Multipolar Migration: The Third Mode of Radial Neuronal Migration in the Developing Cerebral Cortex (original) (raw)
Abstract
Two distinct modes of radial neuronal migration, locomotion and somal translocation, have been reported in the developing cerebral cortex. Although these two modes of migration have been well documented, the cortical intermediate zone contains abundant multipolar cells, and they do not resemble the cells migrating by locomotion or somal translocation. Here, we report that these multipolar cells express neuronal markers and extend multiple thin processes in various directions independently of the radial glial fibers. Time-lapse analysis of living slices revealed that the multipolar cells do not have any fixed cell polarity, and that they very dynamically extend and retract multiple processes as their cell bodies slowly move. They do not usually move straight toward the pial surface during their radial migration, but instead frequently change migration direction and rate; sometimes they even remain in almost the same position, especially when they are in the subventricular zone. Occasionally, the multipolar cells jump tangentially during their radial migration. Because the migration modality of these cells clearly differs from locomotion or somal translocation, we refer to their novel type of migration as “multipolar migration.” In view of the high proportion of cells exhibiting multipolar migration, this third mode of radial migration must be an important type of migration in the developing cortex.
Keywords: cerebral cortex, neuronal migration, radial glial fiber, multipolar cell, mouse, intermediate zone
Introduction
In the developing cerebral cortex, projection neurons are primarily generated in the ventricular zone (VZ) and then move to the developing cortical plate (CP) by means of “radial migration” (Angevine and Sidman, 1961; Berry and Rogers, 1965; Rakic, 1972). Most GABAergic interneurons, however, originate in ganglionic eminences in mice and enter the developing CP via “tangential migration” (Marin and Rubenstein, 2001). Two distinct modes of radial migration, locomotion and somal translocation, have been reported previously (Nadarajah et al., 2001). Locomotion is characterized by cell migration along a radial fiber of a radial glial cell (Rakic, 1972), the fibers of which traverse the entire thickness of the developing cerebral wall. Neurons migrating in this mode have bipolar cell morphology, with a thick leading process and a thin trailing process, and the entire cell moves along the radial fiber. In somal translocation, as the soma of a cell with a long radially directed leading process that terminates at the pial surface advances toward the pial surface, its leading process becomes progressively shorter, whereas its terminal remains attached to the pial surface (Miyata et al., 2001; Nadarajah et al., 2001; Tamamaki et al., 2001).
In contrast to the bipolar or monopolar morphology of the cells that migrate by locomotion or somal translocation, histological analyses of fixed sections of developing cerebral hemisphere using Golgi staining, electron microscopy, or virus vectors expressing green fluorescent protein (GFP) have demonstrated the presence of multipolar cells in the intermediate zone (IZ) (Stensaas, 1967; Shoukimas and Hinds, 1978; Nowakowski and Rakic, 1979; Gadisseux et al., 1990; Noctor et al., 2001; Tamamaki et al., 2001). The radially oriented bipolar or monopolar morphology of locomotion or somal-translocation cells cannot account for the presence of the large proportion of multipolar cells in the IZ, and yet the behavior of these multipolar cells, including whether they indeed migrate, is unknown, because all of the previous analyses have been performed on fixed sections.
We recently established an in utero gene transfer system for use in mouse brains that allows plasmid vectors to be introduced into the embryonic cerebral VZ (Tabata and Nakajima, 2001, 2002). We used this system to label migrating cells in the developing cortex with GFP or red fluorescent protein (DsRed) and performed time-lapse observations. Here, we report that multipolar cells express neuronal markers and migrate by a novel mode of radial migration that we refer to as “multipolar migration.” Because the majority of the cells in the IZ-subventricular zone (SVZ) are multipolar, this mode of migration must be an important type of migration in the developing cortex.
Materials and Methods
In utero electroporation. All animal experiments were performed according to the guidelines of the Japan Neuroscience Society. Pregnant ICR mice (Japan SLC, Shizuoka, Japan) were deeply anesthetized, and their intrauterine embryos were surgically manipulated as described previously (Nakajima et al., 1997). The enhanced green fluorescent protein (EGFP; Clontech, Cambridge, UK) expression vector with a human elongation factor 1 α (EF1α) promoter (Uetsuki et al., 1989) or with modified chicken β-actin promoter with cytomegalovirus-immediate early enhancer (CAG) promoter (Niwa et al., 1991) was directly introduced into the VZ by in utero electroporation as reported previously (Tabata and Nakajima, 2001). For simplicity, when brains were transfected, for example, with GFP expression vector and EF1α promoter on embryonic day 13 (E13) and killed on E16, it is indicated as “EF1α-GFP/E13:E16” in this paper. Red fluorescent protein (DsRed2 or DsRed-Express; Clontech) expression vectors were used for time-lapse analysis.
