Brassinosteroid Levels Increase Drastically Prior to Morphogenesis of Tracheary Elements (original) (raw)
Abstract
As the first step toward understanding the involvement of endogenous brassinosteroids (BRs) in cytodifferentiation, we analyzed biosynthetic activities of BRs in zinnia (Zinnia elegans L. cv Canary Bird) cells differentiating into tracheary elements. The results of feeding experiments suggested that both the early and late C6-oxidation pathways occur during tracheary element differentiation. Gas chromatography-mass spectrometry analysis revealed that five BRs, castasterone, typhasterol, 6-deoxocastasterone, 6-deoxotyphasterol, and 6-deoxoteasterone, actually existed in cultured zinnia cells and culture medium. Quantification of endogenous BRs in each stage of tracheary element differentiation by gas chromatography-mass spectrometry exhibited that they increased dramatically prior to the morphogenesis, which was consistent with the idea that BRs are necessary for the initiation of the final stage of tracheary element differentiation. Moreover, the proportion of each BR in culture medium was quite different from that in cells, suggesting that specific BRs are selectively secreted into medium and may function outside the cells.
Plant development is regulated by a large number of environmental stimuli and endogenous factors. Among endogenous factors, phytohormones, such as auxins, cytokinins, gibberellins, ethylene, abscisic acid, jasmonic acid, and brassinosteroids (BRs) are well known as essential regulators. BRs are plant steroids whose structure is similar to steroid hormones in animals. Exogenously supplied BRs elicit divergent biological activities including stem elongation, root growth, and leaf bending (Yokota, 1997). Several BR-deficient mutants of Arabidopsis, pea, and tomato have been identified recently (Altmann, 1999; Li and Chory, 1999). All BR-deficient mutants show more or less dwarfism, and some mutants show altered leaf morphology, reduced male fertility, and de-etiolating of dark grown seedlings. These researches have provided evidence that BRs play essential roles in plant growth and morphogenesis.
Up to now, over 40 BRs have been characterized in nearly 60 plant species and have been detected in various plant parts such as pollen, seeds, leaves, stems, roots, and flowers (Fujioka, 1999). The biosynthetic pathways of BRs have also been established by feeding deuterio-labeled compounds of possible BR intermediates to cultured cells of Catharanthus roseus and the careful analysis of their bioconversion products by gas chromatography-mass spectrometry (GC-MS; Sakurai, 1999). These experiments revealed that brassinolide (BL) is biosynthesized from campesterol (CR) via two parallel branched pathways, namely the early and late C6-oxidation pathways (Choi et al., 1997). However, the predominance of either pathway varies among plant species and among tissues. For instance, both the late and early C6-oxidation pathways occur in pea (Nomura et al., 1999) and Arabidopsis (Fujioka et al., 1997), whereas the late C6-oxidation pathway is predominant in tomato (Bishop et al., 1999).
One of the well known functions of BRs is promotion of xylogenesis (Clouse and Sasse, 1998). The BR-deficient Arabidopsis mutants,cpd (Szekeres et al., 1996) and dwf7-1 (Choe et al., 1999), exhibit abnormal xylem. Clouse and Zurek (1991) observed that exogenously supplied BL promoted both tracheary element differentiation and cell division in cultured tuber explants of Jerusalem artichoke. Using a zinnia (Zinnia elegans L. cv Canary Bird) system, in which single mesophyll cells can differentiate directly into tracheary elements, Iwasaki and Shibaoka (1991) found that exogenously supplied uniconazole prevented uncommitted cells from trans-differentiating into tracheary elements without inhibiting cell division and that BL but not gibberellin overcame its inhibition. This finding suggests that synthesized endogenous BRs induce cytodifferentiation into tracheary elements.
The process of the in vitro tracheary element differentiation in zinnia is known to be divided into three stages, stage I, II, and III (Fukuda, 1997). Stage I corresponds to a functional dedifferentiation process from mesophyll cells to dedifferentiated cells. During stage II, dedifferentiated cells may restrict their potency and change into tracheary element precursor cells. Stage III involves secondary wall formation and cell death. Uniconazole suppressed specifically the expression of stage III-specific genes but not of stage I- and stage II-specific genes, and the suppression was overcome by BL (Yamamoto et al., 1997). These experimental data suggested that endogenous BRs initiate the final step of cytodifferentiation. One of the most important questions to be solved has been whether BR levels increase prior to stage III, and if so, what kinds of BRs increase.
In this paper, we established a sensitive bioassay system with zinnia cells and demonstrated the existence of endogenous BRs in differentiating cells by a combination of the assay system and GC-MS. The quantification of intracellular BRs by GC-MS revealed a rapid increase in specific BRs just before stage III. Moreover, we found that relatively large amounts of BRs are accumulated in culture medium and their composition differed from intracellular composition, which indicate the active and selective secretion of specific BRs into the medium.
