Adult-Born and Preexisting Olfactory Granule Neurons Undergo Distinct Experience-Dependent Modifications of their Olfactory Responses In Vivo (original) (raw)
Abstract
Neurogenesis continues throughout adulthood in the mammalian olfactory bulb and hippocampal dentate gyrus, suggesting the hypothesis that recently generated, adult-born neurons contribute to neural plasticity and learning. To explore this hypothesis, we examined whether olfactory experience modifies the responses of adult-born neurons to odorants, using immediate early genes (IEGs) to assay the response of olfactory granule neurons. We find that, shortly after they differentiate and synaptically integrate, the population of adult-born olfactory granule neurons has a greater population IEG response to novel odors than mature, preexisting neurons. Familiarizing mice with test odors increases the response of the recently incorporated adult-born neuron population to the test odors, and this increased responsiveness is long lasting, demonstrating that the response of the adult-born neuron population is altered by experience. In contrast, familiarizing mice with test odors decreases the IEG response of developmentally generated neurons, suggesting that recently generated adult-born neurons play a distinct role in olfactory processing. The increased IEG response is stimulus specific; familiarizing mice with a set of different, “distractor” odors does not increase the adult-born neuron population response to the test odors. Odor familiarization does not influence the survival of adult-born neurons, indicating that the changes in the population response of adult-born neurons are not attributable to increased survival of odor-stimulated neurons. These results demonstrate that recently generated adult-born olfactory granule neurons and older, preexisting granule neurons undergo contrasting experience-dependent modifications in vivo and support the hypothesis that adult-born neurons are involved in olfactory learning.
Keywords: neurogenesis, plasticity, neuronal survival, integration, olfaction, granule cell
Introduction
Although the majority of mammalian neurogenesis is completed by the perinatal period, neurogenesis continues throughout adulthood in the olfactory bulb (Altman, 1969; Lois and Alvarez-Buylla, 1994) and the hippocampal dentate gyrus (Altman and Das, 1965; Eriksson et al., 1998). Adult olfactory precursors divide primarily within the subventricular zone (SVZ), migrate tangentially through the rostral migratory stream (RMS), and then migrate radially into the granule and periglomerular layers, in which they differentiate into neurons (Lois and Alvarez-Buylla, 1994; Lois et al., 1996). Adult-born olfactory neurons display appropriate electrophysiological properties in vitro (Belluzzi et al., 2003; Carleton et al., 2003) and express immediate early genes (IEGs) in response to odors in vivo (Carlen et al., 2002; Huang and Bittman, 2002). Adult-born olfactory granule neurons appear to form reciprocal dendrodendritic synapses with mitral and tufted cells (Doetsch and Hen, 2005), the output neurons of the olfactory bulb, and receive centrifugal cortical inputs (Shepherd, 2004). Thus, the overall population of granule neurons (Yokoi et al., 1995; Mori et al., 1999) and adult-born granule neurons are thought to be important in tuning the receptive fields of olfactory output neurons (Yokoi et al., 1995; Mori et al., 1999).
Although it is conceivable that these adult-born neurons could simply replace or function similarly to developmentally generated neurons, the continuous generation (Petreanu and Alvarez-Buylla, 2002; Winner et al., 2002) of large numbers of neurons suggests that adult-born neurons serve a unique role in the olfactory bulb. Adult-born neurons extend new processes and form new synapses, potentially contributing to olfactory plasticity. Rates of neurogenesis correlate with performance on memory tasks (Nilsson et al., 1999; Shors et al., 2001; Rochefort et al., 2002) and olfactory discrimination tasks (Gheusi et al., 2000; Enwere et al., 2004), suggesting a role for adult-born neurons in learning.
Simple exposure to an odor is a powerful stimulus for rodents that results in several types of learning. Rodents exposed to an odor just once ignore similar odors and explore novel odors. Familiarization induces perceptual learning, enhancing the ability to distinguish the odor from other similar odors (Rabin, 1988; Jehl et al., 1995). Olfactory granule neurons likely play an important role in perceptual odor discrimination, and adult-born neurons might contribute elements of plasticity to the system (Cecchi et al., 2001).
To assess the response of adult-born neurons to novel and familiar odors, we examined the expression of c-fos, c-jun, and early growth response 1 (EGR-1)/zif268 in olfactory granule neurons in response to physiological odorant stimulation. IEGs are often expressed as a result of electrophysiological activation (Greenberg et al., 1986; Sagar et al., 1988) and have been used as measures of synaptic activation in a variety of neural systems (Sagar et al., 1988; Rosen et al., 1992; Pinaud et al., 2003). Olfactory IEG expression (Guthrie et al., 1993) yields patterns of odor-evoked activity similar to those measured with electrophysiological (Wilson and Leon, 1988) and optical imaging techniques (Meister and Bonhoeffer, 2001). Analysis of IEG expression allows simultaneous examination of the activity of thousands of adult-born and preexisting neurons in mice that underwent physiologically relevant stimulation while awake.
To definitively establish a role for recently generated adult-born granule neurons in olfactory learning, several criteria, similar to those necessary for implicating LTP in learning and memory (Martin et al., 2000), must be fulfilled. The first of these criteria is that tasks that induce learning should induce modifications of adult-born neurons. To understand whether the responses of adult-born neurons are modified by physiological odorant experience in vivo, we exposed mice to novel and familiar olfactory stimuli and assessed their responses using IEG expression. We show, for the first time, that adult-born neurons undergo experience-dependent modification in response to odor familiarization in alert animals.
Materials and Methods
Bromodeoxyuridine administration
Eight- to 12-week-old female C57BL/6 mice received either ∼225 mg/kg bromodeoxyuridine (BrdU) daily via drinking water or 100 mg/kg BrdU intraperitoneal injections twice daily. Mice were administered BrdU for 4 d, from day -1.5 to day +1.5; the average cell was generated on day 0 (D0). We administered BrdU via drinking water (Magavi and Macklis, 2002) when possible to avoid exposure of the mice to extraneous odorant stimulation and to minimize their pain and distress. We administered BrdU via intraperitoneal injection to standardize quantification of levels of neuronal survival in the experiments described in Figure 5.
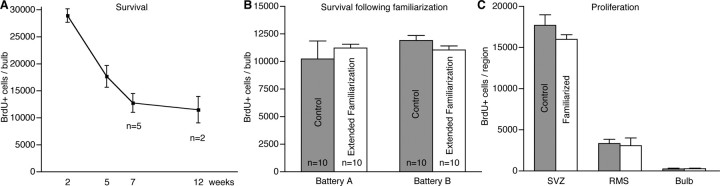
Figure 5.
Odor exposure does not influence the long-term survival of adult-born olfactory granule neurons in normal, wild-type mice, indicating that the enhanced population response observed after odor familiarization is not attributable to increased survival of odor-stimulated neurons. A, The number of adult-born granule neurons decreases during the first 2 months but is relatively stable thereafter. B, Extended familiarization to either odor battery A or odor battery B does not enhance the survival of adult-born olfactory granule neurons (data shown at 7 weeks). Control and experimental mice were housed individually under clean air conditions to limit their exposure to external odors. C, Repeated odor familiarization does not influence the proliferation of endogenous progenitors in the SVZ, RMS, or olfactory bulb. Error bars indicate SD. n = 3 mice examined, unless otherwise indicated.
Odor exposure paradigms
Novel odor stimulation. The IEG response of granule neurons was assessed in mice that had no previous experience with the test odors, “odor battery A.” Mice were administered BrdU for 4 d and allowed to mature for 1, 2, 3, 4, 5, 7, or 16 weeks. The mice were exposed to the odors as described below (see below, Odor stimulation) and perfused 1 h later (Fig. 1 A).