Time-lapse imaging. Coronal brain slices (200 μm thick) from the anterior one-third of the cortex were placed on a Millicell-CM (pore size, 0.4 μm; Millipore, Bedford, MA), mounted in collagen gel, and cultured in Neurobasal medium containing B27 (Invitrogen, San Diego, CA). The dishes were then mounted in a CO2 incubator chamber (5% CO2 at 37°C) fitted onto a confocal microscope [LSM510 (Zeiss, Oberkochen, Germany) or FV300 (Olympus Optical, Tokyo, Japan)]. The dorsomedial or lateral region of the cortex was analyzed, and essentially the same results were obtained in both regions. Approximately 10-20 optical Z sections were obtained automatically every 30 min, and ∼20 focal planes (∼50 μm thickness) were merged to visualize the shape of the entire cell.
Immunostaining. Tissue samples were prepared as described previously (Tabata and Nakajima, 2001). To detect GABA, the animals were perfused with 4% paraformaldehyde with 0.1% glutaraldehyde. In other cases, 4% paraformaldehyde without glutaraldehyde was used. The primary antibodies used in this study were anti-Hu (1:200; Molecular Probes, Eugene, OR), which recognizes HuC and HuD, anti-calbindin (1:1000; Swant, Bellinzona, Switzerland), anti-GABA (1:500; Sigma, St. Louis, MO), anti-nestin (Rat 401; 1:1000; BD Biosciences, San Jose, CA), anti-neurofilament M (1:200; Chemicon, Temecula, CA), anti-βIII-tubulin (TuJ1; 1:1000; Babco, Richmond, CA) and anti-Tbr1 (1:1000) (Hevner et al., 2001). The dorsomedial region in the anterior one-third of the cortex was analyzed. Images were acquired with confocal microscopes [LSM410 or LSM510 (Zeiss) or FV300 (Olympus Optical)].
Results
Abundant multipolar neurons are present in the intermediate and subventricular zones
To examine the morphology of migrating neurons, we directly introduced the GFP or DsRed expression vectors into the cells of the cortical VZ in utero by electroporation (Tabata and Nakajima, 2001). In the EF1α-EGFP/E14:E17 brains (see Materials and Methods for the abbreviation), GFP-positive migrating cells were found in the developing CP, IZ, and SVZ (Fig. 1_A,B_). The GFP-expressing cells in the CP in both the dorsomedial cortex (Fig. 1_A_) and the lateral cortex (Fig. 1_B_) assumed a radially oriented bipolar shape with a thick leading process extending toward the pial surface and a thin trailing process, representing the typical locomotion-cell morphology (Fig. 1_C_). In contrast, many of the GFP-positive cells within the IZ-SVZ of both the dorsomedial and lateral cortices exhibited multipolar morphology (Fig. 1_D_), and the multipolar cells were especially abundant in the lower IZ and SVZ. The strong Hu immunoreactivity in the cytoplasm of the multipolar cells (Fig. 1_D_), as well as the positive staining with TuJ1 (Fig. 1_E-G_), indicated that the cells were indeed neurons. To investigate the relationship between the multipolar cells and the radial fibers, we subsequently stained the radial fibers with an anti-nestin antibody (Fig. 1_H_). Although some of the thin processes of the multipolar cells were apposed to the radial fibers (Fig. 1_H_, arrowhead), most of them extended independently from the radial fibers, suggesting that the multipolar cells are not associated highly with the radial fibers, unlike locomotion cells.
