Heterozygous FOXN1 Variants Cause Low TRECs and Severe T Cell Lymphopenia, Revealing a Crucial Role of FOXN1 in Supporting Early Thymopoiesis (original) (raw)
Abstract
FOXN1 is the master regulatory gene of thymic epithelium development. FOXN1 deficiency leads to thymic aplasia, alopecia, and nail dystrophy, accounting for the nude/severe combined immunodeficiency (nu/SCID) phenotype in humans and mice. We identified several newborns with low levels of T cell receptor excision circles (TRECs) and T cell lymphopenia at birth, who carried heterozygous loss-of-function FOXN1 variants. Longitudinal analysis showed persistent T cell lymphopenia during infancy, often associated with nail dystrophy. Adult individuals with heterozygous FOXN1 variants had in most cases normal CD4+ but lower than normal CD8+ cell counts. We hypothesized a FOXN1 gene dosage effect on the function of thymic epithelial cells (TECs) and thymopoiesis and postulated that these effects would be more prominent early in life. To test this hypothesis, we analyzed TEC subset frequency and phenotype, early thymic progenitor (ETP) cell count, and expression of FOXN1 target genes (Ccl25, Cxcl12, Dll4, Scf, Psmb11, Prss16, and Cd83) in Foxn1 nu/+ (nu/+) mice and age-matched wild-type (+/+) littermate controls. Both the frequency and the absolute count of ETP were significantly reduced in nu/+ mice up to 3 weeks of age. Analysis of the TEC compartment showed reduced expression of FOXN1 target genes and delayed maturation of the medullary TEC compartment in nu/+ mice. These observations establish a FOXN1 gene dosage effect on thymic function and identify FOXN1 haploinsufficiency as an important genetic determinant of T cell lymphopenia at birth.
Keywords: FOXN1, T cell receptor excision circles, SCID, newborn screening, T lymphocytes, thymus, thymopoiesis
Introduction
The thymus is the central organ for the development of adaptive immunity, since it houses all stages of T cell differentiation from bone marrow-derived early thymic progenitors (ETPs) to fully mature T cells ready for export to the periphery. Development of a functional thymus is strictly dependent on the expression of FOXN1, a member of the FOX family of transcription factors that regulates the development of epithelial cells in the thymus and skin.1, 2, 3 The earliest stages of thymus development from the third pharyngeal pouch are independent of Foxn1 during early stages of mouse embryogenesis (E9.5–E11 days), whereas Foxn1 expression is necessary for the development of thymic epithelial cells (TECs) from day E11.5.1 In humans, FOXN1 expression has been reported in the third pharyngeal pouch at week 6 of embryonic development, and expression throughout the thymic lobes has been documented at week 8.4 In particular, the FOXN1 protein induces the expression of genes involved in TEC differentiation and early lymphoid progenitor migration from the bone marrow, such as Ccl25, Cxcl12, Dll4, and Scf.5, 6 More recently, FOXN1 has been shown to directly regulate the expression of genes involved in antigen processing and thymocyte selection, such as Psmb11, Prss16, and Cd83.5 After the initial stages of TEC lineage specification, continued FOXN1 expression is required for all the next phases of TEC differentiation in embryonic life and early after birth, as well as in adult animals to avoid thymic involution.6, 7, 8
Biallelic loss-of-function (LoF) FOXN1 mutations result in complete athymia, congenital alopecia, and nail dystrophy in both mice and humans (MIM: 601705).9 This phenotype was originally identified in nude mice, which lack thymus and fur and have a severe combined immune deficiency (SCID) phenotype.2 A homozygous Foxn1 mutation was found to be responsible for the nude phenotype in mice, but it was only in 1996 that the first two human siblings with a similar phenotype were described10 and 3 more years were required for their homozygous FOXN1 p.Arg255∗ mutation to be identified.11 Subsequently, several additional individuals carrying homozygous FOXN1 mutations and presenting with the nude/SCID phenotype have been described.12, 13, 14, 15
Until now, heterozygous LoF FOXN1 gene variants in humans have been associated only with nail dystrophy in a proportion of individuals,16 but no immunological studies were reported in these subjects. Thymus size and weight are reduced and decrease in the ratio of medullary thymic epithelial cells (mTECs) to cortical TECs (cTECs) is accelerated in mice carrying heterozygous Foxn1 LoF mutations, similar to what is observed in wild-type mice with aging,15, 16 but without major consequences on T lymphocyte development.5, 7
We report here that heterozygous LoF FOXN1 gene variants are associated with low levels of T cell receptor excision circles (TRECs) and T cell lymphopenia at birth and increased susceptibility to infections and persistent lymphopenia during infancy affecting predominantly CD8+ T cells. Analysis of 22 adult subjects carrying heterozygous LoF FOXN1 variants revealed normal T cell counts, aside from CD8+ T cell lymphopenia, and nail dystrophy in several of them. To obtain mechanistic insights into the possible effects of FOXN1 gene dosage, we studied the thymus of Foxn1_nu/+_ mice at prenatal and postnatal age and observed a dramatic reduction in the frequency and absolute count of ETPs and a severe reduction in the expression of all main transcriptional targets of FOXN1 in the thymus during the first weeks of life, with a trend toward normalization over time. Taken together, the study of humans and mice with heterozygous FOXN1 LoF mutations indicates that FOXN1 gene dosage is most critical during embryonic and early post-natal life, so that FOXN1 haploinsufficiency causes low TRECs and T cell lymphopenia at birth that progressively improves with age.
Material and Methods
Human Subjects
Twenty-one newborns with low TREC levels on dried blood spots collected during newborn screening for SCID (P1–P21 in Tables 1 and S1) were identified and managed according to local guidelines. DNA sequencing and targeted analysis of SCID-associated genes identified heterozygous FOXN1 variants (reported in Table 1 as compared to FOXN1 RefSeq accession number GenBank: NM_003593 and NG_007260.1). Genetic studies identified heterozygous FOXN1 variants in four additional infants (P22–P25 in Table 1) with T cell lymphopenia and a history of serious infections in three of them (P22–P24). Peripheral blood samples were obtained from 5 parents (P26–P30 in Table 1) who carried the same heterozygous FOXN1 variant as their offspring and from 17 adult subjects from the village of Acerno (Italy) who had been previously found to carry the heterozygous FOXN1 p.Arg255∗ mutation.16 Written informed consent was obtained from the adult subjects and, in the case of minors, from their parents and/or legal guardians, in compliance with protocols approved by the local Institutional Review Board and the NIH protocol 18-I-0041. Normal range and median values for each peripheral blood cell subset, at different age ranges, were obtained from Shearer et al.17
Table 1.
