Dorsolateral Prefrontal Cortex Promotes Long-Term Memory Formation through Its Role in Working Memory Organization (original) (raw)
Abstract
Results from neuroimaging studies have shown that the dorsolateral prefrontal cortex (DLPFC) implements processes critical for organizing items in working memory (WM). Based on its role in WM, we hypothesized that the DLPFC should contribute to long-term memory (LTM) formation by strengthening associations among items that are organized in WM. We conducted an event-related functional magnetic resonance imaging (fMRI) study to test this hypothesis by investigating prefrontal activity during performance of two different WM tasks: on “rehearse” trials, participants actively maintained triplets of words during a brief delay, whereas on “reorder” trials, participants actively organized each triplet during the delay. After scanning, subjects performed an LTM test on words presented during both WM conditions. Behavioral results showed that WM processing in the reorder condition enhanced LTM by strengthening inter-item associations. fMRI results showed that DLPFC activity specifically during reorder trials was predictive of subsequent LTM. In contrast, activity in the posterior ventrolateral prefrontal cortex was predictive of LTM for words studied on both reorder and rehearse trials. These results support the view that the DLPFC contributes to LTM formation through its role in organization of information in WM.
Keywords: prefrontal, encoding, memory, organization, dorsolateral, fMRI
Introduction
Neuropsychological studies suggest that the prefrontal cortex (PFC) implements control processes that contribute to working memory (WM) and episodic long-term memory (LTM) (Shimamura, 1995; Ranganath and Knight, 2003). Neuroimaging results additionally support the idea that different prefrontal subregions differentially contribute to WM. Specifically, activation in ventrolateral prefrontal areas [ventrolateral prefrontal cortex (VLPFC)], at or near Brodmann's areas (BA) 6, 44, 45, and 47, has been observed during a variety of tasks that require WM maintenance. More dorsolateral prefrontal areas [dorsolateral prefrontal cortex (DLPFC)], at or near BA 9 and 46, are additionally recruited during tasks requiring organization of items that are active in WM (D'Esposito et al., 1999; Petrides, 2000).
Given that both WM maintenance (Greene, 1987; Davachi et al., 2001; Dobbins et al., 2004; Ranganath et al., 2005) and organizational processing (Tulving and Pearlstone, 1966; Bower, 1970; Sternberg and Tulving, 1977; Hunt and Einstein, 1981; Davachi and Wagner, 2002) promote LTM formation, both DLPFC and VLPFC activity at encoding should be correlated with successful LTM performance. Figure 1 and Table 1 summarize results from functional magnetic resonance imaging (fMRI) studies that compared lateral prefrontal activity during encoding of items that were remembered on a subsequent memory test with activity elicited by subsequently forgotten items. Of 30 studies reporting subsequent memory effects, 27 reported local maxima in the VLPFC. In contrast, only seven studies reported local maxima within the DLPFC. Furthermore, five studies reported increased DLPFC activity for subsequently forgotten than for remembered items.
Figure 1.
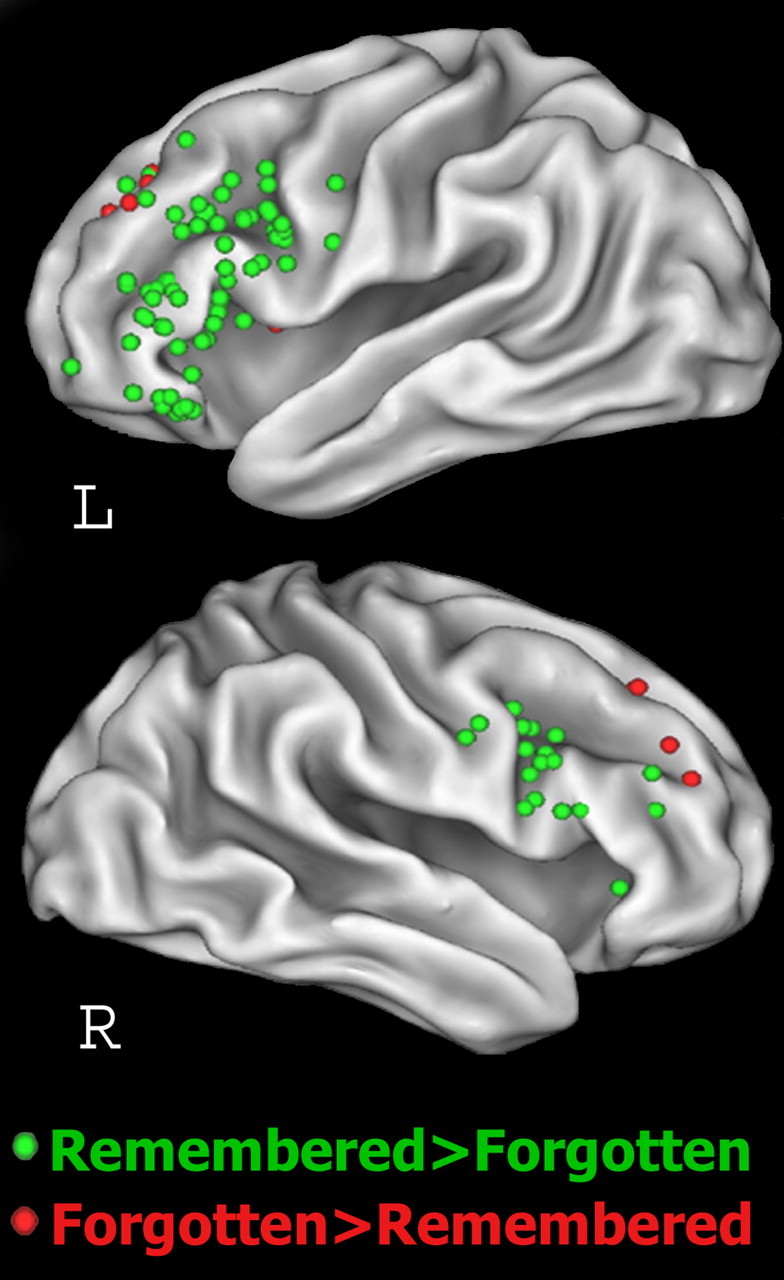
Plot of local maxima within the lateral PFC reported in 33 fMRI studies of LTM formation. Local maxima plotted in green are those associated with subsequent memory (remembered>forgotten). Local maxima plotted in red are those associated with subsequent forgetting (forgotten>remembered). Of the 116 local maxima associated with subsequent memory, only 10 fall within the DLPFC (BA 46 and 9) compared with 106 in the VLPFC (BA 6, 44, 45, and 47). In contrast, 10 of the 11 local maxima associated with subsequent forgetting fall within the DLPFC. L, Left; R, right.
Table 1.
