Protective Autoimmunity Is a Physiological Response to CNS Trauma (original) (raw)
Abstract
Primary damage caused by injury to the CNS is often followed by delayed degeneration of initially spared neurons. Studies in our laboratory have shown that active or passive immunization with CNS myelin-associated self-antigens can reduce this secondary loss. Here we show, using four experimental paradigms in rodents, that CNS trauma spontaneously evokes a beneficial T cell-dependent immune response, which reduces neuronal loss. (1) Survival of retinal ganglion cells in rats was significantly higher when optic nerve injury was preceded by an unrelated CNS (spinal cord) injury. (2) Locomotor activity of rat hindlimbs (measured in an open field using a locomotor rating scale) after contusive injury of the spinal cord (T8) was significantly better (by three to four score grades) after passive transfer of myelin basic protein (MBP)-activated splenocytes derived from spinally injured rats than in untreated injured control rats or rats similarly treated with splenocytes from naive animals or with splenocytes from spinally injured rats activated ex vivo with ovalbumin or without any ex vivo activation. (3) Neuronal survival after optic nerve injury was 40% lower in adult rats devoid of mature T cells (caused by thymectomy at birth) than in normal rats. (4) Retinal ganglion cell survival after optic nerve injury was higher (119 ± 3.7%) in transgenic mice overexpressing a T cell receptor (TcR) for MBP and lower (85 ± 1.3%) in mice overexpressing a T cell receptor for the non-self antigen ovalbumin than in matched wild types. Taken together, the results imply that CNS injury evokes a T cell-dependent neuroprotective response.
Keywords: protective autoimmunity, CNS injury, spinal cord injury, optic nerve injury, neuroprotection, degeneration, encephalitogenic T cells
Axonal injury in the CNS initiates processes of retrograde and anterograde degeneration in fibers that suffered direct damage (Aguayo et al., 1991; Villegas-Perez et al., 1993; Peinado-Ramon et al., 1996). In cases of partial injury, the primary insult is followed by a self-propagating process of secondary degeneration, mediated by various toxic agents and resulting in a lateral spread of damage to fibers that had escaped the primary lesion (Ransom et al., 1990; Waxman, 1993; Behrmann et al., 1994; Lynch and Dawson, 1994; Agrawal and Fehlings, 1996; Yoles and Schwartz, 1998a). Pharmacological methods aimed at preventing or slowing down secondary degeneration have included neutralizing the mediators of toxicity, competing with their receptors, or increasing the resistance of spared neurons to the toxic environment (Yoles and Schwartz, 1997, 1998a).
The role of the immune system in the post-traumatic spread of damage has long been a subject of controversy (Hirschberg and Schwartz, 1995;Popovich et al., 1996, 1997, 1998; Cohen and Schwartz, 1999; Schwartz et al., 1999a,b). Anti-inflammatory compounds, such as methylprednisolone (Constantini and Young, 1994) and dexamethasone (Hirschberg and Schwartz, 1995), and anti-inflammatory cytokines (Bethea et al., 1999) have been found beneficial in restricting the spread of damage at an early post-traumatic stage; yet, paradoxically, inflammatory cells (macrophages) are needed for regrowth of the injured nerves (Lazarov-Spiegler et al., 1996; Prewitt et al., 1997; Rabchevsky and Streit, 1997; Rapalino et al., 1998). The discovery that macrophages are needed for CNS repair set the stage for a reconsideration of the traditional view of immune activity, and especially autoimmunity, as having only a destructive influence in the context of nerve trauma (Schwartz et al., 1999a,b). Recent studies from our laboratory (Moalem et al., 1999, 2000a,b; Hauben et al., 2000a,b) showed, for example, that autoimmune T cells directed against CNS myelin basic protein (MBP) promoted recovery after crush injury of the optic nerve or contusion of the spinal cord in rats. It was demonstrated that T cells produce neurotrophic factors (Ehrhard et al., 1993; Kerschensteiner et al., 1999) and that the secretion of neurotrophic factors by memory T cells is antigen dependent (Moalem et al., 2000b). It was also shown, however, that injury to the rat spinal cord evokes an autoimmune response directed against myelin and that transfer of MBP-activated lymphocytes from spinally injured rats causes the monophasic paralytic disease experimental autoimmune encephalomyelitis (EAE) (Popovich et al., 1997, 1998). These apparently conflicting findings raised a key question: does injury awaken only a harmful autoimmune response or also a beneficial response? If injury can indeed trigger a protective immune response, this would imply that injury acts as a stress signal that evokes beneficial autoimmunity in the form of a corrective immune response directed against self (Schwartz, 2000).
