Overexpression of 5-HT1B Receptor in Dorsal Raphe Nucleus Using Herpes Simplex Virus Gene Transfer Increases Anxiety Behavior after Inescapable Stress (original) (raw)
Abstract
5-HT1B autoreceptors have been implicated in animal models of stress and are regulated selectively by serotonin-selective reuptake inhibitors such as fluoxetine. These terminal autoreceptors regulate serotonin release from dorsal raphe nucleus (DRN) projections throughout rat forebrain. However, it has not been previously possible to manipulate 5-HT1B autoreceptor activity selectively without also changing 5-HT1B activity in other neurons mediating different behavioral responses. Therefore, we have developed a viral-mediated gene transfer strategy to express hemagglutinin-tagged 5-HT1B and manipulate these autoreceptors in DRN. Green fluorescent protein (GFP) was coexpressed from a separate transcriptional unit on the same amplicon to assist in monitoring infection and expression. We confirmed the expression and biological activity of both transgenic proteins in vitro. When injected directly into DRN using stereotaxic procedure, HA-5-HT1B receptors were expressed in serotonergic neurons and translocated to the forebrain. The effect of DRN expression of HA-5-HT1B on stress-induced behaviors was compared with control rats that received GFP-only amplicons. There was no change in immobility in the forced swim test. However, HA-5-HT1B expression significantly reduced entrances into the central region of an open-field arena after water-restraint stress without altering overall locomotor activity, but not in the absence of stress exposure. HA-5-HT1B expression also reduced entries into the open arms of the elevated plus maze after water restraint. Because these tests are sensitive to increases in anxiety-like behavior, our results suggest that overactivity of 5-HT1Bautoreceptors in DRN neurons may be an important mediator of pathological responses to stressful events.
Keywords: herpes simplex virus, HSV, dorsal raphe nucleus, autoreceptor, hemagglutinin, forced swim test, open-field test, elevated plus maze
5-HT1Bautoreceptors are localized in the terminals of serotonergic axonal projections from midbrain raphe nuclei throughout the rat forebrain (Jacobs and Azmitia, 1992). These inhibitory autoreceptors respond to extracellular serotonin (5-HT) by reducing release of 5-HT from axonal terminals acutely and by reducing the amount of 5-HT synthesized over time (Hoyer and Middlemiss, 1989; Hjorth et al., 1995). Therefore, 5-HT1B autoreceptors constitute a negative feedback system that regulates 5-HT neurotransmission on the basis of local conditions at the site of release.
5-HT1B receptors are also synthesized in many other neurons widely distributed throughout the forebrain, where they inhibit the release of various other neurotransmitters such as acetylcholine, glutamate, and GABA (Barnes and Sharp, 1999). Because there are only ∼20,000 serotonergic neurons in rat brain (Wiklund et al., 1981), the proportion of 5-HT1B receptors in rat CNS that are autoreceptors in serotonergic neurons is very small. Indeed, chemical lesioning of the serotonergic system has small and inconsistent effects on total 5-HT1B binding density (Verge et al., 1986; Offord et al., 1988; Sexton et al., 1999).
Pharmacological and null mutation (“knock-out”) strategies have implicated 5-HT1B receptors in a number of physiological processes and complex behaviors (Hen et al., 1993). However, in most cases it has not been possible to ascribe the 5-HT1B effects to a particular population of neurons, because 5-HT1B receptors on various neuronal terminals are intermingled in practically all forebrain areas. This has made it difficult to study the specific cellular mechanisms by which 5-HT1B receptors are involved in brain functions.
5-HT1B autoreceptors in forebrain are coded for by messenger RNA expressed primarily in dorsal raphe nucleus (DRN) (Hamblin et al., 1992; Neumaier et al., 1996b, 2000; Roberts et al., 1998). Although these neurons project very diffusely to forebrain cortical and subcortical structures, the cell bodies are closely packed in a small, midline nucleus (Kosofsky and Molliver, 1987). 5-HT1B terminal autoreceptors appear to be involved in the adaptation of DRN neurons to serotonin-selective antidepressants, which are also effective in many anxiety disorders (Bergqvist et al., 1999; Sayer et al., 1999). 5-HT1B mRNA is selectively downregulated in DRN but not in hippocampus, striatum, or frontal cortex by either fluoxetine or paroxetine in a time-dependent and reversible manner (Neumaier et al., 1996a; Anthony et al., 2000). Learned helpless rats (an animal model of depression involving inescapable stress) have a reversible deficit in 5-HT release in prefrontal cortex (Sherman and Petty, 1980; Petty et al., 1992) and increased 5-HT1B mRNA in DRN (Neumaier et al., 1997). These observations suggest that increased 5-HT1Bautoreceptor activity induces depressive and related anxiety symptoms and that downregulation of DRN 5-HT1Bautoreceptors by antidepressants may be important in normalizing serotonergic neurotransmission and relieving symptoms of depression or anxiety (Briley and Moret, 1993).
We propose to test the hypothesis that 5-HT1Bautoreceptor overactivity alters the behavioral responses to inescapable stress. In this study we manipulated the 5-HT1B autoreceptors selectively while avoiding direct effects on the 5-HT1B heteroreceptors expressed in nonserotonergic neurons throughout the forebrain. To accomplish this we used replication-deficient herpes simplex virus type 1 (HSV) (Geller et al., 1990; Neve and Geller, 1995; Neve, 1999a) to overexpress 5-HT1B autoreceptors in DRN neurons. In this study we describe the development of a dual expression vector carrying both epitope-tagged 5-HT1B receptors and green fluorescent protein (GFP) on separate transcriptional cassettes and have characterized the effects of 5-HT1B gene transfer into DRN neurons on behavioral responses to inescapable stress using the forced swim test (FST), open-field test (OFT), and elevated plus maze (EPM) test.
MATERIALS AND METHODS
Plasmid construction
To introduce a hemagglutinin (HA) epitope tag into the N terminus of the rat 5-HT1B gene, plasmid MGIIB (Hamblin et al., 1992) was used as a template to PCR clone the rat 5-HT1B full-length sequence using an upstream primer (5′-TTCTAGAGCTATGTACCCATATGACGTCCCAGACTACGCCGAGGAGCAGGGTA-3′) that introduced an _Xba_I site and an in-frame HA epitope. The downstream primer (5′-GAGATGCATGATGGAAGCAGT-3′) corresponded to the single _Nsi_I site downstream of the translation start point. The resulting fragment was cloned into pCR-Script AMP SK+ (Stratagene, La Jolla, CA) as described by the manufacturer. This sequence was confirmed in its entirety by automated DNA sequencing. The_Xba_I/_Nsi_I fragment of this plasmid was then ligated into the _Xba_I/_Nsi_I fragment of an intermediate plasmid created by ligation of the _Hin_dIII fragment of the rat 5-HT1B cDNA, MG11B (Hamblin et al., 1992), into _Hin_dIII cut pGEM3Zf+ (Promega, Madison, WI). The resulting plasmid, pHA1B, was cut with _Hin_dIII, blunted with Klenow fragment, cut with _Eco_RI, and ligated into _Eco_RI/_Sma_I cut pCI (Promega). This plasmid, pCI-HA1B, produces hemagglutinin-tagged 5-HT1B under control of the CMV promoter/enhancer. To produce a version of this plasmid that coexpresses enhanced GFP (EGFP), plasmid pEGFP-C1 (Clontech) was cut with _Bam_HI/_Bgl_II and recircularized to eliminate most of the polylinker. The resulting plasmid was then cut with_Xba_I, blunted with Klenow fragment, and cut with_Nhe_I, and the GFP cDNA was isolated and ligated into_Nhe_I/_Sma_I cut pCI (Promega). This plasmid, pCMV-GFP, produces GFP under control of the CMV promoter/enhancer. pCMV-GFP was cut with _Bgl_II/_Bam_HI, and the 3.3 kb fragment was isolated. This fragment was ligated into _Bam_HI cut pCI (Promega) to generate pCIGFP, containing two transcriptional units in tandem, with the first possessing a polylinker for introduction of a desired gene and the second expressing GFP. The pCIGFP was cut with_Eco_RI/_Sma_I, and the HA-5-HT1B fragment used to produce pCI-HA1B was ligated into it to produce pCIGFP-HA1B. All plasmids were identified by multiple restriction cuts.
Amplicon construction and packaging
To produce an HSV amplicon producing both HA-5-HT1B and GFP, pCIGFP-HA1B was cut with _Xba_I and_Bgl_II. This digest was then partially digested with_Bam_HI, and the 3.8 kb fragment was isolated. The resulting fragment was ligated into _Xba_I/_Bam_HI cut pHSV-PrPUC (generously provided by Dr. Rachael Neve, McLean Hospital, Boston, MA). This reconstructed amplicon contains two transcriptional units terminated by SV40 polyadenylation sites, the first producing HA-5-HT1B from an HSV promoter/enhancer and the second producing GFP from a CMV promoter/enhancer. To make an amplicon producing only GFP, the _Nhe_I/blunted _Xba_I fragment of pCMV-GFP was ligated into_Xba_I/(blunted)_Bam_HI cut pHSV-PrPUC. Each plasmid was identified by multiple restriction cuts. HSV amplicons were then packaged either by Dr. Rachael Neve or in our laboratory as described previously (Neve, 1999b).
Cell culture infections and transfections
PC12 cells were infected with packaged HSV amplicons as described previously (Neve, 1999b). Briefly, PC12 cells were grown to ∼80% confluence in DMEM containing 10% fetal bovine serum and penicillin/streptomycin/amphotericin B (100 U/ml, 100 μg/ml, and 0.25 μg/ml, respectively). Cells were harvested by a brief trypsin/EDTA treatment and passed through a 21 gauge syringe to dissociate aggregates. After counting on a hemocytometer, 3 × 105 cells were plated onto poly-d-lysine-coated 24-well cell culture dishes, grown for 24 hr, and then treated with varying concentrations of packaged HSV amplicon stocks (maximum of 1 μl of virus per milliliter of medium). After 24 hr, cells were fixed in 4% paraformaldehyde/sodium phosphate buffer for later fluoromicroscopy and immunocytochemistry. Viral titer was determined from the number of GFP or hemagglutinin/GFP-positive cells.
HeLa, COS7, and CA77 cells were maintained as described previously (Hamblin et al., 1992; Clark et al., 1995; Zhukovskaya and Neumaier, 2000). HeLa cells were transfected essentially as described previously (Tverberg and Russo, 1992). Briefly, cells were grown to ∼80% confluence and harvested by a brief trypsin/EDTA treatment. Cells (∼1 × 106 cells per chamber) were suspended in ice-cold Ca2+/Mg2+-free PBS, chilled 10 min on ice, and electroporated (0.300 kV, 1000 μF) in an Electroporator 2 (Invitrogen, Carlsbad, CA) in the presence of 20 μg of transfection DNA. After electroporation, cells were mixed very gently and placed on ice for another 10 min before plating. COS7 cells were similarly transfected, except that the electroporation conditions were 0.330 kV at 500 μF. CA77 cells were grown to 80% confluence and infected with 3 ml of viral particles per 3 ml of medium per well in six-well dishes. Twenty-four hours after transfection or infection, cells were fixed in 4% paraformaldehyde or collected by centrifugation at 500 × g for 10 min for subsequent RNA extraction.