Summary of previous event-related fMRI studies of memory formation
| Study | Prefrontal regionsa | Study descriptionb |
|---|---|---|
| Remember>forgottenc | ||
| Baker et al. (2001) | VLPFC, DLPFC | Semantic versus structural |
| Brewer et al. (1998) | DLPFC | Photographs |
| Buckner et al. (2001) | VLPFC | Encoding during retrieval |
| Chee et al. (2002) | VLPFC | Word frequency |
| Clark and Wagner (2003) | VLPFC | Novel word learning |
| Davachi et al. (2001) | VLPFC | Maintenance versus elaborative rehearsal |
| de Zubicaray et al. (2005) | VLPFC | Word frequency and strength effects |
| Dolcos et al. (2004) | VLPFC | Arousal, valence, and emotion |
| Erk et al. (2003) | VLPFC | Emotional processing |
| Fletcher et al. (2003) | VLPFC | Semantic versus phonological |
| Garoff et al. (2005) | VLPFC | Specific versus general |
| Henson et al. (1999) | VLPFC | Recollection and familiarity |
| Jackson and Schacter (2004) | VLPFC | Associative memory |
| Johnson et al. (2004) | DLPFC | Refreshing |
| Kirchhoff et al. (2000) | VLPFC | Novelty and content dependency |
| Macrae et al. (2004) | VLPFC | Self-referential processing |
| Maril et al. (2003) | VLPFC | Feeling of knowing |
| Morcom et al. (2003) | VLPFC | Effect of aging |
| Otten and Rugg (2001b) | VLPFC | Content dependency |
| Otten et al. (2002) | VLPFC | Item versus task processing |
| Ranganath et al. (2004a) | VLPFC, DLPFC | Recollection and familiarity |
| Raye et al. (2002) | DLPFC | Refreshing |
| Reber et al. (2002) | VLPFC | Encoding effort |
| Reynolds et al. (2004) | VLPFC | Item versus task processing |
| Sergerie et al. (2005) | VLPFC, DLPFC | Emotional processing of faces |
| Sommer et al. (2005) | VLPFC, DLPFC | Object—location associations |
| Sperling et al. (2003) | VLPFC | Face–name associations |
| Uncapher and Rugg (2005) | VLPFC | |
| Wagner et al. (1998) | VLPFC | Semantic processing |
| Weis et al. (2004) | VLPFC | Photographs |
| Forgotten>rememberedd | ||
| Clark and Wagner (2003) | DLPFC | Novel word learning |
| Daselaar et al. (2004) | VLPFC, DLPFC | Associative memory |
| Kensinger and Schacter (2005) | DLPFC | Reality monitoring |
| Otten and Rugg (2001a) | DLPFC | Content dependency |
| Wagner et al. (1998) | DLPFC | Semantic processing |
These neuroimaging results are consistent with at least two possibilities. One possibility is that the DLPFC implements processes that contribute to WM but do not promote successful LTM formation (Wagner and Davachi, 2001). Another possibility is that the DLPFC contributes to LTM formation, but previous studies were insensitive to detecting these contributions. Relevant to this latter possibility, virtually every previous study of LTM formation has examined encoding of individual items in isolation. We propose that the DLPFC is critical for organizing items that are currently active in WM (Ranganath and D'Esposito, 2005) and that this processing promotes LTM by strengthening inter-item associations (Tulving and Pearlstone, 1966; Bower, 1970; Bower and Winzenz, 1970; Sternberg and Tulving, 1977; Hunt and Einstein, 1981).
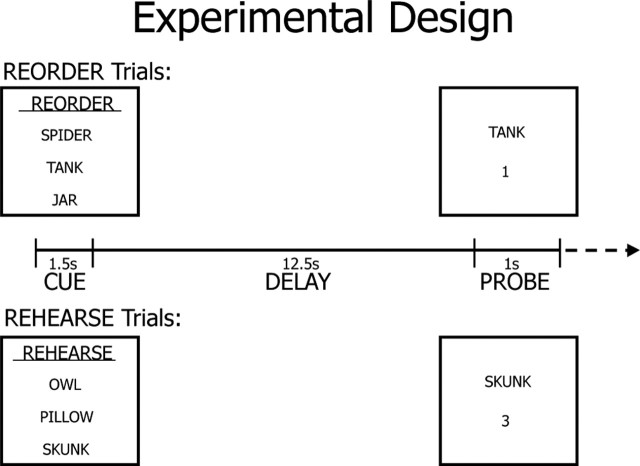
Here, we used event-related fMRI to test this hypothesis by examining prefrontal activity during two WM tasks (Fig. 2). On “rehearse” trials, participants subvocally rehearsed a list of three words across a memory delay. On “reorder” trials, participants mentally rearranged a list of three words based on the weight of the objects that the words referred to. After scanning, we administered a surprise LTM recognition test on words processed in both conditions. With this design, we were able to examine prefrontal activity related to WM processing and as a function of performance on the subsequent LTM test. Because reorder trials require participants to organize multiple items in WM, we predicted that (1) DLPFC activity during the delay period should be greater for reorder trials than for rehearse trials and (2) DLPFC activity during the delay period of reorder trials should be related to subsequent LTM performance.
Figure 2.
Example stimuli and task timing for WM trials.
Materials and Methods
Participants. Thirteen (seven males; age, 22–30 years) participants were recruited from the University of California at Davis (UC Davis) student community. Participants gave informed consent before the experiment and were paid for their participation. One participant was excluded from the reorder subsequent memory analysis, and data from one other subject was excluded from rehearse subsequent memory analysis. Each of these subjects did not have an adequate number of subsequent memory trial bins in either the reorder or the rehearse condition to allow for a meaningful analysis.
Materials. Stimuli in this experiment consisted of 504 words selected from the Medical Research Council Psycholinguistic database (http://www.psych.rl.ac.uk/MRC_Psych_Db.html). The words were 2–13 letters in length (mean, 5.5; SD, 1.8) and moderate frequency (Kucera-Francis written frequency mean 40), highly concrete (mean concreteness rating of 588), and highly imageable (mean imageability rating of 584). These words were used to construct three separate lists of 168 words matched for length, frequency, concreteness, and imageability. Two of the word lists were used for the WM tasks, whereas words from the third list were used as foils for the subsequent LTM recognition test. For the two WM word lists, unique word triplets were constructed in a psuedo-randomized manner. The authors inspected the triplets to ensure that there was no ambiguity in terms of the relative referent weight or pronunciation of the words. The two WM word lists were counterbalanced between subjects and WM condition.
Behavioral procedure. During the scanning phase, participants first performed a visuomotor response task, the results of which were used to empirically estimate a subject-specific hemodynamic response function (HRF; see below). Next, participants performed a total of 56 reorder and 56 rehearse trials (Fig. 2). On rehearse trials, participants were presented with a cue (1.5 s) containing three nouns arranged vertically, and they were instructed to subvocally rehearse the list in sequence across a 12.5 s delay. After the delay, a probe noun from the cue list and a number (1–3) were presented to the participants (1 s). Participants were to press one of two buttons on a response box to indicate whether the probe word was presented in the serial position indicated by the number. The probe word and number were arranged vertically on the screen. During the cue and delay period, the instruction word “REHEARSE” appeared underlined at the topmost region of the screen. On reorder trials, participants were presented with a cue (1.5 s) containing a column of three nouns, and they were instructed to mentally rearrange this list according to the actual weight of the objects during a 12.5 s delay. After the delay, a probe word from the cue list and a number (1–3) were presented to the participants for 1 s. Participants were to press one of two buttons on a response box to indicate whether the number corresponded to the serial position of the probe word in the rearranged list. During the cue and delay period, the instruction word “REORDER” appeared underlined at the topmost region of the screen. Each trial was followed by a variable 10–14 s intertrial interval. Seven reorder trials and seven rehearse trials were presented in a pseudo-random sequence in each scanning run. There were eight scanning runs.
After the scanning session, participants were given a surprise recognition memory test to assess LTM for all of the words that were shown as cue stimuli in the scanner (168 reorder and 168 rehearse plus 168 novel foils). In this test, participants were shown each word individually on a computer screen and were instructed to judge whether they vividly recollected the word (“remember”), felt that word was familiar although they could not recollect the word (“know”), or the word was not studied in the scanner (“new”).
MRI acquisition and processing. MRI data were collected on a 1.5T GE Signa scanner at the UC Davis Imaging Research Center. A gradient echoplanar imaging (EPI) sequence was used (repetition time, 2000 ms; echo time, 40 ms; field of view, 220; 64 × 64 matrix; voxel size, 3.4375 × 3.4375 × 5 mm) to acquire functional images, with each volume consisting of 24 axial slices. Coplanar and high-resolution T1-weighted images were also acquired. fMRI data preprocessing was performed with Statistical Parametric Mapping (SPM99) software for all subjects. EPI images were sinc interpolated in time to correct for between-slice timing differences in image acquisition, realigned using a six-parameter, rigid-body transformation algorithm, spatially normalized to the template from the International Consortium for Brain Mapping Project (Cocosco et al., 1997), resliced into 3.5 mm isotropic voxels, and spatially smoothed with an 8 mm full-width at half-maximum Gaussian filter.