In the present study we addressed this key question by conducting three experiments in rats and one in mice. In rats, we first examined whether the outcome of a CNS lesion (in the optic nerve) is better or worse if preceded by a lesion at a different CNS site (spinal cord). Next we examined whether spleen cells transferred from spinally injured rats to newly injured rats exhibit neuroprotective effects. In the third experiment we examined whether the outcome of crush injury to the rat optic nerve, with or without previous contusion of the spinal cord, is worse in rats subjected to thymectomy at birth than in nonthymectomized controls.
The experiment with mice was conducted with transgenic mice overexpressing a T cell receptor (TcR) for MBP (Chen et al., 1996). Using the mouse model of optic nerve injury, we showed previously in our laboratory that active immunization with myelin-associated proteins reduces the rate of retinal ganglion cell (RGC) death (Fisher et al., 2000). This finding prompted us to use the mouse model in the present study to determine whether mice in which >90% of the endogenous T cells bear the receptor for MBP are more resistant to injurious CNS conditions than normal mice.
MATERIALS AND METHODS
Animals
Rats. Adult Sprague Dawley rats (8−10 weeks old, 250−300 gm) were supplied by the Animal Breeding Center of The Hebrew University of Jerusalem. Inbred adult Lewis rats (10–12 weeks old, 200–250 gm) were supplied by the Animal Breeding Center of The Weizmann Institute of Science. The rats were housed in a light- and temperature-controlled room and matched for age in each experiment.
Mice. Transgenic mice overexpressing a TcR to the CNS self-antigen MBP or to the non-self antigen ovalbumin (OVA) and their matched wild types (B10.PL and BALB/c, respectively) were supplied by Harvard Medical School. The anti-MBP TcR transgenic mice were generated by the injection of rearranged TcR a and b chain constructs derived from an encephalitogenic T cell clone specific for MBP 1–9/IAu. The constructs were injected into C57BL/6 fertilized oocytes, and the mice were crossed onto B10.PL mice.
Antigens
MBP was prepared from the spinal cords of guinea pigs (Moalem et al., 2000a,b) or purchased from Sigma (St. Louis, MO). OVA was purchased from Sigma.
Spinal cord contusion
Rats were anesthetized and their spinal cords were exposed by laminectomy at the level of T8. One hour after induction of anesthesia, a 10 gm rod was dropped onto the laminectomized cord from a height of 50 mm, using the NYU impactor (Basso et al., 1995, 1996). Sham-operated rats were laminectomized but not contused.
Preparation of peritoneal exudate cells
Adult rats were injected intraperitoneally with 1 ml of PBS containing 100 μg concanavalin A (Con A; Sigma). Two days later, peritoneal exudate cells (PECs) were harvested by thorough washing of the peritoneal cavity with 40 ml of PBS.
Preparation of activated splenocytes
The rats were killed 3, 7, and 14 d after spinal cord injury, and their spleens were excised and pressed through a fine wire mesh. The washed cells (2 × 106/ml) were activated with the antigen (15 μg/ml) and PECs (1 × 105/ml) or irradiated thymocytes (2000 rad, 2 × 106 cells/ml) in proliferation medium containing DMEM supplemented withl-glutamine (2 mm), 2-mercaptoethanol (5 × 10−5m), sodium pyruvate (1 mm), penicillin (100 IU/ml), streptomycin (100 μg/ml), nonessential amino acids, and autologous rat serum 1% (v/v). After incubation for 72 hr at 37°C, 90% relative humidity and 7% CO2, cells were washed with PBS, counted, and injected intraperitoneally into autologous rats within 1 hr after spinal cord contusion.
