Spontaneous Calcium Transients in Developing Cortical Neurons Regulate Axon Outgrowth (original) (raw)
Abstract
Growth cones of cortical axons pause for many hours in preparation for axon branching. They become large and complex compared with small advancing growth cones. We wanted to investigate whether calcium transients regulate the advance of mammalian CNS growth cones. We found that spontaneous calcium transients in developing cortical neurons have characteristic patterns, frequencies, and amplitudes. Importantly, neurons with large paused growth cones exhibit high-frequency spontaneous calcium transients, which are rare in those with small advancing growth cones. The incidence, frequencies, and amplitudes of calcium transients are inversely related to rates of axon outgrowth. The transients are mediated primarily by L-type voltage-gated calcium channels, and silencing them with channel blockers promotes axon outgrowth. Thus calcium transients regulate growth cone advance by direct effects on the growth cone.
Keywords: calcium transients, growth cone, cortical development, axon guidance, axon branching, L-type calcium channels
Introduction
Axons are guided to their targets by changes in the behaviors of their terminal growth cones. In the mammalian CNS, however, interstitial axon branches rather than terminal growth cones often innervate target neurons (O'Leary et al., 1990). Previously we found that growth cones demarcate the locations of axon branch points. In the corpus callosum of living cortical slices, axonal growth cones develop large complex morphologies and pause for many hours in regions from which branches later emanate (Halloran and Kalil, 1994). In contrast, rapidly extending growth cones have small simple morphologies. Similarly, growth cones of dissociated cortical neurons become large and complex during stalling behaviors that can last for hours or days. When the growth cone re-extends, it leaves behind filopodial or lamellipodial remnants from which axon branches subsequently extend (Szebenyi et al., 1998). These findings link pausing behaviors of the growth cone to branching by the axon (Kalil et al., 2000). Large complex growth cones are found consistently at choice points in the nervous system where they make decisions about growth in new directions (Mason and Erskine, 2000) including branching. In the central regions of pausing growth cones, microtubules form prominent loops (Tsui et al., 1984; Sabry et al., 1991; Tanaka and Kirschner, 1991; Dent et al., 1999), but their role in regulating growth cone advance is unclear.
Calcium has been shown to be an important regulator of neurite extension (Gomez and Spitzer, 2000; Spitzer et al., 2000). Axon outgrowth occurs within optimal levels of intracellular calcium but slows or ceases when calcium is above or below these levels (Kater and Mills, 1991). Recent studies have shown that transient as opposed to sustained levels of intracellular calcium are important in regulating growth cone advance. In growth cones of dissociated chick DRG and_Xenopus_ spinal neurons (Gomez et al., 1995; Gu and Spitzer, 1995; Gomez and Spitzer, 1999), the frequencies of spontaneous Ca2+ transients were shown to be inversely related to rates of axon outgrowth. In in vivo studies in_Xenopus_ spinal cord (Gomez and Spitzer, 1999), imposition or suppression of Ca2+ transients in specific growth cones was sufficient to slow or accelerate axon outgrowth, respectively.
Given the importance of growth cone pausing for axon guidance and development of axon branches, we wanted to investigate whether Ca2+ transients might also regulate the advance of mammalian CNS growth cones. Spontaneous Ca2+ transients have been documented in cortical neurons within living slices during development of cortical circuitry (Yuste et al., 1992; Garaschuk et al., 2000; Mao et al., 2001). Ca2+ transients also play a role in dendritic development (Lohmann et al., 2002; Redmond et al., 2002). However, the nature of Ca2+ transients in cortical neurons has not been extensively characterized, and their role in regulating axon outgrowth is not known. In the present study, we imaged Ca2+ transients in developing cortical neurons. For the first time we report that Ca2+ transients regulate the advance of mammalian CNS growth cones.
Materials and Methods
Dissociated cell cultures. Cultures were prepared from cortical tissues obtained from embryonic day 14 (E14) and postnatal day 0 (P0)–P3 golden Syrian hamsters (Mesocricetus auratus) as described previously (Dent et al., 1999; Dent and Kalil, 2001; Szebenyi et al., 2001). Glass coverslips were coated with poly-d-lysine. We used etched grid glass coverslips (Bellco, Vineland, NJ) to provide landmarks for locating the positions of neuronal processes over time.
Time-lapse Ca2+ imaging of cortical neurons. Twenty-four hours after plating, cortical neurons were loaded with 2–4 μm fluo-4 AM (Molecular Probes, Eugene, OR) predissolved in 0.01% pluronic acid (Molecular Probes) and 0.1% dimethylsulfoxide (DMSO) for 30 min. Excess dye was washed out with three to five rinses of serum-free medium. The coverslips containing the neurons were then enclosed in a chamber consisting of a 15 mm glass ring (Thomas Scientific, Swedesboro, NJ) and a 25 mm round coverslip (Fisher, Itasca, IL). The dishes were returned to the incubator for 30–60 min. Fluorescence imaging of intracellular Ca2+dynamics for periods ranging from 10 min to 1 hr was performed with a Nikon (Tokyo, Japan) TE300 Quantum inverted epifluorescence microscope equipped with a Princeton Instruments (Trenton, NJ) MicroMax 512BFT cooled CCD camera containing a back-thinned, frame-transfer EEV CCD57-10 chip (Roper Scientific). The imaging system was controlled byMetamorph Software (Universal Imaging, West Chester, PA). Neurons were imaged in time lapse with a 60× magnification, 1.4 numerical aperture (NA) Plan Apo CF160 objective (Nikon). We were interested in the relationship between axon outgrowth and Ca2+ activities. Therefore we selected for study neurons with large pyramidal morphologies, because in vivo these neurons have long efferent axons. Images were captured every 1–15 sec, with 300–500 msec exposures, and under low-light level conditions. Images were collected at a slow transfer rate, which reduces background noise, and binned (2 × 2). In some experiments, differential interference contrast (DIC) images were taken in rapid succession with fluorescent Ca2+ images to monitor the behaviors of the growth cones.
Pharmacological agents. Stock solutions were prepared by solubilizing drugs in DMSO or methanol according to the manufacturer's recommendations. The following drugs in stock solutions were diluted in serum-free medium and bath applied to cultures: the general voltage-gated Ca2+ channel (VGCC) blocker Ni2+ (2 mm; Sigma, St. Louis, MO), the L-type VGCC antagonists nifedipine (Calbiochem, La Jolla, CA) and nimodipine (Calbiochem), the P/Q-type VGCC antagonist ω-agatoxin IVA (Calbiochem), the N-type VGCC antagonist ω-conotoxin GVIA (Calbiochem), the sodium channel blocker tetrodotoxin (TTX; Sigma), the endoplasmic reticulum Ca2+ ATPase blocker thapsigargin (Alomone Labs, Jerusalem, Israel), and the ryanodine receptor antagonist dantrolene (Alomone Labs).
Long-term drug treatment and immunocytochemistry. For long-term treatments, 20 μm nifedipine was added to the cultures 15 hr after plating. Cultures were fixed at 48 hr after plating. Fixation and immunocytochemistry were performed as described previously (Dent and Kalil, 2001). Primary antibodies to the α 1C and 1D subunits of the L-type Ca2+channel (Calbiochem) were diluted 1:100 in blocking buffer and incubated with cortical neurons at 4°C overnight, followed by application of a Cy3-conjugated anti-rabbit secondary antibody (Jackson ImmunoResearch, West Grove, PA) at a dilution of 1:500 in blocking buffer for 1 hr. In some experiments quantifying and comparing the expression of L-type Ca2+channels in embryonic and postnatal neurons, the E14 and P1 cultures were prepared on the same day. They were fixed nearly at the same time after plating and processed with first and secondary antibodies simultaneously. The images were taken on the same day and under the same conditions including exposure time, illumination, and scaling.
Image processing and data analysis. To evaluate changes in [Ca2+]i, average fluorescent pixel intensity of the entire growth cone in each image was digitally quantified with Metamorph software. This value was subtracted from background and normalized to baseline fluorescence intensity with Microsoft (Redmond, WA) Excel software. Fluorescence increases exceeding 150% of baseline were characterized as Ca2+ transients (Gu and Spitzer, 1994). This was reconfirmed by frame-by-frame examination of the time-lapse movie (available at http://kalil.anatomy.wisc.edu). Transients were readily distinguished from spurious fluctuations arising from environmental factors, because fluctuations had very low amplitudes, occurred randomly, had no particular pattern, and were not sustained. The kinetics of Ca2+ transients was estimated in the time-lapse images captured every second. The rise time of Ca2+ transients was measured as the interval between initiating baseline and peak signal, and the decay time of Ca2+ transients was defined as the time from peak signal to baseline. Fluorescence intensity of immunostained neurons was quantified with Metamorph software.
