The β-Amyloid Precursor Protein of Alzheimer’s Disease Enhances Neuron Viability and Modulates Neuronal Polarity (original) (raw)
Abstract
β-Amyloid precursor protein (βPP) can reside at neuron and glial cell surfaces or undergo proteolytic processing into secreted fragments. Although βPP has been studied extensively, its precise physiological role is unknown. A line of transgenic knock-out mice selectively deficient in βPP survive and breed but exhibit motor dysfunction and brain gliosis, consistent with a physiological role for βPP in neuron development. To elucidate these functions, we cultured hippocampal neurons from wild-type and βPP-deficient mice and compared their ability to attach, survive, and develop neurites. We found that hippocampal neurons from βPP-deficient mice had diminished viability and retarded neurite development. We also compared the effects of βPP secretory products, released from wild-type astrocytes, on process outgrowth from wild-type and βPP-deficient hippocampal neurons. Outgrowth was enhanced at 1 d in the presence of wild-type astrocytes, as compared with βPP-deficient astrocytes. However, by 3 d, neurons had shorter axons but more minor processes with more branching when cocultured with wild-type astrocytes, as compared with βPP-deficient astrocytes. Our data demonstrate that cell-associated neuronal βPP contributes to neuron viability, axonogenesis, and arborization and that βPP secretory products modulate axon growth, dendrite branching, and dendrite numbers.
Keywords: arborization, astrocytes, axonogenesis, Aβ, βPP, βPPs, knock-out mice, neurite outgrowth, neuron survival
β-Amyloid precursor protein (βPP) is a type I integral membrane protein that shares homology with glycosylated membrane receptors (Kang et al., 1987) and is present on the surface of neuronal and glial cells (Shivers et al., 1988; Breen et al., 1991; Yamazaki et al., 1997). βPP also can be processed proteolytically by a number of enzymes referred to as “secretases” into soluble βPP fragments including βPPs, the large N-terminal secreted fragment, and Aβ, the major protein component of senile plaques in Alzheimer’s disease (for review, seeSelkoe, 1994). Because Aβ deposition may be central to Alzheimer’s disease pathogenesis, much research has focused on the generation of this small peptide from its full-length precursor. βPP is expressed abundantly by embryonic neurons and astrocytes (Ferreira et al., 1993;Moya et al., 1994; Yamazaki et al., 1995). Although βPP is expressed normally in the CNS throughout life and has been studied for years, the precise functions of full-length cell-associated βPP and its secreted fragments are still unclear. βPP has been implicated in cell adhesion (Schubert et al., 1989), cell growth (Saitoh et al., 1989), neurite outgrowth (Milward et al., 1992; Qiu et al., 1995), and neuroprotection (Goodman and Mattson, 1994). One means of evaluating the role of βPP is by studying neuronal cells derived from βPP-deficient mice (Zheng et al., 1995) and comparing them with wild-type neuronal cells. Some of the phenotypic abnormalities associated with βPP-deficient mice (i.e., reactive gliosis in hippocampus and neocortex plus a reduced forelimb grip strength and altered locomotion) imply that βPP is important for normal CNS function. Furthermore, neuronal cells with diminished βPP expression, whether transfected with antisense βPP (LeBlanc et al., 1992) or treated with antisense oligonucleotides (Majocha et al., 1994; Allinquant et al., 1995), have altered process growth indicating that βPP is important to neuron development.
Although Aβ can be neurotrophic (Whitson et al., 1989; Yankner et al., 1990), fibrillar Aβ is toxic to neurons in vitro and may cause neuron loss in the brains of Alzheimer’s patients (for review, see Yankner, 1996). Because βPP is the precursor to Aβ, knowledge of the normal role of βPP in the nervous system may clarify our understanding of βPP-associated pathology found in Alzheimer’s disease and help to direct potential therapeutic strategies. To this end, hippocampal neurons from wild-type and βPP-deficient mice were evaluated for differences in cell attachment, survival, and neurite outgrowth in cocultures with astrocytes from wild-type and βPP-deficient mice. The use of astrocytes from wild-type and βPP-deficient mice provided a unique means for measuring differences in neuron development associated with secreted factors released into the astrocyte conditioned media. Our results show the importance of both cell-associated βPP and secreted βPP products on neurite differentiation, with implications for normal brain development.
MATERIALS AND METHODS
Transgenic knock-out mice. βPP-deficient knock-out mice, generated in the C57BL6/129 hybrid background, have been described (Zheng et al., 1995). Homozygous βPP-deficient mice survive and breed but have motor dysfunction and develop brain gliosis, as compared with wild-type control mice generated in the same C57BL6/129 hybrid background. Mice used in these studies were handled in accordance with the United States Public Health Policy in Humane Care and Use of Laboratory Animals and National Institutes of Health guidelines.
Primary astrocyte cultures. Glial cultures enriched in type 1 astrocytes (>95%) were prepared as described (Tawil et al., 1993) after dissection, using the protocol of Banker and Goslin (1991). Postnatal day 1 cortical tissues from wild-type and βPP-deficient mice were dissected free of choroid plexus and meninges and dissociated after trypsinization (trypsin–EDTA; Life Technologies, Gaithersburg, MD) and trituration. Astrocytes grown in 75 mm tissue culture flasks with minimal essential medium (MEM), 10% horse serum, and 0.6% glucose (glia–MEM) at 37°C with 5% CO2 were confluent by 10 d. The use of a rotary shaker eliminated other cell types. Astrocytes were plated onto 60 mm plastic Petri dishes to form supportive monolayers.
Primary hippocampal cultures. Hippocampi were dissected from embryonic day 16 (E16) mice and prepared as described (Banker and Goslin, 1991). Briefly, dissected hippocampi were suspended in trypsin–EDTA for 15 min at 37°C, washed three times with calcium- and magnesium-free HBSS and triturated with a fire-polished glass pipette to dissociate cells. Hippocampal cells cultured with serum-free Neurobasal medium with B27 supplement (Life Technologies) were grown in six-well tissue culture plates or on 12 mm glass coverslips (Carolina Biological Supply, Burlington, NC) that were pretreated with poly-l-lysine hydrobromide (1 mg/ml; Sigma, St. Louis, MO) in 0.1 m borate buffer, pH 8.5. Hippocampal cultures in B27-supplemented defined media were plated at high cell density (68,000–102,000 cells/well in six-well plates or 200,000–300,000 cells/60 mm Petri dish on coverslips)._Low_-density hippocampal cultures for morphometric analyses were plated at 100,000 cells/60 mm Petri dish onto poly-l-lysine-treated coverslips with paraffin dots on the plating surfaces. Cells were allowed to attach for 3–5 hr in MEM/10% horse serum/0.6% glucose at 37°C with 5% CO2. Then cells plated at low density on coverslips were transferred to astrocyte monolayers in serum-free N2-supplemented MEM (Bottenstein and Sato, 1979) containing ovalbumin (0.1%) and pyruvate (0.01 mg/ml) with neurons facing, but not in direct contact with, astrocytes.