Genetic and Clinical Features of 47 Individuals with Heterozygous FOXN1 Variants
| Pt ID | Sex | Current Age (years) | Varianta | Protein Effect | MAF | CADD Score | Thymic Shadow | Nail Dystrophy | Infections | Other Clinical Manifestations, Treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | M | 1 | c.505G>A | p.Glu169Lys | 0 | 31 | present | N | rhinovirus, enterovirus | none |
| P2 | M | 2 | c.907_907delG | p.Glu303Serfs∗247 | 0 | 35 | ND | Y | none | none |
| P3 | M | 1 | c.958C>T | p.Arg320Trp | 3.19e−5 | 35 | small | N | rhinovirus, URTI | none |
| P4 | F | 4 | c.961C>A | p.His321Asn | 0 | 29.4 | small | Y | rhinovirus, otitis | atopic dermatitis, HSCT |
| P5 | F | 3 | c.1009_1009delG | p.Gly337Glufs∗213 | 0 | 34 | ND | N | URTI | skin rash |
| P6 | M | 1 | c.1086_1087insA | p.Trp363Metfs∗118 | 0 | 34 | present | N | URTI | none |
| P7 | F | 2 | c.1135+5G>C | NA | 3.21e−5 | 3.25 | absent | N | gastroenteritis | foot polydactyly, abnormal skin |
| P8 | F | 3 | c.1168_1168delG | p.Glu390Lysfs∗160 | 0 | 32 | ND | Y | RSV, influenza, AOM, pneumonia | none |
| P9 | F | 4 | c.1201_1216del16 | p.Pro401Alafs∗144 | 0 | 35 | absent | N | URTI | none |
| P10 | M | 2 | c.1201_1216del16 | p.Pro401Alafs∗144 | 0 | 35 | ND | N | none | atopic dermatitis |
| P11 | F | 2 | c.1201_1216del16 | p.Pro401Alafs∗144 | 0 | 35 | ND | N | none | atopic dermatitis |
| P12 | F | 2 | c.1201_1216del16 | p.Pro401Alafs∗144 | 0 | 35 | absent | N | none | eczema |
| P13 | M | 4 | c.1201_1216del16 | p.Pro401Alafs∗144 | 0 | 35 | ND | Y | none | none |
| P14 | M | 3 | c.1205_1205delC | p.Pro402Leufs∗148 | 4.049e−6 | 27.9 | ND | Y | RSV, rhinovirus, pneumonia | thin hair, eosinophilia |
| P15 | M | 2 | c.1206delT | p.Leu404Cysfs∗146 | 0 | 25.6 | ND | Y | none | none |
| P16 | M | 1 | c.1315_1315delC | p.Leu439Cysfs∗111 | 0 | 32 | present | N | URTI | VSD |
| P17 | M | 2 | c.1315_1315delC | p.Leu439Cysfs∗111 | 0 | 32 | absent | N | URTI | VSD |
| P18 | F | 1 | c.1316_1316delT | p.Leu439Argfs∗111 | 0 | 32 | ND | N | none | none |
| P19 | F | 4 | c.1418_1418delC | p.Pro473Hisfs∗77 | 0 | 34 | small | Y | rhinovirus, norovirus | thin hair, HSCT |
| P20 | F | 1 | c.1420C>T | p.Gln474∗ | 0 | 37 | ND | Y | URTI | none |
| P21 | M | 2 | c.1584_1584delC | p.Leu529Trpfs∗21 | 0 | 27.8 | ND | N | rhinorrhea, diarrhea | none |
| P22 | M | 2 | c.974T>C | p.Leu325Pro | 0 | 27.2 | small | N | norovirus, RSV, rhinovirus, sapovirus, C. difficile, rotavirus, Parainfluenzae pneumonia, corynebacterium | arthritis, hypertension,anemia, failure to thrive,skin rashes, proteinuria, thrombocytopenia, bleedings |
| P23 | F | 9 | c.1392_1401del10 | p.Pro465Argfs∗82 | 0 | 34 | small | Y | bronchitis, omphalitis, Candidiasis, CMV, Molluscum | DLBCL (EBV+), malabsorption |
| P24 | M | 5b | c.1392_1401del10 | p.Pro465Argfs∗82 | 0 | 34 | small | Y | enterocolitis, pneumonia, CMV, HHV6 | HSCT (deceased 2y later) |
| P25 | M | 4 | c.1850_1854del5 | p.Tyr617Cysfs∗157 | 0 | 35 | ND | N | URTI | TEF |
| P26 | F | 22 | c.1201_1216del16 | p.Pro401Alafs∗144 | 0 | 35 | ND | N | otitis, pneumonia | none |
| P27 | F | 30 | c.907_907delG | p.Glu303Serfs∗247 | 0 | 35 | ND | N | none | none |
| P28 | F | 37 | c.1206delT | p.Leu404Cysfs∗146 | 0 | 25.6 | ND | Y | none | none |
| P29 | F | 40 | c.1201_1216del16 | p.Pro401Alafs∗144 | 0 | 35 | ND | Y | none | none |
| P30 | F | 40 | c.1168_1168delG | p.Glu390Lysfs∗160 | 0 | 32 | ND | Y | RRTI | none |
| P31 | M | 29 | c.763C>T | p.Arg255∗ | 3.98e−6 | 38 | ND | Y | none | none |
| P32 | F | 38 | c.763C>T | p.Arg255∗ | 3.98e−6 | 38 | ND | Y | none | seborrheic dermatitis, pituitary adenoma |
| P33 | F | 39 | c.763C>T | p.Arg255∗ | 3.98e−6 | 38 | ND | Y | sinusitis | eczema, multiple dental cavities |
| P34 | F | 40 | c.763C>T | p.Arg255∗ | 3.98e−6 | 38 | ND | N | none | eczema, multiple dental cavities |
| P35 | F | 41 | c.763C>T | p.Arg255∗ | 3.97e−6 | 38 | ND | N | none | osteoporosis |
| P36 | F | 42 | c.763C>T | p.Arg255∗ | 3.98e−6 | 38 | ND | Y | otitis, sinusitis | none |
| P37 | F | 45 | c.763C>T | p.Arg255∗ | 3.98e−6 | 38 | ND | N | none | eczema, rhinoconjunctivitis |
| P38 | F | 48 | c.763C>T | p.Arg255∗ | 3.98e−6 | 38 | ND | Y | none | multiple dental cavities |
| P39 | F | 53 | c.763C>T | p.Arg255∗ | 3.98e−6 | 38 | ND | Y | candidiasis, sinusitis, gastroenteritis | multiple dental cavities, melanoma, cardiac arrhythmias |
| P40 | M | 55 | c.763C>T | p.Arg255∗ | 3.98e−6 | 38 | ND | Y | none | androgenic, hair loss |
| P41 | M | 57 | c.763C>T | p.Arg255∗ | 3.98e−6 | 38 | ND | Y | none | mental retardation, multiple dental cavities, androgenic |
| P42 | M | 58 | c.763C>T | p.Arg255∗ | 3.98e−6 | 38 | ND | N | none | rosacea, multiple dental cavities |
| P43 | F | 59 | c.763C>T | p.Arg255∗ | 3.98e−6 | 38 | ND | N | none | none |
| P44 | F | 59 | c.763C>T | p.Arg255∗ | 3.98e−6 | 38 | ND | Y | none | none |
| P45 | M | 60 | c.763C>T | p.Arg255∗ | 3.98e−6 | 38 | ND | Y | none | androgenic, hair loss |
| P46 | M | 62 | c.763C>T | p.Arg255∗ | 3.98e−6 | 38 | ND | Y | none | eczema, multiple dental cavities, ocular thrombosis |
| P47 | F | 69 | c.763C>T | p.Arg255∗ | 3.98e−6 | 38 | ND | Y | otitis | Chiari malformation |
Mice
Heterozygous Foxn1 nu/+ mice (obtained from Jackson Laboratories) were bred with wild-type (WT) C57BL/6 mice to generate mixed litters of Foxn1 nu/+ (hereafter called “_nu/+”) and Foxn1 wild-type (hereafter called “+/+_”) mice. Most experiments were conducted blindly as genotype was often determined after the flow cytometry and qPCR data were obtained. Animal work was conducted in specific-pathogen-free conditions, in accordance to the USPHS Policy on Humane Care and Use of Laboratory Animals with an animal study protocol approved by the NIAID Animal Care and Use Committee (protocol LCIM 6E).