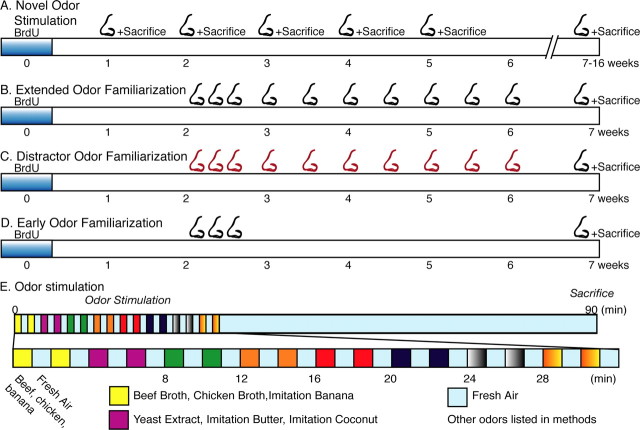
Figure 1.
Olfactory stimulation and familiarization. A, Novel odor stimulation. Mice were administered BrdU and allowed to mature, and their response to the test odors was assessed from 1 to 16 weeks later. B, Extended odor familiarization. Mice were repetitively exposed to the test odors from 2 to 6 weeks. At 7 weeks, their response to those same test odors was assessed. The black nose indicates test odors. C, Distractor odor familiarization. Mice were exposed to a distractor battery of odors, odor battery B (red nose), from 2 to 6 weeks before their response to odor battery A was assessed at 7 weeks. D, Early odor familiarization. Mice were exposed to the test odors on D15, D16, and D17 and allowed to mature for 3, 4, 5, or 7 weeks, and then their response to the test odors was assessed. E, Odor stimulation. Individually housed, alert mice received 12 L/min of clean, HEPA-filtered air (see Materials and Methods) for 8-24 h. They were exposed to eight sets of three odors. They were exposed to each set of odors for two intervals of 1 min separated by a 1 min exposure to clean air, to reduce potential effects of desensitization. They were perfused 1 h after odorant exposure to allow expression of IEGs. Odor battery A consisted of 24 primarily food-based odors, and odor battery B, the distractor odors, consisted of 24 primarily floral odors. Control mice received no olfactory stimulation.
Extended odor familiarization. To examine whether the population response of adult-born neurons is modified by experience, we familiarized mice with a battery of odors and examined the IEG response to those same odors (Fig. 1 B). Mice were repetitively exposed to either odor battery A or odor battery B from 2 to 6 weeks after BrdU administration. At 7 weeks, the population response of adult-born and preexisting granule neurons to the same battery of familiarized odors was examined. Odor battery A was used in the novel odor familiarization and extended familiarization experiments, making the results directly comparable.
Distractor odor familiarization. To examine whether extended familiarization increases the response of adult-born neurons specifically or nonspecifically, we familiarized the mice with odor battery B before testing their response to odor battery A (Fig. 1_C_). Mice were repetitively exposed to odor battery B from 2 to 6 weeks after BrdU administration. At 7 weeks, the population response of adult-born and preexisting granule neurons to odor battery A was examined.
Early odor familiarization. To examine whether the effects of odor familiarization are long lasting, we examined the responses of adult-born neurons after an early, brief period of odor familiarization (Fig. 1 D). Mice were exposed to odor battery A on D15, D16, and D17, after BrdU administration. They were allowed to mature until 3, 4, 5, or 7 weeks, and then their response to odor battery A was assessed.
Odor stimulation
In the experiments assessing the effects of novel and familiarized odors on adult-born granule neuron activation, mice were housed in groups of 3-5 mice per cage, and they were familiarized to the odorants in the presence of other mice. Mice were housed under standard conditions, with routine exposure to a variety of animal colony odors between periods of stimulation (except for the experiments described in Fig. 5_B_). To better control the odor environment, the awake and alert mice underwent the final odor stimulation individually in the absence of food and water. Mice were housed individually in microisolator cages receiving 12 L/min of humidified, HEPA-filtered air for 8-24 h to reduce background activity caused by previous odor exposure. In experiments using c-fos as a measure of activation, mice received 8-12 h of clean air. In experiments using c-jun and EGR-1/zif268 as measures of activation, mice received 24 h of clean air. EGR-1/zif268 and, in particular, c-jun had higher levels of background expression than c-fos and required a significantly longer period of clean air exposure. Control and experimental mice were odor stimulated in parallel, and their brains were fixed, sectioned, and stained together to reduce variability.
Mice were exposed to eight sets of three odors. They were exposed to each set of odors for two intervals of 1 min separated by 1 min of exposure to clean air, to reduce potential effects of desensitization. 12 L/min of HEPA-filtered humidified air was blown over filter paper moistened with the odorants. The test odors (odor battery A) consisted of McCormick (Hunt Valley, MD) food flavorings, food-based odorants, and aliphatic aldehydes, including the following: (1) McCormick Imitation Banana Flavor, Campbell's (Camden, NJ) Beef Broth, and Campbell's Chicken Broth; (2) 2 g/ml yeast extract (Sigma, St. Louis, MO), Imitation Butter, and Imitation Coconut; (3) vanilla extract, anise extract, and almond extract; (4) Imitation Rum, Imitation Brandy, and Imitation Orange; (5) lemon extract, peppermint extract, and Imitation Strawberry flavor; (6) oyster-flavored sauce (China Bowl, Westport, CT), sesame oil (China Bowl), and Root Beer Flavor; (7) Pentanal (Sigma), hexanal (Sigma), and heptanal (Sigma); (8) octanal (Sigma), nonanal (Sigma), and decanal (Sigma). Aliphatic aldehydes were dissolved 1:10 in mineral oil. Three hundred microliters of the food-based odorants and 15 μl of the aliphatic aldehydes were used to stimulate the mice.
The second battery of odors (odor battery B), used in both the alternate familiarization and the distractor familiarization experiments, consisted of qualitatively distinct floral odors of an intensity similar to the test odors. They predominantly included perfume odors from The Body Shop (Wake Forest, NC), including the following: (1) Boyajian (Canton, MA) chili oil, basil oil, and garlic oil; (2) The Body Shop Juba, Mango, and Jasmin; (3) Japanese Musk, White Musk, and Mari Mari; (4) Satsuma, balsamic vinegar, and soy sauce; (5) Cedarwood, Woody Sandal-wood, and Cucumber; (6) Oceanus, Fuzzy peach, and Tea Rose; (7) Dewberry, Lilac, and Spirit of Moonflower; (8) Patchouli, Ananya, and olive oil.
In the experiments presented in Figure 5_B_, investigating extended familiarization and survival, we housed mice in a reduced-odor environment. Mice were housed individually in positive-pressure microisolator cages receiving ∼5 L/min of HEPA-filtered air from days 7 to 49 after BrdU administration, limiting their odor exposure. Standard rodent colony conditions contain a variety of odors from other animals and strongly aromatic cleaning and antiseptic solutions, which could potentially confound the survival experiments.
Retroviral labeling of adult-born neurons
A retrovirus encoding wheat germ agglutinin (WGA), a trans-synaptic tracer, and green fluorescent protein (GFP) was used to elucidate the morphology of adult-born neurons and attempt to examine whether they formed synapses with their putative synaptic partners. WGA was cloned into the chicken matrix metalloproteinase vector (CMMP)-internal cytomegalovirus (ICMV)-internal ribosomal entry site (IRES)-enhanced GFP (eGFP) vector [gift from R. Mulligan, Harvard Medical School, Boston, MA (Klein et al., 2000)] at the 5′_Sac_II and 3′_Bgl_II restriction sites to yield CMMP-ICMV-lectin-IRES-eGFP. The internal CMV promoter within the 5′ viral long-terminal repeat and the internal ribosome entry sequence yields bicistronic expression of both WGA and eGFP after integration into the target cell genome. For viral production, 150 mm plates of 293T cells underwent standard calcium phosphate transfection with 35 μg of pMD-gag-pol, 35 μg of pMD-G, which encodes vesicular stomatitus virus protein G (Niederman et al., 2002), and 40 μg of CMMP-ICMV-lectin-IRES-eGFP. Viral supernatant was passed through a 0.45 μm filter (Nalge, Hereford, UK) 28 h after transfection, concentrated by centrifugation (SW28 rotor at 25,000 × g, 4°C, for 1.5 h), and resuspended in 250 μl of a solution containing Tris-HCl, NaCl, and ethylenediaminetetraacetic acid. The titer of concentrated viral stock on NIH 3T3 cells was ∼3 × 108 infectious particles per milliliter as determined by fluorescence-activated cell sorter analysis.
