Glutamatergic Signaling in the Central Nervous System: Ionotropic and Metabotropic Receptors in Concert (original) (raw)
. Author manuscript; available in PMC: 2019 Apr 26.
Abstract
Glutamate serves as both the mammalian brain’s primary excitatory neurotransmitter and as a key neuromodulator to control synapse and circuit function over a wide range of spatial and temporal scales. This functional diversity is decoded by two receptor families: ionotropic glutamate receptors (iGluRs) and metabotropic glutamate receptors (mGluRs). The challenges posed by the complexity and physiological importance of each of these subtypes has limited our appreciation and understanding of how these receptors work in concert. In this review, by comparing both receptor families with a focus on their crosstalk, we argue for a more holistic understanding of neural glutamate signaling.
To accommodate the complex roles of glutamate as a neurotransmitter, neuromodulator, and gliotransmitter, a diverse array of glutamate receptors (GluRs) is found throughout the nervous system. Ionotropic GluRs (iGluRs) are ligand-gated ion channels that produce excitatory glutamate-evoked currents while metabotropic GluRs (mGluRs) are G protein-coupled receptors (GPCRs) that control cellular processes via G protein signaling cascades. iGluR and mGluR signaling has been well studied in isolation, which has revealed an astonishing complexity within both systems. Nevertheless, glutamate does not distinguish between these receptor families that are both present in many cell types and subcellular compartments. It is important to understand how these receptors signal in concert. Do they talk to each other? Do their signals converge? And when are their effects synergistic or antagonistic? In this review, we focus on the similarities and differences between iGluR and mGluR activation (iGluRs and mGluRs Are Diverse Subfamilies with Functional Overlap), the various modes of crosstalk between these families (GluR Crosstalk by Various Mechanisms on Different Levels), and the implications for synaptic function and disease-related dysfunction (iGluR/mGluR Crosstalk in Synaptic Function and Dysfunction). With a more holistic approach to understanding GluR function, many challenging research questions emerge, for which we discuss new approaches (Overcoming Technical Challenges in Studying GluR Crosstalk) that aid in deciphering the intricacies of concerted glutamatergic signaling.
iGluRs and mGluRs Are Diverse Receptor Families with Functional Overlap
Distinct GluR classes were functionally identified based on the observation of glutamate-evoked excitation (Curtis et al., 1959) and inositol phosphate production (Sladeczek et al., 1985). The first iGluR and mGluR members were cloned in 1989 (Hollmann et al., 1989) and 1991 (Masu et al., 1991), and subsequent biophysical and structural work has given an increasingly detailed understanding of their molecular properties (Figure 1).
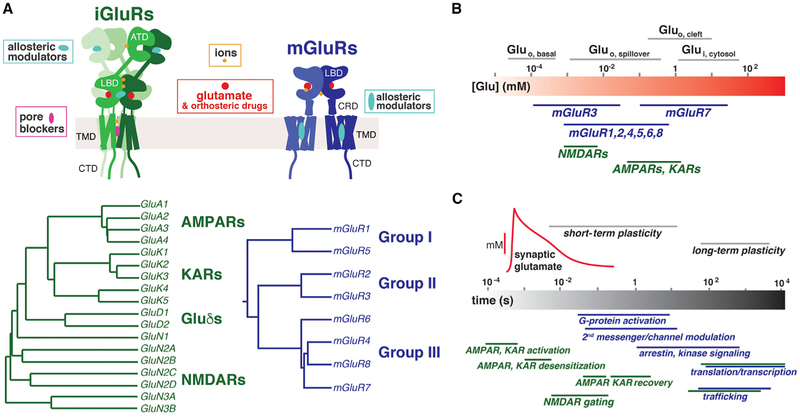
Figure 1. iGluRs and mGluRs: Structural Organization, Glutamate Sensitivity, and Kinetics.
(A) Top: domain organization of iGluRs (green) and mGluRs (blue). Glutamate and orthosteric ligands bind in the inter-lobe cleft of the ligand binding domains (LBDs). In both receptor classes, various ions bind near the orthosteric site, in the inter-LBD dimer interface, and the amino terminal domain (ATD) of iGluRs. Positive or negative allosteric modulators of mGluRs bind in the transmembrane domain (TMD), whereas allosteric modulators of iGluRs bind in the ATD or LBD, and pore blockers bind within the ion channel-forming TMD. Bottom: phylogenetic trees showing the iGluR and mGluR subfamilies and their subunits.
(B) Top: diagram summarizing synaptically relevant glutamate concentrations. Bottom: glutamate sensitivities (centered around EC50) of mGluRs and iGluRs cover a broad range and show a major overlap.
(C) Timescales of synaptic transmission and plasticity (top) and the associated timing of GluR gating and downstream signaling (bottom). The fastest iGluRs, AMPARs and KARs, mediate millisecond responses and enable high-frequency synaptic transmission. Both mGluRs and iGluRs allow sensing and signal transduction (timescale of tens of milliseconds) to control many forms of short-term (seconds to minutes) and long-term synaptic plasticity (hours). Precisely defining these timescales remains an important challenge, particularly for mGluRs in the synaptic context where few high-resolution, direct measurements have been made.
Specific iGluR agonists revealed the existence of AMPA (AMPARs; GluA subunits), kainate (KARs; GluK subunits), and _N_-methyl-D-aspartate (NMDA) (NMDA receptors [NMDARs]; GluN subunits) receptors as the three main subfamilies (Hollmann and Heinemann, 1994; Figure 1A). iGluRs assemble as tetramers that consist of four intertwined subunits forming a non-selective cation channel (Traynelis et al., 2010). Each subunit comprises an extracellular amino-terminal domain (ATD), a ligand binding domain (LBD), a common pore-forming transmembrane domain (TMD), and an intracellular C-terminal domain (CTD). GluA and GluK subunits (Contractor et al., 2011; Greger et al., 2017) form homo- or heterotetrameric receptors that are rapidly activated by glutamate binding to the LBD, which also triggers their desensitization. NMDARs are obligatory heterotetramers of two GluN1 subunits that bind glycine or D-serine and two GluN2 subunits that bind glutamate (Paoletti et al., 2013). The GluN2 subunits can be replaced by GluN3 subunits that bind glycine or D-serine, and triheteromeric GluN1/N2/N3 receptors may serve to curtail the effect of GluN2 signaling or as a platform for metabotropic signaling pathways (Pérez-Otaño et al., 2016). Another important but poorly understood iGluR subfamily are the δ-receptors, which are not directly activated by ligands but are important for _trans_-synaptic organization and plasticity (Yuzaki and Aricescu, 2017). The expression pattern of different iGluR subtypes varies in different brain regions, but their expression is widespread, and individual cells typically express multiple different iGluR subtypes (Hadzic et al., 2017).
The eight mGluRs, mGluR1–mGluR8, are family C GPCRs that exist as constitutive dimers (Doumazane et al., 2011; Levitz et al., 2016a). They differ structurally from other GPCRs by the presence of a large extracellular LBD that is linked to the 7-helix TMD via a cysteine-rich domain (CRD) (Figure 1A). mGluRs are divided into group I, II, or III subfamilies based on sequence homologies and their preferred Gα protein signaling partners (Figure 1A; Niswender and Conn, 2010). Group I mGluRs are primarily Gq-coupled, whereas group II and III mGluRs are Gi/o-coupled. With the exception of mGluR6, which is primarily expressed in retinal ON bipolar cells, mGluRs are widely expressed throughout the nervous system, with distinct but highly overlapping patterns for each subtype (Ferraguti and Shigemoto, 2006).
The molecular diversity of both iGluRs and mGluRs is increased by a number of mechanisms. This includes formation of heteromers within each subfamily (Traynelis et al., 2010, Doumazane et al., 2011, Levitz et al., 2016a), and RNA splicing and editing. The intracellular CTDs, which provide binding sites for crosstalk with intracellular signaling partners, are subject to many posttranslational modifications (Niswender and Conn, 2010; Traynelis et al., 2010). The function of iGluRs is further modulated by association with auxiliary subunits (Schwenk et al., 2012).
Biophysical Activation Mechanisms
iGluRs and mGluRs are typically viewed as receptor families with distinct functions and properties, but both detect glutamate via their extracellular, clamshell-like LBDs, which originate from ancestral bacterial periplasmic binding proteins (Nakanishi et al., 1990; O’Hara et al., 1993) and close upon ligand binding (Armstrong et al., 1998; Kunishima et al., 2000). This initial conformational change is transferred to the TMDs via partially understood mechanisms, which results in either channel opening or G protein activation, respectively. Crystal structures of isolated domains (Armstrong et al., 1998; Doré et al., 2014; Jin et al., 2009; Kunishima et al., 2000; Wu et al., 2014) have provided key pieces of the atomic-level picture of these receptors and have informed mechanistic and pharmacological studies. Various forms of inter-subunit cooperativity and regulation have been revealed in both iGluRs and mGluRs. For instance, in iGluRs, the LBD dimer interface is critical for desensitization (Sun et al., 2002), whereas in mGluRs, this interface modulates basal conformational dynamics and agonist efficacy (Levitz et al., 2016a). Although no full-length mGluR structures have been reported to date, full-length iGluR structures solved by X-ray crystallography or cryoelectron microscopy (Karakas and Furukawa, 2014; Meyerson et al., 2016; Sobolevsky et al., 2009) have begun to give a more complete picture of their overall architecture in different activation states. A major challenge that remains is understanding how the complex structural and biophysical properties of GluRs manifest in the context of synaptic signaling dynamics.
Sensitivity to Glutamate and Other Ligands
GluRs have co-evolved with glutamate release and re-uptake systems to meet the multifaceted needs of synaptic transmission. Consistent with the complex concentration profile of synaptic and extra-synaptic glutamate (Bergles et al., 1999), the glutamate sensitivities of GluRs span a wide concentration range (Figure 1B). Most mGluRs respond to glutamate in the micromolar to tens of micromolar range, whereas mGluR3 has an apparent sub-micromolar half maximal effective concentration (EC50) and mGluR7 an extremely low apparent affinity with a nearly millimolar EC50 (Niswender and Conn, 2010). NMDARs have high glutamate sensitivity with apparent EC50 values between 0.4–4 μM (Paoletti et al., 2013), whereas AMPARs and KARs show values in the hundreds of micromolar to millimolar range (Traynelis et al., 2010).
In addition to being glutamate sensors, GluRs integrate various signals as they are modulated by cations, anions, and other endogenous ligands (Kubo et al., 1998; Traynelis et al., 2010; Vergnano et al., 2014) as well as membrane voltage or lipid composition (Paul et al., 2013; Ohana et al., 2006). A prominent example of signal coincidence detection is provided by NMDARs, whose glutamate-induced currents are strongly facilitated when pore block by external Mg2+ ions is relieved by depolarization (Mayer et al., 1984).
Overlapping Activation and Signaling Kinetics
Gating and subsequent signaling of GluRs occurs over a wide range of timescales; from less than milliseconds to hours (Figure 1C). AMPARs and KARs are extremely fast receptors; at high glutamate concentrations, they open within less than 1 ms to produce fast excitatory currents and deactivate or desensitize within a few milliseconds when glutamate concentrations rapidly drop or remain elevated, respectively (Traynelis et al., 2010). In contrast, NMDARs respond more slowly, with activation times in the 10 ms range and deactivation on the order of hundreds of milliseconds, depending on the GluN subtype. Moreover, NMDARs show no fast ligand-induced desensitization and have a higher Ca2+ permeability than AMPARs and KARs, which allows them to potently induce downstream signaling cascades. Although, classically, GPCRs are thought to signal over timescales of seconds to minutes, mGluR signaling can also occur much more rapidly on nearly the same timescale as iGluRs (Figure 1C). Conformational studies have shown large-scale, activation-associated inter-subunit motions in mGluRs on millisecond timescales (Hlavackova et al., 2012; Vafabakhsh et al., 2015). Although evidence exists that, at least for some mGluR subtypes, responses are diminished upon extended or repeated activation (Gereau and Heinemann, 1998; Jin et al., 2007), little is known about the timing and mechanisms of mGluR desensitization. Furthermore, some mGluRs also likely produce basal glutamate-independent activity that may provide a modulatory tone to the synapse (Ango et al., 2001; Vafabakhsh et al., 2015). Overall, much further work is needed to assess the signaling kinetics of GluRs in physiological settings, especially for mGluRs.
GluR Crosstalk Occurs by Various Mechanisms on Different Levels
Because iGluRs and mGluRs respond to the same ligand and are co-expressed at the sub-cellular compartment level, crosstalk is a likely scenario that can shape the computation and signaling outputs of a neuron. In this section, we categorize the molecular mechanisms that can lead to iGluR/mGluR crosstalk.
Physical Interactions: Direct Binding or Scaffold-Mediated Interactions
Direct iGluR/mGluR Interaction.
GluRs are integral membrane proteins with large extracellular domains and intracellular C-terminal extensions, which provide many surfaces for potential interactions with other proteins. In the confined environment of the synapse, this naturally raises the possibility of direct interaction between GluRs (Figure 2A), but evidence for direct iGluR-mGluR interactions is scarce. Based on bioluminescence resonance energy transfer (BRET) and functional measurements in HEK cells, a dynamic interaction between the CTDs of mGluR5 and GluN1a/2B receptors has been reported that results in bidirectional inhibition (Perroy et al., 2008), and a follow-up study showed BRET between heterologously-expressed mGluR5 and NMDARs in dendritic spines of hippocampal neurons (Moutin et al., 2012). However, future work is needed to characterize the physical nature of this interaction and whether direct crosstalk between mGluR5 and NMDARs is a physiologically relevant phenomenon. Notably, Yu et al. (1997) found a form of group I mGluR-mediated inhibition of NMDAR open probability that was maintained in excised patches from cortical neurons. This indicates that mGluR/iGluR crosstalk may occur in a local, membrane-delimited way, possibly through direct or indirect physical interactions.
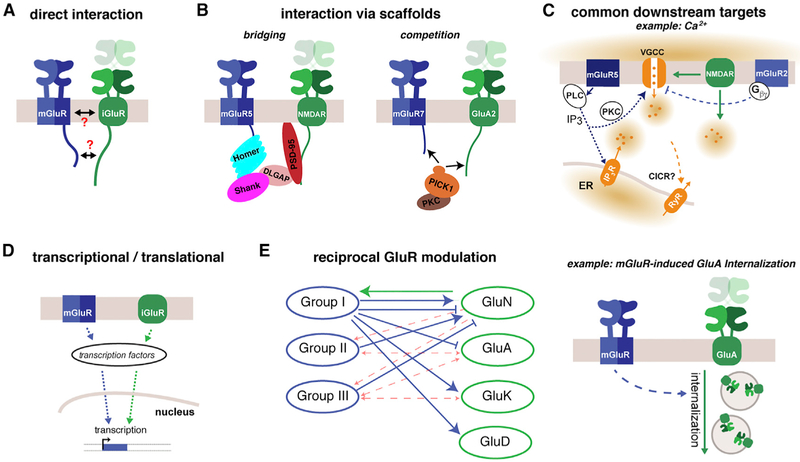
Figure 2. Potential Mechanisms of Crosstalk between iGluRs and mGluRs.
(A) iGluRs and mGluRs may interact directly to modulate each other, as exemplified by potential direct interaction between the CTDs of group I mGluRs and NMDARs..
(B) Synaptic scaffolding proteins that interact with both GluR classes provide ample opportunities for crosstalk in the form of bridging between different receptor complexes (e.g., for group I mGluRs and NMDARs via Homer-SHANK-DLGAP-PSD-95) or competition for the same scaffold (e.g., PICK1, which contains a common binding site for mGluR7 and AMPARs)..