Figure 1.
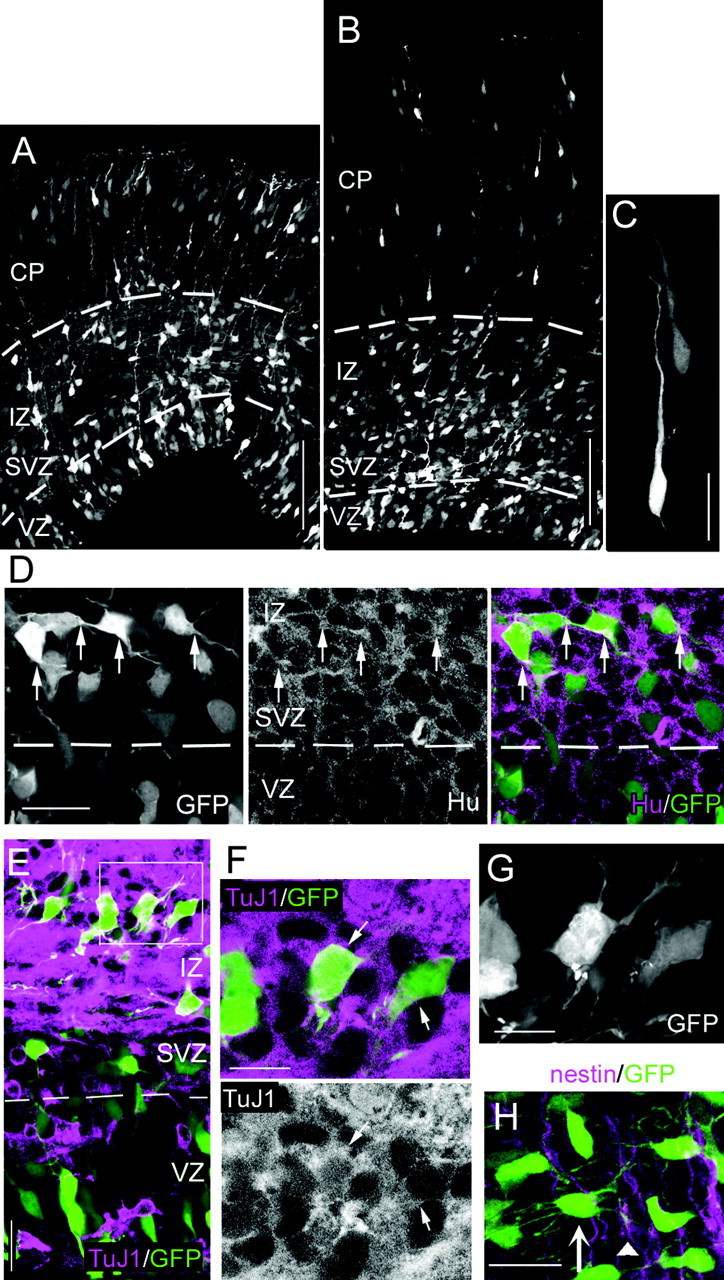
Histological features of multipolar cells in mouse developing cerebral cortex. A, B, Coronal sections of brains transfected with EF1α-GFP/E13:E16. In both the dorsomedial cortex (A) and the lateral cortex (B), most of the GFP-expressing cells in the IZ and SVZ exhibited multipolar cell morphology, whereas cells in the CP they had a radially oriented bipolar cell morphology. The dashed lines indicate the border between VZ and SVZ, or IZ and CP. C, High-magnification views of the GFP-positive cells in the CP. D-G, In the CAG-GFP/E14.5:E16 brains, the GFP-positive cells exhibiting multipolar cell morphology in the IZ-SVZ (D, left panel; E, F; extended focus view of the confocal image in F is shown in G) expressed the neuron markers Hu (D, middle and right panels) and TuJ1 (E, F). The dashed line in D and E indicates the border between VZ and SVZ. The arrows in D and E indicate the cytoplasm of multipolar cells. H, Although some of the thin processes of the multipolar cells were apposed to the radial fibers (arrowhead), which were stained with anti-nestin, most of them extended independently from the radial fibers. EF1α-GFP/E13:E16 brains were analyzed. Scale bars: A, B, 100 μm; C-E, H, 20 μm; F, G, 10 μm.
Multipolar neurons express a marker of projection neurons but not of interneurons
To determine whether the GFP-labeled multipolar neurons were derived from the cortical VZ instead of the ganglionic eminences, we then introduced the vectors into the dorsomedial region of the cortical VZ specifically and not into the ganglionic eminences by positioning the cathode ventrolaterally (Fig. 2_A_). Even in these experiments, however, abundant multipolar cells were still observed within the cortical IZ-SVZ (Fig. 2_B_). Moreover, the GFP-positive multipolar cells did not stain positive for calbindin (n = 0 of 96) (Fig. 2_C_) or GABA (n = 0 of 103) (Fig. 2_D_), two markers expressed by tangentially migrating neurons originating in ganglionic eminences (Anderson et al., 1997). Finally, the GFP-positive multipolar cells in the IZ-SVZ of the CAG-EGP/E12.5:E13.5 brains expressed Tbr1, a marker for subplate (SP) and early generated cortical projection neurons derived from the cortical VZ (Fig. 2_E_) (Hevner et al., 2001). These results suggest strongly that the GFP-positive multipolar cells we investigated in this study were derived from the cortical VZ. However, we have not ruled out the possibility that the tangentially migrating cells derived from the ganglionic eminences may also assume multipolar morphology in the cortical IZ-SVZ, because those cells were not visualized in this study.
Figure 2.