Concentrated retroviral particles (2.5 μl) were injected bilaterally into each lateral ventricle 2-6 weeks before analysis. A Drummond Scientific (Broomall, PA) Nanoject Variable oocyte injector with a glass micropipette was used to inject the retrovirus 1 mm lateral to midline, 0 mm anterior/posterior to bregma, and 1.8 mm below the surface of cortex.
Immunocytochemistry
Immunocytochemistry was performed using primary antibodies against GFP (A11122; anti-rabbit; 1:1000; Invitrogen, Eugene, OR); synaptophysin (MAB368; anti-mouse; 1:1000; Chemicon, Temecula, CA); c-fos (ab-5; anti-rabbit; 1:2500; Oncogene Research, San Diego, CA); c-jun (sc-1694; rabbit, 1:750; Santa Cruz Biotechnology, Santa Cruz, CA); EGR-1/zif-268 (sc-110; anti-rabbit; 1:1500; Santa Cruz Biotechnology); BrdU (OBT0030; anti-rat, 1:400; Accurate Chemical, Westbury, NY); and neuronal-specific nuclear protein (NeuN) (MAB 377; anti-mouse; 1:500; Chemicon). Highly cross-adsorbed secondary antibodies were used to minimize cross-reactivity. We used Invitrogen secondary antibodies, including Alexa 488 anti-rabbit (A11034; 1:250); Alexa 546 antimouse (A11030; 1:750); Alexa 488 anti-rat (A11006; 1:250); Alexa 546 anti-rabbit (A11035; 1:750); Alexa 633 anti-mouse (A21052; 1:333). Vibratome-cut sections (30-μm-thick) were incubated in primary antibodies overnight and secondary antibodies for 2 h at 4°C. Antibodies were diluted in blocking solution containing 8% goat serum, 0.3% bovine serum albumin, and 0.3% Triton-X 100 dissolved in PBS. The BrdU antigen was exposed by incubating sections in 2 m HCl for 2 h. We used Hoechst 33258 (Sigma) as a nuclear stain. We stained control and experimental sections in parallel 24 h after perfusion. Sections were mounted on gel-subbed slides and coverslipped in PBS, which preserved the three-dimensional structure of the sections and avoided compression artifacts.
Microscopy
Confocal images were obtained using a Zeiss (Oberkochen, Germany) LSM 510 laser-scanning confocal microscope. Fluorescence images were obtained using a Zeiss Axioplan and a SPOT cooled CCD digital camera.
Quantification
The population response of adult-born BrdU+ and preexisting NeuN+ neurons was assessed by observers blind to the conditions of the experiment. A randomly placed 100 μm grid was used to systematically sample a random population of BrdU+ or NeuN+ cells from every 12th section through the olfactory bulb, to determine whether they expressed IEGs. Only cells intersecting the grid lines were counted, evenly and randomly sampling cells throughout the granule neuron layer and eliminating observer bias. Cells were identified as granule neurons by their location within the granule neuron layer. To examine whether recently generated adult-born neurons responding to an odor are clustered with the preexisting neurons that respond to the odor, we examined the percentage of adult-born neurons expressing c-fos in activated regions of the granule layer and in surrounding regions. Mice were exposed to octanal, and the activated subregion was defined as encompassing the resulting group of c-_fos_-expressing cells along the dorsolateral aspect of the granule cell layer 1.8 mm posterior to the tip of the olfactory bulb. To assess the effects of olfactory experience on survival, an observer blind to the conditions of the experiment counted BrdU-positive cells in square fields 50 μm on a side, spaced 100 μm apart in every 12th section through the olfactory bulb. Partially stained or sectioned cells were not counted.
Results
Adult-born olfactory neurons are most responsive to novel odors soon after differentiating and synaptically integrating
Adult-born neurons rapidly migrate through the RMS, differentiate into neurons and integrate into the olfactory bulb (Altman, 1969; Luskin, 1993; Lois and Alvarez-Buylla, 1994; Petreanu and Alvarez-Buylla, 2002). To examine the morphology and synaptic connections of adult-born olfactory neurons, we infected adult-born cells with a bicistronic retrovirus encoding both WGA, an anterograde trans-synaptic tracer, and GFP. Adult-born olfactory granule neurons labeled with this virus migrated into the granule layer, differentiated into neurons, adopted a neuronal morphology, and extended complex processes (Fig. 2) (Altman, 1969; Luskin, 1993; Lois and Alvarez-Buylla, 1994; Petreanu and Alvarez-Buylla, 2002). We did not observe conclusive trans-synaptic WGA transport, most likely because of dilution of the WGA. Individual granule neurons form synapses with many mitral cells and may not be able to generate enough WGA to definitively label all of their synaptic partners. However, the processes of adult-born neurons exhibit varicosities that are suggestive of synaptic boutons (Fig. 2). The majority of these presumptive synaptic boutons formed by adult-born olfactory neurons are closely apposed to synaptophysin-rich presynaptic densities, supporting previous findings suggesting that adult-born olfactory granule neurons form functional synapses (Belluzzi et al., 2003; Carleton et al., 2003). Interestingly, like developmentally generated neurons (Shepherd, 2004), adult-born granule neurons appear to form synapses not only in the external plexiform layer but also in the granule cell layer, which has received somewhat less attention (Fig. 2_B,C_).
Figure 2.
Adult-born granule neurons differentiate into neurons and appear to form synapses both in the external plexiform layer and the granule cell layer itself. A, Hoechst (blue) stains nuclei (arrowheads) and reveals the granule cell layer (GCL), the mitral cell layer (MCL), and glomeruli (Glom). Intraventricular injection of a GFP-encoding (green) retrovirus labels adult-born granule neurons (arrow), which extend processes into the external plexiform layer (EPL) and the GCL and form boutons (B, arrowheads) that are closely apposed to synaptophysin (red)-rich presynaptic densities (C, dashed circles), suggesting the existence of synapses.
By 2 weeks after BrdU administration, 92 ± 1.2% (n = 2 mice; 391 ± 120 cells examined/mouse) of adult-born cells in the granule cell layer express NeuN, a mature neuronal marker; after 3 weeks, 96.6 ± 0.6% express NeuN (n = 11 mice; 3-7 weeks of age; 346 ± 95 cells examined/mouse). Thus, consistent with previous results using rigorous confocal analysis (Winner et al., 2002), almost all adult-born, BrdU-positive cells in the olfactory bulb display immunochemical markers of mature neurons. BrdU+/NeuN+ adult-born neurons express c-fos in response to odor exposure (Fig. 3_F-I_). Additionally, after odor stimulation, >99% of c-_fos_-positive cells in the granule cell layer are neurons as indicated by NeuN expression (n = 5 mice; 321 ± 63 cells examined/mouse), suggesting that non-neuronal cells make little contribution to counts of c-_fos_-positive cells (Fig. 3_H,I_). Because almost all BrdU+ cells in the granule cell layer differentiate into neurons, we assessed the neuronal population response by examining the percentage of BrdU-positive cells that express IEGs.
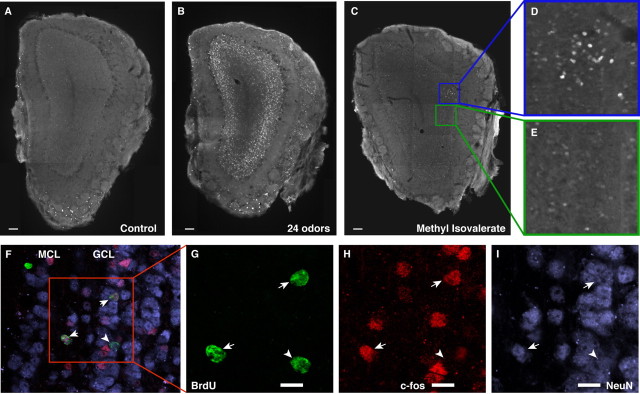
Figure 3.