(C) Downstream signals that are activated by iGluRs and mGluRs via overlapping or distinct mechanisms allow crosstalk, as illustrated by the shared second messenger Ca2+ (orange circles). Glutamate-evoked channel opening of iGluRs produces Ca2+ influx, whereas group I mGluRs induce Ca2+ release from intracellular stores. Both iGluRs and mGluRs can modulate the function of voltage-gated Ca2+ channels (VGCCs), and elevations in cytosolic Ca2+ can lead to Ca2+-induced Ca2+ release (CICR) from the ER, providing many opportunities for crosstalk where Ca2+ signals act cooperatively to control cellular processes..
(D) Transcription and translation are controlled by iGluR and mGluR signaling. Regulating the expression of receptor subtypes, key scaffolds, regulators, or effectors provides a powerful form of positive or negative synergism.
(E) By initiating a number of second messenger and phosphorylation-mediated signaling cascades, mGluRs and iGluRs serve as mutual effectors of each other. Left: summary of major paths of inter-class receptor regulation. Solid lines indicate potentiation or inhibition that has been clearly demonstrated, whereas dotted red lines indicate that insufficient analysis has been performed for this combination. Right: one example of reciprocal regulation is group I mGluR-mediated control of AMPAR internalization, which occurs via a kinase cascade that has not been fully elucidated.
Interaction via Scaffold Intermediaries.
Scaffolding is crucial for defining the highly ordered complex of signaling proteins in the presynaptic and postsynaptic compartments and for aligning these networks across the synapse (Biederer et al., 2017; Haucke et al., 2011; Zhu et al., 2016). Scaffold proteins, many of which contain PDZ domains that recognize interaction motifs on GluRs, modulate receptor function by defining receptor localization and embedding them into spatially organized signaling networks. This can lead to two major forms of scaffold-mediated crosstalk: “bridging,” where scaffolds mediate an interaction between an iGluR and an mGluR, or “competition,” where an iGluR and an mGluR bind the same motif on a scaffold protein.
PSD-95, SHANK, DLGAP (also known as SAPAP or GKAP), and Homer are highly abundant and multivalent postsynaptic scaffolding proteins that have been proposed to bridge group I mGluRs and NMDARs (Figure 2B; Tu et al., 1999). The EVH1 domain of Homer interacts with a proline-rich motif within the CTD of group I mGluRs, whereas a coiled-coil tetramerization domain in Homer-1b, 1c, 2, or 3 allows for the formation of large assemblies; for example, with SHANK binding to another Homer EVH1 domain. Because SHANK, via DLGAPs, also interacts with PSD-95, this complex forms a potential link to NMDARs, which interact with PSD-95 via PDZ motifs in their CTD. KARs and AMPARs may also be part of such a complex because several GluK subunits and the AMPAR auxiliary subunit stargazin (_γ_2), a member of the transmembrane AMPAR-regulating proteins (TARPs), directly interact with PSD-95 (Mehta et al., 2001; Bats et al., 2007). In addition, the Homer EVH1 domain can bias mGluR coupling by binding effector proteins, including the inositol trisphosphate (IP3) receptor (Tu et al., 1998) and TrpC channels (Yuan et al., 2003), and to modulate the basal activity of mGluR5 (Ango et al., 2001). Yang et al. (2004) found that disruption of either NMDAR-PSD-95 or mGluR5-Homer interactions in striatal neurons abolished synergistic ERK activation, demonstrating a direct effect on signaling of this complex. Notably, Homer-1a, which is an immediate-early gene product that binds to the same proline-rich domain as the long isoforms but lacks the coiled-coil domain to multimerize and couple to PSD-95 (Brakeman et al., 1997), may provide a mechanism for dynamic coupling and uncoupling of mGluR5 and NMDARs. Unfortunately, the mGluR-Homer-Shank-DLGAP-PSD-95-iGluR complex has not been well characterized biophysically. Detailed investigations of the endogenous complex would enhance the understanding of the relative arrangement of group I mGluRs and NMDARs in the post-synaptic density, especially in light of studies suggesting higher-order complexes containing SHANK and Homer (Hayashi et al., 2009). A potentially relevant observation is that iGluR CTDs undergo conformational changes upon gating (Aow et al., 2015; Zachariassen et al., 2016), which may affect scaffolds and effectors.
Another important scaffold that may serve as a site for iGluR/mGluR competition as well as bridging is PICK1, which interacts with GluA2, 3, GluK2, mGluR7, and mGluR3 (Hirbec et al., 2002; Xia et al., 1999; Dev et al., 2000). PICK1 is a Ca2+ sensor, contains a membrane curvature-sensing BAR domain (Peter et al., 2004), and can bind protein kinase C (PKC) and, thereby, modulate the availability of this central signaling enzyme. PICK1 contains one PDZ binding domain, providing the possibility for competition between mGluRs and iGluRs (Figure 2B), but PICK1 multimerization raises the alternative possibility of receptor bridging. PICK1 binding has been shown to be required for presynaptic inhibition by mGluR7 (Perroy et al., 2002) and for the anti-epileptic role of mGluR7 in thalamocortical circuits (Bertaso et al., 2008). Although PICK1 also plays a key role in the plasticity-associated trafficking of AMPARs (Xia et al., 1999) and auxiliary AMPAR subunits (Kunde et al., 2017), PICK1-mediated crosstalk between AMPARs and mGluRs has not been assessed.
Detailed studies of scaffolding interactions remain important, particularly as proteomic approaches (Schwenk et al., 2012) begin to widen our view on GluR interactomes. New labeling strategies that allow performing proteomic analyses on specific cell types (Alvarez-Castelao et al., 2017) or local protein environments (Han et al., 2017) should yield a more detailed view of synaptic composition and may allow the identification of new iGluR and mGluR interaction partners.
Crosstalk via Common Downstream Targets
Despite initially distinct mechanisms, iGluRs and mGluRs feed into many of the same downstream signaling systems, where various forms of crosstalk, such as signal summation or cooperativity, ceiling effects or even suppression, as well as subtle changes in spatio-temporal signaling patterns may occur (Figure 2C).
Second Messengers.
Synergistic activation of a second messenger system by iGluRs and mGluRs was first observed in striatal neurons prior to the cloning of mGluRs. Dumuis et al. (1990) found that arachidonic acid release was only observed after co-activation of “quisqualate ionotropic receptors,” which were likely AMPARs, and “quisqualate metabotropic receptors,” which were likely group I mGluRs. Subsequent work on GluR-mediated second messenger signaling crosstalk has focused primarily on Ca2+ and, to a lesser extent, inositol and phospholipids (Kim et al., 2015).
Ca2+ is arguably the most important second messenger in the brain because of its pivotal roles in presynaptic neurotransmitter release, postsynaptic responses, and plasticity induction. iGluRs and mGluRs can generate intracellular Ca2+ signals, albeit by different mechanisms, whose crosstalk has not been thoroughly explored (Figure 2C). iGluRs allow the influx of extracellular Ca2+ upon pore opening. This is widely acknowledged for NMDARs, which have a high Ca2+ conductance, but Ca2+ flux through AMPARs and KARs can still be substantial. Ca2+ permeability is abolished in receptors containing GluA2, GluK1, and GluK2 subunits after RNA editing of a pore residue (Q to R) in these subunits (Traynelis et al., 2010). In contrast, group I mGluRs increase the intracellular Ca2+ concentration via a classical Gq-mediated mechanism that triggers release from intracellular stores through IP3 receptors. Sustained activation of mGluR5 can produce Ca2+ oscillations that are mediated by phosphorylation and de-phosphorylation of the mGluR5 CTD (Kawabata et al., 1996). Furthermore, mGluRs can potentiate (group I; Kato et al., 2012) or inhibit (group II and III; Takahashi et al., 1996) voltage-gated Ca2+ channels, whose activation can also be controlled by iGluR-mediated depolarization, providing another convergence point for Ca2+ regulation by mGluRs and iGluRs. Indeed, such a synergism has been described for hippocampal oriens-alveus interneurons, where group I mGluRs, Ca2+-permeable AMPARs and T-type Ca2+ channels together raise intracellular Ca2+ to levels sufficient for induction of long-term potentiation (Nicholson and Kullmann, 2017).
The complexity of Ca2+ signals within the confined environment of the synapse raises many questions. For instance, how do mGluR- and iGluR-induced pools of cytosolic Ca2+ combine, what are the precise dynamics of Ca2+ nanodomains near glutamate receptors, and what are their key effectors? More work is also needed on GluR-induced Ca2+ signals within organelles, including the nucleus, the endoplasmic reticulum (ER), and mitochondria, as well as in glial compartments (Rusakov et al., 2014). Improved genetically encoded Ca2+ sensors, such as those used by de Juan-Sanz et al., 2017 in the ER of axons that cover the relevant concentration changes and can be targeted to distinct compartments, should allow these questions to be addressed with the requisite precision.
G Protein-Mediated Signaling.
mGluRs are bona fide GPCRs that primarily activate either Gq or Gi/o pathways. However, a growing body of work suggests that iGluRs can also activate G proteins to produce metabotropic signaling (Valbuena and Lerma, 2016). For both KARs and AMPARs, pertussis toxin-sensitive Gi/o-like signals have been observed in neurons (Rozas et al., 2003; Wang et al., 1997); e.g., in CA1 pyramidal cells, where KARs reduce the slow phase of afterhyperpolarization via phospholipase C (PLC) and PKC activation (Melyan et al., 2002). Most recently, a proteomics-based approach identified Go as an interaction partner for GluK1, and evidence for functional coupling was provided in HEK cells using a BRET assay (Rutkowska-Wlodarczyk et al., 2015). It remains unclear how these signaling cascades are activated and whether metabotropic effects are induced independently of ion flux. Nevertheless, the ability of iGluRs to engage in G protein signaling cascades provides many opportunities for competition and synergy with mGluRs.
Convergence of Second Messenger Signaling: Kinases and Phosphatases.
Regulation of cellular function through protein phosphorylation is another important convergence point of GluR signaling. For instance, both iGluRs and mGluRs are linked to Ca2+/calmodulin-dependent protein kinase II (CaMKII), a synaptically enriched kinase whose activation is a key step in the molecular events underlying learning and memory (Lisman et al., 2012). CaMKII forms a dodecameric complex that is autoinhibited at rest and shows a highly cooperative activation by Ca2+/CaM (Chao et al., 2011), providing ample opportunity for signal integration. CaMKII directly and dynamically binds to GluRs and, thereby, controls synaptic plasticity, as shown most comprehensively for the GluN2B CTD (Barria and Malinow, 2005; Strack and Colbran, 1998). Ca2+ influx leads to CaMKII autophosphorylation at Thr286, which enhances CaMKII binding to GluN2B (Strack and Colbran, 1998; Bayer et al., 2001) and leads to subsequent phosphorylation of GluN2B at Ser1303, which may allow CaMKII to dissociate (Strack et al., 2000) and phosphorylate other downstream targets, such as AMPARs and KARs (Carta et al., 2013; Lu et al., 2014). Although less studied in this context, group I mGluRs have also been found to activate CaMKII, and CaMKII can directly bind to intracellular domains of mGluR1 and mGluR5 to alter receptor function (Jin et al., 2013; Mockett et al., 2011).
This overlap in CaMKII signaling and mGluR/iGluR regulation raises critical questions. Can the same holoenzyme respond to different GluR-induced Ca2+ sources or are there distinct iGluR-versus mGluR-coupled CaMKII pools? What are the relative affinities of GluR binding sites? And does binding to different GluRs alter the downstream targets of CaMKII? Intriguingly, activation-induced exchange of CaMKII subunits may contribute to integration of the phosphorylation signal between GluR subtypes Stratton et al., 2014).
Additional downstream kinases are also suited to provide regulatory feedback between iGluRs and mGluRs (Kim et al., 2008; Lussier et al., 2015). It has been suggested that PKC activation in dendrites depends on both diacylglycerol produced by PLC after activation of group I mGluRs and on Ca2+ signals originating from NMDARs, akin to a coincidence detection mechanism (Codazzi et al., 2006), whereas other studies have suggested that NMDAR-induced Ca2+ signals alone may be sufficient to cause PLC-mediated PIP2 depletion in spines (Horne and Dell’Acqua, 2007). Interestingly, PKC shares downstream targets with CaMKII, which also phosphorylates GluA1 Ser831 (Kristensen et al., 2011; Roche et al., 1996) and GluN2B Ser1303 (Liao et al., 2001; Strack et al., 2000), and can regulate mGluR function (Gereau and Heinemann, 1998; Suh et al., 2008). Another channel of crosstalk is provided by protein kinase A (PKA), which is inhibited by cyclic AMP (cAMP) reduction by Gi/o-coupled group II and III mGluRs and acts on both iGluRs and mGluRs (Esteban et al., 2003; Kim et al., 2008; Skeberdis et al., 2006). GluR interaction with phosphatases is another means for crosstalk. For example, group I mGluRs interact with the calcineurin inhibitor protein (Ferreira et al., 2009), which regulates the activity of calcineurin, a Ca2+/CaM-dependent phosphatase, which, in turn, regulates iGluR activation (Banke et al., 2000; Tong et al., 1995; Traynelis and Wahl, 1997).
Improvements in proteomic techniques offer the opportunity to obtain the needed quantitative information on the synaptic phosphatome. Newly developed genetically encoded optical sensors for kinase activity (Tang and Yasuda, 2017) now allow dissection of the temporal dynamics and subcellular spatial extent of downstream signaling from GluRs. For example, a recent study found noncanonical activation of PKA through Gq-coupled receptors, including group I mGluRs, using an optogenetic sensor in hippocampal neurons (Chen et al., 2017).
Neuronal Excitability.
As ligand-gated ion channels, iGluRs depolarize neurons on fast (millisecond) and slow (second) timescales to promote spike firing. In addition to classical excitatory postsynaptic potentials (EPSPs) with ~5- to 50-ms duration, KARs can suppress afterhyperpolarization currents (_I_AHP) (Melyan et al., 2002) and increase neuronal excitability over longer timescales (~50–500 ms). mGluR signaling is also aimed at controlling neuronal excitability by modulating ion channels. Group I mGluR activation typically induces depolarizing inward currents in most neurons by either opening cation channels, such as TRP channels (Gee et al., 2003), or by inhibiting K+ channels (Charpak et al., 1990). However, mGluR1 can also activate Ca2+-activated K+ channels to produce hyperpolarization (Fiorillo and Williams, 1998). Whether group I mGluR activation is inhibitory or excitatory is, thus, context-dependent. In a powerful study, Graves et al. (2012) found two distinct populations of CA1 neurons that responded differentially, in an excitatory or inhibitory way, to mGluR1 or mGluR5 activation, implying that cellular heterogeneity may be a defining feature of mGluR-mediated regulation of excitability. In contrast, activation of group II and III mGluRs typically produces hyperpolarization via K+ channel activation, including, most prominently, GIRK channels (Dutar et al., 2000; Watanabe and Nakanishi, 2003). Thus, Gi/o-coupled mGluRs provide a cellular signal that is inherently opposed to iGluR-mediated excitation to provide feedback inhibition. An exception to the hyperpolarizing effect of group II and III activation was reported by Ster et al. (2011), who characterized the ability of mGluR3 to activate an unidentified depolarizing cation conductance in CA3 neurons.
The effects of mGluR activation on excitability and how those effects summate with iGluR-mediated EPSPs deserve more attention, especially in the context of dendritic integration (Stuart and Spruston, 2015). Methods to optically control iGluRs and mGluRs (Overcoming Technical Challenges in Studying GluR Crosstalk) in combination with technical advances to optically measure membrane potential changes (Xu et al., 2017) should be particularly well-suited for answering these questions with enhanced spatial resolution at many locations across a neuron; i.e., within a dendritic tree.
Transcriptional and Translational Crosstalk.
