Neural Systems Supporting the Control of Affective and Cognitive Conflicts (original) (raw)
. Author manuscript; available in PMC: 2019 Jun 11.
Published in final edited form as: J Cogn Neurosci. 2009 Sep;21(9):1842–1855. doi: 10.1162/jocn.2009.21129
Abstract
Although many studies have examined the neural bases of controlling cognitive responses, the neural systems for controlling conflicts between competing affective responses remain unclear. To address the neural correlates of affective conflict and their relationship to cognitive conflict, the present study collected whole-brain fMRI data during two versions of the Eriksen flanker task. For these tasks, participants indicated either the valence (affective task) or the semantic category (cognitive task) of a central target word while ignoring flanking words that mapped onto either the same (congruent) or a different (incongruent) response as the target. Overall, contrasts of incongruent > congruent trials showed that bilateral dorsal ACC, posterior medial frontal cortex, and dorsolateral pFC were active during both kinds of conflict, whereas rostral medial pFC and left ventrolateral pFC were differentially active during affective or cognitive conflict, respectively. Individual difference analyses showed that separate regions of rostral cingulate/ventromedial pFC and left ventrolateral pFC were positively correlated with the magnitude of response time interference. Taken together, the findings that controlling affective and cognitive conflicts depends upon both common and distinct systems have important implications for understanding the organization of control systems in general and their potential dysfunction in clinical disorders.
INTRODUCTION
From reading a book on a noisy train to finding the best word to express a thought, we rely everyday on the ability to attend to and respond to some stimuli while ignoring others. In the past decade, cognitive neuroscience research has taken great strides toward understanding the neural bases of this ability. Across imaging, electro-physiological, and lesion studies, dorsal regions of the cingulate and the pFCs have been shown to be essential for monitoring conflicts between and selecting among competing perceptual or semantic inputs and their associated responses (Ullsperger & von Cramon, 2004; Botvinick, Braver, Barch, Carter, & Cohen, 2001; Milham et al., 2001; Miller & Cohen, 2001; van Veen, Cohen, Botvinick, Stenger, & Carter, 2001; Carter et al., 1998). Importantly, this work has provided a foundation for translational work on clinical disorders, such as schizophrenia, that has helped clarify the mechanisms by which breakdowns in cognitive control may contribute to dysfunctional behavior (Ochsner, 2008; Kerns et al., 2005; MacDonald et al., 2005).
Comparatively less attention has been paid, however, to the neural systems important for controlling how one attends to and responds to affectively charged stimuli (Ochsner & Gross, 2005, 2008). This is surprising, given that behavioral studies have shown that deficits in this ability characterize numerous clinical disorders, ranging from chronic pain to anxiety, panic, and posttraumatic stress disorder (PTSD; Vythilingam et al., 2007; Wilson & MacLeod, 2003; Eccleston & Crombez, 1999). In recognition of these facts, a growing number of functional imaging studies have begun to investigate the neural mechanisms supporting the ability to control attention to affective inputs. The majority of these studies have asked participants to engage in a primary cognitive task or judgment while resisting interference from task-irrelevant affective information. Although their results have been somewhat mixed, they generally have been consistent with either one of two hypotheses.
The first is that rostral medial regions may play a special role in controlling attention to emotional information, in part because of their interconnections with subcortical structures involved in emotional responding (Ongur, Ferry, & Price, 2003; Ongur & Price, 2000). Consistent with this hypothesis, some studies have found activity in rostral cingulate (rCC) and medial prefrontal (mPFC) cortices when participants make simple judgments about neutral target stimuli or neutral stimulus dimensions (such as color) while ignoring affective stimuli or affective stimulus dimensions (Bishop, Duncan, Brett, & Lawrence, 2004; Compton et al., 2003; Shin et al., 2001; Bush, Luu, & Posner, 2000; Whalen et al., 1998). Interpreting the meaning of this activity is complicated, however, by the fact many of these studies failed to show behavioral evidence that the affective distracters interfered or conflicted with performance of the primary cognitive task. This raises the possibility that rCC activation reflects the extent to which affective stimulus properties are monitored or attended (hence reach awareness) rather than control over cognition–emotion response conflicts per se, an interpretation that is consistent with findings of rCC/mPFC activation when participants explicitly attend to and judge their emotional states (Ochsner et al., 2004; Phan et al., 2003; Gusnard, Akbudak, Shulman, & Raichle, 2001; Lane, Fink, Chau, & Dolan, 1997).
The second hypothesis is that screening out affective distracters may depend upon domain general systems important for controlling conflicts between various kinds of stimuli, regardless of their type. These regions include the dorsal anterior cingulate cortex (dACC) and the posterior medial frontal cortex (pMFC) thought to be involved in monitoring response conflicts, and the dorsolateral pFC (dlPFC) implicated in goal maintenance and response control (Botvinick et al., 2001; MacDonald, Cohen, Stenger, & Carter, 2000). Consistent with this hypothesis, two studies have found dACC and dlPFC activity in paradigms showing behavioral evidence that the presentation of an affective word or image may interfere with cognitive judgments of a subsequently presented stimulus (Blair et al., 2007; Luo et al., 2007).
As important as the aforementioned studies have been for understanding conflicts between cognition and emotion, it is notable that none of them directly investigated conflicts within the emotional domain, that is, the ability to control competition between different kinds of affective responses. This ability may be important whenever situations engender conflicts among or between different kinds of positive and negative emotional responses—an ability that may pose particular problems for clinical disorders, such as borderline personality disorder, anxiety, and PTSD, that are characterized by emotional instability and affect dysregulation (Vythilingam et al., 2007; Constans, McCloskey, Vasterling, Brailey, & Mathews, 2004; Lang, Davis, & Öhman, 2000; Linehan et al., 1999).
To date, only three studies have investigated this issue. All found behavioral evidence of response conflict using a variant of the Stroop task in which participants paid attention to facial expressions and ignored emotion words printed across them that were either incongruent or congruent with the expressions (Egner, Etkin, Gale, & Hirsch, 2007; Etkin, Egner, Peraza, Kandel, & Hirsch, 2006; Haas, Omura, Constable, & Canli, 2006). Imaging results were mixed, however. Although the incongruent versus congruent contrast activated dACC in all studies, only two reported an additional dlPFC activity and an inverse relationship between rCC and amygdala during conflict (Egner et al., 2007; Etkin et al., 2006). One of these articles compared their affective distracter task to a variant in which the distracter words were nonaffective gender labels that either were or were not consistent with the gender of the attended face (Egner et al., 2007). Both affective and nonaffective distracters produced dlPFC activity, but only the affective distracters recruited rCC.
Whatever the reasons for the differences in results of these studies turn out to be, the fact that the stimuli used in this type of task differ in terms of both valence and stimulus type raises the possibility that these activations reflected not only the need to control affective conflicts but also processes specifically related to the types of stimuli used. For example, face stimuli are among the most reliable activators of the amygdala, which could explain its activation here (Phan, Wager, Taylor, & Liberzon, 2002). In addition, these tasks could involve cross-domain conflicts between competing verbal/ semantic and pictorial representations. These conflicts (e.g., seeing a fearful face with the word HAPPY printed over it) may have heightened ambiguity about the meaning of the depicted facial expressions, which itself may be a source of conflict and cingulate (Botvinick et al., 2001) as well as amygdala (Whalen, 1998) activation.
Taken together, extant work suggests that the neural systems important for controlling processing conflicts created by affective information may depend upon dorsal cingulate and lateral prefrontal systems implicated in domain general cognitive control, upon rostral medial systems implicated in attention to emotion, or some combination of both. These conclusions are tentative, however, because the factors determining if and when each type of system may come into play are not yet clear. Some variability may have to do with the different processing demands of the diverse paradigms employed across studies as well as the lack of behavioral evidence of conflict in some studies. Perhaps most salient, however, is the fact that few studies have examined the neural systems for controlling conflict between competing affective responses, and that none have directly compared them to the systems important for controlling cognitive conflicts. As a consequence, important questions about the nature of affective conflict and its relationship to cognitive conflict remain.
