Prospects for a MERS-CoV spike vaccine (original) (raw)
. Author manuscript; available in PMC: 2019 Aug 9.
Abstract
Introduction:
Six years have passed since Middle East respiratory syndrome (MERS) coronavirus (MERS-CoV), a newly emerging infectious virus, was first reported in 2012. Although MERS-CoV has had a consistently high mortality rate in humans, no vaccines have been approved to prevent MERS-CoV infection in humans. MERS-CoV spike (S) protein is a key target for development of MERS vaccines.
Areas covered:
In this review, we illustrate the structure and function of S protein as a vaccine target, describe available animal models for evaluating MERS vaccines, and summarize recent progress on MERS-CoV S-based vaccines, focusing on their ability to elicit antibody and/or cellular immune responses, neutralizing antibodies, and protection against MERS-CoV infection in different models. Prospects for future MERS-CoV S-based vaccines are discussed.
Expert commentary:
The majority of MERS vaccines under development are based on MERS-CoV S protein, including full-length S, S1, and receptor-binding domain (RBD). While it is essential to evaluate the safety of full-length S and S1-based MERS vaccines, further improvement of the efficacy of RBD-based vaccines using novel strategies would be necessary. Overall, this review provides informative guidance for designing and developing safe and effective MERS vaccines based on viral S protein.
Keywords: MERS, MERS-CoV, Spike protein, Receptor-binding domain, Vaccine, Immune responses, Neutralizing antibodies, Protection
1. Introduction
Middle East respiratory syndrome (MERS) coronavirus (MERS-CoV), a newly emerging infectious virus, was first identified in Saudi Arabia in June 2012 [1]. Since then, MERS-CoV has infected 2,229 humans, including 791 deaths (mortality rate ~35%), in at least 27 countries, including Saudi Arabia, South Korea, Iran, Jordan, Qatar, United Kingdom, United States, China, and Thailand [2]. Of note, Saudi Arabia has the largest number of MERS cases, covering 80% of all cases, while in 2015, South Korea reported the largest outbreak of MERS-CoV infection in humans outside Saudi Arabia [3,4]. MERS-CoV has been added to the WHO Blueprint list of priority viruses that cause public health emergency but still lack efficacious drugs and vaccines [5].
MERS-CoV is a zoonotic viral pathogen. It likely uses bats as its natural host, but MERS-CoV replicates in bats without showing clinical signs of disease [6,7]. Dromedary camels are evidenced as the intermediate host of MERS-CoV. The virus is able to transmit from camels to camels, and dromedary camels demonstrate high seropositivity to MERS-CoV [8–10]. Transmission of MERS-CoV from camels to humans occurs, and a number of risk factors, such as direct contact with MERS-CoV-infected dromedary camels, have been identified in camel workers [11–13]. Although MERS-CoV is less able to cause human-to-human transmission compared to severe acute respiratory syndrome coronavirus (SARS-CoV), another coronavirus which led to the 2003 outbreak in humans [14] and is in the same β-genus as MERS-CoV, it does lead to human infections among healthcare workers and patients through healthcare or household-acquired infection [12,15,16]. MERS-CoV continues to cause cases and outbreaks in the Middle East, constituting an ongoing risk to global health security [13,17]. Therefore, effective medical countermeasures, such as vaccines and therapeutic agents, are urgently needed to block camel-to-camel and camel-to-human transmission of MERS-CoV and to prevent and treat MERS-CoV infection in humans.
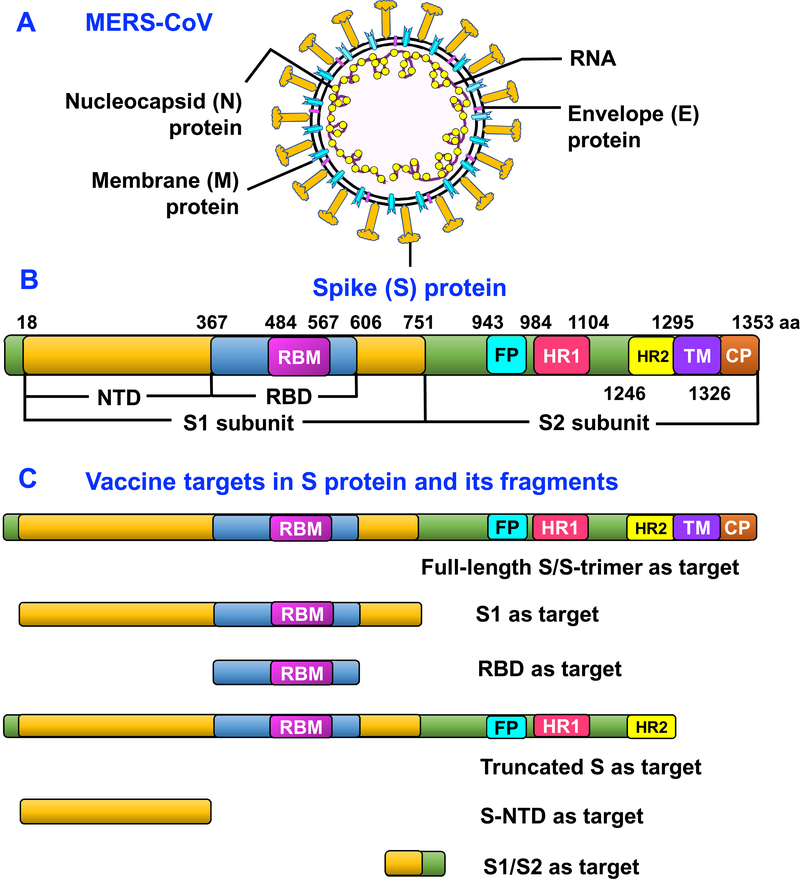
The MERS-CoV RNA genome encodes four structural proteins, including spike (S), membrane (M), nucleocapsid (N), and envelope (E) (Fig. 1A), as well as accessory open reading frame (ORF) proteins, such as non-structural proteins (nsp1–16) and ORF3, 4a, 4b, 5, and 8b [18,19]. Although several MERS-CoV proteins still have unknown functions in the process of MERS-CoV life cycle, infection, or pathogenesis, progress has been made on the structures and/or functions of MERS-CoV accessory proteins, some of which are directly or indirectly related to MERS-CoV pathogenesis [20,21]. For example, MERS-CoV accessory ORF3, 4a, 4b, and 5, as well as nsp16, proteins are necessary for viral infection and pathogenesis. The deletion or attenuation of ORF3–5 proteins dysregulates host responses and augments inflammation, while mutation of nsp16 significantly attenuates MERS-CoV infection in cell cultures and in vivo [22,23].
Figure 1.
MERS-CoV and S protein structures and vaccine targets in MERS-CoV S protein. (A) Schematic structure of MERS-CoV and its key structural proteins, including S, N, M, and E proteins. (B) Schematic structure of MERS-CoV S protein and its functional regions. S protein is composed of S1 and S2 subunits. NTD, N-terminal domain. RBD, receptor-binding domain. RBM, receptor-binding motif. FP, fusion peptide. HR1 and HR2, heptad repeat 1 and 2 regions. TM, transmembrane. CP, cytoplasmic tail. (C) Key vaccine targets in MERS-CoV S protein and its fragments.