In addition to acute signaling events, GluRs can profoundly affect cellular function over longer timescales through transcription and translation. Both iGluRs (Lerea and McNamara, 1993; Kaltschmidt et al., 1995) and mGluRs (Wang and Zhuo, 2012) can induce transcription of immediate-early genes, which are likely downstream of both spike firing and Ca2+ influx. A key open question is whether iGluRs and mGluRs lead to transcription of an overlapping set of genes (Figure 2D), including transcription of GluRs themselves. iGluR or mGluR activation can also induce translation via a variety of pathways, including locally at the synapse (Hsu et al., 2015; Huber et al., 2000). Notably, dysregulated local protein synthesis following mGluR activation forms a central mechanism of the mGluR theory of fragile X syndrome (FXS) (Bhakar et al., 2012). Despite extensive analysis of mGluR and NMDAR-mediated control of local translation, minimal work has addressed bidirectional translational control between GluRs. With studies suggesting that mGluR5 can control PSD-95 (Todd et al., 2003), AMPAR (Muddashetty et al., 2007), or GluN2A translation (Darnell et al., 2011), there is impetus for further study.
Bidirectional Modulation: mGluRs as iGluR Effectors and Vice Versa
GluRs also act as mutual effectors of each other (Figure 2E) by a number of different mechanisms, indicating that multiple modulatory effects may occur concomitantly or in a context-dependent manner.
mGluR Regulation of iGluRs.
After the cloning of mGluRs and iGluRs, functional measurements of their crosstalk were made, with a focus on the downstream effects of group I mGluR activation on NMDARs. Studies in heterologous and native systems found that group I mGluR agonists can potentiate activation of GluN2A/2B containing NMDARs on the timescale of minutes, likely by upregulating surface expression (Fitzjohn et al., 1996; Kelso et al., 1992; Lan et al., 2001; Benquet et al., 2002). This potentiating effect on NMDARs is contradicted by previously mentioned studies (Perroy et al., 2008; Yu et al., 1997) that observed that group I mGluR-mediated NMDAR inhibition is unlikely to be mediated by trafficking effects. Interestingly, NMDAR potentiation was observed primarily in hippocampal neurons, whereas inhibition was found in cortical neurons, suggesting potential cell type dependence. Consistent with the notion of context dependence, Grishin et al., 2004 found that group I mGluR activation results in depression of NMDAR currents in CA3 neurons but potentiation in CA1 neurons. Matta et al. (2011) showed that mGluR5 activation drives the exchange of GluN2B to GluN2A subunits, indicating that modulation by group I mGluRs may depend on the NMDAR subtype and developmental stage.
Unfortunately, minimal progress on deciphering the pathways between group I mGluR activation and NMDAR regulation has been made. Rook et al. (2015) reported an mGluR5 positive allosteric modulator (PAM), VU0409551, that enhanced mGluR5 activation without potentiating NMDAR currents in CA1 neurons. This “biased” PAM has yet to provide mechanistic insight into mGluR5/NMDAR crosstalk but holds great potential for disentangling the role of mGluR5-mediated NMDAR potentiation in neurophysiological processes.
Receptor internalization is a classical form of regulation and has been extensively studied for AMPARs in the context of synaptic plasticity (iGluR/mGluR Crosstalk in Synaptic Function and Dysfunction). Group I mGluR activation leads to AMPAR internalization through a variety of kinase and phosphatase-controlled mechanisms (Moult et al., 2006; Snyder et al., 2001). Interestingly, the initial study that showed mGluR-induced iGluR internalization found clear effects on both AMPARs and NMDARs (Snyder et al., 2001), although subsequent work has largely focused on AMPARs. Moreover, group I mGluRs are also able to potentiate GluK5-containing heteromeric KARs through PKC-mediated phosphorylation (Rojas et al., 2013). A final example of group I mGluR modulation of iGluRs pertains to the GluD subfamily. Group I mGluR activation of Gq proteins has been recently shown to gate GluD1 or GluD2 to produce inward currents in heterologous and native systems (Ady et al., 2014; Benamer et al., 2018). This exciting finding provides a means of glutamate-triggered activation of GluDs and motivates further studies of the mechanisms and its physiological relevance.
Compared with group I mGluRs, less work has addressed group II and III mGluR-mediated regulation of iGluRs. However, recent studies support the notion that NMDARs can be downstream effectors of Gi/o-coupled mGluRs. For example, mGluR7 can downregulate NMDARs through an actin-dependent mechanism (Gu et al., 2012), and agonists of mGluR2 and 3 can potentiate NMDARs via kinase-mediated pathways (Rosenberg et al., 2016). Unfortunately, limited studies have addressed the effects of group II and III mGluR activation on AMPARs or KARs. Notable recent work has identified a non-canonical form of synaptic longterm depression via mGluR3-induced AMPAR internalization (Joffe et al., 2017). Many more mechanisms of reciprocal regulation may exist because iGluRs are affected by signals downstream of mGluR activation, such as arachidonic acid (Nishikawa et al., 1994), PIP2 (Michailidis et al., 2007), phosphatidylinositol 3,4,5-trisphosphate (PIP3) (Arendt et al., 2010), cAMP (Gutierrez-Castellanos et al., 2017), and activated CaMKII (Lisman et al., 2012).
iGluR Regulation of mGluRs.
The aforementioned studies show that mGluR activation modulates iGluR function, but less is known about regulation in the opposite direction. Many of the signals controlled by iGluRs have been proposed to modulate mGluRs, including voltage (Ohana et al., 2006), extracellular ions(Kubo et al., 1998), intracellular Ca2+ (O’Connor et al., 1999), and kinase signaling (Kim et al., 2008). Notably, it has been shown, in Xenopus oocytes and cortical neurons, that NMDAR activation can reverse mGluR5 desensitization via calcineurin-mediated dephosphorylation of mGluR5 at a PKC site (Ser890) (Alagarsamy et al., 1999). Future work is needed to elucidate these mechanisms and to analyze regulation of mGluRs by iGluRs in heterologous and native systems.
iGluR/mGluR Crosstalk in Synaptic Function and Dysfunction
Glutamate Sensing by iGluRs and mGluRs at the Tripartite Synapse
For a mechanistic understanding of concerted glutamate signaling, it is important to define which cells express GluRs, where they are located within the synapse, and under what conditions they are activated.
GluR Expression Patterns.
It is firmly established from RNA in situ hybridization, transcriptome data, radio-ligand binding, immunohistochemistry, and functional studies that both iGluR and mGluR expression is widespread and highly overlapping throughout nearly all brain regions (Ferraguti and Shigemoto, 2006; Hadzic et al., 2017). However, their precise expression patterns remain partially characterized at the level of individual cells. In the cortex and hippocampus, iGluRs and mGluRs are found in both excitatory glutamatergic and inhibitory GABAergic interneurons, with distinct patterns for different receptor subtypes (Figure 3A). As large-scale single cell RNA sequencing reveals an increasingly large diversity of excitatory and inhibitory neuron types (Paul et al., 2017; Zeisel et al., 2015), the need to define the precise expression levels of each GluR variant in each cell type only increases.
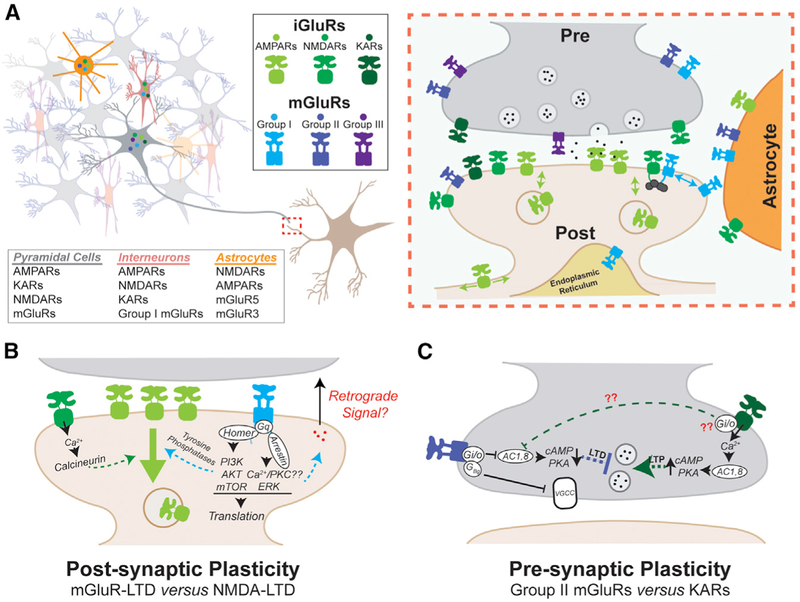
Figure 3. Concerted GluR Signaling at the Synapse.
(A) Left: iGluRs and mGluRs show overlapping expression patterns in all major neuronal cell types as well as astrocytes. The exact expression levels and receptor subtypes are not known and may vary between cells but also between individual synapses. Expression of iGluRs in glia is particularly ill-defined and appears to be regional. Glial NMDARs, which might include GluN2C/D and GluN3 subunits most prominently, remain contro-versial. Right: iGluRs and mGluRs are present in all synaptic compartments; i.e., the presynaptic terminal, the postsynaptic density, perisynaptic regions, and astrocytic processes. The precise ultrastructural localization determines the timing and concentration of glutamate-induced activation and defines both downstream signaling and the potential for functional crosstalk.
(B) GluR crosstalk in the induction of postsynaptic plasticity. This schematic focuses on the distinct pathways employed by NMDARs and mGluR5 in the induction of LTD in hippocampal neurons. Both pathways converge on the internalization of AMPARs but may target distinct populations.
(C) GluR contribution to presynaptic forms of plasticity, as illustrated by the dual roles of group II mGluRs and KARs in presynaptic LTD and LTP at hippocampal mossy fiber synapses and their convergence on regulation of release probability. The factors that determine whether KARs are excitatory or inhibitory and the signaling pathway downstream of PKA remain unclear.
GluRs are also found in various glial cell types. Among mGluRs, mGluR3 and mGluR5 are highly expressed in astrocytes, with mGluR5 expression drastically reduced in cortical and hippocampal astrocytes following development (Sun et al., 2013). Prominent examples of iGluRs in glia are AMPARs in neocortical astrocytes and cerebellar Bergmann glia (Iino et al., 2001; Chai et al., 2017) and NMDARs likely expressed in cortical astrocytes, although a consensus has not been reached (Dzamba et al., 2013). iGluRs and mGluRs are also expressed in microglia (Noda et al., 2000; Taylor et al., 2005). Overall, our understanding of iGluR and mGluR overlap in expression levels and cell type localization remains very limited and qualitative in neurons and glia, especially at the protein level.
Subcellular Localization of GluRs.
The precise nanoscale localization of GluRs will determine when activation occurs and which downstream pathways are initiated and their potential crosstalk. Classically, iGluRs and group I mGluRs are thought to localize to the postsynapse, whereas group II and III mGluRs are concentrated in the presynapse. However, a wealth of work has shown post-synaptic group II and III mGluRs, presynaptic iGluRs, and group I mGluRs as well as glial GluRs, which greatly increases the sites where GluR signaling and crosstalk may occur (Figure 3A). iGluRs are often located within the postsynaptic density (PSD) directly opposing the presynaptic release sites (Petralia and Wenthold, 1992; Matsuda et al., 2016; Biederer et al., 2017). However, iGluRs are also found in membrane regions adjacent to the PSD, perisynaptically, or extrasynaptically (Figure 3A). Exchange between extra-synaptic and intracellular AMPAR pools is thought to allow rapid changes in synaptic strength by diffusing or trafficking in and out of the synapse (Penn et al., 2017; Wu et al., 2017). In contrast, the sub-synaptic localization and dynamics of postsynaptic mGluRs remain less clear. mGluR1 and mGluR5 appear to be located at the periphery of the PSD (Nusser et al., 1994; Luján et al., 1997), but this conclusion is based on relatively few studies at a subset of relevant synapses. The apparent discrepancy in localization between synaptic iGluRs and perisynaptic or extrasynaptic mGluRs raises the question of where physical interactions between GluR classes may occur. For instance, do mGluR1 and mGluR5 exclusively interact with perisynaptic or extrasynaptic NMDARs, or do scaffolding networks bring group I mGluRs directly into the PSD? Similar to AMPARs, post-synaptic mGluR5 localization is dynamic (Sergé et al., 2002), and intracellular populations may mediate plasticity-associated signaling (Purgert et al., 2014). Less is known about the sub-synaptic localization of post-synaptic group II mGluRs, but a recent electron microscopy (EM) study in the prefrontal cortex of primates found mGluR3 directly in the synapse of thin dendritic spines (Jin et al., 2018).
Within a single cell, there is extreme heterogeneity in terms of synapse morphology, organelle population, and signaling. Single-synapse heterogeneity is especially important postsynaptically because dendritic spines provide semi-autonomous biochemical (Yasuda, 2017) and electrical (Beaulieu-Laroche and Harnett, 2018) signaling compartments. Unfortunately, limited data with single-synapse resolution have addressed the co-localization of iGluRs and mGluRs. Different iGluRs are present to varying degrees in synapses of different levels of maturation or localization relative to the soma (O’Rourke et al., 2012; Shipman et al., 2013), but mGluR variability and density across synapses types is not known. Of particular interest are silent synapses, which lack AMPARs but can be converted into active synapses following NMDAR activation (Kerchner and Nicoll, 2008). mGluR activation is proposed to promote silent synapse formation (Gomperts et al., 2000), but a direct analysis of mGluR localization to silent synapses is needed.
iGluRs and mGluRs are both also localized to the presynaptic terminal (Pinheiro and Mulle, 2008). Group II and III mGluRs are thought to serve primarily as presynaptic auto-receptors that, with the exception of the low-affinity mGluR7, are localized outside of active zones (Shigemoto et al., 1996, 1997; Figure 3A). The placement of mGluRs outside of the active zone raises the question of when pre-synaptic mGluRs are activated and how they are able to regulate release at a distance. On the other hand, iGluRs have been reported in both the active zone and perisynaptic locations. Both receptor families also exist in glial processes, where they can mediate structural plasticity (Lavialle et al., 2011), but a detailed picture of localization within glia relative to neuronal synapses does not yet exist.
High-resolution imaging techniques hold great promise for giving a more complete picture of the spatial arrangement of GluRs in individual synapses. Expansion microscopy (Chen et al., 2015) enhances the resolution of fluorescence immunohistochemistry-based approaches in intact tissue, and super-resolution imaging techniques allow live tracking of single molecules and have begun to give insight into the organization of synaptic signaling proteins (Dani et al., 2010; Tang et al., 2016). Gene editing techniques that allow addition of epitopes or fluorescent tags to endogenous proteins in post-mitotic neurons (Nishiyama et al., 2017) may further improve these experiments by avoiding overexpression, which is frequently associated with mislocalization. Excitingly, the introduction of time-resolved freezing techniques to EM (Watanabe et al., 2013) opens the door to follow GluR localization relative to receptor activation or neurotransmitter release.
GluR Activation Is Controlled by Complex Glutamate Dynamics.
The activation of different GluR populations is determined by the complex changes in glutamate concentration and the precise synaptic localization, affinity, and gating properties of each receptor subtype. Although it is experimentally difficult to characterize the nanoscale and sub-millisecond changes in glutamate concentration, indirect deductions from well characterized iGluR currents and simulations suggest that the millimolar peak concentration in the synaptic cleft after synchronized vesicular release drops rapidly with a time constant of 1 ms because of diffusion and eventual re-uptake (Figures 1B and 1C; Bergles et al., 1999). The situation is more complex for extrasynaptic glutamate, which may result from spillover from nearby or distant synapses, low transporter densities in some areas, or non-vesicular release from neurons or glia. Because of these uncertainties, it is difficult to predict, for even the best characterized iGluR subtypes, how individual receptors will respond to a given stimulus; i.e., whether full occupancy and maximal activation will occur, whether desensitization is triggered, and how recovery may be delayed by residual amounts of glutamate. For instance, Lu et al. (2017) recently attributed a slow EPSC lasting hundreds of milliseconds to a delayed AMPAR response, which is controlled by slow glutamate uptake and the presence of stargazin. For mGluRs, for which very little kinetic information has been collected, it remains unclear whether single events are even sufficient to elicit a response; the classical view is that mGluR activation requires pulse trains to produce homo- or heterosynaptic glutamate spillover (Viaene et al., 2013; Mitchell and Silver, 2000). Enhancing our quantitative understanding of mGluR activation dynamics at the synapse is one of the major necessities of the broader field of glutamatergic neurotransmission and is needed for an accurate temporal picture of GluR crosstalk. Existing work suggests that synaptic GluRs are activated in response to different firing frequencies, although some extrasynaptic iGluR populations may also be activated by spillover (Castillo et al., 1997), and evidence exists for volume transmission of glutamate in some brain regions (Szapiro and Barbour, 2007).