Olfactory stimulation specifically increases the number of granule neurons expressing c-fos. A, Mice receiving only clean, HEPA-filtered air have very few c-_fos_-positive (white) granule neurons. The specks in the ventral glomerular layer are autofluorescent fixation artifacts and are easily distinguished from genuine cellular labeling at higher magnification. B, Novel odor exposure dramatically increases the number of c-_fos_-positive granule, mitral, and periglomerular neurons. C-E, Exposure to a single odorant, such as methyl-isovalerate, reproducibly activates specific bilaterally symmetric subregions of the olfactory bulb. D, An activated subregion at high magnification. E, An unactivated subregion immediately adjacent to D. F-I, Confocal imaging reveals that BrdU+ (green) adult-born cells in the granule layer (GCL) differentiate into mature NeuN-expressing neurons (blue) and express c-fos (red) in response to olfactory stimulation. The arrows indicate activated adult-born neurons. The arrowhead indicates an inactive adult-born neuron. Scale bar, 10 μm.
Adult-born olfactory granule neurons specifically and appropriately respond to odors in awake, alert mice (Fig. 3). To examine whether adult-born neurons respond to odors, we labeled adult-born cells, allowed the mice to mature, exposed them to a battery of odors, perfused them 1 h later, and examined their IEG expression. Odorant stimulation increases the expression of the IEGs c-fos, c-jun, and EGR-1 in both preexisting and adult-born olfactory bulb granule neurons (Fig. 3_B_). In contrast, control mice, exposed to only fresh filtered air, have few activated neurons in the granule cell layer (Fig. 3_A_). After odor stimulation, the fraction of adult-born neurons expressing c-fos is greatly increased (Fig. 4_A_)(t test, t = 16.581, p = 0.0005). Two weeks after BrdU administration, 23.3 ± 1.8% (n = 3 mice; 1103 ± 191 cells examined/mouse) of BrdU-labeled, adult-born olfactory granule neurons express c-fos in response to novel odorant stimulation. In the absence of olfactory stimulation, 2 weeks after BrdU administration, only 0.9 ± 0.3% (n = 2 mice; 1113 ± 27 cells examined/mouse) of adult-born neurons express c-fos (Fig. 4_A_). Regardless of the level of maturity of the adult-born neurons, in the absence of olfactory stimulation, very few adult-born neurons express c-fos (1.3 ± 0.5%; n = 10 mice; 2-7 weeks of age; 1100 ± 176 cells examined/mouse; ANOVA, p = 0.1677, F = 2.534, df = 9). Periglomerular neurons were also active in the presence of odors and inactive in the absence of odors.
Figure 4.
Odor familiarization specifically increases the response of adult-born neurons but depresses the response of the overall population of granule neurons. A, Adult-born neurons are activated by odor stimulation, assessed by c-fos expression. B, Adult-born neurons are most responsive soon after they synaptically integrate; as they mature, they become less responsive. Preexisting granule neurons maintain a stable response. C, Extended odor familiarization (Ext. familiar.) increases the percentage of adult-born neurons that respond to the same odors, indicating that in vivo experience modifies the response of the adult-born neuron population. In contrast, the population of preexisting neurons becomes less responsive after odor familiarization, indicating that adult-born and preexisting neurons undergo distinct modifications in response to olfactory experience. D, Assessing activity with alternate IEG markers of activation confirms that extended odor familiarization increases the response of adult-born neurons. Independent experiments, using c-jun or EGR-1/zif268, demonstrate that extended odor familiarization increases the percentage of adult-born neurons activated by the familiarized odors. E, These experience-dependent changes are not unique to one set of odors; an alternate battery of qualitatively different odors also increases the response of the population of adult-born neurons. F, Alternate odor familiarization does not increase the response of the adult-born neuron population to the test odors, demonstrating that the change in response is specific to the familiarized odors. G, Brief, early odor familiarization yields a long-lasting increase in the response of the adult-born granule neuron population. Adult-born neurons exposed to novel odors on days 15, 16, and 17, during their period of synaptic integration, have an increased response to those same odors at least 5 weeks later. H, Early familiarization immediately depresses the response of the overall granule neuron population. IEG expression examined in n = 3 mice per data point (unless otherwise indicated) in ∼1100 BrdU+ cells and ∼950 NeuN+ cells per mouse in a blinded, systematic manner (see Materials and Methods). Two sets of data are displayed as components of multiple panels for clarity of presentation; these data are illustrated in either red or green. *p < 0.05, **p < 0.01, and ***p < 0.001. Error bars indicate SD.
To further examine the specificity of the responses of the adult-born neurons, we exposed mice to a single odorant. Exposure to an individual odorant, such as methyl-isovalerate, at low concentrations induced c-fos expression in distinct and reproducible subregions of the olfactory granule layer (Fig. 3_C-E_), confirming previous findings (Guthrie et al., 1993; Guthrie and Gall, 1995). Adult-born granule neurons activated by single-odorant stimulation are clustered with activated preexisting granule neurons adjacent to activated periglomerular cells. In active subregions of the bulb, 16.3 ± 4.6% of adult-born neurons (129 cells examined in n = 5 mice) express c-fos, whereas only 2.3 ± 2.2% (1000 cells; n = 5 mice) of adult-born neurons located outside of active subregions express c-fos (t test, t = 5.965, p = 0.0003). These results confirm that adult-born olfactory granule neurons functionally integrate into circuitry of the olfactory bulb (Carlen et al., 2002; Huang and Bittman, 2002) and further demonstrate that they appropriately respond to individual odorants in vivo.
The IEG response of adult-born olfactory granule neurons to novel odors varies as a function of maturity. We administered BrdU to the mice and allowed them to mature for 1-16 weeks before exposing the alert mice to a battery of novel odorants and examining the IEG response of their adult-born granule neurons (Fig. 1_A_). One week after BrdU administration, very few BrdU-labeled adult-born neurons have migrated into the granule cell layer, and even fewer have formed any synaptic connections. At 1 week, only 0.4 ± 0.5% of adult-born neurons in the granule cell layer expressed c-fos in response to odorant stimulation (n = 3 mice; 291 ± 43 cells examined/mouse) (Fig. 4_B_). Adult-born cells migrating through the rostral migratory stream did not express detectable levels of c-fos after odor stimulation. These results demonstrate that odor exposure induces IEG expression only after synaptic integration into the olfactory bulb has begun, indicating that the IEG responses examined here are the result of synaptic activation.
Adult-born neurons are most responsive to novel odors soon after they synaptically integrate into the circuitry of the olfactory bulb. Three weeks after BrdU administration, 1 week after their initial synaptic integration, 25.6 ± 3.0% of adult-born neurons (n = 3 mice; 1276 ± 46 cells examined/mouse) respond to a battery of novel odors, as assessed by c-fos expression. The population of adult-born neurons soon becomes less responsive; 7 weeks after BrdU administration, only 14.5 ± 2.5% of adult-born neurons (n = 8 mice; 1212 ± 141 cells examined/mouse) respond to the battery of novel odorants. Changes in IEG expression as a function of maturity may not be caused only by electrophysiological differences; they could also reflect changes in the threshold for expression of IEG and thus downstream cellular responses. Adult-born neurons continue to respond to novel odors for at least 4 months after their generation (Fig. 4_B_). Thus, the population of adult-born neurons undergoes a brief period of heightened response to novel odors before its response declines to baseline levels (ANOVA, F = 46.538, df = 10, p < 0.0001). At the peak of their responsiveness, 2-3 weeks after their generation, recently generated, adult-born olfactory granule neurons are significantly more responsive to novel odors than the overall population of granule neurons (two-tailed t test, t = 3.357, p = 0.0284). These experiments, assessing the IEG response of adult-born neurons as a function of maturity, provide a baseline for our subsequent experiments investigating experience-dependent modification. All subsequent comparisons of recently generated neurons were made between birth-dated olfactory granule neurons assessed at the same level of maturity, eliminating variability attributable to developmental state.
