Mice Lacking Dopamine D2 and D3 Receptors Have Spatial Working Memory Deficits (original) (raw)
Abstract
Mice deficient for dopamine D2 and D3receptors exhibit blunted c-fos responses to D1 agonist stimulation. Stereologic cell counting revealed decreased numbers of medial prefrontal cortex neurons that express Fos immunoreactivity in all layers, particularly in the prelimbic and anterior cingulate subregions. Pretreatment of these mutants with a single, low dose of methamphetamine (METH) led to a sustained increase in the number of neurons that express Fos immunoreactivity in response to a D1 agonist challenge, which was most significant in prelimbic and anterior cingulate subregions. The increased c-fos responses reached wild-type-like levels in METH-pretreated D2 mutants but remained submaximal in METH-pretreated D3 mutants. Additional studies tested the performance of wild type and mutants in a delayed alternation test, a cognitive task critically dependent on optimal activation of prefrontal cortical D1 receptors by synaptically released dopamine. Both D2 and D3 mutants exhibited deficits in their spatial working memory, with increasing impairments at increasing delays. Whereas METH pretreatment rescued the spatial working memory of D2 mutants, it had no effect on D3 mutants. These data suggest that the sustained improvement of spatial working memory in METH-pretreated D2 mutants is attributable to D1 receptor-mediated mechanisms.
Keywords: dopamine D1 receptors, D2-receptor knock-out, D3-receptor knock-out, prefrontal cortex, stereology, c-fos, working memory
The prefrontal cortex (PFC) is a set of neocortical areas involved in temporary storage of information (short-term or working memory) and the implementation of executive processes needed for voluntary, goal-directed behavior (for review, seeMiller, 1999, 2000; Smith and Jonides, 1999; Fuster, 2001). In humans, the effects of lesions of the PFC are most apparent under test conditions that require cognitive control (Miller, 2000), and working memory deficits have also been documented for dopamine-depleted rhesus monkeys (Brozoski et al., 1979). The latter observation gave rise to the conclusion that a decrease in prefrontal cortical dopaminergic neurotransmission is also responsible for the working memory deficits seen in patients with Parkinson's disease (Gotham et al., 1988; Levin et al., 1989) and schizophrenia (Weinberger et al., 1986; Fukushima et al., 1988; Park and Holzman, 1992) and in children at high risk for schizophrenia (Erlenmeyer-Kimling et al., 2000).
Working memory requires activation of prefrontal cortical dopamine D1 receptors (Williams and Goldman-Rakic, 1995). Although studies have shown that the D1 receptor dependence is a “U-shaped” function and that normal cognitive function requires the optimal activation of D1receptors (Williams and Goldman-Rakic, 1995; Zahrt et al., 1997), more recent studies point to the importance of an intricately balanced activity of D1 and D2-like receptors (D2, D3, and D4). For example, Castner et al. (2000) showed that monkeys chronically treated with a neuroleptic drug known to block D2-like receptors exhibit impaired working memory, and molecular studies on knock-out mice deficient for D2 and D3 receptors revealed a decreased agonist-stimulated D1receptor activity in the forebrain (Jung and Schmauss, 1999; Schmauss, 2000) despite unaltered expression of D1 receptor radioligand binding sites in such mutants (Baik et al., 1995; Xu et al., 1997). Moreover, a single dose of either a full D1 agonist or methamphetamine (METH) (Schmauss, 2000) or intermittent D1 agonist stimulation (Castner et al., 2000) led to a sustained rescue of the deficits described in these studies.
To explore further the roles of D2 and D3 receptors in modulating D1 receptor activation in the PFC, the present study investigated agonist-stimulated D1 receptor activity in D2 and D3receptor knock-out mice (Jung et al., 1999). We performed quantitative immunocytochemical studies to analyze prefrontal cortical c-fos responses to a systemically administered D1 agonist and behavioral studies to test the performance of the mutants in a spatial working memory task. The results revealed significantly blunted D1agonist-stimulated c-fos responses in the PFC of both mutants. Moreover, both mutants exhibit significant deficits in spatial working memory. A single dose of METH rescues the blunted prefrontal cortical c-fos responses in D2 mutants but has only a partial effect in D3 mutants. METH also rescues the spatial working memory deficits of D2 mutants. The impaired working memory of D3 mutants, however, is unaffected by METH.
MATERIALS AND METHODS
Animals. All experiments were performed with the fifth generation of homozygous, congenic C57BL/6 D2 and D3 mutants and their wild-type littermates. The generation of the mutant mice is described by Jung et al. (1999). For all studies, male mice at postnatal day 60 (P60) to P90 were used. Animals were group housed and had access to food and water ad libitum unless otherwise indicated. All procedures involving the animals were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use committees at Columbia University and the New York State Psychiatric Institute.
Drugs. All drugs were dissolved in saline and injected intraperitoneally. The D1 agonists SKF82958 and SKF81297 (administered at 1 and 2 mg/kg, respectively) and_S_-methamphetamine (5 mg/kg) were purchased from Sigma (St. Louis, MO).
RNA extraction and Northern blotting. Animals were decapitated, their brains were rapidly removed, and the forebrain was dissected as described by Schmauss (2000). In some experiments, additional dissections of the anterior 4 mm of the forebrain (frontal cortex) were performed. RNA was extracted using guanidine–cesium chloride ultracentrifugation. Ten micrograms of total RNA were loaded onto 1.2% formaldehyde–agarose gels and transferred to nylon membranes. Membranes were hybridized to a32P-radiolabeled, random-primed 540-nucleotide-long mouse c-fos cDNA as described previously (Schmauss, 2000).
In situ hybridization. Sixteen-micrometer-thick cryosections of Freon-frozen brains of wild type and D2/D3 double mutants were thaw mounted onto gelatin-coated slides, dried for 2 min at 37°C, and then refrozen at −80°C. Each slide contained sections of wild type and D2/D3 double mutants that were collected at similar interaural coordinates. In situ hybridizations were performed as described previously (Schmauss et al., 1992), and c-fos mRNA was hybridized to a35S-labeled antisense riboprobe (1 × 106 cpm/300 μl) comprising 540 nucleotides of the mouse c-fos mRNA (Schmauss, 2000). Air-dried slides were exposed to Kodak MR film (Eastman Kodak, Rochester, NY) for 14 hr. Images on film were digitized using the microcomputer imaging device (MCID) image analysis system (Imaging Research, St. Catharines, Ontario, Canada) and colorized uniformly to highlight c-fos signal intensities.
Immunocytochemistry and stereological analysis. The expression of Fos immunoreactivity was analyzed in 16 μm cryosections and in 40 μm microtome sections obtained from brains of drug-naive and METH-pretreated wild type and D2 and D3 single mutants. Slide-mounted cryosections of fresh-frozen brains were postfixed (10 min in 4% paraformaldehyde in 0.1 m phosphate buffer, pH 7.4) immediately after sectioning and processed as described below. Microtome sections (free-floating) were obtained from immersion-fixed brains (6 hr in the fixative described above) and used exclusively for stereological analysis. After fixation, nonspecific staining was reduced by incubating sections for 5 min in hydrogen peroxide [0.3% in 0.1m Tris-buffered saline (TBS), pH 7.6], and then for 30 min in TBS containing 0.5% bovine serum albumin (BSA). Incubations with primary antibody were performed overnight at room temperature in 0.1% BSA and 0.25% Triton X-100 in TBS. A rabbit polyclonal anti-c-fos antibody (Ab-5; 1:7500; Oncogene Sciences, Boston, MA) was used to detect Fos immunoreactivity. Adjacent sections of immersion-fixed tissues were also incubated with a mouse monoclonal antibody directed against tyrosine hydroxylase (1:5000; Diasorin, Stillwater, MN) or a mouse monoclonal anti-neuronal-specific nuclear protein (NeuN) antibody (1:10,000; Chemicon, Temecula, CA). After incubation with primary antibody, sections were incubated for 30 min with biotinylated goat anti-rabbit or goat anti-mouse IgG (1:400 in TBS containing 0.1% BSA; Vector Laboratories, Burlingame, CA), followed by a 30 min incubation in avidin–biotin–peroxidase complex (Vectastain Elite kit; 1:100 in TBS; Vector Laboratories). Bound immunoperoxidase was visualized by incubation for 6 min in 0.022% 3,3′-diaminobenzidine (Aldrich, Milwaukee, WI) and 0.003% hydrogen peroxide in TBS. Sections were rinsed in TBS between incubations and in 0.1 mphosphate buffer at the end of staining. Free-floating sections were mounted onto gelatin-coated slides and lightly counterstained in 0.25% thionin. For photographic purposes, images were captured digitally at 4 and 10× magnification using a SPOT camera (Diagnostic Instruments, Sterling Heights, MI).
