [Google Scholar]
- 16.Ishida K, Haudenschild DR (2013) Interactions between FGF21 and BMP-2 in osteogenesis. Biochem Biophys Res Commun 432(4):677–682. doi: 10.1016/j.bbrc.2013.02.019 [DOI] [PubMed] [Google Scholar]
- 17.Salisbury EA, Lazard ZW, Ubogu EE, Davis AR, Olmsted-Davis EA (2012) Transient brown adipocyte-like cells derive from peripheral nerve progenitors in response to bone morphogenetic protein 2. Stem Cells Transl Med 1:874–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee P, Swarbrick MM, Ho KK (2013) Brown adipose tissue in adult humans: a metabolic renaissance. Endocr Rev 34:413–438 [DOI] [PubMed] [Google Scholar]
- 19.Lee P, Werner CD, Kebebew E, Celi FS (2013) Functional thermogenic beige adipogenesis is inducible in human neck fat. Int J Obes (Lond). doi: 10.1038/ijo.2013.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
suppl