Regulating access to glutamate is one of the major forms of iGluR/mGluR crosstalk. Presynaptic GluRs can modulate spontaneous or evoked release and directly affect the activation of all receptors across the synapse (Pinheiro and Mulle, 2008). In particular, group II and III mGluRs serve as major mediators of feedback inhibition throughout the brain, although defining when presynaptic mGluRs are activated at different synapse types remains to be clarified. Glial GluRs likely also play an important role in shaping glutamatergic signaling because glia provide the main route of glutamate uptake and can release glutamate and other transmitters as part of activity-evoked gliotransmission (Araque et al., 2014). At CA1 synapses, it has been shown that even single synaptic events will, by local activation of mGluR5 on astrocytic processes, activate gliotransmitter release and enhance synaptic activity (Panatier et al., 2011). Notably, glia may receive distinct glutamatergic inputs, as has been demonstrated for Bergman glia, where AMPARs are activated by ectopic glutamate release (Matsui et al., 2005). Basal glutamate levels in and outside of the synaptic cleft remain controversial. Slice measurements indicate that extracellular glutamate at rest is as low as 25 nM (Herman and Jahr, 2007), whereas in vivo microdialysis measurements suggest concentrations up to μM (Dash et al., 2009), which would activate most mGluR subtypes tonically and partially desensitize many iGluR subtypes (Figure 1B). Slice studies have shown basal activity NMDARs (Le Meur et al., 2007) and mGluRs (Rodríguez-Moreno et al., 1998), but in vivo basal activity remains to be demonstrated.
Although the examination of synaptic and extrasynaptic glutamate levels has relied predominantly on electrophysiology, optical sensing approaches provide a new avenue for describing the dynamics of glutamate (Marvin et al., 2013) and, potentially, coagonists and modulatory ions, in more detail. Precise knowledge of when, where, and to what extent GluRs are activated during patterns of activity is key to fully model and understand synaptic transmission, plasticity, and dysfunction.
Opposing and Synergistic Roles in Synaptic Plasticity
Synaptic plasticity is a key property of neural circuits and has been intensively studied for glutamatergic synapses, with particular focus on those of the hippocampus, neocortex, and cerebellum. iGluRs and mGluRs contribute to long-lasting changes in synaptic strength in response to particular patterns of activation through overlapping pre- and postsynaptic mechanisms.
Long-Term Potentiation.
One of the most well-studied forms of synaptic plasticity is tetanus-induced Hebbian long-term potentiation (LTP), which was first demonstrated in the hippocampus and depends on NMDAR-mediated Ca2+ influx, which initiates a CaMKII-dependent signaling cascade that leads to increased postsynaptic AMPAR function (Herring and Nicoll, 2016). Consistent with studies showing group I mGluR-mediated potentiation of NMDARs (Bidirectional Modulation: mGluRs as iGluR Effectors and Vice Versa), group I mGluR activation contributes to the amplitude of LTP in CA1 (mGluR5) (Bashir et al., 1993; Lu et al., 1997; but see Selig et al., 1995), and CA3 (mGluR1) (Conquet et al., 1994). Notably, co-application of NMDA and a group I mGluR agonist produces a chemically induced form of LTP (Kotecha et al., 2003) similar to tetanus-induced LTP, supporting a collaborative role for mGluRs and iGluRs. However, mechanistic questions remain about the contribution of mGluRs to LTP, in part because of subtle changes in experimental conditions between studies, including previous activation of mGluRs (Bortolotto et al., 1994) and stimulus conditions (Wilsch et al., 1998). This work is consistent with the interpretation that mGluRs fine-tune various stages of NMDAR-driven LTP and associated spatial learning processes (Mukherjee and Manahan-Vaughan, 2013). The underlying basis of this crosstalk is unclear, given the many possible mechanisms (GluR Crosstalk by Various Mechanisms on Different Levels), but may, in part, be mediated by a cooperative combination of Ca2+ and CaMKII activation.
Long-Term Depression: mGluR-LTD versus NMDAR-LTD.
There are two major forms of postsynaptic long-term depression (LTD) at glutamatergic synapses, mGluR-LTD and NMDAR-LTD, which have been extensively characterized in the hippocampus (Schaffer collateral-CA1 synapses) but also occur in the cerebellum (parallel fiber-Purkinje cell synapses) and in sub-cortical regions (Lüscher and Huber, 2010). In the hippocampus, NMDAR- and mGluR-LTD can be induced through sparse electrical stimulation of Schaffer collaterals (typically 1 Hz for ~10 min) but are more often studied by using either NMDAR or group I mGluR agonists (Lee et al., 1998; Palmer et al., 1997). Hippocampal mGluR-LTD has been intensively studied in large part because its enhancement is the major endophenotype associated with FXS (Bhakar et al., 2012). mGluR-LTD has been found to be Gq-dependent (Kleppisch et al., 2001); to engage a number of kinase signaling pathways, including those involving phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR (Hou and Klann, 2004), ERK (Gallagher et al., 2004), and β-arrestins (Stoppel et al., 2017); and to require dendritic protein synthesis (Huber et al., 2000). Contradictory conclusions have been drawn about the requirement of Ca2+ elevations for mGluR-LTD (Lüscher and Huber, 2010), whereas NMDAR-LTD is thought to be triggered by a moderate initial Ca2+ influx that results in AMPAR dephosphorylation to facilitate their endocytosis (Lee et al., 2000; Mulkey et al., 1993). This Ca2+-dependent mechanism has been questioned by the controversial observation that NMDARs might induce LTD in the presence of the GluN1 antagonist 7CK or the pore blocker MK-801, which should abolish ion flow, and suggests a metabotropic pathway rather than entry of Ca2+ being in control (Nabavi et al., 2013; Stein et al., 2015; but see Babiec et al., 2014). This proposal is supported by studies showing that ligand binding alone can drive conformational changes in the CTD that affect the interaction with kinases and phosphatases (Aow et al., 2015; Vissel et al., 2001; Weilinger et al., 2016).
Unlike the collaborative nature of group I mGluR and NMDAR signaling in forms of LTP, NMDAR-LTD and mGluR-LTD are thought to be mechanistically distinct. In a particularly elegant study, Oliet et al. (1997) found that mGluR-LTD and NMDAR-LTD do not occlude each other and involve different signaling cascades that require T-type Ca2+ channels and PKC or phosphatase activity, respectively. Remarkably, the different role of mGluRs in LTP versus LTD induction suggests that mGluR5 activation, at least in CA1 neurons, is context-dependent. If NMDARs are co-active, then LTP can be induced, and if NMDARs are not co-active, then NMDAR-independent LTD can be induced. This leads to the hypothesis that, depending on the extent of NMDAR activation, mGluR5 can couple to different effector pathways, which engage either LTP- or LTD-coupled mechanisms. Importantly, mGluR-LTD requires interaction with Homer (Ronesi and Huber, 2008), indicating that precise scaffolding plays an important role. Similarly highlighting the importance of molecular organization, AKAP150-mediated recruitment of calcineurin and PKA is required for GluA1 regulation during NMDAR-LTD but not mGluR-LTD (Jurado et al., 2010; Sanderson et al., 2016), whereas inhibition of tyrosine phosphatases, such as striatal-enriched protein phosphatase (STEP), revealed a role for tyrosine de-phosphorylation on GluA2 in mGluR-LTD but not NMDAR-LTD (Moult et al., 2006). Ultimately, NMDAR and mGluR-LTD converge on reduced surface expression of AMPARs, but further resolution of the distinct pathways and, potentially, different pools of AMPARs that are involved (Casimiro et al., 2011; Figure 3B) is required. In addition, the potential age- and brain region-dependent presynaptic expression of mGluR- and NMDAR-LTD (i.e., the decrease in release probability) (Fitzjohn et al., 2001; Zakharenko et al., 2002; Xu et al., 2013) is a topic under debate.
A question that naturally arises is this: why employ two distinct LTD mechanisms if both are gated by low-frequency glutamate release? A potential answer comes from single-synapse imaging studies that suggest that large ER-containing synapses are particularly susceptible to mGluR-LTD, whereas smaller, newer synapses are more accessible to NMDAR-LTD (Holbro et al., 2009; Oh et al., 2013). Interestingly, Oh et al. (2013) found that activation of group I mGluRs is required in addition to NMDAR activation in the shrinkage of large spines. Consistent with a complex dynamic interplay between synaptic NMDAR and mGluR activation, group I mGluRs have emerged as secondary coincidence detectors in cortical spike timing-dependent LTD (Bender et al., 2006). Together, these observations highlight the need for a deeper understanding of the underlying synaptic activation dynamics of mGluRs and iGluRs.
Although post-synaptic LTD is typically expressed via the reduction of AMPAR-mediated transmission, NMDAR responses can also show a plastic decrease upon low-frequency stimulation (Hunt and Castillo, 2012) triggered by group I mGluRs (Bhouri et al., 2014). This mGluR-induced form of LTD constitutes a form of metaplasticity because the reduction of synaptic NMDARs is strong enough to increase the subsequent stimulation threshold required for NMDAR-dependent LTP. More work is needed to delineate the conditions that produce this form of LTD and the mGluR-dependent mechanisms that produce either AMPAR or NMDAR downregulation.
Presynaptic Plasticity.
mGluRs and iGluRs also mediate presynaptic forms of long-term plasticity. Pre-synaptic LTD induced by Gi/o-coupled receptors is found throughout the brain (Atwood et al., 2014), with a prominent example being NMDAR-independent, group II mGluR-mediated LTD at hippocampal mossy fiber (MF)-CA3 synapses (Kobayashi et al., 1996). Compared with post-synaptic LTD, less is known about the downstream signaling mechanisms, but downregulation of cAMP/PKA signaling (Monday and Castillo, 2017; Tzounopoulos et al., 1998) induces persistent changes in release probability (Figure 3C). Curiously, low-frequency (~0.1 Hz) pre-synaptic stimulation is required during agonist of mGluR2 and 3 application to facilitate LTD induction (Tzounopoulos et al., 1998), suggesting an uncharacterized form of coincidence detection.
Presynaptic KARs also contribute to short- and long-term presynaptic plasticity, including at MF-CA3 synapses (Nicoll and Schmitz, 2005). KAR blockade or knockout (KO) of GluK2 reduces, but does not fully prevent, presynaptic MF LTP (Contractor et al., 2001; Wallis et al., 2015), which, similar to MF LTD, is thought to be mediated by changes in presynaptic cAMP levels. Evidence exists that residual LTP is mediated by group I mGluRs (Contractor et al., 2001; Wallis et al., 2015). Consistent with differential regulation of cAMP signaling, group II mGluR activation reverses the induction of MF LTP (Tzounopoulos et al., 1998), indicating fundamentally opposed roles for group II mGluRs and KARs. However, KARs have also been found to contribute to presynaptic depression at MF-CA3 synapses via a mechanism that may involve activation of Gi/o proteins (Contractor et al., 2000; Frerking et al., 2001). Furthermore, Lyon et al. (2011) found intact MF-CA3 LTD in mGluR2 and 3 double KO mice that was blocked by the AMPAR and KAR antagonist 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX). Together, these observations motivate further analysis of presynaptic KAR/mGluR crosstalk, especially with regard to pertussis toxin (PTX)-sensitive G protein coupling. An attractive hypothesis is that KAR pore opening promotes LTP via Ca2+ influx to activate Ca2+-sensitive adenylyl cyclases, whereas KAR-mediated metabotropic signaling promotes LTD by inhibiting cAMP production through direct activation of G proteins or modulation of nearby GPCRs (Figure 3C).
Finally, presynaptic NMDARs play a highly debated role in long-term synaptic plasticity (Pinheiro and Mulle, 2008; Bouvier et al., 2018). Evidence exists that NMDAR-mediated Ca2+ flux at presynaptic terminals at a number of synapses can induce long-term changes in spontaneous or evoked release probability (Abrahamsson et al., 2017; Park et al., 2014). Further work is needed to delineate precisely which synapses contain presynaptic NMDARs and decipher the mechanisms of regulation and their potential overlap with group II and III mGluR-mediated presynaptic inhibition.
GluR Crosstalk in Neuropsychiatric Disease
A common feature of many cognitive and neuropsychiatric disorders is disruption of glutamatergic synapse function, which leads to excitation/inhibition imbalances (Volk et al., 2015). Consistent with this, glutamate receptors and the many proteins GluRs interact with physically or functionally are thought to be involved in both the underlying pathophysiology and potential treatment of many disorders. Despite many remaining mechanistic questions, both iGluRs and mGluRs have emerged as potential drug targets for a number of disorders, including schizophrenia, depression, and addiction.
The striking observation that administration of ketamine, a non-competitive NMDAR antagonist, works as an antidepressant with rapid onset and long-lasting effects has motivated extensive study of GluR-mediated synaptic plasticity in depression (Duman et al., 2016). Depression is thought to involve deficits in both the number and strength of glutamatergic synapses, particularly in the prefrontal cortex and ventral hippocampus, and, although the mechanisms of ketamine action remain controversial (Zanos and Gould, 2018), antagonism of either group I (Hughes et al., 2013) or group II (Chaki, 2017) mGluRs shows similar rapid anti-depressant effects in mouse models, raising the possibility of overlapping mechanisms. Schizophrenia is also associated with an imbalance in prefrontal cortex glutamate signaling, which is induced or exacerbated by NMDAR hypofunction (Moghaddam and Javitt, 2012), which may be alleviated by group I or II mGluR agonism or potentiation (Foster and Conn, 2017; Moghaddam and Adams, 1998) or AMPAR modulation (Ranganathan et al., 2017). Crosstalk between mGluRs and iGluRs is also relevant to the aberrant synaptic plasticity within the mesocorticolimbic system that underlies various forms of addiction. For example, cocaine use leads to an increase in Ca2+-permeable AMPAR function and an increase of GluN3-containing NMDARs in dopaminergic neurons of the ventral tegmental area, which can be re-normalized by group I mGluR signaling (Creed et al., 2016; Lüscher, 2016). Furthermore, it has been proposed that an imbalance of glutamate homeostasis upon drug use results in elevated extracellular glutamate levels in the ventral striatum, which may be restored by inhibiting release through group II and III mGluR activation (Kalivas, 2009).
The possibility of using mGluR modulators to treat neuropsychiatric disorders is promising, but a deeper understanding of the underlying GluR signaling and circuitry will be critical for higher-precision treatments. Targeting iGluRs and mGluRs in parallel may allow both mechanistic insight and enhanced treatment efficacy while potentially minimizing the side effects of iGluR agonists and antagonists.
Overcoming Technical Challenges in Studying GluR Crosstalk
The technical challenges posed by investigating the role of large, diverse receptor families activated by the same neurotransmitter at the same time is a major reason for our incomplete understanding of GluR crosstalk. Pharmacological tools to agonize, antagonize, or allosterically modulate receptor function are the most widely used means to examine GluR function (Figure 1A; Niswender and Conn, 2010; Traynelis et al., 2010). However, specificity for single receptor subtypes is rare, and poor spatial and temporal control make pharmacological agents ill-suited for studying spatially limited and rapid GluR signaling. An underappreciated issue is the potential promiscuity of drugs between iGluRs and mGluRs, which contain structurally homologous orthosteric binding sites. For instance, many commonly used mGluR drugs, such as the group II agonist DCG-IV, show antagonism or partial agonism at NMDARs (Contractor et al., 1998; Wilsch et al., 1994), limiting the usable concentration ranges and potentially confounding interpretation of experiments. Fortunately, new drugs continue to be developed, such as those with enhanced specificity or sophisticated allosteric mechanisms (Foster and Conn, 2017), including allosteric nanobodies (Scholler et al., 2017), or those that target auxiliary subunits (Maher et al., 2017).