To obtain a quantitative estimate of the numbers of nuclei expressing the Fos protein in the PFC of wild type and D2and D3 single mutants and to test whether differences exist between the three genotypes in the numbers of neurons or glia, a stereologic counting method was used. For this analysis, aZeiss (Oberkochen, Germany) Axioplan 2 photomicroscope equipped with a Dage-MTI (Michigan City, IN) DC-330 CCD camera and Lud1 motorized stage and interfaced with a Gateway Athlon computer and StereoInvestigator(MicroBrightField, Colchester, VT) were used.
The stereologic analysis was conducted on brain sections obtained from five to nine drug-naive and METH-pretreated animals per genotype. For each case, three adjacent series of 40-μm-thick sections, collected at an intraseries interval of 200 μm, were used. These series were processed to label the following: (1) Fos immunoreactivity in thionin-counterstained sections, (2) NeuN immunoreactivity, or (3) tyrosine hydroxylase immunoreactivity. The latter two series were used to identify the boundaries between layers II/III and V/VI. Total numbers of neurons, glia, and Fos-positive nuclei were determined in three subregions, the infralimbic (IL), prelimbic (PL), and anterior cingulate (AC) cortices (Hof et al., 2000) in three (IL) or six (PL and AC) sections from the series, using an unbiased stereologic method, the optical fractionator (West et al., 1991). Thus, a total of six regions (layers II/III and layers V/VI of IL, PL, and AC) were analyzed. Neurons and glia were identified using morphological criteria that characterize the nuclei of both cell types (Vaughan, 1984; Peters et al., 1991). Optical dissector frames and counting grid sizes of 30 and 100 μm2, respectively, were chosen to permit systematic random sampling of three to five neurons within an 8 μm focusing range for each sampling field and 200–600 neurons for each region. These parameters allowed for intrasample coefficients of error (CE), calculated as described previously (Schmitz and Hof, 2000), that averaged 0.06 ± 0.01 for neurons and 0.08 ± 0.01 for glia and Fos-labeled nuclei for all regions. There were no significant differences in CE values across genotypes or treatment groups. All regions were sampled at high magnification in Koehler illumination conditions using a Zeiss 63× Plan-Apochromate objective. The number of Fos-labeled neurons was subsequently expressed as a percentage of the total number of neurons counted within each region. The volume of the different laminar domains of interest in each of the three PFC regions was estimated using the Cavalieri principle. For statistical analysis of the stereologic data, a one-way ANOVA (threshold of significance, α = 0.05) was performed, and significance of differences were analyzed post hoc by a Student's t test.
Spatial delayed alternation. For these experiments, the body weight of the animals was gradually reduced (over a period of 10–14 d) until the animals reached 80% of their individual starting body weight. During the entire course of the experiments, their body weights were monitored daily to adjust the amount of food (Prolab Isopro RMH 3000; PMI Nutrition, Brendwood, MO) provided (usually 2.5 gm/animal per day).
Delayed alternation tests were performed using a T-maze designed for rodents. The maze was constructed from 0.6-cm-thick Plexiglas. Its main alley (58 × 11 × 18.5 cm) was connected to two side arms (30 × 11 × 18.5 cm), which contained sliding doors used to close off the arms manually. Small dishes containing food pellets were positioned at the end of these arms. A holding box (13 × 11 × 18.5 cm) contained a manually operated sliding door used to close off the entrance to the main alley of the T-maze.
Experiments were performed with 32 animals and conducted in two series. In one series, eight homozygous D3 mutants were tested in parallel with eight wild-type littermates, and, in the other series, eight D2 mutants were tested in parallel with eight wild-type animals. At the end of the gradual weight reduction period, mice were exposed for 3 consecutive days to the T-maze, which had both arms open, and baited with food (one mouse at a time). Then, the mice were subjected to a 3 d period of “forced alternation runs,” i.e., one arm was closed off and the food reward was positioned in the other (open) arm (Verma and Moghaddam, 1996). Next, animals were trained at a 5 sec retention time, allowing 11 trials of continuous alternation per day with both arms open. In the first run, animals were allowed to explore both arms until they found the food pellet located in one of them. In the following 10 trials, they were rewarded only for the alternate (correct) selection of arms. After each arm entry (correct or incorrect), animals remained in the arm for 12 sec and were then placed back into the holding box. The training period ended after wild-type animals made >70% correct choices on 2 consecutive days. In both series of experiments, all wild-type animals reached this criterion. Animals were then tested for their performance at 15, 20, or 30 sec delay periods.
In all experiments, the T-maze was located in the same position so that potential spatial cues never changed. All experiments were performed between 1:00 P.M. and 4:30 P.M. A statistical comparison of data were performed by an ANOVA, and significant main effects were analyzed further by post hoc comparisons of means using the Bonferroni's multiple comparisons test.
RESULTS
Blunted c-fos mRNA responses to dopamine D1 agonists in mice deficient for D2 and D3 receptors
The induction of expression of the immediate-early gene c-fos is a sensitive indicator of neuronal activity stimulated by D1 agonists. Previous results of RNA and protein studies indicate that knock-out mice deficient for dopamine D2 and D3receptors exhibit blunted forebrain c-fos responses to administration of the full D1 agonist SKF82958 (Jung and Schmauss, 1999; Schmauss, 2000). As shown in Figure1, forebrain c-fos mRNA expression induced by SKF82958 is critically dependent on D1 receptor expression. D1knock-out mice (generated by Drago et al., 1994) fail to exhibit c-fos mRNA responses to this drug. As further shown in Figure 1, blunted c-fos mRNA responses of both mutants are also detected with another full D1 agonist, SKF81297, and, consistent with results of a previous study that used SKF82958 (Schmauss, 2000), the blunted c-fos responses to SKF81297 treatment can also be reversed in a long-term manner by a single dose of METH. Thus, the results shown in Figure 1, as well as results of studies using SKF82958 in wild-type animals pretreated with a D2-like antagonist (Jung and Schmauss, 1999), indicate that SKF82958 has no agonist effects on D2-like receptors. A more detailed characterization of the basal and SKF82958-stimulated forebrain c-fos mRNA expression levels of drug-naive and METH-pretreated wild type and D2 and D3 mutants can be found elsewhere (Schmauss, 2000).
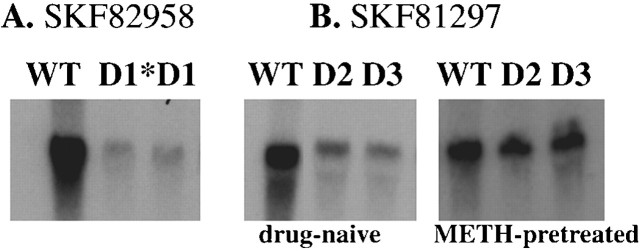
Fig. 1.
D1 agonist-stimulated forebrain c-fos mRNA expression. Northern blot of c-fos mRNA extracted from wild-type and D1, D2, and D3knock-out mice 60 min after injection of two full D1agonists SKF82958 (1 mg/kg) or SKF81297 (2 mg/kg). Each_lane_ contains 10 μg of total RNA extracted from tissues pooled from two animals per genotype. A, SKF82958-stimulated c-fos mRNA expression in wild-type (WT) and D1 −/− mice. The_lane_ marked D1* shows basal levels of c-fos mRNA detected in D1 mutants, and the_lane_ marked D1 shows c-_fos_mRNA detected in these mutants after SKF82958 injection. Note the absence of c-fos mRNA induction in SKF-treated D1 knock-out mice. B, Like SKF82958 (for a quantitative comparison, see Schmauss, 2000), the full D1 agonist SKF81297 also elicits blunted c-fos mRNA responses in D2 −/− (lanes marked D2) and D3−/− mice (lanes marked D3) that can be reversed by METH pretreatment (5 mg/kg). In this experiment, METH was administered 8 d before SKF81297 injection.