Thymus Cell Isolation
Thymus was isolated from littermate nu/+ and +/+ mice of various ages (from E15.5 embryos to 5-week-old mice). For the isolation of thymocytes, thymic tissue was mashed in FACS Buffer, made with PBS (GE Healthcare Bio-Sciences) supplemented with 2% FBS (Gemini Bio-Products) and 0.2% Primocin (InvivoGen). Cells were then filtered through 40 μm cell strainers (VWR), counted, and kept on ice until use. To isolate TECs, the thymus was dissected free from fat and stromal tissue and then digested at 37°C (three steps of 5 min each) with a solution containing Liberase TL (Sigma-Aldrich) and DNase I (Sigma-Aldrich). Digested thymic cells were recovered in IMDM (Iscove’s Modifed Dulbecco Medium, GIBCO, Thermo Fisher Scientific) supplemented with 10% FBS and Primocin. Thymic epithelial cells (TECs) were further enriched by depleting CD45+ cells with the AutoMACS ProSeparator (Miltenyi Biotec), after incubating digested thymic cells with anti-CD45 microbeads (Miltenyi Biotec). TECs were used immediately for staining and FACS analysis or pelleted and stored at −80°C for gene expression studies.
Flow Cytometry
For flow cytometry analysis, thymocytes were stained with the following antibodies (all from BioLegend): anti-CD4 FITC (clone RM4-5), anti-CD8 APC (clone 53-6.7), anti-CD44 PE-Cy7 (clone IM7), anti-CD117 APC-Cy7 (clone 2B8), anti-CD25 PE (clone PC61), and anti-mouse Lineage cocktail Pacific Blue (17A2/RB6-8C5/RA3-6B2/Ter-119/M1/70).
To evaluate TEC phenotype, MACS-purified CD45-negative cells were incubated with the following antibodies: anti-CD45 PE-Cy7 (clone 30-F11, BioLegend), anti-EpCam PerCP-Cy5.5 (clone G8.8, BioLegend), anti-Ly51 PE (clone 6C3, Miltenyi Biotec), anti-MHC class II APC-Cy7 (clone M5/114.15.2, BioLegend), and anti-Ulex Europeaus Agglutinin I (UEA I) (clone FL-1061, Vector Laboratories) antibodies. Live/Dead Fixable Yellow (Thermo Fisher Scientific) was added to the antibody mix to exclude dead cells.
Events were acquired on an LSR Fortessa flow cytometer (BD Biosciences) and analyzed using FlowJo software v.10.5.2 (FlowJo).
Gene Expression
RNA was extracted from TECs using the RNeasy Micro kit (QIAGEN) and stored at −80°C until used. Reverse transcription of mRNA was performed with the Quantabio qScript cDNA Synthesis kit (Quanta BioSciences). Real-time PCR for EpCam, Ccl25, Cxcl12, Dll4, Scf, Psmb11, Prss16, and Cd83 gene expression was performed with Perfecta SYBR Green FAST Mix LowRox (Quanta BioSciences) in MicroAmp Optical 96-well reaction plates (Applied Biosystems) in a final volume of 20 μL and run on the 7500 Real-Time PCR machine (Applied Biosystems). Relative quantification of gene expression was performed with the 2-ΔΔCt method, normalized to Epcam and expressed as fold change relative to Epcam expression. Subsequently, for each gene we calculated the average value obtained in all samples from +/+ mice at each time point, and relative gene expression was calculated by dividing values in individual +/+ and nu/+ mice for the average value in +/+ mice.
Statistical Analyses
All datasets were analyzed using GraphPad Prism software and expressed as mean ± SD. Statistical significance was assessed using the Mann-Whitney test, as indicated in each panel. p values < 0.05 were considered significant.
Results
Heterozygous FOXN1 Variants in Newborns with Low TRECs and T Cell Lymphopenia at Birth
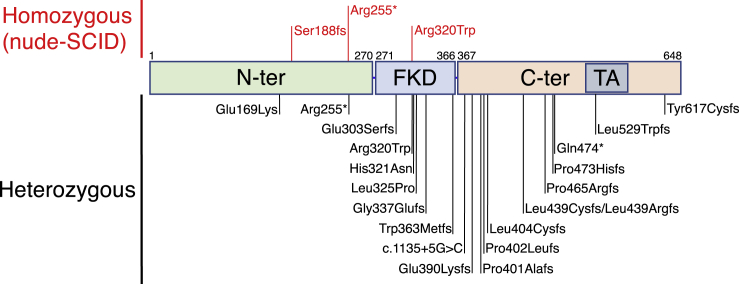
We report here 25 pediatric subjects carrying heterozygous FOXN1 variants (P1 through P25 in Table 1). Twenty-one of them (P1–P21) were identified through abnormally low TREC levels at newborn screening for SCID and were confirmed to have T cell lymphopenia (Table S1), while the remaining four pediatric subjects (P22–P25) were identified based on a clinical phenotype of severe or recurrent, early-onset infections and T cell lymphopenia. The heterozygous FOXN1 variants were identified by DNA sequencing when these infants were investigated for SCID-associated gene variants, according to policies in place at the referring institutions. No disease-causing variants in other SCID-associated genes were identified. Twenty-two adult subjects (P26 through P47, Table 1) with heterozygous FOXN1 variants were also studied and included 5 parents of the 25 pediatric subjects, as well as 17 individuals from the village of Acerno, Italy, known to be heterozygous for the FOXN1 p.Arg255∗ mutation.16 In total, 20 distinct FOXN1 gene variants were identified (Table 1 and Figure 1). Three of these variants have been reported at very low frequency (minor allele frequency < 3.19 × 10−5) in the general population. The remaining 17 variants have not been reported in GnomAD or in 1000 Genomes (Table 1). The majority (n = 13) of FOXN1 variants (65%) were frameshift, four (20%) were missense, and the remaining three were either nonsense (n = 2, 10%) or splice-site (n = 1, 5%) variants. As shown in Figure 1, the variants spanned FOXN1, but 6 of them were clustered in the forkhead domain (FKD, amino acid 271–366) and 12 in the C-terminal domain. One of the latter (p.Leu529Trpfs) is located in the transactivation domain.
Figure 1.
FOXN1 Domains with Location of the Mutations
Mutations previously published as causing nude/SCID phenotype in subjects are indicated in red in the upper part of the figure. Heterozygous variants identified in the subjects included in this study are indicated in black in the lower part of the figure. Numbers on top of the domains indicate the amino acid number in FOXN1 protein. Abbreviations: N-term, N-terminal; FKD, Forkhead domain; C-term, C-terminal; TA, transactivation domain.
Athymia has been reported in individuals carrying homozygous LoF FOXN1 mutations.7 In our cohort of 25 infants with heterozygous FOXN1 variants, presence of the thymic shadow on chest X-ray was evaluated in 13 individuals. Among these, 10 showed absent or reduced thymic shadow, while a normal-sized thymic shadow was detected in 3 infants (Table 1). Infections were reported in 18 FOXN1+/− infants (72%) and were of viral origin in most of them (Table 1). Upper respiratory tract infections were particularly common. Five infants had a history of pneumonia, and protracted watery diarrhea due to norovirus infection was recorded in two other infants. Finally, recurrent, severe infections occurred in five individuals (P8, P14, P22–P24), and one of them (P23) developed EBV-positive diffuse large B cell lymphoma. By contrast, a significant history of recurrent infections was documented only in a minority (6/22, 27.3%) of adult individuals with heterozygous FOXN1 variants. Only one subject (P22) presented with autoimmunity (severe thrombocytopenia). Nail dystrophy is another typical feature of FOXN1 deficiency.9 In our cohort, nail dystrophy was documented in 10/25 (40%) infants and in 15/22 (68.2%) adults with heterozygous FOXN1 variants. No infants had alopecia universalis (another common feature of FOXN1 deficiency), but thin hair was reported in two infants and hair loss in two adults. Eczema and other cutaneous manifestations of atopic dermatitis were documented in seven infants and five adults. Most infants were managed conservatively. However, three of them (P4, P19, and P24) received HSCT before the FOXN1 variants were identified. In P4 and P19, HSCT was motivated by the extreme T cell lymphopenia (14 and 11 CD3+ cells/μL in P4 and P19, respectively) that prompted a diagnosis of T− B+ NK+ SCID. In spite of stem cell engraftment, both individuals remained T cell lymphopenic after transplantation. P24 was referred to HSCT because of a history of severe infections and died of transplant-related complications 2 years after HSCT.