MRI data analysis. Activity changes during each phase of each trial were deconvolved using multiple regression (Courtney et al., 1997; Zarahn et al., 1997; Postle et al., 2000; Rowe et al., 2000; Ranganath and D'Esposito, 2001; Munk et al., 2002; Sakai et al., 2002; Curtis et al., 2004; Ranganath et al., 2004b). The basic assumption behind this approach is that the time course of blood oxygenation level-dependent (BOLD) signal changes on any given trial can be considered as a linear combination of temporally distinct neural activity patterns that are each convolved with the HRF. More specifically, covariates modeling BOLD signal changes associated with the cue, delay, and probe periods of each trial were constructed by convolving vectors of expected neural activity associated with each of these components with a subject-specific HRF estimated from responses in the central sulcus during the visuomotor response task (Handwerker et al., 2004). Data from the visuomotor response task were not available for one subject, and for this subject, covariates were constructed by convolving the vector of expected neural activity with the “canonical” HRF included in SPM99. The onset and offset of delay period vectors were spaced apart from the cue and probe period vectors to minimize the possibility that neural activity limited to the cue and probe periods would load on the delay covariate (Zarahn et al., 1997; Ranganath and D'Esposito, 2001) (for additional details, see supplemental Fig. 1, available at www.jneurosci.org as supplemental material).
BOLD responses were modeled separately for each phase of reorder and rehearse trials. Additional analyses modeled each phase of each trial type according to subsequent memory performance. In the reorder condition, task phases of trials with high recollection (two to three words endorsed as remember in a trial) and task phases of trials with low recollection (zero to one word endorsed as remember in a trial) were modeled with separate covariates. In the rehearse condition, task phases of trials with high recognition (two to three nouns endorsed as remember or know in a trial) and task phases of trials with low recognition (zero to one noun endorsed as remember or know in a trial) were modeled with separate covariates (for the number of trials in each subsequent memory bin, see supplemental material, available at www.jneurosci.org). These covariates only modeled responses for trials that were associated with correct match/nonmatch decisions on the probe. Trials associated with incorrect WM decisions were modeled with separate covariates. Additional nuisance covariates modeled global signal changes that could not be accounted for by variables in the design matrix (Desjardins et al., 2001), trial-specific baseline shifts, and an intercept. The convolution matrix included a time-domain representation of the 1/f power structure (Aguirre et al., 1997; Zarahn et al., 1997) and filters to remove frequencies >0.25 Hz and <0.02 Hz.
After single-subject analyses, images of parameter estimates for each contrast of interest (i.e., linear combinations of regression coefficients derived from the general linear model) were entered into a second-level, one-sample t test, in which the mean estimate across participants at each voxel was tested against zero. Significant regions of activation were identified using an uncorrected one-tailed threshold of p < 0.001. For visualization purposes, thresholded statistical parametric maps were overlaid on T1-weighted images using MRIcro software (Rorden and Brett, 2000) and Caret software (Van Essen et al., 2001; Van Essen, 2002). Additional analyses were performed on regions of interest (ROIs), which were defined by selecting all contiguous, significantly active voxels in anatomically constrained areas.
Results
Behavioral results
Accuracy was high on both rehearse (97.8% correct) and reorder (92.7% correct) trials. The 5.1% performance difference between conditions was statistically significant (t(12) = 5.13; p < 0.0005). Additionally, mean reaction times (RTs) were significantly faster on rehearse (1.43 s) than on reorder (1.49 s) trials (t(12) = 2.57; p < 0.05).
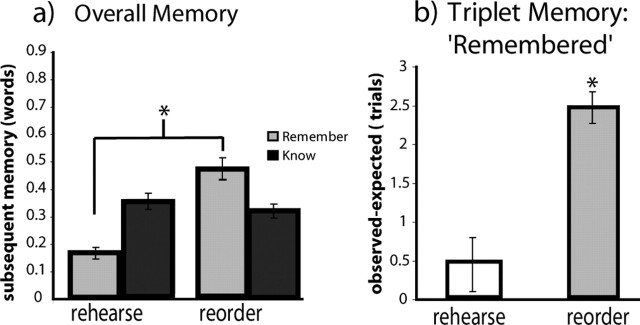
Performance on the subsequent LTM test for words encoded on rehearse and reorder trials is shown in Figure 3_a_. Of the words encoded on reorder trials, 46.5% were judged “remembered” and 31.7% were given a “know” response. Of the words encoded on rehearse trials, 16.3% were judged “remembered” and 34.7% were given a “know” response. For unstudied foil items, 3.0% were judged “remembered” and 27.4% were given a “know” response. Rates of remember (R) responses and overall hits (R+K) were significantly higher than the corresponding false-alarm rates for items studied on reorder (R rate: t(12) = 10.74, p < 0.0001; overall hit rate: _t_(12) = 11.47, _p_ < 0.0001) and rehearse (R rate: _t_(12) = 7.12, _p_ > 0.0001; overall hit rate: t(12) = 6.51, p < 0.0001) trials. Words encoded on reorder trials were significantly more likely to be judged as remembered compared with words encoded on rehearse trials (t(12) = 11.31; p < 10–7). The proportion of know responses was not significantly different between encoding conditions (t(12) = 0.90; p < 0.38).
Figure 3.
Subsequent LTM performance for words studied during reorder and rehearse trials. a, Proportion of remember (gray bars) and know (black bars) hits for words studied in reorder and rehearse conditions. Error bars depict the SEM across subjects, and the asterisk denotes a significant difference in remember rates between reorder and rehearse trials. b, Difference between observed and expected numbers of recollected triplets from each memory set. The mean difference between the observed number of trials for which all three words were successfully judged as remembered and the expected number of such trials given the overall hit rate is separately plotted for reorder and rehearse trials. A positive difference indicated that subsequent memory performance was benefited by enhanced inter-item associations. Error bars depict the SEM across subjects, and the asterisk denotes that the observed expected difference was statistically significant for reorder trials.
In addition to the analyses of overall item memory presented above, we performed analyses to characterize the number of items from a given trial that were remembered. To the extent that subjects simply encoded each word in isolation, one would expect that successful memory for each item should be independent and thus well described by the overall item hit rates. However, to the extent that subjects actively formed associations among words in the memory set, we would expect the number of trials in which all three words from the memory set were subsequently recollected (i.e., given R judgments) to exceed the expected value given by the hit rates. We predicted that organization of memory-set items during reorder trials should result in strengthened associative links between these items (Tulving and Pearlstone, 1966; Bower, 1970; Bower and Winzenz, 1970; Hunt and Einstein, 1981; Greene, 1987). To test this prediction, we calculated the proportion of trials on which we would expect subjects to successfully recollect all three words from the memory set based on the overall hit rates from the reorder and rehearse conditions (Fig. 3_b_). A positive difference between the observed versus expected proportions of recollected triplets would indicate that subsequent LTM performance was supported by enhanced associations between the elements of a memory set. Results showed that, during encoding in reorder trials, every subject showed a larger proportion of subsequently recollected word triplets than what would be expected based on the overall hit rate (sign test; p < 0.0005). In contrast, on rehearse trials, the proportion of subsequently recollected triplets was not significantly different from what would be expected based on the overall hit rates (sign test; _p_ > 0.2256). The results for rehearse trials were essentially unchanged when LTM performance was analyzed by collapsing across remember and know responses. In summary, these analyses suggest that the reorder task enhanced subsequent LTM performance, in part through strengthening of inter-item associations.
fMRI results
Our first analyses tested the hypothesis that the DLPFC is involved in organizing information in WM. Based on this hypothesis, we predicted that DLPFC activity during the delay period should be greater on reorder trials (when subjects were actively organizing a sequence of words) than on rehearse trials (when subjects were maintaining a sequence of words). To test this prediction, parameter estimates indexing early and late delay period activity were contrasted between reorder and rehearse trials. Results from this contrast, shown in Figure 4, were consistent with our prediction: bilateral regions of the DLPFC, at or around BA 9 and 46 in the middle frontal gyrus, showed increased delayperiod activation during reorder trials relative to rehearse trials. Additionally, increased delay-period activity during reorder trials was also observed in the bilateral VLPFC at or near BA 6, 44, 45, and 47 in the inferior frontal gyrus.