Immunocytochemistry, antibodies, neuron attachment, and neuron survival. For immunocytochemistry, cultures were fixed for 20 min with prewarmed 4% paraformaldehyde/4% sucrose in PBS, washed three times with PBS, and preincubated with 10% bovine serum albumin in PBS. Primary antibodies included a monoclonal antibody raised against a class III β-tubulin (clone TuJ1; Frankfurter et al., 1986) and the anti-α-tubulin monoclonal antibody (clone DM1A; Sigma). Secondary antibodies included anti-mouse IgG-FITC for fluorescence microscopy (Jackson ImmunoResearch, West Grove, PA) and anti-mouse IgG-HRP (Amersham, Arlington Heights, IL) for bright-field microscopy. To determine initial relative numbers of neurons, we performed TuJ1 (1:250; Ferreira and Caceres, 1992) labeling of low-density hippocampal cultures in three experiments. Total cell counts by phase-contrast analysis were compared with TuJ1-stained neurons via fluorescence microscopy. The ratio of TuJ1-positive cells to total cell number was determined at time 0 (3–5 hr after plating) in 30 random 0.25 × 0.25 mm square microscopic fields per condition.
Neuron survival was assessed by two different measures. For high-density neuronal cultures grown in B27-supplemented medium, cells in six-well plastic tissue culture plates were immunolabeled with DM1A (1:250) and HRP-conjugated secondary antibody (1:250), followed by 3,3′-diaminobenzidine reaction. Uniform grids were scratched onto the plastic with a “pin rake” (Tyler Research Instruments, Edmonton, Alberta, Canada). Neurite-bearing cells were counted in 50 random 0.25 mm2 grids, using an inverted phase microscope. For low-density neurons in coculture experiments, viability was assessed directly, as reported previously (Canoll et al., 1996), by counting the numbers of live neurons in cultures, using the Live/Dead Viability/Cytotoxicity Kit according to the manufacturer (Molecular Probes, Eugene, OR). Intact (live) cells stained green with calcein-AM, whereas dead cells stained red by ethidium dimer intercalation into DNA of cells with compromised plasma membranes. Total cell counts were obtained via phase-contrast illumination. Total cell numbers and live neuron counts were obtained for 50 nonoverlapping microscopic fields for each coculture condition in three independent experiments.
_Normal hippocampal neuron development in astrocyte coculture.Cocultured hippocampal neurons derived from rats (Caceres et al., 1984) or mice (Chin et al., 1995) recapitulate some of their_in vivo differentiation by establishing axonal and dendritic polarity in vitro. By 1 d in vitro, neurons at “stage 3” (Dotti et al., 1988) have a recognizable axon, which by definition is the single longest neurite extending from the cell body. Because the nonaxonal dendritic minor processes do not compartmentalize MAP2 protein until ∼3–5 d in vitro(Caceres et al., 1984), they are referred to as “minor processes.”
Quantitative morphometry. Neurons plated at low density were fixed and stained with DM1A (see above). Forty neurons per coculture condition were evaluated at 1 and at 3 d after plating. Isolated stage 3 neurons with a distinct axon were selected at random from nonoverlapping fields, and the cell body and all processes were traced from an inverted phase microscope image projected onto a monitor via video camera. Quantitative measurements were obtained with a digitizing pad, as previously described (Brandt and Lee, 1993). Neurons were evaluated for axon outgrowth, minor process outgrowth, axon and minor process branching, minor process numbers, and cell body diameters.Axon outgrowth refers to the single longest neurite extending from the neuronal cell body plus the sum of the lengths of all small processes that emanate laterally from that single longest process. Each neuron analyzed had a single axon. Minor process outgrowth consists of the sum of the lengths of all nonaxonal processes emanating directly from the cell body, including the sum of the lengths of all small lateral processes arising from those minor processes. Total outgrowth is the sum of axonal and minor process outgrowth. Branches refers to the small processes that emanate laterally from the axon (axon branches) or minor processes (minor process branches) of each neuron. Minor process number refers to the total number of processes emanating directly from the neuronal cell body, minus one. _Cell body diameters_represent of the average of three diameter measures across each neural cell body.
Statistical analyses. All data were analyzed with the Instat program (GraphPad Software, San Diego, CA), using Student’s_t_ tests when two sets of data were compared or ANOVA for comparisons of the four neuron/astrocyte coculture conditions.Post hoc analyses of significant ANOVA data were analyzed by the Tukey–Kramer method. All data are averages ± SEM.
RESULTS
Neurons lacking βPP expression attach efficiently to poly-l-lysine-treated substrates but have diminished viability in vitro
The relative numbers of neurons from wild-type and βPP-deficient mice hippocampal dissections were evaluated at 3–5 hr after plating. Fixed cells were immunolabeled for neuronal class III–β-tubulin with antibody TuJ1 (described above), and both total cell number and neurons were counted. At this time point, equal numbers of neurons were stained in low-density cultures from both wild-type (10.3 ± 0.46 neurons/0.25 × 0.25 mm square field) or βPP-deficient (11.6 ± 0.49 neurons/0.25 × 0.25 mm square field) hippocampal dissections that contained 85–95% neurons. Having determined that plating of neurons was equivalent and that both wild-type and βPP-deficient neurons attached equally well, we next assessed neuron survival.
Neuron survival was assessed initially for high cell density cultures grown on poly-l-lysine-treated tissue culture plastic in B27-supplemented Neurobasal medium. Process outgrowth from both wild-type and βPP-deficient neurons was slower to develop in this medium; therefore, neuron counts were performed at 3 d when axons were clearly developed. At 3 d, for high-density cultures plated at two- to threefold higher cell density than the low-density cultures described above, we observed more surviving neurons in wild-type cultures (22 ± 0.69 neurons/0.25 × 0.25 mm square field) than in βPP-deficient cultures (16.1 ± 0.71 neurons/0.25 × 0.25 mm square field). This cell loss was 27% greater for βPP-deficient neurons (t = 5.9; p < 0.0001) than for wild-type neurons, although cultures were plated at the same density from pools with equivalent numbers of neurons.
Because neurite development was slower for neurons in the B27-supplemented medium, as compared with neurons cocultured with astrocytes (Dotti, 1988), we chose to use cocultures for subsequent experiments. Wild-type and βPP-deficient hippocampal neurons on coverslips were cocultured above astrocytes derived from wild-type and βPP-deficient mice. This produced the following four conditions: (1) wild-type neurons with wild-type astrocytes (WT/WT), (2) βPP-deficient knock-out neurons with wild-type astrocytes (KO/WT), (3) wild-type neurons with βPP-deficient astrocytes (WT/KO), and (4) βPP-deficient neurons with βPP-deficient astrocytes (KO/KO).
