Neuronally Restricted RNA Splicing Regulates the Expression of a Novel GABAA Receptor Subunit Conferring Atypical Functional Properties (original) (raw)
Abstract
We report the isolation and characterization of a cDNA encoding a novel member of the GABA receptor gene family, ε. This polypeptide is 506 amino acids in length and exhibits its greatest amino acid sequence identity with the GABAA receptor γ3 subunit (47%), although this degree of homology is not sufficient for it to be classified as a fourth γ subunit. The ε subunit coassembles with GABAA receptor α and β subunits in Xenopus laevis oocytes and transfected mammalian cells to form functional GABA-gated channels. α1β1ε GABAAreceptors, like α1β1γ2s receptors, are modulated by pentobarbital and the steroid 5α-pregnan-3α-ol-20-one but, unlike α1β1γ2s receptors, are insensitive to flunitrazepam. Additionally, α1β1ε receptors exhibit rapid desensitization kinetics, as compared with α1β1 or α1β1γ2s. Northern analysis demonstrates widespread expression of a large ε subunit transcript in a variety of non-neuronal tissues and expression of a smaller transcript in brain and spinal cord. Sequence analysis demonstrated that the large transcript contained an unspliced intron, whereas the small transcript represents the mature mRNA, suggesting regulation of expression of the ε subunit via neuronally restricted RNA splicing. In situ hybridization and immunocytochemistry reveal a pattern of expression in the brain restricted primarily to the hypothalamus, suggesting a role in neuroendocrine regulation, and also to subfields of the hippocampus, suggesting a role in the modulation of long term potentiation and memory.
Keywords: GABAA receptor, ε subunit, function, RNA splicing, hypothalamus: hippocampus
GABA is the major inhibitory neurotransmitter of the vertebrate CNS. It modulates inhibitory tone throughout the CNS by activating two classes of receptors, GABAA and GABAB. The latter are G-protein-coupled receptors, and their molecular structure recently has been elucidated (Kaupmann et al., 1997). GABAA receptors are ligand-gated ion channels and are part of the same gene super family as nicotinic receptors (Schofield et al., 1989). The binding of the agonist GABA to the receptor complex results in the rapid opening of the intrinsic anion channel through which anions, primarily chloride, flow into the cell. This leads to hyperpolarization of the cell membrane and an increase in the inhibitory tone at that synapse. This receptor is the target for a number of classes of drugs, including the benzodiazepines, barbiturates, general anesthetics, neurosteroids, and alcohols (for review, see Macdonald and Angelotti, 1993; Sieghart, 1995; Whiting et al., 1995).
Molecular biological approaches have revealed that the GABAA receptor exists as a gene family of polypeptides. These are divided into subcategories on the basis of their relative sequence identities: α1–α6, β1–β4, γ1–γ4, δ, and ρ1–ρ2 (see Darlison and Albrecht, 1995; Sieghart 1995; Whiting et al., 1995). β4 and γ4 have not been identified in mammals. The unique pharmacology of receptors formed by ρ1 or ρ2 has led to suggestions that they should be classified as GABACreceptors (Johnston, 1996). Each GABAA receptor subunit has a unique pattern of expression in the mammalian brain, although the γ2 subunit is the most ubiquitous (Laurie et al., 1992; Wisden et al., 1992). Subunits are thought to coassemble as pentamers to form a family of receptor subtypes that are expressed differentially throughout the mammalian brain. Experiments in a number of laboratories that use subunit-specific antibodies to characterize native GABAA receptors indicate that the minimum subunit composition of native receptors is thought to be αβγ or αβδ (see Darlison and Albrecht, 1995; McKernan and Whiting, 1996). Studies using recombinant receptors have demonstrated that the subunit composition determines the pharmacology of the receptor and the affinity for GABA (see Ebert et al., 1994; Whiting et al., 1995) and perhaps the targeting of the receptor to different subcellular domains (Connolly et al., 1996). However, relatively little is known about the biophysical properties of the various GABAA receptor subtypes.
Here we report the identification of a novel GABA receptor subunit, ε, which coassembles with α and β GABAA receptor subunits to form a functional receptor with unique properties. The expression of the mature form of ε mRNA transcript seems to be regulated via a neuronally restricted RNA splicing mechanism. The limited expression of this subunit in hypothalamic and hippocampal regions suggests a specialized role in inhibitory neurotransmission.
MATERIALS AND METHODS
Isolation and sequencing of a cDNA encoding the GABAA receptor ε subunit. The Merck.EST (Expressed Sequence Tag) database was searched with the human GABAAreceptor β1 subunit-deduced (GenBank accession number X14767) primary amino acid sequence, using the BLAST search tool (Altschul et al., 1990), and a number of EST sequences were identified. Two of these,R07883 and R49718, were investigated in more detail. PCR was performed to determine whether the two ESTs encoded the same gene product. For PCR, a sense primer was generated from the R07883 sequence (5′ ctgttggagtttggtgtgctcaac 3′), and an antisense primer was generated from the R49718 sequence (5′ accagctggtacctacaagttaag 3′). PCR was performed under standard conditions (Whiting et al., 1990), using human subthalamic nucleus cDNA (Clontech, Cambridge, UK) as a template.
cDNA sequences 5′ of the R07883 sequence were obtained by 5′ rapid amplification of cDNA ends (RACE), using the human brain Marathon cDNA cloning kit (Clontech) according to the manufacturer’s protocols. The nested antisense primers that were used were derived from the R07883 sequence (AS1, 5′ catcgtggtcacggaagaagggac 3′; AS2, 5′ gccaaaccgcctgctcacattgaa 3′). PCR products were subcloned into pMOS vector (Amersham, Braunschweig, Germany), using standard techniques, and sequenced by using an Applied Biosystems (Foster City, CA) 373 DNA sequencer and dye terminator chemistry. One of the PCR products was found to extend far enough to contain a sequence encoding a putative initiating methionine and a 5′ untranslated region (UT).
A full-length cDNA was generated by PCR with primers derived from sequences in the 5′ UT of the RACE PCR product and the 3′ UT sequences in R49718 (5′ caggtggtgcggccgctctccgcggaaatgttgt 3′ and 5′ ccacagggcggccgctggtacctacaagttaag 3′, both incorporating a_Not_I site for subcloning). PCR products (1550 bp) were subcloned into pCDNAI/Amp and sequenced completely on both strands by primer walking. Sequence analysis was performed with Inherit (Applied Biosystems) and Genetics Computer Group (University of Wisconsin) computer programs.
Northern blot analysis. Northern blots containing poly(A+) RNA from various human tissues were obtained from Clontech. The R07883 intron probe was generated as a 283 bp _Eco_RI–_Bsp_HI fragment by digestion of R07883 cDNA, and the R07883 exon probe was generated as a 365 bp_Bsp_HI–_Pac_I fragment. 32P-labeled probes were generated by random priming (Prime-It kit, Stratagene, La Jolla, CA), and blots were probed under high stringency in 5× SSPE (1× SSPE is 0.18 m NaCl, 10 mm Na phosphate, pH 7.4, and 1 mm EDTA) containing 50% formamide at 42°C. Filters were washed in 0.3× SSPE at 65°C and exposed to Kodak XAR film for 2 d at −70°C, using Cronex QIII intensifying screens.
_Characterization of placental ε subunit transcript._Placental cDNA (Clontech) was used as a template for PCR reactions. To generate PCR products 5′ and 3′ of the intron, we used the following oligonucleotide primers: for the 5′ PCR, the sense primer was 5′ gcggccgctctccgcggaaatgttgt 3′ (bp 1–24) and the antisense primer was 5′ gggttgtgaattatttcagtt 3′ (bp 776–795); for the 3′ PCR, the sense primer was 5′ aactatgtcccttcttccgtg 3′ (bp 867–887) and the antisense primer was 5′ gcggccgctggtacctacaagttaag 3′ (bp 1532–1552). PCR was performed under standard conditions (Whiting et al., 1990), and products were resolved by electrophoresis through 1% agarose gels stained with ethidium bromide.
To generate PCR products containing the intron sequence, we used the following oligonucleotide primers: sense, 5′ agaactcctggaagctcttccagt 3′ (bp 727–750) and antisense, 5′ ccaaaccgcctgctcacattgaa 3′ (bp 828–850). PCR was performed with Expand Long Template PCR (Boehringer Mannheim, Mannheim, Germany). PCR was performed by using buffer 1, supplied by manufacturers, with a final concentration of 350 μm deoxynucleotide triphosphates, ∼10 μmof each primer, and 0.75 U of enzyme. PCR conditions were as follows: 2 min initial denaturation, followed by 35 cycles of 94°C for 20 sec, 60°C for 1 min, and 68°C for 3 min. PCR products were resolved by electrophoresis through 0.8% agarose gels stained with ethidium bromide. PCR products were subcloned into pMOS vector (Amersham), using standard methodologies and partial sequencing performed by primer walking, as above.