A single dose of METH (5 mg/kg) results in a long-term reversal of the blunted D1 receptor activity in both D2 and D3 single mutants (Schmauss, 2000). To test whether METH pretreatment can also reverse the blunted c-fos mRNA responses of homozygous D2/D3 double mutants, we performed in situ hybridization experiments using forebrain sections of wild type and double mutants that contained the PFC. As shown in Figure 2, SKF82958-stimulated expression of c-fos mRNA is prominent in wild type and appears highest in PL and IL cortices, as well as in deeper layers of the AC cortex. In corresponding anatomic regions of drug-naive D2/D3 double mutants, c-fos mRNA expression is drastically reduced. Consistent with results of our previous study (Schmauss, 2000), the blunted SKF-induced c-fos mRNA levels are still higher then the basal levels of either drug-naive or METH-pretreated double mutants (data not shown). METH pretreatment of D2/D3 double mutants, however, leads to wild-type-like levels of c-fos mRNA in response to an SKF82958 challenge.
Fig. 2.
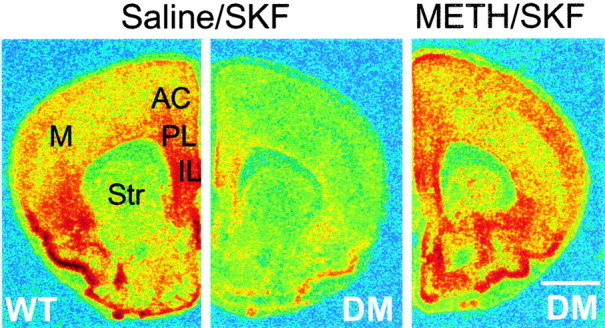
SKF82958-stimulated c-fos mRNA expression in drug-naive and METH-pretreated D2/D3 double mutants. In situ hybridization of c-fos mRNA expressed 60 min after SKF82958 (1 mg/kg) administration to drug-naive (saline-pretreated) wild type (WT) and drug-naive and METH-pretreated homozygous D2/D3double mutants (DM). METH (5 mg/kg) was administered 1 week before SKF administration. Sections were hybridized to a 35S-labeled antisense riboprobe comprising 540 nucleotides of the mouse c-fos mRNA. Images on film were digitized using the MCID image analysis system and colorized uniformly to highlight c-fos signal intensities. Note the blunted SKF-stimulated c-fos mRNA expression in drug-naive double mutants that is completely reversed by METH pretreatment.M, Motor cortices; Str, striatum. Scale bar, 1 mm.
Blunted Fos protein expression in the PFC of D1agonist-treated D2 and D3 mutants
Although the expression of c-fos mRNA is a sensitive indicator of (acutely stimulated) neuronal activity, c-_fos_mRNA levels are not a perfectly reliable indicator for Fos protein levels. c-fos gene transcription is rapidly shut off (within minutes) after induction (Greenberg and Belasco, 1993), and the rapid degradation of c-fos mRNA is a process that is tightly coupled to translation (Chen et al., 1994; Grosset et al., 2000). In fact, a common feature of all immediate early genes is that their stability is profoundly increased when translational activity is low (Greenberg and Belasco, 1993). Hence, regional c-fos mRNA levels may not correlate with corresponding protein levels. Therefore, as a more sensitive readout of the functional consequences of transcriptional c-fos induction, we performed a quantitative analysis of the expression of Fos immunoreactivity in neurons of the PFC.
The top panels of Figure 3illustrate the anatomic topography of the subregions of the mouse PFC (IL, PL, and AC) (Fig. 3, top left) and their neuronal cytoarchitecture revealed by NeuN immunolabeling of coronal sections taken 5.5 mm rostral to the interaural line (Fig. 3, top right). The bottom panels of Figure 3 show the expression patterns of Fos immunoreactivity in coronal cryosections obtained from wild type. These sections were collected from fresh-frozen forebrains and processed in parallel. In contrast to mice receiving only saline, the administration of SKF82958 induces a robust expression of Fos immunoreactivity, which is distributed diffusely in all layers of the PFC and in a defined band of nuclei outlining the outermost extent of layer II. No obvious differences in the expression of SKF82958-stimulated Fos immunoreactivity are evident between drug-naive and METH-pretreated wild type.
Fig. 3.
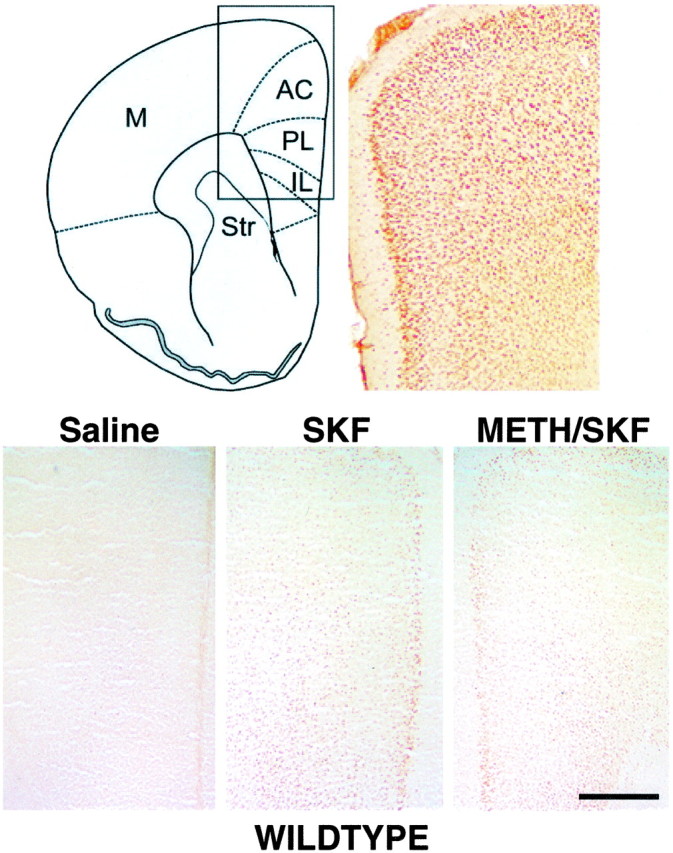
SKF82958-induced expression of Fos immunoreactivity in the PFC of wild type. Top, Schematic illustration of the regional extent of the mouse PFC in a cross section taken 5.5 mm rostral to the interaural line (Hof et al., 2000) and its cytoarchitecture visualized in a photomicrograph of an NeuN-immunolabeled section. Bottom, Expression of Fos immunoreactivity detected 60 min after saline or SKF82958 administration to wild type. In animals treated with saline, only a few nuclei express Fos immunoreactivity. SKF82958 induces a robust nuclear expression of Fos immunoreactivity in both the deep and superficial layers of the PFC. There is no obvious difference in the expression of SKF-stimulated Fos immunoreactivity between drug-naive and METH-pretreated wild type. M, Motor cortices; Str, striatum. Scale bar, 0.5 mm.
Figure 4 compares the Fos immunoreactivity induced by SKF82958 in cryosections of the prelimbic subregion of the PFC obtained from fresh-frozen forebrains of drug-naive wild type and mutants that were processed in parallel. It also illustrates the Fos immunolabeling of neuronal nuclei of this subregion of drug-naive wild-type and mutant mice in thionin-counterstained microtome sections at 100× magnification (Fig.4, bottom). Although the anatomic topography of the expression of Fos immunoreactivity is comparable with wild type, the quantity of Fos-immunoreactive nuclei is reduced in drug-naive D2 and D3 mutants (Fig. 4). Consistent with our previous study (Jung and Schmauss, 1999), the blunted SKF-stimulated c-fos responses of the mutants are still substantially higher then corresponding responses seen in saline-treated mutants (data not shown).
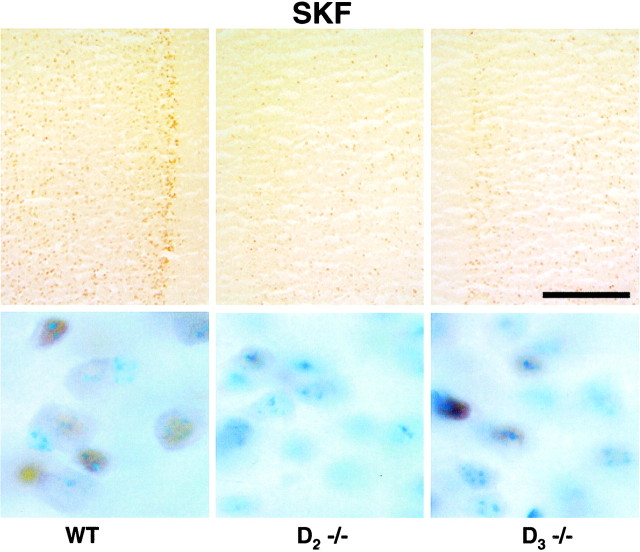
Fig. 4.