Reverse transcribed-PCR
Total RNA from fresh CA77 cells infected with either pHSV-HA1B/GFP or pHSV-GFP was purified using RNeasy columns (Qiagen, Valencia CA) and DNase I treatment. Total RNA from DRN was prepared from a 1 mm tissue punch containing DRN from a 2-mm-thick fresh brain slice that contained the anterior DRN (approximately −6.5 to −8.5 mm relative to bregma). The punched tissue was processed in RNAlater (Ambion, Austin, TX), and total RNA was isolated as described for CA77 cells, using the manufacturer's recommended procedures followed by DNase I treatment. RNA was quantified with RiboQuant (Molecular Probes, Eugene, OR), and control DNA was quantified with PicoGreen assays (Molecular Probes). Total RNA (1.5 μg for CA77 cells; 0.25 μg for DRN) was reverse transcribed into first-strand cDNA using oligo-dT primer and Moloney murine leukemia virus (Promega) in a final volume of 20 μl. HA-5-HT1B was selectively amplified by 35 cycles of PCR using a pair of primers that are specific for the hemagglutinin tag (5′-ACCCATATGACGTCCCA-3′) and the 5-HT1B sequence (5′-ACCGTGTACATGGTGCT-3′), yielding a 350 nucleotide PCR product. Total 5-HT1B reverse transcribed (RT)-PCR was similarly amplified using primers 5′-GGTCTTTTCACAGGTAGGTCAA-3′ (upstream) and 5′-TTGACCTACCTGTGAAAAGACC-3′ (downstream), yielding a 578 nucleotide PCR product. PCR products were resolved using 1.3% Agarose gels and stained with SYBR Gold (Molecular Probes) before photography.
Quantitative reverse transcribed-PCR
5-HT1B mRNA was quantified from first-strand cDNA prepared from DRN as described above using real time quantitative PCR with a LightCycler Instrument (Roche, Indianapolis, IN) with SYBR Green detection of PCR product. A 61 nucleotide PCR product was amplified using primers 5′-CCAAAAGGGCGGCCA-3′ (upstream) and 5′-TGGCAGCGAAATCGAGATG-3′ (downstream) from 1 μl of template containing either first-strand cDNA or known amounts of MG11B control template (1 × 10−7 − 1 × 10−4 ng per reaction). The thermal cycling procedures and quantitation procedures were based on the manufacturer's recommendations. Briefly, a standard curve constructed from the control template reactions was used to calculate the amount of first-strand cDNA present in the samples. Each duplicate determination was analyzed in three independent assays to calculate the relative amount of first-strand cDNA from each tissue sample in a blinded manner. Total 5-HT1B mRNA determinations from each brain sample were standardized using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) RT-PCR quantitation from the same preparation, using the following primers: 5′-AACGACCCCTTCATTGAC-3′ (upstream) and 5′-TCCACGACATACTCAGCAC-3′ (downstream). After the code was broken, treatment group averages were calculated and are expressed as percentage of control (pHSV-GFP). The efficiency of the RT reaction was not calculated, but all samples were prepared in parallel at each step.
cAMP determination
cAMP levels were assayed as described previously (Kohen et al., 1996). Briefly, JEG-3 cells were grown in DMEM supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin under 10% CO2. Cells were seeded into 24-well plates and grown to a density of ∼50,000 cells per well. One to four hours before transfection, the medium was replaced with 250 μl of DMEM supplemented with 10% dialyzed fetal bovine serum and 1% penicillin–streptomycin, after which the cells were switched to 5% CO2. Cells were transiently transfected by a calcium phosphate precipitation method as described previously (Heidmann et al., 1998). Transfected DNA consisted of 1 ng of 5-HT1B (MG11B) or pHSV-HA1B/GFP plasmid (except for controls in which no receptor was transfected), 50 ng of Rous sarcoma virus (RSV)-β-galactosidase plasmid, 2.5 ng RSV-cAMP responsive element (CRE)-luciferase plasmid (Mellon et al., 1989), and plasmid Bluescript II KS(−) (Stratagene) as carrier DNA for a total of 250 ng of DNA in 25 μl per well. Twenty hours after transfection, cells were washed once with PBS, supplemented with 500 μl of serum- and serotonin-free medium (Complete Medium, Cellgro, Herndon, VA) with 1% penicillin–streptomycin, and switched back to 10% CO2. After another 24 hr, triplicate wells were supplemented with 25 μl of forskolin (Calbiochem, San Diego, CA) for a final concentration of 1 mm, and with 25 μl of 2 mm ascorbic acid alone or ascorbic acid containing 5-HT (Sigma, St. Louis, MO) for a final concentration of 1 × 10−11m to 1 × 10−6m. Five hours later, cells were harvested in 100 μl of lysis buffer containing 100 nm KPO4, 6 mmMgSO4, 1 mm dithiothreitol, and 0.1% Triton X-100. To 350 μl of luciferase assay buffer (100 nm KPO4, 4 mm ATP, 6 mm MgSO4), 25 μl of cell extract was added and incubated at room temperature for 30 min. Luciferase activity was then assayed using an Autolumat LB 953 luminometer (EG and G Berthold, Bundoora, Australia) as described elsewhere (Migeon and Nathanson, 1994). Data were analyzed using the program Prism (GraphPad Software, San Diego, CA).
Stereotaxic injections and animal care
All animal procedures were approved by this institution's animal care committee and handled in accordance with National Institutes of Health guidelines. Male Sprague Dawley rats (180–250 gm) were anesthetized with pentobarbital (0.9 mg/kg, i.p.) or isoflurane (2–3% in oxygen), the scalp fur was shaved, the animal was placed in a Stoelting stereotaxic device, and the surgical site was cleaned with betadine. After scalp incision, skull landmarks were visualized by scraping of the periosteum. A small hole was bored at the site of injection. To avoid penetration of the third ventricle, the DRN (−7.7 from bregma, midline, 6.6 mm deep) was approached from an angle 20 or 25° off midline. The needle was slowly advanced over the course of 5 min, and 2 μl of viral particles (∼200,000 infective units) was injected from a Hamilton syringe (#30 needle) over 10 min using a microprocessor-controlled pump (World Precision Instruments, Sarasota, FL). The needle was left in place for 10 min after the injection and then withdrawn slowly over 10 min. This injection volume and procedure correspond to previous studies with pHSV-PrPUC-based amplicons (Carlezon et al., 1997; Song et al., 1998). The skin was closed with surgical methylacrylate glue, and in later injections the closure was augmented with sterile 3-0 monofilament nylon sutures (Ethicon); the rats were monitored until they recovered spontaneous movement. Animals were allowed to recover for 48–96 hr before being killed. For immunohistochemistry, rats were injected with heparin (1000 U, i.p.), deeply anesthetized with pentobarbital, and intracardially perfused with Tyrode's solution followed by 4% paraformaldehyde. The brains were removed, post-fixed for 2 hr in 4% paraformaldehyde, and stored in PBS at 4°C 1–2 d before being processed further. For immunoblot analysis, fresh tissue was harvested, immediately frozen, and stored at −70°C. For RT-PCR, fresh tissue was processed as described below. For all elevated plus maze and pre-stress open-field testing, injection location was confirmed in a blinded manner on perfused 40 μm tissue slices prepared on a vibratome. Animals were excluded if >50% of GFP-expressing neurons were outside the DRN or if there was any evidence of trauma distorting any anatomic structures nearby.
To determine the number of infected neurons, 40 μm sequential vibratome sections through the entire DRN were cut from perfused tissue and mounted on slides. GFP-positive cell bodies within the DRN were then counted manually. Although counting of the same GFP-positive cell body in two sequential sections was theoretically possible, the thickness of the slices relative to cell body size suggests that the error introduced by counting a single cell twice is small relative to the number of total neurons infected.
Immunocytochemistry, immunohistochemistry, and microscopy
For immunocytochemistry of cell cultures, the medium was aspirated, and cultures were rinsed briefly in PBS and fixed in 4% paraformaldehyde in PBS for 30 min at room temperature. Cells were rinsed briefly in PBS blocked with 0.3% gelatin (bovine) in PBS–0.025% Triton X-100 for 1 hr to overnight. Wells were incubated with mouse monoclonal anti-hemagglutinin antibody (1:1000 in 0.3% gelatin PBS–0.025% Triton X-100) (HA.11, Babco, Richmond, CA), rinsed three times for 10 min with PBS–0.025% Triton X-100, and then incubated with a goat anti-mouse Cy3-conjugated antibody (1:500 in 0.3% gelatin PBS–0.025% Triton X-100) (Jackson ImmunoResearch, West Grove PA) for 1 hr at 37°. Wells were then rinsed three times for 10 min with PBS–0.025% Triton X-100 and rinsed briefly with deionized water. Excess water was removed, and the bottoms of the wells were coated with Gel/Mount (Biomeda, Foster City, CA). Immunofluorescence was visualized with a Nikon inverted fluorescence microscope using an FITC filter for detection of GFP fluorescence and a rhodamine filter for detection of Cy3.
For immunohistochemistry, free-floating sections (40 μm) were prepared on a Leica VT1000S and rinsed in PBS. Sections were permeabilized in PBS–0.5% Triton X-100 for 30 min and then blocked with 0.3% gelatin (bovine) in PBS–0.025% Triton X-100 for 1 hr at room temperature or overnight at 4°C. They were then incubated with one or more primary antibodies concurrently: mouse monoclonal anti-hemagglutinin antibody (HA.11, Babco,), guinea pig anti-5-HT1B (Chemicon, Temecula, CA), and guinea pig anti-5-HT1A (Chemicon). All antibodies were diluted 1:1000 in 0.3% gelatin in PBS–0.025% Triton X-100 and incubated overnight at room temperature with gentle agitation. Sections were then rinsed three times for 10 min with PBS–0.025% Triton X-100 and incubated with secondary antibodies [goat anti-mouse Alexa-633 conjugate and/or goat anti-guinea pig Alexa-568 conjugate (Molecular Probes)], again concurrently, in 0.3% gelatin PBS–0.025% Triton X-100 for 1 hr at room temperature. Secondary antibodies were diluted as follows: Alexa-568 conjugate diluted to 5 μg/ml for anti-5-HT1A and to 10 μg/ml for anti-5-HT1B, and Alexa-633 conjugate diluted to 20 μg/ml. Sections were then rinsed three times for 10 min with PBS–0.025% Triton X-100, rinsed briefly with deionized water, and mounted on glass slides with Prolong Antifade mounting medium (Molecular Probes). The sections were analyzed using a Bio-Rad Radiance 2000 confocal system (Bio-Rad, Hercules, CA) and an associated Nikon fluorescence microscope using an argon/krypton laser and red laser diode with appropriate Performance filters (Bio-Rad) for detection of GFP, Alexa-568, and Alexa-633 fluorescence.