RESULTS
Bioactivities of BR Intermediates on Tracheary Element Differentiation
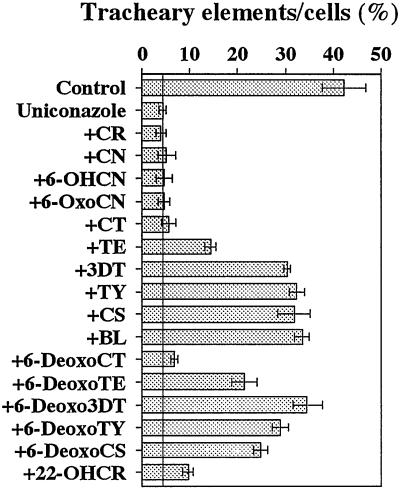
The previous papers indicated that uniconazole suppressed tracheary element differentiation in zinnia cells through the inhibition of BR biosynthesis (Iwasaki and Shibaoka, 1991; Yamamoto et al., 1997). As the first step toward understanding endogenous BR biosynthetic activity in differentiating cells, we examined whether intermediates of BR biosynthesis could rescue the uniconazole inhibition of tracheary element differentiation. BL or its biosynthetic intermediates together with 5 μm uniconazole were fed to isolated zinnia mesophyll cells at the onset of the culture, and the number of tracheary elements was counted at 96 h of culture (Fig. 1). BL at 10 nm overcame tracheary element differentiation to 80% of control. 3-Dehydroteasterone (3DT) and its subsequent intermediates belonging to the early C6-oxidation pathway (6-OxoBRs) as well as 3-dehydro-6-deoxoteasterone (6-Deoxo3DT) and its subsequent intermediates belonging to the late C6-oxidation pathway (6-DeoxoBRs) restored tracheary element differentiation as efficiently as BL. Teasterone (TE), 6-deoxoteasterone (6-DeoxoTE), and 6-deoxocathasterone (6-DeoxoCT), although less efficiently than BL, also overcame the uniconazole suppression of tracheary element differentiation. In contrast, cathasterone (CT) and its precursors at 10 nm did not prevail over the uniconazole inhibition.
Figure 1.
Biological activities of BL and its biosynthetic intermediates in cultured zinnia cells. All intermediates at the concentration of 10 nm were fed with 5 μm uniconazole at the start of culture, and rates of tracheary element formation (%) were counted at 96 h of culture. Abbreviations of intermediates are shown in the text except 6-OHCN, 6α-hydroxycampestanol. Each data point presents the mean of four replicates ± sd.
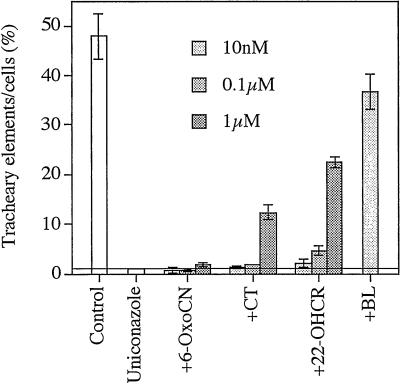
When 6-oxocampestanol (6-OxoCN) and CT were re-examined at higher concentrations, CT at 1 μm prevailed over the uniconazole inhibition partially, but 6-OxoCN did not (Fig.2). 22α-Hydroxycampesterol (22-OHCR) also prevailed over the uniconazole inhibition, and higher concentrations of the agent resulted in larger recoveries (Figs. 1 and2). In general, BL and castasterone (CS) are active BRs and other BRs function after being metabolized to BL or CS (Yokota, 1997; Sakurai, 1999). Our results, therefore, suggest that both the early and late C6-oxidation pathways occur during tracheary element differentiation in zinnia cells.
Figure 2.
Biological activities of 6-OxoCN, CT, and 22-OHCR in cultured zinnia cells. These intermediates at the concentration of 10 nm, 0.1 μm, and 1 μm were fed with 5 μm uniconazole at the start of culture, and rates of tracheary element formation were counted at 96 h of culture. The activity of 10 nm BL was also indicated as the maximum control of recovery. Each data point presents the mean of four replicates ± sd.
Identification of BRs in Cultured Cells and Medium
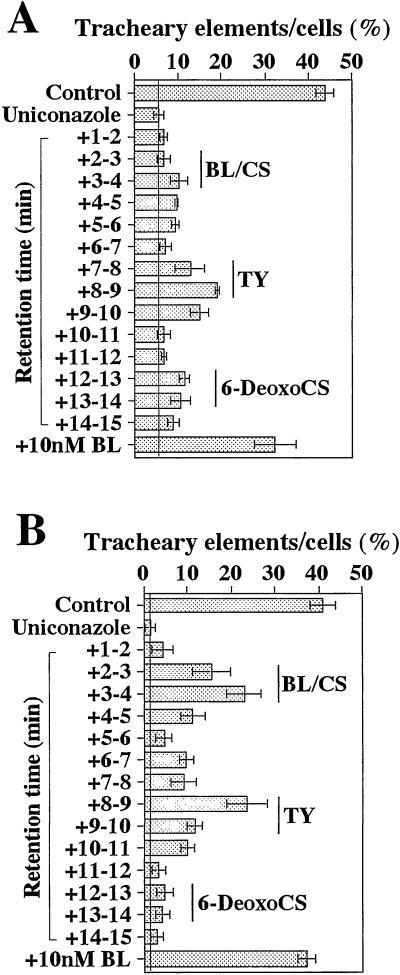
Because the above result clearly indicated that exogenously supplied BR intermediates could restore the inhibitory effect of uniconazole on tracheary element differentiation in zinnia cells, we thought that tracheary element differentiation in zinnia cells can be used as a bioassay system for measuring endogenous BRs. Then, the fractions extracted with chloroform from cells (3 g fresh weight) and medium (500 mL) at 54 h of culture were subjected to silica gel chromatography and the resultant 7% (v/v) methanol fractions were separated by reversed-phase HPLC. Each fraction of HPLC together with 5 μm uniconazole was fed to zinnia mesophyll cells at the start of culture, and the rate of tracheary element differentiation was determined at 96 h of culture (Fig. 3).