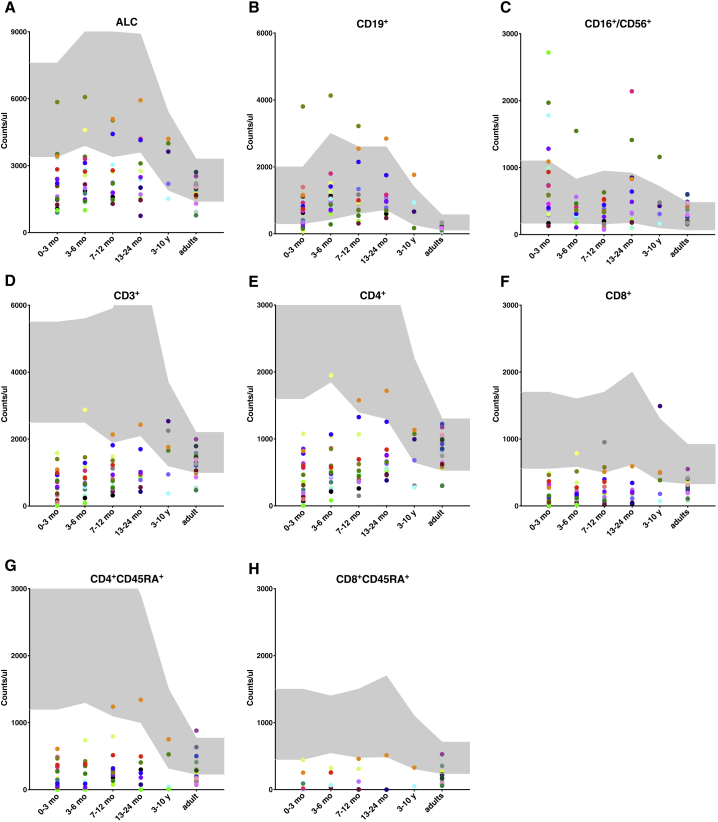
Immunological investigations showed that the lymphopenia was present in the majority of FOXN1+/− infants at diagnosis (Figure 2A and Table S1) and reflected a lower than normal number of CD3+ T cells that included both CD4+ and CD8+ T cells, and their naive (CD45RA+) subsets (Figures 2D–2H). By contrast, no abnormalities were observed in the number of CD19+ B cells and CD3−CD56/16+ NK cells (Figures 2B and 2C). Longitudinal analyses of T cell counts over time in the pediatric cohort showed that CD4+ T cell lymphopenia persisted in most infants, but the severity of the defect was less pronounced beyond the first 2 years of age, while CD8+ T cell counts remained stably below the normal range in most infants (Figures 2E and 2F). While the absolute lymphocyte count (ALC) and total CD3+ and CD4+ T cell counts were within the normal range in most of the adults with heterozygous LoF FOXN1 variants (Figures 2A, 2D, and 2E), the absolute count of CD8+ T cells remained lower than normal in most (Figure 2F). Consistent with these data, the proportion of individuals with lower than normal ALC, CD3+, and CD4+ T cell counts progressively declined with age and eventually resolved (Figures 2E and S1), whereas normalization of CD8+ T cell counts occurred in only a minority of subjects, and most adults remained with a low number of naive CD8+ T cells (Figures 2H and S1). Finally, around 30% of the adults had a lower than normal proportion of naive CD45RA+CD4+ cells (Figure 2G), suggesting that normalization of the total CD4+ T cell counts in adulthood is contributed by homeostatic proliferation. Together, these data indicate that FOXN1 haploinsufficiency is associated with T cell lymphopenia in infancy and that the CD8+ T cell compartment is principally affected.
Figure 2.
Lymphocyte Counts in Individuals Carrying Heterozygous LoF FOXN1 Variants
The graphs show total absolute lymphocyte counts (ALC) (A), CD19+ (B), CD16+/CD56+ (C), CD3+ (D), CD4+ (E), CD8+ (F), CD4+CD45RA+ (G), and CD8+CD45RA+ (H) cell counts indicated as counts/μL at different ages (0–3 months, 3–6 months, 7–12 months, 13–24 months, 3–10 years, adult). Gray areas indicate normal ranges. Each subject is represented by a dot of the same color at the different age ranges.
Nu/+ Mice Have Dramatically Reduced Frequencies and Absolute Numbers of ETPs Only in the First 3 Weeks of Life
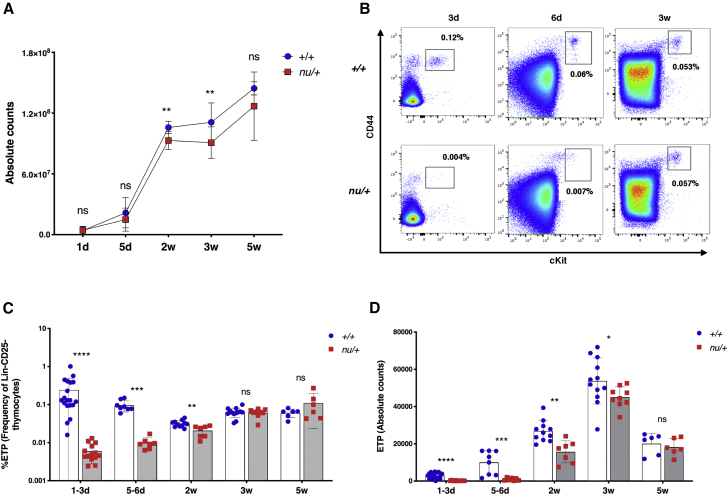
To determine whether the immune status of nu/+ mice at birth recapitulates the findings in FOXN1+/− infants, we analyzed thymocyte counts in nu/+ mice and age-matched littermate wild-type (+/+) controls at various time points after birth: 1–3 days, 5–6 days, 2 weeks, 3 weeks, and 5 weeks. Thymocyte numbers were only slightly reduced at 2 and 3 weeks after birth in nu/+ mice (Figure 3A). However, as compared to +/+ littermate controls, nu/+ mice had a dramatic reduction in the frequency and absolute count of ETPs (identified as CD45+Lin−CD25−CD44+cKit+ cells) (Figures 3B and 3C), indicative of reduced migration of lymphoid progenitors to the thymus through 5 weeks of age, when both the frequency and the count of thymic ETPs were comparable in nu/+ and +/+ mice (Figures 3C and 3D).
Figure 3.
Total Thymocyte Counts and Early Thymic Progenitor (ETP) Counts and Frequency in nu/+ and +/+ Mice
(A) Graph shows thymocyte absolute counts in nu/+ (red squares) and +/+ mice (blue dots) at different ages (d, day; w, weeks). Number of mice analyzed at each time point: 1d (+/+, n = 13; nu/+, n = 11), 5d (+/+, n = 8; nu/+, n = 7), 2w (+/+, n = 14; nu/+, n = 8), 3w (+/+, n = 13; nu/+, n = 9), 5w (+/+, n = 6; nu/+, n = 6). Mean ± SD are represented. ns, not significant, ∗∗p < 0.01; Mann-Whitney test.
(B) Representative flow cytometry plots of early thymic progenitors (ETPs), identified as CD44+cKit+, gated on Lineage−CD25− thymocytes, at different ages (3 days, 6 days, 3 weeks) in +/+ mice (upper row) and in nu/+ mice (lower row). Numbers on the plots indicate the frequency of ETPs.
(C and D) Graphs show ETP frequencies (C) and absolute counts (D) for all +/+ and nu/+ mice analyzed at the different ages. Number of mice analyzed at each time point: 1d (+/+, n = 19; nu/+, n = 14), 5d (+/+, n = 8; nu/+, n = 7), 2w (+/+, n = 11; nu/+, n = 7), 3w (+/+, n = 12-13; nu/+, n = 9), 5w (+/+, n = 6; nu/+, n = 6). Mean ± SD are represented. ns, not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001; Mann-Whitney test.