The overall population of granule neurons maintains a stable response to the set of novel odors at all of the times examined (ANOVA, F = 0.3795, df = 6, p = 0.88) (Fig. 4_B_). These data also serve as a control, confirming that mice at each of the times examined received similar levels of odor stimulation. The overall population of granule neurons consists of both developmentally generated neurons and adult-born neurons. However, developmentally generated neurons and already mature adult-born neurons (as opposed to recently born) form the overwhelming majority of this population. The contrast between the stable IEG response of the overall population of granule neurons and the early peak responsiveness of adult-born granule neurons suggests that adult-born granule neurons have a unique role in olfactory processing and plasticity.
Odor familiarization increases the population response of adult-born neurons
To examine whether the population response of adult-born neurons is modified by experience, we familiarized mice to odor battery A, consisting of food-based odors, and examined the IEG response of granule neurons to those same odors. Control mice were not familiarized with the odors before testing their response to the odors; thus, the odors were novel for these mice. The same battery of odors was used in the novel odor-exposure experiments described above and in these familiarization experiments, making the results directly comparable.
Extended odor familiarization significantly increases the percentage of adult-born neurons that express c-fos in response to the test odors (Fig. 4_C_) (two-tailed t test, t = 9.619, p = 0.0001). By 7 weeks, 14.5 ± 2.5% of adult-born neurons respond to the battery of novel odors; however, after familiarization, 25.8 ± 2.1% of adult-born neurons respond (n = 8 mice; 1218 ± 224 cells/mouse). The response of both control and experimental mice was assessed at 7 weeks, controlling for variation in IEG expression resulting from differences in developmental state. A qualitative inspection of the samples suggested that adult-born granule neurons appear to have brighter, more intense c-fos immunostaining in response to stimulation after familiarization than they do to novel odor exposure.
In contrast to adult-born neurons, the overall granule neuron population becomes less responsive after extended odor familiarization (Woo et al., 1996; Allingham et al., 1999) (two-tailed t test, t = 5.217, p = 0.0001) (Fig. 4_C_). The overall population of granule neurons consists predominantly of perinatally generated and already mature adult-born neurons; recently generated adult-born neurons form a small fraction of all granule neurons (Petreanu and Alvarez-Buylla, 2002; Winner et al., 2002). This overall reduction in olfactory response after familiarization, which has been characterized previously using both IEG-based (Woo et al., 1996; Allingham et al., 1999) and electrophysiological techniques (Buonviso and Chaput, 2000), is thought to represent tuning of olfactory receptive ranges, resulting in a reduction of nonspecific activity. Consistent with this interpretation, we observed that, after familiarization, the pattern of activation in the granule and periglomerular layers is more distinct and less diffuse (S. S. P. Magavi, unpublished results). Our results indicate that adult-born olfactory granule neurons and preexisting neurons have contrasting responses to familiarization.
To assess the effects of familiarization on adult-born neurons using different measures of activity, we examined the effects of extended odor familiarization using both c-jun and EGR-1/zif268 expression, IEGs that are also upregulated by electrophysiological activation (Cole et al., 1989; Baba et al., 1997). Experiments using these independent markers of activation confirmed that a significantly greater percentage of adult-born neurons are activated in odor-familiarized mice than in control mice (Fig. 4_D_); thus, odor familiarization increases the adult-born granule neuron population response (two-tailed t test, c-jun, t = 5.972, p = 0.0094; EGR-1/zif268, t = 4.465, p = 0.0209). These experiments were performed independently of the c-fos experiments, in separate groups of mice. The different IEGs have different thresholds for detection and/or protein expression, contributing to the different numbers of cells activated in each case (Fig. 4_D_). These data demonstrate that the adult-born olfactory granule neuron population undergoes experience-dependent modification of its IEG response to physiological odorant stimuli in awake mice.
To examine whether familiarization with different types of odors can also increase the IEG response of adult-born neurons, we familiarized mice with an alternate battery of odors and examined their IEG response to that alternate battery of odors. The alternate set of odors, odor battery B, consists primarily of perfume-based floral odors (see Materials and Methods) that smell qualitatively different from the food- and spice-based odors in odor battery A. Theoretically, the odors used in odor battery A could have had unique properties that contribute to the changes in the adult-born neuron population response induced by familiarization. We observed the same result with battery B as with battery A: familiarization with odor battery B increased the response of the adult-born neurons to odor battery B (Fig. 4_E_) (two-tailed t test, t = 7.307, p < 0.0001; n = 5 mice per group). The dramatic qualitative differences between the two batteries of odors suggest that the increase in population IEG response observed in recently generated adult-born neurons can be induced with a variety of odors, not only those used in our experiments. Attributable simply to differences between the two odor batteries, odor battery B activated a greater percentage of the adult-born neuron population than did odor battery A in the control, novel odor exposure paradigm (unpaired t test, t = 6.372, df = 10. p < 0.001). These experiments indicate that experience-dependent modification of the adult-born neuron population response generalizes across a broad range of odors; familiarization with a variety of odors increases the IEG response of adult-born neurons to those same odors.
The effects of odor familiarization are odor-battery specific
We next examined whether the effects of odor familiarization are specific: whether extended familiarization increases the response of adult-born neurons specifically, only to the odors with which the mice are familiarized, or nonspecifically to all odors. To test the specificity of the increased adult-born neuron response, we familiarized the mice to an alternate battery of 24 different “distractor” odors, the primarily floral-based odors of odor battery B, before assessing their IEG response to the food-based test odors we used previously, in odor battery A (Fig. 1_C_). Although the two odor batteries may have some chemical overlap in component odorants, to human observers, the odor batteries smell quite different.
Extended familiarization with distractor odors does not increase the response of adult-born neurons to the test odors (t test, t = 1.088, p = 0.3081) (Fig. 4_F_). We found that 14.1 ± 3.7% of adult-born olfactory granule neurons in control mice responded to the test odors (n = 5 mice; 983 ± 59 cells examined/mouse), as did 12.1 ± 1.2% of adult-born neurons in mice familiarized with the distractor odors (n = 5 mice, 1033 ± 68 cells examined/mouse), a statistically insignificant difference (two-tailed t test, t = 1.088, p = 0.3081). The increased response of recently adult-born neurons after odor familiarization is restricted to the battery of odors used to familiarize the mice, demonstrating that adult-born neurons undergo a stimulus-specific form of experience-dependent modification.
Although adult-born neurons undergo a stimulus-specific modification after odor familiarization, preexisting granule neurons undergo a nonspecific reduction in their response after familiarization. Extended familiarization with the distractor odors results in a statistically significant decrease in the number of preexisting neurons that respond to the alternate set of “test” odors (two-tailed t test, t = 2.939, p = 0.0424) (Fig. 4_F_). This result, which is consistent with previous reports (Woo et al., 1996; Allingham et al., 1999; Buonviso and Chaput, 2000), indicates that exposure to a broad variety of odors nonspecifically decreases the response of the preexisting olfactory granule neurons. Although these effects on preexisting neurons are nonspecific, the experience-dependent modification of responses of recently generated adult-born neurons that are induced by odor familiarization is quite specific. These experiments demonstrate that recently generated adult-born neurons respond quite distinctly from preexisting granule neurons to olfactory stimuli in vivo.