Analysis of neurite length. For measurements of axon and branch length, images of neurons were acquired at 48 hr with a 20× magnification, 0.7 NA Neofluor CF160 objective. The distance from the cell body to the distal extent of the central region of the growth cone was measured as the axon length. To measure the rate of advance of growth cones, images of neurons were acquired at 24 hr, and their positions were recorded by etched markings on the coverslips. At 3 and 25 hr after drug application, the same population of neurons was again imaged. The outgrowth of the growth cone was determined by subtracting the axon length measured at 24 hr in vitro from the axon length measured at 27 or 49 hr. Statistical analysis was performed using Sigmastat (Jandel Scientific, Corte Madera, CA). Graphs were created in Sigmaplot (SPSS, Chicago, IL). Images were processed with Metamorph 4.62 and Photoshop 6.0 (Adobe Systems, Mountain View, CA). Images shown in the figures were enhanced using the unsharp mask filter and brightness–contrast adjustment functions in Adobe Photoshop. Time lapse images were assembled into QuickTime movies (Premiere; Adobe Systems).
Results
Spontaneous Ca2+ transients occur in developing cortical neurons
To determine whether spontaneous fluctuations in [Ca2+]i occur in dissociated cortical neurons, we loaded early postnatal (0–3 d) cortical neurons with the Ca2+ indicator dye fluo-4 and imaged the neurons for periods of 10–60 min at intervals ranging from every second to every 15 sec. These procedures did not cause detectable damage to the neurons, and their dendritic and axonal processes maintained motility during the entire imaging period. Imaging was performed on cultures 24 hr after plating at low density. At this stage of development, neurons were still extending processes, but the single long axon was already distinguishable from the uniformly short dendrites. Because most of the processes were not in contact with other cells, there was probably little synaptic activity among cortical neurons. A large percentage (57.4%) of the neurons in the cultures showed spontaneous fluctuations in levels of [Ca2+]i. These Ca2+ transients occurred rapidly and appeared instantaneously throughout the cell body, dendrites, the axon, and the entire growth cone, including filopodia. However, even in neurons with axons longer than 300 μm, transients were propagated too rapidly to determine whether they originated in the cell body or the growth cone.
Ca2+ transients are most prevalent in cortical neurons with large paused growth cones
To determine whether transient elevations in [Ca2+]i are related to axon outgrowth, we focused on measurements of Ca2+ transients in axonal growth cones. In previous studies, we found that cortical growth cone morphologies are well correlated with their rates of extension (Halloran and Kalil, 1994; Szebenyi et al., 1998). Small, simple, bullet-shaped growth cones usually extend rapidly whereas, large complex growth cones with expanded lamellipodia and numerous filopodia pause for many hours. These growth cones develop prominent microtubule loops in their central region before re-extension of the axon and development of branches (Dent et al., 1999; Dent and Kalil, 2001). Therefore, we compared the prevalence of Ca2+ transients in growth cones with morphologies characteristic of each of these stages in outgrowth, pausing, and branching (Fig. 1and Table 1). Small simple growth cones showed very few transient elevations in [Ca2+]i (Fig.1A), whereas all of the growth cones with large complex morphologies showed frequent Ca2+transients (Fig. 1B–F). As illustrated in Figure 1, during the 30 sec imaging periods that were part of longer (20–60 min) sequences, large complex growth cones showed at least one Ca2+ transient. Moreover, the amplitude relative to baseline of the Ca2+transients appeared highest in the large paused growth cones that contained a prominent microtubule loop (Fig. 1D). This growth cone also showed two Ca2+transients in 30 sec, in contrast to the one transient exhibited by the other growth cones shown in Figure 1. We found several examples (Fig.1G) in which a large paused growth cone exhibited frequent large Ca2+ fluctuations, whereas within the same microscopic field, two nearby small growth cones showed no Ca2+ transients (see the movie available at http://kalil.anatomy.wisc.edu). To confirm that growth cone morphologies reflected rates of extension in these experimental conditions, we measured the areas and rates of advance of 45 growth cones (15 growth cones in each group) included in the Ca2+ measurements in Table 1 (Fig.1H). Growth cones with prominent microtubule loops were the largest, more than twice the area of those lacking microtubule loops. Measurements of the rates of extension of growth cones of different morphologies showed that large complex growth cones maintained motility but failed to advance during the 10–60 min imaging period, whereas small simple growth cones extended at an average rate of 14.6 μm/hr. Together, these results show that Ca2+ transients are most prevalent in large pausing growth cones but occur only rarely in small advancing growth cones.
Fig. 1.
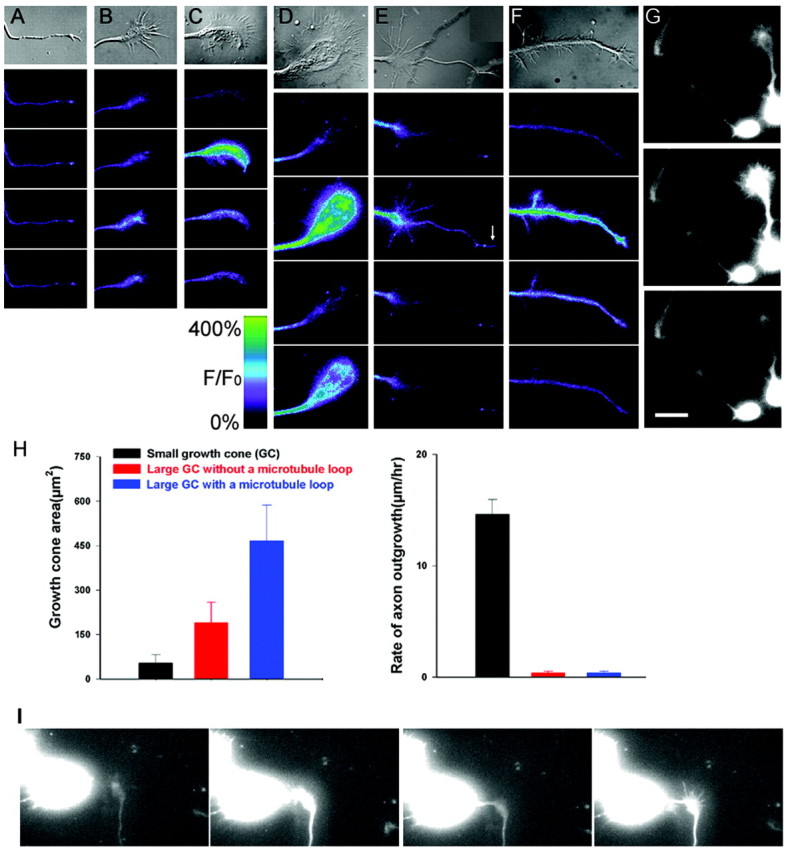
Ca2+ transients occur preferentially in growth cones with large complex morphologies.A–F, Ca2+ transients in cortical growth cones with morphologies representative of progressive stages in growth cone extension, pausing, and branching. Top, DIC images of each growth cone followed by pseudocolor fluorescence images of [Ca2+]i in the growth cone at 10 sec intervals. A, A small simple growth cone shows no detectable Ca2+ fluctuations. B, A pausing growth cone without a central microtubule loop exhibits a single Ca2+ transient of relatively low amplitude.C, A large paused growth cone with a partially formed microtubule loop (a large “transitional” growth cone) shows a single high-amplitude Ca2+ transient at 10 sec.D, A large paused growth cone with a prominent microtubule loop has the highest amplitude Ca2+transients, which occur at 10 and 30 sec. E, A pausing growth cone from which an axon is re-extending exhibits a single transient of moderate amplitude at 10 sec. The growth cone at the tip of the new axon (arrow) shows a simultaneous Ca2+ transient. F, A branching axon exhibits a single high-amplitude Ca2+ transient at 10 sec. G, Sequence of fluorescence images at 10 sec intervals showing changes in Ca2+ levels in three different cortical neurons in close proximity (see supplemental movie 1; available at http://kalil.anatomy.wisc.edu). The smaller growth cones to the left and center show little fluctuation in Ca2+ levels, in contrast to the large paused growth cone, which shows a very large Ca2+transient at 10 sec. The scale shows relative fluorescence intensity over baseline in pseudocolor images. H, Left, Average areas of growth cones of three different morphologies, exemplified by growth cones in A, B, and_D_. Right, Average growth rates of these growth cones. I, Sequence of fluorescence images of a growth cone showing increase in calcium levels as the growth cone changes from extending (0 min) to pausing (6, 10, and 16 min) in response to contact with another cell in the dish (see supplemental movie 2; available at http://kalil.anatomy.wisc.edu). Scale bar: (in_G_), 10 μm.