To help clarify these issues, the goal of the present study was to provide the first direct test of whether the control of affective and cognitive (i.e., nonaffective) conflicts depends upon common or distinct neural systems. To achieve this goal, we developed two versions of the Eriksen flanker task in which participants attended to and judged a target word while ignoring distracting flanker words presented above and below the target. In the affective version of the task, differences in valence between target and flanker words created response conflict. In the cognitive version of the task, differences in semantic category membership between affectively neutral target and flanker words (kinds of metal or fruit) created response conflict. In contrast to the methods used in prior work, a key feature of this paradigm was that for each task variant, the target and the distracter stimuli differed in terms of either their affective valence or their semantic category membership but never both at once and never in terms of their representational format (see Figure 1). The aim here was to isolate processes related to within-domain conflicts that were affective or nonaffective and could not be attributed to the differences in the valence or stimulus type between targets and distracters that have been present in prior work.
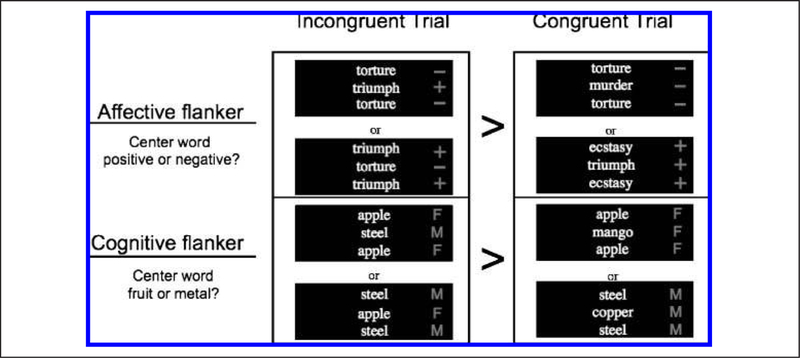
Figure 1.
Diagrammatic representation of the two main trial types used in the affective (top) and cognitive (bottom) flanker tasks. Each black square shows example stimuli that could be present on screen during a given incongruent or congruent trial. During the affective flanker task, target stimuli differ in valence from the flanking stimuli that appear above and below it. During the cognitive flanker task, target and flanker stimuli differ not in valence but in their semantic category. The main comparisons of interest are response time, and brain activation increases on incongruent as compared with congruent trials, as illustrated by the “>“ symbols separating the panels representing each trial type. The +/− and M/F symbols shown to the right of each sample screen are not shown during performance of the actual task and are included here to clearly illustrate the differences between target and flanker stimuli in each task.
Using these tasks, we sought to test two hypotheses about the relationship between affective and cognitive conflicts. First, motivated by prior work, we hypothesized that monitoring of both types of conflict may be mediated by prefrontal and cingulate regions whose activation during conflicts between many types of cognitive and perceptual responses (e.g., Bunge, Hazeltine, Scanlon, Rosen, & Gabrieli, 2002; Botvinick et al., 2001; Ullsperger & von Cramon, 2001; van Veen et al., 2001; Carter et al., 1998) suggests they may play a general role in mediating affective response conflicts as well (Haas et al., 2006; Botvinick, Cohen, & Carter, 2004; Eisenberger & Lieberman, 2004; Hazeltine, Bunge, Scanlon, & Gabrieli, 2003). Second, we hypothesized that whereas affective conflict might differentially depend upon rostral medial regions associated with awareness and selection of affective responses (Etkin et al., 2006; Bishop et al., 2004; Cato et al., 2004; Ochsner et al., 2004), the kind of semantic or meaning-based cognitive conflict studied here might differentially depend upon lateral prefrontal systems implicated in selecting goal-relevant representations from semantic memory (Badre & Wagner, 2007; Thompson-Schill, 2003).
Two kinds of analyses were used to address these hypotheses. First, we identified regions more active on incongruent than on congruent trials for each task and then compared them to determine whether the control of affective and cognitive conflicts depends upon common or distinct patterns of activity. Second, for each task, across participants we correlated conflict-related brain activity with our behavioral index of response conflict—the magnitude of response time slowing on incongruent as compared with congruent trials. This was done in recognition of the fact that group-averaged contrasts only identify regions active in all participants and consequently may fail to detect regions predictive of task performance that are active only in those individuals who perform poorly or well.
METHODS
Participants
Sixteen right-handed participants (9 women; mean age = 21.22 years) were recruited, gave informed consent in compliance with Stanford University human subjects regulation, and were paid $60 for completion of this study. All were screened for any medications or psychological/ neurological conditions that might influence the measurement of CBF.
Behavioral Paradigm
As graphically illustrated in Figure 1, participants completed two modified versions of the Eriksen flanker task that assessed affective and cognitive conflicts, respectively. On the ‘‘affective flanker’’ task, participants indicated whether a central ‘‘target’’ word was positive or negative while ignoring flanking stimuli of either the same (congruent trials) or the opposite valence (incongruent trials) that appeared above and below the central target word for the duration of the presentation. On the ‘‘cognitive flanker’’ task, participants indicated whether a central ‘‘target’’ word was a metal or a fruit while ignoring stimuli from either the same (congruent) or a different (incongruent) semantic category that flanked the central ‘‘target’’ word above and below it.
During each trial, the target and the flanking words remained on the screen for 2 sec followed by a 2-sec fixation cross, for a total trial length of 4 sec. Using a four-button response box, participants used their dominant right hand to indicate the affective or the cognitive category of the central target word by pushing the response button assigned to each category and were instructed to respond as quickly as possible without sacrificing accuracy. Response times were recorded for each trial. From top to bottom, the central target and the flanking stimuli subtended approximately 4.0 vertical degrees of visual angle in the center of the participants’ field of vision.
Because the primary goal of this study was to identify regions involved in monitoring affective and/or cognitive conflict, we did not design the flanker tasks to systematically examine the effects of prior trial interference that may influence the amount of conflict resolution required on the current trial (Etkin et al., 2006; Carter et al., 1998). Instead, we sought to avoid conflict adaptation effects attributable to repetition of trial types (Mayr, Awh, & Laurey, 2003) by intermixing congruent and incongruent trials with filler trials of no theoretical interest (where an affective or semantic target was flanked by XXXXXs). For each task, participants completed two blocks of 168 total trials. Each block was comprised of equal numbers (n = 52) of congruent, incongruent, and filler trials that were randomly inter-mixed with the 12 fixation trials that involved presentation of a fixation cross rather than a word stimulus.
Across tasks, words were matched for mean length (5.53 letters), number of syllables (1.81), and mean frequency (stimulus words averaged 0.00002% of the written or spoken words in the British National Corpus norms). A separate pilot norming study (n = 11) collected ratings of valence (1 = negative, 9 = positive) and arousal (1 = not arousing,9= highly arousing) for all stimuli. These ratings confirmed (a) that positive and negative words differed in valence (positive = 8.16, negative = 1.75; p < .05) but were equated in arousal (arousal: positive = 6.74, negative = 6.75, p = ns), and (b) that these affective words were more valenced and more arousing (all p < .05) than were the fruit and the metal words used on the cognitive task, which were selected to be of comparatively neutral valence (fruit = 6.30, metal = 4.65) and lower arousal (fruit = 3.52, metal = 2.51).