Proliferation assay
Spleen cells from contused rats, excised and pooled 3, 7, and 14 d after spinal cord contusion (n = 3), were cultured in triplicate in flat-bottomed microtiter wells in 0.2 ml of proliferation medium (described above). The cells were cultured in the presence of irradiated thymocytes (2000 rad, 1 × 105/ml cells per well) together with MBP (15 μg/ml), OVA (15 μg/ml), or Con A (1.25 μg/ml). The proliferative response was determined by measuring the incorporation of [3H]thymidine (1 μCi per well), which was added for the last 16 hr of a 72 hr culture.
Passive transfer of cells
Within 1 hr of contusion rats were injected intraperitoneally, on a random basis, with spleen cells or with PBS. Sham-operated rats (laminectomized but not contused) were also injected with spleen cells and examined daily for EAE symptoms (Ben-Nun and Cohen, 1982).
In the spinally contused rats, bladder expression was performed manually at least twice a day (particularly during the first 48 hr after injury, when it was done three times a day), until the end of the second week, by which time automatic voidance had been recovered. The rats were carefully monitored for evidence of urinary tract infection or any other sign of systemic disease. During the first week after contusion and in any case of hematuria after this period, they received a course of sulfamethoxazole (400 mg/ml) and trimethoprim (8 mg/ml) (Resprim, Teva Laboratories, Jerusalem, Israel), administered orally with a tuberculin syringe (0.3 ml of solution per day). Daily inspections included examination of the laminectomy site for evidence of infection and assessment of the hindlimbs for signs of autophagia or pressure.
Assessment of recovery from spinal cord contusion
Behavioral recovery was scored on a scale of 0 (complete paralysis) to 21 (complete mobility) (Behrmann et al., 1994; Basso et al., 1995, 1996, Beattie et al., 1997) by observers blinded to the treatment received by each rat. Approximately once a week, the locomotor activities of the trunk, tail, and hindlimbs were evaluated in an open field by placing each rat for 4 min in the center of a circular enclosure made of molded plastic with a smooth, nonslip floor (90 cm diameter, 7 cm wall height). Before each evaluation, rats were carefully examined for perineal infection, wounds in the hindlimbs, or tail and foot autophagia.
Partial crush injury of the rat optic nerve
The optic nerve was subjected to crush injury as described previously (Yoles and Schwartz, 1998a). Briefly, rats were deeply anesthetized by intraperitoneal injection of Rompun (xylazine, 10 mg/kg; Vitamed, Jerusalem, Israel) and Vetalar (ketamine, 50 mg/kg; Fort Dodge Laboratories, Fort Dodge, IA). With use of a binocular operating microscope, the conjunctiva of the right eye was incised laterally to the cornea. After separation of the retractor bulbi muscles, the optic nerve was exposed intraorbitally by blunt dissection. Using calibrated cross-action forceps, the optic nerve was subjected to a crush injury 1−2 mm from the eye. The left contralateral nerve was left undisturbed.
Measurement of secondary degeneration after crush injury of the rat optic nerve by retrograde labeling of retinal ganglion cells
Secondary degeneration of the optic nerve axons and their attached RGCs was measured after post-injury application of the fluorescent lipophilic dye 4-(4-(didecylamino)styryl)-_N_-methylpyridinium iodide (4-Di-10-Asp) (Molecular Probes Europe BV, Amsterdam, The Netherlands), distally to the lesion site, 2 weeks after crush injury. Application of the dye distally to the lesion site after 2 weeks ensures that only axons that have survived both the primary damage and the secondary degeneration will be counted, because only axons that are intact can transport the dye back to their cell bodies. This approach thus enables us to differentiate between neurons that are still functionally intact and neurons in which the axons are injured but the cell bodies are still viable. With this method, the number of labeled RGCs reliably reflects the number of still-functioning neurons.
Labeling and measurement were performed as follows: the right optic nerve was exposed for the second time, again without damaging the retinal blood supply. Complete axotomy was performed 1−2 mm from the distal border of the injury site, and solid crystals (0.2−0.4 mm diameter) of 4-Di-10-Asp were deposited at the site of the newly formed axotomy. The rats were killed 5 d after dye application. The retina was detached from the eye, prepared as a flattened whole mount in 4% paraformaldehyde solution, and examined for labeled RGCs by fluorescence microscopy. In each retina four to five fields (0.78 mm2 per field), located at the same distance from the optic disc, were counted.