Figure 3.
Distribution of biological activity after ODS-HPLC of the extract from zinnia cultured cells (A) and the medium (B). A part of the extracts corresponding to 3 g fresh weight cells and 500 mL of medium at 54 h were evaluated by the zinnia bioassay. Each data point presents the mean of four replicates ±sd.
This bioassay system revealed that the tracheary element-promoting activity from cell extracts exhibited one major peak in fractions 7 through 10 min with a retention time that corresponds to typhasterol (TY) and two minor peaks in fractions 3 through 6 min and 12 through 14 min with retention times that correspond to BL/CS and 6-deoxocastasterone (6-DeoxoCS), respectively. BR fractions from culture medium also had three similar peaks, but the BL/CS peak was as large as the TY peak.
To elucidate whether these bioactivities were derived from endogenous BRs, the HPLC fractions were analyzed by GC-MS. Based on the full mass spectrum and GC retention time, three BRs were identified: CS was in the fraction 3 through 4 from the medium, TY was in the fractions 8 through 9 and 9 through 10 from both the cells and the medium, and 6-DeoxoCS was in the fraction 12 through 13 from the cells and in the fractions 12 through 13 and 13 through 14 from the medium (TableI).
Table I.
Identification of BRs in cultured zinnia cells and medium
| Source | Compound | Rt in GC | Characteristic Ions m/z (relative intensity %) |
|---|---|---|---|
| Cells | TYa | 11′25" | 544[M+](45), 515(100), 454(52), 229(65), 155(82) |
| 6-DeoxoCSb | 10′58" | 498[M+](28), 273(100), 155(46) | |
| Medium | CSb | 12′50" | 512[M+](63), 358(17), 287(20), 155(100) |
| TYa | 11′57" | 544[M+](63), 515(100), 454(31), 229(22), 155(58) | |
| 6-DeoxoCSb | 11′17" | 498[M+](55), 273(100), 155(43) |
Quantification of BRs in Cultured Cells and Medium
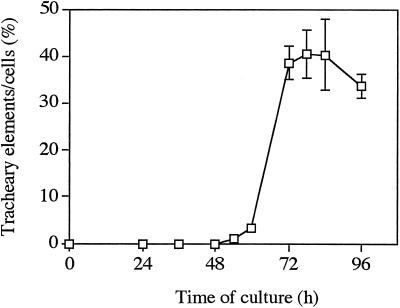
For quantitative analysis of endogenous BRs, we collected the cultured cells and culture medium at 0, 30, 54, and 78 h of culture from seven different cultures that showed similar time courses of tracheary element differentiation. A typical time course is shown in Figure 4; tracheary elements with visible secondary walls appeared at 54 h of culture, and the percentage of differentiated cells reached a maximum (40%) at 78 h.
Figure 4.
A typical time course of tracheary element formation. Cultured cells and the culture medium were collected after 0, 30, 54, and 78 h of culture.
Quantitative analysis of BRs was performed by GC-MS and/or gas chromatography-selected ion monitoring with deuterated internal standards of seven BRs, BL, CS, TY, TE, 6-DeoxoCS, 6-deoxotyphasterol (6-DeoxoTY), and 6-DeoxoTE (Table II). In addition to CS, TY, and 6-DeoxoCS (Table I), 6-DeoxoTY and 6-DeoxoTE were newly identified. However, BL and TE were below the detectable level both in cells and medium at every time point. Freshly isolated mesophyll cells contained very small amounts of every BRs measured. Endogenous levels of CS, TY, 6-DeoxoCS, 6-DeoxoTY, and 6-DeoxoTE in cultured cells increased drastically between 30 and 54 h of culture and thereafter decreased slightly. In particular, the increase in 6-DeoxoCS and 6-DeoxoTY was conspicuous and their amounts reached 44.5 ng g−1 fresh weight and 320.5 ng g−1 fresh weight at maximum, respectively. These 6-DeoxoBRs were 15 to 600 times more abundant than the 6-OxoBRs, CS, and TY. The drastic increase in intercellular BRs preceded formation of tracheary elements, which first appeared at 54 h of culture.
Table II.