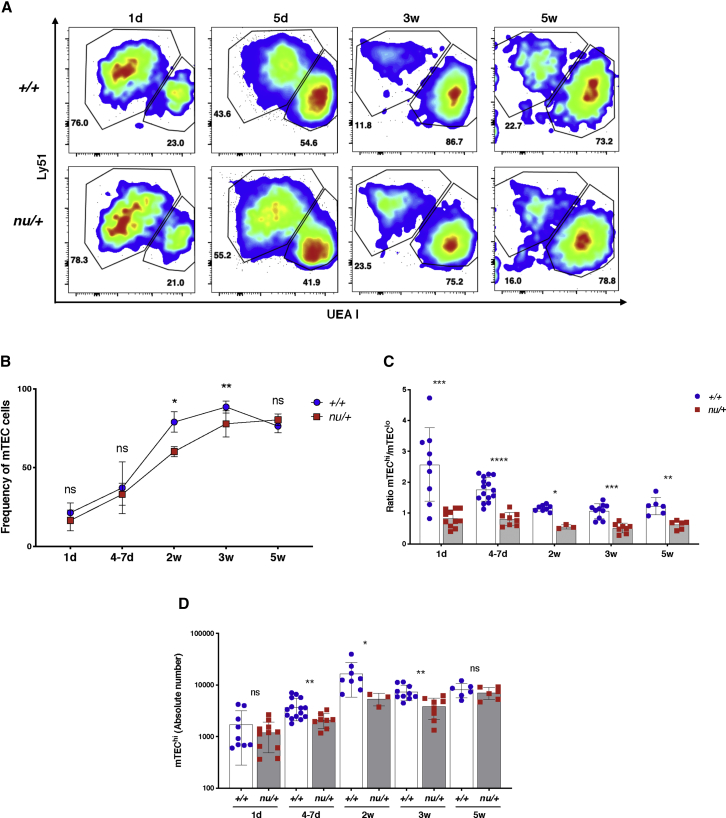
TECs of nu/+ Mice Display an Immature Phenotype and Express Significantly Lower Levels of FOXN1 Target Genes during Embryonic Development and Early in Post-natal Life
To investigate the impact of Foxn1 haploinsufficiency on TEC maturation and function, we set out to analyze the TEC compartment in nu/+ mice and +/+ littermate controls during embryonic development and at various time points early after birth. After digestion of thymic tissue and enrichment for TEC by CD45+ cell depletion, we used flow cytometry to characterize TEC phenotype and quantitative PCR (qPCR) to evaluate their gene expression profile. TECs were identified as CD45−EpCam+ cells and further divided into Ly51+UEA I− cTECs and Ly51−UEA I+ mTECs (Figure 4A). Compared to +/+ littermate controls, nu/+ mice showed a slight reduction in mTEC frequency, which was significant at 2 and 3 weeks of age (Figure 4B). The density of MHC class II (MHC-II) antigen expression has been used to distinguish mTECs at different stages of differentiation. In particular, in post-natal life mTECs expressing lower levels of MHC-II antigens (mTEClo) are considered to represent a less mature stage of differentiation as compared to mTECs expressing high levels of MHC-II (mTEChi).18, 19 In nu/+ mice, the reduction of mTEC numbers was more apparent in the subset of mTEChi cells, as shown by the reduced ratio of mTEChi to mTEClo cells (Figure 4C) and by the reduced absolute count of mTEChi cells (Figure 4D) as compared with +/+ littermate controls. These data indicate that TECs from nu/+ mice have a less mature phenotype than the +/+ mice.
Figure 4.
TEC Phenotype in nu/+ and +/+ Mice
(A) Representative flow cytometry plots of TECs gated on CD45−EpCam+ cells in +/+ (upper row) and nu/+ mice (lower row). Cortical TEC (cTEC) are identified as Ly51+UEA I− and medullary TEC (mTEC) as Ly51−UEA I+. Numbers in the plots indicate the frequency of cTEC and mTEC at the different ages (d, day; w, weeks).
(B) Graph shows the frequency of mTEC in all +/+ and nu/+ mice analyzed at the different ages. Mean ± SD are represented. ns, not significant, ∗p < 0.05, ∗∗p < 0.01; Mann-Whitney test.
(C) Graph shows the ratio between mTEChi and mTEClo in +/+ and nu/+ mice at each age analyzed. Mean ± SD are represented. ∗p < 0.05, ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001; Mann-Whitney test.
(D) Graph shows the absolute counts of mTEChi in +/+ and nu/+ mice at each age analyzed. Mean ± SD are represented. ns, not significant, ∗p < 0.05, ∗∗p < 0.01; Mann-Whitney test.
(B–D) Number of mice analyzed at each age: 1d (+/+, n = 9; nu/+, n = 11), 4–7d (+/+, n = 15; nu/+, n = 8), 2w (+/+, n = 8; nu/+, n = 3), 3w (+/+, n = 10; nu/+, n = 8), 5w (+/+, n = 6; nu/+, n = 6).
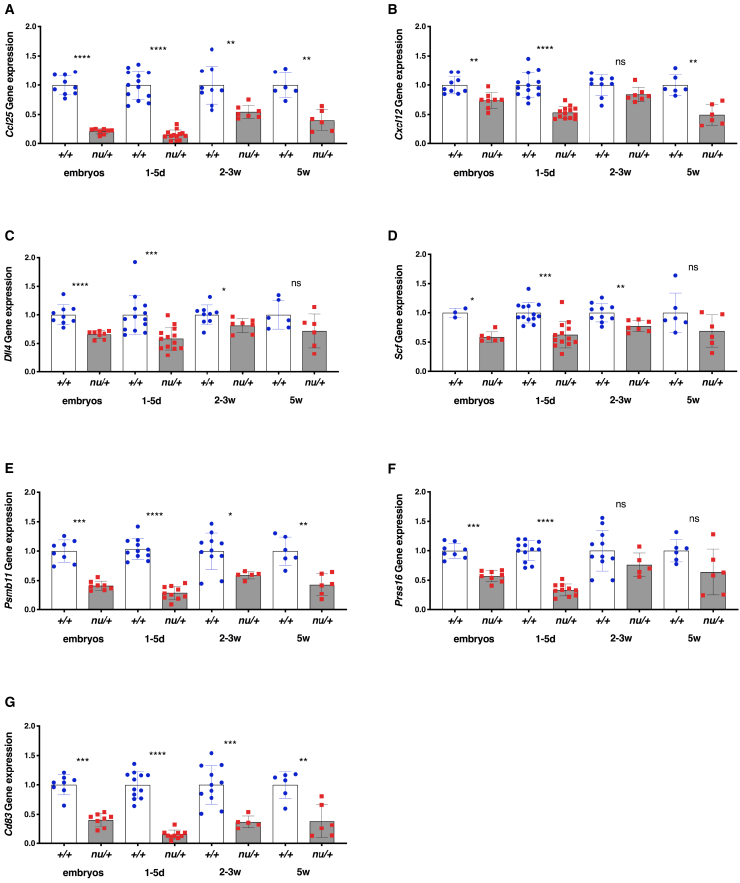
We then analyzed the expression profile of several key FOXN1-target genes (Ccl25, Cxcl12, Dll4, Scf, Psmb11, Prss16, and Cd83) whose expression is critical for TEC function, including recruitment of ETPs and positive selection of CD8+ and CD4+ thymocytes. As shown in Figure 5, for all of these genes, during embryonic development, nu/+ mice had lower levels of expression than +/+ littermate controls. For several genes (Ccl25, Cxcl12, Psmb11, and Cd83), this difference remained significant even at the last time point (week 5 of life), and a similar trend was observed for Dll4, Scf, and Prss16, albeit without reaching statistical significance. In summary, our data indicate that mouse Foxn1 haploinsufficiency has important effects on TEC maturation and function and that these effects are most evident during embryonic development and in the first weeks of life.