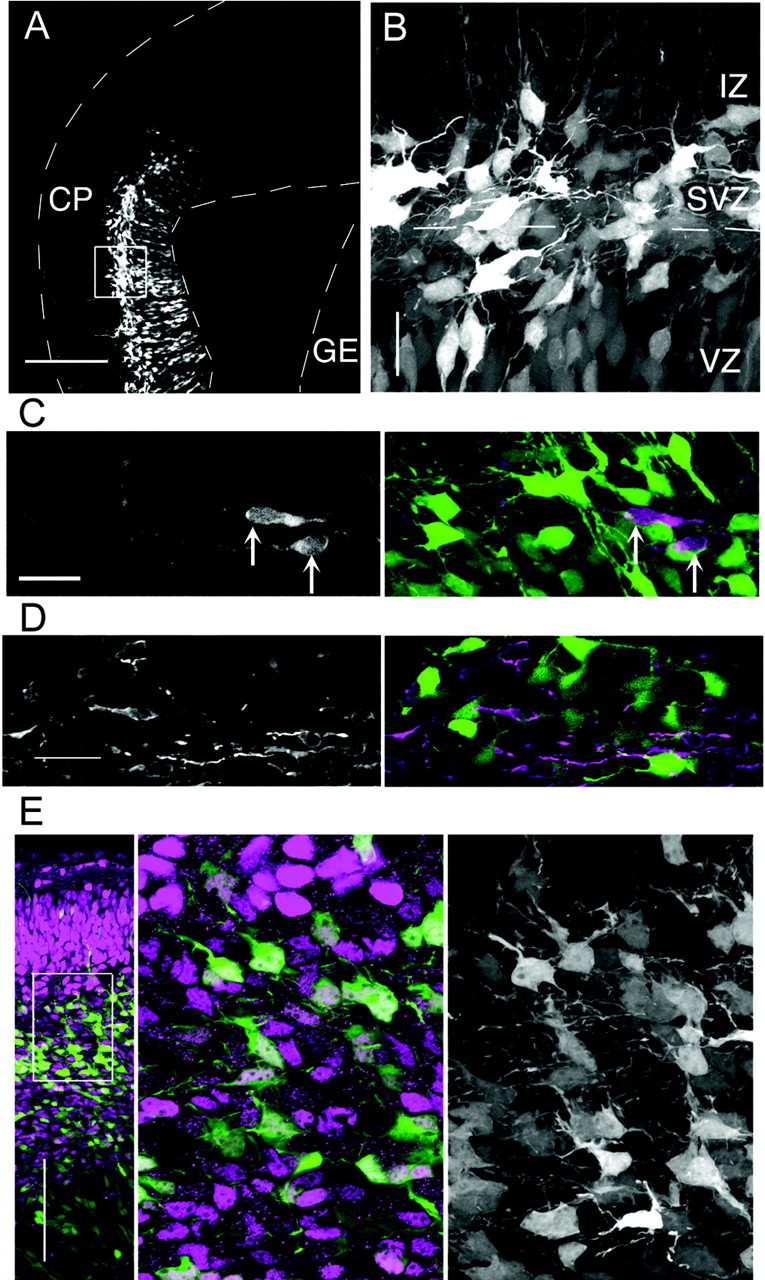
The GFP-labeled multipolar cells originated from the cortical VZ. A, Plasmid DNA was injected into the lateral ventricle on one side, and an anode was placed on the opposite side of the injected hemisphere so that the dorsomedial region was labeled selectively (CAG-GFP/E14.5:E16). The dashed lines indicate the margin of the tissue. GE, Ganglionic eminence. B, High magnification of the boxed region in A revealed that many of the GFP-expressing cells in the IZ-SVZ exhibited multipolar cell morphology, whereas the progenitor cells in the GE were not labeled (A). The dashed line indicates the border of VZ and SVZ. C, D, The GFP-positive multipolar cells (C and D, green in the right panels) were calbindin negative (C, left panel and purple in the right panel) and GABA negative (D, left panel and purple in the right panel). The calbindin-positive cells are indicated by the arrows. The EF1α-GFP/E13:E16 brains (C) and CAG-GFP/E14.5:E16 brains (D) were analyzed. E, Tbr1 immunostaining on the CAG-GFP/E12.5:E13.5 brains. High expression of Tbr1 was seen in the CP, and low expression was detected in the IZ (purple in the left and middle panels). A single confocal section of the boxed region in the left panel is shown in the middle panel. The GFP-positive (green) cells were Tbr1 positive (purple). The extended-focus view of the green channel of the middle panel revealed that the GFP-positive cells exhibited a multipolar cell morphology (right panel). Scale bars: A, E, 200 μm; B, 100 μm; C, D, 20 μm.