Neuron survival, which was less for βPP-deficient cultures at high cell density in B27-supplemented media, again was evaluated for low-density hippocampal neurons in astrocyte cocultures. At 3 d, wild-type hippocampal cocultures had more viable neurons (Fig.1A,C) than βPP-deficient hippocampal cocultures (Fig. 1B,C). In these low-density cultures wild-type neurons survived similarly when cocultured with wild-type (8.1 ± 0.43 neurons/ 0.25 × 0.25 mm square field, Fig. 1A,C) or βPP-deficient astrocytes (7.5 ± 0.51 neurons/0.25 × 0.25 mm square field, Fig. 1C). However, βPP-deficient neurons had equally diminished viability in coculture with wild-type (6.36 ± 0.31 neurons/0.25 × 0.25 mm square field, Fig. 1B) and βPP-deficient astrocytes (6.39 ± 0.34 neurons/0.25 × 0.25 mm square field, Fig. 1C), comparable to the loss observed in high-density cultures with B27-supplemented medium.
Fig. 1.
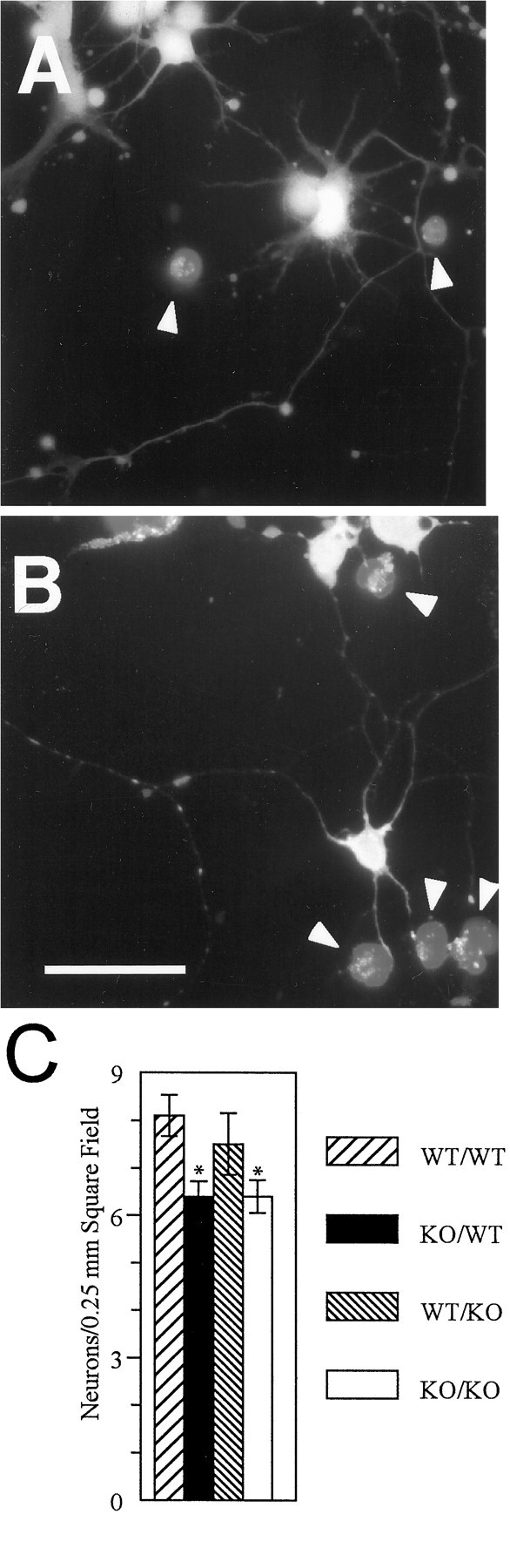
Live cells and dead cells in neuron/astrocyte cocultures at 3 d in vitro. Intact wild-type (A) and βPP-deficient (B) hippocampal neurons (shown here cocultured with wild-type astrocytes) are among dead cells (at arrowheads in A, B). Live neurons appear brightly stained with calcein-AM and have well developed neurites. Dead cells, labeled by ethidium dimer, are rounded and appear faintly stained (at arrowheads in_A, B_). C, βPP-deficient hippocampal cultures had significantly fewer live neurons per field whether cocultured with wild-type astrocytes or βPP-deficient astrocytes than did wild-type hippocampal neurons cocultured with either wild-type or βPP-deficient astrocytes in 50 random fields. WT, Wild-type;KO, βPP-deficient knock-out. WT/WT, Wild-type neurons cocultured with wild-type astrocytes;KO/WT, βPP-deficient neurons cocultured with wild-type astrocytes; WT/KO, wild-type neurons cocultured with βPP-deficient knock-out astrocytes; KO/KO, βPP-deficient neurons cocultured with βPP-deficient astrocytes. ANOVA, p < 0.0001. *Significantly different from WT/WT cocultures. Scale bar, 60 μm.
We also measured neuronal cell bodies to assess potential effects of βPP expression on cell body attachment and spreading. Average cell body diameters were measured for 40 neurons per coculture condition in three independent experiments. Although variability was observed in all conditions, the somatic diameters were not significantly different at 1 d (WT/WT = 15.18 μm ± 0.42; KO/WT = 15.73 μm ± 0.39; WT/KO = 15.07 μm ± 0.40; and KO/KO = 14.67 μm ± 0.41) or at 3 d (WT/WT = 26.4 μm ± 1.7; KO/WT = 30.1 μm ± 2.13; WT/KO = 26.64 μm ± 1.7; and KO/KO = 27.7 μm ± 2.04), indicating that the absence of cell-associated βPP did not diminish cell body attachment and spreading.