Cloning from human genomic DNA of ε subunit sequences containing the unspliced intron. PCR was performed with rTth DNA polymerase (XL PCR, Perkin-Elmer, Oak Brook, IL) and 500 ng of human genomic DNA (Clontech) as a template. Buffer was as supplied by the manufacturer; the final concentration of other constituents was 200 μm deoxynucleotide triphosphates, 1.1 mmmagnesium acetate, ∼10 μm of each primer, and 2 U of enzyme. Conditions for PCR were as follows: 1 min denaturation at 94°C, followed by 35 cycles each of 30 sec at 94°C and 3 min at 68°C. PCR products were resolved by electrophoresis through 0.8% agarose gels stained with ethidium bromide. For subcloning, PCR products were blunt-ended, using T4 DNA polymerase, purified by electrophoresis, and ligated into _Eco_RV cut pBluescript (Stratagene). The PCR product was sequenced completely by primer walking, as above.
Localization of the ε subunit in monkey brain by in situ_hybridization._ Antisense oligonucleotide probes to the human ε subunit sequence were generated on an Applied Biosystems Model 394 DNA synthesizer and purified by preparative polyacrylamide electrophoresis: probe 1, 5′ ggtgacaatcaggcacacaaaagcttcctgatgttggcggg caca 3′ (antisense to bp 1206–1250); probe 2, 5′ cctgctgccaggtactgccctcacaatcggg gaccatgcagaagt 3′ (antisense to bp 1384–1428). Each oligonucleotide was 3′ end-labeled with [35S]deoxyadenosine 5′-(thiotriphosphate) in a 30:1 molar ratio of 35S-isotope/oligonucleotide, using terminal deoxynucleotidyl transferase for 15 min at 37°C in the reaction buffer supplied (Boehringer Mannheim). Radiolabeled oligonucleotide was separated from unincorporated nucleotides, using Sephadex G50 spin columns. The specific activities of the labeled probes in several labeling reactions varied from 1.2–2.3 × 109 cpm/mg. Monkey brains were removed and fresh-frozen in 1 cm blocks. Sections of 12 μm were taken and fixed for in situ hybridization. Hybridization of the sections was performed according to the method of Sirinathsinghji and Dunnett (1993). Briefly, sections were removed from alcohol and air-dried; 5 × 105 cpm of each 35S-labeled probe in 100 μl of hybridization buffer was applied to each slide. Labeled “antisense” probe also was used in the presence of an excess (100×) concentration of unlabeled antisense probe to define nonspecific hybridization. Parafilm coverslips were placed over the sections that were incubated overnight (∼16 hr) at 37°C. After hybridization the sections were washed for 1 hr at 57°C in 1× SSC and then rinsed briefly in 0.1× SSC, dehydrated in a series of alcohols, air-dried, and exposed to Amersham Hyperfilm βmax x-ray film. Autoradiographs were analyzed by a microcomputer imaging device (MCID) computerized image analysis system (Image Research, Ontario, Canada).
Localization of the ε subunit in monkey brain and in transfected HEK 293 cells by immunohistochemistry. A rabbit antiserum was generated to the synthetic peptide I-V-T-T-E-G-S-D-G-E-E-R-P-S-C-S-A-Q-Q that was coupled to keyhole limpet hemocyanin (Affiniti Research Products, Exeter, UK). This sequence is residues 409–427 of the ε subunit, located in the putative cytoplasmic loop between TM3 and TM4. For immunohistochemistry, antibodies were affinity-purified by using the synthetic peptide coupled to Sepharose.
A squirrel monkey was deeply anesthetized with ketamine and sodium pentobarbitone and transcardially perfused with saline, followed by 10% formalin in 0.1 m PBS. The brain was removed, post-fixed in 10% formalin in PBS for 24 hr, and sliced into coronal blocks, which then were embedded in paraffin wax. Coronal sections (10 μm) were cut on a rotary microtome, deparaffinized, rinsed in PBS, and treated with 0.3% H2O2 for 30 min (to block endogenous peroxidase activity). Background staining was blocked by incubating the sections in 3% normal horse serum for 1 hr. Sections were incubated overnight at 4°C with the anti-ε subunit rabbit polyclonal antibody (1:500 dilution). Immunostaining was visualized by using the ABC elite system (Vector Laboratories, Peterborough, UK), followed by development in diaminobenzidine (DAB). Finally, sections were counterstained in Gill’s hematoxylin, dehydrated, and mounted for microscopical examination. For immunofluorescent labeling of transiently transfected HEK 293 cells, the procedure was, in general, similar to that used for brain sections. Cells were transfected with cDNAs as described below and 48 hr later were processed for immunocytochemistry. The cells were fixed in 4% paraformaldehyde, rinsed in PBS, and incubated with the anti-ε subunit antibody at a dilution of 1:1000. Goat anti-rabbit fluorescein isothiocyanate (FITC; 1:100 dilution) was used as the detection system.
Expression in Xenopus oocytes. Adult female_Xenopus laevis_ were anesthetized by immersion in a 0.4% solution of 3-aminobenzoic acid ethylester for 30–45 min (or until unresponsive). Ovary tissue was removed via a small abdominal incision, and Stage V and Stage VI oocytes were isolated with fine forceps. After mild collagenase treatment to remove follicle cells (Type IA, 0.5 mg/ml, for 8 min), the oocyte nuclei were injected directly with 10–20 nl of injection buffer (88 mm NaCl, 1 mm KCl, and 15 mm HEPES, at pH 7, filtered through nitrocellulose) or sterile water containing different combinations of human GABAA subunit cDNAs (20 ng/ml) engineered into the expression vector pCDM8 or pcDNAI/Amp. After incubation for 24–72 hr, oocytes were placed in a 50 μl bath and perfused at 4–6 ml/min with modified Barth’s medium (MBS) consisting of (in mm): 88 NaCl, 1 KCl, 10 HEPES, 0.82 MgSO4, 0.33 Ca(NO3)2, 0.91 CaCl2, and 2.4 NaHCO3, at pH 7.5. Cells were impaled with two 1–3 MΩ electrodes containing 2m KCl and voltage-clamped between −40 and −70 mV.
In all experiments, drugs were applied in the perfusate until the peak of the response was observed. Noncumulative concentration–response curves to agonists were constructed, allowing at least 3 min between each agonist application to prevent desensitization. Curves were fit by using a nonlinear square-fitting program to the equation _f(x) = B_MAX/[1+(EC50/x)n_], where x is the drug concentration, EC50 is the concentration of drug eliciting a half-maximal response, and_n is the Hill coefficient. The effects of GABAAreceptor modulators were examined on control GABA EC20responses with a preapplication time of 30 sec.
Whole-cell patch clamp of HEK 293 cells transiently transfected with human GABAA receptors. Experiments were performed on HEK 293 cells transiently transfected with human cDNA combinations α1β1, α1β1ε, and α1β1γ2s (6 μg of cDNA total per coverslip), using calcium phosphate precipitation (Chen and Okayama, 1988) as previously described (Hadingham et al., 1993). Glass coverslips containing the cells in a monolayer culture were transferred to a chamber on the stage of a Nikon Diaphot inverted microscope. Cells were perfused continuously with a solution containing (in mm): 124 NaCl, 2 KCl, 2 CaCl2, 1 MgCl2, 1.25 KH2PO4, 25 NaHCO3, and 11 d-glucose, pH 7.2, and observed with phase-contrast optics. Patch pipettes were pulled with an approximate tip diameter of 2 μm and a resistance of 4 MΩ with borosilicate glass and were filled with (in mm): 130 CsCl, 10 HEPES, 10 EGTA, and 3 Mg+-ATP, pH-adjusted to 7.3 with CsOH. Cells were patch-clamped in whole-cell mode with a List LM-EPC 7 patch-clamp amplifier (List Biologic, Campbell, CA). Drug solutions were applied by a double-barreled pipette assembly, which was controlled by a stepping motor attached to a Prior manipulator, enabling rapid equilibration around the cell. Increasing GABA concentrations were applied for 10 sec pulses with a 30 sec interval between applications.