Behavior of multipolar neurons
Because the morphology of multipolar cells is very different from cells that migrate by the modes reported previously (locomotion and somal translocation), we attempted to determine whether the multipolar cells were indeed migrating by transfecting mouse brains with a DsRed expression vector in utero on E12.5 and performing time-lapse observations of the dorsomedial cortex in slice cultures on E14 (EF1α-DsRed/E12.5:E14). Many multipolar cells were found in the IZ at this stage of development, and in contrast to locomotion or somal-translocation cells, they did not exhibit fixed cell polarity and were extending and retracting thin processes in various directions in a very dynamic manner (Fig. 3_A,B_) (movie files, available at www.jneurosci.org). Many of the multipolar cells advanced slowly toward the pial surface (Fig. 3_A_, arrow in B); however, some of them remained in almost the same position while dynamically moving their processes (Fig. 3_B_, arrowhead). In the examples shown in Figure 3, A and B, the multipolar cells migrated radially at a rate of 2.3 μm/hr (Fig. 3_A_) or 1.8 μm/hr (Fig. 3_B_, arrow). The behavior of the multipolar cells in the lateral cortex was basically similar to their behavior in the dorsomedial cortex (data not shown). However, in CAG-DsRedExpress/E14.5:E18 brains, we observed that many of the migrating cells in the CP had radially oriented bipolar morphology (Fig. 3_C_, cells a-c) (movie file, available at www.jneurosci.org), and these cells migrated toward the pial surface linearly by locomotion. The migration rate of locomotion cells (9-12 μm/hr) was faster than that of multipolar migration cells (the migration rates of cells a-c were 12.0, 9.6, and 12.0 μm/hr, respectively). In the upper IZ or the SP, multipolar cells extended major leading processes radially toward the pial surface (Fig. 3_D_). These cells may have been in the process of transforming into the locomotion cells. Because the movement of multipolar cells resembled neither locomotion nor somal translocation, we dubbed this novel type of migration multipolar migration.
Figure 3.
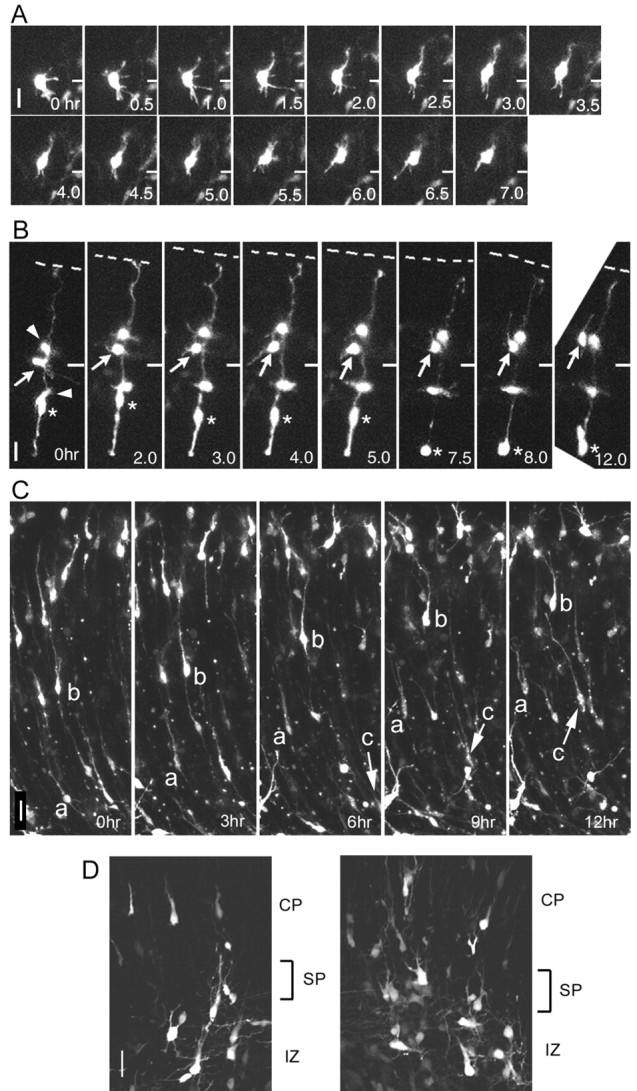
Time-lapse observation of multipolar migration. A, B, The movement of the multipolar cells was observed on living slices prepared from EF1α-GFP/E12.5:E14 brains. A, Multipolar cells advanced toward the pial surface (top) very slowly, while extending and retracting multiple processes dynamically. B, DsRed-positive multipolar cells were colocalized frequently with DsRed-positive radial glial cells. In this specimen, three labeled multipolar cells (arrowheads and arrow) were colocalized closely with a labeled radial glial cell, the body of which (asterisk) was undergoing mitosis (M phase at t = 7.5 hr). One of the multipolar cells, indicated by the arrow, advanced toward the pial surface (dashed line) by means of the dynamic movement of its processes and passed another multipolar cell. The short horizontal bars on the right in A and B represent the initial position (t = 0 hr) of the cell observed. C, The locomotion cells within the CP were observed on the living slices taken from the CAG-GFP/E14.5:E18 brains. The migration rates of cells a-c were measured (see Results) (movie files, available at www.jneurosci.org). The position of cell c is indicated by the arrow. D, The multipolar cells near the border between IZ and CP tend to extend major leading processes toward the pial surface. Scale bars, 20 μm.