SKF82958-stimulated expression of Fos immunoreactivity in the prelimbic subregion of the PFC of D2 and D3 single mutants. Top, Detection of Fos immunoreactivity expressed 60 min after SKF82958 administration to drug-naive wild type (WT) and D2 (_D_2 −/−) and D3 (_D_3 −/−) mutants. Saline-treated mice of all genotypes expressed comparably low levels of Fos immunoreactivity (data not shown). In response to equivalent doses of SKF, drug-naive D2 and D3 mutants express blunted levels of Fos immunoreactivity throughout the prelimbic cortex.Bottom, Detection of Fos immunoreactivity (brown immunoperoxidase labeling) in neuronal nuclei (blue thionin counterstain) of the PFC of drug-naive wild-type and D2 and D3 mutants visualized at 100× magnification. Larger neurons were identified by nuclear size, and smaller neurons typically have only one or two thionin-labeled nuclear densities, a feature distinct from astrocytes. Scale bar, 0.25 mm.
Consistent with results of our previous study on c-fos mRNA expression in METH-pretreated wild type and mutants in the absence of a D1 agonist challenge (Schmauss, 2000), we found very low basal levels of Fos immunoreactivity in METH pretreated animals of all three genotypes (on average, in all subregions of the PFC, fewer than 10 labeled neuronal nuclei were detected in a single tissue section; data not shown). However, as shown in Figure5, compared with drug-naive mutants, METH-pretreated mutants express substantially more Fos immunoreactivity in response to a challenge dose of SKF82958. Altogether, the results of the immunocytochemical studies shown in Figures 4 and 5 are in perfect agreement with the results of studies that measured c-_fos_mRNA (rather than protein) levels in the mutants (Fig. 2) (Schmauss, 2000), and they suggest that the D1 receptor responsiveness to agonist stimulation is substantially blunted in the PFC of drug-naive (but not METH-pretreated) D2and D3 mutants. To ensure that these results are not attributable to differences in the numbers of prefrontal cortical neurons and to obtain a quantitative estimate of the magnitude of differences that are seen in Figures 4 and 5, we used the optical fractionator to estimate the total number of Fos-immunoreactive neurons in layers II/III and V/VI of the IL, PL, and AC. Forty-micrometer-thick sections, processed to detect Fos immunoreactivity on a thionin-counterstained background, were subjected to this analysis (see Materials and Methods). The microscopic inspection of these sections revealed no differences between genotypes and treatment groups in the cytoarchitectures of the regions analyzed.
Fig. 5.
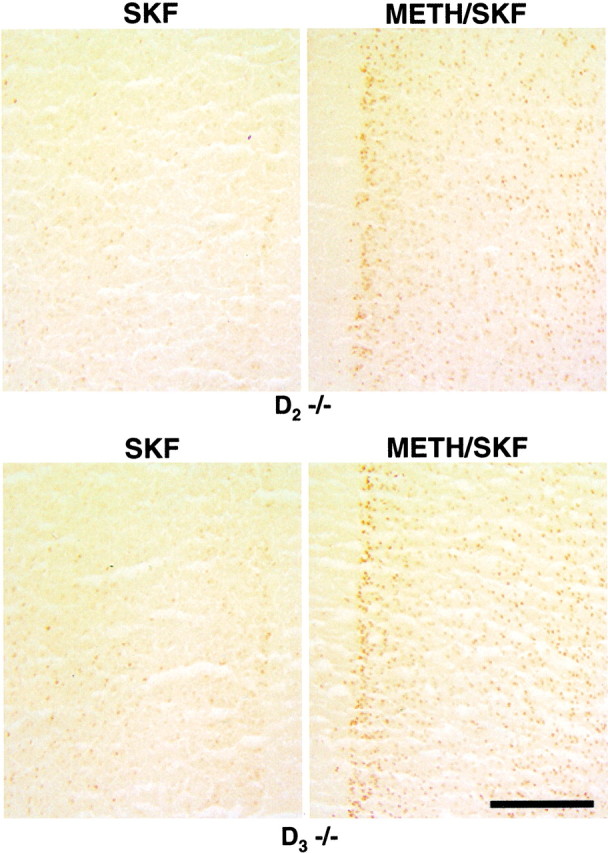
SKF82958-stimulated expression of Fos immunoreactivity in the prelimbic subregion of the PFC of drug-naive and METH-pretreated D2 and D3 single mutants. The blunted c-fos responses of drug-naive D2(D 2 −/−) and D3(_D_3 −/−) mutants are reversed when mutants were treated with METH (5 mg/kg) 1 week before an SKF82958 challenge. Scale bar, 0.25 mm.
The stereological analysis of two laminar territories (layers II/III and V/VI) of each of the three subregions of the PFC (IL, PL, and AC) revealed no significant differences in the number of neurons and glia, as well as the volumes of the regions measured. Thus, the cellular packing density was similar across genotypes and treatment groups. Figure 6 summarizes the mean counts of Fos-immunoreactive neurons expressed as a percentage of the total number of neurons determined for each of the two laminar territories of each of the three prefrontal cortical subregions. In all targeted areas, no significant differences were found in the percentages of Fos-immunoreactive neurons counted in sections of drug-naive and METH-pretreated wild type. In the superficial (II/III) and deep (V/VI) layers of the IL cortex, however, the percentages of neurons expressing Fos immunoreactivity determined for both mutants were lower compared with wild type, and these reductions were slightly more severe in D3 than in D2 mutants (Fig.6, top). In the superficial layers of the IL cortex of drug-naive D3 and D2 mutants, the mean percentage of neurons expressing Fos immunoreactivity was only 67% (p < 0.05) and 80% (nonsignificant), respectively, of that determined for wild type. Similar reductions were observed in the deeper layers of the IL cortex [D2, 67%; D3, 71% of wild type (nonsignificant)]. In METH-pretreated mutants that received a D1 agonist challenge, the percentages of neurons expressing Fos immunoreactivity in both superficial and deep layers of the IL cortex were only slightly increased. Although the increase was larger in METH-pretreated D2 mutants (90% in layers II/III and 92% in layers V/VI of wild type) than in D3 mutants (75% in layers II/III and 77% in layers V/VI of wild type), neither increase reached statistical significance when compared with drug-naive mutants.
Fig. 6.