MRI Data Acquisition
Whole-brain imaging data were collected on a 3T GE Scanner (GE Signa LX Horizon Echospeed Scanner). Twenty-eight 4-mm axial slices were acquired using a T2*-sensitive gradient-echo spiral-in/out pulse sequence (30 msec TE, 2000 msec TR, two interleaves, 608 flip angle, 24 cm field of view, 64 × 64 data acquisition matrix) following high-order shimming (Glover & Law, 2001; Glover, 1999). Anatomical scans were acquired for each participant using T2-weighted flow-compensated spin-echo scans (2000 msec TR, 85 msec TE). Stimulus presentation and response time collection were controlled by an Apple computer running the experimental presentation program Psyscope. Stimuli were back projected onto a screen attached to a custom-built head coil. Participants made their responses by pressing one of two buttons on a four-button box with the index and middle fingers of their dominant right hand. Head motion was limited by a bite bar attached to the head coil and by foam padding around participants’ heads. Participants completed a short training session before being placed in the scanner to ensure that activation effects were due neither to task novelty nor to incomplete understanding of the task.
Data Analysis
Preprocessing and basic statistical analyses were conducted using SPM2 (Wellcome Department of Cognitive Neurology). Slice time correction, realignment (motion correction), and normalization were performed on the functional images, after which the anatomical images were coregistered to the mean functional image. The anatomical images were then normalized and smoothed to a standard template brain, and the normalized functional images were interpolated to 2 × 2 × 2-mm voxels and smoothed with a Gaussian filter (6 mm at full-width half-maximum).
Individual participants’ data were modeled as fixed effects using the general linear model, with blood flow responses to each trial type modeled as events producing a canonical hemodynamic response at the onset of each 4-sec trial. Contrast images for each participant summarizing differences between trial types were used to create SPM{T} maps for the group using a random-effects model. Statistical maps for group contrasts were thresholded at p < .001 uncorrected for multiple comparisons with an extent threshold of five voxels. Maxima are reported in ICBM152 coordinates as in SPM2.
To examine sources of individual variability in conflict-related activations, we used robust regression analyses (Wager, Keller, Lacey, & Jonides, 2005) to correlate individual differences in response time interference with brain activity during either affective or cognitive conflict. Robust regression is useful for examining questions about individual differences because it down-weights potential outliers that could exert undue leverage on results. The goal of these analyses was to identify regions that were correlated with performance to a greater extent during one task as compared with the other. To accomplish this goal, we used custom Matlab scripts (courtesy Tor Wager) to correlate conflict-related response time differences (i.e., the incongruent – congruent RT difference) with activity in the incongruent–congruent contrast for both the affective and the cognitive flanker tasks. We then used the method of Steiger (1980) to identify regions more significantly correlated (at p < .05) with conflict-related brain activity during one task as compared with the other. These regressions were conducted only within mPFC and IPFC regions and cingulate regions previously implicated in cognitive control (Botvinick et al., 2001; Miller & Cohen, 2001) attention to emotion and emotion regulation (Olsson & Ochsner, 2008; Ochsner & Gross, 2005; Bishop et al., 2004; Ochsner et al., 2004; Lane et al., 1998; Lane, Ahern, Schwartz, & Kaszniak, 1997), which included Brodmann’s areas 8, 9, 10, 23, 24, 25, 32, and 44–47. This was done using an mPFC mask constructed and defined by the coordinates of the mPFC regions enumerated above as given by the Talairach atlas and transformed into MNI space. Resulting activation clusters falling within the structural mask were treated as functional ROIs from which beta values from peak voxels data were extracted to illustrate relationships between brain activity and task performance.
RESULTS
Behavioral Results
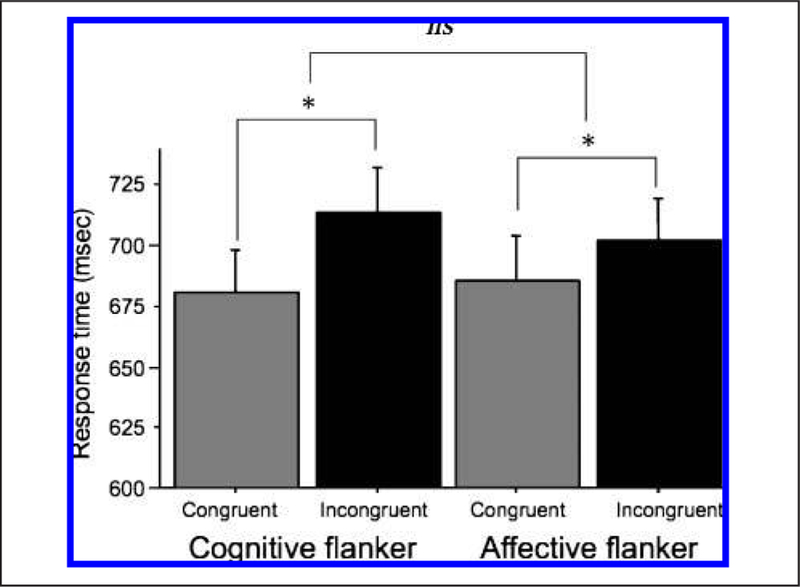
Accuracy was at or above 98% for all trial types. Hence, all analyses were conducted on response times for correct trials. A repeated measures ANOVA with type of task (Cognitive or Affective), type of trial (Incongruent or Congruent), and type of target stimulus (Negative or Fruit, or Positive or Metal) as within-participant factors was computed on response times. The only significant effect was for type of trial, F(1, 15) = 23.72, p < .001, with response times slower on incongruent (_M_ = 708.33 msec) than on congruent (_M_ = 682.90 msec) trials for both tasks. The interaction of task and type of trial was not significant ( _p_ > .1). Planned comparisons verified that each task showed significant incongruent (I) > congruent (C) response time differences (cognitive: I > C, RTs = 714.22 vs. 680.32, F(1, 15) = 29.35, p < .001; affective: I > C, RTs = 702.44 vs. 685.48, F(1, 15) = 7.34, p < .02). These data are shown in Figure 2.
Figure 2.
Graph showing response times for congruent and incongruent trials for both the affective and the cognitive flanker tasks. As can be seen here, response times are significantly and equivalently slower on incongruent trials for both tasks. Inc = incongruent; Con = congruent. *Effect of incongruence significant for each task at p < .05; ns = nonsignificant interaction effect.
Imaging Results
Because behavioral data indicated that were no significant effects of stimulus target type, imaging analyses focused on effects of trial type, task type, and their interaction.
Regions Involved in Both Cognitive and Affective Conflicts
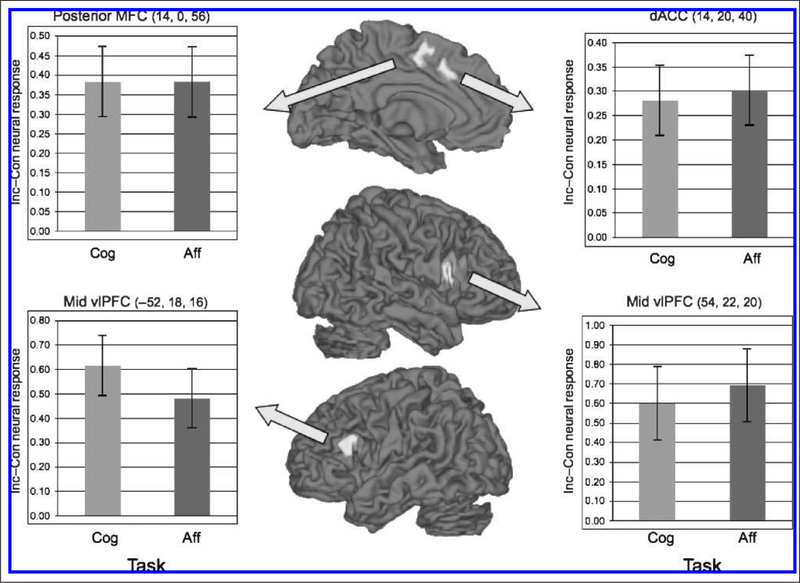
To identify regions involved in controlling conflicts between competing affective and competing cognitive responses, we first computed contrasts of incongruent > congruent (I > C) trials for the affective and cognitive flanker tasks. These contrasts showed activation in bilateral dACC, pMFC and dlPFC, left precuneus, and superior parietal cortex during cognitive conflict (Table 1) and activation of bilateral dACC and pMFC, right dlPFC, and right precuneus during affective conflict (Table 2). The method of Kampe, Frith, and Frith (2003; see also Ochsner et al., 2004) was then used to identify regions active during conflict for both the affective and the cognitive tasks. The I > C contrast for the affective flanker task computed at a threshold of p < .01 was used to generate a mask image for computing the I > C contrast for the cognitive flanker task, which was then thresholded at p < .01. Using the Fisher method of combined probability, the resulting contrast image reflects a joint probability of <.001 that a given region would be activated in both tasks (Ochsner et al., 2004; Kampe et al., 2003). This analysis indicated that dACC, pMFC, and dlPFC were activated bilaterally for the I > C contrast in both tasks (Table 3, Figure 3). To verify that these regions were equivalently active during conflict in both tasks, we extracted parameter estimates for conflict-related (i.e., I–C) activity from each commonly active dACC, MFC, and dlPFC cluster and used planned t tests to compare activity for each task in each of these ROIs. As can be seen from the illustrative graphs in Figure 3, no significant effects were observed: Conflict-related activity in each region was statistically equivalent for each task (all p values >.50). Finally, it should be noted that we used correlation analyses (described in the next section) to look for regions whose magnitude of conflict-related activity correlated with the magnitude of response conflict in both tasks.