To determine further the migration profile of the multipolar cells, we plotted the movements of each multipolar neuron in slice culture (Fig. 4_A_). These experiments revealed that the multipolar cells moved in various directions and changed direction frequently, although they generally tended to move toward the pial surface ultimately. In addition, their migration rates differed from cell to cell and changed over time. Many of the cells occasionally remained in nearly the same position for several hours during their migration (Fig. 4_A_, dotted circles). Thus, whereas the mean migration rate of these multipolar cells was 4.4 μm/hr (1.6-6.4 μm/hr), the mean net change in positions per hour in the radial direction was 2.2 μm [-14 (sometimes they migrated backward) to 55 μm in 10 hr]. These results indicate that the characteristics of multipolar migration are unique and different from those of locomotion or somal translocation.
Figure 4.
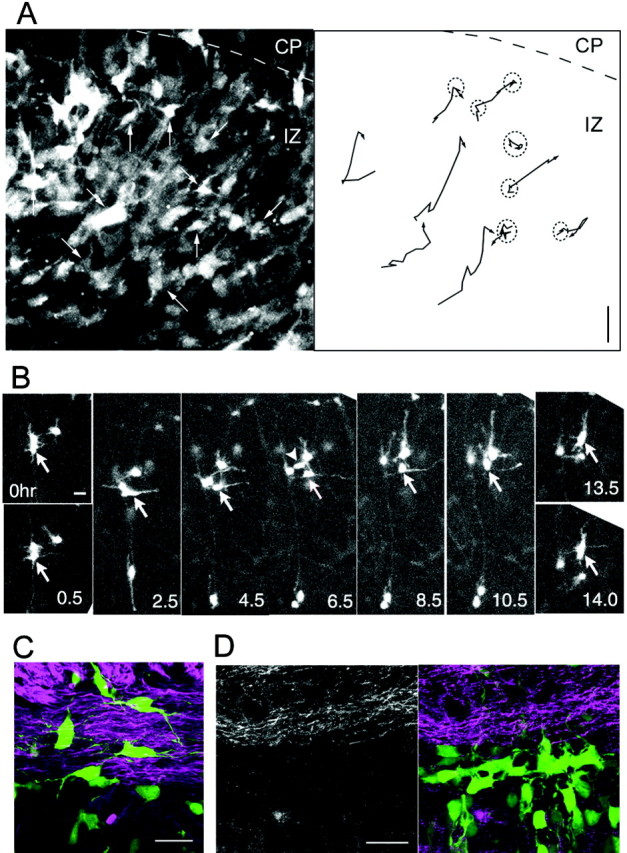
Migratory course of the multipolar neurons. A, Tracing of multipolar cell movement. The slices were prepared from CAG-GFP/E12.5:E14 brains, and time-lapse observations were made in the IZ. The position of 10 individual multipolar cells was plotted at 1 hr intervals for 10 hr. The image taken 2 hr after the first time point is shown in the left panel, and the trajectory is shown in the right panel. Many of the multipolar cells occasionally remained in nearly the same positions for several hours during their migration (dotted circles). The border between the IZ and CP is indicated by a dashed line. The traced cells are indicated by the arrows. B, Tangential jump of multipolar cells. One multipolar cell (arrow) temporarily assumed locomotion cell-like morphology by extending a thick process tangentially and jumping in that direction (t = 2.5-8.5 hr). The cell then retracted the thick tangential process and extended a radially oriented thick process. Finally, the cell resumed radial multipolar migration toward the pial surface (top) (t = 10.5-14 hr). The other cell (arrowhead; t = 6.5) also jumped tangentially (t = 4.5-8.5 hr) (movie file, available at www.jneurosci.org). C, D, The cell bodies and tangential processes of the multipolar cells (green) tended to be oriented in parallel with the NF-positive axon bundles (purple). CAG-GFP/E14.5:E16 (C) or CAG-GFP/E16:E17.5 (D) brains were analyzed. Scale bars, 20 μm.
While observing the behavior of the multipolar cells, we found that they occasionally jumped tangentially (Fig. 4_B_) (movie file, available at www.jneurosci.org). One of the tangentially oriented processes of these cells thickened transiently, and their cell bodies then translocated in the direction of the thickened major process. Finally, the cells retracted the tangential process and reassumed a multipolar morphology to continue their radial migration toward the pial surface. Thus, multipolar cells may have some affinity for radial fibers during migration, but the affinity did not appear to be very strong.
Because the cell bodies and processes of the multipolar cells tended to be oriented tangentially (mediolateral orientation) when they appeared above the VZ (Figs. 1_A,B_, 2_B_), we hypothesized that the tangential axon bundles in the IZ-SVZ affected multipolar cell orientation and served as the initial target of multipolar migration. To test this hypothesis, we visualized the axons by staining with anti-neurofilament (NF) antibody (Fig. 4_C,D_). The multipolar cells were observed to orient along the axon bundles, suggesting that they might interact with each other (Fig. 4_C_). However, when examined at a later stage, the multipolar cells began to orient tangentially even before they reached the strongly NF-positive structure, suggesting that they may share some unknown directional cues in the lower IZ-SVZ instead (Fig. 4_D_).