RESULTS
Identification of a novel member of the GABA receptor family
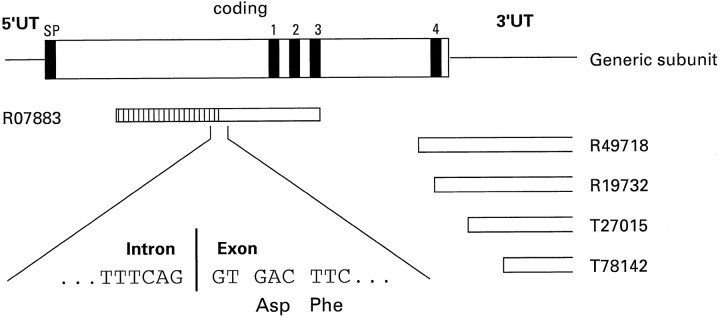
So that new GABA receptor subunits genes could be identified, the Expressed Sequence Tag (EST) database was searched, using the primary amino acid sequence of the human GABAA receptor β1 subunit (Schofield et al., 1989) as a query. Two ESTs, R07883 (from a human fetal liver/spleen cDNA library) and R49718 (from a human infant brain cDNA library), were identified that exhibited significant deduced amino acid sequence identity with the β1 subunit but that were not any known GABA receptor subunit. Further searching of the EST database using R49718 as a query revealed three more ESTs that overlapped withR49718 (Fig. 1). PCR experiments that used primers derived from R07883 and R49718 demonstrated that they were derived from the same gene (data not shown). EST R07883 is 650 bp in length, and further analysis suggested that the 5′ 270 bp of this cDNA was either a cloning artifact or, more likely, an intron sequence. Indeed, the putative intron–exon boundary (Fig. 1) is at exactly the same position as the 5′ splice site of exon 7 of other GABAA receptor genes such as β1 (Kirkness et al., 1991) and δ (Sommer et al., 1990), and subsequent cloning of genomic DNA confirmed this 270 bp to be an intron sequence (see below). 5′ RACE from human brain cDNA was used to obtain cDNA sequences of the 5′ end of the R07883 gene product. Then a full-length cDNA was obtained by PCR with oligonucleotide primers derived from the 5′ untranslated region sequence of 5′ RACE-generated cDNAs and the 3′ untranslated region nucleotide sequence from R49718. Two cDNAs were sequenced in full on both strands, as well as numerous RACE cDNA products.
Fig. 1.
Schematic representation of a GABAAreceptor subunit indicating the relative positions of the ESTs.SP, Signal peptide. 1–4, Putative transmembrane regions 1–4. The hatched area of the_box_ representing EST R07883 is an intron sequence. The boxes open at one end representing ESTs_R49718_, R19732, T27015, and T78142 indicate that the 3′ end of these sequences has not been determined.
Primary structure of the ε subunit
The deduced primary amino acid sequence of the R07883 gene product, which we have termed ε, is shown in Figure2A, aligned with other members of the GABAA receptor gene family. The ε subunit has an open reading frame of 506 amino acids containing the motifs expected of a member of the ligand-gated ion channel super family exemplified by the nicotinic receptor: an 18 residue signal peptide [using the prediction of von Heijne (1986)], two cysteine residues separated by 13 amino acids, and four hydrophobic regions, which are putative transmembrane-spanning domains (TM1–TM4). The putative extracellular domain contains three potential N-glycosylation sites (Asn residues 134, 252, and 272). The most conserved regions are the putative transmembrane domains, whereas the putative cytoplasmic loop between TM3 and TM4 is completely divergent. The ε subunit has its highest amino acid sequence identity with the γ subunits (42–47% identity with γ1–γ3) (Pritchett et al., 1989; Ymer et al., 1990; Hadingham et al., 1995). It exhibits 49% sequence identity with chicken γ4 (Harvey et al., 1993); the human homolog of this avian subunit has yet to be identified. However, because the amino acid sequence conservation of other GABAA receptor subunits between species is >90%, e.g., between chicken γ2 (Glencorse et al., 1990) and human γ2 (Pritchett et al., 1989) it is 92%, the ε subunit is not the human homolog of γ4. The analysis shown in Figure 2_B_indicates that ε represents a novel subfamily of GABA receptor subunits.
Fig. 2.
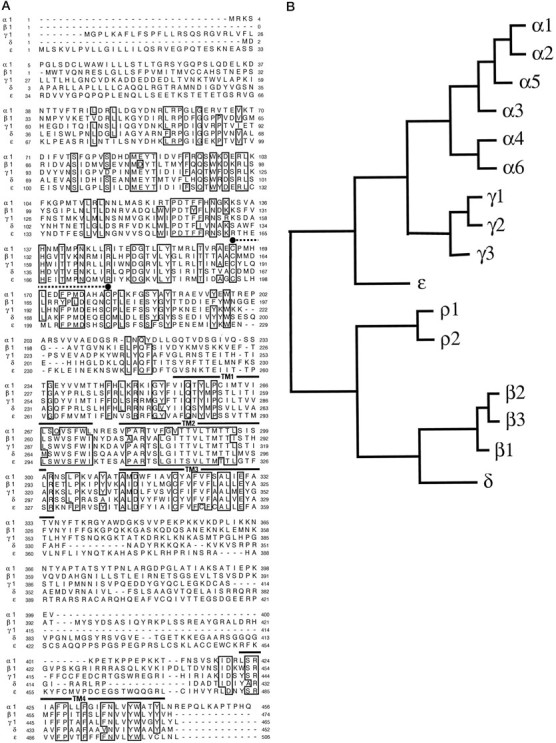
Comparison of the deduced amino acid sequence of the ε subunit with sequences of other GABAA receptor subunits. A, Alignment of the deduced amino acid sequences of the human GABAA receptor α1 (Schofield et al., 1989), β1 (Schofield et al., 1989), γ1 (Ymer et al., 1990), δ (P. Whiting, unpublished data), and ε subunits. Positions in which amino acid residues are conserved in four or more sequences are_boxed_. Numbering of amino acids is given by assigning the initiating methionine as 1. Putative transmembrane regions TM1–TM4 are indicated by a_solid line_, and the two cysteine residues conserved in the ligand-gated ion channel family are indicated by filled circles joined by a dotted line.B, Dendrogram of the deduced amino acid sequences of the GABAA receptor family, including the ε subunit. The analysis was performed by using PileUp (Genetics Computer Group, University of Wisconsin). The distance along the horizontal is proportional to the differences between the sequences.
Characterization of ε subunit transcripts
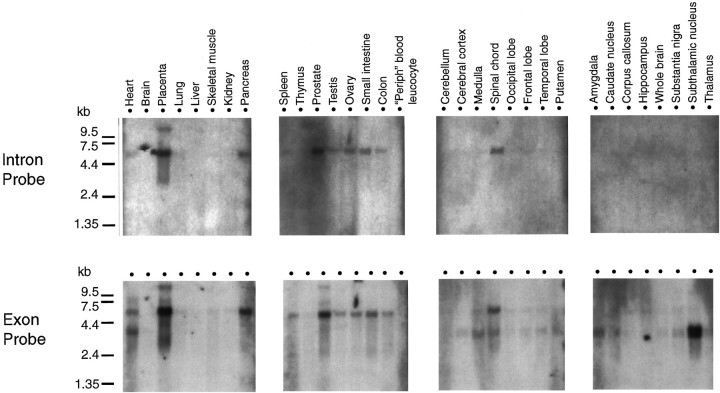
Northern blot analysis (Fig. 3) that used a probe derived from the coding region (i.e., exon sequences) of the ε subunit revealed expression of a large transcript (∼5.5 kb) in numerous peripheral tissues (placenta, pancreas, spleen, prostate, testis, ovary, and intestine) as well as a smaller transcript (∼3.5 kb) in various brain regions, although it was not detectable in whole-brain mRNA. Spinal cord and also the heart appear to express both transcripts. When Northern blots were probed with the intron sequence identified in the R07883 EST (Fig. 1), only the large transcript was identified, suggesting that the large mRNA species represents incompletely spliced transcripts.
Fig. 3.
Expression of ε subunit transcripts in human tissues by Northern blot analysis. Each lane contains 2 μg of poly(A+) RNA purified from various human tissues and resolved through 1.2% agarose in formaldehyde (Clontech). Blots were hybridized with 32P-labeled intron or exon cDNA probes, as detailed in Materials and Methods. RNA size marker bands are indicated on the left of the figure.