The effects of odor stimulation are long lasting
To examine whether the effects of odor familiarization are long lasting, we examined the responses of adult-born neurons after an early, brief period of odor familiarization. These experiments also serve to characterize how the response of adult-born neurons evolves over time. An early, brief period of odor familiarization is sufficient to produce long-lasting changes in the population response of adult-born neurons (Fig. 4_G_). To examine the effects of early, brief olfactory familiarization, we administered BrdU to mice, familiarized them with the test odors on days 15, 16, and 17, and then examined the responses of adult-born neurons at weeks 3-7 (Fig. 1_D_). Adult-born neurons are highly responsive to novel odors on days 15-17, soon after they synaptically integrate. At week 3, there is no significant difference in the response of adult-born neurons to novel versus familiar stimuli (two-tailed t test, t = 1.129, p = 0.3221). However, as the adult-born neurons mature, their response to the familiarized odors becomes significantly greater than their response to novel odors (two-tailed t test, t = 4.403, p = 0.006). Even 7 weeks after early familiarization, 19.6 ± 2.1% of recently adult-born neurons still respond to the familiarized odors (n = 8 mice, 1195 ± 126 cells/mouse), compared with only 14.5 ± 2.6% of adult-born neurons responding to novel odors. These results demonstrate that even a brief period of odor familiarization induces long-lasting changes in the recently generated adult-born neuron population.
Early, brief odor familiarization results in an immediate decrease in the response of the overall population of granule neurons that lasts for several weeks (Fig. 4_H_). Just a few days after familiarization, at week 3, the response of the overall population of neurons is significantly decreased (t test, t = 4.520, p = 0.01). The responses of the overall population of granule neurons to novel and familiarized odors are no longer significantly different by week 7. Together, these data further illustrate that early, brief familiarization has opposite effects on recently generated adult-born neurons versus preexisting neurons.
Lack of effect of odor familiarization on neuronal survival
Activity is thought to be an important influence on the survival of immature neurons, suggesting the hypothesis that familiarization may enhance the survival of newly generated adult-born neurons. To directly test this hypothesis and investigate the effects of olfactory experience on adult-born neuron survival, we examined the survival of adult-born neurons in mice undergoing either brief or extended odor familiarization. We labeled adult-born cells with BrdU, exposed mice to a battery of odors once daily on days 15, 16, and 17 after neuron birth (Fig. 1_D_), or repeatedly from 2 to 6 weeks after neuron birth (Fig. 1_B_), and perfused the mice at week 7. Odor familiarization did not result in a significant increase in the number of adult-born neurons surviving at week 7 (ANOVA, p = 0.1740, F = 2.030, df = 14).
We hypothesized that the variety of strong odors present under normal animal facility conditions could be confounding our results. The background odors present might cause a saturating level of survival, and additional odor exposure or familiarization might not have a detectable effect. To reduce potentially confounding odor stimulation, we reared mice individually in cages receiving ∼5 L/min of HEPA-filtered air, thus limiting their odor exposure to their own food, bedding, and waste. Previous olfactory experience appears to modify the threshold for activation of neurons throughout the olfactory bulb, because previous exposure to odors significantly decreases the number of neurons activated by odor stimulation (Woo et al., 1996; Allingham et al., 1999; Buonviso and Chaput, 2000) (Fig. 4_C_). To normalize the effects of previous olfactory experience on neuronal activity, and to provide a standard threshold for activity, we exposed control and experimental mice to an alternate battery of 24 odors before administering BrdU at the beginning of the experiment. Experimental mice were exposed to a battery of 24 complex odors at least five times per week from weeks 2 through 6 and were perfused at week 7.
We did not observe a statistically significant difference in neuronal survival after odor familiarization with either of the odor batteries (Fig. 5_B_). We conducted two independent trials with odor battery A, the food-based odors, and two independent trials with odor battery B, the perfume-based odors; pooled results for each odor battery are shown (n = 5 mice in each group per trial; a total of 40 mice were examined). Our results indicate that, whereas odor familiarization strongly modifies the response of adult-born neurons, it does not significantly influence the long-term survival of adult-born neurons.
To examine the possibility that odor exposure modifies proliferation, we familiarized mice with the test odors for 5 d, administered four 50 mg/kg intraperitoneal BrdU injections at 2 h intervals, and quantified BrdU-positive cells in the olfactory granule cell layer, the RMS, and the anterior SVZ. There were no differences between the rates of proliferation in control and experimental mice (Fig. 5_C_). These results, which build on previous findings (Corotto et al., 1994; Petreanu and Alvarez-Buylla, 2002; Rochefort et al., 2002), demonstrate that odor exposure does not influence the generation of adult-born olfactory neurons in normal wild-type mice.
Discussion
We demonstrate here that the response of adult-born olfactory granule neurons is altered by olfactory experience in alert mice in a stimulus-specific manner. Adult-born neurons are most responsive to novel odors soon after they synaptically integrate, suggesting that their function changes as they mature. Familiarization with a battery of test odors increases the population response of adult-born neurons to the test odors. This increase in response is specific to the familiarized odor battery; familiarization with alternate odors does not increase the response of adult-born neurons to the test odors. Preexisting neurons, in contrast, become less responsive after odor familiarization, indicating that recently generated, adult-born neurons play a distinct role in olfactory circuitry. Together, these results demonstrate that the population of adult-born neurons undergoes experience-dependent modification in response to physiological odorant stimuli and provide additional evidence implicating adult-born neurons in olfactory learning.
Adult-born neuron response varies as a function of maturity
The population of adult-born neurons is most responsive to novel odors soon after the neurons undergo synaptic integration. Three weeks after their generation, adult-born neurons are more likely to express IEGs in response to novel odorants than are preexisting neurons. As they mature, adult-born neurons become less responsive, but they reach a plateau by ∼2 months and continue to respond to novel odors for at least 4 months. Changes in the IEG response of recently generated adult-born neurons may reflect modifications including changes in neurotransmitter receptors, electrophysiological characteristics of the neurons, or the threshold for IEG activation. These experiments form a baseline for our other experiments; familiarization-induced changes in IEG responses of adult-born neurons were assessed at the same time after neuronal birth, reducing variability attributable to developmental state. Our data indicate that adult-born granule neurons have a unique role in olfactory processing; there is a striking contrast between the stable IEG response of the overall population of granule neurons and the early peak responsiveness of adult-born neurons. Because adult-born olfactory neurons are continuously being generated, there is always a cohort of highly responsive, recently generated neurons, providing a source of plasticity in olfactory circuitry.
Our results indicate that recently generated neurons and mature neurons have differing properties in vivo and are compatible with in vitro experiments showing that these populations of neurons have differing electrophysiological properties. Recently generated periglomerular olfactory neurons have a greater Na+ current than more mature neurons (Belluzzi et al., 2003). Recently generated adult-born hippocampal granule neurons have a lower threshold for initiating action potentials and LTP than more mature neurons (Schmidt-Hieber et al., 2004). Surgically induced olfactory deprivation preferentially modifies the morphology and increases the excitability of newly generated olfactory neurons in vitro (Saghatelyan et al., 2005). Adult-born neurons also differ from neonatal neurons in their tendency to be located deeper rather than more superficially within the granule cell layer (Lemasson et al., 2005). Thus, both previous data and our results, gathered in response to physiological odor stimulation in vivo, indicate that adult-born neurons have distinctive properties.
The population response of adult-born neurons is modified by experience
Odor familiarization modifies the response properties of adult-born olfactory granule neurons in vivo. Familiarizing mice with test odors increases the response of the recently generated adult-born neuron population to the test odors. Brief exposure to the test odors is sufficient to induce long-lasting changes in the adult-born neurons' response. In these experiments, we made comparisons between adult-born neurons at the same stages of maturity, eliminating potential differences resulting from maturational influences on IEG expression. These results demonstrate, in vivo, that the response of adult-born neurons is modified by olfactory experience in alert mice.
The increased response of adult-born olfactory granule neurons after familiarization with test odors might be specific to the battery of test odors, or it might be nonspecific. In theory, environmental enrichment could lower the threshold for activation in response to all odors, or otherwise allow them to respond more strongly to any odors. However, familiarization with distractor odors does not increase the IEG response of adult-born neurons to the original test odors. These results demonstrate that the increase in adult-born neuron responsiveness is stimulus specific, strongly supporting the interpretation that adult-born neurons become modified to preferentially respond to a particular set of stimuli.