Endogenous levels of BRs in cultured zinnia cells and medium during differentiation
| BRs | BR Level | ||||||
|---|---|---|---|---|---|---|---|
| 0 h | 30 h | 54 h | 78 h | ||||
| Cells | Cells | Medium | Cells | Medium | Cells | Medium | |
| ng g−1 fresh wt | ng L−1 | ng g−1 fresh wt | ng L−1 | ng g−1fresh wt | ng L−1 | ||
| BL | NDa | ND | ND | ND | ND | ND | ND |
| CS | 0.2 | 0.2 | ND | 0.5 | 25.2 | 0.6 | 31.7 |
| TY | ND | ND | 0.4 | 3.3 | 30.0 | 3.8 | 36.0 |
| TE | ND | ND | ND | ND | ND | ND | ND |
| 6-DeoxoCS | 1.8 | 9.4 | 10.0 | 44.5 | 58.6 | 21.0 | 30.5 |
| 6-DeoxoTY | 0.4 | 8.5 | 1.5 | 320.5 | 31.0 | 289.7 | 25.0 |
| 6-DeoxoTE | 0.3 | 0.3 | 2.0 | 1.2 | 1.7 | 1.0 | 1.4 |
In the medium, drastic increases in CS, TY, 6-DeoxoCS, and 6-DeoxoTY also occurred between 30 and 54 h of culture. Thereafter CS and TY increased, whereas 6-DeoxoCS and 6-DeoxoTY decreased. The levels of these four BRs, CS, TY, 6-DeoxoCS, and 6-DeoxoTY were almost the same in the medium at 54 h of culture. Averaged fresh weights of cultured cells per liter of medium were 4.0, 4.0, 5.0, and 6.3 g at 0, 30, 54, and 78 h of culture, respectively. At 54 h, therefore, 1 L of culture includes 2.5 ng of CS, 16 ng of TY, 222 ng of 6-DeoxoCS, and 1,602 ng of 6-DeoxoTY in cells and 25 ng of CS, 30 ng of TY, 58 ng of 6-DeoxoCS, and 31 ng of 6-DeoxoTY in the medium. These values clearly indicate that cells preferentially release 6-OxoBRs into the culture medium prior to tracheary element formation.
DISCUSSION
6-DeoxoBRs Are as Active as 6-OxoBRs in the Zinnia System
The biological activities of intermediates in the biosynthesis of BL were demonstrated by several BR bioassay systems (Wada et al., 1984;Fujioka et al., 1998). It is generally understood that the biological activity of intermediates increases according to their order in the biosynthetic pathway, thus, BL and CS are active BRs and other BRs function after being metabolized to BL or CS (Yokota, 1997; Sakurai, 1999). However, the relative activities of exogenously supplied 6-OxoBRs and of 6-DeoxoBRs vary among plant species, among tissues, and under light conditions. In Arabidopsis, 6-DeoxoBRs are more active than 6-OxoBRs in the light, whereas in the dark 6-DeoxoBRs are less active (Fujioka et al., 1997; Choe et al., 1998). In tomato, however, 6-DeoxoBRs are more active than 6-OxoBRs both in the light and in the dark (Koka et al., 2000), and in pea, 6-OxoBRs are more active than 6-DeoxoBRs in the light (Nomura et al., 1999). These results have suggested that activities of exogenously supplied BRs are modulated by intracellular BR synthetic activity, which varies among tissues and under light conditions. Our experiments about feeding of BL and its biosynthetic intermediates to uniconazole-treated zinnia cells revealed that the differentiation-inducing activities of intermediates tended to increase with their proximity to BL in the biosynthetic pathway, but 3DT, TY, CS, 6-Deoxo3DT, 6-DeoxoTY, and 6-DeoxoCS were as active as BL in cultured zinnia cells (Fig. 1). This result implies that these BRs are converted to the active form efficiently in cultured zinnia cells.
We used uniconazole to prevent BL biosynthesis and the resulting tracheary element differentiation in cultured zinnia cells. Uniconazole is a member of huge family of molecules containing pyridine, pyrimidine, imidazole, or triazole groups that inhibit cytochrome P450 enzymes (P450s) (Benveniste, 1986; Bolwell et al., 1994). P450s are ubiquitous hemeproteins that catalyze mono-oxigenase reactions in all phyla (Stegeman and Livingstone, 1998). Some P450s catalyze oxidation steps in the biosynthetic pathway of plant sterols and BRs (Halkier, 1996; Chapple, 1998), whereas others catalyze hydroxylation or oxidation steps in the conversion from campesterol to BL. Yet another P450 protein, CYP72B1, inactivates BL through C26-hydroxylation (Neff et al., 1999). In zinnia cells, 3DT, 6-Deoxo3DT, and their downstream BRs completely reversed the uniconazole inhibition, whereas CT, TE, 6-DeoxoCT, 6-DeoxoTE, and 22-OHCR only partially overcame the uniconazole inhibition. However 6-OxoCN and campestanol (CN) did not prevail over the inhibition. These results suggest that a target site of uniconazole may be C22α-hydroxylation catalyzed by a DWF4 type of P450. BR bioassays of Arabidopsis, cress, and rice have also revealed that the target site of brassinazole, a new inhibitor of BR biosynthesis, is the hydroxylation of both C22 and C23 positions of BRs catalyzed by P450s encoded by DWF4 and CPD (Asami and Yoshida, 1999; Min et al., 1999). The difference between the two results may be due to the difference of specificity of the two inhibitors or to the difference of susceptibility to inhibitors among plant species or tissues.