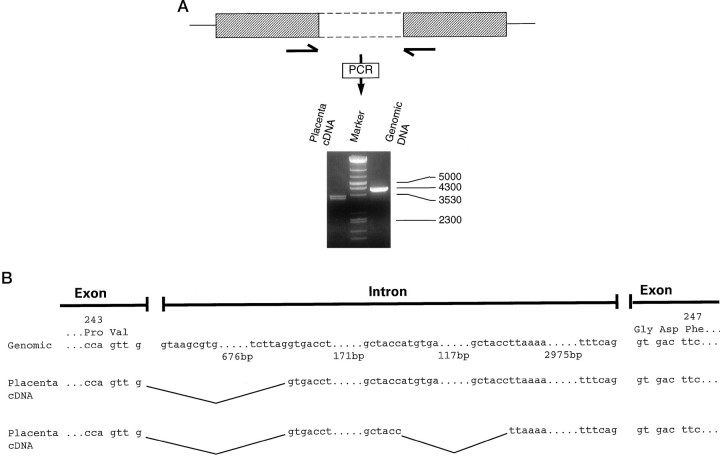
The widespread expression of the incompletely spliced ε RNA species and the more restricted expression of the smaller RNA species suggested that the expression of the mature ε transcript is controlled, at least in part, via a tissue-specific RNA splicing mechanism. To investigate this further, we characterized the genomic sequence around the putative incompletely spliced intron sequence, as well as the large transcript expressed in placenta. PCR from genomic DNA with primers derived from the exon sequence on either side of the putative intron yielded a product of ∼4000 bp (Fig.4A). Complete sequencing of the PCR product (Fig. 4B) indicated that the intron is 3991 bp and that the 3′ splice site is identical to the sequence identified in the EST R07883. PCR from placenta cDNA with the same primer pair yielded two products of ∼3300–3500 bp (Fig. 4A). In both of these cDNAs the intronic sequence starts at a presumed cryptic splice site 692 bp from the 5′ end of the genomic intron sequence. The smaller PCR product also contains an additional deletion of 129 bp, as compared with the genomic intron sequence (Fig.4B). These data suggest that in peripheral tissues such as placenta the 5′ splice donor of this intron is used, whereas the 3′ splice acceptor is not. As may be expected, the intron sequence retained in the placental transcripts contained numerous stop codons, indicating that it would not lead to translation of a GABAAreceptor subunit open reading frame. Additional PCR experiments were performed with primer pairs flanking the intron–exon splice site and in the 5′ or 3′ untranslated region to determine the structure of the 5′ and 3′ end of the placental ε subunit RNA. A 5′ PCR product of ∼800 bp was generated, which is the predicted size (798 bp) if this region were spliced appropriately so as to contain only exon sequences (data not shown). Similarly, a 3′ PCR product of ∼700 bp was generated, in agreement with the 686 bp size predicted if this region were spliced appropriately so as to contain only exon sequences (data not shown). These data suggest that in placental RNA only the intron between exons tentatively assigned as 6 and 7 has not been spliced out.
Fig. 4.
Cloning of ε subunit genomic sequences containing the intron between exons 6 and 7 and characterization of placental ε subunit transcripts. A, Generation of products containing unspliced intron by PCR, using placenta cDNA or genomic DNA as a template. PCR primers (indicated by_arrows_) were derived from exon sequences flanking the intron. DNA size markers are indicated to the right of the figure. The single line represents 5′ and 3′ UT, the_hatched box_ represents the coding region, and the_open box_ with dashed lines represents the intron. B, Nucleotide sequence of the intron between exons 6 and 7 cloned from genomic DNA and from two cDNAs cloned from placental mRNA. The deduced amino acid sequence of the surrounding exon is shown also, with the amino acid number indicated_above_ it.
The regional expression of ε subunit transcripts in the brain
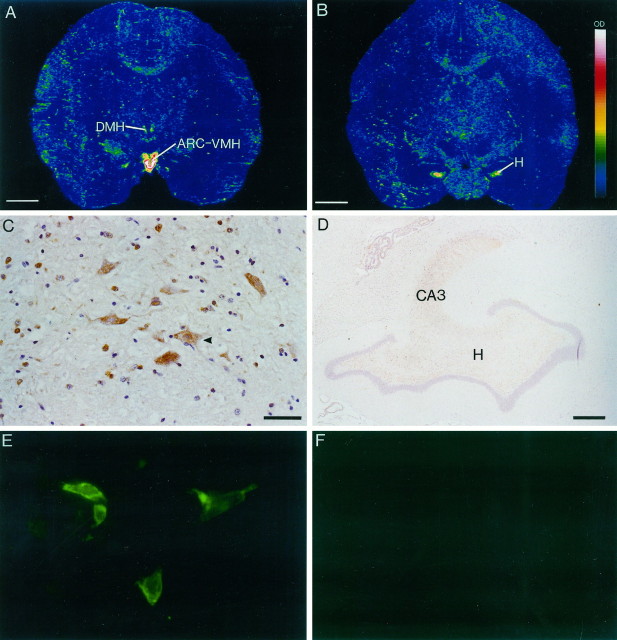
In the absence of human brain material, the expression of ε transcripts was determined in the squirrel monkey brain by in situ hybridization with 35S-labeled oligonucleotide probes. PCR and sequencing experiments demonstrated that nucleotide sequences chosen for synthesis of oligonucleotide probes were conserved between human and monkey (data not shown). The ε subunit transcript was highly localized, primarily in the hypothalamus, with high densities of expression principally within the arcuate–ventromedial area (ARC–VMH); much weaker expression of the transcript was detected in the dorsomedial hypothalamus (Fig. 5A). Dense mRNA expression also was found in the hilus of the dentate gyrus of the hippocampus (Fig. 5B). It is of interest to note that no expression was detected in other hippocampal fields nor in the amygdala or subthalamic nucleus, two regions that, according to the Northern blot analysis, contained transcripts. This discrepancy may reflect the difficulties in accurately dissecting human brain regions for RNA purification. Immunohistochemical labeling of squirrel monkey brain sections was performed with a polyclonal antiserum generated to a synthetic peptide derived from amino acids 409–427 of the ε subunit sequence. Immunohistochemical staining with the ε subunit antibody showed a distribution of immunoreactivity corresponding with the mRNA localization (Fig. 5C,D). Figure 5C shows labeling of magnocellular cells and numerous smaller cells within the dorsomedial hypothalamus, and Figure 5D shows labeling of cells within the hilus and CA3 region of the hippocampus. Other subfields of the hippocampus showed minimal specific labeling. The immunohistochemical staining patterns obtained with the ε subunit antibody were specific because preabsorption experiments on adjacent brain sections with the peptide gave no labeling (data not shown). Similarly, immunofluorescent labeling with the ε subunit antibody of HEK 293 cells transiently transfected with α1, β1, and ε subunits showed positively labeled cells (Fig. 5E), the pattern of staining indicating membrane association of the subunit. Nontransfected cells gave no labeling (Fig. 5F).
Fig. 5.
In situ hybridization autoradiograms and immunohistochemical staining of squirrel monkey brain sections and transfected HEK 293 cells showing expression of the expression of ε subunit. A, Dense mRNA expression in the arcuate–ventromedial area (ARC–VMH) and weaker expression in the dorsomedial hypothalamus (DMH). The signal intensity is indicated in the scale bar in B, with white representing the strongest signal. B, Dense mRNA expression in the hilus (H) of the dentate gyrus of the hippocampus. C, Immunoreactive cells in the DMH.Arrowhead indicates a labeled magnocellular neuron.Brown is the immunostaining reaction product, and_purple_ is the hematoxylin counterstain.D, Immunoreactivity in the hilus of the dentate gyrus and in the CA3 region of the hippocampus.E, ε subunit immunofluorescent labeling of HEK 293 cells transiently transfected with α1, β1, and ε subunits.F, Lack of labeling in untransfected HEK 293 cells. Scale bars: A, B, 0.5 cm;C, 10 μm; D, 100 μm.E, Magnification, 100×.