Table 1.
Group Activations for Incongruent > Congruent for Semantic Flanker Task
| Region of Activation | Brodmann | Coordinates | Z Score | Volume (mm3) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Middle frontal gyrus | L9 | −36 | 22 | 28 | 3.48 | 56 |
| Middle frontal gyrus | R6 | 32 | −6 | 44 | 4.33 | 1168 |
| Middle frontal gyrus | R6 | 10 | −10 | 58 | 4.05 | 472 |
| Middle frontal gyrus | R9 | 14 | 40 | 30 | 4.04 | 88 |
| Inferior frontal gyrus | L45/9 | −56 | 20 | 24 | 3.91 | 720 |
| Insula/inferior frontal gyrus | R13 | 36 | 22 | 16 | 3.53 | 40 |
| Precentral/inferior frontal gyri | L6 | −38 | −6 | 42 | 4.36 | 1072 |
| Precentral gyrus | R6 | 34 | 2 | 28 | 3.93 | 560 |
| Precentral gyrus | R6 | 58 | −10 | 44 | 3.70 | 144 |
| Medial frontal gyrus | L32 | −16 | 12 | 50 | 3.67 | 40 |
| Cingulate gyrus | L24/32 | −16 | 14 | 34 | 5.18 | 392 |
| Cingulate gyrus | L32 | −8 | 22 | 46 | 4.09 | 400 |
| Cingulate gyrus | L24 | −6 | −6 | 40 | 3.47 | 120 |
| Superior parietal lobule | L7 | −40 | −66 | 50 | 3.62 | 136 |
| Parahippocampal gyrus | L | −38 | −26 | −16 | 3.75 | 56 |
| Precuneus | L39 | −32 | −66 | 36 | 3.39 | 80 |
Table 2.
Group Activations for Incongruent > Congruent Trials for Affective Flanker Task
| Region of Activation | Brodmann | Coordinates | Z Score | Volume (mm3) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Group Contrast | ||||||
| Superior frontal gyrus | L6 | 14 | 2 | 58 | 3.58 | 184 |
| Middle frontal gyrus | L8 | −8 | 38 | 44 | 3.33 | 144 |
| Middle frontal gyrus | L6/9 | −10 | 30 | 38 | 3.18 | 40 |
| Middle frontal/cingulate gyri | L8/32 | −6 | 16 | 52 | 3.93 | 42 |
| Inferior frontal gyrus/insula | L47/13 | −34 | 16 | −8 | 3.56 | 48 |
| Inferior frontal gyrus | R45 | 58 | 22 | 20 | 3.18 | 40 |
| Cingulate gyrus | L32/24 | −4 | 10 | 22 | 3.52 | 40 |
| Cingulate gyrus | R32/24 | 16 | 16 | 32 | 3.76 | 72 |
| Cingulate gyrus | R32 | 16 | 20 | 44 | 3.51 | 160 |
| Cingulate gyrus | R23 | 12 | −16 | 32 | 3.47 | 48 |
| Superior temporal gyrus | R38/22 | 50 | 14 | −8 | 3.65 | 72 |
| Precuneus | R7 | 14 | −64 | 38 | 3.49 | 120 |
| Caudate | R | 6 | 2 | 22 | 3.38 | 48 |
Table 3.
Group Activations Common to Affective (Incongruent > Congruent) and Cognitive (Incongruent > Congruent) Conflicts
| Region of Activation | Brodmann | Coordinates | Z Score | Volume (mm3) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Anterior cingulate cortex | R24 | 26 | −20 | 34 | 3.81 | 464 |
| Anterior cingulate cortex | R32 | 14 | 18 | 46 | 3.37 | 384 |
| Posterior medial frontal | R6 | 14 | 2 | 58 | 3.58 | 640 |
| Posterior medial frontal | L8 | −6 | 16 | 52 | 3.93 | 448 |
| Posterior medial frontal | R6 | 32 | 6 | 28 | 3.20 | 96 |
| Inferior frontal gyrus | R45 | 56 | 22 | 20 | 3.25 | 416 |
| Inferior frontal gyrus | L44 | −52 | 16 | 16 | 3.31 | 576 |
Figure 3.
Regions showing greater conflict-related activity in the incongruent–congruent contrast for both the affective and the cognitive flanker tasks. Graphs at the left and the right show the magnitude of this activity for selected functional ROIs shown in the center panels. As can be seen in these graphs, these regions are equivalently activated (all pairwise p values = ns) during both affective and cognitive conflicts. dACC = dorsal anterior cingulate cortex; MFC = medial frontal cortex; vlPFC = ventrolateral pFC. MNI coordinates for each region are shown in parentheses.
Regions Differentially Involved in Cognitive or Affective Conflict
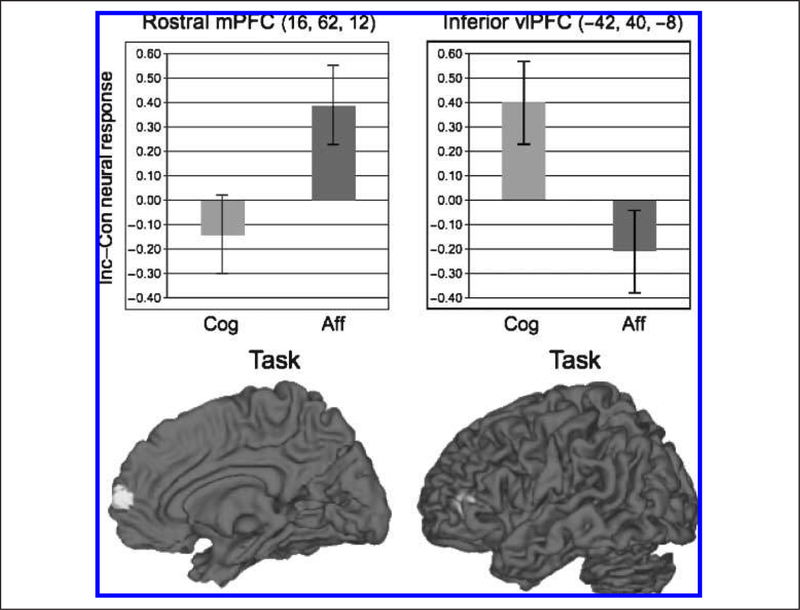
To identify regions differentially involved in affective as compared with cognitive conflict, we computed an interaction contrast by subtracting the I > C contrast for the cognitive flanker task from the I > C contrast for the affective flanker task [Affective (I > C) – Cognitive (I > C)]. Affective conflict did not selectively activate any regions at p < .001. However, given a prior interest in the role of medial frontal cortex in affective conflict, we relaxed the threshold to _p_ < .005 in this region only and found that affective conflict did selectively recruit a region of right rostral mPFC. By contrast, cognitive conflict [Cognitive (I > C) – Affective (I > C)] did not differentially activate any medial frontal regions. Instead, it selectively recruited left ventrolateral pFC (vlPFC) and left parietal cortex (Table 4, Figure 4). To verify this selectivity in frontal regions, we extracted parameter estimates for conflict-related (i.e., I–C) activity from the functionally defined medial and lateral frontal ROIs and entered them into an ANOVA with type of task (Affective and Cognitive) and ROI (mPFC and vlPFC) as factors. We found a significant interaction, F(1, 15) = 49.41, p < .001, and no other significant effects, as illustrated in Figure 4.