Figure 4.
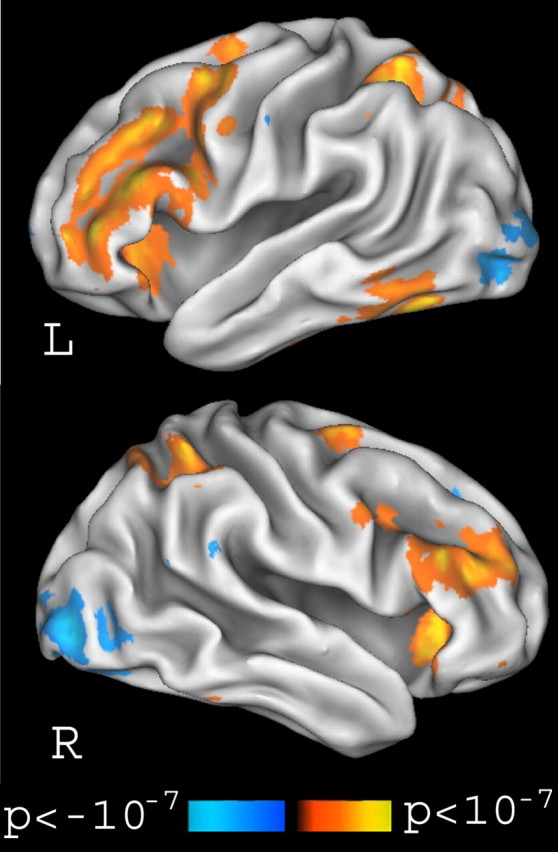
Cortical regions showing greater delay-period activity on reorder than on rehearse trials. Bilateral regions in the DLPFC (BA 9 and 46) and VLPFC (BA 44, 45, and 47) were activated in this contrast. L, Left; R, right.
The above results are consistent with the idea that the DLPFC is involved in organizing items that are active in WM. Results from previous behavioral studies, (Tulving and Pearlstone, 1966; Bower, 1970; Bower and Winzenz, 1970; Hunt and Einstein, 1981; Greene, 1987), along with the analyses of behavioral results presented above, showed that organizing items in WM facilitates LTM by strengthening inter-item associations. Accordingly, our next set of analyses tested the prediction that DLPFC activity during the delay period of reorder trials would correlate with enhancements of inter-item associations in LTM. To test this second hypothesis, we used the results from the above fMRI analyses to functionally define ROIs in the left and right DLPFC (BA 46 and 9) and examined activity in these ROIs as a function of subsequent LTM performance. Additionally, we examined activity in relatively anterior (aVLPFC: BA 45 and 47) and relatively posterior (pVLPFC: BA 6 and 44) regions within the VLPFC, because these prefrontal regions have been implicated in numerous studies of successful item memory encoding (Table 1).
To assess subsequent memory effects on reorder trials, we contrasted delay-period activity between reorder trials for which two or three words from each triplet were recollected on the subsequent LTM test (“high recollection”) and trials for which only zero or one word was recollected (“low recollection”). The reasoning behind this contrast is that organizing information in WM on reorder trials should promote LTM formation by strengthening associations between the items in each triplet. On high-recollection trials, given that participants were subsequently able to recollect details of either two or three words from a given triplet, it is likely that LTM was supported in part through inter-item associations (Mandler, 1980). Thus, if the DLPFC contributes to LTM formation through its role in organizing items in WM, we would expect delay activation to be greater on high-recollection trials than on low-recollection trials, on which memory would be less likely to be supported by inter-item associations. Trial-averaged time courses in the left hemisphere ROIs are shown in Figure 5. Consistent with our prediction, both left and right DLPFC ROIs showed greater delay-period activity for high-recollection trials relative to low-recollection trials (left: t(11) = 2.82, p > 0.05; right: t(11) = 3.73, p < 0.05). Outside of the DLPFC, we also investigated whether activity in ventrolateral prefrontal areas identified by the reorder>rehearse contrast also contributed to LTM formation on reorder trials. Results showed that ROIs in the aVLPFC and pVLPFC demonstrated greater activity on high-recollection trials than on low-recollection trials (left aVLPFC: t(11) = 3.90, p < 0.05; right aVLPFC: t(11) = 3.73, p < 0.05; left pVLPFC: t(11) = 3.45, p < 0.05).
Figure 5.
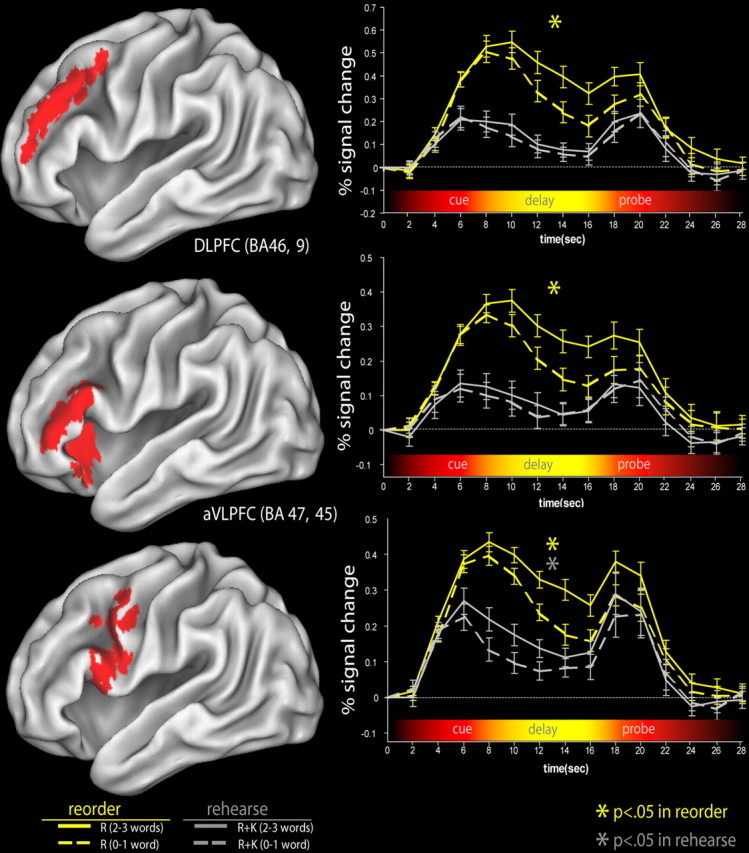
Time course of activation in prefrontal ROIs. The activity in the reorder and rehearse task is plotted separately for the left DLPFC, left aVLPFC, and left pVLPFC. These results show that delay-period activity in the DLPFC and aVLPFC was correlated with subsequent LTM performance specifically during reorder trials. In contrast, delay-period activation in the pVLPFC was predictive of subsequent LTM on both rehearse and reorder trials. The error bars in the time courses reflect the SEM at each time point for the reorder and rehearse tasks for each ROI.