Table 1.
The occurrence of calcium transients in growth cones of different morphologies
| Growth cone (GC) type | Number of neurons with calcium transients | Number of neurons without calcium transients | % of neurons with calcium transients |
|---|---|---|---|
| Small GC | 20 | 89 | 18% |
| Large GC without a microtubule loop | 29 | 27 | 52% |
| Large transitional GC | 124 | 50 | 71% |
| Large GC with a microtubule loop | 89 | 18 | 83% |
| Large GC re-extending | 28 | 12 | 70% |
| Branching axon (GC of primary axon) | 35 | 8 | 81% |
Cortical growth cones undergo transitions from paused to growth states over long time periods of up to several days (Szebenyi et al., 1998). Thus it was not possible to monitor fluctuations in Ca2+ levels in the same growth cone for many hours or several days as growth cones underwent transitions from pausing to extending. However, in one fortuitous case, we monitored Ca2+ activity in a growth cone that went from extending to pausing after contact with another cell (see the movie available at http://kalil.anatomy.wisc.edu). While the growth cone was advancing at 15 μm/hr, it showed no Ca2+ activity (Fig. 1I). Within minutes after contact with a cell body, the growth cone stopped advancing and exhibited high Ca2+ activity (one transient per minute) that continued during the rest of the 1 hr imaging period. As shown in the image at 16 min, the growth cone also enlarged. This observation demonstrates that the transition from growth cone advancing to pausing is accompanied by a rapid development of persistent Ca2+ transients.
Ca2+ transients in growth cones have characteristic patterns, frequencies, and amplitudes
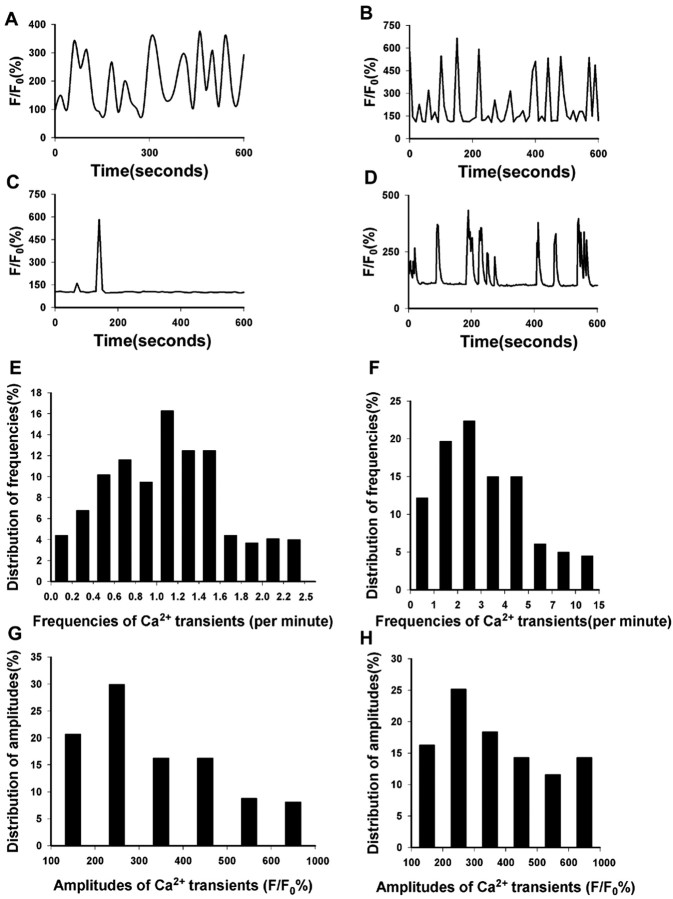
Characterization of Ca2+ transients in pausing growth cones (n = 420) revealed that patterns of Ca2+ oscillations occurred with varying temporal patterns in different neurons. As shown in Figure2A–D, these repetitive patterns of Ca2+ transients were characteristic for individual growth cones and remained constant during the entire 20–60 min imaging period. Measurements of the frequencies and amplitudes of Ca2+ transients also showed wide variation among different growth cones. When imaging intervals of 10–15 sec were used, frequencies varied from 0.1 to 2.5 transients per minute. Analysis of frequency distributions in 295 neurons showed an average frequency of 1.0 ± 0.1 transient per minute (Fig. 2E). However, imaging neurons every 1–5 sec (n = 147) revealed a more accurate average frequency of 3.2 ± 0.1 per minute (Fig. 2F). Amplitudes of the Ca2+ transients also varied widely, ranging from 50 to 1000% above baseline (Fig.2G,H). To determine the kinetics of Ca2+ transients, we measured their rise and decay times in 35 large paused growth cones at intervals of 1 sec. Ca2+ transients had a rapid rise time of 2.14 ± 0.2 sec and a decay time of 5.5 ± 0.3 sec. Ca2+ transients with a rapid rise and short duration are consistent with Ca2+spikes observed in other systems (Gu et al., 1994).
Fig. 2.
Ca2+ transients in growth cones have characteristic temporal patterns, frequencies, and amplitudes.A–D, Examples of characteristic temporal patterns of Ca2+ transients in four different large paused growth cones. Changes in fluorescence intensity are shown relative to baseline (_F/F_0%). Note that, over the 10 min imaging period shown, the pattern of Ca2+fluctuations is consistent and repetitive. These patterns persist for up to 1 hr (data not shown). E, F, Distribution of frequencies of Ca2+ transients per minute in the entire population of growth cones included in this study.E, Ca2+ imaging was performed at 10–15 sec intervals in growth cones of varying morphologies (n = 295). F, Ca2+ imaging was performed at 1–5 sec intervals in growth cones of varying morphologies (n = 147).G, H, Distribution of amplitudes of Ca2+ transients in percentages of growth cones with various morphologies. Amplitudes are expressed as a percentage of baseline fluorescence. The growth cone population in G_and H corresponds to that in E and_F, respectively. Values for amplitudes were obtained from the peak amplitudes in plots of transients for individual growth cones, as shown in A–D.
Although Ca2+ transients observed in cortical growth cones generally reflected those in the cell body, we found several examples in which patterns of Ca2+ transients appeared to be endogenous to the growth cone. As shown in Figure3A, two growth cones branched from a single axon. Although the Ca2+transients were initiated simultaneously and had similar frequencies in both growth cones, the characteristics of the rise and decay times and the peak amplitudes were different in each growth cone (Fig.3C). In several other cases, we found growth cones that had been severed from the cell body. One such growth cone (Fig.3B) had a large complex morphology and exhibited high-frequency Ca2+ transients, although their rise and decay times were faster than in normal attached growth cones (Fig. 3D). The growth cone appeared healthy and remained motile for several hours. These results suggest that growth cones can have endogenous Ca2+ transients independent of the cell body.
Fig. 3.