Theoretically, the increased response of adult-born granule neurons after familiarization might be solely attributable to an increased response in neurons “upstream” of the olfactory granule neurons. Increased upstream activity would lead to increased activity of all olfactory granule neurons. However, the increased response after familiarization is unique to recently generated granule neurons, and the response of preexisting granule neurons is decreased. Because recently generated granule neurons and preexisting granule neurons have contrasting responses to familiarization with the same stimuli, the changes we observed must be occurring at the level of granule neurons. The two most likely possibilities for the changes we observe in the response of adult-born neurons are that there is (1) a modification in the adult-born granule neurons themselves or (2) a synaptic modification that occurs only between mitral, tufted, or centrifugal cells and adult-born granule neurons but not between these cells and preexisting granule neurons.
Adult neurogenesis might simply contribute new neurons with the same properties as preexisting, mature neurons. These new neurons might be simply replacing older, damaged neurons or contributing greater numbers of functionally identical units to the olfactory bulb. Alternatively, recently generated adult-born neurons might make distinct contributions to olfactory circuitry. In contrast to recently generated adult-born neurons, the overall granule neuron population becomes less responsive after odor familiarization. The decreased overall response after familiarization suggests that the receptive fields of granule neurons are being tuned, reducing nonspecific activity. Thus, it is likely that olfactory bulb plasticity occurs both via integration of adult-born neurons and via modification of preexisting neurons. The contrasting effects of odor familiarization on adult-born and preexisting neurons indicate that recently generated neurons have unique properties that distinguish them from older neurons.
Can selective survival explain changes in the population response of adult-born neurons?
Neuronal activity is thought to potently enhance the survival of immature neurons, raising the possibility that odor familiarization could modify the circuitry of the olfactory bulb by increasing the survival of subsets of recently generated neurons. Adult-born olfactory neurons activated by odor exposure might be more likely to survive than inactive neurons. The population of neurons surviving after odor familiarization would then consist predominantly of neurons that respond to the familiarized odors, altering the circuitry of the olfactory bulb. Consistent with this hypothesis, odor familiarization increases the percentage of adult-born neurons that respond to the familiarized odors.
However, when we examined the survival of adult-born olfactory granule neurons in control and odor-familiarized mice, we did not find significant differences. We initially examined the survival of adult-born neurons in mice reared under normal mouse colony conditions, which involves exposure to a variety of odors from other mice, equipment, and cleaning solutions. Because background odors might be confounding our results, we repeated our experiments using mice housed individually and reared under low odor conditions. Despite using two batteries of odors in four independent trials, we did not detect any differences in granule neuron survival between control and familiarized mice. These data indicate that adult-born olfactory granule neuron survival is not centrally dependent on olfactory experience.
Intriguingly, although olfactory deprivation depresses neuronal survival, our data demonstrate that olfactory enrichment does not enhance long-term survival. Previous studies show a reduction of adult-born neuron survival in mice that are completely deprived of odor stimuli, via cauterization of the nostril (Corotto et al., 1994; Yamaguchi and Mori, 2005), olfactory receptor axotomy (Mandairon et al., 2003), or genetically modified olfactory “knock-out” mice (Petreanu and Alvarez-Buylla, 2002), and not simply reared in a reduced odor environment. Even complete odor deprivation did not completely abolish adult-born neuron survival. Odor exposure has short-term effects on neuronal integration; olfactory enrichment increases the number of adult-born neurons 3 weeks after they are generated, soon after integration, but before the majority of adult-born cell death has occurred (Rochefort et al., 2002; Miwa and Storm, 2005). A burst of neurogenesis that does not result in a long-lasting increase in neuronal survival could have an interesting role in olfactory function. Together with our data, these results indicate that a very low level of olfactory stimulation (or even spontaneous activity in normal olfactory circuitry) may be sufficient to achieve a saturating level of long-term neuronal survival; enrichment beyond this baseline may not enhance survival.
The ability of new neurons to integrate into mature neural circuitry and undergo experience-dependent plasticity might have implications for attempts to repopulate diseased neuronal circuitry in other systems. To replace neurons lost to disease, neurons generated from either endogenous (Magavi et al., 2000; Arvidsson et al., 2002; Nakatomi et al., 2002; Parent et al., 2002; Chen et al., 2004) or transplanted (Shin et al., 2000; Fricker-Gates et al., 2002) neural precursors must survive, integrate, and become functional. Environmental stimulation may be essential to promote the appropriate integration of new neurons in the adult brain.
Together, our results demonstrate that the IEG response to odors of the population of adult-born olfactory granule neurons is modified in a stimulus-specific manner in alert mice. Familiarization has opposite effects on mature, preexisting neurons and adult-born neurons, suggesting a distinct role for adult-born neurons in the olfactory bulb. These data demonstrate that the population of adult-born neurons undergoes experience-dependent modification in response to odorant stimulation, supporting the hypothesis that adult-born neurons are involved in learning and memory. Additional investigation of the functional role of adult-born neurons could broaden our understanding of how recently generated neurons integrate into complex existing circuitry of the adult brain and reveal unique roles of adult neurogenesis.
Footnotes
This work was supported by National Institutes of Health (NIH) Grants NS45523, NS41590, and NS49553 (J.D.M.), Mental Retardation Research Center Grant HD18655, an NIH predoctoral training grant, a Leopold Schepp Foundation Fellowship (S.S.P.M.), and postdoctoral fellowships from the LifeBridge Foundation and the United Sydney Association (B.D.M.).We thank Paola Arlotta, Jinhui Chen, Jason Emsley, Brad Molyneaux, Hande Ozdliner, and other members of the Macklis laboratory for helpful discussion and comments on this manuscript.
Correspondence should be addressed to Jeffrey D. Macklis, Departments of Neurosurgery and Neurology, Massachusetts General Hospital-Harvard Medical School Center for Nervous System Repair, Edwards Research Building, Mail Code EDR 410, 50 Blossom Street, Boston, MA 02114. E-mail: jeffrey_macklis@hms.harvard.edu.