The findings described above potentially suggest a role for the DLPFC in strengthening inter-item associations, but they also could reflect a role for these regions in simply enhancing item strength. To rule out this possibility, additional analyses were performed to investigate the relationship between prefrontal activation and subsequent memory for items encoded on rehearse trials. There were too few rehearse trials with two or three subsequently recollected items to conduct the same type of subsequent memory analysis that was performed for reorder trials (see supplemental material, available at www.jneurosci.org). This is not surprising, because previous studies have shown that rote maintenance rehearsal promotes memory performance primarily by building item strength in a manner that supports overall recognition, rather than recollection (Greene, 1987; Dobbins et al., 2004). We therefore investigated the relationship between prefrontal activity during the memory delay on rehearse trials and subsequent item recognition memory, collapsing across remember and know responses. Specifically, for each PFC ROI, we contrasted delay-period activation between rehearse trials for which two or three words were subsequently recognized (high recognition) with delay-period activity during rehearse trials in which one or zero words were recognized (low recognition). We reasoned that regions that contribute to LTM formation by enhancing overall item strength should be expected to show increased activation during the delay period of high-recognition trials relative to low-recognition trials.
As shown in Figure 5, DLPFC ROIs did not show activity differences between high- and low-recognition trials (left: t(11) = 1.54, p > 0.05; right: t(11) = 1.27, p > 0.05). The left and right aVLPFC and right pVLPFC did not show significant activity differences between high- and low-recognition trials (all t < 1.7; all _p_ > 0.20). However, the left pVLPFC, corresponding to BA 44 and BA 6 (t(11) = 2.78; p < 0.05) did show significantly greater activation on high-recognition trials than on low-recognition trials (Fig. 5).
The results described above suggest that left DLPFC activity was correlated with subsequent memory performance specifically on reorder trials, whereas left pVLPFC activity was correlated with subsequent memory on both reorder and rehearse trials. To statistically test whether this pattern of results reflected a dissociation between the two regions, we compared left DLPFC and left pVLPFC activation as a function of subsequent memory on rehearse trials. We performed a region (DLPFC, pVLPFC)-by-memory (high recognition, low recognition) ANOVA on delayperiod activation on rehearse trials. These analyses revealed a marginal region-by-memory interaction (F(1,11) = 3.30; p = 0.09). This finding suggests that although the DLPFC and pVLPFC exhibited different patterns of subsequent memory effects in our experiment, differences between these two regions may be more graded than absolute.
Alternative explanations for DLPFC activity
The results described above are consistent with the idea that the DLPFC can promote successful LTM formation through its role in WM organization, but they are also open to alternative interpretations. We therefore conducted additional analyses to rule out alternative accounts for the pattern of results observed in the DLPFC. For example, reorder trials were more difficult (as indexed by RT and accuracy) than rehearse trials, raising the possibility that DLPFC WM activity and subsequent memory effects could solely reflect nonspecific effects related to making a more difficult decision. Our analysis methods minimized this possibility in the following ways. First, our analyses only included trials that were associated with correct WM decisions, excluding the possibility that effects might have been driven by incorrect responses on the more difficult task. Second, our event-related design allowed us to separate activation occurring during the delay period from activation related to processing of the cue and probe stimuli. If the observed differences were related to simply making a more difficult judgment, such differences would occur during the probe phase, rather than the delay period, which was the focus of the present analyses.
We conducted additional analyses to verify that the DLPFC subsequent memory effect was not confounded with task difficulty. First, we compared RTs for reorder trials with high (2–3 R) and low (0–1 R) levels of subsequent recollection. We found no significant RT differences between the two trial types (reorder high, 1.48 s; reorder low, 1.51 s; t(11) = 0.62; p > 0.05), suggesting that reorder trials with high versus low levels of subsequent recollection were associated with equivalent levels of task difficulty. Additionally, we found no significant correlation between individual RT differences between high- and low-recollection trials and DLPFC subsequent memory effects (left DLPFC: t(11) = –0.37, p > 0.05; right DLPFC: t(11) = –1.47, p > 0.05). The results from these analyses demonstrate that DLPFC subsequent memory effects were not confounded with task difficulty.
Another potential explanation of DLPFC subsequent memory effects is that DPLFC activation might be generally associated with encoding that leads to subsequent recollection regardless of the type of process that is engaged during encoding. According to this account, the DLPFC showed a subsequent memory effect on reorder trials because these analyses contrasted activation as a function of the number of items per trial that were subsequently recollected (i.e., received an R response). To test this account, we performed two additional analyses. First, we compared delay-period activation between rehearse trials on which one or more items were subsequently given a remember response against rehearse trials that were associated with no subsequently recollected items. If DLPFC activation is associated with any form of encoding that leads to recollection, then we would expect to observe DLPFC activation in this contrast. However, this analysis revealed no significant difference in the left or right DLPFC ROI (t(11) = 0.84; p > 0.05).
To further rule out the possibility that DLPFC activation is generally associated with encoding that leads to recollection, we conducted another subsequent memory analysis on results from rehearse trials. In this analysis, we contrasted DLPFC activation on reorder trials that were associated with low levels of recollection (zero or one R response) with activation on rehearse trials with high levels of overall recognition memory (two to three items receiving R or K responses). A direct comparison revealed that these two trial types were matched with respect to the mean proportion of items associated with R responses per trial (t(11) = 0.63; p > 0.05). Accordingly, contrasting these trial types allowed us to assess DLPFC activation associated with organizational processing while controlling for differences in subsequent recollection. If DLPFC activation is associated with any form of encoding that leads to recollection, then we would not expect to find DLPFC activation in this contrast (because both trial types were associated with similar levels of subsequent recollection). If, on the other hand, DLPFC activation specifically reflects the engagement of organizational processing during encoding, we would expect to see activation in this contrast. Consistent with the latter view, delay-period activation in the left DLPFC ROI was significantly higher for reorder than for rehearse trials (t(12) = 2.90; p < 0.01), although the trial types were matched for subsequent recollection. Together, these results rule out the possibility that DLPFC activity during encoding is generally related to subsequent recollection and supports the view that the DLPFC more specifically supports subsequent memory through its role in WM organization.
Finally, a third potential explanation of DLPFC subsequent memory effect is that it was driven by the demand to refresh previously active representations on reorder trials (Raye et al., 2002; Johnson et al., 2004). If the DLPFC contributes to LTM formation through refreshing, we would expect that DLPFC activation during the early part of the delay period of rehearse trials should be correlated with subsequent LTM performance. To test this hypothesis, we examined subsequent memory effects on rehearse trials separately for covariates modeling activity during the early and late delay periods. These analyses did not reveal reliable subsequent memory effects during either task period in the left (early delay: t(11) = 1.37, p > 0.05; late delay: t(11) = 1.38, p > 0.05) or right (early delay: t(11) = 1.39, p > 0.05; late delay: t(11) = 1.29, p > 0.05) DLPFC ROIs. This suggests that refreshing in the absence of organization is not sufficient to elicit subsequent memory effects in the DLPFC.
Discussion
We tested the hypothesis that the DLPFC promotes successful LTM formation by virtue of its role in WM organization. By “organization” we mean modifying preexisting relationships or establishing a new relationship among items that are currently active in WM. Consistent with our hypotheses, we observed the following: (1) DLPFC activity was disproportionately increased during the delay period of reorder trials, relative to rehearse trials; (2) DLPFC activity during the delay period of reorder trials was greater for trials in which two to three items were later recollected compared with trials in which zero or one item was later recollected; and (3) DLPFC activity during rehearse trials that did not emphasize organizational processing was not significantly related to subsequent LTM performance.
DLPFC and WM processing
Our findings are consistent with several studies that reported increased DLPFC activation during “manipulation” tasks that required participants to modify the sequential relationships among items that are currently active in WM (D'Esposito et al., 1999, 2000; Postle et al., 1999; Wagner et al., 2001b; Barde and Thompson-Schill, 2002). DLPFC activation has also been reported in studies investigating “chunking,” which involves processing of relationships to build higher-level groupings among items that are active in WM (Bor et al., 2004). Reasoning tasks, which entail extraction and integration of relationships among verbal propositions (Bunge et al., 2005), mathematical operations (Prabhakaran et al., 2001), or complex visual objects (Christoff et al., 2001; Kroger et al., 2002) active in WM have also been shown to activate the DLPFC. These findings highlight the fact that the DLPFC is consistently active during tasks that require the establishment or modification of relationships among items in WM.