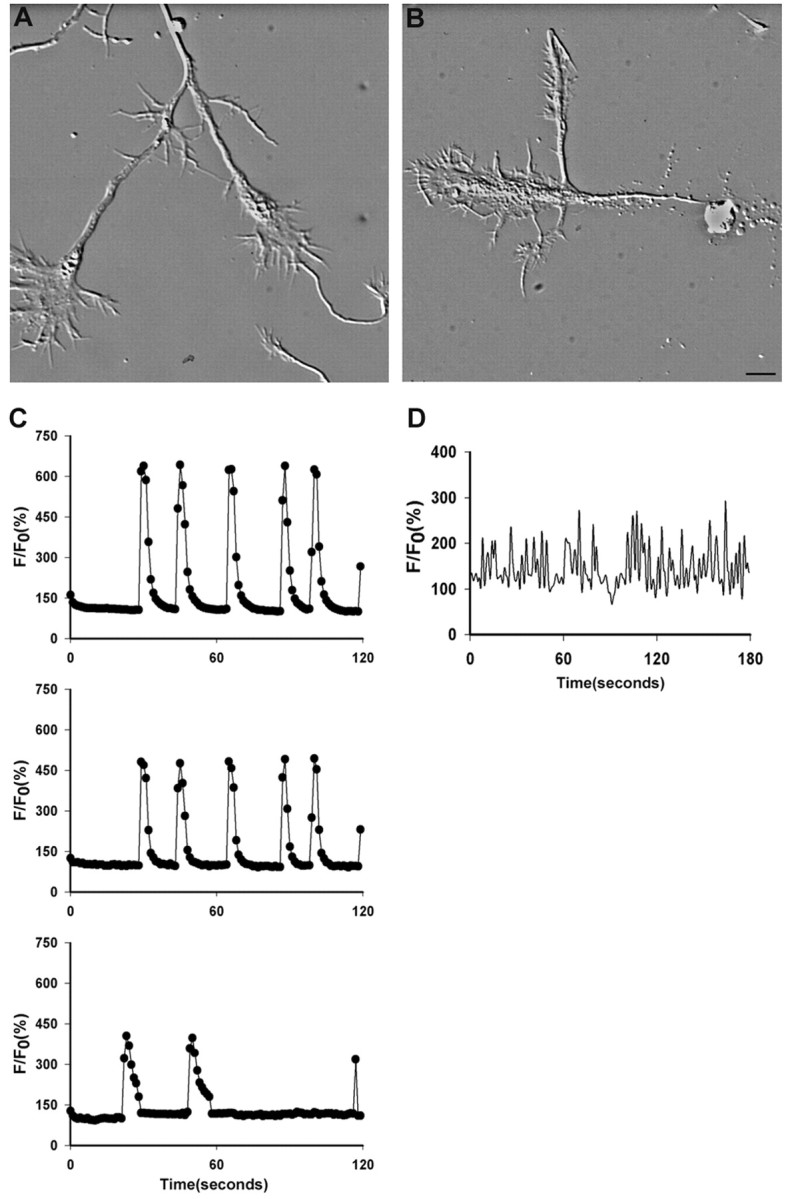
Ca2+ transients can show endogenous patterns in growth cones. A, Two large complex growth cones branching from a single axon (top) and a small simple growth cone (bottom).B, A complex branching growth cone that has been severed from the cell body. Scale bar: (in B) A, B, 10 μm. C, Patterns of Ca2+ transients at the top,middle, and bottom were measured in the growth cones in A at the left (branching growth cones), right (primary growth cone), and_bottom_ (small advancing growth cone), respectively. Growth cones branching from the same axon show the same frequency but different amplitudes. The large complex growth cones show higher frequencies than the small growth cone. D, The pattern of Ca2+ transients imaged every second in the severed growth cone has a very high frequency and faster than normal kinetics.
Frequency and amplitude of Ca2+ transients are inversely proportional to rates of axon outgrowth
To determine whether the frequency and amplitude of Ca2+ transients are correlated with growth cone morphologies and rates of axon outgrowth, we plotted the average frequencies and amplitudes of Ca2+transients in growth cones (n = 249) of different morphologies (Fig. 4). The rates of outgrowth of a subset of these growth cones are shown in Figure1H. Large paused growth cones with prominent microtubule loops (n = 77) had the highest average frequencies of Ca2+ transients (1.2 ± 0.1 per minute) when images were acquired every 10 sec. Imaging another group of large paused growth cones with similar morphologies at 1 sec intervals (n = 56) revealed even higher average frequencies (5.0 ± 0.5 per minute). In some large growth cones, frequencies could reach 14 transients per minute. For large growth cones with re-extending axons (n = 28), the average frequency of the transients (0.8 ± 0.1 per minute) was significantly less than in large paused growth cones. For smaller but complex growth cones without microtubule loops (n = 29) that extended slowly, the frequency of Ca2+ transients averaged 0.8 ± 0.1 per minute. In contrast, small simple growth cones (n = 20) that were extending relatively rapidly had average frequencies of 0.5 ± 0.1 transients per minute. Amplitudes of the Ca2+ transients were also well correlated with growth cone morphologies and rates of outgrowth. Plotting the maximum amplitudes of the same growth cones shown in the frequency plots demonstrated that amplitudes of Ca2+transient were highest in large paused growth cones and lowest in small advancing growth cones (Fig. 4B). Thus, measurements of Ca2+ transients in large numbers of growth cones of different morphologies and rates of extension demonstrate that the frequency and amplitude of Ca2+ transients are inversely correlated with rates of growth cone advance.
Fig. 4.
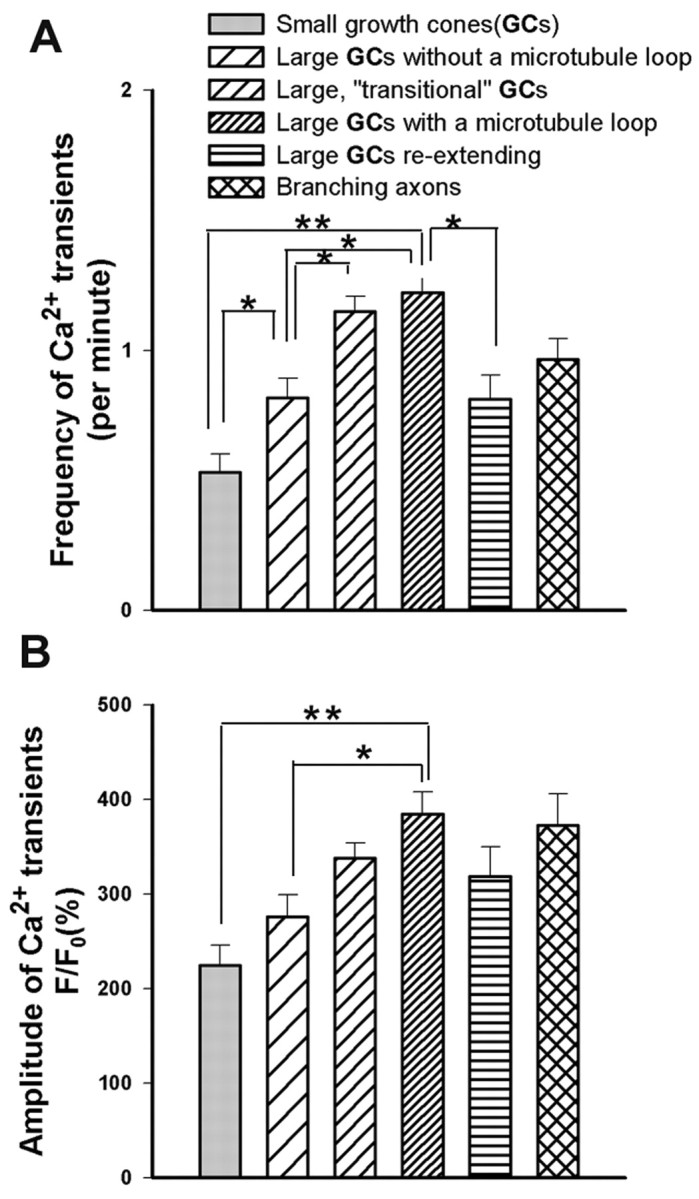
Frequency and amplitude of Ca2+transients are inversely proportional to rates of axon outgrowth.A, B, Histograms plotting frequencies (A) and amplitudes (B) of growth cones (GCs) with different morphologies included in Table 1 and illustrated and described in Figure 1. Growth cones of progressively larger size and greater complexity (corresponding to slower rates of outgrowth) show increasingly higher frequencies and amplitudes of Ca2+ transients. *p < 0.05 (t test); **p < 0.001 (t test).
Ca2+ transients are mediated primarily by L-type voltage-gated channels
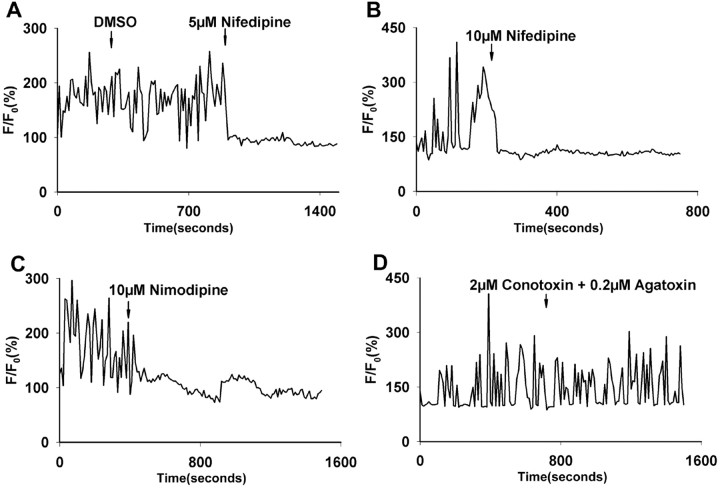
To determine whether Ca2+ transients are related to electrical activity, we tested the effects of the voltage-gated sodium channel blocker TTX. We chose large paused growth cones with microtubule loops for study, because these growth cones had the highest incidence of Ca2+ transients with the highest frequencies and amplitudes. Application of 1 μm TTX to the cortical cultures completely abolished Ca2+ transients within a few seconds (n = 20 growth cones; data not shown). To identify the channels mediating the Ca2+ transients, we first applied 2 mmNi2+, which at this concentration is a general calcium channel blocker. Ni2+immediately abolished all Ca2+ transients (n = 6 growth cones; data not shown), suggesting a role for VGCCs in generating Ca2+ transients. We subsequently applied blockers specific for L-type (nimodipine and nifedipine), N-type (ω-conotoxin), and P/Q-type (ω-agatoxin) VGCCs. Application of conotoxin or agatoxin did not have any effect on Ca2+ transients (Fig.5D). We tested a range of concentrations for conotoxin (1–5 μm) and agatoxin (0.1–0.5 μm) (n = 10), all of which gave the same results. In contrast, both nimodipine and nifedipine eliminated Ca2+ transients in a dose-dependent manner.