Figure 5.
Gene Expression Profile of TEC in nu/+ and +/+ Mice
Graphs show gene expression of Ccl25 (A), Cxcl12 (B), Dll4 (C), Scf (D), Psmb11 (E), Prss16 (F), and Cd83 (G) in TEC enriched after thymus digestion and CD45-positive cell depletion in +/+ and nu/+ mice at different ages (d, day; w, weeks). Relative quantification of gene expression was performed with the 2-ΔΔCt method, normalized to EpCam and expressed as fold change relative to EpCam. Subsequently, for each gene we calculated the average value obtained in all samples from +/+ mice at each age, and relative gene expression was calculated by dividing values in individual +/+ and nu/+ mice for the average value in +/+ mice. Number of mice analyzed at each time point: embryos (+/+, n = 3-8; nu/+, n = 6–8), 1–5d (+/+, n = 11–13; nu/+, n = 10–13), 2–3w (+/+, n = 9–11; nu/+, n = 5–7), 5w (+/+, n = 6; nu/+, n = 6). Mean ± SD are represented. ns, not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001; Mann-Whitney test.
Discussion
We present here evidence that rare heterozygous LoF variants in the FOXN1 gene are associated with low TRECs and T cell lymphopenia at birth and early in childhood.
The FOXN1 gene is considered loss-of-function intolerant with a pLI score of 0.94 in the genome aggregation database (GnomAD). To date, ten subjects carrying three distinct homozygous FOXN1 mutations have been reported and they all manifested a complete nude phenotype.10, 12, 13, 14, 15 The mutation found in the original two individuals and subsequently in six additional infants is a nonsense mutation at position 255.10, 12, 13, 15 The other two mutations were found only in one subject each and include a frameshift mutation at position 18814 and a missense mutation at position 320 (p.Arg320Trp).13 Both the p.Arg255∗ and the p.Ser188fs mutations result in loss of protein expression due to mRNA decay.11, 14 The missense p. Arg320Trp mutation falls in the FKD and affects an amino acid residue that is highly conserved among species20, 21 and that plays a critical role in binding at the GACGC FOXN1 consensus sequence.22
In this manuscript, we have reported a total of 19 distinct FOXN1 heterozygous variants in 25 infants. One of these variants (p.Arg320Trp) was previously described in one individual with biallelic FOXN1 mutation and a nude phenotype.13 Among the remaining new 18 variants, the majority (13 out 18, 72.2%) were frameshift mutations, one affected a splice site, and one was nonsense. Because of their nature, all of these are predicted to be deleterious. The remaining three variants were missense: two (p.His321Asn and p.Leu325Pro) are also located in the FKD, like the p.Arg320Trp mutation. In particular, FOXN1 residue His321 has been recently shown to be directly involved, together with Arg320, in binding of the FOXN1 DNA consensus sequence.20 The last missense variant (c.505G>A [p.Glu169Lys]) is located in the N-terminal domain, and its impact on FOXN1 expression and function remains to be investigated. Overall, the nature of the FOXN1 variants identified in this series suggests that they are most likely LoF. However, the possibility that alleles disrupting the C-terminal domain but retaining the FKD domain intact might act as dominant-negative mutants23 cannot be formally excluded.
Most of the infants with heterozygous FOXN1 variants included in this study were identified at birth following detection of out-of-range TREC levels during newborn screening for SCID and were confirmed to have T cell lymphopenia. Longitudinal follow up of these infants during the first years of life revealed persistence of CD8+ T cell lymphopenia and a trend toward improvement of the CD4+ T cell count. In some individuals, these immunological abnormalities were associated with recurrent infections, which were particularly severe in a few. The nail dystrophy was initially overlooked in most of the subjects and was recognized only in a proportion of them after identification of FOXN1 haploinsufficiency prompted a more careful physical examination. In a previous study of adults heterozygous for the FOXN1 p.Arg255∗ mutation, the nail dystrophy was not immediately obvious on examination and was often restricted to toenails.16 To better assess the immunological and clinical impact of FOXN1 haploinsufficiency in adulthood, we studied 22 adults who are heterozygous for the FOXN1 c.763C>T (p.Arg255∗) mutation or for the same variant identified in their offspring. Our data demonstrate that most of these individuals had a normal CD4+ T cell count and were not unduly susceptible to recurrent infections; however, most of them had a low CD8+ T cell count and 60% of them showed nail dystrophy. These data indicate that FOXN1 haploinsufficiency causes a marked T cell lymphopenia early in life, followed by progressive normalization of the CD4+ T cell count but persistence of CD8+ T cell lymphopenia that may extend into adulthood. The mechanisms accounting for a differential effect of FOXN1 haploinsufficiency on CD4+ and CD8+ T cell numbers with age are unclear. One possibility is that FOXN1 gene dosage has a more stringent role on the expression of genes involved in development of CD8+ T cells, such as PSMB11. Alternatively, homeostatic proliferation might be more prominent for CD4+ than for CD8+ T cells. There is evidence that the requirements for homeostatic proliferation are different for CD4+ versus CD8+ T cells.24 The observation that most adults with heterozygous LOF FOXN1 variants had a normal CD4+ T cell count but a lower than normal proportion of naive (CD45RA+) CD4+ cells is consistent with the hypothesis that normalization of total CD4+ cell count with age is contributed by homeostatic proliferation.
Studies in mice have shown that the initial stages of TEC specification during embryogenesis are independent of FOXN1,20, 25 while all the subsequent steps of TEC differentiation and growth are dependent on the continuous expression of Foxn1, both during embryonic and in post-natal life.6, 7, 8 Persistence of Foxn1 expression during adulthood is necessary to avoid or delay thymic involution.7, 8 Previous studies have analyzed expression of FOXN1-target genes in mice carrying hypomorphic Foxn1 alleles that allow lower expression levels than in nu/+ mice.5, 6, 7 We report here evidence of reduced expression of FOXN1-target genes during embryonic development and early after birth in Foxn1 haploinsufficient mice. In particular, we detected a dramatic decrease in the frequency and absolute counts of ETPs that was limited to the first 3 weeks of life. FOXN1 is a well-known inducer of CCL25, CXCL12, and SCF secretion by TECs.5 Of these, CCL25 and CXCL12 are critical attractants for lymphoid progenitors migrating from the bone marrow to the thymus,5, 26 while SCF is a major growth factor driving expansion of immature thymocytes.27, 28 Expression of all these three genes was strongly reduced in nu/+ mice. In addition, the expression of Dll4, a direct target of FOXN15 and a critical factor for the commitment of early thymic progenitors to T cell development,29, 30 was also greatly reduced in nu/+ animals.
Finally, FOXN1 is also involved in the induction of genes that play an important role in later steps of TEC-thymocyte cross-talk, such as antigen processing (Psmb11 and Prss16) and presentation (Cd83).5 Expression of all these genes was also greatly reduced in TEC from nu/+ mice. Importantly, the most significant differences in expression levels were found in TEC isolated from nu/+ mice embryos and in the first week of life. These data may also account for a more significant impact of Foxn1 haploinsufficiency on ETP count in the first weeks of life. In addition to reduced gene expression in TECs of nu/+ mice, we also detected a reduced frequency of mTECs, affecting in particular mTEChi cells. A similar effect on mTEChi cells had previously been reported in other mouse models expressing decreased levels of FOXN1.5, 7 Together, our observations in humans and mice suggest that the levels of FOXN1 needed for optimal thymic function and for CD4+ and CD8+ T cell development are different in embryonic versus early post-natal life and adulthood. A possible explanation for the time-dependent effects of FOXN1 gene dosage comes from the recent observation that FOXN1 binding to the DNA consensus sequence GACGC in the promoter of target genes is influenced by the methylation status of the CpG dinucleotide, such that binding occurs significantly less tightly if the CpG is methylated.20 Since increased promoter methylation is known to occur with aging,31 the altered epigenetic status of FOXN1 target sequences may play a role for a less pronounced FOXN1 gene dosage effect in older individuals.