BRs Rapidly Increase before Entry into the Final Stage
Demura and Fukuda (1994) and Fukuda (1997), have presented a hypothesis that the process of differentiation of zinnia mesophyll cells into tracheary elements is divided into three stages; stage I (0–24 h of culture); stage II (24–48 h of culture); and stage III (48–96 h of culture); stage III involves tracheary element-specific events including secondary wall thickenings and cell death.
Iwasaki and Shibaoka (1991) examined the time at which exogenous BL is required if zinnia cells are to differentiate into tracheary elements and indicated that the BL-requiring stage is late stage II. We have demonstrated that BL is a prerequisite for the expression of stage III-specific genes but not for that of stage I- or stage II-related genes (Yamamoto et al., 1997). These results imply that BRs may be required for the entry of cells into stage III.
In this paper, we succeeded in detecting five BRs, CS, TY, 6-DeoxoCS, 6-DeoxoTY, and 6-DeoxoTE, in cultured cells differentiating into tracheary elements, but not BL and TE. The identified five BRs increased rapidly between 30 and 54 h of culture, although they were below detectable levels in freshly isolated cells (Table II). In particular, the increases in 6-DeoxoTY and 6-DeoxoCS were conspicuous and reached 320 ng/g fresh weight and 44 ng/g fresh weight at maximum, respectively. These amounts are comparable with those in rich sources of BRs (Clouse and Sasse, 1998). The timing of BR biosynthesis coincides with the time when BRs are required for tracheary element differentiation. Therefore one can consider that BRs are synthesized in late stage II and the synthesized BRs direct the transition of cells from stage II to stage III in which secondary wall thickenings and programmed cell death occur.
There are few reports documenting BRs in vascular tissues in plants. Although, the existence of BRs in vascular cells of pine tree has been reported (Kim et al., 1990), it is still unknown if BRs are biosynthesized in the vascular tissues themselves. Mathur and others indicated that a chimeric CPD promoter-uid gene is strongly expressed in palisade cells, suggesting that a key enzyme for synthesizing BRs is expressed in mesophyll cells (Mathur et al., 1998). Although they did not refer to the expression of the chimeric gene in the vasculature, some pictures appear to show a preferential expression of the gene in vascular networks in leaves. To understand BR biosynthesis in vascular tissues, we need to elucidate the expression of a set of BR biosynthesis-related enzymes in vascular cells in both culture and plants.
BRs Are Secreted Actively in Culture Medium
In culture medium where zinnia cells were differentiating into tracheary elements, high levels of five BRs were also identified (TableII). The quantification of BRs in medium revealed that levels of all of the identified BRs in medium rapidly elevated just before tracheary element formation, similarly to those of intracellular BRs. However, the proportion of each BR in culture medium surprisingly was quite different from that in cells. These results clearly indicate that cells release preferentially 6-OxoBRs into culture medium prior to tracheary element formation. In particular, it is interesting that a high level of CS, one of most active BRs, is secreted into medium, but not accumulated in cells, suggesting a possibility that BRs function out of the cells via BR-receptors. In zinnia cells, at least 0.03 to 0.3 nm BL can promote tracheary element formation. The sum of BRs in medium where cells had been cultured for 54 h was approximately 0.3 nm. Therefore, one can suppose that BRs in the medium function positively in tracheary element differentiation.
A BR-insensitive mutant bri1 in Arabidopsis shows severe pleiotropic effects on plant development (Clouse et al., 1996). Recently the BRI1 gene was isolated (Li and Chory, 1997) and revealed to encode a Leu-rich repeat receptor kinases that function at the cell surface to transduce BR signals (Freidrichsen et al., 2000; He et al., 2000). Our results indicating active release of BRs out of the cell are consistent with the hypothesis that BRs function outside the cell.
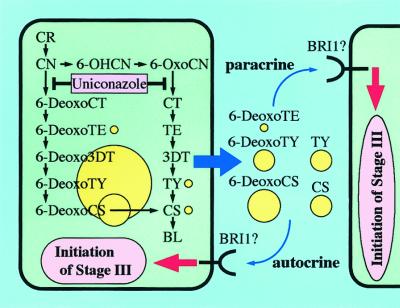
Figure 5 illustrates a possible BR biosynthetic and signaling pathway in zinnia cell culture. BRs are actively synthesized via two parallel branched pathways prior to stage III, and 6-OxoBRs are preferentially secreted out of the cell, whereas 6-DeoxoBRs are mostly accumulated within the cell. The secreted active BRs such as CS may transduce the BR signal through a BRI1-like receptor on the plasma membrane of the cells that are secreting BRs (autocrine) and/or that are not secreting them (paracrine), which initiates the entry into stage III. Efforts are under way to inspect this hypothesis.
Figure 5.
A possible BR biosynthetic and signaling pathway in zinnia cell culture. BRs are synthesized via two parallel branched pathways during stage II of tracheary element differentiation. Uniconazole may inhibit the step of 22α-hydroxylation. Among BRs, 6-DeoxoBRs are mostly accumulated within the cell but 6-OxoBRs are preferentially secreted out of the cell. The secreted active BRs may bind to a BRI1-like receptor on the plasma membrane of the cells that are secreting BRs (autocrine) and/or that are not secreting BRs (paracrine), and induce the initiation of stage III in which secondary wall formation and cell death occur. Yellow circles indicate the levels of BRs semiquantitatively.