Functional expression of ε subunit containing GABAA receptor
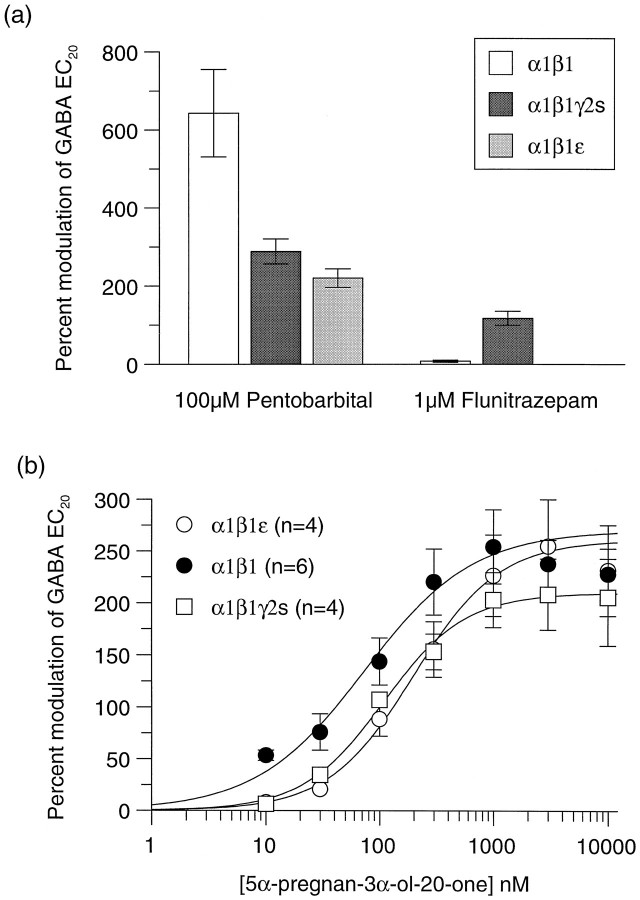
To investigate whether the ε subunit could form functional GABA gated channels, we expressed it in _Xenopus_oocytes, either alone or in combination with other members of the GABAA receptor gene family. When expressed alone, ε failed to show any response to applied GABA, indicating that this subunit is unable to form functional homomeric channels. Similarly, no functional channels were formed when ε was coexpressed in oocytes with an α1 subunit or a β1 subunit. Coexpression with both an α1 and a β1 subunit resulted in the formation of functional GABA-gated channels. However, because α1 and β1 are able to coassemble to form functional receptors, further pharmacological analysis was required. Maximum current sizes for all three subunit combinations were similar (Table 1). GABA concentration–effect curves were generated to α1β1, α1β1γ2s, and α1β1ε (Fig.6a). The EC50 for GABA was similar for both α1β1 and α1β1ε subunit combinations [5.9 ± 1.1 μm (n = 7) and 4.0 ± 1.2 μm (n = 8), respectively]. The Hill slope, however, was significantly lower for receptors containing an ε subunit (0.85 ± 0.08 compared with 1.32 ± 0.06 for α1β1; Table 1). This was found for receptors expressed in oocytes or in HEK 293 cells and is in contrast to that observed for native GABAA receptors (Sakmann et al., 1983) and other recombinant GABAA receptor subtypes so far examined (Horne et al., 1993; Verdoorn, 1994; Hadingham et al., 1996; Saxena and Macdonald, 1996), for which a Hill slope of >1 is observed. This suggested that ε was coassembling with α1 and β1. Definitive evidence for coassembly of the ε subunit came from examining the inhibition of various subunit combinations by zinc. Zn2+ ions are 175-fold more potent at αβ than at αβε combinations (Fig. 6b, Table 1), demonstrating that ε was coassembling with α1 and β1 to form a GABAA receptor with low affinity for zinc. Additionally, the zinc inhibition curve could be fit to a single site, suggesting that a homogeneous population of αβε receptors (rather than a mixed population of αβ and αβε) was being formed.
Table 1.
Comparison of the properties of α1β1, α1β1ε, and α1β1γ2s GABAA receptors expressed in_Xenopus_oocytes
| α1β1 | α1β1ε | α1β1γ2s | |
|---|---|---|---|
| GABA EC50 | 5.9 ± 1.1 μm (n = 7) | 4.0 ± 1.2 μm (n = 8) | 19.9 ± 4.6 μm (n = 7) |
| Hill slope | 1.32 ± 0.06 | 0.85 ± 0.08 | 1.36 ± 0.18 |
| Mean maximum current (nA) | 1307 ± 224 (n = 13) | 973 ± 78 (n = 15) | 1519 ± 134 (n = 10) |
| Zinc IC50 | 0.24 ± 0.12 μm (n = 4) | 41.9 ± 3.6 μm (n = 4) | 712 ± 296 μm (n = 6) |
| 5α-Pregnan-3α-ol-20-one EC50 | 78 ± 10.9 nm1-b | 194 ± 12 nm | 114 ± 21 nm |
| 5α-Pregnan-3α-ol-20-one potentiation1-a | 265 ± 38% (n = 6) | 260 ± 45 (n = 4) | 214 ± 35 (n = 4) |
Fig. 6.
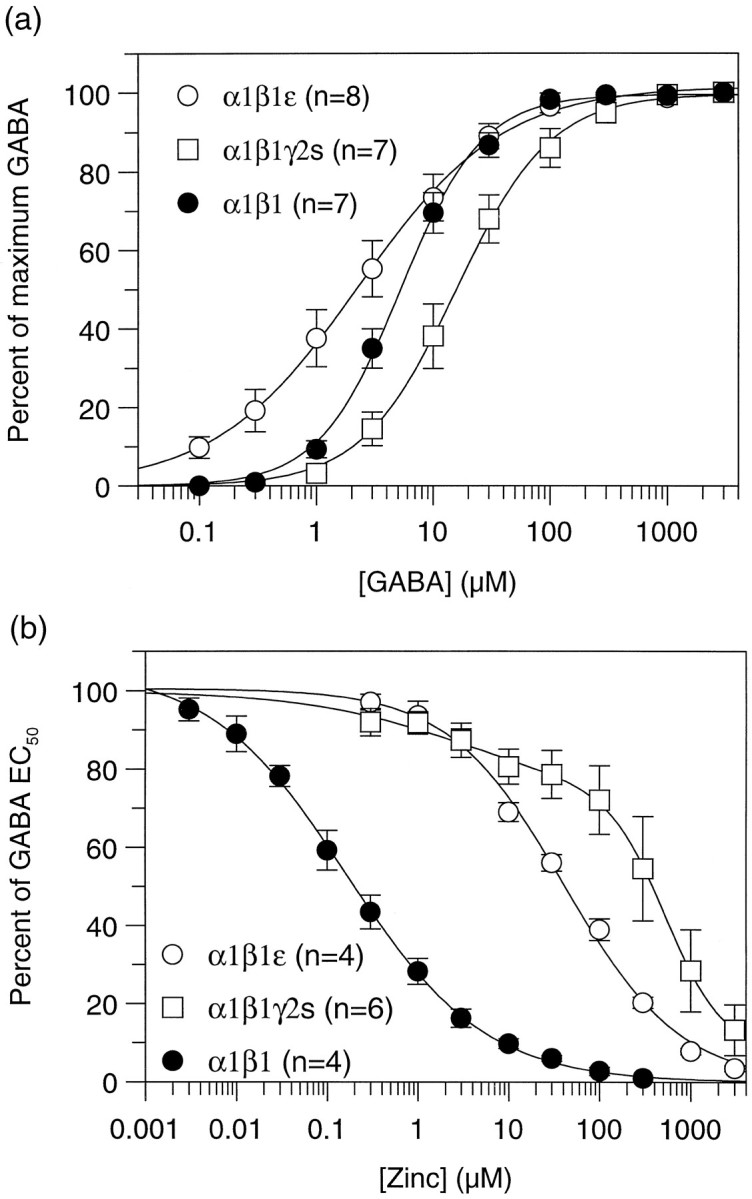
Functional expression of receptors containing the ε subunit. a, Concentration–response curves to GABA of α1β1, α1β1γ2s, and_α1β1ε_ GABAA receptors expressed in_Xenopus_ oocytes. b, Concentration–response curves for inhibition of an EC50concentration of GABA by increasing concentrations of zinc. Data represent mean curves from the number of cells indicated for each subtype. Curves were fit as described in Materials and Methods, with the exception of α1β1γ2s, which was best fit with two components of inhibition.
The ability of different GABAA receptor modulators to act at α1β1ε was investigated (Fig. 7). Unlike receptors containing a γ2s subunit, the GABA currents of α1β1ε receptors were not potentiated by the benzodiazepine flunitrazepam (1 μm). The barbiturate pentobarbital (100 μm) potentiated α1β1ε to the same extent as α1β1γ2s (200–300% of GABA EC20), but this was significantly lower than that observed with α1β1 receptors (Fig. 7a). Full concentration–response curves for the steroid 5α-pregnan-3α-ol-20-one on all three receptor subunit combinations revealed a similar maximum potentiation of ∼250% on all, but a significantly higher affinity of 78 nm(p < 0.05) on α1β1, as compared with 194 nm on α1β1ε and 114 nm on α1β1γ2s (Fig. 7b, Table 1).