Consistent with the observation that the thymus is smaller in nu/+ than in +/+ mice,32, 33 we have documented mild defects in thymocyte numbers in nu/+ mice. A more significant reduction in thymocyte counts has been reported in mice with hypomorphic Foxn1 variants allowing lower levels of protein expression than nu/+ mice.5, 6, 7 In any case, the lymphopenia at birth in individuals carrying heterozygous LoF FOXN1 variants was more profound than that of nu/+ mice, suggesting that FOXN1 haploinsufficiency is responsible for a more severe phenotype in humans than in mice.
Both thymus transplantation and HSCT have been previously used to treat individuals with bi-allelic FOXN1 mutations and a nude phenotype. Three individuals received unmanipulated T cell-replete HSCT from HLA-matched siblings. Reconstitution of the T cell count was observed in one, who was reported to be alive and infection-free 6 years after transplantation; however, CD4+ T cells were unable to proliferate.34 The other two individuals who received HSCT died of complications post-transplant.14, 15 Two individuals received thymus transplantation; both had a slower T cell reconstitution, but eventually attained normal T cell counts, with capacity to generate naive CD4+ T cells, normal in vitro T cell proliferative responses to mitogens, and reconstitution of antibody production. These subjects were reported to be alive and infection-free at 3 and 5 years after transplantation, respectively,13, 35 although one of them developed autoimmune hypothyroidism and vitiligo.36
Three of the infants included in this study underwent HSCT either before the genotype was known or because it was thought that FOXN1 haploinsufficiency was not a likely cause of the T cell lymphopenia. However, none of them normalized the T cell count or reversed the clinical phenotype after transplantation, indicating that the defect was extrinsic to the hematopoietic lineage.
Our results have important implications for the diagnosis and clinical management of infants with T cell lymphopenia at birth. The wider implementation of newborn screening for SCID is likely to increase the number of babies with low TRECs and T cell lymphopenia at birth, some of whom will be heterozygous for LoF FOXN1 variants. Our study shows that these subjects can have a sustained CD8+ T cell lymphopenia and may be at higher risk of infections, especially in early childhood. For most, however, the clinical and immunological abnormalities tend to improve with time. Three of the subjects reported in this study received HSCT, but none of them attained T cell reconstitution, and one died of transplant-related toxicity. These data indicate that HSCT is not a curative treatment for individuals with FOXN1 haploinsufficiency, consistent with the thymus-intrinsic role of the molecule. However, the observation that a few babies with heterozygous LoF FOXN1 variants developed serious infections suggests careful clinical monitoring and adoption of preventive measures, as indicated in babies with severe T cell lymphopenia at birth. In particular, in our series, recurrent and/or serious infections were documented in 2 out of 21 infants identified with a positive newborn screening (P8, P14), but in all 3 children in whom heterozygous FOXN1 variants were identified only later in life, based on a positive clinical history and documented T cell lymphopenia. It is possible that in the first group of infants the prompt use of adequate preventive measures helped reduce the risk of infections. Future prospective studies may better define the reasons underlying phenotypic heterogeneity of FOXN1 haploinsufficiency and possibly guide treatment on a case-by-case basis. Whether thymus transplantation may be needed in extremely severe cases remains to be seen. Furthermore, studies in mice indicate that decreased Foxn1 expression is associated with accelerated loss of thymic architecture and function.5, 7 Whether FOXN1 haploinsufficiency in aging humans will lead to accelerated thymic involution, restriction of the T cell repertoire, and increased risk of infections and autoimmunity remains to be studied.
Declaration of Interests
The authors declare no competing interests.
Acknowledgments
This work was supported in part by the Intramural Research Program of the DIR, NIAID, NIH, by Center for Clinical and Translational Research at Brown University (GM115677), the Center for Cancer Research, National Cancer Institute, National Institutes of Health, and by the Jeffrey Modell Diagnostic and Research Center for Primary Immunodeficiencies at St. Louis Children’s Hospital. C.B. is supported by a Michael Smith Foundation for Health Research Health Professional-Investigator Award.
Published: August 22, 2019
Footnotes
Web Resources
- GenBank, https://www.ncbi.nlm.nih.gov/genbank/
- OMIM, https://www.omim.org/
Supplemental Data
Document S1. Figure S1 and Table S1
Document S2. Article plus Supplemental Information
References
- 1.Gordon J., Bennett A.R., Blackburn C.C., Manley N.R. Gcm2 and Foxn1 mark early parathyroid- and thymus-specific domains in the developing third pharyngeal pouch. Mech. Dev. 2001;103:141–143. doi: 10.1016/s0925-4773(01)00333-1. [DOI] [PubMed] [Google Scholar]
- 2.Flanagan S.P. ‘Nude’, a new hairless gene with pleiotropic effects in the mouse. Genet. Res. 1966;8:295–309. doi: 10.1017/s0016672300010168. [DOI] [PubMed] [Google Scholar]
- 3.D’Assante R., Fusco A., Palamaro L., Giardino G., Gallo V., Cirillo E., Pignata C. Unraveling the Link Between Ectodermal Disorders and Primary Immunodeficiencies. Int. Rev. Immunol. 2016;35:25–38. doi: 10.3109/08830185.2015.1010724. [DOI] [PubMed] [Google Scholar]
- 4.Farley A.M., Morris L.X., Vroegindeweij E., Depreter M.L.G., Vaidya H., Stenhouse F.H., Tomlinson S.R., Anderson R.A., Cupedo T., Cornelissen J.J., Blackburn C.C. Dynamics of thymus organogenesis and colonization in early human development. Development. 2013;140:2015–2026. doi: 10.1242/dev.087320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Žuklys S., Handel A., Zhanybekova S., Govani F., Keller M., Maio S., Mayer C.E., Teh H.Y., Hafen K., Gallone G. Foxn1 regulates key target genes essential for T cell development in postnatal thymic epithelial cells. Nat. Immunol. 2016;17:1206–1215. doi: 10.1038/ni.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nowell C.S., Bredenkamp N., Tetélin S., Jin X., Tischner C., Vaidya H., Sheridan J.M., Stenhouse F.H., Heussen R., Smith A.J.H., Blackburn C.C. Foxn1 regulates lineage progression in cortical and medullary thymic epithelial cells but is dispensable for medullary sublineage divergence. PLoS Genet. 2011;7:e1002348. doi: 10.1371/journal.pgen.1002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L., Xiao S., Manley N.R. Foxn1 is required to maintain the postnatal thymic microenvironment in a dosage-sensitive manner. Blood. 2009;113:567–574. doi: 10.1182/blood-2008-05-156265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng L., Guo J., Sun L., Fu J., Barnes P.F., Metzger D., Chambon P., Oshima R.G., Amagai T., Su D.-M. Postnatal tissue-specific disruption of transcription factor FoxN1 triggers acute thymic atrophy. J. Biol. Chem. 2010;285:5836–5847. doi: 10.1074/jbc.M109.072124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallo V., Cirillo E., Giardino G., Pignata C. FOXN1 Deficiency: from the Discovery to Novel Therapeutic Approaches. J. Clin. Immunol. 2017;37:751–758. doi: 10.1007/s10875-017-0445-z. [DOI] [PubMed] [Google Scholar]