Immunoblot analysis
Frozen tissue was crushed on dry ice, placed into boiling 5% SDS/50 mm Tris-HCl, pH 8.0, for 5 min, sonicated for 10 sec, and spun at 10,000 × g for 10 min. The supernatant was retained and assayed for protein concentration by the BCA Protein Assay (Pierce Biochemical, Rockford, IL). Protein samples (50–100 μg) were separated on a 10% SDS-PAGE gel. Western blotting using nitrocellulose was performed at 4°C in a Bio-Rad blotting apparatus at 15 mV overnight or 35 mV for 2 hr. Blots were blocked in 5% instant milk in 50 mm Tris–0.9% saline containing 0.025% Tween 20 (TBST) for 1–2 hr, incubated in anti-hemagglutinin primary antibody (1:1000 in 5% non-fat instant milk-TBST for 1 hr at room temperature) (HA.11, Babco), and rinsed three times for 10 min in TBST. Blots were incubated in anti-mouse-HRP-conjugated secondary antibody (1:1000 in 5% non-fat instant milk–TBST for 1 hr at room temperature) and rinsed three times for 10 min in TBST. Blots were developed with SuperSignal West Pico chemiluminescent substrate (Pierce Biochemicals) for 5 min and exposed to film. Biotin-conjugated molecular weight markers were visualized by further incubating the blot in anti-biotin-HRP-conjugated antibody (New England Biolabs, Beverly, MA) at 1:1000 for 1 hr, rinsing three times for 10 min in TBST, and redeveloping in chemiluminescent substrate.
Behavioral testing procedures
Forced swim test. The FST was performed as described previously (Porsolt et al., 1977; Detke et al., 1995). The FST container was a 40-cm-tall Plexiglas cylinder with a 20-cm-diameter base, mounted on a Plexiglas base. It was filled with tap water (25°C) to 30 cm, a level deep enough to prevent the rat from resting on its extended tail. The forced swim stress session consisted of placing the rat in the chamber for 15 min, followed by towel drying the rat under warm lamps and returning it to the home cage. The following day (between the hours of 9 and 11 A.M.), the test session was performed by putting the rat into the water and videotaping its behavior for 5 min. Each cylinder was cleaned between animals. FST behaviors were scored in a blinded manner, by a different experimenter (J.N.), using a time-sampling method (Detke et al., 1995). Every 5 sec the animal's behavior was scored as climbing, swimming, or immobile; a total of 60 observations were made during the 5 min test session. Statistical comparison of between group differences was performed with the Mann–Whitney U test using GB-Stat software, with p ≤ 0.05 considered significant. Increased immobility time was considered to represent behavioral depression or behavioral despair but could also be operationally defined as a behavioral pattern that is preventable by treating the animal with antidepressants (Porsolt, 2000).
Water-restraint stress. Some animals were stressed for 15 min by water restraint on the third day after viral particle injection, as modified from a previously described procedure (Pare, 1994). Animals were loosely restrained in an envelope constructed of plastic mesh so that they could not make gross body movements, and they were suspended to the level of their necks in 25°C water for 15 min (using an FST chamber). The animals were then wiped briefly with a towel, dried under a lamp, and returned to their home cage.
Open-field test. Animals were either tested 3 d after viral particle injection (no stress exposure) or 24 hr later after exposure to water-restraint stress. The open-field test (OFT) was performed using a 45-cm-square black Plexiglas enclosure with 30-cm-tall walls set on a nonreflective black plastic base divided into a grid of nine equal squares. The OFT arena was located in a small, quiet, light-proof room with video monitoring so that the researcher could leave the room immediately after placing the rat in the center of the arena. Animals were tested between 4 and 6 P.M. under low illumination red light, to which Sprague Dawley rats are blind, thereby simulating darkness and increasing locomotor activity. Behavioral data were collected by videotape for 10 min; the tape was scored by a different experimenter (J.N.) in a blinded manner. The number of entries into the central square over the first 3 min and the total squares entered over 10 min were counted. The procedure and analysis used were based on previous studies suggesting that centroid entering on initial placement in the maze was most sensitive to stress-induced anxiety states (Pare, 1994; Izumi et al., 1997; Durand et al., 1999). Furthermore, factor analysis suggests that center entering assesses approach/avoidance toward aversive stimuli, which is considered a reliable index of fearfulness/anxiety and responds to anxiolytic agents (Ramos et al., 1997). Statistical comparison of between group differences was performed with the Mann–Whitney U test using GB-Stat software, with p ≤ 0.05 considered significant.
Elevated plus maze. The EPM apparatus was constructed in this lab from black Plexiglas with nonreflective painted surfaces. The maze consisted of four runways (10 × 40 cm) joined by a central 10 × 10 cm square, 50 cm above the floor. Opposing arms were either open (having only a 0.5 cm lip) or enclosed by 40-cm-high walls. The maze was illuminated by a dim lamp above the apparatus (12 lux). Experimental methodology was based on previously published studies of the EPM (Handley and McBlane, 1993; Hogg, 1996). Percentage open arm entries was the key parameter assessed, because factor analysis had previously shown this index to be associated with fearfulness/anxiety (Ramos et al., 1997). Animals were introduced into the center square facing an open arm, and behavior was video recorded for 5 min and analyzed using the SMART computer analysis program (San Diego Instruments, San Diego, CA). The number of entries into open or closed arms and total distance traveled were measured. Statistical comparison of between group differences was performed with the Mann–Whitney U test using GB-Stat software, with_p_ ≤ 0.05 considered significant. Open arm time, closed arm time, open entries, and closed entries were also recorded. The center square was not considered to be part of either the open or closed arms. To confirm our ability to detect anxiolytic and anxiogenic effects in our EPM apparatus, we used the protocol of Grahn et al. (1995) to assess our methodology. Compared with vehicle alone, we found that diazepam (2 mg/kg, with 4 d pretreatment to allow tolerance to motor effects of the drug) increases and methyl 6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate (a benzodiazepine inverse agonist, 0.4 mg/kg,) decreases percentage open arm entries while not altering total locomotion, as seen with our viral injections (data not shown). This finding indicates that we can detect both anxiolytic and anxiogenic effects in our EPM apparatus.
RESULTS
Coexpression of HA-5-HT1B and GFP in vitro using multiple promoter/enhancer elements
Because 5-HT1B autoreceptors in DRN neurons are translocated to axon terminals in forebrain (Hoyer and Middlemiss, 1989; Boschert et al., 1994; Ghavami et al., 1999), they may be difficult to detect in the cell body. Accordingly, it would be useful to express both the receptor and a marker protein that can be detected in the cytosol of the transfected cell. GFP, which can be detected easily both in vivo and in vitro, can provide such a cytosolic marker.
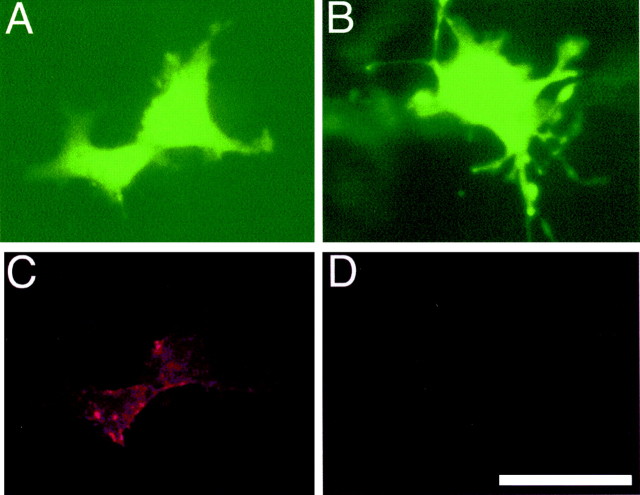
To accomplish our goal, we first introduced a hemagglutinin epitope into the N terminus of the rat 5-HT1B cDNA to facilitate detection of expressed transgenic receptor with commercially available antibodies. The modified cDNA was then cloned into the multiple cloning site of plasmid pCIGFP, in which a complete expression unit containing the GFP gene driven by the CMV immediate/early (IE) promoter/enhancer, and terminated with the SV40 polyadenylation site, was inserted into the single_Bam_HI site of pCI (Fig. 1). The resulting plasmid expressed both GFP and HA-5-HT1B, each from a separate transcriptional unit. To determine whether this plasmid, pCIGFP-HA1B, did indeed express both genes, it was transfected into COS7 cells by electroporation. After 72 hr, all cells that displayed GFP fluorescence also displayed HA immunoreactivity, which appeared to be concentrated at the cell membrane (Fig.2A,C). In contrast, cells transfected with pCIGFPdisplayed only GFP fluorescence (Fig.2B,D). Transfection of pCIGFP-HA1B also led to expression of both gene products in HeLa cells (data not shown).
Fig. 1.
Amplicon maps used for HA-5-HT1B and GFP expression. The plasmids pHSV-HA1B/GFP and pHSV-GFP were constructed as described in Materials and Methods and were confirmed by sequence analysis. Note that either GFP alone or the HA-5-HT1B and GFP sequences were inserted into the pHSV-PrPUC backbone provided by Dr. Rachael Neve (Neve and Geller, 1995). In the latter case, the HA-5-HT1B and GFP gene sequences were interrupted by an SV40 polyadenylation signal; the two genes have different promoter/enhancers controlling expression (HSV IE 4/5 and CMV IE, respectively), to reduce competition effects.ori S, HSV origin of replication; AmpR, ampicillin resistance gene.
Fig. 2.
COS cells transfected with pCIGFP-HA1B show dual expression. Cells were transfected with pCIGFP-HA1B or pCIGFP by electroporation as described. A and C show pCIGFP-HA1B-transfected cells; B and_D_ show pCIGFP-transfected cells.A and B show GFP fluorescence;C and D show hemagglutinin immunoreactivity. The HA-tagged 5-HT1B receptor could be detected only in the pCIGFP-HA1B-transfected cells, and there was no apparent interaction between GFP and HA-5-HT1Bexpression in the same cells. Scale bar, 20 μm.
HA-5-HT1B receptors coexpressed with GFP decrease cAMP accumulation and forskolin-stimulated CRE activity
In mammalian cell culture, the rodent 5-HT1Breceptor is known to inhibit adenylate cyclase via activation of Giα (Barnes and Sharp, 1999), thereby reducing the production of cAMP. To determine whether the HA-tagged 5-HT1B receptor retains this activity when coexpressed with GFP, the ability of the receptor to suppress forskolin-stimulated CRE-mediated expression of a reporter gene (Mellon et al., 1989) was determined. As can be seen in Figure3, transfection into JEG-3 cells with either pCIGFP-HA1B or MG11B (wild-type receptor) produced 5-HT-responsive reductions in luciferase expression with an EC50 of 5.1 and 4.8 nm, respectively, agreeing with previously published values of ∼6 nm(Hamblin et al., 1992). Therefore, the HA epitope tag on the 5-HT1B amino terminus and coexpression of GFP do not appear to change the apparent affinity or coupling efficiency of the receptor.