Copyright © 2005 Society for Neuroscience 0270-6474/05/2510729-11$15.00/0
References
- Allingham K, Brennan PA, Distel H, Hudson R (1999) Expression of c-fos in the main olfactory bulb of neonatal rabbits in response to garlic as a novel and conditioned odour. Behav Brain Res 104: 157-167. [DOI] [PubMed] [Google Scholar]
- Altman J (1969) Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol 137: 433-457. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD (1965) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124: 319-335. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O (2002) Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 8: 963-970. [DOI] [PubMed] [Google Scholar]
- Baba K, Ikeda M, Houtani T, Nakagawa H, Ueyama T, Sato K, Sakuma S, Yamashita T, Tsukahara Y, Sugimoto T (1997) Odor exposure reveals non-uniform expression profiles of c-Jun protein in rat olfactory bulb neurons. Brain Res 774: 142-148. [DOI] [PubMed] [Google Scholar]
- Belluzzi O, Benedusi M, Ackman J, LoTurco JJ (2003) Electrophysiological differentiation of new neurons in the olfactory bulb. J Neurosci 23: 10411-10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonviso N, Chaput M (2000) Olfactory experience decreases responsiveness of the olfactory bulb in the adult rat. Neuroscience 95: 325-332. [DOI] [PubMed] [Google Scholar]
- Carlen M, Cassidy RM, Brismar H, Smith GA, Enquist LW, Frisen J (2002) Functional integration of adult-born neurons. Curr Biol 12: 606-608. [DOI] [PubMed] [Google Scholar]
- Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM (2003) Becoming a new neuron in the adult olfactory bulb. Nat Neurosci 6: 507-518. [DOI] [PubMed] [Google Scholar]
- Cecchi GA, Petreanu LT, Alvarez-Buylla A, Magnasco MO (2001) Unsupervised learning and adaptation in a model of adult neurogenesis. J Comput Neurosci 11: 175-182. [DOI] [PubMed] [Google Scholar]
- Chen J, Magavi SS, Macklis JD (2004) Neurogenesis of corticospinal motor neurons extending spinal projections in adult mice. Proc Natl Acad Sci USA 101: 16357-16362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole AJ, Saffen DW, Baraban JM, Worley PF (1989) Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature 340: 474-476. [DOI] [PubMed] [Google Scholar]
- Corotto F, Henegar J, Maruniak J (1994) Odor deprivation leads to reduced neurogenesis and reduced neuronal survival in the olfactory bulb of the adult mouse. Neuroscience 61: 739-744. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Hen R (2005) Young and excitable: the function of new neurons in the adult mammalian brain. Curr Opin Neurobiol 15: 121-128. [DOI] [PubMed] [Google Scholar]
- Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S (2004) Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci 24: 8354-8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH (1998) Neurogenesis in the adult human hippocampus. Nat Med 4: 1313-1317. [DOI] [PubMed] [Google Scholar]
- Fricker-Gates RA, Shin JJ, Tai CC, Catapano LA, Macklis JD (2002) Late-stage immature neocortical neurons reconstruct interhemispheric connections and form synaptic contacts with increased efficiency in adult mouse cortex undergoing targeted neurodegeneration. J Neurosci 22: 4045-4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheusi G, Cremer H, McLean H, Chazal G, Vincent JD, Lledo PM (2000) Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc Natl Acad Sci USA 97: 1823-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ME, Ziff EB, Greene LA (1986) Stimulation of neuronal acetyl-choline receptors induces rapid gene transcription. Science 234: 80-83. [DOI] [PubMed] [Google Scholar]
- Guthrie KM, Gall CM (1995) Functional mapping of odor-activated neurons in the olfactory bulb. Chem Senses 20: 271-282. [DOI] [PubMed] [Google Scholar]
- Guthrie KM, Anderson AJ, Leon M, Gall C (1993) Odor-induced increases in c-fos mRNA expression reveal an anatomical “unit” for odor processing in olfactory bulb. Proc Natl Acad Sci USA 90: 3329-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Bittman EL (2002) Olfactory bulb cells generated in adult male golden hamsters are specifically activated by exposure to estrous females. Horm Behav 41: 343-350. [DOI] [PubMed] [Google Scholar]
- Jehl C, Royet JP, Holley A (1995) Odor discrimination and recognition memory as a function of familiarization. Percept Psychophys 57: 1002-1011. [DOI] [PubMed] [Google Scholar]
- Klein C, Bueler H, Mulligan RC (2000) Comparative analysis of genetically modified dendritic cells and tumor cells as therapeutic cancer vaccines. J Exp Med 191: 1699-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasson M, Saghatelyan A, Olivo-Marin JC, Lledo PM (2005) Neonatal and adult neurogenesis provide two distinct populations of newborn neurons to the mouse olfactory bulb. J Neurosci 25: 6816-6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A (1994) Long-distance neuronal migration in the adult mammalian brain. Science 264: 1145-1148. [DOI] [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A (1996) Chain migration of neuronal precursors. Science 271: 978-981. [DOI] [PubMed] [Google Scholar]
- Luskin MB (1993) Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron 11: 173-189. [DOI] [PubMed] [Google Scholar]
- Magavi SS, Macklis JD (2002) Identification of newborn cells by BrdU labeling and immunocytochemistry in vivo. In: Neural stem cells: methods and protocols (Zigova T, Sanchez-Ramos J, eds), pp 283-290. Totowa, NJ: Humana. [DOI] [PubMed]
- Magavi SS, Leavitt BR, Macklis JD (2000) Induction of neurogenesis in the neocortex of adult mice. Nature 405: 951-955. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Jourdan F, Didier A (2003) Deprivation of sensory inputs to the olfactory bulb up-regulates cell death and proliferation in the subventricular zone of adult mice. Neuroscience 119: 507-516. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG (2000) Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci 23: 649-711. [DOI] [PubMed] [Google Scholar]
- Meister M, Bonhoeffer T (2001) Tuning and topography in an odor map on the rat olfactory bulb. J Neurosci 21: 1351-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa N, Storm DR (2005) Odorant-induced activation of extracellular signal-regulated kinase/mitogen-activated protein kinase in the olfactory bulb promotes survival of newly formed granule cells. J Neurosci 25: 5404-5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Nagao H, Yoshihara Y (1999) The olfactory bulb: coding and processing of odor molecule information. Science 286: 711-715. [DOI] [PubMed] [Google Scholar]
- Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M (2002) Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 110: 429-441. [DOI] [PubMed] [Google Scholar]
- Niederman TM, Ghogawala Z, Carter BS, Tompkins HS, Russell MM, Mulligan RC (2002) Antitumor activity of cytotoxic T lymphocytes engineered to target vascular endothelial growth factor receptors. Proc Natl Acad Sci USA 99: 7009-7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS (1999) Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol 39: 569-578. [DOI] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM (2002) Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol 52: 802-813. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Alvarez-Buylla A (2002) Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J Neurosci 22: 6106-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaud R, Vargas CD, Ribeiro S, Monteiro MV, Tremere LA, Vianney P, Delgado P, Mello CV, Rocha-Miranda CE, Volchan E (2003) Light-induced Egr-1 expression in the striate cortex of the opossum. Brain Res Bull 61: 139-146. [DOI] [PubMed] [Google Scholar]
- Rabin MD (1988) Experience facilitates olfactory quality discrimination. Percept Psychophys 44: 532-540. [DOI] [PubMed] [Google Scholar]
- Rochefort C, Gheusi G, Vincent JD, Lledo PM (2002) Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci 22: 2679-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen KM, McCormack MA, Villa-Komaroff L, Mower GD (1992) Brief visual experience induces immediate early gene expression in the cat visual cortex. Proc Natl Acad Sci USA 89: 5437-5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar SM, Sharp FR, Curran T (1988) Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science 240: 1328-1331. [DOI] [PubMed] [Google Scholar]
- Saghatelyan A, Roux P, Migliore M, Rochefort C, Desmaisons D, Charneau P, Shepherd GM, Lledo PM (2005) Activity-dependent adjustments of the inhibitory network in the olfactory bulb following early postnatal deprivation. Neuron 46: 103-116. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J (2004) Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429: 184-187. [DOI] [PubMed] [Google Scholar]
- Shepherd GM (2004) The synaptic organization of the brain. Oxford: Oxford UP.
- Shin JJ, Fricker-Gates RA, Perez FA, Leavitt BR, Zurakowski D, Macklis JD (2000) Transplanted neuroblasts differentiate appropriately into projection neurons with correct neurotransmitter and receptor phenotype in neocortex undergoing targeted projection neuron degeneration. J Neurosci 20: 7404-7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E (2001) Neurogenesis in the adult is involved in the formation of trace memories. Nature 410: 372-376. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Leon M (1988) Spatial patterns of olfactory bulb single-unit responses to learned olfactory cues in young rats. J Neurophysiol 59: 1770-1782. [DOI] [PubMed] [Google Scholar]
- Winner B, Cooper-Kuhn CM, Aigner R, Winkler J, Kuhn HG (2002) Long-term survival and cell death of newly generated neurons in the adult rat olfactory bulb. Eur J Neurosci 16: 1681-1689. [DOI] [PubMed] [Google Scholar]
- Woo CC, Oshita MH, Leon M (1996) A learned odor decreases the number of Fos-immunopositive granule cells in the olfactory bulb of young rats. Brain Res 716: 149-156. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Mori K (2005) Critical period for sensory experience-dependent survival of newly generated granule cells in the adult mouse olfactory bulb. Proc Natl Acad Sci USA 102: 9697-9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi M, Mori K, Nakanishi S (1995) Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc Natl Acad Sci USA 92: 3371-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]