Labeling of mouse retinal ganglion cells
Mice were anesthetized and placed in a stereotactic device. The skull was exposed and kept dry and clean. The bregma was identified and marked. The designated point of injection was at a depth of 2 mm from the brain surface, 2.92 mm behind the bregma in the anteroposterior axis and 0.5 mm lateral to the midline. A window was drilled in the scalp above the designated coordinates in the right and left hemispheres. The neurotracer dye FluoroGold (5% solution in saline; Fluorochrome, Denver, CO) was then applied (1 μl, at a rate of 0.5 μl/min) using a Hamilton syringe, and the skin over the wound was sutured.
Crush injury of the mouse optic nerve
The mice were anesthetized 72 hr after RGC labeling and subjected to severe crush injury in the intraorbital portion of the optic nerve, 1−2 mm from the eyeball. With the aid of a binocular operating microscope, the conjunctiva was incised, and the optic nerve was exposed. Using cross-action forceps and taking special care not to interfere with the blood supply, the nerve was crushed for 2 sec (Fisher et al., 2000; Verbin-Levkovich et al., 2000).
Assessment of retinal ganglion cell survival in mice
One week after crush injury the mice were given a lethal dose of pentobarbitone (170 mg/kg). Their eyes were enucleated, and the retinas were detached and prepared as flattened whole mounts in 4% paraformaldehyde solution. Labeled cells from six to eight fields of identical size (0.076 mm2), located at approximately the same distance from the optic disk, were counted under the fluorescence microscope and averaged.
Isolation of cells from the injured spinal cord
Infiltrating cells were collected from rat spinal cords 7 d after severe contusion. The entire spinal cord was excised, and a single-cell suspension was prepared by passage of the dissociated spinal cord through a 200-mesh stainless steel sieve. The cell suspension was mixed with isotonic Percoll (density 1.129 gm/ml; Pharmacia Biotech AB, Uppsala, Sweden) in a 2:1 ratio (30% Percoll). The cells were then resuspended, transferred to a conical centrifuge tube, underlaid with a 60% Percoll solution, and spun for 20 min at 600 × g at 4°C. The cells at the interface above the 60% Percoll solution were collected, washed twice with PBS, and resuspended in an RNA isolation reagent (TRI Reagent, Molecular Research Center, Cincinnati, OH).
RT-PCR
Total RNA was extracted using TRI Reagent. The first-strand cDNA synthesis reaction was performed as follows. RNA was incubated at 65°C for 5 min, chilled on ice, and then reverse-transcribed in the presence of oligo-dT primer, 50 mm Tris−HCl, pH 8.3, 75 mm KCl, 3 mm MgCl2, 20 mm DTT, 0.5 mm dNTP mixture, and 200 U of SuperScript II Rnase Reverse Transcriptase (Life Technologies, Rockville, MD_)_ at 42°C for 1 hr. The cDNA generated was amplified with 0.6 U of DyNAzyme II DNA polymerase (Finnzymes Oy, Rihitontuntie, Finland) in the presence of 50−70 pmol of primers, 0.1 mm dNTP mixture, 10 mmTris−HCl, pH 8.8, 1.5 mmMgCl2, 50 mm KCl, and 0.1% Triton X-100. The cycling conditions were 30 sec at 94°C for denaturation, 1 min at 60°C for annealing, 2 min at 72°C for extension, and 7 min at 72°C after the last cycle. cDNA samples were amplified for 27 cycles for IL-10 and IFNγ, 32 cycles for GATA3, and 21 cycles for L19. PCR products were visualized after electrophoresis on 1.5% agarose gels by staining with ethidium bromide. The following primers were used: IFNγ (rat): 5′-ATG AGT GCT ACA CGC CGC GTC TTG G, 5′-GAG TTC ATT GAC TTT GTG CTG G; IL-10 (rat): 5′-GAG TGA AGA CCA GCA AAG GC, 5′-TCG CAG CTG TAT CCA GAG G; and GATA 3 (mouse): 5[prime-ATG TAA GTC GAG GCC CAA G, 5′-GTC ATG CAC CTT TTT GCA C.