Fig. 5.
Ca2+ transients are mediated by L-type VGCCs. A, B, Effects of 5 μm(A) and 10 μm(B) nifedipine on silencing Ca2+ transients. C, A 10 μm concentration of nimodipine also silenced Ca2+ transients in the growth cone.D, Conotoxin and agatoxin, which block N-type and P/Q-type channels, respectively, had no effect.
As shown for the growth cone in Figure 5A, 5 μm nifedipine is capable of silencing Ca2+ transients within seconds. However, in five additional growth cones tested, we found that 5 μm nifedipine completely silenced Ca2+ transients in only one and partially decreased the amplitudes of Ca2+transients in the remaining growth cones. Higher concentrations of nifedipine more reliably silenced Ca2+transients. At 10 μm (Fig. 5B), calcium activity was completely silenced in one-half the growth cones tested (n = 6) and only partially attenuated in the others. At concentrations of 20 μm(n = 6), Ca2+ activity was completely blocked in all six growth cones examined. For nimodipine (Fig. 5C), another L-type channel blocker, 10 μm nimodipine completely blocked all activity (n = 3). These results demonstrate that the L-type VGCCs are the major channels involved in mediating Ca2+ transients in cortical neurons. However, the observation that concentrations of 20 μm were necessary to silence Ca2+ transients completely in some neurons suggests that nifedipine at higher concentrations may also block other VGCCs.
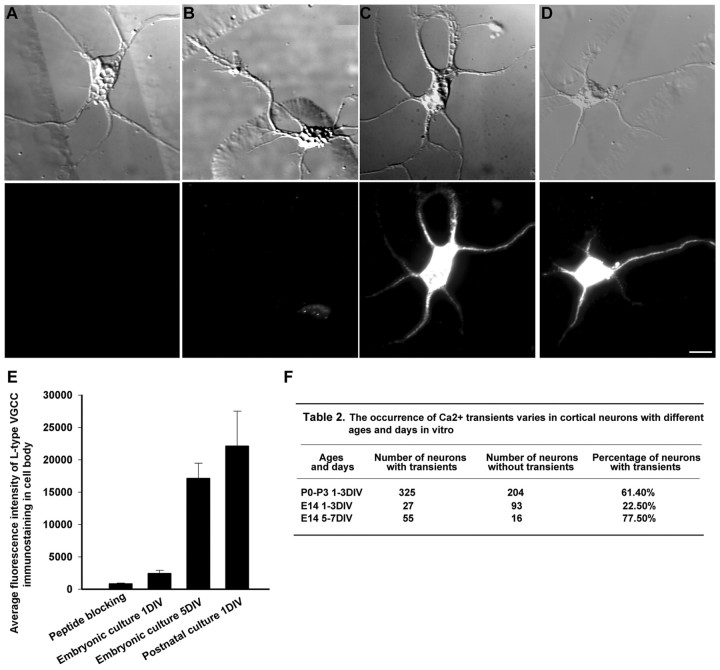
Previous studies have demonstrated the presence of L-type VGCCs on developing cortical and hippocampal neurons (Hell et al., 1993;Dolmetsch et al., 2001; Timmermann et al., 2002). To determine the distribution of L-type channels on cortical neurons in our cultures, we stained late embryonic and early postnatal neurons with antibodies to the α 1C and 1D subunits of the L-type Ca2+ channel. As shown in Figure6D, L-type Ca2+ channels were distributed on the entire postnatal neuron, including the dendritic and axonal processes. However, L-type Ca2+ channels were very sparse on embryonic neurons but did appear after several days in culture (Fig. 6B,C). Interestingly, in comparison with postnatal cortical neurons (0–3 d) E14 neurons showed very few Ca2+ transients (Fig.6F). Postnatal neurons (n = 529) had a much higher incidence of Ca2+ transients in their growth cones (61.4%) than did E14 neurons (_n_= 120) (22.5%) (Fig. 6F), and amplitudes of Ca2+ transients were also greater in postnatal neurons. However, when embryonic neurons were allowed to mature for 5–7 d in vitro (DIV), they expressed L-type channels and concomitantly a large percentage (77.5%) of their axonal growth cones exhibited Ca2+ transients. These results demonstrate that the developmental expression of L-type channels is well correlated with the incidence of Ca2+ transients.
Fig. 6.
The incidence of Ca2+transients correlates with developmental expression of L-type VGCCs.A–D, Images of cortical neurons (top) and fluorescence images (bottom) after staining with antibodies to the L-type VGCC. A, Control staining of a postnatal neuron with preabsorbed antibody. B, Staining of an embryonic neuron after 1 DIV showing very weak expression.C, Staining of an embryonic neuron after 5 DIV.D, Staining of a postnatal neuron after 1 DIV. Scale bar, 10 μm. E, Fluorescence intensity of neurons at different ages and days in vitro after staining with antibodies to L-type VGCCs. Fluorescence intensity of immunostained neurons is expressed as grayscale values relative to the maximum levels that the camera can acquire. F, Table comparing the incidence of Ca2+ transients at different ages and days in vitro. The ages at which Ca2+transients occur corresponds to development of L-type VGCCs.
In addition to Ca2+ entry through L-type Ca2+ channels, Ca2+ is also known to be released from intracellular stores. To determine whether intracellular stores contributed to Ca2+ transients in cortical neurons, we applied thapsigargin and dantrolene, which block the endoplasmic reticulum Ca2+ ATPases and ryanodine receptors, respectively. Low concentrations of these blockers significantly reduced the amplitude (∼40%) but not the frequency of the Ca2+ transients (data not shown), demonstrating that at least some of the rise in [Ca2+]i is attributable to release from intracellular stores.