MATERIALS AND METHODS
Reagents
Uniconazole was kindly supplied by Sumitomo Chemical (Takarazuka, Hyogo, Japan). BL was purchased from Fuji Chemical Industry Ltd (Takaoka, Toyama, Japan). Stock solutions of uniconazole and BRs were prepared in dimethyl sulfoxide (DMSO), stored at −20°C, and added to cultures at appropriate concentrations. Final concentrations of DMSO in the medium was 0.5% (v/v) in all samples, and at this level it has no effect on tracheary element differentiation or cell division (Fukuda and Komamine, 1981).
Plant Material and Culture Methods
Seeds of zinnia (Zinnia elegans L. cv Canary Bird) were sown in vermiculite and grown for about 2 weeks. A small scale of zinnia cell culture was carried out according to Fukuda and Komamine (1982). For a large scale of culture, mesophyll cells were isolated mechanically from 400 of the first leaves as described inSugiyama and Fukuda (1995) and suspended at a cell density of approximately 8 × 104 cells mL−1 in 2 L of Fukuda-Komamine's medium (Fukuda and Komamine, 1980) containing at 0.1 mg L−1 naphthylacetic acid and 0.2 mg L−1 benzyladenine. Each 30 mL of the suspension cells was distributed into culture tubes (30 × 200 mm) and cultured. The rate of tracheary element formation (%) was determined as the number of tracheary elements per the number of living cells plus tracheary elements. The number of cells was counted in three or four samples under a microscope, and at least 500 cells were examined in each sample.
Feeding Experiments (Bioassay)
Biosynthetic intermediates and HPLC fractions corresponding to 3 g fresh weight cells and 500 mL medium were diluted to the desired concentration with DMSO. They were fed into 10-mL culture medium with 5 μm uniconazole at the start of culture. After 96 h of culture, the rate of tracheary element formation (%) was counted.
HPLC Fractionation and Identification of BRs
Cultured cells and medium were collected at approximately 54 h when the rate of tracheary element formation was approximately 5% and then stored at −80°C. The stored cells (total 63.4 g fresh weight) were sonicated and extracted three times with methanol. The medium (total 1,500 mL) was filtrated with a filter paper (qualitative filter paper No. 1, ADVANTEC, Higashiosaka, Osaka, Japan) and extracted three times with chloroform. After evaporation of both solvents in vacuo, the crude extract was partitioned two times between chloroform and water. The chloroform soluble fractions were subjected to silica gel chromatography (Sep-Pak Vac 10 g/35 cc for the elute derived from the cells, Sep-Pak Vac 1 g/6 cc for the elute derived from the medium; Sap-Pak Vac, Waters, Milford, MA). The column was subsequently eluted with chloroform, 2% (v/v) methanol in chloroform, and 7% (v/v) methanol in chloroform. Both the 7% (v/v) methanol fractions were subjected to octadecylsilane (ODS)-HPLC (Senshu Pak, ODS-1151-D, 4.6 × 150 mm) at a flow rate of 1 mL min−1 with the solvents of 65% (v/v) acetonitrile. HPLC fractions were collected every 1 min. HPLC fractions corresponding to 3 g fresh weight cells and 500 mL medium were subjected to bioassay as described above. The rest of HPLC fractions were analyzed by full-scan GC-MS analysis after derivatization as described byFujioka et al. (1997).
Quantification of Endogenous BRs
Cells (5 g fresh weight) and the medium (1 L) from each stage of culture were collected, and BR fractions were extracted from them as described above. The crude extracts were spiked with 100 ng of each2H6-labeled internal standards, [2H6]BL, [2H6]CS, [2H6]TY, [2H6]TE, [2H6]6-DeoxoCS, [2H6]6-DeoxoTY, and [2H6]6-DeoxoTE. After chloroform extraction, each of the chloroform soluble fractions extracted as above was subjected to silica gel chromatography (Sep-Pak Vac 1 g/6 cc). The column was subsequently eluted with 20 mL of chloroform, 2% (v/v) methanol in chloroform, and 7% (v/v) methanol in chloroform. The elutes were subjected to ODS-HPLC at a flow rate of 1 mL min−1 with the solvents of 80% (v/v) acetonitrile for the elutes derived from the 2% (v/v) methanol fraction and of 65% (v/v) acetonitrile for the elutes derived from the 7% (v/v) methanol fraction. HPLC purification from the 2% (v/v) methanol fraction yielded the 6-DeoxoTE fraction (retention time from 9–14 min) and the 6-DeoxoTY fraction (retention time from 17–22 min), and HPLC purification from the 7% (v/v) methanol fraction yielded the BL fraction (retention time from 2–3 min), the CS fraction (retention time from 3–4.5 min), the TE fraction (retention time from 5.5–7.5 min), the TY fraction (retention time from 7.5–10.5 min), and the 6-DeoxoCS fraction (retention time from 11.5–14 min). Each fraction was derivatized and analyzed by GC-MS and/or gas chromatography-selected ion monitoring as described Fujioka et al. (1997). The endogenous levels of BRs were determined as the ratio of the peak area of molecular ions for the internal standard to that of the endogenous steroid.