RESULTS
CNS injury evokes a systemic neuroprotective response
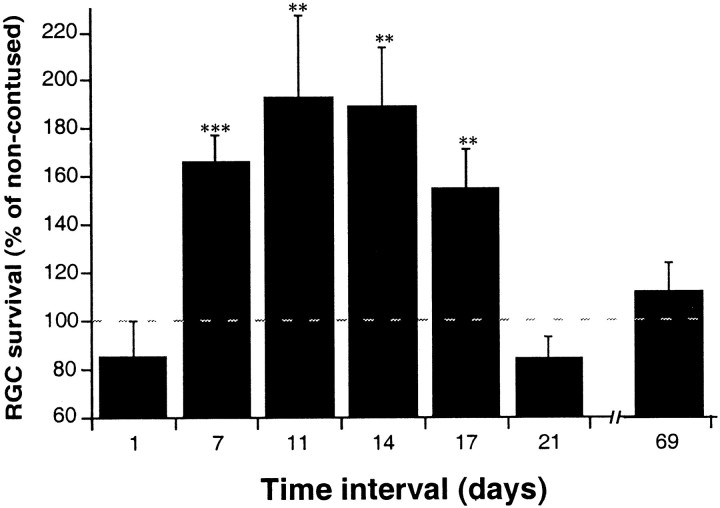
To determine whether the endogenous systemic response awakened by a spinal cord injury in the rat has a beneficial effect, we first examined whether the number of surviving neurons after an optic nerve injury is higher if preceded by a spinal cord injury. A controlled contusive injury was inflicted on the spinal cords of anesthetized male SPD rats by dropping a 10 gm weight from a height of 50 mm onto the laminectomized cord at the level of T8 (Hauben et al., 2000a,b). After 1, 7, 11, 14, 17, 21, or 69 d, the rats (n = 8−14 at each time point) were anesthetized again and subjected to a controlled unilateral partial crush of the optic nerve (Yoles and Schwartz, 1998a). The latter injury was shown previously to serve as a reliable model for the assessment of primary and secondary degeneration and the effects of neuroprotective intervention (Yoles et al., 1992,1996; Yoles and Schwartz, 1997, 1998a,b; Schwartz and Yoles, 1999). At the same time as the experimental rats underwent spinal cord contusion, control rats were anesthetized but not injured. They were subsequently reanesthetized and subjected to partial crush injury of the optic nerve, as in the experimental rats (n = 9−17; same time points as for the experimental rats). Two weeks after the optic nerve crush, neuronal survival in the injured optic nerve of each rat of both groups was assessed in terms of the number of neurons that had escaped both the initial lesion and the subsequent secondary degeneration. For assessment of surviving axons, a dye was applied distally to the lesion site (see Materials and Methods). Counting of labeled RGCs yielded the number of neurons that were still intact.
In the rats subjected to spinal cord contusion 7, 11, 14, or 17 d before optic nerve crush, the number of surviving optic neurons was significantly higher (by 40% on average) than in rats with crushed optic nerves without previous contusion (Fig.1). An interval of 1 d between the two lesions was found to be too short for development of an effective response, and ≥21 d appeared to be too long to maintain it. These results suggest that CNS injury evokes a systemic neuroprotective response, detectable here by the improved recovery of a subsequent CNS lesion at another site.
Fig. 1.
Systemic neuroprotection evoked by CNS injury in male rats. Male SPD rats were subjected to unilateral partial crush injury of the optic nerve 1−69 d after spinal cord contusion. Two weeks after the crush injury the number of surviving neurons was determined by retrograde labeling of their RGCs. The numbers of rats used at each time point (contused/control) were as follows: day 1, 14/17; day 7, 11/10; day 11, 10/11; day 14, 10/10; day 17, 14/15; day 21, 8/9; day 69, 9/12. At each time point, the ratio between the mean number of RGCs in the retinas of contused rats (i.e., where the optic nerve lesion was preceded by a spinal cord contusion) and the mean number of RGCs in control rats (where the optic nerve lesion was preceded at the corresponding time point by anesthesia only) was calculated. The results are expressed as mean percentages ± SE. The mean numbers of viable fibers (reflected by labeled RGCs) in all the control groups were similar, regardless of the interval between anesthesia and optic nerve crush (50.6, 57.3, 42.53, 58.17, 45.52, 43.25; mean ± SEM for all experiments, 48.85 ± 3.05). The time lapse between the two injuries significantly affected neuronal survival after the optic nerve injury (p < 0.008; ANOVA). Comparison between the groups revealed significantly better neuronal survival when the time lapse between the two injuries was 7–17 d.