As a complement to pharmacological studies, genetic KO models are an important tool for addressing GluR physiology. However, many GluR KOs show only modest effects, indicating functional overlap and, possibly, genetic compensation, and, given the widespread nature of GluRs in the brain, the coarseness of these perturbations may often fail to capture the distinct and sometimes opposing roles of different populations of the same receptor subtype. Recent years have seen advancements in the ability to target genetic KO or knockin of specific mutations, improving the utility of such approaches. For example, Incontro et al. (2014) established the CRISPR/Cas9 system for knockdown of either GluN1 or GluA2, and Barnes et al. (2015) developed a targeted KO mouse to identify a possible role for mGluR5 in parvalbumin-positive interneurons in neurodevelopmental disorders.
An appealing approach is to combine pharmacological approaches with optical control to manipulate GluR populations with high spatial and temporal resolution. To facilitate such experiments, caged or photoswitchable ligands have been developed for improving spatiotemporal control of native GluRs (Pittolo et al., 2014; Reiner et al., 2015). However, the soluble nature of these compounds constrains their precision because they are subject to diffusion and slow unbinding kinetics, and full subtype specificity is rarely achieved. Furthermore, the lack of genetic targeting limits the interpretation of experiments based on such compounds in native systems.
Optogenetic approaches have the advantage of being able to target distinct cell types or brain regions, which offers exciting possibilities for addressing GluR crosstalk on both the cellular and circuit level. Photoswitchable tethered ligands (PTLs), which attach directly to the receptor of interest, have permitted extremely rapid, reversible, targeted control of KARs (Volgraf et al., 2006), NMDARs (Berlin et al., 2016), and group II mGluRs (Levitz et al., 2013) with complete subtype specificity and genetic targeting capability. PTLs have facilitated biophysical work on receptor gating mechanisms Levitz et al., 2016a; Reiner and Isacoff, 2014) as well as neurophysiological studies both ex vivo and in vivo (Hou et al., 2011; Levitz et al., 2016b). Most recently, photoswitchable orthogonally and remotely tethered ligands (PORTLs) have been developed, which allow optical control of group II and III mGluRs via attachment to genetically encoded labeling tags (i.e., SNAP or CLIP) with improved specificity, orthogonality, and efficiency (Broichhagen et al., 2015, Levitz et al., 2017). Crucial for their utility in probing crosstalk, the flexibility and orthogonality of the PORTL approach, along with spectral tuning of photoswitches, has allowed multiplexing optical control of multiple GluR subtypes (Berry et al., 2017; Broichhagen et al., 2015). The ability to activate multiple GluR subtypes with defined temporal patterns relative to each other in overlapping or distinct cellular or synaptic populations will allow direct testing of the collaborative or opposing roles of receptors in different processes, such as modulation of neuronal excitability or plasticity induction.
Ideally, the PTL and PORTL approaches can be expanded to all GluR subtypes to yield a genetically targetable toolkit that can be efficiently multiplexed. Other promising complementary optogenetic approaches include the ability to optically control targeting of GluRs to the PSD (Sinnen et al., 2017) and the use of photoswitchable unnatural amino acids to inactivate or allosterically gate GluRs (Klippenstein et al., 2017). Innovative methods to perturb native GluR signaling have recently been developed, including antibody-mediated targeted ablation of AMPARs (Takemoto et al., 2017), genetically targeted delivery of AMPAR antagonists (Shields et al., 2017), or targeted visualization and ablation of native synaptic proteins (Gross et al., 2016). Together with the aforementioned methods for localizing and measuring GluRs and their downstream effectors, these technologies continue to push the field closer to the ultimate goal of pairing genetic targeting and high-resolution optical manipulation to control native receptor sub-populations.
The plethora of new approaches and the vast base of knowledge on GluRs that has been accumulated from decades of research leave the field of GluR biology poised to make major advances that will help unify our understanding of the coordinated function of iGluRs and mGluRs at the molecular, synapse, and circuit levels. mGluRs and iGluRs should be thought of as signaling partners that work in concert to balance synaptic excitation and feedback inhibition. Greater insight into iGluR/mGluR coordination will also be relevant for the general phenomena of crosstalk between ion channel and GPCRs, which is prevalent throughout the nervous system within and between neurotransmitter and neuromodulator systems.
ACKNOWLEDGMENTS
We thank Francis Lee, Jeremy Dittman, and Margaret Stratton for helpful discussions. A.R. is supported by the NRW Rückkehrerprogramm, and J.L. is supported by an R35 grant from the National Institute of General Medical Sciences (1 R35 GM124731).
REFERENCES
- Abrahamsson T, Chou CYC, Li SY, Mancino A, Costa RP, Brock JA, Nuro E, Buchanan KA, Elgar D, Blackman AV, et al. (2017). Differential regulation of evoked and spontaneous release by presynaptic NMDA receptors. Neuron 96, 839–855.e5. [DOI] [PubMed] [Google Scholar]
- Ady V, Perroy J, Tricoire L, Piochon C, Dadak S, Chen X, Dusart I, Fagni L, Lambolez B, and Levenes C (2014). Type 1 metabotropic glutamate receptors (mGlu1)triggerthe gating of GluD2 delta glutamate receptors. EMBO Rep. 15, 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagarsamy S, Marino MJ, Rouse ST, Gereau RW 4th, Heinemann SF, and Conn PJ (1999). Activation of NMDA receptors reverses desensitization of mGluR5 in native and recombinant systems. Nat. Neurosci. 2, 234–240. [DOI] [PubMed] [Google Scholar]
- Alvarez-Castelao B, Schanzenbächer CT, Hanus C, Glock C, Tom Dieck S, Dörrbaum AR, Bartnik I, Nassim-Assir B, Ciirdaeva E, Mueller A, et al. (2017). Cell-type-specific metabolic labeling of nascent proteomes in vivo. Nat. Biotechnol. 35, 1196–1201. [DOI] [PubMed] [Google Scholar]
- Ango F, Prézeau L, Muller T, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, and Fagni L (2001). Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature 411, 962–965. [DOI] [PubMed] [Google Scholar]
- Aow J, Dore K, and Malinow R (2015). Conformational signaling required for synaptic plasticity by the NMDA receptor complex. Proc. Natl. Acad. Sci. USA 112, 14711–14716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, and Volterra A (2014). Gliotransmitters travel in time and space. Neuron 81, 728–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt KL, Royo M, Fernández-Monreal M, Knafo S, Petrok CN, Martens JR, and Esteban JA (2010). PIP3 controls synaptic function by maintaining AMPA receptor clustering at the postsynaptic membrane. Nat. Neurosci. 13, 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong N, Sun Y, Chen GQ, and Gouaux E (1998). Structure of a glutamate-receptor ligand-binding core in complex with kainate. Nature 395, 913–917. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Lovinger DM, and Mathur BN (2014). Presynaptic long-term depression mediated by Gi/o-coupled receptors. Trends Neurosci. 37, 663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiec WE, Guglietta R, Jami SA, Morishita W, Malenka RC, and O’Dell TJ (2014). Ionotropic NMDA receptor signaling is required for the induction of long-term depression in the mouse hippocampal CA1 region. J. Neurosci. 34, 5285–5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banke TG, Bowie D, Lee H, Huganir RL, Schousboe A, and Traynelis SF (2000). Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J. Neurosci. 20, 89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SA, Pinto-Duarte A, Kappe A, Zembrzycki A, Metzler A, Mukamel EA, Lucero J, Wang X, Sejnowski TJ, Markou A, and Behrens MM (2015). Disruption of mGluR5 in parvalbumin-positive interneurons induces core features of neurodevelopmental disorders. Mol. Psychiatry 20, 1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, and Malinow R (2005). NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron 48, 289–301. [DOI] [PubMed] [Google Scholar]
- Bashir ZI, Bortolotto ZA, Davies CH, Berretta N, Irving AJ, Seal AJ, Henley JM, Jane DE, Watkins JC, and Collingridge GL (1993). Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature 363, 347–350. [DOI] [PubMed] [Google Scholar]
- Bats C, Groc L, and Choquet D (2007). The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron 53, 719–734. [DOI] [PubMed] [Google Scholar]
- Bayer KU, De Koninck P, Leonard AS, Hell JW, and Schulman H (2001). Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature 411, 801–805. [DOI] [PubMed] [Google Scholar]
- Beaulieu-Laroche L, and Harnett MT (2018). Dendritic spines prevent synaptic voltage clamp. Neuron 97, 75–82.e3. [DOI] [PubMed] [Google Scholar]
- Benamer N, Marti F, Lujan R, Hepp R, Aubier TG, Dupin AAM, Frébourg G, Pons S, Maskos U, Faure P, et al. (2018). GluD1, linked to schizophrenia, controls the burst firing of dopamine neurons. Mol. Psychiatry 23, 691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender VA, Bender KJ, Brasier DJ, and Feldman DE (2006). Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J. Neurosci. 26, 4166–4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benquet P, Gee CE, and Gerber U (2002). Two distinct signaling pathways upregulate NMDA receptor responses via two distinct metabotropic glutamate receptor subtypes. J. Neurosci. 22, 9679–9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Diamond JS, and Jahr CE (1999). Clearance of glutamate inside the synapse and beyond. Curr. Opin. Neurobiol. 9, 293–298. [DOI] [PubMed] [Google Scholar]
- Berlin S, Szobota S, Reiner A, Carroll EC, Kienzler MA, Guyon A, Xiao T, Trauner D,and Isacoff EY (2016). A family of photoswitchable NMDA receptors. eLife 5, e12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry MH, Holt A, Levitz J, Broichhagen J, Gaub BM, Visel M, Stanley C Aghi K, Kim YJ, Cao K, et al. (2017). Restoration of patterned vision with an engineered photoactivatable G protein-coupled receptor. Nat. Commun. 8, 1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertaso F, Zhang C, Scheschonka A, de Bock F, Fontanaud P, Marin P, Huganir RL, Betz H, Bockaert J, Fagni L, and Lerner-Natoli M (2008). PICK1 uncoupling from mGluR7a causes absence-like seizures. Nat. Neurosci. 11, 940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakar AL, Dölen G, and Bear MF (2012). The pathophysiology of fragile X (and what it teaches us about synapses). Annu. Rev. Neurosci. 35, 417–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhouri M, Farrow PA, Motee A, Yan X, Battaglia G, Di Menna L, Riozzi B, Nicoletti F, Fitzjohn SM, and Bashir ZI (2014). mGlu1 receptor-induced LTD of NMDA receptor transmission selectively at Schaffer collateral-CA1 synapses mediates metaplasticity. J. Neurosci. 34, 12223–12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederer T, Kaeser PS, and Blanpied TA (2017). Transcellular Nanoalignment of Synaptic Function. Neuron 96, 680–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolotto ZA, Bashir ZI, Davies CH, and Collingridge GL (1994). A molecular switch activated by metabotropic glutamate receptors regulates induction of long-term potentiation. Nature 368, 740–743. [DOI] [PubMed] [Google Scholar]
- Bouvier G, Larsen RS, Rodríguez-Moreno A, Paulsen O, and Sjöström PJ (2018). Towards resolving the presynaptic NMDA receptor debate. Curr. Opin. Neurobiol. 51, 1–7. [DOI] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O’Brien R, Roche K, Barnes CA, Huganir RL, and Worley PF (1997). Homer: a protein that selectively binds metabotropic glutamate receptors. Nature 386, 284–288. [DOI] [PubMed] [Google Scholar]
- Broichhagen J, Damijonaitis A, Levitz J, Sokol KR, Leippe P, Konrad D, Isacoff EY, and Trauner D (2015). Orthogonal optical control ofa G protein-coupled receptor with a SNAP-tethered photochromic ligand. ACS Cent. Sci. 1, 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Opazo P, Veran J, Athane A, Choquet D, Coussen F, and Mulle C (2013). CaMKII-dependent phosphorylation of GluK5 mediates plasticity of kainate receptors. EMBO J. 32, 496–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro TM, Sossa KG, Uzunova G, Beattie JB, Marsden KC, and Carroll RC (2011). mGluR and NMDAR activation internalize distinct populations of AMPARs. Mol. Cell. Neurosci. 48, 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Malenka RC, and Nicoll RA (1997). Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature 388, 182–186. [DOI] [PubMed] [Google Scholar]
- Chai H, Diaz-Castro B, Shigetomi E, Monte E, Octeau JC, Yu X, Cohn W, Rajendran PS, Vondriska TM, Whitelegge JP, et al. (2017). Neural circuit-specialized astrocytes: transcriptomic, proteomic, morphological, and functional evidence. Neuron 95, 531–549.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaki S (2017). mGlu2/3 receptor antagonists as novel antidepressants. Trends Pharmacol. Sci. 38, 569–580. [DOI] [PubMed] [Google Scholar]
- Chao LH, Stratton MM, Lee IH, Rosenberg OS, Levitz J, Mandell DJ, Kortemme T, Groves JT, Schulman H, and Kuriyan J (2011). A mechanism for tunable autoinhibition in the structure of a human Ca2+/calmodulin- dependent kinase II holoenzyme. Cell 146, 732–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpak S, Gähwiler BH, Do KQ, and Knöpfel T (1990). Potassium conductances in hippocampal neurons blocked by excitatory amino-acid transmitters. Nature 347, 765–767. [DOI] [PubMed] [Google Scholar]
- Chen F, Tillberg PW, and Boyden ES (2015). Optical imaging. Expansion microscopy. Science 347, 543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Granger AJ, Tran T, Saulnier JL, Kirkwood A, and Sabatini BL (2017). Endogenous Galphaq-coupled neuromodulator receptors activate protein kinase A. Neuron 96, 1070–1083.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codazzi F, Di Cesare A, Chiulli N, Albanese A, Meyer T, Zacchetti D, and Grohovaz F (2006). Synergistic control of protein kinase Cgamma activity by ionotropic and metabotropic glutamate receptor inputs in hippocampal neurons. J. Neurosci. 26, 3404–3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conquet F, Bashir ZI, Davies CH, Daniel H, Ferraguti F, Bordi F, Franz-Bacon K, Reggiani A, Matarese V, Condé F, et al. (1994). Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature 372, 237–243. [DOI] [PubMed] [Google Scholar]
- Contractor A, Gereau RW 4th, Green T, and Heinemann SF (1998). Direct effects of metabotropic glutamate receptor compounds on native and recombinant N-methyl-D-aspartate receptors. Proc. Natl. Acad. Sci. USA 95, 8969–8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor A, Swanson GT, Sailer A, O’Gorman S, and Heinemann SF (2000). Identification of the kainate receptor subunits underlying modulation of excitatory synaptic transmission in the CA3 region of the hippocampus. J. Neurosci. 20, 8269–8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor A, Swanson G, and Heinemann SF (2001). Kainate receptors are involved in short- and long-term plasticity at mossy fiber synapses in the hippocampus. Neuron 29, 209–216. [DOI] [PubMed] [Google Scholar]
- Contractor A, Mulle C, and Swanson GT (2011). Kainate receptors coming of age: milestones of two decades of research. Trends Neurosci. 34, 154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed M, Kaufling J, Fois GR, Jalabert M, Yuan T, Lüscher C, Georges F, and Bellone C (2016). Cocaine Exposure Enhances the Activity of Ventral Tegmental Area Dopamine Neurons via Calcium-Impermeable NMDARs. J. Neurosci. 36, 10759–10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis DR, Phillis JW, and Watkins JC (1959). Chemical excitation of spinal neurones. Nature 183, 611–612. [DOI] [PubMed] [Google Scholar]