To quantitate the population of cells exhibiting multipolar migration, we then counted the multipolar migration cells on the time-lapse sequences in living slices from EF1α-DsRed/E12.5:E14 brains. Among the 122 cells that were observed to move within the IZ of the dorsomedial cortex, in 107 cells, the mode was multipolar migration (88%). Together, these in vitro observations and in vivo data from fixed brains indicate that multipolar migration must be used by the major population of migrating cells in the cortical IZ and SVZ.
Discussion
Multipolar migration is a mode of migration distinct from locomotion or somal translocation
We found that the major mode of migration in the IZ-SVZ was multipolar migration, a mode of migration that is distinct from locomotion and somal translocation. The mean net change in positions per hour of the multipolar migration cells (2.2 μm/hr) was much smaller than that of the locomotion cells in the CP (9-12 μm/hr). A previous study involving sequential labeling experiments in vivo with [3H]thymidine and bromodeoxyuridine showed that mean net change in positions per hour of migrating neurons in the lower IZ-SVZ of E14.5 mouse embryos was 2 μm/hr (Takahashi et al., 1996), a finding that is consistent with our observation of multipolar migration cells. The migration rate by locomotion that we observed in this study (9-12 μm/hr) was comparable with that observed by O'Rourke et al. (1992) (11 μm/hr) but slower than reported by Nadarajah et al. (2001) (35 μm/hr). This discrepancy may be attributable to the difference in experimental conditions in the slice culture. Takahashi et al. (1996) reported an in vivo migration rate of E14-born cells in the CP of 6.4 μm/hr. Because most migrating cells in the CP seemed to migrate by locomotion (Figs. 1_A-C_, 2_B_), our estimate (9-12 μm/hr) in our slice culture system may be closer to, but still faster than, the in vivo migration rate. This may reflect the difference between the in vivo and the in vitro environment of the migrating neurons.
The three modes of migration may differ in their dependency on the guidance cues for radial migration. In locomotion, the radial fibers provide the direction of migration and act as the scaffold. If the leading process extending toward the pial surface were maintained, the locomotion cells might be able to migrate along the radial fibers without any additional positional cues. In somal translocation, the ascending process from the cell body reaches the final destination so that migrating neurons can arrive at the final destination without any additional positional cues. In contrast, in multipolar migration, neurons do not associate closely with the radial fibers, and they sometimes jump tangentially during their radial migration. Therefore, it is likely that the multipolar cells sense some directional cue when they resume radial migration. These findings suggest that some environmental factor that directs migrating neurons toward the pial surface exists in the developing cortex.
Biological meaning of multipolar migration
During multipolar migration, multipolar cells repeatedly extend and retract their processes in a very dynamic manner. This kind of behavior is also observed during the pathfinding activity of axonal growth cones while in the “decision region,” in which the growing axon changes direction (Godement et al., 1990; Halloran and Kalil, 1994). These observations suggest that multipolar movement may be needed to search for environmental cues related to the direction of axon growth or radial migration.
Although locomotion cells and somal translocation cells are restricted in their tangential dispersion because of their dependence on radial fibers (locomotion) and long radial processes (somal translocation), multipolar cells seem to be free to migrate in tangential directions (Fig. 4_A,B_). This flexibility may contribute to passing through the IZ when several potential obstacles to radial migration exist, such as afferent and efferent fibers, tangentially migrating neurons, and previous radially migrating neurons.
Although we observed that the multipolar cells accounted for the major population of the GFP-labeled migrating cells in the IZ-SVZ, we did not find multipolar migration cells in the CP, in which most migrating cells exhibited locomotion morphology (Figs. 1_A-C_, 3_C_). These locomotion cells in the CP may have migrated by locomotion all the way from the cortical VZ, and they may be a population independent from the multipolar cells in the IZ-SVZ. Another possibility is that the locomotion CP cells may have derived from multipolar IZ-SVZ cells. If the latter is true, the multipolar cells must have transformed into locomoting cells before entering the CP. When we investigated the morphology of the migrating cells in the IZ-SVZ, we found only a small population of cells exhibiting locomotion (Fig. 1_A,B_). In addition, the multipolar cells generally ultimately migrated toward the pial surface (Fig. 4_A_), expressed neuronal markers (Figs. 1_D-G_, 2_E_), and exhibited locomotion cell-like morphology beneath the CP in vivo (Fig. 3_D_); in addition, at least most of them were not apoptotic, as revealed by terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling staining (n = 0 of 107; data not shown) (Thomaidou et al., 1997). These results suggest that the multipolar cells are likely to enter the CP as locomotion cells. Additional experiments, however, will be required to draw a final conclusion.
Footnotes
This work was supported by the Japan Science and Technology Corporation, the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Japan Society for the Promotion of Science, a Keio University Special grant-in-aid for innovative collaborative research projects, and the Sumitomo Foundation. We thank S. Nagata for the EF1α promoter, J. Miyazaki for the CAG promoter, and R. Hevner for anti-Tbr1.