In a similar experiment using female SPD rats, nine rats underwent spinal cord contusion and seven were sham operated (the spinal cord was exposed and laminectomized without contusion), and after 1 week all 16 rats were subjected to a partial crush injury of the optic nerve. The number of surviving neurons, 2 weeks later, was determined as described above. In the rats with previous spinal injury, the number of labeled RGCs (expressed as a percentage of the mean number of RGCs in the retinas of injured nerves of the sham-operated rats) was 177 ± 36 (mean ± SE). The difference between the two groups was significant (p < 0.05, t test). The use of sham-operated rats rules out the possibility that a beneficial effect is derived from the procedure itself rather than from the response evoked by the damaged neurons.
The systemic beneficial activity evoked by CNS injury is transferable by splenocytes
If the neuroprotective activity elicited by the spinal cord injury is indeed an immune-associated response, in principle it could be mediated either by reactive lymphocytes or by humoral factors such as antibodies, cytokines, or growth factors. In view of previous studies from our laboratory demonstrating that autoimmune T cells can have a beneficial effect on traumatized CNS neurons (Moalem et al., 1999a,2000a,b; Hauben et al., 2000a,b), it was of interest to determine whether lymphocytes transferred from spinally injured rats to rats with fresh CNS injuries would be neuroprotective. Successful lymphocyte-mediated transfer of the beneficial immune activity after CNS injury would strongly support the claim that the neuroprotective activity manifested after CNS injury is evoked by T cells.
Splenocytes from the spinally injured rats were exposed in vitro for 3 d to MBP together with PECs. The MBP-activated splenocytes were then washed, counted, and administered by systemic injection at a dosage of 4.5 × 107cells to rats newly subjected to spinal cord contusion. The injected lymphocytes had a significant neuroprotective effect in the newly injured rats, manifested by recovery of locomotor activity assessed in an open field using the Basso, Beattie, and Bresnahan (BBB) test (Fig. 2A). The mean maximal behavioral score in rats treated with MBP-activated splenocytes derived from spinally injured rats (n = 5) was 6.5 ± 1.2, compared with 2.3 ± 0.3 in PBS-injected rats (n = 5). In both groups of spinally contused rats, behavioral recovery as a function of time after contusion was compared by two-way repeated measures ANOVA (group, p < 0.02; time, p < 0.0001). It should be noted here that the full scale of scores on the BBB test in rats ranges from 0 (complete paralysis of the hindlimbs) to 21 (full mobility). However, because studies in our laboratory have shown that the effect mediated by the T cells is neuroprotective (Moalem et al., 1999, 2000a,b; Hauben et al., 2000a,b), the relevant range is limited to scores that reflect only the activity of neurons that survived the contusion and can potentially be rescued from degeneration. This range is from 0 (total paralysis) to 6 or 7 (maximal locomotor function reflecting maximal activity of spared fibers) (Hauben et al., 2000a,b). On the latter scale, a difference of 3 or 4 points therefore represents a very large difference in terms of functional recovery. An increase in the score from 3 to 7, for example, represents a change from almost complete paralysis with only slight movement of one joint to extensive movement of all hindlimb joints (Basso et al., 1995, 1996).
Fig. 2.