Fig. 3.
HA-5-HT_1B_receptors inhibit adenylate cyclase in JEG-3 cells. Adenylate cyclase activity was assayed using a luciferase reporter gene assay as described in Materials and Methods. Data points represent SD of triplicate determinations; two replicate assays were performed. The curve fits and EC50 determinations were calculated using Prism 2.0. HA tagging of the 5-HT1B receptor did not appear to reduce its ability to inhibit adenylate cyclase activity, and coexpression of GFP from the same plasmid did not impair the level of HA-5-HT1B expression or function. RLU, Relative light units.
HA-5-HT1B and GFP can be coexpressed from a single HSV amplicon in vitro
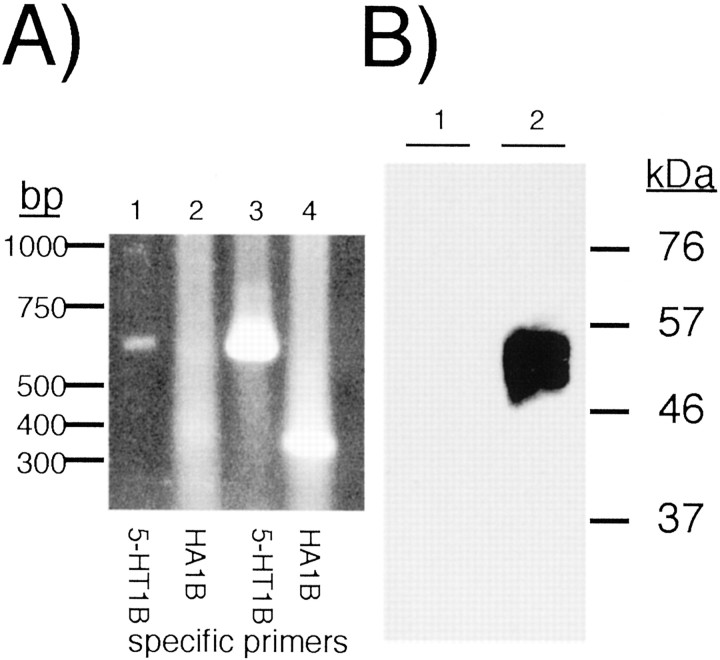
To produce an HSV amplicon from pCIGFP-HA1B, the 3.8 kb restriction fragment containing 5′-HA-5-HT1B–SV40 polyA–CMV I/E–GFP–SV40 polyA-3′ was ligated into _Xba_I/_Bam_HI cut pHSV-PrPUC. In the resulting plasmid, pHSV-HA1B/GFP, HA-5-HT1B cDNA is expressed from the HSV IE 4/5 promoter/enhancer, and GFP is expressed from the CMV promoter/enhancer transferred from pCIGFP-HA1B (Fig. 1). The amplicon was then packaged using replication-deficient HSV as described previously (Neve and Geller, 1995; Neve, 1999b). The recombinant viral particles carrying pHSV-HA1B/GFP were titered using PC-12 cells by assaying for HA immunocytochemistry and GFP fluorescence, and they typically contained 1–2 × 108infective units per milliliter. CA77 cells were infected with 3 μl of viral particles per well, with 50–90% infection rates. Infected CA77 cells were examined for the presence of 5-HT1BRNA. RT-PCR amplification of polyadenylated RNA from cells infected with pHSV-HA1B/GFP produced a robust RT-PCR product, whereas those infected with pHSV-GFP RNA had low levels of endogenous 5-HT1B mRNA (Clark et al., 1995), similar to that in vehicle-treated cells (Fig.4A). The HA-specific RT-PCR product was evident only in pHSV-HA1B/GFP-infected cells. HA specific antibodies labeled a single band of 65 kb only in pHSV-HA1B/GFP infected cells (Fig. 4B). Taken together, these in vitro studies showed that high levels of functional HA-5-HT1B and GFP could be expressed from separate promoter/enhancer elements using HSV amplicons.
Fig. 4.
In vitro expression of pHSV-HA1B/GFP in CA77 cells. A, Twenty-four hours after infection with pHSV-GFP (lanes 1, 2) or pHSV-HA1B/GFP (lanes 3, 4) (3 μl per well in six-well tissue culture plates; 1–2 × 108 infective units per milliliter), CA77 cells were harvested and processed for either immunoblot analysis or poly-A RNA extraction. GFP expression and HA immunoreactivity were robustly detected in ∼50–90% of cells (data not shown). RT-PCR of poly-A RNA from these cells showed the presence of 5-HT1B mRNA using total 5-HT1B primers for amplification (lanes 1, 3) and HA-specific primers (lanes 2, 4). Vehicle and pHSV-GFP-treated CA77 cells express low levels of 5-HT1B RNA (lane 1) and no HA epitope (lane 2). pHSV-HA1B/GFP-treated cells showed dramatically more total 5-HT1B message (lane 3) and a strong HA-5-HT1B-specific PCR product (lane 4). Vehicle-treated CA77 cells showed low levels of 5-HT1B mRNA, similar to pHSV-GFP (data not shown).B, Protein samples (5 μm) from pHSV-GFP (lane 1)- or pHSV-HA1B/GFP (lane 2)-infected CA77 cells were separated by PAGE and immobilized on membranes by Western blot. HA-specific immunoreactive protein was detected only in pHSV-HA1B/GFP-infected cells.
HSV amplicons are capable of expressing HA-5-HT1B and GFP gene products in vivo
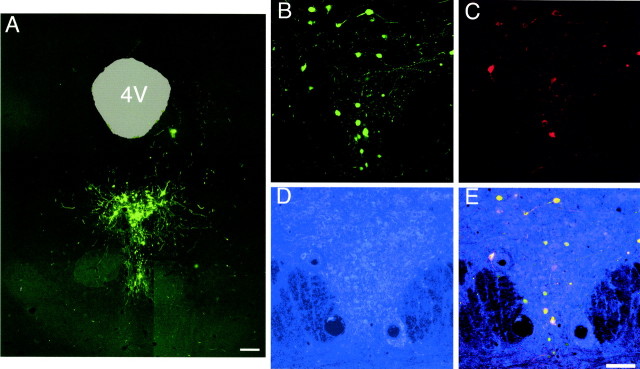
To determine whether pHSV-HA1B/GFP is capable of infecting mammalian brain and inducing gene expression of both HA-5-HT1B and GFP, we injected viral particles into the rat DRN by stereotaxic surgery. After microinjection into the DRN, animals were returned to home cages for 4 d and then euthanized. The brains were either prepared for immunocytochemistry or rapidly frozen for subsequent Western blot. Tissue sections were examined for GFP fluorescence and immunostained for the presence of the HA epitope. Large numbers of GFP-positive neurons were detected at the sites of injection (Fig. 5A). In six consecutive brains examined 4 d after gene transfer, 930 ± 160 GFP-positive cells were counted (mean ± SEM). These counts may be an underestimate of total transgene expression because gene expression peaks on day 3 and declines gradually thereafter (Carlezon et al., 2000a; Pliakas et al., 2001), but these numbers are comparable to previous studies that used gene transfer with this vector or similar strategies (Carlezon et al., 1997; Fabre et al., 2000; Pliakas et al., 2001). There are ∼11,000–15,000 serotonergic neurons in the full rostral–caudal extent of the nucleus (Wiklund and Bjorklund, 1980; Vertes and Crane, 1997). Therefore, we estimate that ∼10% of serotonergic neurons in the anterior dorsal raphe nucleus, the area targeted in the injections, expressed HA-5-HT1B. As shown in confocal micrographs, individual DRN neurons infected with pHSV-HA1B/GFP coexpressed GFP and HA-5-HT1B signals (Fig.5B,C). Furthermore, most of the transgene-expressing DRN neurons were serotonergic, as indicated by 5-HT1A immunostaining (Fig.5D,E). In some cases, either GFP or anti-HA label was apparent in a particular neuron or a specific confocal plane, but in most cases both signals colocalized in the same neurons (Fig. 5E). Specifically, immunolabeling was most intense near the surfaces of the tissue section, likely because of antibody penetration, whereas GFP expression was equally intense at all depths within a tissue section.
Fig. 5.
Coexpression of HA-5-HT_1B_ and GFP in vivo. pHSV-HA1B/GFP viral particles were injected stereotaxically into DRN, and animals were killed 4 d later for evaluation of transgene expression by immunostaining and confocal microscopy. A, A composite image of several 10× fields shows clear GFP localization within the anatomic region of the DRN. The fourth ventricle (4V) has been colored light green for clarity. Scale bar, 100 μm. B, A 20× image of DRN shows GFP fluorescence in cells and beaded fibers.C, HA-5-HT1B immunostaining of the same region shown in B. D, 5-HT1Aimmunostaining of the same region shown in B and_C_. Because of the lower laser strength available for the secondary dye used (Alexa-633), the intensity of positive staining, typically visible as rings around darker nuclei, is relatively low.E, A composite image of all three signals displays cells positive for GFP, HA-5-HT1B, and 5-HT1A, demonstrating that serotonergic neurons have been infected and that these neurons produce both viral transgene products in vivo. A count of cells positive for both GFP and 5-HT1A in images obtained for this study showed that 80% of GFP-positive neurons were also positive for 5-HT1A. Scale bar, 100 μm.
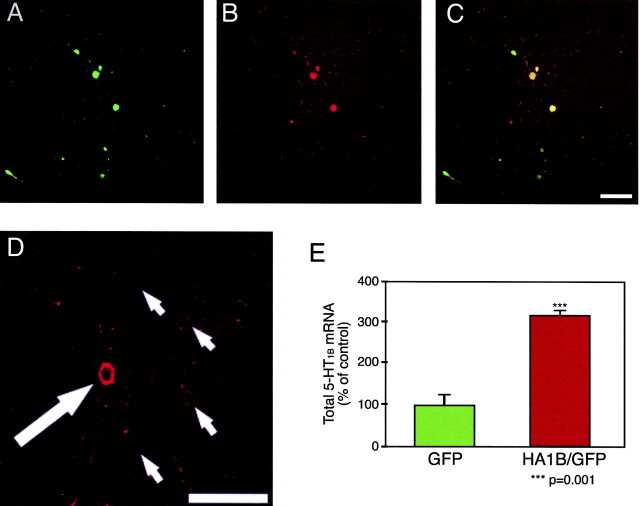
5-HT1B immunostaining was much more intense in GFP-expressing cells than in other serotonergic cells expressing only endogenous 5-HT1B receptor within the DRN (Fig.6A_–_D). In a subset of injected brains, the DRN was removed by punch, total RNA was extracted, and total 5-HT1B mRNA was quantified by real-time RT-PCR. This was normalized for GAPDH mRNA. DRNs that were injected with pHSV-HA1B/GFP had approximately threefold more 5-HT1B mRNA than pHSV-GFP-injected brains (Fig.6E). This reflects total expression in the DRN; therefore, the infected neurons probably had significantly higher levels of expression than suggested above.