Correspondence should be addressed to Dr. Kazunori Nakajima, Department of Anatomy, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan. E-mail: kazunori@sc.itc.keio.ac.jp.
Copyright © 2003 Society for Neuroscience 0270-6474/03/239996-06$15.00/0
References
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JLR ( 1997) Interneuron migration from basal forebrain to neocortex: dependence on Dix genes. Science 278: 474-476. [DOI] [PubMed] [Google Scholar]
- Angevine JB, Sidman RL ( 1961) Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature 192: 766-768. [DOI] [PubMed] [Google Scholar]
- Berry M, Rogers AW ( 1965) The migration of neuroblasts in the developing cerebral cortex. J Anat 99: 691-709. [PMC free article] [PubMed] [Google Scholar]
- Gadisseux JF, Kadhim HJ, van den Bosch de Aguilar P, Caviness VS, Evrard P ( 1990) Neuron migration within the radial glial fiber system of the developing murine cerebrum: an electron microscopic autoradiographic analysis. Brain Res Dev Brain Res 52: 39-56. [DOI] [PubMed] [Google Scholar]
- Godement P, Salaun J, Mason CA ( 1990) Retinal axon pathfinding in the optic chiasm: divergence of crossed and uncrossed fibers. Neuron 5: 173-186. [DOI] [PubMed] [Google Scholar]
- Halloran MC, Kalil K ( 1994) Dynamic behaviors of growth cones extending in the corpus callosum of living cortical brain slices observed with video microscopy. J Neurosci 14: 2161-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner RF, Shi L, Justice N, Hsueh Y, Sheng M, Smiga S, Bulfone A, Goffinet AM, Campagnoni AT, Rubenstein JL ( 2001) Tbr1 regulates differentiation of the preplate and layer 6. Neuron 29: 353-366. [DOI] [PubMed] [Google Scholar]
- Marin O, Rubenstein JL ( 2001) A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci 2: 780-790. [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Okano H, Ogawa M ( 2001) Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron 31: 727-741. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Brunstrom JE, Grutzendler J, Wong RO, Pearlman AL ( 2001) Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci 4: 143-150. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Mikoshiba K, Miyata T, Kudo C, Ogawa M ( 1997) Disruption of hippocampal development in vivo by CR-50 mAb against reelin. Proc Natl Acad Sci USA 94: 8196-8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J ( 1991) Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108: 193-199. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR ( 2001) Neurons derived from radial glial cells establish radial units in neocortex. Nature 409: 714-720. [DOI] [PubMed] [Google Scholar]
- Nowakowski RS, Rakic P ( 1979) The mode of migration of neurons to the hippocampus: a Golgi and electron microscopic analysis in foetal rhesus monkey. J Neurocytol 8: 697-718. [DOI] [PubMed] [Google Scholar]
- O'Rourke NA, Dailey ME, Smith SJ, McConnell SK ( 1992) Diverse migratory pathways in the developing cerebral cortex. Science 258: 299-302. [DOI] [PubMed] [Google Scholar]
- Rakic P ( 1972) Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol 145: 61-84. [DOI] [PubMed] [Google Scholar]
- Shoukimas GM, Hinds JW ( 1978) The development of the cerebral cortex in the embryonic mouse: an electron microscopic serial section analysis. J Comp Neurol 179: 795-830. [DOI] [PubMed] [Google Scholar]
- Stensaas LJ ( 1967) The development of hippocampal and dorsolateral pallial regions of the cerebral hemisphere in fetal rabbits. II. Twenty millimeter stage, neuroblast morphology. J Comp Neurol 129: 71-84. [DOI] [PubMed] [Google Scholar]
- Tabata H, Nakajima K ( 2001) Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscience 103: 865-872. [DOI] [PubMed] [Google Scholar]
- Tabata H, Nakajima K ( 2002) Neurons tend to stop migration and differentiate along the cortical internal plexiform zones in the Reelin signal-deficient mice. J Neurosci Res 69: 723-730. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness Jr VS ( 1996) Interkinetic and migratory behavior of a cohort of neocortical neurons arising in the early embryonic murine cerebral wall. J Neurosci 16: 5762-5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Nakamura K, Okamoto K, Kaneko T ( 2001) Radial glia is a progenitor of neocortical neurons in the developing cerebral cortex. Neurosci Res 41: 51-60. [DOI] [PubMed] [Google Scholar]
- Thomaidou D, Mione MC, Cavanagh JF, Parnavelas JG ( 1997) Apoptosis and its relation to the cell cycle in the developing cerebral cortex. J Neurosci 17: 1075-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetsuki T, Naito A, Nagata S, Kaziro Y ( 1989) Isolation and characterization of the human chromosomal gene for polypeptide chain elongation factor-1 alpha. J Biol Chem 264: 5791-5798. [PubMed] [Google Scholar]