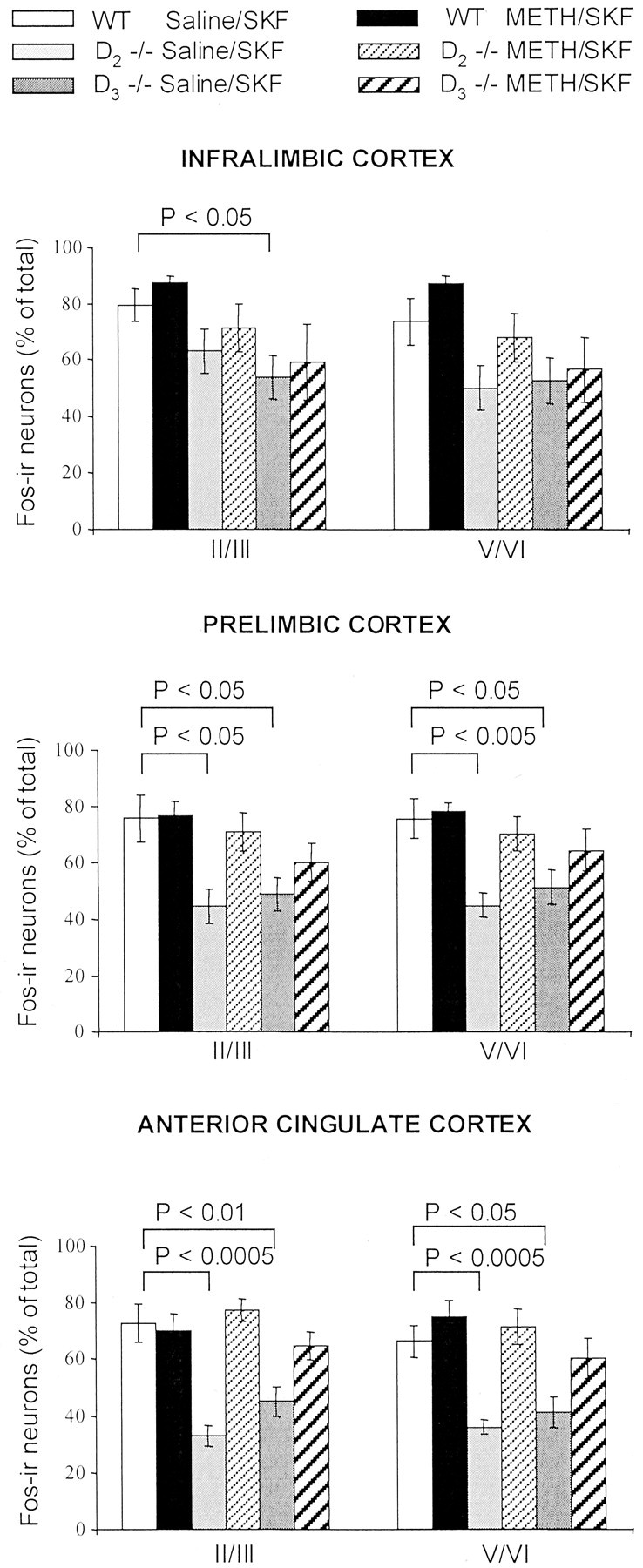
Stereological comparison of the numbers of Fos-immunoreactive (Fos-ir) neurons in the PFC of wild type and D2 and D3 single mutants. Mean ± SEM percentages of neurons expressing SKF-induced Fos immunoreactivity (calculated as percentage of the total number of neurons) in the superficial (II/III) and deep (V/VI) layers of the IL, PL, and AC cortices of saline- or METH-pretreated mice. In D2 and D3 singlemutants, the numbers of neurons expressing Fos immunoreactivity are reduced in all subregions of the PFC compared with wild type (WT) (reductions rank, AC > PL > IL). c-fos responses to SKF82958 are increased by METH pretreatment of the mutants. This increase is larger in D2 mutants but remains submaximal (relative to wild type) in all three subregions of the PFC of D3mutants.
In the PL cortex, drug-naive D2 and D3 mutants had significantly fewer Fos-immunoreactive neurons compared with wild type. These reductions were more severe in D2 [59% of wild type in both superficial (p < 0.05) and deep (p < 0.005) layers] than in D3 mutants [65% in superficial (p < 0.05) and 68% in deep (p < 0.05) layers] (Fig. 6,middle). Corresponding percentages calculated for METH-pretreated mutants, however, did not differ significantly from wild type. In METH-pretreated D2 mutants, the percentages of Fos-immunoreactive neurons were 94% (layers II/III) and 93% (layers V/VI) of wild type, and they were significantly increased compared with corresponding percentages obtained from drug-naive D2 mutants (layers II/III, p < 0.05; layers V/VI, p < 0.01). METH pretreatment of D3 mutants led to a more modest increase in the percentages of neurons that express Fos immunoreactivity [80% (layers II/III) and 85% (layers V/VI) of wild type]. This increase, however, did not reach statistical significance when compared with drug-naive D3 mutants.
The largest reduction in the expression of Fos immunoreactivity was found in the superficial and deep layers of the AC cortex of the mutants, and, similar to the PL cortex, the reduction was greater in D2 than D3 mutants (Fig. 6,bottom). In drug-naive D2 mutants, the mean percentage of neurons expressing Fos immunoreactivity was only 45% (layers II/III; p < 0.005) and 54% (layers V/VI;p < 0.005) of wild type, whereas in drug-naive D3 mutants, the mean percentages were 62% (layers II/III; p < 0.01) and 68% (layers V/VI;p < 0.05) of wild-type mice. METH pretreatment of both mutants significantly increased the number of neurons expressing Fos immunoreactivity. In METH-pretreated D2 mutants, the mean percentages of neurons expressing Fos immunoreactivity slightly exceeded that of wild-type mice (layers II/III, 106%; layers V/VI, 108% of wild type), and they were significantly increased compared with the corresponding numbers determined for drug-naive D2 mutants (layers II/III, p < 0.001; layers V/VI,p < 0.001). In METH-pretreated D3 mutants, the mean percentages of Fos-immunoreactive neurons were 90% (layers II/III) and 85% (layers V/VI) of wild type. Although these estimates did not significantly differ from wild type, when compared with drug-naive D3 mutants, they differed significantly only for numbers obtained from the superficial layers (p< 0.05).
In summary, both D2 and D3mutants exhibit a decreased agonist-stimulated D1receptor activity in the PFC. The magnitude of the long-term rescue of this blunted D1 receptor activity by a single dose of METH was larger in D2 than in D3 mutants.
Spatial delayed alternation
Delayed alternation tasks are considered to be particularly sensitive in demonstrating working memory deficits after lesions of the PFC in all mammalian species (Markowitsch and Pritzel, 1977). In rodents, this task is often performed in a T-maze (Moran, 1993), a valuable tool for evaluating spatial working memory associated with prefrontal cortical function (Van Haaren et al., 1985). Thus, because of the well documented role of prefrontal cortical D1 receptors in the control of working memory (Williams and Goldman-Rakic, 1995; Zahrt et al., 1997), we examined the performance of wild-type and mutant mice in a delayed alternation test performed in a T-maze. In contrast to the above study that relied on systemically administered D1 agonists, the performance in the T-maze is dependent on D1receptors that are activated by physiological concentrations of synaptically released dopamine.
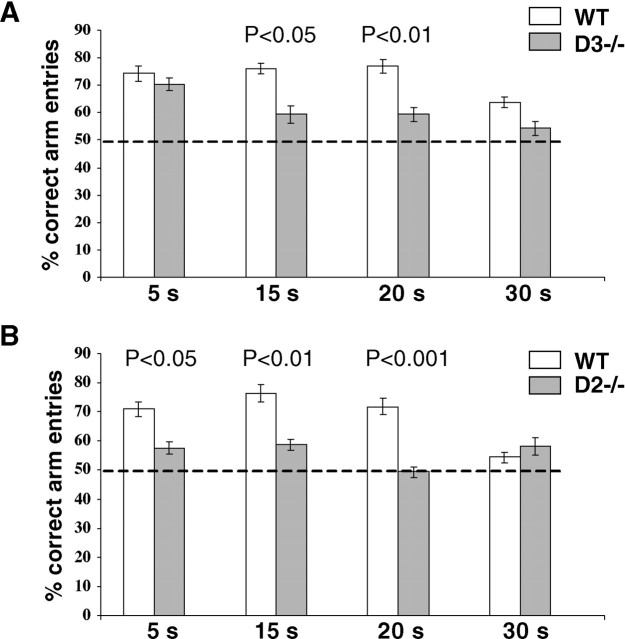
In a first series of experiments, homozygous D3mutants and their wild-type littermates were trained and tested in parallel as described in Materials and Methods. Wild type and D3 mutants exhibited similar learning curves with near-chance level performance (40–50% of correct arm entries) between days 5 and 12 of training (performed with 5 sec intertrial delays; data not shown), followed by a gradual improvement that reached a plateau of >70% correct arm entries on days 16 and 17 of the training (5 sec delay) (Fig. 7A). Retention times in the holding box were then increased to 15, 20, and 30 sec delays. As can be seen in Figure 7A, the performance of wild-type mice remained stable at 15 and 20 sec, with no trend toward improved performance at these delay times, but deteriorated significantly (p < 0.05) as the delay interval increased to 30 sec. The performance of D3 mutants, however, deteriorated gradually with increasing memory load and differed significantly from wild type in tests with 15 (p< 0.05) and 20 (p < 0.01) sec delays (Fig.7A).
Fig. 7.
T-Maze performance of wild type and D2and D3 single mutants at four retention times. The mean ± SEM percentage of correct arm entries was determined from tests using eight animals per genotype. Each delay period was tested twice, and the means of both days were statistically compared (ANOVA) between wild type and mutants. A, Comparison of wild type (WT) and D3 −/− mutants trained in parallel. B, Comparison of second group of wild type trained in parallel with D2 −/− mutants. Delay periods (holding box retention times) are indicated on the_abscissa_. The dashed line across the_bars_ at 50% correct arm entries indicates chance performance.