- 10.Pignata C., Fiore M., Guzzetta V., Castaldo A., Sebastio G., Porta F., Guarino A. Congenital Alopecia and nail dystrophy associated with severe functional T-cell immunodeficiency in two sibs. Am. J. Med. Genet. 1996;65:167–170. doi: 10.1002/(SICI)1096-8628(19961016)65:2<167::AID-AJMG17>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 11.Frank J., Pignata C., Panteleyev A.A., Prowse D.M., Baden H., Weiner L., Gaetaniello L., Ahmad W., Pozzi N., Cserhalmi-Friedman P.B. Exposing the human nude phenotype. Nature. 1999;398:473–474. doi: 10.1038/18997. [DOI] [PubMed] [Google Scholar]
- 12.Adriani M., Martinez-Mir A., Fusco F., Busiello R., Frank J., Telese S., Matrecano E., Ursini M.V., Christiano A.M., Pignata C. Ancestral founder mutation of the nude (FOXN1) gene in congenital severe combined immunodeficiency associated with alopecia in southern Italy population. Ann. Hum. Genet. 2004;68:265–268. doi: 10.1046/j.1529-8817.2004.00091.x. [DOI] [PubMed] [Google Scholar]
- 13.Markert M.L., Marques J.G., Neven B., Devlin B.H., McCarthy E.A., Chinn I.K., Albuquerque A.S., Silva S.L., Pignata C., de Saint Basile G. First use of thymus transplantation therapy for FOXN1 deficiency (nude/SCID): a report of 2 cases. Blood. 2011;117:688–696. doi: 10.1182/blood-2010-06-292490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chou J., Massaad M.J., Wakim R.H., Bainter W., Dbaibo G., Geha R.S. A novel mutation in FOXN1 resulting in SCID: a case report and literature review. Clin. Immunol. 2014;155:30–32. doi: 10.1016/j.clim.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Radha Rama Devi A., Panday N.N., Naushad S.M. FOXN1 Italian founder mutation in Indian family: Implications in prenatal diagnosis. Gene. 2017;627:222–225. doi: 10.1016/j.gene.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 16.Auricchio L., Adriani M., Frank J., Busiello R., Christiano A., Pignata C. Nail dystrophy associated with a heterozygous mutation of the nude/SCID human FOXN1 (WHN) gene. Arch. Dermatol. 2005;141:647–648. doi: 10.1001/archderm.141.5.647. [DOI] [PubMed] [Google Scholar]
- 17.Shearer W.T., Rosenblatt H.M., Gelman R.S., Oyomopito R., Plaeger S., Stiehm E.R., Wara D.W., Douglas S.D., Luzuriaga K., McFarland E.J., Pediatric AIDS Clinical Trials Group Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J. Allergy Clin. Immunol. 2003;112:973–980. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Gray D., Abramson J., Benoist C., Mathis D. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J. Exp. Med. 2007;204:2521–2528. doi: 10.1084/jem.20070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyewski B., Klein L. A central role for central tolerance. Annu. Rev. Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- 20.Nehls M., Pfeifer D., Schorpp M., Hedrich H., Boehm T. New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature. 1994;372:103–107. doi: 10.1038/372103a0. [DOI] [PubMed] [Google Scholar]
- 21.Kaufmann E., Knöchel W. Five years on the wings of fork head. Mech. Dev. 1996;57:3–20. doi: 10.1016/0925-4773(96)00539-4. [DOI] [PubMed] [Google Scholar]
- 22.Newman J.A., Aitkenhead H., Gavard A., Rota I.A., Handel A.E., Hollander G.A., Gileadi O. The structural basis for forkhead box family specificity revealed by the crystal structure of human FOXN1 in complex with DNA. bioRxiv. 2018 doi: 10.1074/jbc.RA119.010365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bajoghli B., Aghaallaei N., Hess I., Rode I., Netuschil N., Tay B.-H., Venkatesh B., Yu J.-K., Kaltenbach S.L., Holland N.D. Evolution of genetic networks underlying the emergence of thymopoiesis in vertebrates. Cell. 2009;138:186–197. doi: 10.1016/j.cell.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Tan J.T., Ernst B., Kieper W.C., LeRoy E., Sprent J., Surh C.D. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nehls M., Kyewski B., Messerle M., Waldschütz R., Schüddekopf K., Smith A.J., Boehm T. Two genetically separable steps in the differentiation of thymic epithelium. Science. 1996;272:886–889. doi: 10.1126/science.272.5263.886. [DOI] [PubMed] [Google Scholar]
- 26.Bleul C.C., Boehm T. Chemokines define distinct microenvironments in the developing thymus. Eur. J. Immunol. 2000;30:3371–3379. doi: 10.1002/1521-4141(2000012)30:12<3371::AID-IMMU3371>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 27.Rodewald H.R., Kretzschmar K., Swat W., Takeda S. Intrathymically expressed c-kit ligand (stem cell factor) is a major factor driving expansion of very immature thymocytes in vivo. Immunity. 1995;3:313–319. doi: 10.1016/1074-7613(95)90116-7. [DOI] [PubMed] [Google Scholar]
- 28.Rodewald H.R., Ogawa M., Haller C., Waskow C., DiSanto J.P. Pro-thymocyte expansion by c-kit and the common cytokine receptor γ chain is essential for repertoire formation. Immunity. 1997;6:265–272. doi: 10.1016/s1074-7613(00)80329-5. [DOI] [PubMed] [Google Scholar]
- 29.Tsukamoto N., Itoi M., Nishikawa M., Amagai T. Lack of Delta like 1 and 4 expressions in nude thymus anlages. Cell. Immunol. 2005;234:77–80. doi: 10.1016/j.cellimm.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Hozumi K., Mailhos C., Negishi N., Hirano K., Yahata T., Ando K., Zuklys S., Holländer G.A., Shima D.T., Habu S. Delta-like 4 is indispensable in thymic environment specific for T cell development. J. Exp. Med. 2008;205:2507–2513. doi: 10.1084/jem.20080134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zampieri M., Ciccarone F., Calabrese R., Franceschi C., Bürkle A., Caiafa P. Reconfiguration of DNA methylation in aging. Mech. Ageing Dev. 2015;151:60–70. doi: 10.1016/j.mad.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Scheiff J.M., Cordier A.C., Haumont S. The thymus of Nu/+ mice. Anat. Embryol. (Berl.) 1978;153:115–122. doi: 10.1007/BF00343368. [DOI] [PubMed] [Google Scholar]
- 33.Kojima A., Saito M., Hioki K., Shimanura K., Habu S. NFS/N-nu/ + mice can macroscopically be distinguished from NFS/N- +/+ littermates by their thymic size and shape. Exp. Cell Biol. 1984;52:107–110. [PubMed] [Google Scholar]
- 34.Pignata C., Gaetaniello L., Masci A.M., Frank J., Christiano A., Matrecano E., Racioppi L. Human equivalent of the mouse Nude/SCID phenotype: long-term evaluation of immunologic reconstitution after bone marrow transplantation. Blood. 2001;97:880–885. doi: 10.1182/blood.v97.4.880. [DOI] [PubMed] [Google Scholar]
- 35.Albuquerque A.S., Marques J.G., Silva S.L., Ligeiro D., Devlin B.H., Dutrieux J., Cheynier R., Pignata C., Victorino R.M.M., Markert M.L., Sousa A.E. Human FOXN1-deficiency is associated with αβ double-negative and FoxP3+ T-cell expansions that are distinctly modulated upon thymic transplantation. PLoS ONE. 2012;7:e37042. doi: 10.1371/journal.pone.0037042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levy E., Neven B., Entz-Werle N., Cribier B., Lipsker D. Vitiligo post-greffe thymique chez un enfant porteur d’un déficit en Foxn1. Ann. Dermatol. Venereol. 2012;139:468–471. doi: 10.1016/j.annder.2012.03.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Document S1. Figure S1 and Table S1
Document S2. Article plus Supplemental Information