Suppression of Ca2+ transients by blocking L-type VGCCs promotes axon outgrowth
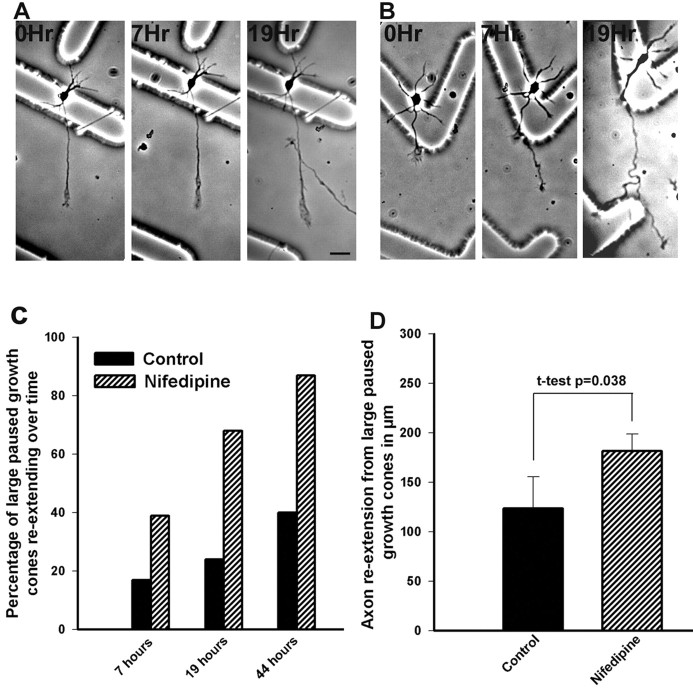
If high-frequency Ca2+ transients are associated with growth cone pausing, then silencing Ca2+ transients should induce axon outgrowth. We therefore applied nifedipine, which at concentrations of 20 μm completely silences Ca2+ transients mediated by L-type VGCCs for as long as antagonist is present. In a few experiments, we used 10 μm nimodipine. First we studied populations of cortical growth cones that were treated with nifedipine at 15 hr after plating. After exposure of the neurons to nifedipine (Fig.7A,C,D) or nimodipine (Fig.7B) for 33 hr, most of the cortical neurons had long axons tipped by small growth cones. This effect was dose dependent, and increasing concentrations of nifedipine resulted in longer axons. As shown in Figure 7C, 5 μm nifedipine had only a small but statistically significant (p = 0.033) effect on axon length, which is consistent with our finding that 5 μmnifedipine only partially eliminated Ca2+transients. Application of 10 μm nifedipine or nimodipine more completely suppressed Ca2+transients and had a greater effect on increasing axon outgrowth (p = 0.002). At higher concentrations (Fig.7C,D), nifedipine resulted in increasing axon lengths. Very few axons (Fig. 7E) had large paused growth cones. In contrast, control cultures treated with DMSO had many large growth cones and fewer long axons. Next we compared rates of axon outgrowth in nifedipine-treated versus control (DMSO) cultures. After nifedipine application at 24 hr after plating, we monitored the outgrowth of 56 axons and their growth cones along with an equal number of controls. For both control and nifedipine conditions, we first imaged the neurons in phase microscopy before treatments and recorded their positions. We then imaged the entire neuron, including the cell body, the axon, and the growth cone at 3 hr and again at 25 hr after treatment. Rates of axon outgrowth were calculated by comparing positions of the axons and growth cones at these two time points. Application of nifedipine almost doubled the average rates of axon outgrowth compared with controls (Fig. 7F). This increase in rate of axon outgrowth was already apparent within the first 3 hr of nifedipine treatment, which suggests that suppression of Ca2+transients has relatively acute effects on promotion of axon extension. In another set of experiments, we followed large pausing growth cones over time to determine changes in individual growth cones (n = 38) induced by nifedipine. As shown in one example (Fig. 8B), at the beginning of the experiment the large growth cone had a prominent microtubule loop and was not advancing. After 7 hr, the axon had advanced 85 μm and the remnants of the paused growth cone remained as an expanded region of the axon. By 19 hr, the axon had extended even further. In comparison, control cultures still had many large paused growth cones and much shorter axons (Fig. 8A). Analysis of all growth cones (Fig. 8C) showed that by 19 hr after nifedipine treatment, 68% of the large paused growth cones (n = 38) had extended, in comparison with only 24% of controls (n = 42). Axon outgrowth of nifedipine-treated neurons at this time point was 181 ± 17 versus 115 ± 26 μm (Fig. 8D). Interestingly, in contrast to control cultures, nifedipine-treated cortical neurons failed to develop collateral branches from regions along the axon in which growth cones had paused. These results demonstrate that Ca2+ transients play a central role in regulating axon outgrowth.
Fig. 7.
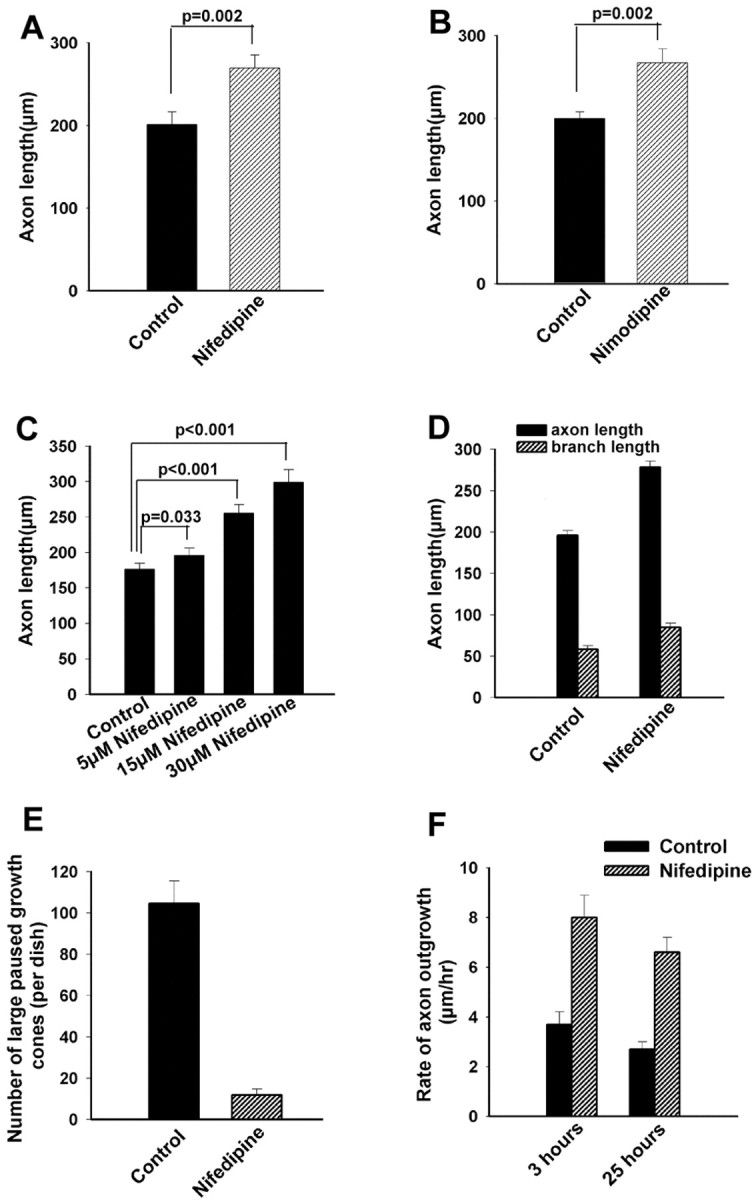
Suppressing Ca2+ transients in cortical growth cones promotes axon outgrowth. A, B, Histograms showing effects of 10 μm nifedipine (A) and 10 μm nimodipine on increasing axon length (n = 34 for each experimental treatment). C, Histogram showing that application of nifedipine 15 hr after plating increased axon length in a dose-dependent manner (n = 32). D, Histogram showing that application of 20 μm nifedipine 15 hr after plating increases axon and branch length (n = 133). E, Application of nifedipine virtually eliminates large paused growth cones after 33 hr.F, Histograms comparing rates of axon outgrowth in nifedipine-treated and control cultures at 3 and 25 hr after addition of 20 μm nifedipine (n = 56 for each condition).
Fig. 8.
Eliminating Ca2+ transients by blocking L-type VGCCs promotes axon outgrowth from large paused growth cones. A, A large pausing growth cone in control conditions imaged at three different time points. With reference to the markings on the coverslip, the growth cone has advanced only slightly during 19 hr. In contrast, at 19 hr a small growth cone has advanced into the field. B, A large pausing growth cone treated with nifedipine imaged at three different time points. At time 0, the growth cone contains a prominent microtubule loop visible even in phase microscopy. By 7 hr, a new axon tipped by a growth cone has extended from the pausing growth cone. By 19 hr, the axon has extended even further. C, Histograms comparing the percentage of large pausing growth cones that have re-extended in control versus nifedipine-treated cultures at successive times after addition of nifedipine or DMSO (control). D, Histograms comparing outgrowth of axons re-extending from large paused growth cones 19 hr after addition of nifedipine or DMSO (control). Scale bar: (in_A_) A, B, 20 μm.
We have interpreted the results of blocking L-type VGCCs as affecting Ca2+ transients. However, it is possible that blocking L-type VGCCs can have other effects on Ca2+, such as lowering basal cytoplasmic levels, or on Ca2+ release from intracellular stores. We have not examined these possibilities. However, even if these indirect effects contributed to our results, they would not obviate our conclusion that eliminating Ca2+ transients promotes axon outgrowth.
Discussion
We demonstrate here that developing cortical neurons in dissociated cultures exhibited spontaneous Ca2+ transients with varying frequencies, amplitudes, and temporal patterns. These transients were most prevalent in large paused growth cones but rare in small advancing growth cones. The frequency and amplitude of Ca2+transients were inversely correlated with rates of axon outgrowth. Ca2+ entry occurred primarily through L-type VGCCs, although other VGCCs may also be involved. Remarkably, within a few hours, silencing Ca2+transients by blocking L-type Ca2+channels caused outgrowth of large paused growth cones that would otherwise remain stationary for many hours or several days. Together these results provide the first evidence that Ca2+ transients in mammalian CNS neurons play a central role in regulating forward advance of the growth cone.