- Dani A, Huang B, Bergan J, Dulac C, and Zhuang X (2010). Superresolution imaging of chemical synapses in the brain. Neuron 68, 843–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, et al. (2011). FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146, 247–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash MB, Douglas CL, Vyazovskiy VV, Cirelli C, and Tononi G (2009). Long-term homeostasis of extracellular glutamate in the rat cerebral cortex across sleep and waking states. J. Neurosci. 29, 620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Juan-Sanz J, Holt GT, Schreiter ER, de Juan F, Kim DS, and Ryan TA (2017). Axonal endoplasmic reticulum Ca2+ content controls release probability in CNS nerve terminals. Neuron 93, 867–881.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev KK, Nakajima Y, Kitano J, Braithwaite SP, Henley JM, and Nakanishi S (2000). PICK1 interacts with and regulates PKC phosphorylation of mGLUR7. J. Neurosci. 20, 7252–7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doré AS, Okrasa K, Patel JC, Serrano-Vega M, Bennett K, Cooke RM, Errey JC, Jazayeri A, Khan S, Tehan B, et al. (2014). Structure of class C GPCR metabotropic glutamate receptor 5 transmembrane domain. Nature 511, 557–562. [DOI] [PubMed] [Google Scholar]
- Doumazane E, Scholler P, Zwier JM, Trinquet E, Rondard P, and Pin JP (2011). A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors. FASEB J. 25, 66–77. [DOI] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK, Sanacora G, and Krystal JH (2016). Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat. Med. 22, 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumuis A, Pin JP, Oomagari K, Sebben M, and Bockaert J (1990). Arachidonic acid released from striatal neurons by joint stimulation of ionotropic and metabotropic quisqualate receptors. Nature 347, 182–184. [DOI] [PubMed] [Google Scholar]
- Dutar P, Petrozzino JJ, Vu HM, Schmidt MF, and Perkel DJ (2000). Slow synaptic inhibition mediated by metabotropic glutamate receptor activation of GIRK channels. J. Neurophysiol. 84, 2284–2290. [DOI] [PubMed] [Google Scholar]
- Dzamba D, Honsa P, and Anderova M (2013). NMDA receptors in glial cells: pending questions. Curr. Neurpharmacol. 11, 250–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, and Malinow R (2003). PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat. Neurosci. 6, 136–143. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, and Shigemoto R (2006). Metabotropic glutamate receptors. Cell Tissue Res. 326, 483–504. [DOI] [PubMed] [Google Scholar]
- Ferreira LT, Dale LB, Ribeiro FM, Babwah AV, Pampillo M, and Ferguson SS (2009). Calcineurin inhibitor protein (CAIN) attenuates Group I metabotropic glutamate receptor endocytosis and signaling. J. Biol. Chem. 284, 28986–28994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorillo CD, and Williams JT (1998). Glutamate mediates an inhibitory post-synaptic potential in dopamine neurons. Nature 394, 78–82. [DOI] [PubMed] [Google Scholar]
- Fitzjohn SM, Irving AJ, Palmer MJ, Harvey J, Lodge D, and Collingridge GL (1996). Activation of group I mGluRs potentiates NMDA responses in rat hippocampal slices. Neurosci. Lett. 203, 211–213. [DOI] [PubMed] [Google Scholar]
- Fitzjohn SM, Palmer MJ, May JE, Neeson A, Morris SA, and Collingridge GL (2001). A characterisation of long-term depression induced by metabotropic glutamate receptor activation in the rat hippocampus in vitro. J. Physiol. 537, 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DJ, and Conn PJ (2017). Allosteric Modulation of GPCRs: New Insights and Potential Utility for Treatment of Schizophrenia and Other CNS Disorders. Neuron 94, 431–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerking M, Schmitz D, Zhou Q, Johansen J, and Nicoll RA (2001). Kainate receptors depress excitatory synaptic transmission at CA3->CA1 synapses in the hippocampus via a direct presynaptic action. J. Neurosci. 21, 2958–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher SM, Daly CA, Bear MF, and Huber KM (2004). Extracellular signal-regulated protein kinase activation is required for metabotropic glutamate receptor-dependent long-term depression in hippocampal area CA1. J. Neurosci. 24, 4859–4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee CE, Benquet P, and Gerber U (2003). Group I metabotropic glutamate receptors activate a calcium-sensitive transient receptor potential-like conductance in rat hippocampus. J. Physiol. 546, 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gereau RW 4th, and Heinemann SF (1998). Role of protein kinase C phosphorylation in rapid desensitization of metabotropic glutamate receptor 5. Neuron 20, 143–151. [DOI] [PubMed] [Google Scholar]
- Gomperts SN, Carroll R, Malenka RC, and Nicoll RA (2000). Distinct roles for ionotropic and metabotropic glutamate receptors in the maturation of excitatory synapses. J. Neurosci. 20, 2229–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves AR, Moore SJ, Bloss EB, Mensh BD, Kath WL, and Spruston N (2012). Hippocampal pyramidal neurons comprise two distinct cell types that are countermodulated by metabotropic receptors. Neuron 76, 776–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger IH, Watson JF, and Cull-Candy SG (2017). Structural and Functional Architecture of AMPA-Type Glutamate Receptors and Their Auxiliary Proteins. Neuron 94, 713–730. [DOI] [PubMed] [Google Scholar]
- Grishin AA, Gee CE, Gerber U, and Benquet P (2004). Differential calcium-dependent modulation of NMDA currents in CA1 and CA3 hippocampal pyramidal cells. J. Neurosci. 24, 350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross GG, Straub C, Perez-Sanchez J, Dempsey WP, Junge JA, Roberts RW, Trinh le A, Fraser SE, De Koninck Y, De Koninck P, et al. (2016). An E3-ligase-based method for ablating inhibitory synapses. Nat. Methods 13, 673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Liu W, Wei J, and Yan Z (2012). Regulation of N-methyl-D-aspartic acid (NMDA) receptors by metabotropic glutamate receptor 7. J. Biol. Chem. 287, 10265–10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Castellanos N, Da Silva-Matos CM, Zhou K, Canto CB, Renner MC, Koene LMC, Ozyildirim O, Sprengel R, Kessels HW, and De Zeeuw CI (2017). Motor learning requires Purkinje cell synaptic potentiation through activation of AMPA-receptor subunit GluA3. Neuron 93, 409–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadzic M, Jack A, and Wahle P (2017). Ionotropic glutamate receptors: which ones, when, and where in the mammalian neocortex. J. Comp. Neurol. 525, 976–1033. [DOI] [PubMed] [Google Scholar]
- Han S, Li J, and Ting AY (2017). Proximity labeling: spatially resolved proteomic mapping for neurobiology. Curr. Opin. Neurobiol. 50, 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haucke V, Neher E, and Sigrist SJ (2011). Protein scaffolds in the coupling of synaptic exocytosis and endocytosis. Nat. Rev. Neurosci. 12, 127–138. [DOI] [PubMed] [Google Scholar]
- Hayashi MK, Tang C, Verpelli C, Narayanan R, Stearns MH, Xu RM, Li H, Sala C, and Hayashi Y (2009). The postsynaptic density proteins Homer and Shank form a polymeric network structure. Cell 137, 159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, and Jahr CE (2007). Extracellular glutamate concentration in hippocampal slice. J. Neurosci. 27, 9736–9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring BE, and Nicoll RA (2016). Long-term potentiation: from CaMKII to AMPA receptor trafficking. Annu. Rev. Physiol. 78, 351–365. [DOI] [PubMed] [Google Scholar]
- Hirbec H, Perestenko O, Nishimune A, Meyer G, Nakanishi S, Henley JM, and Dev KK (2002). The PDZ proteins PICK1, GRIP, and syntenin bind multiple glutamate receptor subtypes. Analysis of PDZ binding motifs. J. Biol. Chem. 277, 15221–15224. [DOI] [PubMed] [Google Scholar]
- Hlavackova V, Zabel U, Frankova D, Bätz J, Hoffmann C, Prezeau L, Pin JP, Blahos J, and Lohse MJ (2012). Sequential inter- and intrasubunit rearrangements during activation of dimeric metabotropic glutamate receptor 1. Sci. Signal. 5, ra59. [DOI] [PubMed] [Google Scholar]
- Holbro N, Grunditz A, and Oertner TG (2009). Differential distribution of endoplasmic reticulum controls metabotropic signaling and plasticity at hippocampal synapses. Proc. Natl. Acad. Sci. USA 106, 15055–15060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, and Heinemann S (1994). Cloned glutamate receptors. Annu. Rev. Neurosci. 17, 31–108. [DOI] [PubMed] [Google Scholar]
- Hollmann M, O’Shea-Greenfield A, Rogers SW, and Heinemann S (1989). Cloning by functional expression of a member of the glutamate receptor family. Nature 342, 643–648. [DOI] [PubMed] [Google Scholar]
- Horne EA, and Dell’Acqua ML (2007). Phospholipase C is required for changes in postsynaptic structure and function associated with NMDA receptor-dependent long-term depression. J. Neurosci. 27, 3523–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, and Klann E (2004). Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J. Neurosci. 24, 6352–6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q, Gilbert J, and Man HY (2011). Homeostatic regulation of AMPA receptor trafficking and degradation by light-controlled single-synaptic activation. Neuron 72, 806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu WL, Chung HW, Wu CY, Wu HI, Lee YT, Chen EC, Fang W, and Chang YC (2015). Glutamate stimulates local protein synthesis in the axons of rat cortical neurons by activating α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors and metabotropic glutamate receptors. J. Biol. Chem. 290, 20748–20760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, and Bear MF (2000). Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science 288, 1254–1257. [DOI] [PubMed] [Google Scholar]
- Hughes ZA, Neal SJ, Smith DL, Sukoff Rizzo SJ, Pulicicchio CM, Lotarski S, Lu S, Dwyer JM, Brennan J, Olsen M, et al. (2013). Negative allosteric modulation of metabotropic glutamate receptor 5 results in broad spectrum activity relevant to treatment resistant depression. Neuropharmacology 66, 202–214. [DOI] [PubMed] [Google Scholar]
- Hunt DL, and Castillo PE (2012). Synaptic plasticity of NMDA receptors: mechanisms and functional implications. Curr. Opin. Neurobiol. 22, 496–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- lino M, Goto K, Kakegawa W, Okado H, Sudo M, Ishiuchi S, Miwa A, Takayasu Y, Saito I, Tsuzuki K, and Ozawa S (2001). Glia-synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science 292, 926–929. [DOI] [PubMed] [Google Scholar]
- Incontro S, Asensio CS, Edwards RH, and Nicoll RA (2014). Efficient, complete deletion ofsynaptic proteins using CRISPR. Neuron 88, 1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Kim SJ, Kim J, Worley PF, and Linden DJ (2007). Long-term depression of mGluR1 signaling. Neuron 55, 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Singh SK, Gu S, Furukawa H, Sobolevsky AI, Zhou J, Jin Y, and Gouaux E (2009). Crystal structure and association behaviour of the GluR2 amino-terminal domain. EMBO J. 28, 1812–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin DZ, Guo ML, Xue B, Fibuch EE, Choe ES, Mao LM, and Wang JQ (2013). Phosphorylation and feedback regulation of metabotropic glutamate receptor 1 by calcium/calmodulin-dependent protein kinase II. J. Neurosci. 88, 3402–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin LE, Wang M, Galvin VC, Lightbourne TC, Conn PJ, Arnsten AFT, and Paspalas CD (2018). mGluR2 versus mGluR3 metabotropic glutamate receptors in primate dorsolateral prefrontal cortex: postsynaptic mGluR3 strengthen working memory networks. Cereb. Cortex 28, 974–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe ME, Santiago CI, Engers JL, Lindsley CW, and Conn PJ (2017). Metabotropic glutamate receptor subtype 3 gates acute stress-induced dysregulation of amygdalo-cortical function. Mol. Psychiatry. . Published online December 21, 2017. 10.1038/s41380-017-0015-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado S, Biou V, and Malenka RC (2010). A calcineurin/AKAP complex is required for NMDA receptor-dependent long-term depression. Nat. Neurosci. 78, 1053–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW (2009). The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 10, 561–572. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt C, Kaltschmidt B, and Baeuerle PA (1995). Stimulation of ionotropic glutamate receptors activates transcription factor NF-kappa B in primary neurons. Proc. Natl. Acad. Sci. USA 92, 9618–9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakas E, and Furukawa H (2014). Crystal structure of a heterotetrameric NMDA receptor ion channel. Science 844, 992–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato HK, Kassai H, Watabe AM, Aiba A, and Manabe T (2012). Functional coupling of the metabotropic glutamate receptor, InsP3 receptor and L-type Ca2+ channel in mouse CA1 pyramidal cells. J. Physiol. 590, 3019–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata S, Tsutsumi R, Kohara A, Yamaguchi T, Nakanishi S, and Okada M (1996). Control of calcium oscillations by phosphorylation of metabotropic glutamate receptors. Nature 888, 89–92. [DOI] [PubMed] [Google Scholar]
- Kelso SR, Nelson TE, and Leonard JP (1992). Protein kinase C-mediated enhancement of NMDA currents by metabotropic glutamate receptors in Xenopus oocytes. J. Physiol. 449, 705–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, and Nicoll RA (2008). Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat. Rev. Neurosci. 9, 813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Lee J, Lee JY, and Roche KW (2008). Metabotropic glutamate receptors: phosphorylation and receptor signaling. J. Neurosci. Res. 86, 1–10. [DOI] [PubMed] [Google Scholar]
- Kim HH, Lee KH, Lee D, Han YE, Lee SH, Sohn JW, and Ho WK (2015). Costimulation of AMPA and metabotropic glutamate receptors underlies phospholipase C activation by glutamate in hippocampus. J. Neurosci. 85, 6401–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleppisch T, Voigt V, Allmann R, and Offermanns S (2001). G(alpha)q-deficient mice lack metabotropic glutamate receptor-dependent long-term depression but show normal long-term potentiation in the hippocampal CA1 region. J. Neurosci. 21, 4943–4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klippenstein V, Hoppmann C, Ye S, Wang L, and Paoletti P (2017). Optocontrol of glutamate receptor activity by single side-chain photoisomerization. eLife 6, e25808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Manabe T, and Takahashi T (1996). Presynaptic long-term depression at the hippocampal mossy fiber-CA3 synapse. Science 278, 648–650. [DOI] [PubMed] [Google Scholar]
- Kotecha SA, Jackson MF, Al-Mahrouki A, Roder JC, Orser BA, and MacDonald JF (2003). Co-stimulation of mGluR5 and N-methyl-D-aspartate receptors is required for potentiation of excitatory synaptic transmission in hippocampal neurons. J. Biol. Chem. 278, 27742–27749. [DOI] [PubMed] [Google Scholar]
- Kristensen AS, Jenkins MA, Banke TG, Schousboe A, Makino Y, Johnson RC, Huganir R, and Traynelis SF (2011). Mechanism of Ca2+/calmodulin-dependent kinase II regulation of AMPA receptor gating. Nat. Neurosci. 14, 727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo Y, Miyashita T, and Murata Y (1998). Structural basis for a Ca2+-sensing function of the metabotropic glutamate receptors. Science 279, 1722–1725. [DOI] [PubMed] [Google Scholar]
- Kunde SA, Rademacher N, Zieger H, and Shoichet SA (2017). Protein kinase C regulates AMPA receptor auxiliary protein Shisa9/CKAMP44 through interactions with neuronal scaffold PICK1. FEBS Open Bio 7, 1234–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, and Morikawa K (2000). Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 407, 971–977. [DOI] [PubMed] [Google Scholar]