2. MERS-CoV S protein
Located at the MERS-CoV surface, S protein engages with viral cellular receptor dipeptidyl peptidase 4 (DPP4, e.g., CD26), mediating viral attachment to host cells and subsequent virus-cell membrane fusion [24–26]. MERS-CoV S protein consists of S1 and S2 subunits. The receptor-binding domain (RBD), a functional domain in S1, is divided into a core subdomain and receptor-binding motif (RBM), while the latter contains the motif and key residues to bind DPP4 receptor on the host cell membrane [27,28]. After binding to cellular receptor, MERS-CoV S protein undergoes significant conformational changes, exposing heptad repeat 1 (HR1) and 2 (HR2) regions in S2 subunit, further forming a 6-helix bundle fusion core, leading to membrane fusion [29]. Recent structural studies on MERS-CoV S-trimer also suggest a potential mechanism for initiating the fusion process through receptor-induced triggering which involves sequential activation of protomers [30]. The schematic structure of MERS-CoV S protein and its functional regions are described in Figure 1B.
Theoretically, MERS-CoV S1 and S2 subunits, as well as their functional regions, such as RBD, play different roles in MERS-CoV infection and pathogenesis. Therefore, S1, RBD, and/or full-length S protein all serve as good targets to develop MERS-CoV vaccines and anti-MERS-CoV therapeutic antibodies, while S2, particularly its HR1 region, is an ideal target to develop anti-MERS-CoV fusion inhibitors [18,19]. Indeed, MERS-CoV S protein is able to induce strong antibody responses and/or cellular immune responses in immunized animals, in which S-specific neutralizing antibodies play a key role in preventing MERS-CoV infection [31–33]. It has been shown that an anti-MERS-CoV polyclonal antibody produced from S protein nanoparticle-immunized transchromosomic (Tc) cattle inhibits MERS-CoV infection in mice and demonstrates safety and tolerability in human Phase I trials [34,35]. Also, monoclonal antibodies or nanobodies targeting MERS-CoV S protein significantly protect against MERS-CoV infection in different animal models, including transgenic mice, rabbits, and non-human primates (NHPs) [18,36–38]. These studies highlight the importance of S protein-based vaccines and therapeutics in preventing and treating MERS-CoV infection. Previous reviews have summarized anti-MERS-CoV therapeutics targeting S protein [18]. This review will focus on the MERS-CoV S protein-based vaccines. The key vaccine targets in MERS-CoV S protein are illustrated in Figure 1C.
3. Animal models available for evaluating the efficacy of MERS vaccines
MERS vaccines, including those based on the S protein, must be evaluated in suitable animal models to prove their efficacy before moving to large-scale production and/or human clinical trials. Other than dromedary camels, MERS-CoV has shown susceptibility to llamas, pigs, and rabbits, but not to goats, sheep and horses [36,39–41]. MERS-CoV may infect NHPs, including rhesus macaques and marmosets [42–44], although the two appear to have varying degrees of histopathological changes and pneumonia in the lungs after MERS-CoV infection [45]. Thus, NHPs can be used as effective animal models to evaluate protective immunity of MERS candidate vaccines.
MERS-CoV does not naturally infect small animals, including mice, ferrets, and hamsters. Mice expressing human DPP4 (hDPP4), including adenovirus 5 (Ad5)/hDPP4-transduced mice, hDPP4-Tg mice (transgenic for expressing hDPP4), and humanized (HuDPP4) mice (replacing mouse DPP4 with hDPP4), have been developed accordingly and are susceptible to wild-type MERS-CoV strains [46–50]. In addition, a mouse model was generated by modifying the mouse genome encoding two mouse amino acids into corresponding human sequences in the DPP4 receptor, resulting in susceptibility to a mouse-adapted MERS-CoV and viral replication with lung symptoms and diseases [51]. Other mouse models, such as hDPP4-knock-in mice, were also developed, and they are susceptible to a mouse-adapted MERS-CoV clone, showing lung diseases [52]. Compared with large animal models, these small mouse models can be used as convenient and economical vehicles to evaluate the efficacy of MERS vaccines.
4. Advances in the development of MERS-CoV S protein-based vaccines
Like SARS and other viral vaccines, a good MERS vaccine should induce strong immune responses, elicit potent neutralizing antibodies, and demonstrate high protection in vaccinated animals and humans [14,19]. No MERS vaccine is approved for use in humans. Most currently developed vaccines against MERS-CoV are in preclinical stages, the majority of which are based on viral S protein [31,53–55]. These MERS-CoV S-targeting vaccines are summarized in Tables 1 and 2, and they are described later as vaccines based on the full-length S/S-trimer, S1, RBD, and other regions of MERS-CoV S protein, focusing on their ability to induce humoral and/or cellular immune responses and neutralizing antibodies, as well as protection against MERS-CoV infection, in different animal models.
Table 1.
Summary of vaccines based on MERS-CoV full-length S or S-trimer proteinsa
| Vaccines | Categories | Antibody responses and neutralizing antibodies | Cellular immune responses | Protection | Ref. |
|---|---|---|---|---|---|
| Viral vector-based | Ad (human Ad5/ Ad41; simian Ad: ChAdOx1) | Induces MERS-CoV S/RBD-specific systemic and mucosal antibody responses and neutralizing antibodies in mice; neutralizes pseudotyped and live MERS-CoV (EMC2012, most <1:103) and vector Ad | Elicits MERS-CoV S-specific T cell responses in mice | Protects hDPP4-Tg mice from MERS-CoV (EMC2012) challenge; Phase I trial (ChAdOx1) | [56,61,64–66] |
| MVA | Induces MERS-CoV S- and/or vector MVA-specific systemic and/or mucosal antibody responses in mice and camels; induces neutralizing antibodies against live MERS-CoV (EMC2012, ≥1:103), MVA, and camelpox virus | Elicits MERS-CoV S and vector MVA-specific T cell responses in mice | Protects Ad5/hDPP4-transuced mice and camels from MERS-CoV (EMC2012) challenge; protection correlates with serum neutralizing antibodies | [31,62,65,67] | |
| MV | Induces MERS-CoV S- and vector MV-specific antibody responses and neutralizing antibodies in mice; neutralizes live MERS-CoV (EMC2012, <1:103) | Elicits MERS-CoV S- and vector MV-specific T cell responses in mice | Protects Ad/hDPP4-transduced mice from MERS-CoV (EMC2012) challenge | [68] | |
| VSV | Induces MERS-CoV S-specific antibody responses and neutralizing antibodies in mice and NHPs; neutralizes pseudotyped MERS-CoV | Elicits MERS-CoV S-specific T cell responses in NHPs | Not reported | [69] | |
| DNA-based | DNA | Induces MERS-CoV S-specific antibody responses and neutralizing antibodies in mice, camels, and NHPs; neutralizes divergent pseudotyped and/or live MERS-CoV (human/1390, camel/31, camel/39, EMC2012, 1:102–103) | Elicits MERS-CoV S-specific T cell responses in mice and NHPs | Protects NHPs against MERS-CoV (EMC2012) challenge, without demonstrating clinical or radiographic signs of pneumonia; Phase I trial | [55,57,63] |
| DNA/protein-based | DNA prime/ protein (S1) boost | Induces persistent MERS-CoV S-targeting antibody responses and neutralizing antibodies in mice and/or NHPs; neutralizes divergent pseudotyped and live MERS-CoV (Jordan-N3) | Not reported | Protects NHPs from MERS-CoV (Jordan-N3) challenge and virus-induced radiographic pneumonia | [58] |
| VLP-based | VLP | Induces MERS-CoV S-RBD-specific antibody responses and neutralizing antibodies (1:40) in NHPs | Elicits MERS-CoV S-RBD-specific T cell responses in NHPs | Not reported | [59] |
| Nanoparticle-based | Nanoparticle | Induces MERS-CoV S-specific antibody response and neutralizing antibodies (1:102–103) in mice and Tc bovines; neutralizes live MERS-CoV (Jordan) | Not reported | Protects Ad5/hDPP4-transduced mice from MERS-CoV (Jordan or EMC2012) challenge | [34,53,60] |
| S-trimer protein-based | Subunit | Induces neutralizing antibodies in mice; neutralizes variant pseudotyped MERS-CoV | Not reported | Not reported | [30] |
Table 2.