In a second series of experiments, homozygous D2mutants and their wild-type littermates were trained and tested in parallel. In this experiment, the learning curve and the test results obtained from wild type during the training period were in excellent agreement with results obtained from wild type tested in the first series involving D3 mutants (data not shown). At days 16 and 17 of training, wild-type mice made >70% correct choices, and their performance remained stable with no trend of additional improvement at 15 and 20 sec delays, and, at 30 sec delay, their performance deteriorated significantly (p < 0.01) (Fig. 7B). In D2 mutants, however, a significantly impaired performance is evident at all retention intervals, with increasing impairment at increasing delays (5 sec delay, p < 0.05; 15 sec delay, p< 0.01; 20 sec delay, p < 0.001).
In summary, drug-naive D3 and D2 mutants exhibited significant spatial working memory deficits. These deficits are not attributable to pronounced motivational impairments of the mutants. Both wild type and mutants exhibited “omission errors” (i.e., they visited the correct arm but did not take the food) only at the very beginning of the training but not subsequently (data not shown). Moreover, in all tests involving increasing delay periods, all mice started running immediately after the door of the holding box was opened, found the food, and ate it rapidly. Thus, mutants were able and motivated to perform the task, but their spatial working memory is reduced compared with wild type. Finally, although D2 mutants run slightly slower than controls during training [a consequence of their impaired locomotor activity (Jung et al., 1999)], differences in running speed cannot account for the lack of improvement in their working memory performance at the end of the training (Aultman and Moghaddam, 2001).
After baseline performance at all delay periods were established for the three drug-naive genotypes, all mice received a single injection of METH (5 mg/kg), and their working memory performance was monitored on days 1, 3, 5, and 6 after injection using the 5 sec delay paradigm. During this test period, the performance of wild-type mice tested in the first series fluctuated somewhat [percentage of correct arm entries: 71.4 ± 7.4 (day 1); 65 ± 6 (day 3); 70 ± 5.4 (day 5); and 76.3 ± 5.7 (day 6)] but did not statistically differ from corresponding results obtained from the same wild type before METH injection. The level of performance of D3 mutants was decreased (percentage of correct arm entries: 61.4 ± 8; 56.7 ± 5.6; 57.1 ± 4.7; and 61.4 ± 8.6). This decrease, however, did not reach statistical significance. A slightly, but nonsignificantly, decreased performance level was also detected in the group of wild-type animals tested in the second series of experiments (65.8 ± 4.6%; 71.3 ± 3.5%; 71.3 ± 4.4; and 65.7 ± 6.1%). Interestingly, the performance of D2 mutants gradually increased. On day 1 after METH, animals made 58.8 ± 3.5% correct choices, a result almost identical to their drug-naive performance. On days 3, 5, and 6 after METH, however, their performance improved and reached 65.7 ± 3.5, 62.5 ± 5.6, and 65.0 ± 3.3%, respectively, correct choices.
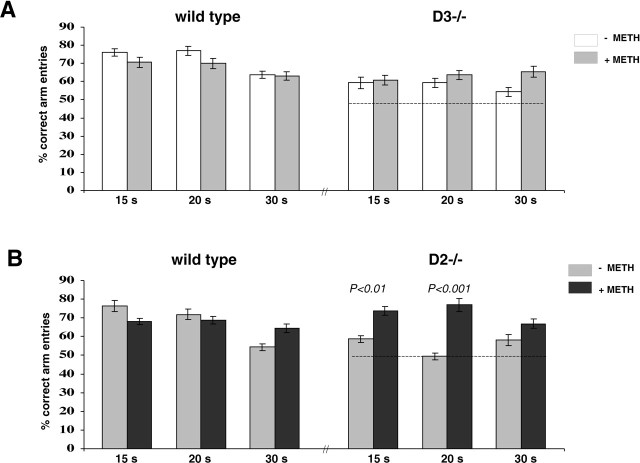
On days 7–12 after METH injection, animals were tested using the 15, 20, and 30 sec delay paradigms. Each delay period was tested twice (on 2 different days), and the means ± SEM, shown in Figure8, represent all data obtained in these 2 d. For each delay period, the performance of all METH-pretreated controls did not differ from their drug-naive performance. The performance of METH-pretreated D3 mutants also did not differ significantly from their drug-naive performance (Fig.8A). METH-pretreated D2mutants, however, significantly outperformed their drug-naive performance at 15 (p < 0.01) and 20 (p < 0.001) sec delays (Fig.8B). In fact, the performance of METH-pretreated D2 mutants recorded at 15, 20, and 30 sec delay periods differed neither from drug-naive nor METH-pretreated wild type. Thus, METH pretreatment rescues the working memory deficits of D2, but not D3, mutant mice.
Fig. 8.
Comparison of the T-maze performance of drug-naive and METH-pretreated wild type and D2 and D3mutants at three retention times. A, Comparison of drug-naive and METH-pretreated wild type tested in parallel with drug-naive and METH-pretreated D3 mutants.B, Comparison of drug-naive and METH-pretreated wild type tested in parallel with drug-naive and METH-pretreated D2 mutants. Delay periods are indicated on the_abscissa_, and the dashed line across the_bars_ at 50% correct arm entries indicates chance performance. Each delay period was tested twice, and the means of both days were statistically compared (ANOVA) between genotypes and treatment groups. Statistical differences are indicated on_top_ of the corresponding bars. In both series of experiments and for all three delay periods, no statistical differences were found between drug-naive and METH-pretreated wild type (wild type–D3 series, p = 0.09,p = 0.32, and p = 0.22 at 15, 20, and 30 sec delay, respectively; wild type–D2 series,p = 0.09, p = 0.10, and_p_ = 0.32 at 15, 20, and 30 sec delay, respectively).
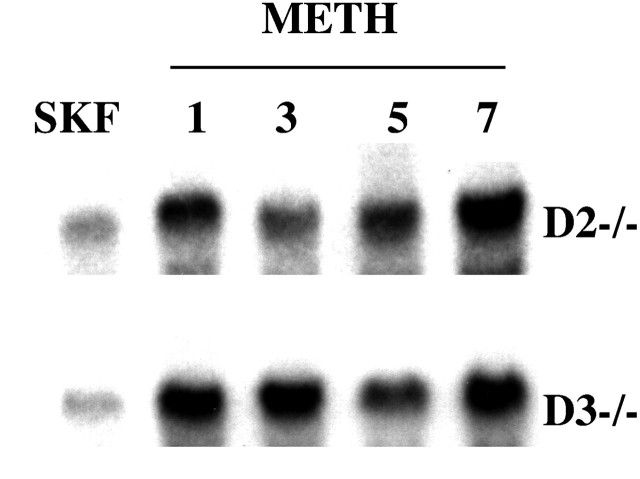
The gradually improved performance of METH-pretreated D2 mutants in the spatial working memory test, which peaked on day 8 after METH injection (and remained stable on the following days), suggested the possibility that SKF82958-stimulated c-fos responses of METH-pretreated mutants also increase gradually. However, results of Northern blots of frontal cortical RNA, shown in Figure 9, revealed that the reversal of the blunted responsiveness of D1receptors to agonist stimulation is already evident 24 hr after METH injection. Thus, in D2 mutants, the onset of the rescued agonist-stimulated D1 receptor activity and the onset of rescued cognitive performance are separated by a delay of ∼7 d. This time may be needed to reactivate neuronal circuitries that are critically involved in spatial working memory.
Fig. 9.
SKF82958-stimulated c-fos mRNA expression detected 1–7 d after METH pretreatment. Northern blot of frontal cortical c-fos mRNA expressed 60 min after injection of SKF82958 (1 mg/kg) in drug-naive (lanes marked SKF) and METH-pretreated D2 (D2 −/−) and D3 (D3 −/−) mice. Lanes_marked 1, 3, 5, and_7 illustrate SKF-stimulated c-fos mRNA levels detected 1, 3, 5, and 7 d, respectively, after METH (5 mg/kg) pretreatment.
DISCUSSION
The present study shows blunted prefrontal cortical c-fos responses to D1 agonist stimulation in mice deficient for D2 and D3 receptors and an impaired performance of these mutants in a spatial working memory task.