Fig. 6.
Injection of pHSV-HA1B/GFP increases 5-HT1B expression in vivo. Sections through the DRN were taken from animals injected at that site with pHSV-HA1B/GFP viral particles, immunostained for 5-HT1B, and examined by confocal microscopy.A, GFP-containing cells and associated fibers may be clearly seen in the DRN, as shown previously. B, 5-HT1B immunoreactivity is detected within both GFP- and non-GFP-containing cells. C, When GFP fluorescence and 5-HT1B immunoreactivity are overlapped, GFP-positive cells are shown to typically display intense 5-HT1Bimmunoreactivity, as indicated by the yellow-green coloration of these cells. A–C, Magnification: 20×. Scale bar, 100 μm. D, In another focal plane from the same section shown in A_–_C, 5-HT1B immunoreactivity is much more intense in a GFP-positive cell (indicated by a large white arrow) compared with endogenously expressed 5-HT1B protein in non-GFP-positive cells (small arrows). The GFP-positive cell displays much greater 5-HT1B immunoreactivity, although both types of cells display what appears to be membrane-bound localization of 5-HT1B signal. Magnification: 40×. Scale bar, 100 μm. E, DRN punches from animals injected with either pHSV-GFP or pHSV-HA1B/GFP viral particles were analyzed by quantitative RT-PCR for 5-HT1B message content (n = 9 for each group). 5-HT1B mRNA levels, normalized for GAPDH mRNA expression, were 3.16-fold times higher in animals injected with pHSV-HA1B/GFP than in animals injected with pHSV-GFP (***p = 0.001; Student's_t_ test).
Because the 5-HT1B receptor is translocated to axon terminals, only a small proportion of 5-HT1Bprotein would be expected in the soma, whereas the majority would be expected to be present diffusely among the termination zones of DRN axonal projections such as frontal cortex and striatum (Molliver, 1987). To determine whether midbrain DRN infection with pHSV-HA1B/GFP and pHSV-GFP induces gene expression and protein translocation to forebrain, immunocytochemistry was performed on striatal sections for HA epitope. HA immunoreactive fibers with morphology characteristic of DRN projections (Kosofsky and Molliver, 1987) are seen in the striatum of pHSV-HA1B/GFP-injected animals (Fig.7A) but not pHSV-GFP-injected animals (Fig. 7B). To confirm this data, protein samples from frontal cortex and striatum were subjected to PAGE and immunoblot analysis. As can be seen in Figure 7C, rat forebrain contains an HA-immunoreactive band at 65 kDa, approximately the same size as photoaffinity-labeled CNS 5-HT1Breceptor (Hamblin et al., 1988) and slightly larger than that seen_in vitro_ (Fig. 4B). The HA immunoreactivity was more intense in striatum than frontal cortex, perhaps reflecting the greater density of 5-HT terminals in striatum. These findings strongly suggest that HA-5-HT1Breceptors introduced by viral-mediated gene transfer are translocated from DRN to serotonergic axon terminals in forebrain.
Fig. 7.

HA-5-HT_1B_is translocated to the forebrain. Striatal sections of animals injected in the DRN with either pHSV-HA1B/GFP or pHSV-GFP viral particles were immunostained to detect the presence of HA-5-HT1B immunoreactivity and examined by confocal microscopy. A, In animals injected with pHSV-HA1B/GFP, beaded fibers with anti-HA immunoreactivity may be clearly seen. The fibers demonstrate typical pleiomorphic varicosities, suggesting multiple sites of neurotransmitter release that are characteristic of DRN axons (Kosofsky and Molliver, 1987). B, In animals injected with pHSV-GFP, only background is present. In neither case was GFP detected (data not shown). Images shown are flattened, 60× confocal stacks. Scale bar, 50 μm. C, Western blot of HA-immunostained protein from terminals field of DRN axonal projections to forebrain. Protein samples from frontal cortex (lanes 1, 2) or striatum (lanes 3,4) after DRN injection of pHSV-GFP (−) or pHSV-HA1B/GFP (+) viral particles. The single immunoreactive HA-5-HT1B band migrated at an apparent size of 65 kDa, perhaps reflecting glycosylation and/or other posttranslational modifications of the 49 kDa predicted protein.
HA-5-HT1B overexpression in rat DRN alters anxiety-related behavior after an inescapable stressor
5-HT1B terminal autoreceptors are downregulated by antidepressants (Artigas et al., 1996; Sayer et al., 1999) and may be upregulated in learned helpless rats (Edwards et al., 1991; Neumaier et al., 1997) but are difficult to manipulate in behavioral models without also impinging on 5-HT1B heteroreceptors. Therefore we sought to determine whether increasing 5-HT1B mRNA in DRN would induce behaviors relevant to the symptoms of depression and anxiety. We first hypothesized that because antidepressants reduce immobility in the Porsolt FST (Porsolt et al., 1977), overexpression of 5-HT1B autoreceptors would increase immobility in the same test. Animals received stereotaxic injections of viral particles containing either pHSV-HA1B/GFP or pHSV-GFP into DRN and were subjected to forced swim 3 d later and tested the following morning. We used the pHSV-GFP amplicon as a control treatment because it controlled for the surgical procedure, infection with viral particles, the presence of viral particle constituents, transgenic RNA expression, and expression of GFP. We believe that this represents a good control strategy for viral-mediated gene transfer studies in rat brain. The animals' behavior was coded as climbing, swimming, or immobile, as described previously (Detke et al., 1995). There were no statistically significant changes in any of these behaviors in control or experimental animals (Fig. 8).
Fig. 8.
HA-5-HT_1B_expression in DRN neurons did not alter immobility in the forced swim test. Animals received injections of pHSV-HA1B/GFP (n = 11) or pHSV-GFP (n = 8) in DRN and were subjected to the standard FST procedure on days 3 and 4 as described in Materials and Methods. Behaviors were counted as swimming, climbing, or immobile as described in Materials and Methods. Numerical data are as follows and are presented as mean ± SEM (climb: GFP 14 ± 2.0, HA1B/GFP 17 ± 2.5;swim: GFP 14 ± 2.9, HA1B/GFP 12 ± 2.1;tread: GFP 31 ± 4.5, HA1B 31 ± 3.4). There were no significant differences in these behaviors between treatment groups.
The OFT has been used to model “emotionality” or behavioral anxiety in rodents, whose open-field behavior is altered by both stressors and antidepressants (Stockert et al., 1988; Kelly and Leonard, 1994; Pare, 1994; Meerlo et al., 1996; Izumi et al., 1997; Ramos et al., 1997;Durand et al., 1999). Factor analysis suggests that center entries are most related to approach/avoidance toward potentially aversive stimuli, which responds more strongly to anxiolytic drugs such as diazepam and is considered an index of anxiety/fearfulness (Ramos et al., 1997). Thus, we hypothesized that open entries would be altered in animals overexpressing 5-HT1B in the DRN. Animals were injected in DRN with viral particles carrying either pHSV-HA1B/GFP or pHSV-GFP, housed in routine conditions for 3 d without specific stress exposure, and then tested with the OFT. In the absence of specific stress exposure, pHSV-HA1B/GFP-treated animals showed greater exploration of the center of the open field, with 35% more entries into the center square as compared with pHSV-GFP-treated animals (Fig.9A) (_p_= 0.05). There was no difference in total locomotor activity between groups as shown by total square entries (Fig. 9B), indicating that overexpression of 5-HT1Breceptors in DRN did not alter general locomotor activity. In a separate experiment, animals received viral injections followed by water-restraint stress 3 d later and were then tested in the OFT 24 hr later. This stress paradigm differs from the FST in that it prevents gross body movements (Pare, 1994). The pHSV-HA1B/GFP-injected animals entered the central region of the arena 30% less frequently than the pHSV-GFP-treated animals (Fig. 9C) (p = 0.044). Total locomotor activity, as determined by total square entries, was not affected by HA-5-HT1B expression (Fig. 9D). This suggests that the animals that received pHSV-HA1B/GFP were more sensitive to water-restraint stress than GFP controls.
Fig. 9.
HA-5-HT1B expression in DRN neurons increased avoidance of the center of an open field only after water-restraint stress. Animals received injections of pHSV-HA1B/GFP or pHSV-GFP and either were tested in the OFT 3 d later (A, B) or subjected to water-restraint stress on day 3 and tested in the OFT 24 hr later (C,D). The numbers of entries into the central square during the first 3 min were counted and are shown as mean ± SEM (A, GFP 4.5 ± 0.56, HA1B/GFP 6.5 ± 0.64;C, GFP 8.4 ± 0.86, HA1B/GFP 5.9 ± 0.58). HA-5-HT1B expression increased central square entries in the absence of a specific stress exposure (#p = 0.05) but reduced entries into the central region after stress by 30% (*p = 0.044). The total number of zone crossings, shown as mean ± SEM (B, GFP 102 ± 9, HA1B/GFP 123 ± 10; D, GFP 160 ± 13, HA1B/GFP 162 ± 20), was not different between pHSV-HA1B/GFP and pHSV-GFP.n = 8–14 animals in each treatment condition.
To further examine the effects of 5-HT1Boverexpression in DRN on stress-induced behaviors, we used the EPM, another commonly used test for anxiety-like behaviors (Handley and McBlane, 1993; Hogg, 1996). As in the OFT, we assessed behavior most associated with indices of anxiety. On the basis of factor analysis (Ramos et al., 1997) and response to anxiolytics (Handley and McBlane, 1993; Hogg, 1996), we chose to examine percentage open arm entries as our primary measure. Indeed, our preliminary studies with diazepam and the benzodiazepine inverse agonist methyl 6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate (Grahn et al., 1995) demonstrated that this parameter was the most robust and least variable indicator of anxiety in our protocol (data not shown). Animals were exposed to water-restraint stress 3 d after viral vector injection into DRN and were tested in the EPM 24 hr later. Animals treated with pHSV-HA1B/GFP demonstrated greater anxiety-like behavior than pHSV-GFP controls, as indicated by a 20% reduction in percentage entries into the open arms of the maze (Fig.10A) (p = 0.047). 5-HT1Boverexpression did not significantly affect total distance traveled in the EPM (Fig. 10B).
Fig. 10.
HA-5-HT_1B_expression in DRN reduced open arm entries in the EPM 24 hr after water-restraint stress. Animals received injections of pHSV-HA1B/GFP (n = 13) or pHSV-GFP (n = 8), were subjected to water-restraint stress 3 d later, and were tested in the EPM 24 hr later. The rat's behavior was recorded and analyzed by computer-assisted video monitoring. A shows the percentage of open arm/total arm entries; HA-5-HT1B-expressing animals had significantly reduced percentage of entries into open arms (GFP 55 ± 4%; HA1B/GFP 44 ± 3%; mean ± SEM shown); *p = 0.047. B, There was no significant difference in total distance traveled between treatment groups (GFP 415 ± 38 cm; HA1B/GFP 450 ± 40 cm; mean ± SEM shown). Other parameters measured include percentage open time (GFP 29.6 ± 8.9; HA1B/GFP 22.3 ± 4.0), percentage closed time (GFP 51.5 ± 8.1, HA1B/GFP 54.2 ± 5.2), open entries (GFP 8.2 ± 1.0, HA1B/GFP 7.2 ± 1.1), and closed entries (GFP 7.4 ± 1.5, HA1B/GFP 9.1 ± 1.2); mean ± SEM shown. Although these parameters did not reach statistical significance (p > 0.05), all trends are consistent with anxiogenic effects in HA-5-HT1B-expressing animals.