Summary of vaccines based on MERS-CoV S fragmentsa
| Vaccines | Categories | Antibody responses and neutralizing antibodies | Cellular immune responses | Protection | Ref. |
|---|---|---|---|---|---|
| Vaccines based on MERS-CoV S1 | |||||
| Viral vector-based | Ad (Ad5) | Induces MERS-CoV S-specific antibody responses and neutralizing antibodies (>1:103) in mice; neutralizes live MERS-CoV (EMC2012) | Not reported | Not reported | [56] |
| RABV | Induces MERS-CoV S- and vector RABV-specific antibody responses and neutralizing antibodies (>1:103) in mice; neutralizes live MERS-CoV (Jordan-N3) | Not reported | Protects Ad5/hDPP4-transuced mice from MERS-CoV (Jordan-N3) challenge | [70] | |
| DNA-based | DNA | Induces MERS-CoV S/RBD-specific antibody responses and neutralizing antibodies (1:102–104) in mice; neutralizes live MERS-CoV (EMC2012, human/1390, camel/31, and camel/39) | Elicits MERS-CoV S-RBD-specific T cell responses in mice | Protects Ad5/hDPP4-transuced mice from MERS-CoV (EMC2012) challenge | [55,71] |
| Protein-based | Subunit | Induces MERS-CoV S-targeting antibody responses and neutralizing antibodies in mice and NHPs; neutralizes divergent pseudotyped and live MERS-CoV (Jordan-N3) | Not reported | Protects NHPs from MERS-CoV (Jordan-N3) challenge | [58] |
| Vaccines based on MERS-CoV RBD | |||||
| VLP-based | VLP | Induces MERS-CoV S-RBD-specific antibody responses and neutralizing antibodies in mice; neutralizes pseudotyped MERS-CoV | Elicits S-RBD-specific T cell responses in mice | Not reported | [72] |
| Protein-based (residues 358–588) | Subunit | Induces MERS-CoV-specific antibody responses and neutralizing antibodies (>1:103) in rabbits | Not reported | Not reported | [79] |
| Protein-based (residues 377–588) | Subunit | Induces MERS-CoV S/RBD-specific antibody responses and neutralizing antibodies in mice and rabbits; neutralizes divergent strains of pseudotyped and live MERS-CoV (EMC2012, London1–2012, >1:103) | Elicits MERS-CoV S-RBD-specific T cell responses in mice | Protects Ad5/hDPP4-transduced mice and hDPP4-Tg mice from MERS-CoV (EMC2012) challenge without causing immuno-pathological effects | [32,33,74–76,81–83] |
| Protein-based (residues 377–662) | Subunit | Induces MERS-CoV S/RBD-specific systemic and mucosal antibody responses and neutralizing antibodies in mice; neutralizes pseudotyped and live MERS-CoV (EMC2012, 1:102–103) | Induces MERS-CoV S-specific T cell responses in mice | Not reported | [77,78,84] |
| Protein-based (residues 367–606) | Subunit | Induces MERS-CoV S-RBD-specific antibody responses and neutralizing antibodies in mice and/or NHPs; neutralizes pseudotyped and live MERS-CoV (EMC2012, <1:103) | Elicits MERS-CoV S-RBD-specific T cell responses in mice | Partially protects NHPs from MERS-CoV (EMC2012) challenge | [73,80] |
| Vaccines based on other S regions of MERS-CoV, including truncated S, S-NTD, and S1/S2 | |||||
| Viral vector-based (truncated S) | MV vector | Induces MERS-CoV S- and vector MV-specific antibody responses and neutralizing antibodies in mice; neutralizes live MERS-CoV (EMC2012, <1:103) and MV | Elicits MERS-CoV S- and vector MV-specific T cell responses in mice | Protects Ad/hDPP4-transduced mice from MERS-CoV (EMC2012) challenge | [68] |
| Protein-based (S-NTD) | Subunit | Induces MERS-CoV S-NTD-specific antibody responses and neutralizing antibodies (1:40–102) in mice and rabbits; neutralizes live MERS-CoV (EMC2012) | Elicits MERS-CoV S-NTD-specific T cell responses in mice | Protects Ad5/hDPP4-transduced mice from MERS-CoV (EMC2012) challenge | [54,79] |
| Protein-based (S1/S2: residues 736–761) | Subunit | Induces low-titer neutralizing antibodies in rabbits; inhibits pseudotyped MERS-CoV entry and membrane fusion | Not reported | Not reported | [85] |
4.1. MERS-CoV full-length S or S-trimer protein-based vaccines
A number of vaccines have been developed using full-length S protein of MERS-CoV as the target (Table 1). These vaccines are categorized as viral vector-, DNA-, nanoparticle-, virus-like particle (VLP)-, and S-trimer protein-based subunit vaccines [30,31,56–60]. Many of the reported full-length S-based vaccines have been tested in suitable animal models, and they demonstrated efficacy against MERS-CoV infection [31,53,57,61,62]. Two of these vaccines, e.g., a full-length S-based simian adenovirus vector vaccine (ChAdOx1) and a DNA vaccine (GLS-5300), are scheduled for clinical trials (Phase I) to test their safety and immunogenicity [63,64].
Viral vector-based vaccines encoding full-length S protein of MERS-CoV have been studied extensively, among which human or simian Ad, modified Vaccinia Ankara (MVA), measles virus (MV), and vesicular stomatitis virus (VSV) can be employed as the vaccine vehicles. For example, MERS-CoV S/RBD-specific systemic, mucosal, and/or cellular immune responses, as well as neutralizing antibodies against pseudotyped and live MERS-CoV, are induced in mice after immunizing them with human Ad5-based and Ad41-based or simian adenovirus vector (ChAdOx1)-based MERS-CoV full-length S-encoding vaccines [56,61,65,66], protecting hDPP4-Tg mice against MERS-CoV infection [61]. Also, MERS-CoV S-specific systemic and mucosal antibody responses and T cell responses, particularly neutralizing antibodies, are elicited in mice and/or camels immunized with MERS-CoV full-length S-expressing MVA vaccines, protecting Ad5/hDPP4-transduced mice and dromedary camels against MERS-CoV infection [31,62,67]. It is noted that MERS-CoV S-specific immune responses and neutralizing antibodies are significantly improved by the Ad (ChAdOx1)-S vaccine priming and MVA-S vaccine boosting approach [65]. In addition, a full-length S-encoding MV vaccine induces MERS-CoV S-specific antibody and T cell responses, as well as MERS-CoV neutralizing antibodies, protecting Ad5/hDPP4-transduced mice from MERS-CoV challenge [68]. Furthermore, a VSV-based MERS-CoV full-length S vaccine is shown to elicit MERS-CoV neutralizing antibodies and T cell responses in mice and/or NHPs [69].