Table 4.
Group Activations Specific to Affective or Cognitive Conflict
| Region of Activation | Brodmann | Coordinates | Z Score | Volume (mm3) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Affective Conflict | ||||||
| Rostral mPFC* | BA 9/10 | 14 | 62 | 12 | 2.80 | 24 |
| Cognitive Conflict | ||||||
| vlPFC | L47 | −42 | 40 | 8 | 3.67 | 136 |
| Lateral parietal | −42 | −72 | 46 | 3.41 | 96 |
Figure 4.
Regions selectively activated for affective (left panel) or cognitive (right panel) conflicts. Comparison of left and right panels makes clear that affective and cognitive conflicts differentially depend upon medial and left lateral prefrontal systems, respectively. mPFC = medial pFC; vlPFC = ventrolateral pFC. MNI coordinates for each region are shown in parentheses.
As described in the Methods section, we then performed a second analysis that contrasted the strength of correlation between brain activity and performance for each flanker task. This analysis was intended to complement the group-averaged contrasts presented above and involved two steps used previously (Zaki, Ochsner, Hanelin, Wager, & Mackey, 2007) that were described in the Methods section. First, we used robust regression analyses to perform a search within a prefrontal mask for regions significantly correlated with performance in one task or the other at p < .01. For this analysis, response time interference (RT for incongruent trials – RT for congruent trials) was correlated with measures of activation in the I > C contrast for both the affective and the cognitive flanker tasks. A liberal threshold was chosen for this step so as to minimize chances of false-negative findings at the next step. Second, we used the method of Steiger (1980) for comparing dependent correlations to determine which of these regions was significantly more correlated with performance in one task as compared with the other. Thus, all regions identified in this analysis must show a correlation with performance at p < .01 for one task, and that correlation must also be greater than that shown in the other task at p < .05 (Steiger, 1980). This analysis revealed that activation of different regions predicted increases in RT interference during performance of each task: Right rostral/subgenual cingulate cortex correlated with interference for the affective flanker task, whereas a region of left ventral pFC correlated with interference for the cognitive flanker task (Table 4, Figure 5).
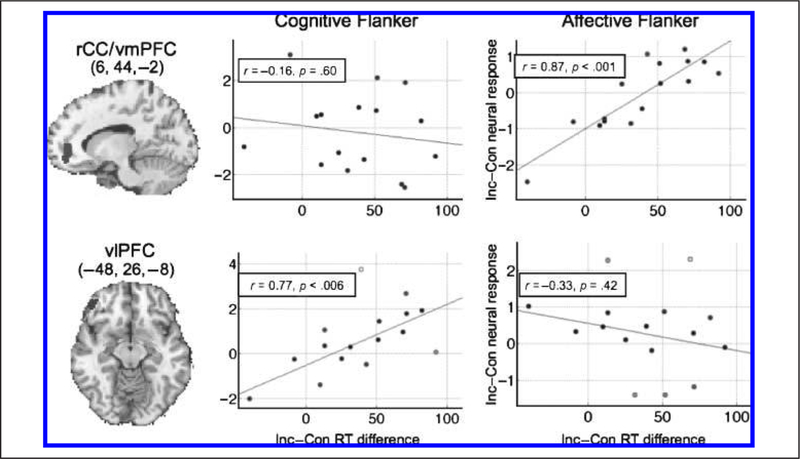
Figure 5.
Regions whose conflict-related activity differentially correlated with the magnitude of response time interference on incongruent as compared with congruent trials during the affective (right panels) or cognitive (left panels) flanker tasks. Each point represents data for a single subject, with gray/partially filled circles representing relative outliers down-weighted by the robust regression algorithm ( Wager et al., 2005) used to compute the observed relationships. Top and bottom panels show medial and ventrolateral regions whose activity differentially correlated positively with the magnitude of behavioral response conflict during either each task. These data dovetail with an extend those shown in Figure 4 by showing additional regions of rostral medial and left inferior vlPFC selectively associated with affective as compared with cognitive conflict, respectively. rCC/vmPFC = rostral cingulate/ventromedial pFC; vlPFC = ventrolateral pFC. Inc = incongruent; Con = congruent.
DISCUSSION
The goal of the present article was to identify common and distinct neural systems underlying the control of affective and cognitive conflicts. Toward that end, we devised affective and cognitive variants of the Eriksen flanker task that produced statistically equivalent levels of conflict-related response time slowing. Contrast analyses of functional imaging data revealed two key findings. First, conflict on both tasks was associated with activity in bilateral regions of dACC, pMFC, and dlPFC. Second, affective and cognitive conflicts differentially recruited rostral mPFC and left vlPFC, respectively. Correlational analyses dovetailed with these findings by demonstrating that the magnitude of behavioral response conflict predicted greater conflict-related activity in rCC and mPFC for the affective task and greater conflict-related activity in left vlPFC for the cognitive task. Taken together, these data provide the first evidence for common and distinct neural systems for controlling conflicts between competing affective or competing cognitive responses.
The finding that both types of conflict activated over-lapping regions of pMFC and dlPFC is consistent with the view that these regions comprise a domain-general system for higher-level behavioral control (Botvinick et al., 2001; Miller & Cohen, 2001). That being said, how best to characterize the functions of these regions remains a topic of debate. Dorsal ACC and related pMFC regions like those activated here have been described as important for conflict monitoring, expectancy violation, error detection, and response selection (van Veen & Carter, 2006; Ridderinkhof, Ullsperger, Crone, & Nieuwenhuis, 2004; Ullsperger & von Cramon, 2004; Botvinick et al., 2001; Milham et al., 2001). Although the present study was not designed to discriminate among these alternatives, we favor the general view that posterior regions on the medial wall of the frontal lobe are important for signaling the need for a change in control processes, which may be motivated by the detection of processing conflicts, expectancy violations, and stimuli that may be salient to current goals (Davis et al., 2005; Eisenberger & Lieberman, 2004; Botvinick et al., 2001; Ochsner et al., 2001).
On this view, lateral prefrontal systems—like those activated here—are important for the implementation of control processes that accomplish tasks goals (Botvinick et al., 2001). These processes could include the maintenance of the goals themselves, which is essential for performance of both the affective and the cognitive flanker tasks (MacDonald et al., 2000). Given the verbal nature of the tasks employed here, they might also include processes involved in the controlled retrieval of information from semantic memory, as suggested by work associating these processes with regions of mid-vlPFC near those activated here (Badre & Wagner, 2007). This account would suggest that the site of common prefrontal activation reflects increased retrieval demands on incongruent as compared with congruent trials, as participants focus on retrieving semantic information about target but not flanker stimuli in both tasks. In future work, it will be important to disentangle these alternative possibilities. Whichever interpretation turns out to be correct, it is noteworthy that our data are consistent with prior work employing flanker tasks, only some of which employed verbal stimuli, that have shown conflict-related activity in similar regions of dACC/pMFC and/or lateral pFC (Hazeltine et al., 2003; Ullsperger & von Cramon, 2001; van Veen et al., 2001; Hazeltine, Poldrack, & Gabrieli, 2000).