Wild-type hippocampal neurons have enhanced axon development and enhanced branching when cocultured with wild-type astrocytes for 1 d
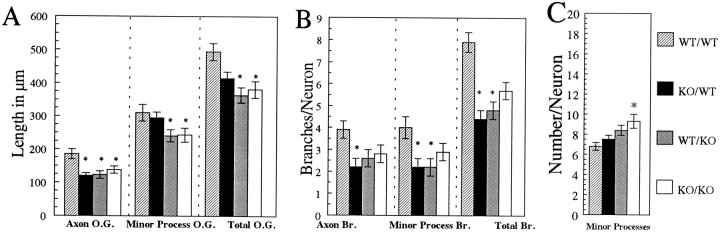
All neurons measured at 1 d had an axon and several minor processes (for detailed descriptions of axonal and minor process outgrowth and branching, refer to Quantitative Morphometry in Materials and Methods). Wild-type neurons cocultured with wild-type astrocytes (Fig. 2A) had greater total outgrowth (Fig. 3A), as compared with neurons in the other three coculture conditions at 1 d. This enhanced outgrowth resulted from significantly longer axons at this time (Fig. 3A). In addition, wild-type neurons cocultured with wild-type astrocytes (Fig.2A) had more branching of axons and minor processes than did neurons in the other three coculture conditions (Figs. 2, 3B). When wild-type hippocampal neurons were cocultured with βPP-deficient astrocytes (Fig. 2C), they had significantly shorter axons (Fig. 3A) with less axon branching (Fig. 3B), as compared with wild-type neurons cocultured with wild-type astrocytes. Although minor process outgrowth (Fig. 3A) and axon and minor process branching (Fig.3B) were greater for wild-type neurons cocultured with wild-type astrocytes, these neurons actually had the least number of minor processes at 1 d (Fig. 3C).
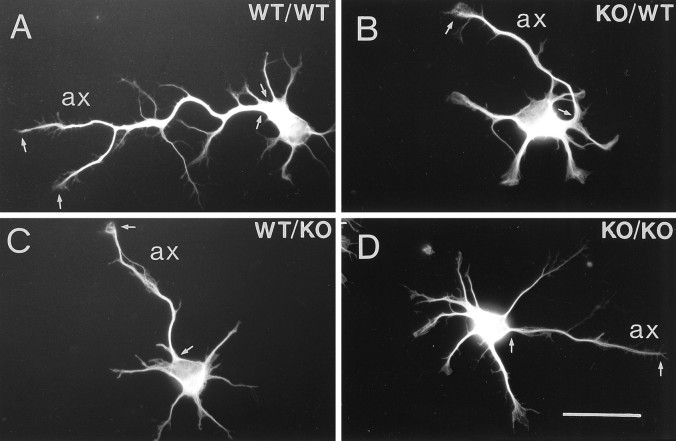
Fig. 2.
Stage 3 hippocampal neurons in astrocyte cocultures for 1 d. Proximal and distal portions of each axon (ax) are marked by small arrows for a wild-type neuron cocultured with wild-type astrocytes (A), a βPP-deficient neuron cocultured with wild-type astrocytes (B), a wild-type neuron cocultured with βPP-deficient astrocytes (C), and a βPP-deficient neuron cocultured with βPP-deficient astrocytes (D). The short neurites emanating from the cell body are predendritic, minor processes. WT/WT, Wild-type neurons cocultured with wild-type astrocytes; KO/WT, βPP-deficient neurons cocultured with wild-type astrocytes;WT/KO, wild-type neurons cocultured with βPP-deficient knock-out astrocytes; KO/KO, βPP-deficient neurons cocultured with βPP-deficient astrocytes. Scale bar, 25 μm.
Fig. 3.
βPP-related effects on neuron morphology for hippocampal neurons cocultured with wild-type and βPP-deficient astrocytes for 1 d. A, Axon, minor process, and total outgrowth were greatest for wild-type neurons cocultured with wild-type astrocytes (WT/WT). Significantly less axon growth was apparent for βPP-deficient knock-out neurons in both astrocyte conditions and for wild-type neurons cocultured with βPP-deficient astrocytes. B, Branching of axons and minor processes was more pronounced for neurons in WT/WT cultures.C, βPP-deficient neurons, which lack cell-surface βPP, had significantly more minor processes when cocultured with βPP-deficient astrocytes, which do not secrete βPPs or Aβ. WT/WT, Wild-type neurons cocultured with wild-type astrocytes; KO/WT, βPP-deficient knock-out neurons with wild-type astrocytes; WT/KO, wild-type neurons with βPP-deficient knock-out astrocytes; KO/KO, βPP-deficient knock-out neurons with βPP-deficient knock-out astrocytes; O.G., outgrowth; Br., branches. Data are averages of 40 neurons ± SEM. *Significantly different from WT/WT cocultures. Histogram legend in _C_applies to A–C.
βPP-deficient neurons cocultured with wild-type (Fig.2B) or βPP-deficient astrocytes (Fig.2D) had diminished total outgrowth that was associated with significantly shorter axons at 1 d (Fig.3A). Minor process outgrowth was similar for βPP-deficient neurons and wild-type neurons cocultured with wild-type astrocytes (Fig. 3A); however, βPP-deficient neurons cocultured with βPP-deficient astrocytes had significantly diminished minor process outgrowth (Fig. 3A). It is noteworthy that a marked reduction in minor process outgrowth was observed for both wild-type and βPP-deficient neurons when they were cocultured with βPP-deficient astrocytes for 1 d (Fig. 3A).
Axon branching was less for βPP-deficient neurons cocultured with wild-type astrocytes and somewhat reduced for βPP-deficient neurons cocultured with βPP-deficient astrocytes at 1 d (Fig.3B). Minor processes were affected similarly. Minor process branching for βPP-deficient neurons in both coculture conditions was less than that observed for wild-type neurons cocultured with wild-type astrocytes (Fig. 3B). Furthermore, although βPP-deficient neurons cocultured with βPP-deficient astrocytes had significantly more minor processes at 1 d (Fig. 3C), these minor processes were significantly shorter than those of wild-type neurons cocultured with wild-type astrocytes (Fig. 3A).
Hippocampal neurons cocultured with wild-type astrocytes for 3 d have shorter axons but enhanced minor process outgrowth
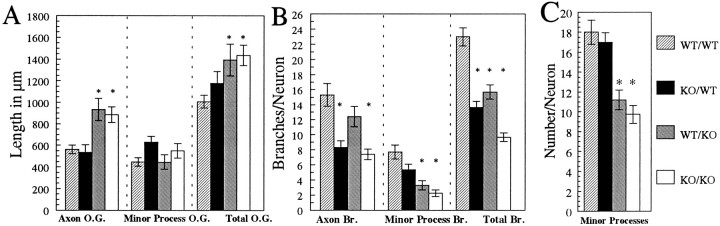
By 3 d in culture wild-type neurons cocultured with wild-type astrocytes (Fig. 4A) and wild-type neurons cocultured with βPP-deficient astrocytes (Fig.4C) had grown considerably. However, the distribution of outgrowth was markedly different, depending on the astrocytes used for coculture. Interestingly, axon outgrowth from wild-type neurons, which had been significantly greater in the presence of wild-type astrocytes at 1 d, was now greater in the presence of βPP-deficient astrocytes at 3 d (Fig.5A). Similarly, βPP-deficient neurons cocultured with βPP-deficient astrocytes also had significantly longer axons, as compared with βPP-deficient neurons cocultured with wild-type astrocytes at 3 d (Fig.5A). Therefore, the growth of both wild-type and βPP-deficient neurons with wild-type astrocytes was found to limit the growth of axons. Both wild-type and βPP-deficient neurons had significantly longer axons and greater total outgrowth when grown with βPP-deficient astrocytes for 3 d (Fig. 5A). Although somewhat greater for βPP-deficient neurons than for wild-type neurons, minor process outgrowth was not significantly different for neurons in the four coculture conditions by 3 d (Fig.5A).