Fig. 7.
Effects of 100 μm pentobarbital, 1 μm flunitrazepam, and 5α-pregnan-3α-ol-20-one on α1β1, α1β1γ2s, and α1β1ε GABAA receptor subunit combinations in Xenopus oocytes.a, Potentiation by 100 μm pentobarbital and 1 μm flunitrazepam. Data represent the percentage of modulation of an EC20 GABA response determined for each individual oocyte and are the mean ± SE of at least four determinations. b, Potentiation of the response to a GABA EC20 by increasing concentrations of 5α-pregnan-3α-ol-20-one on oocytes expressing α1β1 (•), α1β1γ2s (□), and α1β1ε (○) GABAA receptors. Data represent mean ± SE of at least four individual concentration–response curves, and values calculated from this are shown in Table 1.
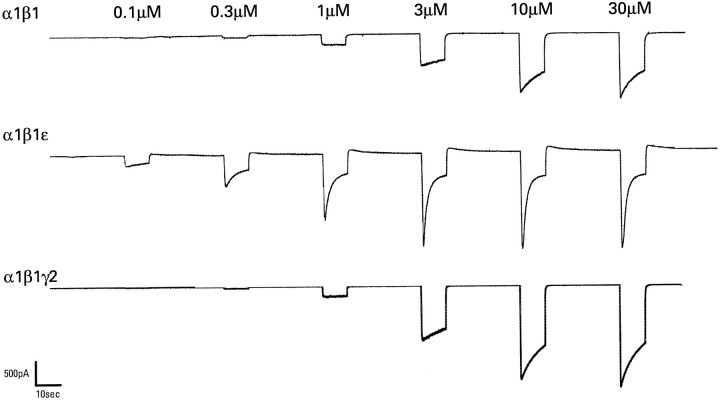
The current–voltage relationship for α1β1ε receptors was not significantly different from receptors consisting of α1β1 (data not shown). Maximum GABA responses in oocytes expressing α1β1ε appeared to show a greater rate of desensitization than those expressing α1β1 or α1β1γ2s. To investigate this further, we transfected these subunits into HEK 293 cells and studied them by using whole-cell patch-clamp techniques in which rapid solution changes allowed a more accurate measure of desensitization kinetics. With this method it was clear that ε was conferring rapid desensitization to the receptor, as compared with α1β1 and α1β1γ2s (Fig.8). At a concentration of 30 μm, which was maximum for all combinations, the peak/steady-state (after 10 sec) ratios were 4.22 ± 0.5 (n = 8) for α1β1ε, 2.17 ± 0.2 (n = 9) for α1β1, and 2.18 ± 0.3 (n = 6) for α1β1γ2s, the α1β1ε being significantly higher than the other two combinations (p < 0.005). Fitting single exponentials to the desensitization phase revealed rate constants of 1.79 ± 0.33, 4.23 ± 0.83, and 4.55 ± 1.2 sec for α1β1ε, α1β1, and α1β1γ2s, respectively. Rapid desensitization may affect the GABA concentration–response curve in oocytes, and the slightly higher affinities determined by using patch clamp suggest that this may be the case; however, the GABA EC50 values and Hill coefficients showed a similar relationship among the different subunit combinations to that observed in oocytes (data not shown).
Fig. 8.
GABA-gated current responses of HEK 293 cells transiently transfected with human α1β1, α1β1ε, and α1β1γ2s GABAAreceptor subunit cDNAs. Recordings were made under whole-cell patch-clamp conditions. The concentration of GABA is indicated_above_ each application. So that peak to steady-state ratios could be determined, applications lasted 10 sec, with at least 30 sec between applications.
DISCUSSION
ε coassembles with other GABAA receptor subunits to form a functional channel
Based on deduced amino acid sequence comparisons, the ε subunit has 32–49% sequence identity with other GABAAreceptor polypeptides and thus is a member of the GABAAreceptor gene family. ε does not form functional channels when expressed alone. Similarly, when coexpressed with other GABAA receptor subunits, it is unable to substitute for an α or β subunit. However, when it is coexpressed with both an α and a β subunit, a channel with a unique pharmacology is formed, demonstrating coassembly of the three subunits into a receptor complex. Most GABAA receptors studied to date are thought to consist of α, β, and γ or δ subunits (see Darlison and Albrecht, 1995;McKernan and Whiting, 1996). Thus ε seems to be able to substitute for a γ or δ subunit. Detailed immunoprecipitation experiments will be required to determine the subunit composition of native ε-containing receptors.
Zinc ions are known to be negative modulators of GABAAreceptor function (Smart and Constanti, 1990), and indeed they have been shown to be more potent at receptors consisting of α and β subunits than those constituted by α, β, and γ subunits (Draguhn et al., 1990; Smart et al., 1991) (Fig. 6b). Similarly, the subunit combination α1β1ε had 175-fold lower affinity for zinc than receptors composed of αβ. A lowering of the potency of zinc is observed also when the δ subunit is coexpressed with α and β; zinc has a 20-fold lower affinity at α6β3δ, as compared with α6β3 receptors (Thompson et al., 1997). Thus, inclusion of a third subunit into a binary αβ complex appears to have the general property of lowering the potency for zinc.
α1β1ε receptors are not modulated by the benzodiazepine flunitrazepam. This is perhaps not surprising because it is thought that the benzodiazepine binding site is made up of determinants from both the α and γ subunits (Pritchett et al., 1989; Stephenson et al., 1990). Similarly, GABAA receptors made up of α, β, and δ subunits are reported not to be modulated by benzodiazepines (Saxena and Macdonald, 1994).
The barbiturate pentobarbital interacts with the GABAAreceptor at both a modulatory site and a directly activating site, and the affinity and efficacy of pentobarbital for direct activation show subunit selectivity, being higher at α6-containing (α6β2γ2s) receptors, as compared with receptors containing other α subunits (Thompson et al., 1996). Here we show that the potentiation by 100 μm pentobarbital was not significantly different between α1β1ε and α1β1γ2s receptors. However, the potentiation at α1β1 receptors by the same concentration of pentobarbital was significantly higher than at either of the trimeric receptors, suggesting that the inclusion of a third subunit lowers the efficacy. Pentobarbital (100 μm) elicited a small response in the absence of GABA at α1β1ε, but not at α1β1 or α1β1γ2s receptors (data not shown).
Concentration–response curves for the steroid 5α-pregnan-3α-ol-20-one demonstrate an equivalent maximum level of potentiation on all three subunit combinations; however, the αβ assembly shows a small but significantly higher affinity for the steroid over α1β1ε or α1β1γ2s. The location of the binding sites for both barbiturates (Thompson et al., 1996) and steroids (Lambert et al., 1995) currently is unknown, but it is clearly not critically dependent on the presence of γ, δ, or ε subunits. The cloning of the ε subunit recently has been reported by Davies et al. (1997), who also reported insensitivity of ε-containing receptors to pentobarbital and pregnenolone; however, the clear potentiation observed here with both of these agents suggests that ε does not confer insensitivity to anesthetics. The reason for this discrepancy is currently unclear, because it is unlikely that the different α and β subunits used in the study could account for this and it is unknown which subunits coassemble with ε in vivo. The deduced amino acid sequence of the subunit reported by Davies et al. (1997)differs at position 102 (an alanine in the sequence of Davies et al. (1997), a serine in the sequence described here), but this difference does not account for the pharmacological inconsistencies (data not shown).
A novel observation was the rapid rate of desensitization of α1β1ε receptors, as compared with α1β1 or α1β1γ2s (Fig.8). The ε subunit clearly confers rapid desensitization kinetics to the GABAA receptor. In contrast, the presence of a δ subunit (in α1β1δ receptors) appears to decrease the rate of GABA-induced desensitization (Saxena and Macdonald, 1994).