DISCUSSION
Validation of HA-5-HT_1B_ and GFP dual expression
Several recombinant viral vectors have been developed for use in the mammalian CNS, including gene transfer systems derived from adenovirus, adeno-associated virus, HSV, and others (Zlokovic and Apuzzo, 1997). The HSV system used here possesses many advantages for delivery of genes into postmitotic neurons, including neurotropic specificity, high infectivity, efficient extrachromosomal gene expression, and low toxicity (Neve and Geller, 1995). HSV amplicons have been used previously to introduce transgenes for the purpose of overexpressing an endogenous protein of interest in discrete structures of the rat brain (Chiocca et al., 1990; Geller et al., 1991; Wood et al., 1994; Carlezon et al., 1997, 2000b; Song et al., 1998; Neve, 1999a). Double infection with two HSV vectors has been used to introduce two genes of interest into primary cortical neurons in culture (Coopersmith and Neve, 1999). Although using two viral amplicons simultaneously can lead to dual infection of some neurons, essentially all neurons that are infected with pHSV-HA1B/GFP should express both genes. This report is the first to use a single amplicon to introduce and express two genes of interest in rat brain. When viral particles were injected into DRN in rat midbrain, expression of HA-5-HT1B and GFP in individual neurons could be detected, and HA immunoreactivity was found in the striatum and frontal cortex only when HA-5-HT1B/GFP was injected into DRN. We have validated that both genes are expressed in vitro and in vivo, preserving protein activity and receptor localization.
This approach made it possible to manipulate 5-HT1B presynaptic autoreceptors separately from 5-HT1B postsynaptic heteroreceptors in forebrain tissue. Such discrimination is critical in examining the role of DRN 5-HT1B autoreceptors in depression and anxiety, because most of the 5-HT1B receptors in the brain are postsynaptic heteroreceptors, located on nonserotonergic neurons, that are intermingled with 5-HT1Bautoreceptor-containing serotonergic fibers (Verge et al., 1986; Offord et al., 1988; Jacobs and Azmitia, 1992; Sexton et al., 1999). Manipulating expression of the autoreceptor population selectively offers the opportunity to examine the behavioral role of a small but very important subpopulation of 5-HT1B receptors that has not been possible using other genetic or pharmacologic techniques. Fabre et al. (2000) recently used nonviral gene transfer to alter serotonin transporter expression in the rat DRN, observing alterations of circadian rhythms in animals transfected with an antisense-expressing plasmid. The nonviral approach transfects both glia and neurons. However, both viral and nonviral methods are well suited to altering gene expression in DRN because of its small size and mostly homogenous neuron type (i.e., 70% serotonergic). Although our infection rate of 10% of DRN neurons appears fairly low, previous studies using viral-mediated gene transfer demonstrating robust biological effects have often targeted larger brain regions than the DRN while infecting approximately as many neurons (Chiocca et al., 1990; Geller et al., 1991; Wood et al., 1994; Carlezon et al., 1997,2000b; Song et al., 1998; Neve, 1999a). Because DRN has only 11,000–15,000 serotonergic neurons and we targeted the anterior section of the nucleus, we achieved an equal or higher proportion of transgene-expressing neurons within the region of interest compared with previous studies. However, our findings do not rule out the possibility of differential involvement of 5-HT1Bautoreceptors in anxiety behaviors between subregions of the DRN or in other raphe nuclei such as the median raphe nucleus. Further investigation will likely prove useful in addressing these issues.
Analyses of behavior after HA-5-HT1B/GFP infection of dorsal raphe nucleus
The second purpose of this study was to characterize the behavioral effects of HA-5-HT1B/GFP expression in DRN neurons. We examined several behavioral paradigms to elucidate the role of 5-HT1B autoreceptors in depression and anxiety. The FST has been used to predict the antidepressant activity of drugs, whereas the OFT and EPM have been used to detect changes in anxiety-like behavior (Porsolt, 2000). We did not detect an effect of HA-5-HT1B/GFP expression in DRN on immobility or struggling behaviors using the FST. This negative result could be explained by several interpretations. First, the FST may be more sensitive in detecting antidepressant activity than prodepressant activity. It is also possible that forced swim stress does not activate DRN mechanisms to the same extent as other stress procedures. Forced swim does cause region-specific changes in 5-HT release and metabolism immediately after forced swim, but no changes were detected 24 hr later (Kirby and Lucki, 1998), suggesting that forced swim either does not alter 5-HT1Breceptor activity or does so at a different time point than we tested using the standard FST procedure. Previously we found 5-HT1B mRNA to be elevated in rats displaying learned helplessness in shuttle box testing after inescapable restraint and tail shock (Neumaier et al., 1997). Tail shock stress alters DRN function for at least 24 hr (Maier et al., 1995; Sutton et al., 1997;Grahn et al., 1999). Different stress paradigms have variable effects on monoamine activity in general and have different impacts on dorsal versus median raphe activity in particular (Adell et al., 1997; Durand et al., 1999). Thus, it is important to consider a range of behavioral measures in assessing the role of 5-HT1Bautoreceptors in depression- and anxiety-related behavior.
Toward this end we also analyzed the effect of water-restraint stress on OFT and EPM behavior after HA-5-HT1B/GFP infection of DRN. We used water restraint because it has previously been found to be useful in a rodent model of stress-induced depression, Wistar Kyoto rats (Pare, 1994), and was similar to our FST procedure. Using this assay, we found a significant reduction in central square entries after an inescapable stressor in the pHSV-HA1B/GFP group as compared with the pHSV-GFP control group. OFT procedures differ widely between different research groups, particularly in the size, shape, and lighting of the testing arena and in the behavioral outcomes measured. In this study, rats were tested in the afternoon to maximize the potential contribution of 5-HT1B autoreceptors (Sayer et al., 1999) and under low intensity red illumination because this leads to greater overall locomotor activity. A number of behaviors have been measured in previous OFT studies, including total locomotor activity, central entries, rearing, defecation, and others (Plaznik et al., 1988; Stockert et al., 1988; Kelly and Leonard, 1994; Pare, 1994;Meerlo et al., 1996; Izumi et al., 1997; Durand et al., 1999). We chose to measured central square entries, which reflects an approach/avoidance conflict, because this was recently shown to be particularly sensitive to stress-induced anxiety states (Ramos et al., 1997; Durand et al., 1999). There was no change in overall animal locomotor activity in the OFT, ruling out a nonspecific change in locomotor activity. Similarly, rats with 5-HT1Boverexpression in DRN who were stressed by water restraint avoided the open arms of the EPM, consistent with increased anxiety-like behavior, but had no greater total locomotor activity than GFP controls. Therefore, 5-HT1B overexpression in DRN combined with exposure to a stressor increased anxiety-like behavior 24 hr later, suggesting that the 5-HT1B overexpression induced an enduring change in behavior after stress.
There are at least two possible interpretations of this data. Increasing 5-HT1B autoreceptor expression in serotonergic DRN neurons either makes the animals more anxious directly or increases the impact of stress on anxiety behaviors. When we examined open-field behavior in the absence of stress exposure, pHSV-HA1B/GFP did not decrease entries into the center of the open field. Indeed, 5-HT1B overexpression in DRN in the absence of stress increased exploration of the central square. Although it is not clear why increased 5-HT1Bautoreceptor expression might lower anxiety behavior in an unstressed animal, we have recently observed that 5-HT1BmRNA is elevated in the stress-resistant group from two models of differential stress susceptibility when the animals had not been stressed (Neumaier et al., 2002). The findings suggest a complex role for the 5-HT1B autoreceptor in modulating anxiety. Our working hypothesis is that the impact of 5-HT1B overexpression in DRN is dependent on context (in this case, exposure to stress). Thus in the absence of stress, 5-HT1B autoreceptor overexpression may increase an approach toward potentially aversive stimuli, whereas in the presence of stress, these stimuli may provoke increased anxious behavior. Because serotonin release is increased or decreased by stress in different brain regions at different time points (Adell et al., 1997; Amat et al., 1998; Kirby and Lucki, 1998), there are likely to be discrete regulatory mechanisms that have yet to be elucidated.
Although OFT, EPM, and FST behaviors may involve different behavioral circuits and do not necessarily change in a unified manner (Plaznik et al., 1988; West and Weiss, 1998; Durand et al., 1999; Page et al., 1999), forced swim appears to have less impact on 5-HT1B autoreceptor mechanisms than inescapable water-restraint (this study) or inescapable restraint with tail shock (Neumaier et al., 1997). Controllability of stress is especially important in activating the DRN (Grahn et al., 1999). This may explain why we detected larger effects of water restraint than forced swim in this study: the combination of water stress and restraint may have potently impacted the dimension of controllability. The behavioral measures used in this study may be less dependent on the amygdala, which receives serotonergic innervation predominantly from DRN, than other models such as learned helplessness, fear conditioning, and social interaction indices of stress (Maier et al., 1993; Gonzalez et al., 1996; Amat et al., 1998). It will be interesting to examine the effect of HA-5-HT1B/GFP expression using other stress paradigms such as inescapable tail shock, and in other testing paradigms, to understand the role of 5-HT1Bautoreceptors more fully.
In summary, we have used a modification of HSV-based gene delivery to overexpress 5-HT1B mRNA in DRN and to express GFP as a vital marker of neuronal infection. The dual expressing amplicon led to functional expression of membrane-bound, epitope-tagged 5-HT1B receptors in vitro and in vivo. Overexpression of HA-5-HT1B/GFP in DRN had marked effects on stress-sensitive behaviors in the open-field paradigm. This manipulation is particularly well suited to studying the 5-HT1B autoreceptor in DRN because only the region of interest is directly altered, and exogenous agonist treatment is not necessary. Because 5-HT1B autoreceptors are thought to be predominantly active in the axon terminals and not in the vicinity of DRN (Pineyro et al., 1995), HA-5-HT1B/GFP effects in animal models of depression or anxiety can be attributed mainly to these axonal projections. Although the cellular specificity of acute gene transfer strategies is still incomplete, it offers some advantages over stem cell approaches (e.g., null mutant or “knock back in” mice) that have either no cellular specificity at all or incomplete regional specificity. Viral-mediated gene transfer also allows one to test hypotheses efficiently in various animal strains with different genetic backgrounds. Combining stem cell and acute gene transfer strategies will help circumvent anatomical complexity and the lack of sufficiently selective drugs to study the cellular basis of the involvement of serotonin in behavior.