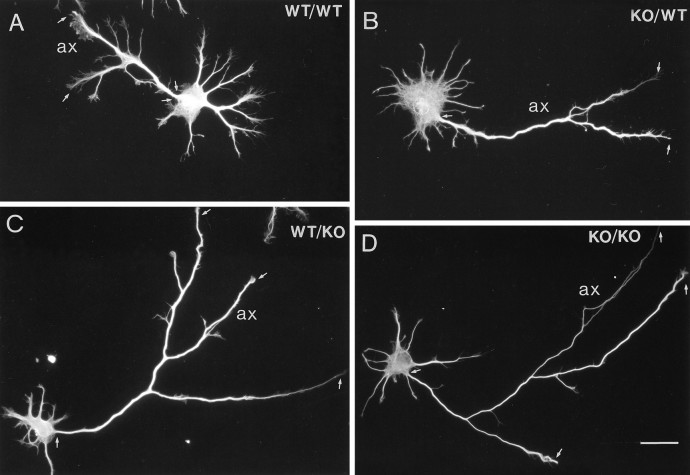
Fig. 4.
Hippocampal neurons cocultured with wild-type and βPP-deficient astrocytes for 3 d. Neurons had extensive axon (ax) and minor process development (small processes emanating from the cell body) by 3 d in vitro. Proximal and distal portions of each axon are indicated by small arrows for a wild-type neuron cocultured with wild-type astrocytes (A), a βPP-deficient neuron cocultured with wild-type astrocytes (B), a wild-type neuron cocultured with βPP-deficient astrocytes (C), and a βPP-deficient neuron cocultured with βPP-deficient astrocytes (D). The developing predendritic, minor processes are more pronounced for neurons cultured with wild-type astrocytes for 3 d (A,B) than for neurons grown with βPP-deficient glia (C, D). WT/WT, Wild-type neurons cocultured with wild-type astrocytes; KO/WT, βPP-deficient neurons cocultured with wild-type astrocytes;WT/KO, wild-type neurons cocultured with βPP-deficient knock-out astrocytes; KO/KO, βPP-deficient neurons cocultured with βPP-deficient astrocytes. Scale bar, 25 μm.
Fig. 5.
βPP-related effects on hippocampal neurons grown in coculture with wild-type and βPP-deficient astrocytes for 3 d. A, Axon outgrowth was significantly less for neurons cocultured with wild-type astrocytes, which secrete βPPsand Aβ. Minor process outgrowth was not significantly different for the four coculture conditions. Total outgrowth, which paralleled axon outgrowth, was greater in the absence of βPP secretory products.B, Wild-type neurons, which express cell-surface βPP, had more axon branching than βPP-deficient neurons in both coculture conditions. Minor process branching was enhanced for neurons grown with wild-type astrocytes, which secrete βPPs and Aβ. Total branching was enhanced for wild-type neurons cocultured with wild-type astrocytes (WT/WT). C, More minor processes were produced by neurons cocultured with wild-type astrocytes, which secrete βPPs and Aβ.WT/WT, Wild-type neurons cocultured with wild-type astrocytes; KO/WT, βPP-deficient knock-out neurons with wild-type astrocytes; WT/KO, wild-type neurons with βPP-deficient knock-out astrocytes; KO/KO, βPP-deficient knock-out neurons with βPP-deficient knock-out astrocytes; O.G., outgrowth; Br., branches. Data are averages of 40 neurons ± SEM. *Significantly different from WT/WT cocultures. Histogram legend in _C_applies to A–C.
Total branching at 3 d was greatest for wild-type neurons cocultured with wild-type astrocytes (Fig.4A) than for neurons in the other three coculture conditions (Figs. 4, 5B). Axon branching was significantly greater for wild-type neurons than for βPP-deficient neurons in both astrocyte coculture conditions (Fig. 5B).Minor process branching was greater for all neurons grown in the presence of wild-type astrocytes for 3 d, with wild-type neurons having the most minor process branching of the four coculture conditions (Fig. 5B). Growth of both wild-type and βPP-deficient neurons with βPP-deficient astrocytes produced less minor process branching. Although minor process branching had nearly doubled for neurons grown with wild-type astrocytes for 3 d, neurons cocultured with βPP-deficient astrocytes had markedly less minor process branching by 3 d (compare Figs. 3B and5B). Minor process branching for wild-type neurons was increased only slightly at 3 d relative to 1 d, and minor process branching was actually decreased by 3 d for βPP-deficient neurons (compare Figs. 3B and5B). Of all the measures obtained in these studies, minor process branching for βPP-deficient neurons cocultured with βPP-deficient astrocytes was the only value that did not increase between 1 and 3 d. Minor process numbers were increased significantly for neurons cocultured in the presence of wild-type astrocytes but only slightly increased for neurons grown with βPP-deficient astrocytes between 1 and 3 d (compare Figs.3C and 5C).
DISCUSSION
Hippocampal neurons from βPP-deficient transgenic knock-out mice and wild-type control animals were analyzed for survival and neurite outgrowth. Neurons express abundant βPP, yet relatively little βPP is processed into secreted products by neurons (Hung et al., 1992;Wertkin et al., 1993; LeBlanc et al., 1996), implying that a larger percentage of neuronal βPP remains intact. Although many biological functions have been attributed to βPPs (Saitoh et al., 1989; Schubert et al., 1989; Mattson et al., 1993; Goodman et al., 1994; Jin et al., 1994; Mattson, 1994; Yamamoto et al., 1994), much less is known about uncleaved βPP function. Full-length βPP is localized at hippocampal neuron cell surfaces (Yamazaki et al., 1995) and can mediate binding to other cell surfaces to enhance adhesion and neurite outgrowth (Qiu et al., 1995). To directly evaluate a role for cell-associated βPP, we measured differences in neuron development attributable to cell-associated βPP present on wild-type neurons, but not on βPP-deficient neurons. In addition, we evaluated the responses of neurons to βPP secreted products by growing hippocampal neurons with wild-type astrocytes that release βPPs and Aβ (LeBlanc et al., 1996). Our data have generated two major findings previously unreported for βPP: (1) that cell-associated βPP plays a role in neuron survival in culture and (2) that both cell-associated βPP and βPP secreted products contribute to axon and dendritic outgrowth and arborization in a complex manner.