Other types of MERS-CoV S-based vaccines, including those based on DNA, S-trimer protein, nanoparticle, and VLP, have shown immunogenicity and/or protective efficacy against MERS-CoV infection in mouse, camel, and NHP models [30,53,57–59]. For instance, an optimized DNA expressing full-length MERS-CoV S protein induces effective neutralizing antibodies and cellular immune responses in mice, camels and NHPs, protecting NHPs from MERS-CoV-caused disease [57]. Immunization of mice and NHPs with full-length S-DNA priming followed by S1 protein boosting elicits sufficient neutralizing antibodies, which protect NHPs against MERS-CoV-induced radiographic pneumonia [58]. A full-length S-based VLP vaccine induces MERS-CoV S-RBD-specific antibody responses and neutralizing antibodies in NHPs in the presence of Alum adjuvant [59]. A baculovirus insect cell-derived full-length S nanoparticle vaccine with Alum or Matrix-M1 adjuvant also induces MERS-CoV neutralizing antibodies in mice and Tc bovines, protecting Ad5/hDPP4-transduced mice from MERS-CoV infection [34,53,60].
Depending on antigen doses, injection doses, or immunization routes, MERS-CoV S/RBD-specific antibody and cellular immune responses and MERS-CoV neutralizing antibody titers induced by viral vectored full-length S vaccines might vary [61,62], while the titer of neutralizing antibodies elicited by other vaccine types, such as nanoparticle vaccines, could be significantly affected by adjuvants, not by antigen doses [53,60]. It is shown that some MERS-CoV full-length S-based DNA (≤1:103) or viral vector (MVA) (≥1:103) vaccines elicit a relatively higher titer of serum neutralizing antibodies than VLP (<1:102) or nanoparticle (1:102–103) vaccines against live MERS-CoV infection [31,57,59,60,62,67]. Here, 50% tissue culture infectious dose (TCID50) or 50% fluorescence-reduction neutralization assay (FRNA50) is utilized to measure neutralizing antibodies (e.g. 50% or 90% neutralization titers). Therefore, the difference of neutralizing antibody titers induced by the aforementioned vaccines might result from variant neutralization assays tested, in addition to different neutralizing immunogenicity of vaccines, MERS-CoV strains, or virus titers. Currently, it appears that there is no international antibody standard to harmonize the data obtained from different neutralization assays. Surely, the breadth and potency of MERS-CoV neutralizing antibodies are improved by a prefusion MERS-CoV S-trimer protein with proline substitutions in the S2-HR1 region [30]. Notably, in addition to inducing MERS-CoV-specific immune responses and neutralizing antibodies, viral vector-based full-length MERS-CoV S vaccines generally elicit anti-vector immune responses and/or neutralizing antibodies [31,62,65,68], a phenomenon that can be easily eliminated by S-based other vaccine types, such as DNA, S-trimer protein, nanoparticle, and VLP, as described earlier.
4.2. MERS-CoV S1-based vaccines
The MERS-CoV S1 subunit has potential as a vaccine target. Several vaccines have been constructed based on this region, which are categorized as viral vectored (Ad and rabies virus: RABV), DNA, and protein vaccines (Table 2). An Ad vector encoding MERS-CoV S1 extracellular domain (Ad5.MERS-S1) and a RABV vector encoding S1 elicit antibody responses and neutralizing antibodies in mice, while the RABV vectored S1 also protects Ad5/hDPP4-transduced mice from MERS-CoV challenge [56,70]. In addition, DNA-based MERS-CoV S1 vaccines elicit antibody and cellular immune responses and neutralizing antibodies capable of protecting the above mouse model from MERS-CoV infection [55,71]. Particularly, S1 protein boost of a full-length MERS-CoV S-DNA or S1 protein elicits similar levels of MERS-CoV neutralizing antibodies in mice, protecting NHPs from MERS-CoV challenge and pulmonary disease. However, a slightly lower titer of such antibodies is seen in S1/S1-vaccinated, compared to S-DNA/S1-vaccinated, NHPs, resulting in a higher peak volume of pulmonary disease in the S1/S1 group after MERS-CoV challenge [58]. Similar to MERS-CoV full-length S-based viral vectored vaccines, MERS-CoV S1-based viral vectored vaccines also induce a varying degree of anti-vector immune responses and neutralizing antibodies [70].
4.3. MERS-CoV RBD-based vaccines
As a short fragment inside the S1 subunit of MERS-CoV S protein, the RBD is applied as a key target for developing MERS vaccines in the categories of VLP and subunit vaccines, and their immunogenicity and efficacy have been tested in mouse, rabbits, and NHP models (Table 2).
One study indicates that a chimeric, spherical VLP (sVLP) expressing MERS-CoV RBD induces RBD-specific antibody and cellular immune responses in mice, neutralizing pseudotyped MERS-CoV entry into target cells [72]. Almost all other MERS-CoV RBD-based vaccines reported thus far are based on recombinant proteins expressed in mammalian or insect cell expression systems [32,73–77]. It has been demonstrated that a mammalian cell-expressed recombinant RBD protein containing residues 377–662 of MERS-CoV S induces robust humoral systemic and mucosal immune responses and neutralizing antibodies in immunized mice [32,78]. Studies have also shown that residues 358–588 and 367–606 of MERS-CoV RBD expressed in mammalian and insect cells, respectively, elicit RBD-specific antibody or cellular immune responses and neutralizing antibodies in mice and/or rabbits [79,80]. By optimizing and comparing five RBD fragments with different lengths, a RBD fragment containing residues 377–588 of MERS-CoV S protein is identified to induce the highest titer of antibody responses and neutralizing antibodies in immunized mice and rabbits with the capability of protecting Ad5/hDPP4 and hDPP4-Tg mice from MERS-CoV challenge with no evidence of immunological toxicity or eosinophilic immune enhancement [32,33,75,76,81]. In addition to its high immunogenicity and strong protection in mice and rabbits, MERS-CoV RBD is also immunogenic and protective in NHPs. This is evidenced by the induction of robust and sustained immune responses and neutralizing antibodies and protection against MERS-CoV challenge with minimal pathological effects in NHPs immunized with a RBD protein [73]. Importantly, MERS-CoV RBD protein-induced antibodies are shown to potently cross-neutralize multiple pseudotyped MERS-CoVs expressing S proteins of human and camel MERS-CoV strains, live human MERS-CoV strains, and mAb escape MERS-CoV mutants [74].
It should be noted that the immunogenicity of MERS-CoV RBD-based subunit vaccines is not significantly affected by antigen dosage, but by injection doses and vaccination intervals. For example, RBD dosage down to one microgram is sufficiently immunogenic to elicit strong immune responses in animals, and a regimen of two doses of RBD at 4-week intervals elicits the strongest antibody responses and neutralizing antibodies against MERS-CoV infection among one dose and two doses at 1-, 2-, and 3-week intervals tested, protecting hDPP4-Tg mice from MERS-CoV challenge [82,83]. Also, intranasal vaccination of the RBD protein induces much stronger local mucosal immune responses and neutralizing antibodies than subcutaneous immunization [84]. In particular, MERS-CoV RBD-elicited immune responses and neutralizing antibodies are significantly improved in the presence of suitable adjuvants, such as MF59, or fusion with appropriate immunopotentiators, such as Fc tag [32]. Compared with the wild-type RBD, a mutant RBD vaccine with a non-neutralizing epitope masked by a glycan probe provided 100% survival of hDPP4-Tg mice against lethal MERS-CoV infection [75]. These studies suggest that the RBD of MERS-CoV is a critical neutralizing domain and a strong immunogen for developing MERS vaccines.