The finding that distinct neural systems were activated during affective and cognitive conflicts joins prior work showing that there may be material-specific effects associated with the stimulus features responsible for processing conflict (Hazeltine et al., 2003). At the broadest remove, the present findings fit with emerging views of the relative processing specializations of medial and lateral prefrontal systems. On these views, medial regions may serve to integrate information about internal mental and visceral states—which are essential components of affective responses—whereas lateral regions may play a greater role in the maintenance and the manipulation of nonaffective information (Lieberman, 2007).
In this context it is important to note that there are two ways in which the conflict induced by the affective flanker task may differ from that in the cognitive flanker task. On one hand, affective conflict involved stimuli that differed in their valence to a greater extent and were of higher arousal than the stimuli that elicited cognitive conflict. Given this, it makes sense that the rostral mPFC region selectively active during affective conflict is quite similar to regions whose activity may covary with the judged arousal or valence of affective responses (Gilbert et al., 2006; Ochsner et al., 2004; Phan et al., 2004; Lane, Fink, et al., 1997). On the other hand, it could be argued that the affective and the cognitive flanker tasks both asked participants to semantically categorize target stimuli, albeit in different ways. Here it is interesting to note that the rostral mPFC identified here also has been activated by tasks that require participants to generate category exemplars for affectively charged as compared with neutral semantic categories (Cato et al., 2004; Crosson et al., 2002). These views are not mutually exclusive, however, and it is possible that the ability to semantically categorize affective information is what underlies the association of mPFC with judgments of experienced valence and arousal (Ochsner et al., 2004).
Either way, the present findings fit with prior research implicating rostral medial regions in cognitive–affective conflicts (Bishop et al., 2004; Whalen et al., 1998) and affective–affective conflicts (Egner et al., 2007; Etkin et al., 2006) but goes beyond these studies in two ways. First, it confirms that activity in mPFC is related to behavioral conflict per se, which has not been shown in some prior studies (Bishop et al., 2004; Whalen et al., 1998). Second, it shows that this conflict-related activity can be based on the affective properties of stimuli and is not dependent on cross-talk between different representational channels related to facial, verbal, visual–spatial, or affective as compared with nonaffective processing (in prior work, targets and distracters differed in valence as well as type—faces vs. words, faces vs. houses, number vs. identity of words).
Whereas the affective flanker task drew more heavily on mPFC systems important for selecting among competing affective representations, the cognitive flanker task drew more heavily on left lateral pFC systems that may be associated in general with selecting stimuli based on their semantic category membership (Badre & Wagner, 2007). As noted above, common activity across flanker tasks was observed in bilateral vlPFC, and we also observed additional activity in left pFC in a region anterior and ventral to the common focus. It has been suggested that recruitment of left inferior vlPFC regions may be important for selecting among semantic representation after they have been retrieved by mid-vlPFC regions (Badre & Wagner, 2007). As applied to the present data, this view suggests that selecting based on semantic category membership in the cognitive flanker task taxes this postretrieval process more heavily than does the affective flanker task, which in turn depends on a similar process mediated in mPFC. Although consistent with the present data, future work will be necessary to more directly test this interpretation.
Another key feature of the present results was the finding that correlational analyses both supported and extended the contrast analyses described above. In general, correlations between measures of task performance and neural activity may identify brain regions more or less active in good or poor performers that are not revealed in group-averaged contrasts. Here we found that conflict-related response time increases and brain activity were positively correlated in different regions for each flanker task: Behavioral evidence of conflict predicted greater activity in rCC and ventromedial pFC for the affective flanker and greater activity in left vlPFC for the cognitive flanker task. The fact that these correlations were positive suggests that as flankers caused more interference across subjects, additional activity in these regions was required to focus on and select the appropriate target response.
The fact that these regions were close to, but distinct from, the medial and the lateral regions identified in contrasts as selective for affective of cognitive conflict could be interpreted in two ways. One interpretation is that the systems identified in the contrasts reveal systems that are consistently necessary for selecting among competing affective and cognitive responses and that the correlational analyses indicate that greater recruitment of essentially similar processes is required in individuals who experience greater response conflict. This interpretation may fit the region showing a performance– activity correlation for the cognitive flanker task, which may fall within the portion of left vlPFC most sensitive to selecting among competing semantic representations (cf. Badre & Wagner, 2007). An alternative interpretation is that correlational analyses reveal not that more of the same kind of processing is needed, but that additional kinds of processes come into play as selection becomes particularly difficult. This interpretation may fit the region showing a performance–activity correlation for the affective flanker task, which fell within a portion of ventral mPFC previously associated with self-referential processing and the so-called ‘‘default state’’ present during uninstructed baseline conditions (Gillihan & Farah, 2005; Gusnard et al., 2001). Although speculative, this may suggest that affective conflict is difficult to the extent that it has personal meaning or elicits personal associations, which makes sense given that computing the affective significance of a stimulus by definition is about its relevance to ones personal goals, wants, and needs (Lazarus, 1991).
One caveat for the present findings is we did not use task manipulations, such as conflict adaptation (Egner et al., 2007; Etkin et al., 2006; Carter et al., 2000), or various means of parametrically manipulating response selection demands (Badre, Poldrack, Pare-Blagoev, Insler, & Wagner, 2005; Durston et al., 2003) that might allow the dissociation of different types of control processes associated with goal maintenance, conflict monitoring, or response selection per se. Here, the emphasis was on taking a first step toward, showing that affective and cognitive conflicts rely upon both common as well as distinct mechanisms. A next step for future work will be to precisely specify which particular affective and cognitive control processes are associated with any given region. In particular, an important goal will be to understand how different portions of the medial prefrontal wall have distinct functional specializations that may be best described as cognitive, affective, or domain general (Olsson & Ochsner, 2008; Lieberman, 2007; Gilbert et al., 2006; Ochsner et al., 2004). Another question concerns the lack of conflict-related amygdala activity reported in some prior studies examining affective conflict (Egner et al., 2007; Etkin et al., 2006). Although the precise reasons for this lack are not known, as noted in the introduction, it is possible that amygdala activity in those studies is attributable to the use of emotionally expressive face stimuli per se and/or the potential for ambiguity in meaning of the faces caused by placing an incongruent expression label over them, both of which have been shown to activate the amygdala (Phan et al., 2002; Whalen, 1998). Future work could serve to determine whether the amygdala’s role in affective conflict is specific to conflicts created by specific types of stimuli, stimulus ambiguity, or some other process.
Finally, it is valuable to consider the implications of the present work for understanding how the mechanisms of cognitive and affective control may break down in psychopathology. During the past decade, models of the neural bases of cognitive control have been applied to understanding the way in which dorsal cingulate and lateral prefrontal function may be abnormal in psychiatric disorders such as schizophrenia and depression (Holmes et al., 2005; MacDonald & Carter, 2003; Cohen, Braver, & O’Reilly, 1996). The present work suggests that dysfunction in these regions may produce problems not only in selecting among competing cognitive responses but also when selecting among competing affective responses. To date, however, this possibility has not been tested because few paradigms have been available to examine deficits in affective response selection per se. The present method may provide a means for determining whether this is the case: To the extent that a given population shows deficits in resolving interference on both the affective and the cognitive flanker tasks, one might infer dysfunction in the dorsal cingulate and the prefrontal systems commonly recruited by both tasks. By contrast, to the extent that a population shows deficits on just one task, it would support the inference that dysfunction lies within either the medial frontal or the left ventrolateral regions differentially associated with controlling affective as opposed to cognitive conflict. A task that could differentially predict deficits in cognitive as compared with affective control may be particularly valuable given that current nonaffective measures of working memory and response selection may predict cognitive deficits shown by a given patient population, but not their deficits in emotional and social functioning (Ochsner, 2005, 2008; Carter & Barch, 2007).