Because βPP plays a role in neuronal cell adhesion (Schubert et al., 1989; LeBlanc et al., 1992; Qiu et al., 1995), we tested βPP-deficient neurons for attachment and spreading. The lack of βPP expression by βPP-deficient neurons did not affect attachment or spreading on poly-l-lysine-treated substrates. This result is not surprising because neurons adhere better to poly-l-lysine-treated substrates than to tissue culture plastic, fibronectin, or laminin, even in the presence of anti-βPP antibodies (Breen et al., 1991). Furthermore, neurons have many alternate adhesion molecules (e.g., NCAM, L1, and Axonin-1; for review, see Fields and Itoh, 1996) that effectively mediate attachment.
Although βPPs has been proposed to contribute to cell survival (Yamamoto et al., 1995), ours are the first data to show that endogenous cell-associated βPP contributes to neuron survival. In both B27-supplemented media and in neuron/astrocyte cocultures there was a significantly greater loss of βPP-deficient neurons, as compared with wild-type neurons. Moreover, βPP-deficient hippocampal cultures had the identical degree of neuron loss whether grown with wild-type or βPP-deficient astrocytes (Fig. 1C). Culturing βPP-deficient neurons in the presence of wild-type astrocyte-conditioned medium (which includes βPPs and Aβ) had no rescuing effect on the βPP-deficient neurons that died during the 3 d of culture. This observation strongly implicates cell-associated βPP in survival of the cultured neurons. Two potential modes of action for cell-associated βPP in neuron survival include (1) a direct promotion of neuron survival (as described below) or (2) a homophilic interaction between βPPs or Aβ and cell-associated βPP, now absent from βPP-deficient neurons. Thus βPP-deficient neurons have provided the first evidence that endogenous cell-associated βPP directly contributes to neuron survival.
The influence of βPP on the development of axonal and dendritic processes is more complex than its effects on neuronal viability described above. Our results showed that both cellular βPP and βPP secreted products contributed to all aspects of neurite outgrowth examined in this study. First, βPP appears to play a role in axon development. This is suggested by βPP-deficient neurons having markedly shorter axons at 1 d. Because βPP-deficient neurons grown with wild-type astrocytes ultimately developed axons equal in length to those of wild-type neurons, it appears that cell-associated βPP primarily contributes to the onset of axon formation. Neither minor process outgrowth nor total outgrowth was affected significantly by the absence of βPP from βPP-deficient neurons grown with wild-type astrocytes, suggesting a specific effect on axon development. Although axons are shorter at 1 d in the absence of neuronal βPP expression, the manner in which this occurs is undetermined. Perhaps in the absence of cell-associated βPP, neurons have difficulty determining which process will become the axon.
Second, cell-associated βPP also contributes to arborization and process formation. Wild-type neurons always had more branching and ultimately had more minor processes than did βPP-deficient hippocampal neurons. Again, the most pronounced effect for cell-associated βPP on branching was observed for axons. Because βPP-deficient neurons had axons that were initially shorter and always less branched, the data suggest that cell-associated βPP contributes to normal axon formation. Dendritic (i.e., minor process) branching also was affected by the absence of βPP. The finding that all processes branched less when neurons lacked βPP suggests that axon/dendritic connectivity may be abnormal in the βPP-deficient mice and may underlie the diminished motor functions observed in these animals (Zheng et al., 1995). Indeed, βPP is abundantly expressed by motor neurons during normal mouse development (Salbaum and Ruddle, 1994).
Taken together, cell-associated neuronal βPP appears to function both in neuronal survival and neurite outgrowth. The mechanisms by which βPP promotes these effects are unclear. Nonetheless, it is interesting to hypothesize that cell-surface βPP specifically mediates these effects. Cell-surface βPP appears to use the region between amino acids 444–592 (βPP695 numbering) for cell adhesion, neurite outgrowth (Qiu et al., 1995), and protection against excitotoxicity (Mattson, 1994). Cell-surface βPP may transduce signals important for neuron growth and survival by an interaction of its C terminus with the heterotrimeric G0-protein (Okamoto, 1995). βPP also may provide a molecular link between the neuronal cytoskeleton (Allinquant et al., 1994) and the extracellular environment and thereby contribute to neurite outgrowth and pathfinding. The recent observation of colocalization of cell-surface βPP with integrins in neural cells (Storey et al., 1996; Yamazaki et al., 1997) is consistent with this hypothesis. Although βPP expression may be most important for particular subsets of neurons (Salbaum and Ruddle, 1994) or during specific neurodevelopmental stages, its significance is substantiated nonetheless by the finding that βPP-deficient neurons survived less well in culture. Therefore, our results confirm the importance of βPP to neuron function and provide compelling evidence that cell-associated, possibly cell-surface βPP mediates neuron survival.
The effects of βPP secretory products were determined by growing hippocampal neurons with astrocytes from wild-type and βPP-deficient mice. Although an earlier study suggested that neural cells secreted little βPPs (Haass et al., 1991), a recent report shows that up to 40% of total astrocytic βPP is processed into βPPs but very little into Aβ (LeBlanc et al., 1996). By 3 d in vitro, wild-type and βPP-deficient neurons cocultured with wild-type astrocytes had significantly_shorter_ axons than neurons cocultured with βPP-deficient astrocytes. It is noteworthy that both wild-type and βPP-deficient hippocampal neurons showed the identical axonal response to both wild-type and βPP-deficient astrocyte conditioned media. Unlike the diminution of axon outgrowth, the number of minor processes and the number of branch points were significantly greater for neurons cultured with wild-type astrocytes. These results suggest that soluble factors present in wild-type astrocyte conditioned medium modulate axon growth and process branching. Neurite growth can be affected by both βPPs (Mattson, 1994) and Aβ (Koo et al., 1993). Dendritic growth and branching in our studies are similar to the effect described by Mattson, using exogenous βPPs (1994); however, we did not assess the effect of βPPs or Aβ on neurite development directly. In addition, other secreted factors besides βPPs or Aβ may have been present or absent from βPP-deficient astrocyte conditioned medium. Alternately, a complexing of βPPs with other cell-surface proteins as described for heparan sulfate proteoglycan (Small et al., 1994) or Aβ interacting with neuronal adhesion molecules (Sabo et al., 1995) also may have contributed to the growth differences observed for neurons cocultured with wild-type astrocytes. Further analysis is required to define the mechanism underlying our observations.
In summary, the data indicate that both cell-associated βPP and secreted βPP products appear to be important for normal neuronal development. Cell-associated βPP, possibly cell-surface βPP, enhances neuron survival, the timely initiation of axon growth, and axon arborization. βPP secreted products contribute to axonal and dendritic growth in a manner that modulates neuronal polarity and appears to limit the growth of axons at the same time dendritic growth is enhanced and may be involved in coordinating the timing of connections in the developing CNS. Future analyses with this rodent model should help to elucidate the complex role of βPP in both the developing and mature CNS.