Previous work from our laboratory has shown that either a single dose of METH or a full D1 agonist leads to a long-term (as much as 2 weeks) reversal of the blunted D1agonist-stimulated c-fos expression in D2 and D3 mutants (Schmauss, 2000). Whereas the present quantitative analysis of expression of Fos immunoreactivity in the PFC of drug-naive and METH-pretreated D2 and D3mutants confirmed these previous findings, it also revealed a number of important quantitative differences between both mutants. Reductions in the neuronal expression of Fos immunoreactivity in drug-naive mutants are evident in all regions examined. They are more significant in the AC than in the PL cortex, and they are only modest in the IL cortex. In the superficial and deep layers of the AC or PL cortices, however, the reduced expression of Fos immunoreactivity is more pronounced in D2 than D3 mutants (although reductions in the IL cortex of D3mutants exceeded that estimated for D2 mutants). Whereas METH pretreatment resulted in an increased number of neurons that expressed Fos immunoreactivity in response to D1 agonist stimulation in both mutants, the rescue is greatest in the AC and PL cortices relative to the IL cortex, and the rescue is greater in D2 than in D3 mutants. In fact, D1agonist-stimulated c-fos responses in the AC and PL subregions of the PFC are indistinguishable from wild type only in METH-pretreated D2 mutants.
It is highly unlikely that differences in the behavioral state of SKF82958-treated drug-naive and METH-pretreated animals contributed to the differences seen in c-fos responses in the PFC. All genotypes exhibit an increased horizontal locomotor activity in response to SKF, and the predominant locomotor response of drug-naive animals is an oral stereotypy (sniffing, nibbling, and licking). Quantitatively, this stereotypic response does not differ between wild type and D3 mutants, but it is significantly enhanced in D2 mutants (Glickstein and Schmauss, 2001). This is unlikely to be attributable to decreased D1 receptor activation in the PFC because both D2 and D3 mutants show significantly reduced prefrontal cortical c-fos responses to SKF. In METH-pretreated animals, the response to SKF is no longer predominantly stereotypic but rather characterized by increased horizontal locomotion (running) that appeared indistinguishable between genotypes.
Blunted activity of D1 receptors in the PFC of D2 and D3 mutants is also evident when the performance of these mutants in a delayed alternation test, a spatial working memory task, is compared with wild type. The performance of D2 mutants is impaired at all delay periods with increasing impairment at increasing delays. In comparison, the performance of D3 mutants is less impaired, and D3 mutants are similar to wild type at the shortest delay (5 sec). These differences parallel the differences between both mutants in the magnitude of the blunted D1-agonist-stimulated c-fos responses in the AC and PL subregions of the PFC. Moreover, METH pretreatment affects the impaired working memory of both mutants differently. It completely restores wild-type-like performance levels in D2 mutants, but it does not alleviate the working memory deficits of D3 mutants. Interestingly, the stereologic analysis of D1 agonist-stimulated c-fos responses revealed that, in AC and PL, c-fos responses of METH-pretreated D3mutants remain submaximal. The correlation between D1 agonist-induced c-fos expression levels and the levels of performance in the spatial working memory task suggest that the sustained improvement of spatial working memory in METH-pretreated D2 mutants is attributable to D1 receptor-mediated mechanisms. However, our study has only established a correlation between the different magnitudes of prefrontal cortical c-fos responses to D1 agonist stimulation and the different levels of performance in working memory tasks. Direct evidence that these c-fos responses are necessary for working memory or, alternatively, that some D1 receptor function is necessary for spatial working memory but independent of c-fos expression levels is still lacking.
The finding of spatial working memory deficits of mice deficient for D2 and D3 receptors is consistent with results of a recent study showing that chronic treatment of monkeys with neuroleptic drugs (that block D2-like receptors) impairs their performance in working memory tests (Castner et al., 2000). We also found that chronic (but not acute or subacute) administration of neuroleptic drugs to wild-type mice decreases their c-fos responses to D1 agonist stimulation (S. B. Glickstein and C. Schmauss, unpublished observation). Moreover, several findings shown in Figures 7 and 8 illustrate clearly that the performance of mice in the T-maze reflects their working memory capacity: (1) the performance of wild type in the delayed alternation task is stable up to 20 sec retention intervals, and the accuracy of their performance is inversely proportional to retention time; (2) during the entire test period, the performance of wild type and mutants remained at a submaximal level (indicating that no “overtraining” occurred that would have required the implementation of longer retention times); (3) the impaired performance of drug-naive D2 and D3 mutants is also sensitive to delay periods, i.e., a gradually decreased performance was detected at increasing delay; and (4) no evidence for a decreased motivation of the mutants to perform the test was obtained. The weight of the present evidence therefore suggests that the spatial working memory deficit of D2 and D3 mutants is attributable to their decreased prefrontal cortical D1 receptor activity. However, direct proof that the working memory deficits described here are attributable to decreased D1 receptor activity remains to be provided.
An unexpected finding of the present study is that, in contrast to METH-pretreated D2 mutants, METH-pretreated D3 mutants show only a submaximal rescue of prefrontal c-fos responses to D1agonist stimulation, and METH pretreatment does not rescue their spatial working memory deficit. The reasons for the resistance of D3 mutants to METH pretreatment are presently unknown. It is possible that the inactivation of D3 receptors does not only lead to blunted prefrontal cortical D1 receptor responses to agonist stimulation but that D3 receptors also play a direct role in the control of working memory, a role that would be abolished in the D3 (but not D2) mutants. It is also possible that different mechanisms lead to the similarly blunted D1receptor activity in both mutants and that METH predominantly affects those mechanisms that are responsible for the phenotype detected in D2 mutants. For example, we reported recently that D2, but not D3, mutants exhibit decreased G-protein activation in response to D1 agonist stimulation and that this G-protein activation is differently affected by inhibition of phosphatases 1/2A and 2B in both mutants. The decreased SKF82958-stimulated G-protein activation of D2 mutants is completely reversed by METH pretreatment, but the abnormal sensitivities to phosphatase inhibitions are unaffected by METH pretreatment of both mutants (Hsiung et al., 2001).
How can a single, low dose of METH exert the long-lasting effects described here? METH has many complex actions, and not all of its targets may yet be known. For example, at the level of neuronal circuitries, METH could have prolonged stimulatory effects on dopamine synthesis in midbrain dopaminergic neurons and, thus, enable increased activation of projection areas (including the PFC) during stimulation. This hypothesis is consistent with the observation that a single dose of amphetamine leads to a sustained increase in electrically evoked release of dopamine in the forebrain (Vanderschuren et al., 1999). The long-lasting effects of METH could also be mediated by mechanisms that operate at the cellular level and that involve, for example, sustained decreases in the kinetics of agonist-provoked receptor internalization. Such decreases would increase the size of the receptor pool available for agonist stimulation. As mentioned above, we have preliminary evidence supporting the hypothesis that D1receptors expressed in the neocortex of D2mutants are hyperphosphorylated, suggesting that a large proportion of these receptors are not available for high-affinity agonist binding (Hsiung et al., 2001). When these mutants are pretreated with METH, however, the amount of agonist-stimulated G-protein activation increases significantly, and we are currently investigating whether METH pretreatment restores the normal phosphorylation state of the receptor expressed in the mutants.
Finally, in view of the potential clinical importance of the present findings that suggest that the chronic treatment of schizophrenic patients with typical neuroleptic drugs (that block the D2 class of dopamine receptors) worsens their cognitive deficits and that the selective blockade of either D2 or D3 receptors would have the same effect, more research is needed to elucidate the different mechanisms that maintain and disrupt the normally balanced activities of D1, D2, and D3 receptors in vivo.
Footnotes
This work was supported by National Institutes of Health Grant MH56123, National Science Foundation Grant IBN9808567, and the Essel Foundation (National Alliance for Research on Schizophrenia and Depression) (C.S.). S.B.G. was supported by Institutional Training Grant T32-MH18870 (Columbia University). We thank Chet Sherwood for help with stereology.
Correspondence should be addressed to Claudia Schmauss, Box 42, 1051 Riverside Drive, New York, NY 10032. E-mail:schmauss@neuron.cpmc.columbia.edu.