The present work may also suggest new ways of understanding prior demonstrations of abnormal rCC and mPFC activity in clinical populations. For example, anxiety and PTSD have been associated with reduced rCC and/or ventral mPFC activity during the perception of affective stimuli (Phan, Britton, Taylor, Fig, & Liberzon, 2006; Shin et al., 2005) and during cognitive–affective conflict (Bishop et al., 2004; Bremner et al., 2004; Shin et al., 2001; Whalen et al., 1998), and patients with PTSD have shown gray matter reductions in ventral cingulate and mPFC as well (Shin et al., 2001). It has been suggested that these functional and structural abnormalities may be associated with deficits in the ability to extinguish affective responses, which has been shown in normals to be associated with the structural integrity and the functional activation of ventral mPFC regions (Milad, Rauch, Pitman, & Quirk, 2006; Quirk & Beer, 2006; Rauch, Shin, & Phelps, 2006; Milad et al., 2005; Phelps, Delgado, Nearing, & LeDoux, 2004). The present results suggest a slightly different interpretation of these data. Namely, that the ability to select task-appropriate affective responses, which is associated with this region, may manifest itself in a variety of ways in both normal and abnormal populations. On this view, extinction is a specific example of needing to select a context-appropriate affective response, and failures to recruit ventral mPFC to support selection of appropriate responses may contribute to a variety of affective disorders, including PTSD. Future work examining cognitive and affective conflicts in clinical populations will be essential for addressing these possibilities.
Acknowledgments
The authors thank grants MH076137 (K. N. O.) and MH58147 ( J. D. E. G. and J. J. G.) from the National Institute of Health for support of this research. We also thank Tor Wager for providing us with custom robust regression and differential correlation scripts.
REFERENCES
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, & Wagner AD (2005). Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron, 47, 907–918. [DOI] [PubMed] [Google Scholar]
- Badre D, & Wagner AD (2007). Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia, 45, 2883–2901. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, & Lawrence AD (2004). Prefrontal cortical function and anxiety: Controlling attention to threat-related stimuli. Nature Neuroscience, 7, 184–188. [DOI] [PubMed] [Google Scholar]
- Blair KS, Smith BW, Mitchell DG, Morton J, Vythilingam M, Pessoa L, et al. (2007). Modulation of emotion by cognition and cognition by emotion. Neuroimage, 35, 430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, & Cohen JD (2001). Conflict monitoring and cognitive control. Psychological Review, 108, 624–652. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, & Carter CS (2004). Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences, 8, 539–546. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Vythilingam M, Afzal N, Schmahl C, Elzinga B, et al. (2004). Neural correlates of the classic color and emotional stroop in women with abuse-related posttraumatic stress disorder. Biological Psychiatry, 55, 612–620. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, & Gabrieli JD (2002). Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage, 17, 1562–1571. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, & Posner MI (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4, 215–222. [DOI] [PubMed] [Google Scholar]
- Carter CS, & Barch DM (2007). Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: The CNTRICS initiative. Schizophrenia Bulletin, 33, 1131–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, & Cohen JD (1998). Anterior cingulate cortex, error detection, and the online monitoring of performance. Science, 280, 747–749. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, et al. (2000). Parsing executive processes: Strategic vs. evaluative functions of the anterior cingulate cortex. Proceedings of the National Academy of Sciences, U.S.A, 97, 1944–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato MA, Crosson B, Gokcay D, Soltysik D, Wierenga C, Gopinath K, et al. (2004). Processing words with emotional connotation: An fMRI study of time course and laterality in rostral frontal and retrosplenial cortices. Journal of Cognitive Neuroscience, 16, 167–177. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Braver TS, & O’Reilly RC (1996). A computational approach to prefrontal cortex, cognitive control and schizophrenia: Recent developments and current challenges. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences, 351, 1515–1527. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Banich MT, Mohanty A, Milham MP, Herrington J, Miller GA, et al. (2003). Paying attention to emotion: An fMRI investigation of cognitive and emotional Stroop tasks. Cognitive, Affective & Behavioral Neuroscience, 3, 81–96. [DOI] [PubMed] [Google Scholar]
- Constans JI, McCloskey MS, Vasterling JJ, Brailey K, & Mathews A (2004). Suppression of attentional bias in PTSD. Journal of Abnormal Psychology, 113, 315–323. [DOI] [PubMed] [Google Scholar]
- Crosson B, Cato MA, Sadek JR, Gokcay D, Bauer RM, Fischler IS, et al. (2002). Semantic monitoring of words with emotional connotation during fMRI: Contribution of anterior left frontal cortex. Journal of the International Neuropsychological Society, 8, 607–622. [DOI] [PubMed] [Google Scholar]
- Davis KD, Taylor KS, Hutchison WD, Dostrovsky JO, McAndrews MP, Richter EO, et al. (2005). Human anterior cingulate cortex neurons encode cognitive and emotional demands. Journal of Neuroscience, 25, 8402–8406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Thomas KM, Worden MS, Tottenham N, Martinez A, et al. (2003). Parametric manipulation of conflict and response competition using rapid mixed-trial event-related fMRI. Neuroimage, 20, 2135–2141. [DOI] [PubMed] [Google Scholar]
- Eccleston C, & Crombez G (1999). Pain demands attention: A cognitive–affective model of the interruptive function of pain. Psychological Bulletin, 125, 356–366. [DOI] [PubMed] [Google Scholar]
- Egner T, Etkin A, Gale S, & Hirsch J (2007). Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cerebral Cortex, 18, 1475–1484. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, & Lieberman MD (2004). Why rejection hurts: A common neural alarm system for physical and social pain. Trends in Cognitive Sciences, 8, 294–300. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, & Hirsch J (2006). Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron, 51, 871–882. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, et al. (2006). Functional specialization within rostral prefrontal cortex (area 10): A meta-analysis. Journal of Cognitive Neuroscience, 18, 932–948. [DOI] [PubMed] [Google Scholar]
- Gillihan SJ, & Farah MJ (2005). Is self special? A critical review of evidence from experimental psychology and cognitive neuroscience. Psychological Bulletin, 131, 76–97. [DOI] [PubMed] [Google Scholar]
- Glover G (1999). 3D Z-shim method for reduction of susceptibility effects in BOLD fMRI. Magnetic Resonance in Medicine, 42, 290–299. [DOI] [PubMed] [Google Scholar]
- Glover GH, & Law CS (2001). Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance in Medicine, 46, 515–522. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, & Raichle ME (2001). Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences, U.S.A, 98, 4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BW, Omura K, Constable RT, & Canli T (2006). Interference produced by emotional conflict associated with anterior cingulate activation. Cognitive, Affective & Behavioral Neuroscience, 6, 152–156. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Bunge SA, Scanlon MD, & Gabrieli JD (2003). Material-dependent and material-independent selection processes in the frontal and parietal lobes: An event-related fMRI investigation of response competition. Neuropsychologia, 41, 1208–1217. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Poldrack R, & Gabrieli JD (2000). Neural activation during response competition. Journal of Cognitive Neuroscience, 12(Suppl. 2), 118–129. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, MacDonald A III, Carter CS, Barch DM, Andrew Stenger V, & Cohen JD (2005). Prefrontal functioning during context processing in schizophrenia and major depression: An event-related fMRI study. Schizophrenia Research, 76, 199–206. [DOI] [PubMed] [Google Scholar]
- Kampe KK, Frith CD, & Frith U (2003). ‘‘Hey John’’: Signals conveying communicative intention toward the self activate brain regions associated with ‘‘mentalizing,’’ regardless of modality. Journal of Neuroscience, 23, 5258–5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, Macdonald AW III, Johnson MK, Stenger VA, Aizenstein H, et al. (2005). Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. American Journal of Psychiatry, 162, 1833–1839. [DOI] [PubMed] [Google Scholar]