Expression of the ε transcript in brain is highly restricted
Only the ρ subunits, the expression of which is primarily retinal (Enz et al., 1995), and α6, the expression of which is limited to cerebellar granule cells (Lüddens et al., 1990;Hadingham et al., 1996), have a more restricted pattern of expression than the ε subunit. The most abundant expression of the mRNA was found within the hypothalamic region, especially within the arcuate–ventromedial area. Other GABAA receptor subunits are known to be expressed in hypothalamic nuclei, primarily, but not exclusively, α2, β3, and γ2 (Wisden et al., 1992; Fenelon and Herbison, 1995; Fenelon et al., 1995). GABAergic transmission is known to play a key role in the hypothalamus, modulating the synthesis or release of vasopressin and oxytocin (Bisset et al., 1990), somatostatin (Gillies and Davidson, 1992), luteinizing hormone-releasing hormone (Mitsushima et al., 1994), and pro-opiomelanocortin (Blazquez et al., 1994). Colocalization studies that use anti-ε subunit and antipeptide hormone antisera will be required to define in detail a role for ε-containing receptors in the modulation of hormonal systems. Interestingly, the other site of high ε subunit mRNA expression was found in the hilus (polymorphic layer) of the dentate gyrus of the hippocampus. There was also low expression in the granule cell layer, but no other hippocampal field showed expression. Immunohistochemical labeling of monkey brain sections showed slight immunoreactivity in cells of the granule layer but dense immunoreactivity in the polymorphic layer and in the CA3 region. This is consistent with labeling of the mossy fibers in the pathway from dentate to CA3 and indicates that the ε subunit may play a specific role in the modulation of this excitatory pathway, with importance to long-term potentiation and memory.
ε subunit expression is regulated in part by tissue-specific RNA splicing
Northern analysis revealed that the ε subunit was expressed as two major polyadenylated transcripts. The larger transcript was expressed in a variety of peripheral tissues, but at barely detectable levels, in the brain. The smaller transcript was expressed within the brain but, with the exception of the heart and spinal cord, was not detectable in peripheral tissues. cDNA cloning revealed that the smaller transcript was appropriately spliced mature mRNA, whereas the larger transcript contained an unspliced intron between putative exons 6 and 7. These data suggest that the molecular mechanism for appropriate splicing of this particular intron is present within neurons but largely absent in peripheral tissues. Furthermore, because there is abundant expression of the unspliced transcript in a number of peripheral tissues, the neuronal-specific expression of mature ε subunit transcript is being regulated via a neuronally restricted RNA splicing mechanism. Other mechanisms, such as alternative splicing (for review, see Lewin, 1994) and RNA editing (Sommer et al., 1991), are known by which expression of the gene product can be regulated at the level of the RNA. Indeed, tissue-specific alternative RNA splicing has been described for the synaptic terminal protein synaptojanin (Ramjaun and McPherson, 1996) and the Pem homeobox gene (Maiti et al., 1996). The alternative splicing of neural cell adhesion molecule (NCAM) mRNA, which leads to the inclusion of exon 18 in differentiated N2a neuroblastoma cells and the omission in undifferentiated cells, has been characterized in detail and shown to depend on the 5′ splice site of the following intron and is controlled presumably by_trans_-acting factors (Tacke and Goridis, 1991).
Splicing is obviously not the only mode of regulation of the ε subunit transcript expression; the discrete regional expression of the ε subunit transcript within the brain presumably is being controlled at the level of the promoter. Two tissues, heart and spinal cord, expressed both the unspliced and the mature mRNA species. The precise cellular localization of the two mRNAs within these tissues is not known, but it is possible that the appropriately spliced mature mRNA is restricted to neuronal cells. GABAergic inhibition via GABAA receptors is known to be important in the spinal cord, and indeed several GABAA receptor subunits, particularly α2, β3, and γ2, are known to be expressed in this tissue (Persohn et al., 1991).
Footnotes
Correspondence should be addressed to Dr. Paul J. Whiting, Neuroscience Research Centre, Merck Sharp & Dohme Research Laboratories, Eastwick Road, Harlow, Essex CM20 2QR, United Kingdom.
REFERENCES
- 1.Altschul SF, Gish W, Miller W, Myers EW, Lipman D. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Blazquez C, Jegou S, Feuillololey M, Rosier A, Vandesande F, Vaudry H. Visualization of γ aminobutyric acid-A receptors on pro-opiomelanocortin-producing neurons in the rat hypothalamus. Endocrinology. 1994;135:2759–2764. doi: 10.1210/endo.135.6.7988468. [DOI] [PubMed] [Google Scholar]
- 3.Bisset GW, Chowdrey HS, Fairhall KM, Gunn LK. Central inhibition by gamma-aminobutyric acid and muscimol of the release of vasopressin and oxytocin by an osmotic stimulus in the rat. Br J Pharmacol. 1990;99:529–535. doi: 10.1111/j.1476-5381.1990.tb12963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen CA, Okayama H. Calcium phosphate-mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. Biotechniques. 1988;6:632–638. [PubMed] [Google Scholar]
- 5.Connolly CN, Wooltorton JRA, Smart TG, Moss SJ. Subcellular localization of γ-aminobutyric acid type A receptors is determined by receptor β subunits. Proc Natl Acad Sci USA. 1996;93:9899–9904. doi: 10.1073/pnas.93.18.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darlison MG, Albrecht BE. GABAA receptors; which, where, and why? Semin Neurosci. 1995;7:115–126. [Google Scholar]
- 7.Davies PA, Hanna MC, Hales TG, Kirkness EF. Insensitivity to anaesthetic agents conferred by a class of GABAA receptor subunit. Nature. 1997;385:820–823. doi: 10.1038/385820a0. [DOI] [PubMed] [Google Scholar]
- 8.Draguhn A, Verdoorn TA, Ewert M, Seeburg PH, Sakmann B. Function and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2+. Neuron. 1990;5:781–788. doi: 10.1016/0896-6273(90)90337-f. [DOI] [PubMed] [Google Scholar]
- 9.Ebert B, Wafford KA, Whiting PJ, Krogsgaard-Larsen P, Kemp JA. Molecular pharmacology of γ-aminobutyric acid type A receptor agonists and partial agonists in oocytes injected with different α, β, and γ receptor subunit combinations. Mol Pharmacol. 1994;46:957–963. [PubMed] [Google Scholar]
- 10.Enz R, Brandstätter JH, Hartveit E, Wässle H, Bormann J. Expression of GABA receptor ρ1 and ρ2 subunits in the retina and brain of the rat. Eur J Neurosci. 1995;7:1495–1501. doi: 10.1111/j.1460-9568.1995.tb01144.x. [DOI] [PubMed] [Google Scholar]
- 11.Fenelon VS, Herbison AE. Characterisation of GABAA receptor gamma subunit expression by magnocellular neurones in the rat hypothalamus. Mol Brain Res. 1995;34:45–56. doi: 10.1016/0169-328x(95)00130-k. [DOI] [PubMed] [Google Scholar]
- 12.Fenelon VS, Sieghart W, Herbison AE. Cellular localisation and differential distribution of GABAA receptor subunit proteins and messenger RNAs within hypothalamic magnocellular neurons. Neuroscience. 1995;64:1129–1143. doi: 10.1016/0306-4522(94)00402-q. [DOI] [PubMed] [Google Scholar]
- 13.Gillies G, Davidson K. GABAergic influences on somatostatin secretion from hypothalamic neurons cultured in defined medium. Neuroendocrinology. 1992;55:248–256. doi: 10.1159/000126122. [DOI] [PubMed] [Google Scholar]
- 14.Glencorse TA, Bateson A, Darlison MG. Sequence of the chicken GABAA receptor gamma 2-subunit cDNA. Nucleic Acids Res. 1990;18:7155–7157. doi: 10.1093/nar/18.23.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadingham KL, Wingrove P, Le Bourdelles B, Palmer KJ, Ragan CI, Whiting PJ. Cloning of cDNA sequences encoding human alpha 2 and alpha 3 gamma-aminobutyric acid-A receptor subunits and characterization of the benzodiazepine pharmacology of recombinant alpha 1-, alpha 2-, alpha 3-, and alpha 5-containing human gamma-aminobutyric acid-A receptors. Mol Pharmacol. 1993;43:970–975. [PubMed] [Google Scholar]