DLPFC activity and LTM formation
Previous neuroimaging studies of LTM formation have examined a range of factors, including semantic processing, phonological control, self-relevance, item-versus state-related processes, accessibility, and emotional valence (Table 1). These studies have consistently reported VLPFC subsequent memory effects, but few have reported DLPFC subsequent memory effects. Most previous studies of memory encoding used tasks that did not require organization and examined encoding of items in isolation. Additionally, some studies that examined encoding of item pairs (Sperling et al., 2003; Prince et al., 2005) required participants to a compare the items in each pair but did not require them to modify or form a new relationship. Our results demonstrate that the DLPFC contributes to LTM formation when participants must organize items during encoding.
One previous fMRI study by Davachi et al. (2001) examined the relationship between WM organization and subsequent LTM performance. In their study, as in ours, DLPFC and VLPFC activation was increased during organization of items in WM, relative to trials in which items were maintained. However, Davachi et al. (2001) did not report a significant relationship between DLPFC activity and subsequent LTM performance. Because their study was primarily intended to investigate neural mechanisms of WM maintenance, there are several key differences between their experiment and the present study. Methodological differences in the types of WM and LTM tests that were used, and the procedures used for analyzing behavioral and imaging results, could account for the discrepancy between Davachi et al. (2001) and the present findings.
As noted above, DLPFC activation during encoding has sometimes been associated with subsequent forgetting. These effects have been interpreted to reflect task-irrelevant processing that is deleterious to LTM formation (Otten and Rugg, 2001a; Wagner and Davachi, 2001) or activity changes in networks that, when deactivated, support successful memory formation (Daselaar et al., 2004). We propose that DLPFC activation can be correlated with subsequent remembering or forgetting, depending on the particular encoding and retrieval conditions that are being investigated. During encoding tasks that emphasize the distinctiveness of single items, incidental processing of inter-item relationships would be task irrelevant and potentially counterproductive. Additionally, if subjects are tested under conditions with high similarity between foils and previously studied items, encoding of inter-item relationships might not support accurate discrimination. In these instances, DLPFC activity during encoding could be associated with item forgetting. Additional research will be necessary to test this hypothesis.
It is important to note that the reorder and rehearse trials differed in a number of ways, potentially raising concerns that the results in the DLPFC could be attributed to processes other than organization. One possibility is that semantic or mental imagery processes occurring during reorder might account for the subsequent memory effect in the DLPFC. This is unlikely given that studies investigating encoding during semantic (Wagner et al., 1998; Davachi et al., 2001; Fletcher and Henson, 2001) or imageability (Chee et al., 2004; Garoff et al., 2005) judgments on one or more items do not find DLPFC subsequent memory effects.
Another possibility is that DLPFC activation reflects encoding processes that lead to recollection regardless of the type of process that is engaged. However, our analyses showed that DLPFC activation was not associated with recollection in the rehearse condition and that it was greater on reorder than rehearse trials even after subsequent recollection was equated. Additionally, several studies have failed to observe DLPFC subsequent memory effects associated with subsequent recollection (Davachi and Wagner, 2002; Davachi et al., 2003; Sperling et al., 2003; Jackson and Schacter, 2004; Ranganath et al., 2004a; Prince et al., 2005). These findings cast doubt on the idea that the DLPFC is generally associated with encoding that leads to recollection.
A third possibility is that DLPFC activation was driven by nonspecific factors correlated with task difficulty (as measured by RT and accuracy). However, the RT on reorder trials did not differ as a function of subsequent memory performance, nor did it correlate with DLPFC subsequent memory effects. These findings are consistent with studies that have reported increased DLPFC activation in contrasts between conditions that were matched for difficulty (Cabeza et al., 2002) or during performance of the less difficult of two tasks (Braver and Bongiolatti, 2002; Bor et al., 2004). Additionally, in the extant encoding literature, four of the five encoding studies that reported significant accuracy differences between encoding tasks do not report DLPFC subsequent memory effects in either the more difficult or the relatively easier condition (Chee et al., 2003; Clark and Wagner, 2003; Reynolds et al., 2004; de Zubicaray et al., 2005) (but see Baker et al., 2001).
A final possibility is that the DLPFC subsequent memory effect was driven by the fact that reorder trials forced subjects to repeatedly refresh previously active representations (Raye et al., 2002; Johnson et al., 2004). However, refreshing was also necessary during the early delay period of rehearse trials, and yet DLPFC activity during this period was not correlated with subsequent memory performance. This finding argues against the strong hypothesis that refreshing in the absence of organization can drive DLPFC subsequent memory effects. Nonetheless, it is possible that the DLPFC works with pVLPFC to coordinate refreshing in the service of organization.
VLPFC subregions involved in encoding
Several neuroimaging studies have implicated the left VLPFC in linguistic control processes (Wagner et al., 2001a; Barde and Thompson-Schill, 2002; Gold and Buckner, 2002). According to one account (Poldrack et al., 1999; Wagner et al., 2001a), the left aVLPFC (BA 45 and 47) implements controlled retrieval and maintenance of semantic information, whereas the left pVLPFC (BA 6 and 44) implements controlled retrieval and maintenance of phonological information [for alternative viewpoints, see Barde and Thompson-Schill (2002) and Gold and Buckner (2002)]. Consistent with this model, we found that the left pVLPFC exhibited delay-period activation during both rehearse and reorder trials (both engaged phonological processing), whereas the left aVLPFC exhibited delay-period activation specifically during reorder trials (which additionally engaged semantic processing). Our results also showed that delay-period activation in the left pVLPFC predicted subsequent memory in both reorder and rehearse trials, whereas delay-period activation in the left aVLPFC specifically predicted subsequent memory on reorder trials. This pattern of results is consistent with findings dissociating anterior and posterior VLPFC subregions (Wagner et al., 2000, 2001a; Otten et al., 2001) and links the roles of these regions in WM with their roles in promoting effective LTM formation (Wagner, 1999).
Finally, it should be emphasized that, although our review and empirical results are consistent with the idea that different prefrontal regions implement different processes, we did not observe strong evidence for dissociations between these regions. The present study was not explicitly designed to dissociate the DLPFC and VLPFC, and indeed WM tasks involving manipulation/organization recruit both of these regions (D'Esposito et al., 1999; Postle and D'Esposito, 1999; Postle et al., 1999). Such results are consistent hierarchical models in which DLPFC processes are implemented through its connections with the VLPFC and posterior cortical areas (Fuster, 1985).
Conclusion
In conclusion, our findings are consistent with the hypothesis that the DLPFC contributes to LTM formation through its role in WM organization. These results converge with neuropsychological studies implicating the DLPFC in strategic organization during encoding (Dellarocchetta and Milner, 1993; Gershberg and Shimamura, 1995) and highlight the important link between WM control processes and LTM formation (Wagner, 1999; Buckner, 2003; Ranganath et al., 2003).
Footnotes
This work was supported by National Institutes of Health Grants RO1 MH068721 and P01NS40813. We thank Teodora Dimtcheva, Sara Trefethen, and Aaron Heller for assistance in data analysis and behavioral piloting. We thank Silvia Bunge, Andrew Yonelinas, Neal Kroll, Carter Wendelken, Mike X. Cohen, Craig Brozinsky, and Linda Murray for lively discussions and helpful feedback.
Correspondence should be addressed to Robert S. Blumenfeld, Center for Neuroscience, University of California at Davis, 1544 Newton Court, Davis, CA 95616. E-mail: roblume@ucdavis.edu.