Footnotes
This work was supported by National Institutes of Health Grants NS28121 (E.H.K.) and 5T32AG00222 (R.G.P.) and the Paul Beeson Physician Faculty Scholar in Aging Research from the American Federation for Aging Research (E.H.K.). We are grateful to Dr. A. Frankfurter for the gift of TuJ1 antibody; Drs. Adriana Ferreira, Tsuneo Yamazaki, and Gloria Lee for technical advice and supportive discussions; and Drs. Willi Halfter, Deborah Watson, and Margaret Kruse for critical reading of this manuscript.
Correspondence should be addressed to Dr. Ruth G. Perez, Allegheny University of the Health Sciences, Neurosciences Research Center, 320 East North Avenue/10th Floor, South Tower, Pittsburgh, PA 15212-4772.
Dr. Koo’s present address: Department of Neurosciences 0691, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093-0691.
REFERENCES
- 1.Allinquant B, Moya KL, Bouillot C, Prochiantz A. Amyloid precursor protein in cortical neurons: coexistence of two pools differentially distributed in axons and dendrites and association with cytoskeleton. J Neurosci. 1994;14:6842–6854. doi: 10.1523/JNEUROSCI.14-11-06842.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allinquant B, Hantraye P, Mailleux P, Moya K, Bouillot C, Prochiantz A. Downregulation of amyloid precursor protein inhibits neurite outgrowth in vitro. J Cell Biol. 1995;128:919–927. doi: 10.1083/jcb.128.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banker G, Goslin K. Rat hippocampal neurons in low-density culture. In: Banker G, Goslin K, editors. Culturing nerve cells. MIT; Cambridge, MA: 1991. pp. 251–281. [Google Scholar]
- 4.Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci USA. 1979;76:514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandt R, Lee G. Functional organization of microtubule-associated protein tau. J Biol Chem. 1993;268:3414–3419. [PubMed] [Google Scholar]
- 6.Breen K, Bruce M, Anderson B. β-Amyloid precursor protein mediates neuronal cell–cell and cell-surface adhesion. J Neurosci Res. 1991;28:90–100. doi: 10.1002/jnr.490280109. [DOI] [PubMed] [Google Scholar]
- 7.Caceres A, Banker GA, Steward O, Binder L, Payne M. MAP2 is localized to the dendrites of hippocampal neurons which develop in culture. Dev Brain Res. 1984;13:314–318. doi: 10.1016/0165-3806(84)90167-6. [DOI] [PubMed] [Google Scholar]
- 8.Canoll PD, Musacchio JM, Hardy R, Reynolds R, Marchionni MA, Salzer JL. GGF/neuregulin is a neuronal signal that promotes the proliferation and survival and inhibits the differentiation of oligodendrocyte progenitors. Neuron. 1996;17:229–243. doi: 10.1016/s0896-6273(00)80155-5. [DOI] [PubMed] [Google Scholar]
- 9.Chin L-S, Lian L, Ferreira A, Kosik K, Greengard P. Impairment of axonal development and of synaptogenesis in hippocampal neurons of synapsin I-deficient mice. Proc Natl Acad Sci USA. 1995;92:9230–9234. doi: 10.1073/pnas.92.20.9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira A, Caceres A. Expression of the class III β-tubulin isotype in developing neurons in culture. J Neurosci Res. 1992;32:516–529. doi: 10.1002/jnr.490320407. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira A, Caceres A, Kosik KS. Intraneuronal compartments of the amyloid precursor protein. J Neurosci. 1993;13:3112–3123. doi: 10.1523/JNEUROSCI.13-07-03112.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fields RD, Itoh K. Neural cell adhesion molecules in activity-dependent development and synaptic plasticity. Trends Neurosci. 1996;19:473–480. doi: 10.1016/S0166-2236(96)30013-1. [DOI] [PubMed] [Google Scholar]
- 14.Frankfurter A, Binder LI, Rebhun L. Limited tissue distribution of a novel β-tubulin isoform. J Cell Biol. 1986;103:273. [Google Scholar]
- 15.Goodman Y, Mattson MP. Secreted forms of β-amyloid precursor protein protect hippocampal neurons against amyloid β-peptide-induced oxidative injury. Exp Neurol. 1994;128:1–12. doi: 10.1006/exnr.1994.1107. [DOI] [PubMed] [Google Scholar]
- 16.Haass C, Hung AY, Selkoe DJ. Processing of β-amyloid precursor protein in microglia and astrocytes favors an internal localization over constitutive secretion. J Neurosci. 1991;11:3783–3793. doi: 10.1523/JNEUROSCI.11-12-03783.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hung AY, Koo EH, Haass C, Selkoe DJ. Increased expression of β-amyloid precursor protein during neuronal differentiation is not accompanied by secretory cleavage. Proc Natl Acad Sci USA. 1992;89:9439–9443. doi: 10.1073/pnas.89.20.9439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin L-W, Ninomiya H, Roch J-M, Schubert D, Masliah E, Otero DA, Saitoh T. Peptides containing the RERMS sequence of amyloid β-protein precursor bind cell surface and promote neurite extension. J Neurosci. 1994;14:5461–5470. doi: 10.1523/JNEUROSCI.14-09-05461.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang J, Lemaire H, Unterbeck A, Salbaum JM, Masters CL, Grzeschik K, Multhaup G, Beyreuther K, Muller-Hill B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 20.Koo EH, Park L, Selkoe DJ. Amyloid β-protein as a substrate interacts with extracellular matrix to promote neurite outgrowth. Proc Natl Acad Sci USA. 1993;90:4748–4752. doi: 10.1073/pnas.90.10.4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeBlanc AC, Kovacs DM, Chen HY, Villare F, Tykocinski M, Autilio-Gambetti L, Gambetti P. Role of amyloid precursor protein (APP): study with antisense transfection of human neuroblastoma cells. J Neurosci Res. 1992;31:635–645. doi: 10.1002/jnr.490310407. [DOI] [PubMed] [Google Scholar]
- 22.LeBlanc AC, Xue R, Gambetti P. Amyloid precursor protein metabolism in primary cell cultures of neurons, astrocytes, and microglia. J Neurochem. 1996;66:2300–2310. doi: 10.1046/j.1471-4159.1996.66062300.x. [DOI] [PubMed] [Google Scholar]