4.4. Vaccines based on other regions of MERS-CoV S protein
As indicated earlier, most MERS-CoV vaccines under development focus on the full-length S protein, S1, or RBD. However, a few MERS vaccines are reported based on other regions of MERS-CoV S protein, such as truncated S, S-NTD, and S1/S2 regions (Table 2). For example, a MV-vectored MERS-S lacking the transmembrane domain (truncated S) is able to elicit MERS-CoV neutralizing antibodies, but the titer is slightly lower than that elicited by a full-length MERS-CoV S vaccine in the same viral vector [68]. Also, a MERS-CoV S-NTD-based protein vaccine is shown to induce favorable antibody and cellular responses, as well as neutralizing antibodies, reducing lung abnormalities in Ad5/hDPP4-transduced mice after MERS-CoV challenge [54]. In addition, a peptide spanning the S1/S2 regions, i.e., residues 736–761, has neutralizing activities by inhibiting pseudotyped MERS-CoV entry and membrane fusion, pointing out the possibility of developing vaccines covering this neutralizing epitope [85]. Nevertheless, the protective efficacy of such vaccines needs to be further confirmed in a lethal challenge model. At present, no vaccines are reported solely based on the MERS-CoV S2 subunit, potentially because of its low immunogenicity and inability to induce strong neutralizing antibodies.
5. Summary and Conclusions
This review introduces the structure and function of MERS-CoV S protein, summarizes current animal models available for evaluating the efficacy of MERS vaccines, and describes recent advances in MERS vaccines based on viral S protein, including those on the full-length S/S-trimer, S1, RBD, and other S regions. These S-based vaccines are further categorized as different types, including viral vector, DNA, VLP, nanoparticle, and protein-based vaccines, and their ability to elicit antibody and/or cellular immune responses, neutralizing antibodies, and protective efficacy against MERS-CoV infection was compared in different models. Such data will provide useful information and important guidance for designing and developing safe and effective MERS-CoV S protein-based vaccines.
6. Expert commentary
Although full-length S-based vaccines show protection against MERS-CoV infection and demonstrate promise for further development, no safety profile has been established. More specifically, some non-neutralizing immunodominant epitopes on the full-length S protein may compete with the neutralizing epitopes to attenuate neutralizing activity or even enhance MERS-CoV infection, potentially causing immunopathological effects or other harmful immune responses, as seen in the case of full-length S-based SARS vaccines [86–88]. In addition, MERS-CoV S1 subunit, which is much longer than the RBD, also contains some non-neutralizing immunodominant epitopes. Such immunodominant epitope in SARS-CoV S1 is shown to elicit epitope sequence-dependent enhancement of viral infection [89]. Therefore, evaluation of the safety and potential immunopathological consequences is essential for full-length S and S1-based MERS vaccines before moving them to large-scale development and beginning clinical trials.
Compared with vaccines based on other targets of MERS-CoV S, such as full-length S protein, RBD-based vaccines have a strong safety profile. The RBD does not contain non-neutralizing epitopes that may cause harmful immune responses, and RBD-based vaccines do not show immunological toxicity and immunopathological effects in the animals tested [81]. Similar to the SARS-CoV RBD, which covers multiple conformation-dependent neutralizing epitopes [90], the RBD of MERS-CoV also contains a critical neutralizing fragment with major neutralizing epitopes capable of inducing highly potent neutralizing antibodies [76]. like SARS-CoV RBD-based vaccines that induce high titers of cross-neutralizing antibodies against divergent strains of human and animal SARS-CoV [91], MERS-CoV RBD-based vaccines also elicit broad-spectrum neutralizing antibodies and cross-protective immunity against infections of divergent MERS-CoV strains from humans, camels, and antibody escape mutants [74]. Future strategies can be applied to improve the potency and breadth of MERS-CoV RBD candidate vaccines by using structure-based rational design to mask unfavorable non-neutralizing immunodominant epitopes and expose neutralizing epitopes within the RBD [75], or incorporating RBD with favorable neutralizing epitopes in the S2 or non-RBD S1 regions. Such vaccination approaches are also expected to improve the efficiency of vaccines against escape antibody mutant virus strains [92].
In some cases, viral vectored vaccines encoding MERS-CoV S1 or S protein, such as RABV-MERS-S1 or MVA-MERS-S vaccines, induce anti-vector responses helpful in preventing host-derived RABV and camelpox virus in immunized mice or camels [31,70]. However, viral vectored vaccines, such as those based on Ad, against other viruses, including HIV, may induce a rapid memory immune response against the vector, enhance virus infection, or elicit limited efficacy in immunized hosts, resulting in early halting of clinical trials [93–95]. Thus, in addition to investigating immunogenicity and protection of viral-vectored MERS-CoV S candidate vaccines against MERS-CoV infection, careful design and selection of suitable viral vectors, comprehensive investigation of the possibility of anti-vector immunity in preventing MERS-CoV-specific immune responses, as well as extensive evaluation of their safety and potential toxicity, are needed before moving such vaccines forward for trials in humans.
Generally speaking, neutralizing antibodies are indispensable for preventing MERS-CoV infection, and the titers of neutralizing antibodies are correlated with protection [33,83]. Therefore, improvement of a vaccine’s neutralizing immunogenicity to induce highly potent neutralizing antibodies would be the key for further development of next-generation MERS-CoV S-based vaccines. In addition to humoral immune responses, cellular immune responses or various cell populations may play different roles in fighting against MERS-CoV infection or regulating pathogenesis. For instance, airway memory CD4+ T cells mediate protective immunity against MERS-CoV, while CD8+ T cells and macrophages regulate MERS-CoV-induced pathology and clinical symptoms of the disease [96,97]. In this regard, cellular immunity of MERS-CoV S vaccines may well complement their humoral immunity to increase the vaccine’s potency in preventing MERS-CoV infection.
As indicated earlier, MERS-CoV S protein serves as a key vaccine target. In addition to S-based vaccines, MERS vaccines can be engineered by targeting other viral structural or non-structural proteins, such as E, ORFs and nsp16, to generate mutant live virus vaccine platforms that lack E, delete ORF3–5, or mutate nsp16 [22,23]. These non-S-based vaccines could be applied as an alternative or complement of S-based vaccines to prevent MERS-CoV infection. Overall, in order to further improve immunogenicity and efficacy, S-based MERS vaccines can be used alone or combined with other types of S or non-S vaccines with complementary effects by priming-boosting vaccination approaches, or conjugating with different adjuvants, and optimizing for doses, routes, or intervals. Such vaccines should be tested in different animal models, including large animal models, such as NHPs and/or camels, to confirm immunogenicity, efficacy, toxicity, and immunopathology before processing to human clinical trials.
7. Five-year view
Six years have passed since MERS-CoV was first reported in 2012. In addition to two candidate vaccines that are scheduled for clinical trials, most MERS-CoV vaccines, including those based on viral S protein, are still in preclinical development or not even processed for testing in large animal models, such as NHPs. Lack of sufficient funds to support such studies is potentially one of the main reasons, in addition to regulations and other issues. A number of MERS-CoV S-based vaccine candidates, such as those based on the RBD, with strong safety profile, immunogenicity, and protective efficacy, but without inducing immunopathology in animals, are expected to also induce potent immune responses and efficacy, as well as maintain good safety profile, in humans. Therefore, it is anticipated that sufficient funds will be invested from governments, private entities, or big pharmaceutical companies and that an increasing number of MERS vaccines, particularly those based on the S and/or RBD proteins, can be tested in large animals, moved to clinical trials, and/or approved to prevent MERS-CoV infection in humans in the next five years.