DOI:10.1523/JNEUROSCI.2353-05.2006
Copyright © 2006 Society for Neuroscience 0270-6474/06/260916-10$15.00/0
References
- Aguirre GK, Zarahn E, D'Esposito M (1997) Empirical analyses of BOLD fMRI statistics. II. Spatially smoothed data collected under null-hypothesis and experimental conditions. NeuroImage 5: 199–212. [DOI] [PubMed] [Google Scholar]
- Baker JT, Sanders AL, Maccotta L, Buckner RL (2001) Neural correlates of verbal memory encoding during semantic and structural processing tasks. NeuroReport 12: 1251–1256. [DOI] [PubMed] [Google Scholar]
- Barde LH, Thompson-Schill SL (2002) Models of functional organization of the lateral prefrontal cortex in verbal working memory: evidence in favor of the process model. J Cogn Neurosci 14: 1054–1063. [DOI] [PubMed] [Google Scholar]
- Bor D, Cumming N, Scott CE, Owen AM (2004) Prefrontal cortical involvement in verbal encoding strategies. Eur J Neurosci 19: 3365–3370. [DOI] [PubMed] [Google Scholar]
- Bower GH (1970) Imagery as a relational organizer in associative learning. J Verb Learn Verb Behav 9: 529–533. [Google Scholar]
- Bower GH, Winzenz D (1970) Comparison of associative learning strategies. Psychon Sci 20: 119–120. [Google Scholar]
- Braver TS, Bongiolatti SR (2002) The role of frontopolar cortex in subgoal processing during working memory. NeuroImage 15: 523–536. [DOI] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD (1998) Making memories: brain activity that predicts how well visual experience will be remembered. Science 281: 1185–1187. [DOI] [PubMed] [Google Scholar]
- Buckner RL (2003) Functional-anatomic correlates of control processes in memory. J Neurosci 23: 3999–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Wheeler ME, Sheridan MA (2001) Encoding processes during retrieval tasks. J Cogn Neurosci 13: 406–415. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wendelken C, Badre D, Wagner AD (2005) Analogical reasoning and prefrontal cortex: evidence for separable retrieval and integration mechanisms. Cereb Cortex 15: 239–249. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Dolcos F, Graham R, Nyberg L (2002) Similarities and differences in the neural correlates of episodic memory retrieval and working memory. NeuroImage 16: 317–330. [DOI] [PubMed] [Google Scholar]
- Chee MW, Hon NH, Caplan D, Lee HL, Goh J (2002) Frequency of concrete words modulates prefrontal activation during semantic judgments. NeuroImage 16: 259–268. [DOI] [PubMed] [Google Scholar]
- Chee MW, Westphal C, Goh J, Graham S, Song AW (2003) Word frequency and subsequent memory effects studied using event-related fMRI. NeuroImage 20: 1042–1051. [DOI] [PubMed] [Google Scholar]
- Chee MW, Goh JO, Lim Y, Graham S, Lee K (2004) Recognition memory for studied words is determined by cortical activation differences at encoding but not during retrieval. NeuroImage 22: 1456–1465. [DOI] [PubMed] [Google Scholar]
- Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, Gabrieli JD (2001) Rostrolateral prefrontal cortex involvement in relational integration during reasoning. NeuroImage 14: 1136–1149. [DOI] [PubMed] [Google Scholar]
- Clark D, Wagner AD (2003) Assembling and encoding word representations: fMRI subsequent memory effects implicate a role for phonological control. Neuropsychologia 41: 304–317. [DOI] [PubMed] [Google Scholar]
- Cocosco C, Kollokian V, Kwan R, Evans A (1997) Brainweb: online interface to a 3D MRI simulated brain database. NeuroImage 5: s425. [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV (1997) Transient and sustained activity in a distributed neural system for human working memory. Nature 386: 608–611. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Rao VY, D'Esposito M (2004) Maintenance of spatial and motor codes during oculomotor delayed response tasks. J Neurosci 24: 3944–3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R (2004) When less means more: deactivations during encoding that predict subsequent memory. NeuroImage 23: 921–927. [DOI] [PubMed] [Google Scholar]
- Davachi L, Wagner AD (2002) Hippocampal contributions to episodic encoding: insights from relational and item-based learning. J Neurophysiol 88: 982–990. [DOI] [PubMed] [Google Scholar]
- Davachi L, Maril A, Wagner AD (2001) When keeping in mind supports later bringing to mind: neural markers of phonological rehearsal predict subsequent remembering. J Cogn Neurosci 13: 1059–1070. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD (2003) Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci USA 100: 2157–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellarocchetta AI, Milner B (1993) Strategic search and retrieval inhibition—the role of the frontal lobes. Neuropsychologia 31: 503–524. [DOI] [PubMed] [Google Scholar]
- Desjardins AE, Kiehl KA, Liddle PF (2001) Removal of confounding effects of global signal in functional MRI analyses. NeuroImage 13: 751–758. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Ballard D, Lease J (1999) Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn 41: 66–86. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Rypma B (2000) Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Exp Brain Res 133: 3–11. [DOI] [PubMed] [Google Scholar]
- de Zubicaray GI, McMahon KL, Eastburn MM, Finnigan S, Humphreys MS (2005) fMRI evidence of word frequency and strength effects during episodic memory encoding. Brain Res Cogn Brain Res 22: 439–450. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Kroll NE, Yonelinas AP (2004) Dissociating familiarity from recollection using rote rehearsal. Mem Cognit 32: 932–944. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R (2004) Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related fMRI study. NeuroImage 23: 64–74. [DOI] [PubMed] [Google Scholar]
- Erk S, Kiefer M, Grothe J, Wunderlich AP, Spitzer M (2003) Emotional context modulates subsequent memory effect. NeuroImage 18: 439–447. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN (2001) Frontal lobes and human memory: insights from functional neuroimaging. Brain 124: 849–881. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Stephenson CM, Carpenter TA, Donovan T, Bullmorel ET (2003) Regional brain activations predicting subsequent memory success: an event-related fMRI study of the influence of encoding tasks. Cortex 39: 1009–1026. [DOI] [PubMed] [Google Scholar]
- Fuster JM (1985) Memory in the cerebral cortex. Cambridge, MA: MIT.