- Lan JY, Skeberdis VA, Jover T, Zheng X, Bennett MV, and Zukin RS (2001). Activation of metabotropic glutamate receptor 1 accelerates NMDA receptor trafficking. J. Neurosci. 21, 6058–6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavialle M, Aumann G, Anlauf E, Pröls F, Arpin M, and Derouiche A (2011). Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc. Natl. Acad. Sci. USA 108, 12915–12919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Meur K, Galante M, Angulo MC, and Audinat E (2007). Tonic activation of NMDA receptors by ambient glutamate of non-synaptic origin in the rat hippocampus. J. Physiol. 580, 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Kameyama K, Huganir RL, and Bear MF (1998). NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron 21, 1151–1162. [DOI] [PubMed] [Google Scholar]
- Lee HK, Barbarosie M, Kameyama K, Bear MF, and Huganir RL (2000). Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature 405, 955–959. [DOI] [PubMed] [Google Scholar]
- Lerea LS, and McNamara JO (1993). Ionotropic glutamate receptor subtypes activate c-fos transcription by distinct calcium-requiring intracellular signaling pathways. Neuron 10, 31–41. [DOI] [PubMed] [Google Scholar]
- Levitz J, Pantoja C, Gaub B, Janovjak H, Reiner A, Hoagland A, Schoppik D, Kane B, Stawski P, Schier AF, et al. (2013). Optical control of metabotropic glutamate receptors. Nat. Neurosci. 16, 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz J, Habrian C, Bharill S, Fu Z, Vafabakhsh R, and Isacoff EY (2016a). Mechanism of Assembly and Cooperativity of Homomeric and Heteromeric Metabotropic Glutamate Receptors. Neuron 92, 143–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz J, Popescu AT, Reiner A, and Isacoff EY (2016b). A Toolkit for Orthogonal and in vivo Optical Manipulation of Ionotropic Glutamate Receptors. Front. Mol. Neurosci. 9, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz J, Broichhagen J, Leippe P, Konrad D, Trauner D, and Isacoff EY (2017). Dual optical control and mechanistic insights into photoswitchable group II and III metabotropic glutamate receptors. Proc. Natl. Acad. Sci. USA 114, E3546–E3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao GY, Wagner DA, Hsu MH, and Leonard JP (2001). Evidence for direct protein kinase-C mediated modulation of N-methyl-D-aspartate receptor current. Mol. Pharmacol. 59, 960–964. [DOI] [PubMed] [Google Scholar]
- Lisman J, Yasuda R, and Raghavachari S (2012). Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 13, 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YM, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM, and Roder JC (1997). Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J. Neurosci. 17, 5196–5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Khatri L, and Ziff EB (2014). Trafficking of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA) receptor subunit GluA2 from the endoplasmic reticulum is stimulated by a complex containing Ca2+/calmodulin-activated kinase II (CaMKII) and PICK1 protein and by release of Ca2+ from internal stores. J. Biol. Chem. 289, 19218–19230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HW, Balmer TS, Romero GE, and Trussell LO (2017). Slow AMPAR synaptic transmission is determined by Stargazin and glutamate transporters. Neuron 96, 73–80.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luján R, Roberts JD, Shigemoto R, Ohishi H, and Somogyi P (1997). Differential plasma membrane distribution of metabotropic glutamate receptors mGluR1 alpha, mGluR2 and mGluR5, relativeto neurotransmitter release sites. J. Chem. Neuroanat. 13, 219–241. [DOI] [PubMed] [Google Scholar]
- Lüscher C (2016). The Emergence of a Circuit Model for Addiction. Annu. Rev. Neurosci. 39, 257–276. [DOI] [PubMed] [Google Scholar]
- Lüscher C, and Huber KM (2010). Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron 65, 445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier MP, Sanz-Clemente A, and Roche KW (2015). Dynamic regulation of N-methyl-d-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxa-zolepropionic acid (AMPA) receptors by posttranslational modifications. J. Biol. Chem. 290, 28596–28603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon L, Borel M, Carrión M, Kew JN, Corti C, Harrison PJ, Burnet PW, Paulsen O, and Rodríguez-Moreno A (2011). Hippocampal mossy fiber long-term depression in Grm2/3 double knockout mice. Synapse 65, 945–954. [DOI] [PubMed] [Google Scholar]
- Maher MP, Matta JA, Gu S, Seierstad M, and Bredt DS (2017). Getting a handle on neuropharmacology by targeting receptor-associated proteins. Neuron 96, 989–1001. [DOI] [PubMed] [Google Scholar]
- Marvin JS, Borghuis BG, Tian L, Cichon J, Harnett MT, Akerboom J, Gordus A, Renninger SL, Chen TW, Bargmann CI, et al. (2013). An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat. Methods 10, 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masu M, Tanabe Y, Tsuchida K, Shigemoto R, and Nakanishi S (1991). Sequence and expression of a metabotropic glutamate receptor. Nature 349, 760–765. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Budisantoso T, Mitakidis N, Sugaya Y, Miura E, Kakegawa W, Yamasaki M, Konno K, Uchigashima M, Abe M, et al. (2016). Transsynaptic modulation of kainate receptor functions by C1q-like proteins. Neuron 90, 752–767. [DOI] [PubMed] [Google Scholar]
- Matsui K, Jahr CE, and Rubio ME (2005). High-concentration rapid transients of glutamate mediate neural-glial communication via ectopic release. J. Neurosci. 25, 7538–7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta JA, Ashby MC, Sanz-Clemente A, Roche KW, and Isaac JT (2011). mGluR5 and NMDA receptors drive the experience- and activity-dependent NMDA receptor NR2B to NR2A subunit switch. Neuron 70, 339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, and Guthrie PB (1984). Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature 309, 261–263. [DOI] [PubMed] [Google Scholar]
- Mehta S, Wu H, Garner CC, and Marshall J (2001). Molecular mechanisms regulating the differential association of kainate receptor subunits with SAP90/PSD-95 and SAP97. J. Biol. Chem. 276, 16092–16099. [DOI] [PubMed] [Google Scholar]
- Melyan Z, Wheal HV, and Lancaster B (2002). Metabotropic-mediated kainate receptor regulation of IsAHP and excitability in pyramidal cells. Neuron 34, 107–114. [DOI] [PubMed] [Google Scholar]
- Meyerson JR, Chittori S, Merk A, Rao P, Han TH, Serpe M, Mayer ML, and Subramaniam S (2016). Structural basis of kainate subtype glutamate receptor desensitization. Nature 537, 567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailidis IE, Helton TD, Petrou VI, Mirshahi T, Ehlers MD, and Logothetis DE (2007). Phosphatidylinositol-4,5-bisphosphate regulates NMDA receptor activity through alpha-actinin. J. Neurosci. 27, 5523–5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, and Silver RA (2000). Glutamate spillover suppresses inhibition by activating presynaptic mGluRs. Nature 404, 498–502. [DOI] [PubMed] [Google Scholar]
- Mockett BG, Guévremont D, Wutte M, Hulme SR, Williams JM, and Abraham WC (2011). Calcium/calmodulin-dependent protein kinase II mediates group I metabotropic glutamate receptor-dependent protein synthesis and long-term depression in rat hippocampus. J. Neurosci. 31, 7380–7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, and Adams BW (1998). Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 281, 1349–1352. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, and Javitt D (2012). From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology 37, 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monday HR, and Castillo PE (2017). Closing thegap: long-term presynaptic plasticity in brain function and disease. Curr. Opin. Neurobiol. 45, 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moult PR, Gladding CM, Sanderson TM, Fitzjohn SM, Bashir ZI, Molnar E, and Collingridge GL (2006). Tyrosine phosphatases regulate AMPA receptor trafficking during metabotropic glutamate receptor-mediated longterm depression. J. Neurosci. 26, 2544–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutin E, Raynaud F, Roger J, Pellegrino E, Homburger V, Bertaso F, Ollendorff V, Bockaert J, Fagni L, and Perroy J (2012). Dynamic remodeling of scaffold interactions in dendritic spines controls synaptic excitability. J. Cell Biol. 198, 251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muddashetty RS, Kelić S, Gross C, Xu M, and Bassell GJ (2007). Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. J. Neurosci. 27, 5338–5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, and Manahan-Vaughan D (2013). Role of metabotropic glutamate receptors in persistent forms of hippocampal plasticity and learning. Neuropharmacology 66, 65–81. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Herron CE, and Malenka RC (1993). An essential role for protein phosphatases in hippocampal long-term depression. Science 261, 1051–1055. [DOI] [PubMed] [Google Scholar]
- Nabavi S, Kessels HW, Alfonso S, Aow J, Fox R, and Malinow R (2013). Metabotropic NMDA receptor function is required for NMDA receptor-dependent long-term depression. Proc. Natl. Acad. Sci. USA 110, 4027–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi N, Shneider NA, and Axel R (1990). A family of glutamate receptor genes: evidence for the formation of heteromultimeric receptors with distinct channel properties. Neuron 5, 569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson E, and Kullmann DM (2017). T-type calcium channels contribute to NMDA receptor independent synaptic plasticity in hippocampal regular-spiking oriens-alveus interneurons. J. Physiol. 595, 3449–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, and Schmitz D (2005). Synaptic plasticity at hippocampal mossy fibre synapses. Nat. Rev. Neurosci. 6, 863–876. [DOI] [PubMed] [Google Scholar]
- Nishikawa M, Kimura S, and Akaike N (1994). Facilitatory effect of docosahexaenoic acid on N-methyl-D-aspartate response in pyramidal neurones of rat cerebral cortex. J. Physiol. 475, 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama J, Mikuni T, and Yasuda R (2017). Virus-mediated genome editing via homology-directed repair in mitotic and postmitotic cells in mammalian brain. Neuron 96, 755–768.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, and Conn PJ (2010). Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 50, 295–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M, Nakanishi H, Nabekura J, and Akaike N (2000). AMPA-kainate subtypes of glutamate receptor in rat cerebral microglia. J. Neurosci. 20, 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Mulvihill E, Streit P, and Somogyi P (1994). Subsynaptic segregation of metabotropic and ionotropic glutamate receptors as revealed by immunogold localization. Neuroscience 61, 421–427. [DOI] [PubMed] [Google Scholar]
- O’Connor V, El Far O, Bofill-Cardona E, Nanoff C, Freissmuth M, Karschin A, Airas JM, Betz H, and Boehm S (1999). Calmodulin dependence of presynaptic metabotropic glutamate receptor signaling. Science 286, 1180–1184. [DOI] [PubMed] [Google Scholar]
- O’Hara PJ, Sheppard PO, Thøgersen H, Venezia D, Haldeman BA, McGrane V, Houamed KM, Thomsen C, Gilbert TL, and Mulvihill ER (1993). The ligand-binding domain in metabotropic glutamate receptors is related to bacterial periplasmic binding proteins. Neuron 11, 41–52. [DOI] [PubMed] [Google Scholar]
- O’Rourke NA, Weiler NC, Micheva KD, and Smith SJ (2012). Deep molecular diversity of mammalian synapses: why it matters and how to measure it. Nat. Rev. Neurosci. 13, 365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh WC, Hill TC, and Zito K (2013). Synapse-specific and size-dependent mechanisms ofspine structural plasticity accompanying synaptic weakening. Proc. Natl. Acad. Sci. USA 110, E305–E312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohana L, Barchad O, Parnas I, and Parnas H (2006). The metabotropic glutamate G-protein-coupled receptors mGluR3 and mGluR1a are voltage-sensitive. J. Biol. Chem. 281, 24204–24215. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Malenka RC, and Nicoll RA (1997). Two distinct forms of longterm depression coexist in CA1 hippocampal pyramidal cells. Neuron 18, 969–982. [DOI] [PubMed] [Google Scholar]
- Palmer MJ, Irving AJ, Seabrook GR, Jane DE, and Collingridge GL (1997). The group I mGlu receptor agonist DHPG induces a novel form of LTD in the CA1 region of the hippocampus. Neuropharmacology 36, 1517–1532. [DOI] [PubMed] [Google Scholar]
- Panatier A, Vallée J, Haber M, Murai KK, Lacaille JC, and Robitaille R (2011). Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 146, 785–798. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Bellone C, and Zhou Q (2013). NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 14, 383–400. [DOI] [PubMed] [Google Scholar]
- Park H, Popescu A, and Poo MM (2014). Essential role of presynaptic NMDA receptors in activity-dependent BDNF secretion and corticostriatal LTP. Neuron 84, 1009–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul SM, Doherty JJ, Robichaud AJ, Belfort GM, Chow BY, Hammond RS, Crawford DC, Linsenbardt AJ, Shu HJ, Izumi Y, et al. (2013). The major brain cholesterol metabolite 24(S)-hydroxycholesterol is a potent allosteric modulator of N-methyl-D-aspartate receptors. J. Neurosci. 33, 17290–17300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A, Crow M, Raudales R, He M, Gillis J, and Huang ZJ (2017). Transcriptional architecture of synaptic communication delineates GABAergic neuron identity. Cell 171, 522–539.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn AC, Zhang CL, Georges F, Royer L, Breillat C, Hosy E, Petersen JD, Humeau Y, and Choquet D (2017). Hippocampal LTP and contextual learning require surface diffusion of AMPA receptors. Nature 549, 384–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Otaño I, Larsen RS, and Wesseling JF (2016). Emerging roles of GluN3-containing NMDA receptors in the CNS. Nat. Rev. Neurosci. 17, 623–635. [DOI] [PubMed] [Google Scholar]
- Perroy J, El Far O, Bertaso F, Pin JP, Betz H, Bockaert J, and Fagni L (2002). PICK1 is required for the control of synaptic transmission by the metabotropic glutamate receptor 7. EMBO J. 21, 2990–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroy J, Raynaud F, Homburger V, Rousset MC, Telley L, Bockaert J, and Fagni L (2008). Direct interaction enables cross-talk between ionotropic and group I metabotropic glutamate receptors. J. Biol. Chem. 283, 6799–6805. [DOI] [PubMed] [Google Scholar]
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, and McMahon HT (2004). BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303, 495–499. [DOI] [PubMed] [Google Scholar]
- Petralia RS, and Wenthold RJ (1992). Light and electron immunocyto-chemical localization of AMPA-selective glutamate receptors in the rat brain. J. Comp. Neurol. 318, 329–354. [DOI] [PubMed] [Google Scholar]
- Pinheiro PS, and Mulle C (2008). Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nat. Rev. Neurosci. 9, 423–436. [DOI] [PubMed] [Google Scholar]
- Pittolo S, Gómez-Santacana X, Eckelt K, Rovira X, Dalton J, Goudet C, Pin JP, Llobet A, Giraldo J, Llebaria A, and Gorostiza P (2014). An allosteric modulator to control endogenous G protein-coupled receptors with light. Nat. Chem. Biol. 10, 813–815. [DOI] [PubMed] [Google Scholar]
- Purgert CA, Izumi Y, Jong YJ, Kumar V, Zorumski CF, and O’Malley KL (2014). Intracellular mGluR5 can mediate synaptic plasticity in the hippocampus. J. Neurosci. 34, 4589–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan M, DeMartinis N, Huguenel B, Gaudreault F, Bednar MM, Shaffer CL, Gupta S, Cahill J, Sherif MA, Mancuso J, et al. (2017). Attenuation of ketamine-induced impairment in verbal learning and memory in healthy volunteers by the AMPA receptor potentiator PF-04958242. Mol. Psychiatry 22, 1633–1640. [DOI] [PubMed] [Google Scholar]
- Reiner A, and Isacoff EY (2014). Tethered ligands reveal glutamate receptor desensitization depends on subunit occupancy. Nat. Chem. Biol. 10, 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Levitz J, and Isacoff EY (2015). Controlling ionotropic and metabotropic glutamate receptors with light: principles and potential. Curr. Opin. Pharmacol. 20, 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, and Huganir RL (1996). Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron 16, 1179–1188. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Moreno A, Sistiaga A, Lerma J, and Sánchez-Prieto J (1998). Switch from facilitation to inhibition of excitatory synaptic transmission by group I mGluR desensitization. Neuron 21, 1477–1486. [DOI] [PubMed] [Google Scholar]