8. Key issues.
- MERS-CoV, a newly emerging infectious coronavirus, continues to infect human populations, particularly in Saudi Arabia, with about 35% mortality rate. As a zoonotic virus, MERS-CoV transmits efficiently among camels and occasionally in people who are in close contact with infected camels and persons, constituting a continual threat to global public health and thus requiring effective medical countermeasures, such as vaccines, to prevent MERS-CoV infection.
- MERS-CoV genome encodes four structural proteins, including S, E, M and N, and a number of accessory proteins. The structures and/or functions of some of these proteins, such as S, ORF3–5, and nsp16, have been extensively studied.
- MERS-CoV S protein consists of S1 and S2 subunits. RBD in S1 is responsible for cellular receptor binding, while HR1 and HR2 regions in S2 mediate virus fusion and entry into the target cell. Receptor binding to the RBD may trigger sequential activation of protomers.
- MERS-CoV can infect dromedary camels, but it does not naturally infect other large animals (e.g., sheep, horses) and small animals (e.g, mice, ferrets and hamsters). Large animal models, including non-human primate models, and small animal models, such as adenovirus/hDPP4-transduced mice, hDPP4-transgenic mice, and hDPP4-knock-in mice, have been developed for testing protective efficacy of MERS vaccines.
- S protein is the primary target for the humoral immune response during infection and thus a key target for developing MERS-CoV vaccines. S-RBD contains a critical neutralizing domain capable of inducing highly potent neutralizing antibodies. S- and RBD-specific neutralizing antibodies play an essential role in preventing MERS-CoV infection, and neutralizing antibody titers are correlated with protection.
- No MERS vaccines have been approved for use in humans. Two MERS-CoV S-based vaccines are scheduled for clinical trials, and all other MERS vaccines are in preclinical development.
- Most MERS vaccines under development are based on viral S protein, including the full-length S/S-trimer, S1, RBD, and other S regions. These vaccines induce MERS-CoV-specific antibody and/or cellular immune responses, in addition to neutralizing antibodies at varying titers, a number of which have been tested in mouse, camel, or NHP models with protective efficacy.
- Each vaccine category has advantages and potential limitations. By comparison, MERS-CoV RBD-based subunit vaccines elicit relatively higher titers of neutralizing antibodies against multiple virus strains, protecting transgenic mice from MERS-CoV challenge without causing immunopathological effects, demonstrating strong safety profile. Clinical trials are warranted if sufficient funds are available.
Acknowledgments
Funding
The study was supported by NIH grants R01AI137472, R01AI139092, and R21AI128311.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
* of interest
** of considerable interest
- 1.Zaki AM, van BS, Bestebroer TM, et al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 2012;367(19):1814–20. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Middle East respiratory syndrome coronavirus (MERS-CoV). [cited 2018]. Available from: http://www.who.int/emergencies/mers-cov/en/.
- 3.Chen X, Chughtai AA, Dyda A, et al. Comparative epidemiology of Middle East respiratory syndrome coronavirus (MERS-CoV) in Saudi Arabia and South Korea. Emerg Microbes Infect 2017;6(6):e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saeed AA, Abedi GR, Alzahrani AG, et al. Surveillance and testing for Middle East respiratory syndrome coronavirus, Saudi Arabia, April 2015-February 2016. Emerg Infect Dis 2017;23(4):682–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. List of Blueprint priority diseases. [cited 2018]. Available from: http://www.who.int/blueprint/priority-diseases/en/.
- 6.Munster VJ, Adney DR, van DN, et al. Replication and shedding of MERS-CoV in Jamaican fruit bats (Artibeus jamaicensis). Sci Rep 2016;6:21878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anthony SJ, Gilardi K, Menachery VD, et al. Further evidence for bats as the evolutionary source of Middle East respiratory syndrome coronavirus. MBio 2017;8(2). pii: e00373–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali MA, Shehata MM, Gomaa MR, et al. Systematic, active surveillance for Middle East respiratory syndrome coronavirus in camels in Egypt. Emerg Microbes Infect 2017;6(1):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falzarano D, Kamissoko B, de WE, et al. Dromedary camels in northern Mali have high seropositivity to MERS-CoV. One Health 2017;3:41–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali M, El-Shesheny R, Kandeil A, et al. Cross-sectional surveillance of Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels and other mammals in Egypt, August 2015 to January 2016. Euro Surveill 2017;22(11). pii: 30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sikkema RS, Farag EABA, Himatt S, et al. Risk factors for primary Middle East respiratory syndrome coronavirus infection in camel workers in Qatar during 2013–2014: A case-control study. J Infect Dis 2017;215(11):1702–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alhakeem RF, Midgley CM, Assiri AM, et al. Exposures among MERS case-patients, Saudi Arabia, January-February 2016. Emerg Infect Dis 2016;22(11):2020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yusof MF, Queen K, Eltahir YM, et al. Diversity of Middle East respiratory syndrome coronaviruses in 109 dromedary camels based on full-genome sequencing, Abu Dhabi, United Arab Emirates. Emerg Microbes Infect 2017;6(11):e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du L, He Y, Zhou Y, et al. The spike protein of SARS-CoV--a target for vaccine and therapeutic development. Nat Rev Microbiol 2009;7(3):226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alfaraj SH, Al-Tawfiq JA, Altuwaijri TA, et al. Middle East respiratory syndrome coronavirus transmission among health care workers: Implication for infection control. Am J Infect Control 2018;46(2):165–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunter JC, Nguyen D, Aden B, et al. Transmission of Middle East respiratory syndrome coronavirus infections in healthcare settings, Abu Dhabi. Emerg Infect Dis 2016;22(4):647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azhar EI, Lanini S, Ippolito G, et al. The Middle East respiratory syndrome coronavirus - A continuing risk to global health security. Adv Exp Med Biol 2017;972:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. *.Du L, Yang Y, Zhou Y, et al. MERS-CoV spike protein: a key target for antivirals. Expert Opin Ther Targets 2017;21(2):131–43. A report summarizing MERS-CoV S protein-based therapeutics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du L, Tai W, Zhou Y, et al. Vaccines for the prevention against the threat of MERS-CoV. Expert Rev Vaccines 2016;15(9):1123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batool M, Shah M, Patra MC, et al. Structural insights into the Middle East respiratory syndrome coronavirus 4a protein and its dsRNA binding mechanism. Sci Rep 2017;7(1):11362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hao W, Wojdyla JA, Zhao R, et al. Crystal structure of Middle East respiratory syndrome coronavirus helicase. PLoS Pathog 2017;13(6):e1006474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menachery VD, Gralinski LE, Mitchell HD, et al. Middle East respiratory syndrome coronavirus nonstructural protein 16 Is necessary for interferon resistance and viral pathogenesis. mSphere 2017;2(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menachery VD, Mitchell HD, Cockrell AS, et al. MERS-CoV accessory ORFs play key role for infection and pathogenesis. MBio 2017;8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu G, Hu Y, Wang Q, et al. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature 2013;500(7461):227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li F Receptor recognition mechanisms of coronaviruses: a decade of structural studies. J Virol 2015;89(4):1954–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raj VS, Mou H, Smits SL, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013;495(7440):251–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Rajashankar KR, Yang Y, et al. Crystal structure of the receptor-binding domain from newly emerged Middle East respiratory syndrome coronavirus. J Virol 2013;87(19):10777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang N, Shi X, Jiang L, et al. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res 2013;23(8):986–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu L, Liu Q, Zhu Y, et al. Structure-based discovery of Middle East respiratory syndrome coronavirus fusion inhibitor. Nat Commun 2014;5:3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. **.Pallesen J, Wang N, Corbett KS, et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc Natl Acad Sci U S A 2017;114(35):E7348–E7357. A report on struture-based design of a MERS-CoV S-trimer protein vaccine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. **.Haagmans BL, van den Brand JM, Raj VS, et al. An orthopoxvirus-based vaccine reduces virus excretion after MERS-CoV infection in dromedary camels. Science 2016;351(6268):77–81. A report about a modified vaccinia virus Ankara (MVA) vectored vaccine with protective efficacy against MERS-CoV and camelpox virus in dromedary camels. [DOI] [PubMed] [Google Scholar]