REFERENCES
- 1.Aultman JM, Moghaddam B. Distinct contributions of glutamate and dopamine receptors to temporal aspects of rodent working memory using a clinically relevant task. Psychopharmacology. 2001;153:353–364. doi: 10.1007/s002130000590. [DOI] [PubMed] [Google Scholar]
- 2.Baik J-H, Picetti R, Salardi A, Thiriet G, Dierich A, Depaulis A, Le Meur A, Borrelli E. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature. 1995;377:424–428. doi: 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- 3.Brozoski T, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkeys. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- 4.Castner SA, Williams GV, Goldman-Rakic PS. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science. 2000;287:2020–2022. doi: 10.1126/science.287.5460.2020. [DOI] [PubMed] [Google Scholar]
- 5.Chen CY, Chen TM, Shyu AB. Interplay of two functionally and structurally distinct domains of the c-fos AU-rich element specifies its mRNA-destabilizing function. Mol Cell Biol. 1994;14:416–426. doi: 10.1128/mcb.14.1.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drago J, Gerfen CR, Lachowicz JE, Steiner H, Hollon TR, Love PE, Ooi GT, Grinberg A, Lee EJ, Huang SP, Bartlett PF, Jose PA, Sibley DR, Westphal H. Altered striatal function in a mutant mouse lacking D1A dopamine receptors. Proc Natl Acad Sci USA. 1994;91:12564–12568. doi: 10.1073/pnas.91.26.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erlenmeyer-Kimling L, Rock D, Roberts SA, Janal M, Cornblatt B, Adamo UH, Gottesman II. Attention, memory, and motor skills as childhood predictors of schizophrenia-related psychosis: The New York High-Risk Project. Am J Psychiatry. 2000;157:1416–1422. doi: 10.1176/appi.ajp.157.9.1416. [DOI] [PubMed] [Google Scholar]
- 8.Fukushima J, Fukushima K, Chiba T, Tanaka S, Yamashita I, Kato M. Disturbances of voluntary control of saccadic eye movements in schizophrenic patients. Biol Psychiatry. 1988;23:670–677. doi: 10.1016/0006-3223(88)90050-9. [DOI] [PubMed] [Google Scholar]
- 9.Fuster JM. The prefrontal cortex—an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 10.Glickstein SB, Schmauss C. Dopamine receptor functions: lessons from knockout mice. Pharmacol Ther. 2001;91:63–83. doi: 10.1016/s0163-7258(01)00145-0. [DOI] [PubMed] [Google Scholar]
- 11.Gotham AM, Brown RG, Marsden CP. “Frontal” cognitive function in patients with Parkinson's disease “on” and “off” levodopa. Brain. 1988;111:299–321. doi: 10.1093/brain/111.2.299. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg ME, Belasco JG. Control of the decay of labile protooncogene and cytokine mRNAs. In: Belasco JG, Brawerman G, editors. Control of messenger RNA stability. Academic; San Diego: 1993. pp. 199–218. [Google Scholar]
- 13.Grosset C, Chen C-YA, Xu N, Sonnenberg N, Jacquemin-Sablon H, Shyu A-B. A mechanism for translationally coupled mRNA turnover: interaction between the poly(A) tail and a c-fos RNA coding determinant via a protein complex. Cell. 2000;29:29–40. doi: 10.1016/s0092-8674(00)00102-1. [DOI] [PubMed] [Google Scholar]
- 14.Hof PR, Young WG, Bloom FE, Belichenko PV, Celio MR. Comparative cytoarchitectonic atlas of the C57Bl/6 and 129/SV mouse brains. Elsevier; Amsterdam: 2000. [Google Scholar]
- 15.Hsiung S-C, Adlersberg M, Schmauss C, Tamir H. Decreased dopamine D1-receptor-activated binding of [35S]GTPγS in the neocortex of mice deficient for D2 receptors. Soc Neurosci Abstr. 2001;27:379.9. [Google Scholar]
- 16.Jung M-Y, Schmauss C. Decreased c-fos responses to D1-receptor agonist stimulation in mice deficient for D3 receptors. J Biol Chem. 1999;274:29406–29412. doi: 10.1074/jbc.274.41.29406. [DOI] [PubMed] [Google Scholar]
- 17.Jung M-Y, Skryabin BV, Arai M, Abbondanzo S, Fu D, Robakis NK, Brosius J, Polites HG, Pintar JE, Schmauss C. Potentiation of the D2-mutant motor phenotype in mice lacking dopamine D2 and D3 receptors. Neuroscience. 1999;91:911–924. doi: 10.1016/s0306-4522(98)00705-2. [DOI] [PubMed] [Google Scholar]
- 18.Levin BE, Labre MM, Weiner WJ. Cognitive impairment associated with early Parkinson's disease. Neurology. 1989;39:557–561. doi: 10.1212/wnl.39.4.557. [DOI] [PubMed] [Google Scholar]
- 19.Markowitsch HJ, Pritzel M. Comparative analysis of prefrontal learning functions in rats, cats, and monkeys. Psychol Bull. 1977;84:817–837. [PubMed] [Google Scholar]
- 20.Miller EK. The prefrontal cortex: complex neuronal properties for complex behavior. Neuron. 1999;22:15–17. doi: 10.1016/s0896-6273(00)80673-x. [DOI] [PubMed] [Google Scholar]
- 21.Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- 22.Moran PM. Differential effects of scopolamine and mecamylamine on working and reference memory in the rat. Neurosci Lett. 1993;138:157–160. doi: 10.1016/0091-3057(93)90502-k. [DOI] [PubMed] [Google Scholar]
- 23.Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- 24.Peters A, Palay SL, Webster H de F. The fine structure of the nervous system—neurons and their supporting cells, Ed 2. Oxford UP; New York: 1991. [Google Scholar]
- 25.Schmauss C. A single dose of methamphetamine leads to a long-term reversal of the blunted dopamine D1-receptor-mediated neocortical c-fos responses in mice deficient for D2 and D3 receptors. J Biol Chem. 2000;275:38944–38948. doi: 10.1074/jbc.M005064200. [DOI] [PubMed] [Google Scholar]
- 26.Schmauss C, Brines ML, Lerner MR. The gene encoding the small nuclear ribonucleoprotein-associated protein N is expressed at high levels in neurons. J Biol Chem. 1992;267:8521–8529. [PubMed] [Google Scholar]
- 27.Schmitz C, Hof PR. Recommendations for straightforward and rigorous methods of counting neurons based on a computer simulation approach. J Chem Neuroanat. 2000;20:93–114. doi: 10.1016/s0891-0618(00)00066-1. [DOI] [PubMed] [Google Scholar]
- 28.Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- 29.Vanderschuren LMJ, Schmidt ED, De Vries TJ, Van Moorsel CAP, Tilders FJH, Schoffelmeer ANM. A single exposure to amphetamine is sufficient to induce long-term behavioral, neuroendocrine, and neurochemical sensitization in rats. J Neurosci. 1999;19:9579–9586. doi: 10.1523/JNEUROSCI.19-21-09579.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Haaren F, De Bruin JP, Heinsbroek RP, Van de Poll NE. Delayed spatial response alternation: effects of delayed-interval duration and lesions of the medial prefrontal cortex on response accuracy of male and female Wistar rats. Behav Brain Res. 1985;18:41–49. doi: 10.1016/0166-4328(85)90167-6. [DOI] [PubMed] [Google Scholar]
- 31.Vaughan DW. The structure of neuroglial cells. In: Jones EG, Peters A, editors. Functional properties of cortical cells. Cerebral cortex, Vol 2. Plenum; New York: 1984. pp. 285–329. [Google Scholar]
- 32.Verma A, Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci. 1996;16:373–379. doi: 10.1523/JNEUROSCI.16-01-00373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinberger DR, Berman KF, Zec RF. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow (rCBF) evidence. Arch Gen Psychiatry. 1986;43:114–125. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- 34.West MJ, Slomianka L, Gundersen HJG. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- 35.Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 36.Xu M, Koeltzow TE, Santiago GT, Moratalla R, Cooper DC, Hu X-T, White NM, Graybiel AM, White FJ, Tonegawa S. Dopamine D3 receptor mutant mice exhibit increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors. Neuron. 1997;19:837–848. doi: 10.1016/s0896-6273(00)80965-4. [DOI] [PubMed] [Google Scholar]
- 37.Zahrt J, Taylor JR, Mathew RG, Arnsten AFT. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]