- 23.Majocha RE, Agrawal S, Tang J-Y, Humke EW, Marotta CA. Modulation of the PC12 cell response to nerve growth factor by antisense oligonucleotide to amyloid precursor protein. Cell Mol Neurobiol. 1994;14:425–437. doi: 10.1007/BF02088829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mattson MP. Secreted forms of β-amyloid precursor protein modulate dendrite outgrowth and calcium responses to glutamate in cultured embryonic hippocampal neurons. J Neurobiol. 1994;25:439–450. doi: 10.1002/neu.480250409. [DOI] [PubMed] [Google Scholar]
- 25.Mattson MP, Cheng B, Culwell A, Esch F, Lieberberg I, Rydel R. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the β-amyloid precursor protein. Neuron. 1993;10:243–254. doi: 10.1016/0896-6273(93)90315-i. [DOI] [PubMed] [Google Scholar]
- 26.Milward EA, Papadopoulos R, Fuller SJ, Moir RD, Small D, Beyreuther K, Masters CL. The amyloid protein precursor of Alzheimer’s disease is a mediator of the effects of nerve growth factor on neurite outgrowth. Neuron. 1992;9:129–137. doi: 10.1016/0896-6273(92)90228-6. [DOI] [PubMed] [Google Scholar]
- 27.Moya KL, Benowitz LI, Schneider GE, Allinquant B. The amyloid precursor protein is developmentally regulated and correlated with synaptogenesis. Dev Biol. 1994;161:597–603. doi: 10.1006/dbio.1994.1055. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto T, Takeda S, Murayama Y, Ogata E, Nishimoto I. Ligand-dependent G-protein coupling function of amyloid transmembrane precursor. J Biol Chem. 1995;270:4205–4208. doi: 10.1074/jbc.270.9.4205. [DOI] [PubMed] [Google Scholar]
- 29.Qiu WQ, Ferreira A, Miller C, Koo EH, Selkoe DJ. Cell-surface β-amyloid precursor protein stimulates neurite outgrowth of hippocampal neurons in an isoform-dependent manner. J Neurosci. 1995;15:2157–2167. doi: 10.1523/JNEUROSCI.15-03-02157.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sabo S, Lambert MP, Kessey K, Wade W, Krafft G, Klein WL. Interaction of β-amyloid peptides with integrins in a human nerve cell line. Neurosci Lett. 1995;184:25–28. doi: 10.1016/0304-3940(94)11159-g. [DOI] [PubMed] [Google Scholar]
- 31.Saitoh T, Sundsmo M, Roch J-M, Kimura N, Cole G, Schenk D. Secreted form of amyloid β protein precursor is involved in the growth regulation of fibroblasts. Cell. 1989;58:615–622. doi: 10.1016/0092-8674(89)90096-2. [DOI] [PubMed] [Google Scholar]
- 32.Salbaum MJ, Ruddle FH. Embryonic expression pattern of amyloid protein precursor suggests a role in differentiation of specific subsets of neurons. J Exp Zool. 1994;269:116–127. doi: 10.1002/jez.1402690205. [DOI] [PubMed] [Google Scholar]
- 33.Schubert D, Jin L-W, Saitoh T, Cole G. The regulation of amyloid β protein precursor secretion and its modulatory role in cell adhesion. Neuron. 1989;3:689–694. doi: 10.1016/0896-6273(89)90237-7. [DOI] [PubMed] [Google Scholar]
- 34.Selkoe DJ. Alzheimer’s disease: a central role for amyloid. J Neuropathol Exp Neurol. 1994;53:438–447. doi: 10.1097/00005072-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Shivers BD, Hilbich C, Multhaup G, Salbaum M, Beyreuther K, Seeburg PH. Alzheimer’s disease amyloidogenic glycoprotein: expression pattern in rat brain suggests a role in cell contact. EMBO J. 1988;7:1365–1370. doi: 10.1002/j.1460-2075.1988.tb02952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Small DH, Nurcombe V, Reed G, Clarris H, Moir R, Beyreuther K, Master CL. A heparin-binding domain in the amyloid protein precursor of Alzheimer’s disease is involved in the regulation of neurite outgrowth. J Neurosci. 1994;14:2117–2127. doi: 10.1523/JNEUROSCI.14-04-02117.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Storey E, Beyreuther K, Masters CL. Alzheimer’s disease amyloid precursor protein on the surface of cortical neurons in primary culture co-localizes with adhesion patch components. Brain Res. 1996;735:217–231. doi: 10.1016/0006-8993(96)00608-7. [DOI] [PubMed] [Google Scholar]
- 38.Tawil N, Wilson P, Carbonetto S. Integrins in point contacts mediate cell spreading: factors that regulate integrin accumulation in point contacts vs focal contacts. J Cell Biol. 1993;120:261–271. doi: 10.1083/jcb.120.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wertkin AM, Turner RS, Pleasure SJ, Golde TE, Younkin SG, Trojanowski JQ, Lee VM-Y. Human neurons derived from a teratocarcinoma cell line express solely the 695-amino acid amyloid precursor protein and produce intracellular β-amyloid or A4 peptides. Proc Natl Acad Sci USA. 1993;90:9513–9517. doi: 10.1073/pnas.90.20.9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitson JS, Selkoe DJ, Cotman CW. Amyloid beta protein enhances the survival of hippocampal neurons in vitro. Science. 1989;243:1488–1490. doi: 10.1126/science.2928783. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto K, Miyoshi T, Yae T, Kawashima K, Araki H, Hanad K, Otero DA, Roch JM, Saitoh T. The survival of rat cerebral cortical neurons in the presence of trophic APP peptides. J Neurobiol. 1994;25:585–594. doi: 10.1002/neu.480250510. [DOI] [PubMed] [Google Scholar]
- 42.Yamazaki T, Selkoe DJ, Koo EH. Trafficking of cell surface β-amyloid precursor protein: retrograde and transcytotic transport in cultured neurons. J Cell Biol. 1995;129:431–442. doi: 10.1083/jcb.129.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamazaki T, Koo EH, Selkoe DJ. Cell surface amyloid β-protein precursor colocalizes with β1 integrins at substrate contact sites in neural cells. J Neurosci. 1997;17:1004–1010. doi: 10.1523/JNEUROSCI.17-03-01004.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yankner BA. Mechanisms of neuronal degeneration in Alzheimer’s disease. Neuron. 1996;16:921–932. doi: 10.1016/s0896-6273(00)80115-4. [DOI] [PubMed] [Google Scholar]
- 45.Yankner BA, Duffy LK, Kirschner DA. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990;250:279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- 46.Zheng H, Jiang M, Trumbauer ME, Sirinathsinghji DJ, Hopkins R, Smith DW, Heavens RP, Dawson GR, Boyce S, Conner MW, Sisodia S, Van der Ploeg L. β-Amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell. 1995;81:525–531. doi: 10.1016/0092-8674(95)90073-x. [DOI] [PubMed] [Google Scholar]