- 32.Zhang N, Channappanavar R, Ma C, et al. Identification of an ideal adjuvant for receptor-binding domain-based subunit vaccines against Middle East respiratory syndrome coronavirus. Cell Mol Immunol 2016;13(2):180–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tai W, Zhao G, Sun S, et al. A recombinant receptor-binding domain of MERS-CoV in trimeric form protects human dipeptidyl peptidase 4 (hDPP4) transgenic mice from MERS-CoV infection. Virology 2016;499:375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luke T, Wu H, Zhao J, et al. Human polyclonal immunoglobulin G from transchromosomic bovines inhibits MERS-CoV in vivo. Sci Transl Med 2016;8(326):326ra21. [DOI] [PubMed] [Google Scholar]
- 35. *.Beigel JH, Voell J, Kumar P, et al. Safety and tolerability of a novel, polyclonal human anti-MERS coronavirus antibody produced from transchromosomic cattle: a phase 1 randomised, double-blind, single-dose-escalation study. Lancet Infect Dis 2018;18(4):410–418. A report about the safety and tolerability of Phase I trial of an anti-MERS-CoV polyclonal antibody. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Houser KV, Gretebeck L, Ying T, et al. Prophylaxis with a Middle East respiratory syndrome coronavirus (MERS-CoV)-specific human monoclonal antibody protects rabbits from MERS-CoV infection. J Infect Dis 2016;213(10):1557–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van DN, Falzarano D, Ying T, et al. Efficacy of antibody-based therapies against Middle East respiratory syndrome coronavirus (MERS-CoV) in common marmosets. Antiviral Res 2017;143:30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao G, He L, Sun S, et al. A novel nanobody targeting Middle East respiratory syndrome coronavirus (MERS-CoV) receptor-binding domain has potent cross-neutralizing activity and protective efficacy against MERS-CoV. J Virol 2018; pii: JVI.00837–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van DN, Hijazeen ZS, Holloway P, et al. High prevalence of Middle East respiratory coronavirus in young dromedary camels in Jordan. Vector Borne Zoonotic Dis 2017;17(2):155–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adney DR, Brown VR, Porter SM, et al. Inoculation of goats, sheep, and horses with MERS-CoV does not result in productive viral shedding. Viruses 2016;8(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adney DR, van DN, Brown VR, et al. Replication and shedding of MERS-CoV in upper respiratory tract of inoculated dromedary camels. Emerg Infect Dis 2014;20(12):1999–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao Y, Bao L, Deng W, et al. An animal model of MERS produced by infection of rhesus macaques with MERS coronavirus. J Infect Dis 2014;209(2):236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falzarano D, de WE, Feldmann F, et al. Infection with MERS-CoV causes lethal pneumonia in the common marmoset. PLoS Pathog 2014;10(8):e1004250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de WE, Rasmussen AL, Falzarano D, et al. Middle East respiratory syndrome coronavirus (MERS-CoV) causes transient lower respiratory tract infection in rhesus macaques. Proc Natl Acad Sci U S A 2013;110(41):16598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu P, Xu Y, Deng W, et al. Comparative pathology of rhesus macaque and common marmoset animal models with Middle East respiratory syndrome coronavirus. PLoS One 2017;12(2):e0172093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agrawal AS, Garron T, Tao X, et al. Generation of a transgenic mouse model of Middle East respiratory syndrome coronavirus infection and disease. J Virol 2015;89(7):3659–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pascal KE, Coleman CM, Mujica AO, et al. Pre- and postexposure efficacy of fully human antibodies against spike protein in a novel humanized mouse model of MERS-CoV infection. Proc Natl Acad Sci U S A 2015;112(28):8738–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao J, Li K, Wohlford-Lenane C, et al. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci U S A 2014;111(13):4970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tao X, Garron T, Agrawal AS, et al. Characterization and demonstration of the value of a lethal mouse model of Middle East respiratory dyndrome coronavirus infection and disease. J Virol 2015;90(1):57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao G, Jiang Y, Qiu H, et al. Multi-organ damage in human dipeptidyl peptidase 4 transgenic mice infected with Middle East respiratory syndrome-coronavirus. PLoS One 2015;10(12):e0145561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cockrell AS, Yount BL, Scobey T, et al. A mouse model for MERS coronavirus-induced acute respiratory distress syndrome. Nat Microbiol 2016;2:16226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li K, Wohlford-Lenane CL, Channappanavar R, et al. Mouse-adapted MERS coronavirus causes lethal lung disease in human DPP4 knockin mice. Proc Natl Acad Sci U S A 2017;114(15):E3119–E3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coleman CM, Venkataraman T, Liu YV, et al. MERS-CoV spike nanoparticles protect mice from MERS-CoV infection. Vaccine 2017;35(12):1586–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiaming L, Yanfeng Y, Yao D, et al. The recombinant N-terminal domain of spike proteins is a potential vaccine against Middle East respiratory syndrome coronavirus (MERS-CoV) infection. Vaccine 2017;35(1):10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Amri SS, Abbas AT, Siddiq LA, et al. Immunogenicity of candidate MERS-CoV DNA vaccines based on the spike protein. Sci Rep 2017;7:44875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim E, Okada K, Kenniston T, et al. Immunogenicity of an adenoviral-based Middle East respiratory syndrome coronavirus vaccine in BALB/c mice. Vaccine 2014;32(45):5975–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muthumani K, Falzarano D, Reuschel EL, et al. A synthetic consensus anti-spike protein DNA vaccine induces protective immunity against Middle East respiratory syndrome coronavirus in nonhuman primates. Sci Transl Med 2015;7(301):301ra132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang L, Shi W, Joyce MG, et al. Evaluation of candidate vaccine approaches for MERS-CoV. Nat Commun 2015;6:7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang C, Zheng X, Gai W, et al. MERS-CoV virus-like particles produced in insect cells induce specific humoural and cellular imminity in rhesus macaques. Oncotarget 2017;8(8):12686–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coleman CM, Liu YV, Mu H, et al. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine 2014;32(26):3169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Munster VJ, Wells D, Lambe T, et al. Protective efficacy of a novel simian adenovirus vaccine against lethal MERS-CoV challenge in a transgenic human DPP4 mouse model. NPJ Vaccines 2017;2:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Volz A, Kupke A, Song F, et al. Protective efficacy of recombinant modified vaccinia virus Ankara (MVA) delivering Middle East respiratory syndrome coronavirus spike glycoprotein. J Virol 2015;89(16):8651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Phase I, Open label dose ranging safety study of GLS-5300 in healthy volunteers. [cited July 27, 2016]. Available from: https://clinicaltrials.gov/ct2/show/NCT02670187?cond=MERS&rank=21.
- 64. *.Safety and immunogenicity of a candidate MERS-CoV vaccine (MERS001). [cited January 16, 2018]. Available from: https://clinicaltrials.gov/ct2/show/NCT03399578?cond=mers-cov&rank=6. A report describing the status of Phase I trial of a viral vectored vaccine based on MERS-CoV full-length S protein.