Footnotes
This work was supported by the National Institute of Mental Health (MH 57049 and 63303) and the University of Washington Royalty Research Fund. We thank Dr. Rachael Neve for her generous gifts of the parent HSV amplicon and related packaging materials as well as her advice and assistance.
Correspondence should be addressed to Dr. John F. Neumaier, Harborview Medical Center, Psychiatry, Box 359911, 325 Ninth Avenue, Seattle, WA 98104-2499. E-mail: neumaier@u.washington.edu.
REFERENCES
- 1.Adell A, Casanovas JM, Artigas F. Comparative study in the rat of the actions of different types of stress on the release of 5-HT in raphe nuclei and forebrain areas. Neuropharmacology. 1997;36:735–741. doi: 10.1016/s0028-3908(97)00048-8. [DOI] [PubMed] [Google Scholar]
- 2.Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Res. 1998;812:113–120. doi: 10.1016/s0006-8993(98)00960-3. [DOI] [PubMed] [Google Scholar]
- 3.Anthony JP, Sexton TJ, Neumaier JF. Antidepressant induced regulation of 5-HT1B mRNA in rat dorsal raphe nucleus reverses rapidly following drug discontinuation. J Neurosci Res. 2000;61:82–87. doi: 10.1002/1097-4547(20000701)61:1<82::AID-JNR10>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 4.Artigas F, Romero L, de Montigny C, Blier P. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci. 1996;19:378–383. doi: 10.1016/S0166-2236(96)10037-0. [DOI] [PubMed] [Google Scholar]
- 5.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 6.Bergqvist PB, Bouchard C, Blier P. Effect of long-term administration of antidepressant treatments on serotonin release in brain regions involved in obsessive-compulsive disorder. Biol Psychiatry. 1999;45:164–174. doi: 10.1016/s0006-3223(98)00154-1. [DOI] [PubMed] [Google Scholar]
- 7.Boschert M, Amara DA, Segu L, Hen R. The mouse 5-hydroxytryptamine1B receptor is localized predominantly on axon terminals. Neuroscience. 1994;58:167–182. doi: 10.1016/0306-4522(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 8.Briley M, Moret C. Neurobiological mechanisms involved in antidepressant therapies. Clin Neuropharmacol. 1993;16:387–400. doi: 10.1097/00002826-199310000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Carlezon WA, Jr, Boundy VA, Haile CN, Lane SB, Kalb RG, Neve RL, Nestler EJ. Sensitization to morphine induced by viral-mediated gene transfer. Science. 1997;277:812–814. doi: 10.1126/science.277.5327.812. [DOI] [PubMed] [Google Scholar]
- 10.Carlezon WA, Jr, Nestler EJ, Neve RL. Herpes simplex virus-mediated gene transfer as a tool for neuropsychiatric research. Crit Rev Neurobiol. 2000a;14:47–67. doi: 10.1080/08913810008443546. [DOI] [PubMed] [Google Scholar]
- 11.Carlezon WA, Jr, Haile CN, Coppersmith R, Hayashi Y, Malinow R, Neve RL, Nestler EJ. Distinct sites of opiate reward and aversion within the midbrain identified using a herpes simplex virus vector expressing GluR1. J Neurosci 20 2000b. RC62(1–5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiocca EA, Choi BB, Cai WZ, DeLuca NA, Schaffer PA, DiFiglia M, Breakefield XO, Martuza RL. Transfer and expression of the lacZ gene in rat brain neurons mediated by herpes simplex virus mutants. New Biol. 1990;2:739–746. [PubMed] [Google Scholar]
- 13.Clark MS, Lanigan TM, Page NM, Russo AF. Induction of a serotonergic and neuronal phenotype in thyroid C-cells. J Neurosci. 1995;15:6167–6178. doi: 10.1523/JNEUROSCI.15-09-06167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coopersmith R, Neve RL. Expression of multiple proteins within single primary cortical neurons using a replication deficient HSV vector. Biotechniques. 1999;27:1156–1160. doi: 10.2144/99276st01. [DOI] [PubMed] [Google Scholar]
- 15.Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology. 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- 16.Durand M, Berton O, Aguerre S, Edno L, Combourieu I, Mormede P, Chaouloff F. Effects of repeated fluoxetine on anxiety-related behaviours, central serotonergic systems, and the corticotropic axis in SHR and WKY rats. Neuropharmacology. 1999;38:893–907. doi: 10.1016/s0028-3908(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 17.Edwards E, Harkins K, Wright G, Henn FA. 5-HT1b receptors in an animal model of depression. Neuropharmacology. 1991;30:101–105. doi: 10.1016/0028-3908(91)90050-l. [DOI] [PubMed] [Google Scholar]
- 18.Fabre V, Boutrel B, Hanoun N, Lanfumey L, Fattaccini CM, Demeneix B, Adrien J, Hamon M, Martres MP. Homeostatic regulation of serotonergic function by the serotonin transporter as revealed by nonviral gene transfer. J Neurosci. 2000;20:5065–5075. doi: 10.1523/JNEUROSCI.20-13-05065.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geller AI, Keyomarsi K, Bryan J, Pardee AB. An efficient deletion mutant packaging system for defective herpes simplex virus vectors: potential applications to human gene therapy and neuronal physiology. Proc Natl Acad Sci USA. 1990;87:8950–8954. doi: 10.1073/pnas.87.22.8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geller AI, During MJ, Neve RL. Molecular analysis of neuronal physiology by gene transfer into neurons with herpes simplex virus vectors. Trends Neurosci. 1991;14:428–432. doi: 10.1016/0166-2236(91)90040-2. [DOI] [PubMed] [Google Scholar]
- 21.Ghavami A, Stark KL, Jareb M, Ramboz S, Segu L, Hen R. Differential addressing of 5-HT1A and 5-HT1B receptors in epithelial cells and neurons. J Cell Sci. 1999;112:967–976. doi: 10.1242/jcs.112.6.967. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez LE, Andrews N, File SE. 5-HT1A and benzodiazepine receptors in the basolateral amygdala modulate anxiety in the social interaction test, but not in the elevated plus-maze. Brain Res. 1996;732:145–153. doi: 10.1016/0006-8993(96)00517-3. [DOI] [PubMed] [Google Scholar]
- 23.Grahn RE, Kalman BA, Brennan FX, Watkins LR, Maier SF. The elevated plus-maze is not sensitive to the effect of stressor controllability in rats. Pharmacol Biochem Behav. 1995;52:565–570. doi: 10.1016/0091-3057(95)00141-i. [DOI] [PubMed] [Google Scholar]
- 24.Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- 25.Hamblin MW, Adriaenssens PI, Ariani K, Tan GL, Ciaranello RD. Photoaffinity labeling of rat brain 5-HT1B receptors with [125I]iodocyanopindolol diazirine. Soc Neurosci Abstr. 1988;14:313. [Google Scholar]
- 26.Hamblin MW, McGuffin RW, Metcalf MA, Dorsa DM, Merchant KA. Distinct 5-HT1B and 5-HT1D serotonin receptors in rat: structural and pharmacological comparison of the two cloned receptors. Mol Cell Neurosci. 1992;3:578–587. doi: 10.1016/1044-7431(92)90070-i. [DOI] [PubMed] [Google Scholar]
- 27.Handley SL, McBlane JW. An assessment of the elevated X-maze for studying anxiety and anxiety-modulating drugs. J Pharmacol Toxicol Methods. 1993;29:129–138. doi: 10.1016/1056-8719(93)90063-k. [DOI] [PubMed] [Google Scholar]
- 28.Heidmann DE, Szot P, Kohen R, Hamblin MW. Function and distribution of three rat 5-hydroxytryptamine 7 (5-HT7) receptor isoforms produced by alternative splicing. Neuropharmacology. 1998;37:1621–1632. doi: 10.1016/s0028-3908(98)00070-7. [DOI] [PubMed] [Google Scholar]
- 29.Hen R, Boschert U, Lemeur M, Dierich A, Ait Amara D, Buhot MC, Segu L, Misslin R, Saudou F. 5-HT1B receptor “knock-out”: pharmacological and behavioral consequences. Soc Neurosci Abstr. 1993;19:632. [Google Scholar]
- 30.Hjorth S, Suchowski CS, Galloway MP. Evidence for 5-HT autoreceptor-mediated, nerve impulse-independent, control of 5-HT synthesis in the rat brain. Synapse. 1995;19:170–176. doi: 10.1002/syn.890190304. [DOI] [PubMed] [Google Scholar]
- 31.Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- 32.Hoyer D, Middlemiss DN. Species differences in the pharmacology of terminal 5-HT autoreceptors in mammalian brain. Trends Pharmacol Sci. 1989;10:130–132. doi: 10.1016/0165-6147(89)90159-4. [DOI] [PubMed] [Google Scholar]
- 33.Izumi J, Washizuka M, Hayashi-Kuwabara Y, Yoshinaga K, Tanaka Y, Ikeda Y, Kiuchi Y, Oguchi K. Evidence for a depressive-like state induced by repeated saline injections in Fischer 344 rats. Pharmacol Biochem Behav. 1997;57:883–888. doi: 10.1016/s0091-3057(96)00455-8. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 35.Kelly JP, Leonard BE. The effect of tianeptine and sertraline in three animal models of depression. Neuropharmacology. 1994;33:1011–1016. doi: 10.1016/0028-3908(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 36.Kirby LG, Lucki I. The effect of repeated exposure to forced swimming on extracellular levels of 5-hydroxytryptamine in the rat. Stress. 1998;2:251–263. doi: 10.3109/10253899809167289. [DOI] [PubMed] [Google Scholar]
- 37.Kohen R, Metcalf MA, Khan N, Druck T, Huebner K, Lachowicz JE, Meltzer HY, Sibley DR, Roth BL, Hamblin MW. Cloning, characterization, and chromosomal localization of a human 5-HT6 serotonin receptor. J Neurochem. 1996;66:47–56. doi: 10.1046/j.1471-4159.1996.66010047.x. [DOI] [PubMed] [Google Scholar]
- 38.Kosofsky BE, Molliver ME. The serotoninergic innervation of cerebral cortex: different classes of axon terminals arise from dorsal and median raphe nuclei. Synapse. 1987;1:153–168. doi: 10.1002/syn.890010204. [DOI] [PubMed] [Google Scholar]
- 39.Maier SF, Grahn RE, Kalman BA, Sutton LC, Wiertelak EP, Watkins LR. The role of the amygdala and dorsal raphe nucleus in mediating the behavioral consequences of inescapable shock. Behav Neurosci. 1993;107:377–388. doi: 10.1037//0735-7044.107.2.377. [DOI] [PubMed] [Google Scholar]