The immune neuroprotection evoked by spinal cord contusion is transferable in SPD rats. A, Rats were subjected to spinal cord contusion. One week later their spleens were excised and incubated in vitro with PECs as antigen-presenting cells and with MBP. After 3 d the activated spleen cells were collected, washed, and counted. The resuspended cell population (4.5 × 107) or PBS was injected into rats newly subjected to spinal contusion (n = 5 for each group). In a separate experiment (B), similarly prepared splenocytes from spinally contused rats activated ex vivo with MBP (Contused-MBP; n = 4) or OVA (Contused-OVA; n = 5), or splenocytes prepared from sham-operated animals activated with MBP (Naive-MBP; n = 5), were transferred to newly contused rats. A group of newly contused rats injected with PBS (n = 5) was also included. No effect was seen with splenocytes from contused rats incubated in vitro_with OVA. Similarly prepared splenocytes, withdrawn from rats 14 d (C) or 3 d (D) after spinal contusion, were activated with irradiated thymocytes in the presence of MBP. After 3 d in vitro the activated spleen cells were collected, washed, and counted. The resuspended cells were injected into six rats newly subjected to spinal contusion (4.5 × 107 cells per rat). In each experiment (C, D), five rats that were subjected to spinal contusion and injected with the vehicle (PBS) served as control. E, Transfer of splenocytes withdrawn 14 d after spinal cord contusion and injected with no previous_ex vivo activation into five rats newly subjected to spinal cord contusion. Five rats that were subjected to spinal contusion and injected with the vehicle (PBS) served as control. Behavioral outcome was evaluated according to a double-blind protocol, using a locomotor test with scores ranging from 0 to 21 (see Materials and Methods). Results are expressed as the mean ± SE at each time point tested. Repeated-measures ANOVA revealed significant effect (p < 0.05) in A–C and no effect in_D_ and E.
To further verify that the observed beneficial effect after splenocyte transfer is related to MBP, we repeated the above experiments with a new set of rats, which were divided into three groups and subjected to spinal cord contusion. Two groups were treated with splenocytes that were obtained from spinally injured rats and activated ex vivo with MBP (group 1) or OVA (group 2). Rats in group 3 were treated with similarly prepared splenocytes that were obtained, however, from rats without spinal cord injury and activated ex vivo with MBP. No significant beneficial effect of the transferred splenocytes was seen in rats from group 2 or 3 (Fig.2B). The mean maximal behavioral score of all the rats in these two groups (n = 15) was 4.3 ± 1.3, and the differences between the groups were not significant. Rats treated with MBP-activated splenocytes (group 1; n = 4) achieved an average behavioral score of 7.6 ± 1.3. Behavioral recovery as a function of time after contusion in group 1 was compared with that in groups 2 and 3 by two-way repeated measures ANOVA (group,p < 0.016; time, p < 0.0001).
In the two experiments described in Figure 2, A and_B_, the splenocytes transferred from the spinally injured rats were activated ex vivo by MBP in the presence of PECs as antigen-presenting cells. To rule out a possible contribution of PECs to the observed recovery, we repeated the experiment using splenocytes obtained from spinally injured rats and subjected to_ex vivo_ MBP activation using irradiated thymocytes as the antigen-presenting cells. Rats were subjected to spinal cord contusion and treated 1 hr later with splenocytes derived from rats that had been spinally injured 14 d earlier and activated ex vivo by incubation for 3 d with MBP and irradiated thymocytes. As shown in Figure 2C, the transferred splenocytes were as effective as similarly derived splenocytes incubated ex vivo for 3 d with MBP and PECs, thus ruling out a possible contribution of the PECs to the recovery shown in Figure 2, A and B. It is interesting to note, however, that no beneficial effect of transferred splenocytes on newly injured rats was observed when the time interval between spinal injury in the donor rats and withdrawal of their splenocytes for ex vivo activation was only 3 d (Fig.2D). Similar transfer of the same number of splenocytes derived from spinally injured rats that were not exposed_ex vivo_ to any antigen had no significant effect, either beneficial or destructive, in the spinally contused recipient rats (Fig. 2E). Because of slight variations in outcome (ranging from 2.3 ± 0.3 to 4.3 ± 1.3) among untreated contused control rats, a control group of untreated contused rats was included in each experiment shown in Figure2A_–_E.
Taken together, the results summarized above support our suggestion that spinal cord injury in the rat triggers a protective systemic response that can be transferred to recipient rats by splenocytes activated ex vivo with MBP. They further suggest, as in the case of the observed beneficial effect of previous spinal injury on recovery from optic nerve injury (when the time lapse between the two injuries was 7−17 d; see Fig. 1), that a similar time lapse is required for effective transfer of the beneficial effect of splenocytes derived from spinally injured rats to newly injured rats. Because only those cells that were exposed ex vivo to MBP could transfer the neuroprotective activity, it seems reasonable to suggest that an MBP-dependent T cell response is evoked physiologically and that its beneficial (neuroprotective) effect can be transferred.