- Lane RD, Ahern GL, Schwartz GE, & Kaszniak AW (1997). Is alexithymia the emotional equivalent of blindsight? Biological Psychiatry, 42, 834–844. [DOI] [PubMed] [Google Scholar]
- Lane RD, Fink GR, Chau PM, & Dolan RJ (1997). Neural activation during selective attention to subjective emotional responses. NeuroReport, 8, 3969–3972. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, & Schwartz GE (1998). Neural correlates of levels of emotional awareness. Evidence of an interaction between emotion and attention in the anterior cingulate cortex. Journal of Cognitive Neuroscience, 10, 525–535. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Davis M, & Öhman A (2000). Fear and anxiety: Animal models and human cognitive psychophysiology. Journal of Affective Disorders, 61, 137–159. [DOI] [PubMed] [Google Scholar]
- Lazarus RS (1991). Cognition and motivation in emotion. American Psychologist, 46, 352–367. [DOI] [PubMed] [Google Scholar]
- Lieberman MD (2007). Social cognitive neuroscience: A review of core processes. Annual Review of Psychology, 58, 259–289. [DOI] [PubMed] [Google Scholar]
- Linehan MM, Schmidt H III, Dimeff LA, Craft JC, Kanter J, & Comtois KA (1999). Dialectical behavior therapy for patients with borderline personality disorder and drug-dependence. American Journal on Addictions, 8, 279–292. [DOI] [PubMed] [Google Scholar]
- Luo Q, Mitchell D, Jones M, Mondillo K, Vythilingam M, & Blair RJ (2007). Common regions of dorsal anterior cingulate and prefrontal-parietal cortices provide attentional control of distracters varying in emotionality and visibility. Neuroimage, 38, 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW III, & Carter CS (2003). Event-related fMRI study of context processing in dorsolateral prefrontal cortex of patients with schizophrenia. Journal of Abnormal Psychology, 112, 689–697. [DOI] [PubMed] [Google Scholar]
- MacDonald AW III, Carter CS, Kerns JG, Ursu S, Barch DM, Holmes AJ, et al. (2005). Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. American Journal of Psychiatry, 162, 475–484. [DOI] [PubMed] [Google Scholar]
- MacDonald AW III, Cohen JD, Stenger VA, & Carter CS (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science, 288, 1835–1838. [DOI] [PubMed] [Google Scholar]
- Mayr U, Awh E, & Laurey P (2003). Conflict adaptation effects in the absence of executive control. Nature Neuroscience, 6, 450–452. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, & Rauch SL (2005). Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proceedings of the National Academy of Sciences, U.S.A, 102, 10706–10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, & Quirk GJ (2006). Fear extinction in rats: Implications for human brain imaging and anxiety disorders. Biological Psychology, 73, 61–71. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, et al. (2001). The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Brain Research, Cognitive Brain Research, 12, 467–473. [DOI] [PubMed] [Google Scholar]
- Miller EK, & Cohen JD (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. [DOI] [PubMed] [Google Scholar]
- Ochsner KN (2005). Characterizing the functional architecture of affect regulation: Emerging answers and outstanding questions. In Cacioppo JT (Ed.), Social neuroscience: People thinking about people (pp. 245–268). Cambridge: MIT Press. [Google Scholar]
- Ochsner KN (2008). The social-emotional processing stream: Five core constructs and their translational potential for schizophrenia and beyond. Biological Psychiatry, 64, 48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, & Gross JJ (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9, 242–249. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, & Gross JJ (2008). Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Current Directions in Psychological Science, 17, 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow D, Hanelin J, Ramachandran T, & Mackey S (2004). Reflecting upon feelings: An fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience, 16, 1746–1772. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Kosslyn SM, Cosgrove GR, Cassem EH, Price BH, Nierenberg AA, et al. (2001). Deficits in visual cognition and attention following bilateral anterior cingulotomy. Neuropsychologia, 39, 219–230. [DOI] [PubMed] [Google Scholar]
- Olsson A, & Ochsner KN (2008). The role of social cognition in emotion. Trends in Cognitive Sciences, 12, 65–71. [DOI] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, & Price JL (2003). Architectonic subdivision of the human orbital and medial prefrontal cortex. Journal of Comparative Neurology, 460, 425–449. [DOI] [PubMed] [Google Scholar]
- Ongur D, & Price JL (2000). The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex, 10, 206–219. [DOI] [PubMed] [Google Scholar]
- Phan KL, Britton JC, Taylor SF, Fig LM, & Liberzon I (2006). Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Archives of General Psychiatry, 63, 184–192. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Decker LR, Noll DC, Nichols TE, et al. (2003). Activation of the medial prefrontal cortex and extended amygdala by individual ratings of emotional arousal: A fMRI study. Biological Psychiatry, 53, 211–215. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, & Liberzon I (2004). Neural correlates of individual ratings of emotional salience: A trial-related fMRI study. Neuroimage, 21, 768–780. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, & Liberzon I (2002). Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage, 16, 331–348. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, & LeDoux JE (2004). Extinction learning in humans: Role of the amygdala and vmPFC. Neuron, 43, 897–905. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, & Beer JS (2006). Prefrontal involvement in the regulation of emotion: Convergence of rat and human studies. Current Opinion in Neurobiology, 16, 723–727. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, & Phelps EA (2006). Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research-past, present, and future. Biological Psychiatry, 60, 376–382. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, & Nieuwenhuis S (2004). The role of the medial frontal cortex in cognitive control. Science, 306, 443–447. [DOI] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, et al. (2001). An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biological Psychiatry, 50, 932–942. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. (2005). A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry, 62, 273–281. [DOI] [PubMed] [Google Scholar]
- Steiger JH (1980). Tests for comparing elements of a correlation matrix. Psychological Bulletin, 85, 211–229. [Google Scholar]
- Thompson-Schill SL (2003). Neuroimaging studies of semantic memory: Inferring ‘‘how’’ from ‘‘where’’. Neuropsychologia, 41, 280–292. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, & von Cramon DY (2001). Subprocesses of performance monitoring: A dissociation of error processing and response competition revealed by event-related fMRI and ERPs. Neuroimage, 14, 1387–1401. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, & von Cramon DY (2004). Neuroimaging of performance monitoring: Error detection and beyond. Cortex, 40, 593–604. [DOI] [PubMed] [Google Scholar]
- van Veen V, & Carter CS (2006). Error detection, correction, and prevention in the brain: A brief review of data and theories. Clinical EEG & Neuroscience, 37, 330–335. [DOI] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, & Carter CS (2001). Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage, 14, 1302–1308. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Blair KS, McCaffrey D, Scaramozza M, Jones M, Nakic M, et al. (2007). Biased emotional attention in post-traumatic stress disorder: A help as well as a hindrance? Psychological Medicine, 37, 1445–1455. [DOI] [PubMed] [Google Scholar]
- Wager TD, Keller MC, Lacey SC, & Jonides J (2005). Increased sensitivity in neuroimaging analyses using robust regression. Neuroimage, 26, 99–113. [DOI] [PubMed] [Google Scholar]
- Whalen PJ (1998). Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science, 7, 177–188. [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, et al. (1998). The emotional counting Stroop paradigm: A functional magnetic resonance imaging probe of the anterior cingulate affective division. Biological Psychiatry, 44, 1219–1228. [DOI] [PubMed] [Google Scholar]
- Wilson E, & MacLeod C (2003). Contrasting two accounts of anxiety-linked attentional bias: Selective attention to varying levels of stimulus threat intensity. Journal of Abnormal Psychology, 112, 212–218. [DOI] [PubMed] [Google Scholar]
- Zaki J, Ochsner KN, Hanelin J, Wager TD, & Mackey S (2007). Different circuits for different pain: Patterns of functional connectivity reveal distinct networks for processing pain in self and others. Social Neuroscience, 2, 276–291. [DOI] [PMC free article] [PubMed] [Google Scholar]