Footnotes
1
This work was supported by Grants-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan (grant nos. 10304063, 10219201, and 10182101 to H.F. and grant no. 10158204 to T.D.), by a Grant-in-Aid from the Japan Society for the Promotion of Science (grant no. JSPS–RFTF96L00605 to H.F.), and the Japan Society for the Promotion of Science Research Fellowships for Young Scientists (to R.Y.).
LITERATURE CITED
- Altmann T. Molecular physiology of brassinosteroids revealed by the analysis of mutants. Planta. 1999;208:1–11. doi: 10.1007/s004250050528. [DOI] [PubMed] [Google Scholar]
- Asami T, Yoshida S. Brassinosteroid biosynthesis inhibitors. Trends Plant Sci. 1999;4:348–353. doi: 10.1016/s1360-1385(99)01456-9. [DOI] [PubMed] [Google Scholar]
- Benveniste P. Sterol biosynthesis. Annu Rev Plant Physiol. 1986;37:275–308. [Google Scholar]
- Bishop GJ, Nomura T, Yokota T, Harrison K, Noguchi T, Fujioka S, Takatsuto S, Jones JDG, Kamiya Y. The tomato DWARF enzyme catalyzes C6-oxidation in brassinosteroid biosynthesis. Proc Natl Acad Sci USA. 1999;96:1761–1766. doi: 10.1073/pnas.96.4.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell GP, Bozak K, Zimmerlin A. Plant cytochrome P450. Phytochemistry. 1994;37:1491–1506. doi: 10.1016/s0031-9422(00)89567-9. [DOI] [PubMed] [Google Scholar]
- Chapple C. Molecular-genetic analysis of plant cytochrome P450-dependent monooxygenases. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:311–343. doi: 10.1146/annurev.arplant.49.1.311. [DOI] [PubMed] [Google Scholar]
- Choe S, Dilkes BP, Fujioka S, Takatsuto S, Sakurai A, Feldmann KA. The DWF4 gene of Arabidopsis encodes a cytochrome P450 that mediates multiple 22α-hydroxylation steps in brassinosteroid biosynthesis. Plant Cell. 1998;10:231–243. doi: 10.1105/tpc.10.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S, Noguchi T, Fujioka S, Takatsuto S, Tissier CP, Gregory BD, Ross AS, Tanaka A, Yoshida S, Tax FE, Feldmann KA. The Arabidopsis dwf7/ste1 mutant is defective in the 916'3frquote 3f7-sterol C-5 desaturation step leading to brassinosteroid biosynthesis. Plant Cell. 1999;11:207–221. [PMC free article] [PubMed] [Google Scholar]
- Choi YH, Fujioka S, Nomura T, Harada A, Yokota T, Takatsuto S, Sakurai A. An alternative brassinolide biosynthetic pathway via late C6-oxidation. Phytochemistry. 1997;44:609–613. [Google Scholar]
- Clouse SD, Langford M, McMorris TC. A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol. 1996;111:671–678. doi: 10.1104/pp.111.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse SD, Sasse JM. Brassinosteroids: essential regulators of plant growth and development. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:427–451. doi: 10.1146/annurev.arplant.49.1.427. [DOI] [PubMed] [Google Scholar]
- Clouse SD, Zurek D. Molecular analysis of brassinolide action in plant growth and development. In: Cutler HG, Yokota T, Adam G, editors. Brassinosteroids: Chemistry, Bioactivity and Applications. Washington, DC: American Chemical Society; 1991. pp. 122–140. [Google Scholar]
- Demura T, Fukuda H. Novel vascular cell-specific genes whose expression is regulated temporally and spatially during vascular system development. Plant Cell. 1994;6:967–981. doi: 10.1105/tpc.6.7.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freidrichsen DM, Joazeiro CAP, Li J, Huinter T, Chory J. Brassinosteroid-insensitive-1 is a ubiquitously expressed leucine-rich repeat receptor serine/threonine kinase. Plant Physiol. 2000;123:1247–1255. doi: 10.1104/pp.123.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka S. Natural occurrence of brassinosteroids in the plant kingdom. In: Sakurai A, Yokota T, Clouse SD, editors. Brassinosteroids: Steroidal Plant Hormones. Tokyo: Springer-Verlag; 1999. pp. 21–45. [Google Scholar]
- Fujioka S, Li J, Choi YH, Seto H, Takatsuto S, Noguchi T, Watanabe T, Kuriyama H, Yokota T, Chory J, Sakurai A. The Arabidopsis deetiolated2 mutant is blocked early in brassinosteroid biosynthesis. Plant Cell. 1997;9:1951–1962. doi: 10.1105/tpc.9.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka S, Noguchi T, Takatsuto S, Yoshida S. Activity of brassinosteroids in the dwarf rice lamina inclination bioassay. Phytochemistry. 1998;49:1841–1848. [Google Scholar]