- Garoff RJ, Slotnick SD, Schacter DL (2005) The neural origins of specific and general memory: the role of the fusiform cortex. Neuropsychologia 43: 847–859. [DOI] [PubMed] [Google Scholar]
- Gershberg FB, Shimamura AP (1995) Impaired use of organizational strategies in free-recall following frontal lope damage. Neuropsychologia 33: 1305–1333. [DOI] [PubMed] [Google Scholar]
- Gold BT, Buckner RL (2002) Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron 35: 803–812. [DOI] [PubMed] [Google Scholar]
- Greene RL (1987) Effects of maintenance rehearsal on human-memory. Psychol Bull 102: 403–413. [Google Scholar]
- Handwerker DA, Ollinger JM, D'Esposito M (2004) Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. NeuroImage 21: 1639–1651. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Rugg MD, Shallice T, Josephs O, Dolan RJ (1999) Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J Neurosci 19: 3962–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt RR, Einstein GO (1981) Relational and item specific information in memory. J Verb Learn Verb Behav 20: 497–514. [Google Scholar]
- Jackson III O, Schacter DL (2004) Encoding activity in anterior medial temporal lobe supports subsequent associative recognition. NeuroImage 21: 456–462. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Mitchell KJ, Raye CL, Greene EJ (2004) An age-related deficit in prefrontal cortical function associated with refreshing information. Psychol Sci 15: 127–132. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL (2005) Emotional content and reality-monitoring ability: fMRI evidence for the influences of encoding processes. Neuropsychologia 43: 1429–1443. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE (2000) Prefrontal-temporal circuitry for episodic encoding and subsequent memory. J Neurosci 20: 6173–6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroger JK, Sabb FW, Fales CL, Bookheimer SY, Cohen MS, Holyoak KJ (2002) Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: a parametric study of relational complexity. Cereb Cortex 12: 477–485. [DOI] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM (2004) Medial prefrontal activity predicts memory for self. Cereb Cortex 14: 647–654. [DOI] [PubMed] [Google Scholar]
- Mandler G (1980) Recognizing—the judgment of previous occurrence. Psychol Rev 87: 252–271. [Google Scholar]
- Maril A, Simons JS, Mitchell JP, Schwartz BL, Schacter DL (2003) Feeling-of-knowing in episodic memory: an event-related fMRI study. NeuroImage 18: 827–836. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Good CD, Frackowiak RS, Rugg MD (2003) Age effects on the neural correlates of successful memory encoding. Brain 126: 213–229. [DOI] [PubMed] [Google Scholar]
- Munk MH, Linden DE, Muckli L, Lanfermann H, Zanella FE, Singer W, Goebel R (2002) Distributed cortical systems in visual short-term memory revealed by event-related functional magnetic resonance imaging. Cereb Cortex 12: 866–876. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD (2001a) When more means less: neural activity related to unsuccessful memory encoding. Curr Biol 11: 1528–1530. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD (2001b) Task-dependency of the neural correlates of episodic encoding as measured by fMRI. Cereb Cortex 11: 1150–1160. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Henson RN, Rugg MD (2001) Depth of processing effects on neural correlates of memory encoding: relationship between findings from across- and within-task comparisons. Brain 124: 399–412. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Henson RN, Rugg MD (2002) State-related and item-related neural correlates of successful memory encoding. Nat Neurosci 5: 1339–1344. [DOI] [PubMed] [Google Scholar]
- Petrides M (2000) The role of the mid-dorsolateral prefrontal cortex in working memory. Exp Brain Res 133: 44–54. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD (1999) Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage 10: 15–35. [DOI] [PubMed] [Google Scholar]
- Postle BR, D'Esposito M (1999) “What-then-where” in visual working memory: an event-related fMRI study. J Cogn Neurosci 11: 585–597. [DOI] [PubMed] [Google Scholar]
- Postle BR, Berger JS, D'Esposito M (1999) Functional neuroanatomical double dissociation of mnemonic and executive control processes contributing to working memory performance. Proc Natl Acad Sci USA 96: 12959–12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Zarahn E, D'Esposito M (2000) Using event-related fMRI to assess delay-period activity during performance of spatial and nonspatial working memory tasks. Brain Res Brain Res Protoc 5: 57–66. [DOI] [PubMed] [Google Scholar]
- Prabhakaran V, Rypma B, Gabrieli JDE (2001) Neural substrates of mathematical reasoning: a functional magnetic resonance imaging study of neocortical activation during performance of the Necessary Arithmetic Operations Test. Neuropsychology 15: 115–127. [DOI] [PubMed] [Google Scholar]
- Prince SE, Daselaar SM, Cabeza R (2005) Neural correlates of relational memory: successful encoding and retrieval of semantic and perceptual associations. J Neurosci 25: 1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, D'Esposito M (2001) Medial temporal lobe activity associated with active maintenance of novel information. Neuron 31: 865–873. [DOI] [PubMed] [Google Scholar]
- Ranganath C, D'Esposito M (2005) Directing the mind's eye: prefrontal, inferior and medial temporal mechanisms for visual working memory. Curr Opin Neurobiol 15: 175–182. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Knight RT (2003) Prefrontal cortex and episodic memory: integrating findings from neuropsychology and event-related functional neuroimaging. In: Memory encoding and retrieval: a cognitive neuroscience perspective (Wilding E, Bussey T, eds), pp 83–99. New York: Psychology.
- Ranganath C, Johnson MK, D'Esposito M (2003) Prefrontal activity associated with working memory and episodic long-term memory. Neuropsychologia 41: 378–389. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D'Esposito M (2004a) Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia 42: 2–13. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Cohen MX, Dam C, D'Esposito M (2004b) Inferior temporal, prefrontal, and hippocampal contributions to visual working memory maintenance and associative memory retrieval. J Neurosci 24: 3917–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Cohen MX, Brozinsky CJ (2005) Working memory maintenance contributes to long-term memory formation: neural and behavioral evidence. J Cogn Neurosci 17: 994–1010. [DOI] [PubMed] [Google Scholar]
- Raye CL, Johnson MK, Mitchell KJ, Reeder JA, Greene EJ (2002) Neuroimaging a single thought: dorsolateral PFC activity associated with refreshing just-activated information. NeuroImage 15: 447–453. [DOI] [PubMed] [Google Scholar]
- Reber PJ, Siwiec RM, Gitelman DR, Parrish TB, Mesulam MM, Paller KA (2002) Neural correlates of successful encoding identified using functional magnetic resonance imaging. J Neurosci 22: 9541–9548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JR, Donaldson DI, Wagner AD, Braver TS (2004) Item- and task-level processes in the left inferior prefrontal cortex: positive and negative correlates of encoding. NeuroImage 21: 1472–1483. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M (2000) Stereotaxic display of brain lesions. Behav Neurol 12: 191–200. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE (2000) The prefrontal cortex: response selection or maintenance within working memory? Science 288: 1656–1660. [DOI] [PubMed] [Google Scholar]
- Sakai K, Rowe JB, Passingham RE (2002) Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nat Neurosci 5: 479–484. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Lepage M, Armony JL (2005) A face to remember: emotional expression modulates prefrontal activity during memory formation. NeuroImage 24: 580–585. [DOI] [PubMed] [Google Scholar]
- Shimamura AP (1995) Memory and the prefrontal cortex. Ann NY Acad Sci 769: 151–159. [DOI] [PubMed] [Google Scholar]
- Sommer T, Rose M, Weiller C, Büchel C (2005) Contributions of occipital, parietal and parahippocampal cortex to encoding of object-location associations. Neuropsychologia 43: 732–743. [DOI] [PubMed] [Google Scholar]
- Sperling R, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL, Albert M (2003) Putting names to faces: successful encoding of associative memories activates the anterior hippocampal formation. NeuroImage 20: 1400–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg RJ, Tulving E (1977) Measurement of subjective organization in free-recall. Psychol Bull 84: 539–556. [Google Scholar]
- Tulving E, Pearlstone Z (1966) Availability versus accessibility of information in memory for words. J Verb Learn Verb Behav 5: 381–391. [Google Scholar]
- Uncapher MR, Rugg MD (2005) Encoding and the durability of episodic memory: a functional magnetic resonance imaging study. J Neurosci 25: 7260–7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC (2002) Windows on the brain: the emerging role of atlases and databases in neuroscience. Curr Opin Neurobiol 12: 574–579. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH (2001) An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc 8: 443–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD (1999) Working memory contributions to human learning and remembering. Neuron 22: 19–22. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Davachi L (2001) Cognitive neuroscience: forgetting of things past. Curr Biol 11: R964–R967. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL (1998) Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science 281: 1188–1191. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Koutstaal W, Maril A, Schacter DL, Buckner RL (2000) Task-specific repetition priming in left inferior prefrontal cortex. Cereb Cortex 10: 1176–1184. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA (2001a) Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron 31: 329–338. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Bjork RA, Schacter DL (2001b) Prefrontal contributions to executive control: fMRI evidence for functional distinctions within lateral prefrontal cortex. NeuroImage 14: 1337–1347. [DOI] [PubMed] [Google Scholar]
- Weis S, Klaver P, Reul J, Elger CE, Fernandez G (2004) Temporal and cerebellar brain regions that support both declarative memory formation and retrieval. Cereb Cortex 14: 256–267. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre G, D'Esposito M (1997) A trial-based experimental design for fMRI. NeuroImage 6: 122–138. [DOI] [PubMed] [Google Scholar]