- 40.Maier SF, Busch CR, Maswood S, Grahn RE, Watkins LR. The dorsal raphe nucleus is a site of action mediating the behavioral effects of the benzodiazepine receptor inverse agonist DMCM. Behav Neurosci. 1995;109:759–766. doi: 10.1037//0735-7044.109.4.759. [DOI] [PubMed] [Google Scholar]
- 41.Meerlo P, Overkamp GJ, Benning MA, Koolhaas JM, Van den Hoofdakker RH. Long-term changes in open field behaviour following a single social defeat in rats can be reversed by sleep deprivation. Physiol Behav. 1996;60:115–119. doi: 10.1016/0031-9384(95)02271-6. [DOI] [PubMed] [Google Scholar]
- 42.Mellon PL, Clegg CH, Correll LA, McKnight GS. Regulation of transcription by cyclic AMP-dependent protein kinase. Proc Natl Acad Sci USA. 1989;86:4887–4891. doi: 10.1073/pnas.86.13.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Migeon JC, Nathanson NM. Differential regulation of cAMP-mediated gene transcription by m1 and m4 muscarinic acetylcholine receptors. Preferential coupling of m4 receptors to Gi alpha-2. J Biol Chem. 1994;269:9767–9773. [PubMed] [Google Scholar]
- 44.Molliver ME. Serotonergic neuronal systems: what their anatomic organization tells us about function. J Clin Psychopharmacol. 1987;7:3S–23S. [PubMed] [Google Scholar]
- 45.Neumaier JF, Root DC, Hamblin MW. Chronic fluoxetine reduces serotonin transporter mRNA and 5-HT1B mRNA in a sequential manner in the rat dorsal raphe nucleus. Neuropsychopharmacology. 1996a;15:515–522. doi: 10.1016/S0893-133X(96)00095-4. [DOI] [PubMed] [Google Scholar]
- 46.Neumaier JF, Szot P, Peskind E, Dorsa DM, Hamblin MW. Serotonergic lesioning differentially effects presynaptic and postsynaptic 5-HT1B receptor mRNA levels in rat brain. Brain Res. 1996b;722:50–58. doi: 10.1016/0006-8993(96)00178-3. [DOI] [PubMed] [Google Scholar]
- 47.Neumaier JF, Petty F, Kramer GL, Szot P, Hamblin MW. Learned helplessness increases 5-hydroxytryptamine 1B receptor mRNA levels in the rat dorsal raphe nucleus. Biol Psychiatry. 1997;41:668–674. doi: 10.1016/S0006-3223(96)00114-X. [DOI] [PubMed] [Google Scholar]
- 48.Neumaier JF, Sexton TJ, Hamblin MW, Beck SG. Corticosteroids regulate 5-HT1A but not 5-HT1B receptor mRNA in rat hippocampus. Mol Brain Res. 2000;82:65–73. doi: 10.1016/s0169-328x(00)00181-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neumaier JF, Edwards E, Plotsky PM (2002) 5-HT1B mRNA regulation in two animal models of altered stress reactivity. Mol Psychiatry, in press. [DOI] [PubMed]
- 50.Neve RL. Overview of gene delivery into cells using HSV-1-based vectors. Current protocols in neuroscience Hall ZW. 1999a, Section 4.12. New York: Wiley. [DOI] [PubMed]
- 51.Neve RL. Generation of high-titer defective HSV-1 vectors. Current protocols in neuroscience Hall ZW. 1999b, Section 4.13. New York: Wiley. [DOI] [PubMed]
- 52.Neve RL, Geller AI. A defective herpes simplex virus vector system for gene delivery into the brain: comparison with alternative gene delivery systems and usefulness for gene therapy. Clin Neurosci. 1995;3:262–267. [PubMed] [Google Scholar]
- 53.Offord SJ, Ordway GA, Frazer A. Application of [125I]iodocyanopindolol to measure 5-hydroxytryptamine1B receptors in the brain of the rat. J Pharmacol Exp Ther. 1988;244:144–153. [PubMed] [Google Scholar]
- 54.Page ME, Detke MJ, Dalvi A, Kirby LG, Lucki I. Serotonergic mediation of the effects of fluoxetine, but not desipramine, in the rat forced swimming test. Psychopharmacology. 1999;147:162–167. doi: 10.1007/s002130051156. [DOI] [PubMed] [Google Scholar]
- 55.Pare WP. Open field, learned helplessness, conditioned defensive burying, and forced-swim tests in WKY rats. Physiol Behav. 1994;55:433–439. doi: 10.1016/0031-9384(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 56.Petty F, Kramer G, Wilson L. Prevention of learned helplessness: in vivo correlation with cortical serotonin. Pharmacol Biochem Behav. 1992;43:361–367. doi: 10.1016/0091-3057(92)90163-a. [DOI] [PubMed] [Google Scholar]
- 57.Pineyro G, de Montigny C, Blier P. 5-HT1D receptors regulate 5-HT release in the rat raphe nuclei. In vivo voltammetry and in vitro superfusion studies. Neuropsychopharmacology. 1995;13:249–260. doi: 10.1016/0893-133X(95)00109-Q. [DOI] [PubMed] [Google Scholar]
- 58.Plaznik A, Tamborska E, Hauptmann M, Bidzinski A, Kostowski W. Brain neurotransmitter systems mediating behavioral deficits produced by inescapable shock treatment in rats. Brain Res. 1988;447:122–132. doi: 10.1016/0006-8993(88)90972-9. [DOI] [PubMed] [Google Scholar]
- 59.Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Porsolt RD. Animal models of depression: utility for transgenic research. Rev Neurosci. 2000;11:53–58. doi: 10.1515/revneuro.2000.11.1.53. [DOI] [PubMed] [Google Scholar]
- 61.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 62.Ramos A, Berton O, Mormede P, Chaouloff F. A multiple-test study of anxiety-related behaviours in six inbred rat strains. Behav Brain Res. 1997;85:57–69. doi: 10.1016/s0166-4328(96)00164-7. [DOI] [PubMed] [Google Scholar]
- 63.Roberts C, Belenguer A, Middlemiss DN, Routledge C. Differential effects of 5-HT1B/1D receptor antagonists in dorsal and median raphe innervated brain regions. Eur J Pharmacol. 1998;346:175–180. doi: 10.1016/s0014-2999(98)00061-2. [DOI] [PubMed] [Google Scholar]
- 64.Sayer TJ, Hannon SD, Redfern PH, Martin KF. Diurnal variation in 5-HT1B autoreceptor function in the anterior hypothalamus in vivo: effect of chronic antidepressant drug treatment. Br J Pharmacol. 1999;126:1777–1784. doi: 10.1038/sj.bjp.0702535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sexton T, McEvoy C, Neumaier J. (+) 3,4-Methylenedioxymethamphetamine (“Ecstasy”) transiently increases striatal 5-HT1B binding sites without altering 5-HT1B mRNA in rat brain. Mol Psychiatry. 1999;4:572–579. doi: 10.1038/sj.mp.4000574. [DOI] [PubMed] [Google Scholar]
- 66.Sherman AD, Petty F. Neurochemical basis of the action of antidepressants on learned helplessness. Behav Neural Biol. 1980;30:119–134. doi: 10.1016/s0163-1047(80)91005-5. [DOI] [PubMed] [Google Scholar]
- 67.Song S, Wang Y, Bak SY, During MJ, Bryan J, Ashe O, Ullrey DB, Trask LE, Grant FD, O'Malley KL, Riedel H, Goldstein DS, Neve KA, LaHoste GJ, Marshall JF, Haycock JW, Neve RL, Geller AI. Modulation of rat rotational behavior by direct gene transfer of constitutively active protein kinase C into nigrostriatal neurons. J Neurosci. 1998;18:4119–4132. doi: 10.1523/JNEUROSCI.18-11-04119.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stockert M, Serra J, De Robertis E. Effect of olfactory bulbectomy and chronic amitryptiline treatment in rats. 3H-imipramine binding and behavioral analysis by swimming and open field tests. Pharmacol Biochem Behav. 1988;29:681–686. doi: 10.1016/0091-3057(88)90187-6. [DOI] [PubMed] [Google Scholar]
- 69.Sutton LC, Grahn RE, Wiertelak EP, Watkins LR, Maier SF. Inescapable shock-induced potentiation of morphine analgesia in rats: involvement of opioid, GABAergic, and serotonergic mechanisms in the dorsal raphe nucleus. Behav Neurosci. 1997;111:816–824. doi: 10.1037//0735-7044.111.4.816. [DOI] [PubMed] [Google Scholar]
- 70.Tverberg LA, Russo AF. Cell-specific glucocorticoid repression of calcitonin/calcitonin gene-related peptide transcription. Localization to an 18-base pair basal enhancer element. J Biol Chem. 1992;267:17567–17573. [PubMed] [Google Scholar]
- 71.Verge D, Daval G, Marcinkiewicz M, Patey A, el Mestikawy S, Gozlan H, Hamon M. Quantitative autoradiography of multiple 5-HT1 receptor subtypes in the brain of control or 5,7-dihydroxytryptamine-treated rats. J Neurosci. 1986;6:3474–3482. doi: 10.1523/JNEUROSCI.06-12-03474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vertes RP, Crane AM. Distribution, quantification, and morphological characteristics of serotonin-immunoreactive cells of the supralemniscal nucleus (B9) and pontomesencephalic reticular formation in the rat. J Comp Neurol. 1997;378:411–424. doi: 10.1002/(sici)1096-9861(19970217)378:3<411::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 73.West CH, Weiss JM. Effects of antidepressant drugs on rats bred for low activity in the swim test. Pharmacol Biochem Behav. 1998;61:67–79. doi: 10.1016/s0091-3057(98)00076-8. [DOI] [PubMed] [Google Scholar]
- 74.Wiklund L, Bjorklund A. Mechanisms of regrowth in the bulbospinal serotonin system following 5,6-dihydroxytryptamine induced axotomy. II. Fluorescence histochemical observations. Brain Res. 1980;191:109–127. [PubMed] [Google Scholar]
- 75.Wiklund L, Leger L, Persson M. Monoamine cell distribution in the cat brain stem. A fluorescence histochemical study with quantification of indolaminergic and locus coeruleus cell groups. J Comp Neurol. 1981;203:613–647. doi: 10.1002/cne.902030405. [DOI] [PubMed] [Google Scholar]
- 76.Wood MJ, Byrnes AP, Kaplitt MG, Pfaff DW, Rabkin SD, Charlton HM. Specific patterns of defective HSV-1 gene transfer in the adult central nervous system: implications for gene targeting. Exp Neurol. 1994;130:127–140. doi: 10.1006/exnr.1994.1192. [DOI] [PubMed] [Google Scholar]
- 77.Zhukovskaya NL, Neumaier JF. Clozapine downregulates 5-hydroxytryptamine(6) (5-HT6) and upregulates 5-HT7 receptors in HeLa cells. Neurosci Lett. 2000;288:236–240. doi: 10.1016/s0304-3940(00)01225-8. [DOI] [PubMed] [Google Scholar]
- 78.Zlokovic BV, Apuzzo ML. Cellular and molecular neurosurgery: pathways from concept to reality–part II: vector systems and delivery methodologies for gene therapy of the central nervous system. Neurosurgery. 1997;40:805–812. doi: 10.1097/00006123-199704000-00028. [DOI] [PubMed] [Google Scholar]