- 65.Alharbi NK, Padron-Regalado E, Thompson CP, et al. ChAdOx1 and MVA based vaccine candidates against MERS-CoV elicit neutralising antibodies and cellular immune responses in mice. Vaccine 2017;35(30):3780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo X, Deng Y, Chen H, et al. Systemic and mucosal immunity in mice elicited by a single immunization with human adenovirus type 5 or 41 vector-based vaccines carrying the spike protein of Middle East respiratory syndrome coronavirus. Immunology 2015;145(4):476–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song F, Fux R, Provacia LB, et al. Middle East respiratory syndrome coronavirus spike protein delivered by modified vaccinia virus Ankara efficiently induces virus-neutralizing antibodies. J Virol 2013;87(21):11950–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Malczyk AH, Kupke A, Prufer S, et al. A highly immunogenic and protective Middle East respiratory syndrome coronavirus vaccine based on a recombinant Measles virus vaccine platform. J Virol 2015;89(22):11654–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu R, Wang J, Shao Y, et al. A recombinant VSV-vectored MERS-CoV vaccine induces neutralizing antibody and T cell responses in rhesus monkeys after single dose immunization. Antiviral Res 2018;150:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wirblich C, Coleman CM, Kurup D, et al. One-Health: a safe, efficient, dual-use vaccine for humans and animals against Middle East respiratory syndrome coronavirus and Rabies virus. J Virol 2017;91(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chi H, Zheng X, Wang X, et al. DNA vaccine encoding Middle East respiratory syndrome coronavirus S1 protein induces protective immune responses in mice. Vaccine 2017;35(16):2069–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang C, Zheng X, Gai W, et al. Novel chimeric virus-like particles vaccine displaying MERS-CoV receptor-binding domain induce specific humoral and cellular immune response in mice. Antiviral Res 2017;140:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lan J, Yao Y, Deng Y, et al. Recombinant receptor binding domain protein induces partial protective immunity in Rhesus Macaques against Middle East respiratory syndrome coronavirus challenge. EBioMedicine 2015;2(10):1438–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. *.Tai W, Wang Y, Fett CA, et al. Recombinant receptor-binding domains of multiple Middle East respiratory syndrome coronaviruses (MERS-CoVs) induce cross-neutralizing antibodies against divergent human and camel MERS-CoVs and antibody escape mutants. J Virol 2017;91(1). A report describing cross-neutralizing ability of a MERS-CoV receptor-binding domain (RBD)-based subunit vaccine agaisnt divergent MERS-CoV strains. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. **.Du L, Tai W, Yang Y, et al. Introduction of neutralizing immunogenicity index to the rational design of MERS coronavirus subunit vaccines. Nat Commun 2016;7:13473 A study introducing a novel approach to design MERS-CoV vaccines with enhanced efficacy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma C, Wang L, Tao X, et al. Searching for an ideal vaccine candidate among different MERS coronavirus receptor-binding fragments--the importance of immunofocusing in subunit vaccine design. Vaccine 2014;32(46):6170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Du L, Zhao G, Kou Z, et al. Identification of a receptor-binding domain in the S protein of the novel human coronavirus Middle East respiratory syndrome coronavirus as an essential target for vaccine development. J Virol 2013;87(17):9939–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang N, Tang J, Lu L, et al. Receptor-binding domain-based subunit vaccines against MERS-CoV. Virus Res 2015;202:151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mou H, Raj VS, van Kuppeveld FJ, et al. The receptor binding domain of the new MERS coronavirus maps to a 231-residue region in the spike protein that efficiently elicits neutralizing antibodies. J Virol 2013;87(16):9379–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lan J, Deng Y, Chen H, et al. Tailoring subunit vaccine immunity with adjuvant combinations and delivery routes using the Middle East respiratory coronavirus (MERS-CoV) receptor-binding domain as an antigen. PLoS One 2014;9(11):e112602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nyon MP, Du L, Tseng CK, et al. Engineering a stable CHO cell line for the expression of a MERS-coronavirus vaccine antigen. Vaccine 2018;36(14):1853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tang J, Zhang N, Tao X, et al. Optimization of antigen dose for a receptor-binding domain-based subunit vaccine against MERS coronavirus. Hum Vaccin Immunother 2015;11(5):1244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Y, Tai W, Yang J, et al. Receptor-binding domain of MERS-CoV with optimal immunogen dosage and immunization interval protects human transgenic mice from MERS-CoV infection. Hum Vaccin Immunother 2017;13(7):1615–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ma C, Li Y, Wang L, et al. Intranasal vaccination with recombinant receptor-binding domain of MERS-CoV spike protein induces much stronger local mucosal immune responses than subcutaneous immunization: Implication for designing novel mucosal MERS vaccines. Vaccine 2014;32(18):2100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang Y, Deng Y, Wen B, et al. The amino acids 736–761 of the MERS-CoV spike protein induce neutralizing antibodies: implications for the development of vaccines and antiviral agents. Viral Immunol 2014;27(10):543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jaume M, Yip MS, Kam YW, et al. SARS CoV subunit vaccine: antibody-mediated neutralisation and enhancement. Hong Kong Med J 2012;18 Suppl 2:31–6. [PubMed] [Google Scholar]
- 87.Weingartl H, Czub M, Czub S, et al. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J Virol 2004;78(22):12672–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Czub M, Weingartl H, Czub S, et al. Evaluation of modified vaccinia virus Ankara based recombinant SARS vaccine in ferrets. Vaccine 2005;23(17–18):2273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Q, Zhang L, Kuwahara K, et al. Immunodominant SARS coronavirus epitopes in humans elicited both enhancing and neutralizing effects on infection in non-human primates. ACS Infect Dis 2016;2(5):361–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.He Y, Lu H, Siddiqui P, et al. Receptor-binding domain of severe acute respiratory syndrome coronavirus spike protein contains multiple conformation-dependent epitopes that induce highly potent neutralizing antibodies. J Immunol 2005;174(8):4908–15. [DOI] [PubMed] [Google Scholar]
- 91.He Y, Li J, Li W, et al. Cross-neutralization of human and palm civet severe acute respiratory syndrome coronaviruses by antibodies targeting the receptor-binding domain of spike protein. J Immunol 2006;176(10):6085–92. [DOI] [PubMed] [Google Scholar]
- 92. **.Wang L, Shi W, Chappell JD, et al. Importance of neutralizing monoclonal antibodies targeting multiple antigenic sites on MERS-CoV spike to avoid neutralization escape. J Virol 2018; pii: JVI.02002–17. A report demonstrating the importance of combining monoclonal antibodies targeting distinct epitopes of MERS-CoV S protein in preventing viral neutralization escape. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sekaly RP. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J Exp Med 2008;205(1):7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clinical alert: Immunizations are discontinued in two HIV vaccine trials. [cited 2018]. https://www.nlm.nih.gov/databases/alerts/hiv_step_study.html.
- 95.Knox R Failure of latest HIV vaccine test: A ‘huge disappointment’. [cited April 26, 2013]. Available from: https://www.npr.org/sections/health-shots/2013/04/26/179231916/failure-of-latest-hiv-vaccine-test-a-huge-disappointment.
- 96.Zhao J, Zhao J, Mangalam AK, et al. Airway memory CD4(+) T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity 2016;44(6):1379–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Coleman CM, Sisk JM, Halasz G, et al. CD8+ T cells and macrophages regulate pathogenesis in a mouse model of Middle East respiratory syndrome. J Virol 2017;91(1). pii: e01825–16. [DOI] [PMC free article] [PubMed] [Google Scholar]