- 16.Hadingham KL, Wafford KA, Palmer K, Whiting PJ. Cloning of cDNAs encoding the human γ-aminobutyric acid type A receptor and characterisation of the pharmacology of γ3-containing receptors. Eur J Pharmacol Mol Pharmacol Sect. 1995;291:301–309. [Google Scholar]
- 17.Hadingham KL, Garrett EM, Wafford KA, Bain C, Heavens RP, Sirinathsinghji DJ, Whiting PJ. Cloning of cDNAs encoding the human γ-aminobutyric acid type A receptor α6 subunit and characterisation of the pharmacology of α6-containing receptors. Mol Pharmacol. 1996;49:253–259. [PubMed] [Google Scholar]
- 18.Harvey RJ, Kim HC, Darlison MG. Molecular cloning reveals the existence of a fourth gamma subunit of the vertebrate brain GABAA receptor. FEBS Lett. 1993;331:211–216. doi: 10.1016/0014-5793(93)80339-v. [DOI] [PubMed] [Google Scholar]
- 19.Horne AL, Macaulay AJ, Harkness PC, Hadingham K, Whiting P, Kemp JA. On the effect of the γ2L subunit on modulation of responses to GABAA receptor activation. Br J Pharmacol. 1993;108:711–716. doi: 10.1111/j.1476-5381.1993.tb12866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston GAR. GABAB receptors: relatively simple transmitter-gated ion channels? Trends Pharmacol Sci. 1996;17:319–323. [PubMed] [Google Scholar]
- 21.Kaupmann K, Huggel K, Heid J, Flor PJ, Bischoff S, Mickel SJ, McMaster G, Angst C, Bittiger H, Froesti W, Bettler B. Expression cloning of GABAB receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386:239–246. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- 22.Kirkness EF, Kusiak JW, Fleming JT, Menninger J, Gocayne JD, Ward DC, Venter JC. Isolation, characterization, and localization of human genomic DNA encoding the β1 subunit of the GABAA receptor (GABRB1). Genomics. 1991;10:985–995. doi: 10.1016/0888-7543(91)90189-l. [DOI] [PubMed] [Google Scholar]
- 23.Lambert JJ, Belilli D, Hill-Venning C, Peters JA. Neurosteroids and GABAA receptor function. Trends Pharmacol Sci. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- 24.Laurie DJ, Seeburg PH, Wisden W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci. 1992;12:1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewin B. Genes, Vol V, pp 845–964. Oxford UP; Oxford: 1994. Eukaryotic transcription and RNA processing. [Google Scholar]
- 26.Lüddens H, Keinanen K, Monyer H, Sprengel R, Seeburg PH. Cerebellar GABAA receptor selective for a behavioral alcohol antagonist. Nature. 1990;346:648–651. doi: 10.1038/346648a0. [DOI] [PubMed] [Google Scholar]
- 27.Macdonald RL, Angelotti TP. Native and recombinant GABAA receptor channels. Cell Physiol Biochem. 1993;3:352–373. [Google Scholar]
- 28.Maiti S, Doskow J, Li-S, Nhim RP, Lindsey JS, Wilkinson MF. The Pem homeobox gene: androgen-dependent and independent promoters and tissue-specific alternative RNA splicing. J Biol Chem. 1996;271:17536–17546. doi: 10.1074/jbc.271.29.17536. [DOI] [PubMed] [Google Scholar]
- 29.McKernan RM, Whiting PJ. Which GABAA receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- 30.Mitsushima D, Hei DL, Terasawa E. γ-Aminobutyric acid is an inhibitory neurotransmitter restricting the release of luteinizing hormone-releasing hormone before the onset of puberty. Proc Natl Acad Sci USA. 1994;91:395–399. doi: 10.1073/pnas.91.1.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Persohn E, Malherbe P, Richards JG. In situ hybridisation histochemistry reveals a diversity of GABAA receptor mRNAs in neurons of the rat spinal cord and dorsal root ganglia. Neuroscience. 1991;42:497–507. doi: 10.1016/0306-4522(91)90392-2. [DOI] [PubMed] [Google Scholar]
- 32.Pritchett DB, Sontheimer H, Shivers BH, Ymer S, Kettenmann H, Schofield PH, Seeburg PH. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- 33.Ramjaun AR, McPherson PS. Tissue-specific alternative splicing generates two synaptojanin isoforms with differential membrane-binding properties. J Biol Chem. 1996;271:24856–24861. doi: 10.1074/jbc.271.40.24856. [DOI] [PubMed] [Google Scholar]
- 34.Sakmann B, Hamill OP, Bormann J. Patch-clamp measurements of elementary chloride currents activated by the putative inhibitory transmitters GABA and glycine in mammalian spinal neurons. J Neural Transm [Suppl] 1983;18:83–95. [PubMed] [Google Scholar]
- 35.Saxena NC, Macdonald RL. Assembly of GABAA receptor subunits: role of the δ subunit. J Neurosci. 1994;14:7077–7086. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saxena NC, Macdonald RL. Properties of putative cerebellar γ-aminobutyric acid-A receptor isoforms. Mol Pharmacol. 1996;49:567–579. [PubMed] [Google Scholar]
- 37.Schofield PR, Pritchett DB, Sontheimer H, Kettenmann H, Seeburg PH. Sequence and expression of human GABAA receptor α1 and β1 subunits. FEBS Lett. 1989;244:361–364. doi: 10.1016/0014-5793(89)80563-0. [DOI] [PubMed] [Google Scholar]
- 38.Sieghart W. Structure and pharmacology of γ-aminobutyric acid-A receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 39.Sirinathsinghji DJS, Dunnett S. Imaging gene expression in neural graft. In: Sharif NA, editor. Molecular imaging in neuroscience: a practical approach. Oxford UP; Oxford: 1993. pp. 43–70. [Google Scholar]
- 40.Smart TG, Constanti A. Studies on the mechanism of action of picrotoxinin and other convulsants at the crustacean muscle GABA receptor. Br J Pharmacol. 1990;99:643–654. doi: 10.1098/rspb.1986.0019. [DOI] [PubMed] [Google Scholar]
- 41.Smart TG, Moss SJ, Xie X, Huganir RL. GABAA receptors are differentially sensitive to zinc dependence on subunit composition. Br J Pharmacol. 1991;103:1837–1839. doi: 10.1111/j.1476-5381.1991.tb12337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sommer B, Poustka A, Spurr NK, Seeburg PH. The murine GABAA receptor δ-subunit gene: structure and assignment to human chromosome 1. DNA Cell Biol. 1990;9:561–568. doi: 10.1089/dna.1990.9.561. [DOI] [PubMed] [Google Scholar]
- 43.Sommer B, Kohler M, Sprengel R, Seeburg PH. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;67:11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- 44.Stephenson FA, Duggan MJ, Pollard S. The γ2 subunit is an integral component of the γ-aminobutyric acid-A receptor, but the α1 polypeptide is the principal site of the agonist benzodiazepine photoaffinity-labeling reaction. J Biol Chem. 1990;265:21160–21165. [PubMed] [Google Scholar]
- 45.Tacke R, Goridis C. Alternative splicing in the neural cell adhesion molecule pre mRNA: regulation of exon 18 skipping depends upon the 5′ splice site. Genes Dev. 1991;5:1416–1429. doi: 10.1101/gad.5.8.1416. [DOI] [PubMed] [Google Scholar]
- 46.Thompson SA, Whiting PJ, Wafford KA. Alpha subunits influence the action of pentobarbital on recombinant GABAA receptors. Br J Pharmacol. 1996;117:521–527. doi: 10.1111/j.1476-5381.1996.tb15221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson SA, Thomas D, Whiting PJ, Wafford KA. Expression and pharmacology of the human GABAA receptor δ subunit. Br J Pharmacol. 1997;120:283P. doi: 10.1111/j.1476-5381.1996.tb15221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verdoorn T. Formation of heteromeric γ-aminobutyric acid type A receptors containing two different α subunits. Mol Pharmacol. 1994;45:475–480. [PubMed] [Google Scholar]
- 49.von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986;14:4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whiting P, McKernan RM, Iversen LL. Another mechanism for creating diversity in γ-aminobutyrate type A receptors: RNA splicing directs expression of two forms of the γ2 subunit, one of which contains a protein kinase C phosphorylation site. Proc Natl Acad Sci USA. 1990;87:9966–9970. doi: 10.1073/pnas.87.24.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whiting PJ, McKernan RM, Wafford KA. Structure and pharmacology of vertebrate GABAA receptor subtypes. Int Rev Neurobiol. 1995;38:95–138. doi: 10.1016/s0074-7742(08)60525-5. [DOI] [PubMed] [Google Scholar]
- 52.Wisden W, Laurie DJ, Monyer HM, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ymer S, Draguhn A, Wisden W, Werner P, Keinanen K, Schofield PR, Sprengel R, Pritchett DB, Seeburg PH. Structural and functional characterisation of the γ1 subunit of GABA benzodiazepine receptors. EMBO J. 1990;9:3261–3267. doi: 10.1002/j.1460-2075.1990.tb07525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]