- Fukuda H. Tracheary element differentiation. Plant Cell. 1997;9:1147–1156. doi: 10.1105/tpc.9.7.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H, Komamine A. Establishment of an experimental system for the study of tracheary element differentiation from single cells isolated from the mesophyll of Zinnia elegans. Plant Physiol. 1980;65:57–60. doi: 10.1104/pp.65.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H, Komamine A. Relationship between tracheary element differentiation and DNA synthesis in single cells isolated from the mesophyll of Zinnia elegans: analysis by inhibitors of DNA synthesis. Plant Cell Physiol. 1981;22:41–49. [Google Scholar]
- Fukuda H, Komamine A. Lignin synthesis and its related enzymes as markers of tracheary-element differentiation in single cells isolated from the mesophyll of Zinnia elegans. Planta. 1982;155:423–430. doi: 10.1007/BF00394471. [DOI] [PubMed] [Google Scholar]
- Halkier BA. Catalytic reactivities and structure/function relationships of cytochrome P450 enzymes. Phytochemistry. 1996;43:1–21. [Google Scholar]
- He Z, Wang ZY, Li J, Zhu Q, Lamb C, Ronald P, Chory J. Perception of brassinosteroids by the extracellular domain of the receptor kinase BRI1. Science. 2000;288:2360–2363. doi: 10.1126/science.288.5475.2360. [DOI] [PubMed] [Google Scholar]
- Iwasaki T, Shibaoka H. Brassinosteroids act as regulators of tracheary-element differentiation in isolated Zinnia mesophyll cells. Plant Cell Physiol. 1991;32:1007–1014. [Google Scholar]
- Kim SK, Abe H, Little CHA, Pharis RP. Identification of two brassinosteroids from the cambial region of Scots pine (Pinus silverstris) by gas chromatography-mass spectrometry, after detection using a dwarf rice lamina inclination bioassay. Plant Physiol. 1990;94:1709–1713. doi: 10.1104/pp.94.4.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koka CV, Cerny RE, Gardner RG, Noguchi T, Fujioka S, Takatsuto S, Yoshida S, Clouse SD. A putative role for the tomato genes DUMPY and CURL-3 in brassinosteroid biosynthesis and response. Plant Physiol. 2000;122:85–98. doi: 10.1104/pp.122.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chory J. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell. 1997;90:929–938. doi: 10.1016/s0092-8674(00)80357-8. [DOI] [PubMed] [Google Scholar]
- Li J, Chory J. Brassinosteroids actions in plants. J Exp Bot. 1999;50:275–282. [Google Scholar]
- Mathur J, Molnar G, Fujioka S, Takatsuto S, Sakurai A, Yokota T, Adam G, Voigt B, Nagy F, Mass C, Schell J, Koncz C, Szekeres M. Transcription of the Arabidopsis CPD gene, encoding a steroidogenic cytochrome P450, is negatively controlled by brassinosteroids. Plant J. 1998;14:593–602. doi: 10.1046/j.1365-313x.1998.00158.x. [DOI] [PubMed] [Google Scholar]
- Min YK, Asami T, Fujioka S, Murofushi N, Yamaguchi I, Yoshida S. New lead compounds for brassinosteroid biosynthesis inhibitors. Bioorg Med Chem Lett. 1999;9:425–430. doi: 10.1016/s0960-894x(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Neff MM, Nguyen SM, Malancharuvil EJ, Fujioka S, Noguchi T, Seto H, Tsubuki M, Honda T, Takatsuto S, Yoshida S, Chory J. BAS1: a gene regulating brassinosteroid levels and light responsiveness in Arabidopsis. Proc Natl Acad Sci USA. 1999;96:15316–15323. doi: 10.1073/pnas.96.26.15316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Kitasaka Y, Takatsuto S, Reid JB, Fukami M, Yokota T. Brassinosteroid/sterol synthesis and plant growth as affected by lka and lkb mutations of pea. Plant Physiol. 1999;119:1517–1526. doi: 10.1104/pp.119.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai A. Brassinosteroid biosynthesis. Plant Physiol Biochem. 1999;37:351–361. [Google Scholar]
- Stegeman JJ, Livingstone DR. Forms and functions of cytochrome P450. Comp Biochem Physiol Part C. 1998;121:1–3. [PubMed] [Google Scholar]
- Sugiyama M, Fukuda H. Plant Tissue Culture Manual, Supplement 5, H2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. Zinnia mesophyll cell culture system to sutudy xylogenesis; pp. 1–15. [Google Scholar]
- Szekeres M, Nemeth K, Koncz-Kalman Z, Mathur J, Kauschmann A, Altmann T, Redei GP, Nagy F, Schell J, Koncz C. Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell. 1996;85:171–182. doi: 10.1016/s0092-8674(00)81094-6. [DOI] [PubMed] [Google Scholar]
- Wada K, Marumo K, Abe H, Morishita T, Nakamura K, Uchiyama M, Mori K. A rice lamina inclination test: a micro-quantitative bioassay for brassinosteroids. Agric Biol Chem. 1984;48:719–726. [Google Scholar]
- Yamamoto R, Demura T, Fukuda H. Brassinosteroids induce entry into the final stage of tracheary element differentiation in cultured Zinnia cells. Plant Cell Physiol. 1997;38:980–983. doi: 10.1093/oxfordjournals.pcp.a029262. [DOI] [PubMed] [Google Scholar]
- Yokota T. The structure, biosynthesis and function of brassinosteroids. Trends Plant Sci. 1997;2:137–143. [Google Scholar]