- Rojas A, Wetherington J, Shaw R, Serrano G, Swanger S, and Dingledine R (2013). Activation of group I metabotropic glutamate receptors potentiates heteromeric kainate receptors. Mol. Pharmacol. 83, 106–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi JA, and Huber KM (2008). Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J. Neurosci. 28, 543–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook JM, Xiang Z, Lv X, Ghoshal A, Dickerson JW, Bridges TM, Johnson KA, Foster DJ, Gregory KJ, Vinson PN, et al. (2015). Biased mGlu5-positive allosteric modulators provide in vivo efficacy without potentiating mGlu5 modulation of NMDAR currents. Neuron 86, 1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N, Gerber U, and Ster J (2016). Activation of group ii metabotropic glutamate receptors promotes LTP induction at Schaffer collateral-CA1 pyramidal cell synapses by priming NMDA receptors. J. Neurosci. 36, 11521–11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozas JL, Paternain AV, and Lerma J (2003). Noncanonical signaling by ionotropic kainate receptors. Neuron 39, 543–553. [DOI] [PubMed] [Google Scholar]
- Rusakov DA, Bard L, Stewart MG, and Henneberger C (2014). Diversity of astroglial functions alludes to subcellular specialisation. Trends Neurosci. 37, 228–242. [DOI] [PubMed] [Google Scholar]
- Rutkowska-Wlodarczyk I, Aller MI, Valbuena S, Bologna JC, Prézeau L, and Lerma J (2015). A proteomic analysis reveals the interaction of GluK1 ionotropic kainate receptor subunits with Go proteins. J. Neurosci. 35, 5171–5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson JL, Gorski JA, and Dell’Acqua ML (2016). NMDA receptor-dependent LTD requires transient synaptic incorporation of Ca2+-permeable AMPARs mediated by AKAP150-anchored PKA and calcineurin. Neuron 89, 1000–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholler P, Nevoltris D, de Bundel D, Bossi S, Moreno-Delgado D, Rovira X, Møller TC, El Moustaine D, Mathieu M, Blanc E, et al. (2017). Allosteric nanobodies uncover a role ofhippocampal mGlu2 receptor homodimers in contextual fear consolidation. Nat. Commun. 8, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk J, Harmel N, Brechet A, Zolles G, Berkefeld H, Meller CS, Bildl W, Baehrens D, Huber B, Kulik A, et al. (2012). High-resolution proteomics unravel architecture and molecular diversity of native AMPA receptor complexes. Neuron 74, 621–633. [DOI] [PubMed] [Google Scholar]
- Selig DK, Lee HK, Bear MF, and Malenka RC (1995). Reexamination of the effects of MCPG on hippocampal LTP, LTD, and depotentiation. J. Neurophysiol. 74, 1075–1082. [DOI] [PubMed] [Google Scholar]
- Sergé A, Fourgeaud L, Hémar A, and Choquet D (2002). Receptoractivation and homer differentially control the lateral mobility of metabotropic glutamate receptor 5 in the neuronal membrane. J. Neurosci. 22, 3910–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields BC, Kahuno E, Kim C, Apostolides PF, Brown J, Lindo S, Mensh BD, Dudman JT, Lavis LD, and Tadross MR (2017). Deconstructing behavioral neuropharmacology with cellular specificity. Science 356, eaaj2161. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kulik A, Roberts JD, Ohishi H, Nusser Z, Kaneko T, and Somogyi P (1996). Target-cell-specific concentration of a metabotropic glutamate receptor in the presynaptic active zone. Nature 381, 523–525. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, and Mizuno N (1997). Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J. Neurosci. 17, 7503–7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipman SL, Herring BE, Suh YH, Roche KW, and Nicoll RA (2013). Distance-dependent scaling of AMPARs is cell-autonomous and GluA2 dependent. J. Neurosci. 33, 13312–13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnen BL, Bowen AB, Forte JS, Hiester BG, Crosby KC, Gibson ES, Dell’Acqua ML, and Kennedy MJ (2017). Optogenetic control of synaptic composition and function. Neuron 93, 646–660.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeberdis VA, Chevaleyre V, Lau CG, Goldberg JH, Pettit DL, Suadicani SO, Lin Y, Bennett MV, Yuste R, Castillo PE, and Zukin RS (2006). Protein kinase A regulates calcium permeability of NMDA receptors. Nat. Neurosci. 9, 501–510. [DOI] [PubMed] [Google Scholar]
- Sladeczek F, Pin JP, Récasens M, Bockaert J, and Weiss S (1985). Glutamate stimulates inositol phosphate formation in striatal neurones. Nature 317, 717–719. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, and Bear MF (2001). Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat. Neurosci. 4, 1079–1085. [DOI] [PubMed] [Google Scholar]
- Sobolevsky AI, Rosconi MP, and Gouaux E (2009). X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature 462, 745–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein IS, Gray JA, and Zito K (2015). Non-ionotropic NMDA receptor signaling drives activity-induced dendritic spine shrinkage. J. Neurosci. 35, 12303–12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ster J, Mateos JM, Grewe BF, Coiret G, Corti C, Corsi M, Helmchen E, and Gerber U (2011). Enhancement of CA3 hippocampal network activity by activation of group II metabotropic glutamate receptors. Proc. Natl. Acad. Sci. USA 108, 9993–9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppel LJ, Auerbach BD, Senter RK, Preza AR, Lefkowitz RJ, and Bear MF (2017). β-Arrestin2 couples metabotropic glutamate receptor 5 to neuronal protein synthesis and is a potential target to treat fragile X. Cell Rep. 18, 2807–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack S, and Colbran RJ (1998). Autophosphorylation-dependent targeting of calcium/ calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl- D-aspartate receptor. J. Biol. Chem. 273, 20689–20692. [DOI] [PubMed] [Google Scholar]
- Strack S, McNeill RB, and Colbran RJ (2000). Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-D-aspartate receptor. J. Biol. Chem. 275, 23798–23806. [DOI] [PubMed] [Google Scholar]
- Stratton M, Lee IH, Bhattacharyya M, Christensen SM, Chao LH, Schulman H, Groves JT, and Kuriyan J (2014). Activation-triggered subunit exchange between CaMKII holoenzymes facilitates the spread of kinase activity. eLife 3, e01610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart GJ, and Spruston N (2015). Dendritic integration: 60 years of progress. Nat. Neurosci. 18, 1713–1721. [DOI] [PubMed] [Google Scholar]
- Suh YH, Pelkey KA, Lavezzari G, Roche PA, Huganir RL, McBain CJ, and Roche KW (2008). Corequirement of PICK1 binding and PKC phosphorylation for stable surface expression of the metabotropic glutamate receptor mGluR7. Neuron 58, 736–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Olson R, Horning M, Armstrong N, Mayer M, and Gouaux E (2002). Mechanism of glutamate receptor desensitization. Nature 417, 245–253. [DOI] [PubMed] [Google Scholar]
- Sun W, McConnell E, Pare JF, Xu Q, Chen M, Peng W, Lovatt D, Han E, Smith Y, and Nedergaard M (2013). Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science 339, 197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szapiro G, and Barbour B (2007). Multiple climbing fibers signal to molecular layer interneurons exclusively via glutamate spillover. Nat. Neurosci. 10, 735–742. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Forsythe ID, Tsujimoto T, Barnes-Davies M, and Onodera K (1996). Presynaptic calcium current modulation by a metabotropic glutamate receptor. Science 274, 594–597. [DOI] [PubMed] [Google Scholar]
- Takemoto K, Iwanari H, Tada H, Suyama K, Sano A, Nagai T, Hamakubo T, and Takahashi T (2017). Optical inactivation of synaptic AMPA receptors erases fear memory. Nat. Biotechnol. 35, 38–47. [DOI] [PubMed] [Google Scholar]
- Tang S, and Yasuda R (2017). Imaging ERK and PKA activation in single dendritic spines during structural plasticity. Neuron 93, 1315–1324.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AH, Chen H, Li TP, Metzbower SR, MacGillavry HD, and Blanpied TA (2016). A trans-synaptic nanocolumn aligns neurotransmitter release to receptors. Nature 536, 210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DL, Jones F, Kubota ES, and Pocock JM (2005). Stimulation of microglial metabotropic glutamate receptor mGlu2 triggers tumor necrosis factor alpha-induced neurotoxicity in concert with microglial-derived Fas ligand. J. Neurosci. 25, 2952–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd PK, Mack KJ, and Malter JS (2003). The fragile X mental retardation protein is required for type-I metabotropic glutamate receptor-dependent translation of PSD-95. Proc. Natl. Acad. Sci. USA 100, 14374–14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong G, Shepherd D, and Jahr CE (1995). Synaptic desensitization of NMDA receptors by calcineurin. Science 267, 1510–1512. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, and Wahl P (1997). Control of rat GluR6 glutamate receptor open probability by protein kinase A and calcineurin. J. Physiol. 503, 513–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, and Dingledine R (2010). Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 62, 405–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, and Worley PF(1998). Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron 21, 717–726. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, and Worley PF (1999). Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron 23, 583–592. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Janz R, Südhof TC, Nicoll RA, and Malenka RC (1998). A role for cAMP in long-term depression at hippocampal mossy fiber synapses. Neuron 21, 837–845. [DOI] [PubMed] [Google Scholar]
- Vafabakhsh R, Levitz J, and Isacoff EY (2015). Conformational dynamics of a class C G-protein-coupled receptor. Nature 524, 497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valbuena S, and Lerma J (2016). Non-canonical signaling, the hidden life of ligand-gated ion channels. Neuron 92, 316–329. [DOI] [PubMed] [Google Scholar]
- Vergnano AM, Rebola N, Savtchenko LP, Pinheiro PS, Casado M, Kieffer BL, Rusakov DA, Mulle C, and Paoletti P (2014). Zinc dynamics and action at excitatory synapses. Neuron 82, 1101–1114. [DOI] [PubMed] [Google Scholar]
- Viaene AN, Petrof I, and Sherman SM (2013). Activation requirements for metabotropic glutamate receptors. Neurosci. Lett. 541, 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissel B, Krupp JJ, Heinemann SF, and Westbrook GL (2001). A use-dependent tyrosine dephosphorylation of NMDA receptors is independent of ion flux. Nat. Neurosci. 4, 587–596. [DOI] [PubMed] [Google Scholar]
- Volgraf M, Gorostiza P, Numano R, Kramer RH, Isacoff EY, and Trauner D (2006). Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat. Chem. Biol. 2, 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk L, Chiu SL, Sharma K, and Huganir RL (2015). Glutamate synapses in human cognitive disorders. Annu. Rev. Neurosci. 38, 127–149. [DOI] [PubMed] [Google Scholar]
- Wallis JL, Irvine MW, Jane DE, Lodge D, Collingridge GL, and Bortolotto ZA (2015). An interchangeable role for kainate and metabotropic glutamate receptors in the induction of rat hippocampal mossy fiber long-term potentiation in vivo. Hippocampus 25, 1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, and Zhuo M (2012). Group I metabotropic glutamate receptor-mediated gene transcription and implications for synaptic plasticity and diseases. Front. Pharmacol. 3, 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Small DL, Stanimirovic DB, Morley P, and Durkin JP (1997). AMPA receptor-mediated regulation of a Gi-protein in cortical neurons. Nature 383, 502–504. [DOI] [PubMed] [Google Scholar]
- Watanabe D, and Nakanishi S (2003). mGluR2 postsynaptically senses granule cell inputs at Golgi cell synapses. Neuron 39, 821–829. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Rost BR, Camacho-Perez M, Davis MW, Sohl-Kielczynski A, Rosenmund C, and Jorgensen EM (2013). Ultrafast endocytosis at mouse hippocampal synapses. Nature 504, 242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weilinger NL, Lohman AW, Rakai BD, Ma EM, Bialecki J, Maslieieva V, Rilea T, Bandet MV, Ikuta NT, Scott L, et al. (2016). Metabotropic NMDA receptor signaling couples Src family kinases to pannexin-1 during excitotoxicity. Nat. Neurosci. 19, 432–442. [DOI] [PubMed] [Google Scholar]
- Wilsch VW, Pidoplichko VI, Opitz T, Shinozaki H, and Reymann KG (1994). Metabotropic glutamate receptor agonist DCG-IV as NMDA receptor agonist in immature rat hippocampal neurons. Eur. J. Pharmacol. 262, 287–291. [DOI] [PubMed] [Google Scholar]
- Wilsch VW, Behnisch T, Jäger T, Reymann KG, and Balschun D (1998). When are class I metabotropic glutamate receptors necessary for long-term potentiation? J. Neurosci. 18, 6071–6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Wang C, Gregory KJ, Han GW, Cho HP, Xia Y, Niswender AM, Katritch V, Meiler J, Cherezov V, et al. (2014). Structure of a class C GPCR metabotropic glutamate receptor 1 bound to an allosteric modulator. Science 344, 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Bacaj T, Morishita W, Goswami D, Arendt KL, Xu W, Chen L, Malenka RC, and Sudhof TC (2017). Postsynaptic synaptotagmins mediate AMPA receptor exocytosis during LTP. Nature 544, 316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Zhang X, Staudinger J, and Huganir RL (1999). Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron 22, 179–187. [DOI] [PubMed] [Google Scholar]
- Xu W, Tse YC, Dobie FA, Baudry M, Craig AM, Wong TP, and Wang YT (2013). Simultaneous monitoring of presynaptic transmitter release and postsynaptic receptor trafficking reveals an enhancement of presynaptic activity in metabotropic glutamate receptor-mediated long-term depression. J. Neurosci. 33, 5867–5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zou P, and Cohen AE (2017). Voltage imaging with genetically encoded indicators. Curr. Opin. Chem. Biol. 39, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Mao L, Tang Q, Samdani S, Liu Z, and Wang JQ (2004). A novel Ca2+-independent signaling pathway to extracellular signal-regulated protein kinase by coactivation of NMDA receptors and metabotropic glutamate receptor 5 in neurons. J. Neurosci. 24, 10846–10857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda R (2017). Biophysics of biochemical signaling in dendritic spines: implications in synaptic plasticity. Biophys. J. 113, 2152–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SP, Sensi SL, Canzoniero LM, Buisson A, and Choi DW (1997). Membrane-delimited modulation of NMDA currents by metabotropic glutamate receptor subtypes 1/5 in cultured mouse cortical neurons. J. Physiol. 499, 721–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S, and Worley PF (2003). Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell 114, 777–789. [DOI] [PubMed] [Google Scholar]
- Yuzaki M, and Aricescu AR (2017). A GluD coming-of-age story. Trends Neurosci. 40, 138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariassen LG, Katchan L, Jensen AG, Pickering DS, Plested AJ, and Kristensen AS (2016). Structural rearrangement of the intracellular domains during AMPA receptor activation. Proc. Natl. Acad. Sci. USA 113, E3950–E3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharenko SS, Zablow L, and Siegelbaum SA (2002). Altered presynaptic vesicle release and cycling during mGluR-dependent LTD. Neuron 35, 1099–1110. [DOI] [PubMed] [Google Scholar]
- Zanos P, and Gould TD (2018). Mechanisms of ketamine action as an antidepressant. Mol. Psychiatry 23, 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel A, Murñoz-Manchado AB, Codeluppi S, Lönnerberg P, La Manno E, Jureus A, Marques S, Munguba H, He L, Betsholtz C, et al. (2015). Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347, 1138–1142. [DOI] [PubMed] [Google Scholar]
- Zhu J, Shang Y, and Zhang M (2016). Mechanistic basis of MAGUK-organized complexes in synaptic development and signalling. Nat. Rev. Neurosci. 17, 209–223. [DOI] [PubMed] [Google Scholar]