Developing CNS neurons exhibit Ca2+ transients related to electrical activity
A number of studies in slice preparations have reported spontaneous Ca2+ transients in developing cortical neurons (Yuste et al., 1992; Owens and Kriegstein, 1998;Garaschuk et al., 2000; Mao et al., 2001). In developing postnatal cortical slices, neurons exhibited high-frequency spontaneous Ca2+ transients that varied in their temporal patterns, but these transients were oscillatory (Mao et al., 2001). In embryonic cortical slices, precursor cells in the ventricular zone displayed intermittent spontaneous Ca2+ transients in the cell bodies that occurred approximately once every 20 min and had slow onsets (Owens and Kriegstein, 1998). In contrast to our results, these transients were not activity dependent and did not involve VGCC activation but instead were mediated by calcium release from intracellular stores. These Ca2+ transients were thought to contribute to the regulation of neurogenesis. Other developing CNS neurons such as cerebellar Purkinje cells also exhibit spontaneous high-frequency Ca2+ transients that are mediated through L-type VGCCs and have been correlated with electrical activity (Liljelund et al., 2000). Although it was suggested that Ca2+ dynamics can influence downstream events such as gene expression, no correlations were made between Ca2+ transients and specific aspects of development.
Ca2+ transients are mediated by VGCCs
Our results suggest that the L-type calcium channel is a major VGCC involved in the Ca2+ transients that we observed. Our results and other studies (Dolmetsch et al., 2001) have shown that L-type channels are abundant in developing cortical neurons, which also contain other VGCCs such as N- and R-type channels (Timmermann et al., 2002). Thus it is possible that other VGCCs in addition to L-type channels are involved in Ca2+ transients in cortical neurons. Our results show that application of blockers to N- and P/Q-type channels at high concentrations failed to block calcium activity. However, we did not test for the involvement of R- and T-type channels. At a concentration of 10 μm, the blocking action of nifedipine is generally regarded as specific to L-type calcium channels. Ca2+ transients in many of the cortical growth cones were completely silenced at this concentration. However, we also found that for some neurons, higher concentrations up to 20 μm were required to completely silence Ca2+ transients, suggesting that at higher concentrations nifedipine may be blocking other VGCCs in addition to L-type channels. This interpretation is consistent with a recent study of calcium activity in developing cortical neurons (Redmond et al., 2002).
Ca2+ transients play a role in regulating axon outgrowth
Previous studies have shown that levels of [Ca2+]i play an important role in regulating neurite outgrowth. Sustained elevation of intracellular Ca2+ concentrations inhibited growth cone advance (Kater et al., 1988), and maximal outgrowth occurred within an optimal range of calcium concentrations (Kater and Mills, 1991). Transient elevations of [Ca2+]i that were either spontaneous or induced by electrical stimulation also regulated neurite extension by slowing growth cone advance. Electrical stimulation of DRG neurons and elevation of [Ca2+]i (Fields et al.,1990) inhibited the growth rate of DRG neurites and induced collapse of the lamellipodia and filopodia. In contrast, our results show that high-frequency spontaneous Ca2+transients did not cause growth cone collapse or retraction. Rather, cortical growth cones stalled in their advance but maintained motility while exhibiting large expanded lamellipodia and active filopodia. These differences may reflect differences in induced versus spontaneous Ca2+ transients. Highly variable temporal patterns of spontaneous Ca2+ transients may be intrinsic to specific types of cortical neurons. However, because large pyramidal neurons rather than small interneurons were selected for imaging, it is not likely that the incidence of Ca2+ transients reflects differences in neuronal cell types. The meaning encoded in these patterns of activity is unknown but may be difficult to mimic by imposed electrical stimulation.
Naturally occurring Ca2+ transients have been shown to regulate growth cone extension (Gomez et al., 1995;Goldberg and Grabham, 1999; Gomez and Spitzer, 1999). Two distinct types of Ca2+ transients were characterized in Xenopus spinal neurons (Gu and Spitzer, 1994; Gu et al., 1995). Spikes are generated by spontaneous calcium-dependent action potentials in an all-or-none bidirectional manner and act through VGCCs. In contrast, waves, which can arise in the growth cone as well as the cell body, are not elicited by depolarization, do not involve VGCCs, and are propagated at a rate consistent with diffusion of Ca2+. Spikes and waves could be generated in the same cell, but waves rather than spikes were shown to regulate axon outgrowth (Gu and Spitzer, 1995).Gomez and Spitzer (1999) found that spontaneous Ca2+ transients regulate the rate of axon outgrowth in vivo in the embryonic Xenopus spinal cord, and that rates of axon outgrowth are inversely proportional to the frequency of Ca2+ transients. Like_Xenopus_ spinal neurons, cortical neurons exhibited Ca2+ transients during growth cone pausing. Similarly, frequencies of Ca2+transients were inversely related to rates of growth cone advance, and suppression of Ca2+ transients in both types of neurons increased rates of axon outgrowth. However, in cortical neurons Ca2+ transients that regulate axon outgrowth have the characteristics of spikes, act through VGCCs, and have frequencies that can reach 14 per minute, in contrast to waves, which have frequencies ranging from 3 to 16 per hour. Ca2+ transients in cortical neurons regulate not only axon outgrowth but also axon branching. Application of nifedipine, which blocks Ca2+transients, interrupted growth cone pausing and promoted axon outgrowth. Consequently, development of axon branches from large paused growth cones failed to occur. Thus, at decision regions in the mammalian CNS, spontaneous Ca2+transients, by slowing growth cone advance, could play an important role in the development of branches at appropriate regions along the axon.
Possible mechanisms for regulation of growth cone advance by Ca2+ transients
The exact mechanisms whereby Ca2+transients regulate growth cone advance have not been identified. It is likely that transient elevation of [Ca2+]i plays a role in the reorganization of the cytoskeleton that underlies changes in growth cone behaviors (Suter and Forscher, 2000). When growth cones slow their advance, they develop prominent microtubule loops (Sabry et al., 1991; Tanaka and Kirschner, 1991). Previously we found that during transitions from paused to growth states, the microtubule loop undergoes splaying and fragmentation (Dent et al., 1999), and initiation of new growth from the growth cone and the formation of axon branches require interaction between dynamic microtubules and actin filaments (Dent and Kalil, 2001). It is therefore intriguing that Ca2+ transients with the highest incidence, frequency, and amplitude were found in large paused growth cones with prominent microtubule loops. Preliminary results (our unpublished observations) show that application of nifedipine causes disruption of microtubule loops in large paused growth cones within 1 hr. This suggests that Ca2+ transients play a role in maintaining the stability of looped microtubules, thus slowing growth cone advance. Consistent with this finding, previous studies have suggested that neurite elongation may be regulated by calcium in part by influencing actin filament stability (Lankford and Letourneau, 1989) and consequently microtubule dynamics. There is recent evidence that Ca2+ transients acting via calcineurin may slow neurite extension by depolymerizing actin filaments (Lautermilch and Spitzer, 2000). Recent studies have shown that Ca2+ transients in localized regions of the growth cone can induce filopodial protrusion by actin polymerization (Lau et al., 1999), slow growth cone advance by reducing filopodial motility (Gomez et al., 2001), and induce growth cone turning behaviors (Hong et al., 2000; Zheng, 2000). In the future, it will be important to examine how induction of Ca2+ transients in localized regions of the growth cone leads to local reorganization of the cytoskeleton to elicit changes in growth cone behaviors and the development of axon branches.
Footnotes
This work was funded by National Institutes of Health Grant NS14428 (K.K.) and by Predoctoral Training Grant Award GM07507 (E.W.D.). We thank Timothy Gomez for helpful advice throughout the course of this study and for comments on this manuscript. We also thank Aileen Barnes for excellent technical assistance and Ian Hutchins for comments on this manuscript.
Correspondence should be addressed to Dr. Katherine Kalil, Department of Anatomy, University of Wisconsin-Madison, 1300 University Avenue, Madison, WI 53705. E-mail: kakalil@facstaff.wisc.edu.
E. W. Dent's current address: Department of Biology, Massachusetts Institute of Technology, Building 68, Room 270, 77 Massachusetts Avenue, Cambridge, MA 02139.
References
- 1.Dent EW, Kalil K. Axon branching requires interactions between dynamic microtubules and actin filaments. J Neurosci. 2001;21:9757–9769. doi: 10.1523/JNEUROSCI.21-24-09757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dent EW, Callaway JL, Szebenyi G, Baas PW, Kalil K. Reorganization and movement of microtubules in axonal growth cones and developing interstitial branches. J Neurosci. 1999;19:8894–8908. doi: 10.1523/JNEUROSCI.19-20-08894.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- 4.Fields RD, Neale EA, Nelson PG. Effects of patterned electrical activity on neurite outgrowth from mouse sensory neurons. J Neurosci. 1990;10:2950–2964. doi: 10.1523/JNEUROSCI.10-09-02950.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garaschuk O, Linn J, Eilers J, Konnerth A. Large-scale oscillatory calcium waves in the immature cortex. Nat Neurosci. 2000;3:452–459. doi: 10.1038/74823. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg DJ, Grabham PW. Braking news: calcium in the growth cone. Neuron. 1999;22:423–425. doi: 10.1016/s0896-6273(00)80697-2. [DOI] [PubMed] [Google Scholar]
- 7.Gomez TM, Spitzer NC. In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature. 1999;397:350–355. doi: 10.1038/16927. [DOI] [PubMed] [Google Scholar]
- 8.Gomez TM, Spitzer NC. Regulation of growth cone behavior by calcium: new dynamics to earlier perspectives. J Neurobiol. 2000;44:174–183. [PubMed] [Google Scholar]
- 9.Gomez TM, Snow DM, Letourneau PC. Characterization of spontaneous calcium transients in nerve growth cones and their effect on growth cone migration. Neuron. 1995;14:1233–1246. doi: 10.1016/0896-6273(95)90270-8. [DOI] [PubMed] [Google Scholar]
- 10.Gomez TM, Robles E, Poo M, Spitzer NC. Filopodial calcium transients promote substrate-dependent growth cone turning. Science. 2001;291:1983–1987. doi: 10.1126/science.1056490. [DOI] [PubMed] [Google Scholar]
- 11.Gu X, Spitzer NC. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature. 1995;375:784–787. doi: 10.1038/375784a0. [DOI] [PubMed] [Google Scholar]
- 12.Gu X, Olson EC, Spitzer NC. Spontaneous neuronal calcium spikes and waves during early differentiation. J Neurosci. 1994;14:6325–6335. doi: 10.1523/JNEUROSCI.14-11-06325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halloran MC, Kalil K. Dynamic behaviors of growth cones extending in the corpus callosum of living cortical brain slices observed with video microscopy. J Neurosci. 1994;14:2161–2177. doi: 10.1523/JNEUROSCI.14-04-02161.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, Snutch TP, Catterall WA. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel α1 subunits. J Cell Biol. 1993;123:949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong K, Nishiyama M, Henley J, Tessier-Lavigne M, Poo M. Calcium signalling in the guidance of nerve growth by netrin-1. Nature. 2000;403:93–98. doi: 10.1038/47507. [DOI] [PubMed] [Google Scholar]
- 16.Kalil K, Szebenyi G, Dent EW. Common mechanisms underlying growth cone guidance and axon branching. J Neurobiol. 2000;44:145–158. [PubMed] [Google Scholar]
- 17.Kater SB, Mills LR. Regulation of growth cone behavior by calcium. J Neurosci. 1991;11:891–899. doi: 10.1523/JNEUROSCI.11-04-00891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kater SB, Mattson MP, Cohan C, Connor J. Calcium regulation of the neuronal growth cone. Trends Neurosci. 1988;11:315–321. doi: 10.1016/0166-2236(88)90094-x. [DOI] [PubMed] [Google Scholar]
- 19.Lankford KL, Letourneau PC. Evidence that calcium may control neurite outgrowth by regulating the stability of actin filaments. J Cell Biol. 1989;109:1229–1243. doi: 10.1083/jcb.109.3.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lau PM, Zucker RS, Bentley D. Induction of filopodia by direct local elevation of intracellular calcium ion concentration. J Cell Biol. 1999;145:1265–1275. doi: 10.1083/jcb.145.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lautermilch NJ, Spitzer NC. Regulation of calcineurin by growth cone calcium waves controls neurite extension. J Neurosci. 2000;20:315–325. doi: 10.1523/JNEUROSCI.20-01-00315.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liljelund P, Netzeband JG, Gruol DL. L-type calcium channels mediate calcium oscillations in early postnatal Purkinje neurons. J Neurosci. 2000;20:7394–7403. doi: 10.1523/JNEUROSCI.20-19-07394.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lohmann C, Myhr KL, Wong RO. Transmitter-evoked local calcium release stabilizes developing dendrites. Nature. 2002;418:177–181. doi: 10.1038/nature00850. [DOI] [PubMed] [Google Scholar]
- 24.Mao BQ, Hamzei-Sichani F, Aronov D, Froemke RC, Yuste R. Dynamics of spontaneous activity in neocortical slices. Neuron. 2001;32:883–898. doi: 10.1016/s0896-6273(01)00518-9. [DOI] [PubMed] [Google Scholar]
- 25.Mason C, Erskine L. Growth cone form, behavior, and interactions in vivo: retinal axon pathfinding as a model. J Neurobiol. 2000;44:260–270. doi: 10.1002/1097-4695(200008)44:2<260::aid-neu14>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 26.O'Leary DD, Bicknese AR, De Carlos JA, Heffner CD, Koester SE, Kutka LJ, Terashima T. Target selection by cortical axons: alternative mechanisms to establish axonal connections in the developing brain. Cold Spring Harb Symp Quant Biol. 1990;55:453–468. doi: 10.1101/sqb.1990.055.01.045. [DOI] [PubMed] [Google Scholar]
- 27.Owens DF, Kriegstein AR. Patterns of intracellular calcium fluctuation in precursor cells of the neocortical ventricular zone. J Neurosci. 1998;18:5374–5388. doi: 10.1523/JNEUROSCI.18-14-05374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redmond L, Kashani AH, Ghosh A. Calcium regulation of dendritic growth via CaM kinase IV and CREB-mediated transcription. Neuron. 2002;34:999–1010. doi: 10.1016/s0896-6273(02)00737-7. [DOI] [PubMed] [Google Scholar]
- 29.Sabry JH, O'Connor TP, Evans L, Toroian-Raymond A, Kirschner M, Bentley D. Microtubule behavior during guidance of pioneer neuron growth cones in situ. J Cell Biol. 1991;115:381–395. doi: 10.1083/jcb.115.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spitzer NC, Lautermilch NJ, Smith RD, Gomez TM. Coding of neuronal differentiation by calcium transients. BioEssays. 2000;22:811–817. doi: 10.1002/1521-1878(200009)22:9<811::AID-BIES6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 31.Suter DM, Forscher P. Substrate-cytoskeletal coupling as a mechanism for the regulation of growth cone motility and guidance. J Neurobiol. 2000;44:97–113. [PubMed] [Google Scholar]
- 32.Szebenyi G, Callaway JL, Dent EW, Kalil K. Interstitial branches develop from active regions of the axon demarcated by the primary growth cone during pausing behaviors. J Neurosci. 1998;18:7930–7940. doi: 10.1523/JNEUROSCI.18-19-07930.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szebenyi G, Dent EW, Callaway JL, Seys C, Lueth H, Kalil K. Fibroblast growth factor-2 promotes axon branching of cortical neurons by influencing morphology and behavior of the primary growth cone. J Neurosci. 2001;21:3932–3941. doi: 10.1523/JNEUROSCI.21-11-03932.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka EM, Kirschner MW. Microtubule behavior in the growth cones of living neurons during axon elongation. J Cell Biol. 1991;115:345–363. doi: 10.1083/jcb.115.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Timmermann DB, Westenbroek RE, Schousboe A, Catterall WA. Distribution of high-voltage-activated calcium channels in cultured GABAergic neurons from mouse cerebral cortex. J Neurosci Res. 2002;67:48–61. doi: 10.1002/jnr.10074. [DOI] [PubMed] [Google Scholar]
- 36.Tsui HT, Lankford KL, Ris H, Klein WL. Novel organization of microtubules in cultured central nervous system neurons: formation of hairpin loops at ends of maturing neurites. J Neurosci. 1984;4:3002–3013. doi: 10.1523/JNEUROSCI.04-12-03002.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuste R, Peinado A, Katz LC. Neuronal domains in developing neocortex. Science. 1992;257:665–669. doi: 10.1126/science.1496379. [DOI] [PubMed] [Google Scholar]
- 38.Zheng JQ. Turning of nerve growth cones induced by localized increases in intracellular calcium ions. Nature. 2000;403:89–93. doi: 10.1038/47501. [DOI] [PubMed] [Google Scholar]