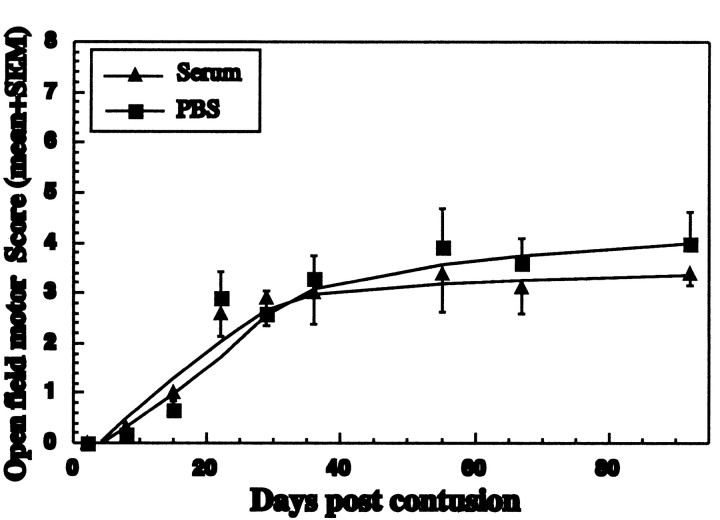
To determine whether the neuroprotective activity can be mediated by anti-MBP antibodies, we collected sera displaying high anti-MBP antibody titers from seven rats and transferred the pooled sera by intravenous injection (2 ml of undiluted sera) to rats newly subjected to spinal cord contusion. No effect was observed on recovery from the spinal cord injury (Fig. 3). We also assayed the anti-MBP antibodies in the sera of spinally contused rats. By 2 weeks after contusion, the presence of antibodies in the recipient rats was barely detectable (data not shown).
Fig. 3.
Passive transfer of antibodies does not confer neuroprotection. Lewis rats were immunized with MBP in incomplete Freund's adjuvant. After 60 d, 2 ml of sera pooled from seven rats was injected intravenously into rats newly subjected to spinal cord contusion. Six control rats were injected with PBS. At the indicated time periods, rats were evaluated by a locomotor test with scores ranging from 0 to 21 (see Materials and Methods). Behavioral outcome was assessed by observers blinded to the treatment received by the rats. Results are expressed as the mean ± SE at each time point tested (n = 6 for each group).
In vitro analysis of the T cell response to spinal cord contusion
We measured the proliferative capacity of splenocytes collected from spleens excised 3, 7, and 14 d after spinal cord contusion (Table 1). All proliferative responses were very low. The proliferative responses to MBP were only slightly higher than to OVA and were higher on days 7 and 14 than on day 3. The fact that the increase in the MBP proliferation index of splenocytes derived from spinally contused rats was slight does not necessarily imply, however, that the incidence of these MBP-specific cells is low. It is possible, for example, that the neuroprotective T cells are a unique type of MBP-specific T cells, characterized by low proliferation and specific cytokine dependency. This possibility is strengthened by the results of our analysis of lymphocytes isolated by Percoll gradient from the contused spinal cords of rats excised 1 week after the injury. Fluorescence-activated cell sorting analysis of these lymphocytes for purity, using CD3 antibodies, showed that 83% of them, on average, were CD3+ (data not shown). RNA extracted from the isolated lymphocytes was subjected to RT−PCR analysis for expression of the cytokines IFNγ and IL-10. Primers for GFAP were used to rule out contamination of the preparation by astrocytes. The extracted RNA was found to express IL-10 (a cytokine characteristic of Th2) and not IFNγ (a cytokine characteristic of Th1). It also expressed GATA-3, a Th2-promoting transcription factor (Rodriguez-Palmero et al., 1999). Selection of the primers used was based on the mouse sequence, and the identity of the transcribed product using rat RNA was verified as GATA-3 (Fig.4). These findings confirm that T cells accumulate at the site of injury after spinal cord contusion and suggests that among the accumulated T cells found at the site of the lesion 1 week after contusion, and presumably contributing to the observed protection, are perhaps cells reminiscent of Th2 helper T cells. The phenotype of the accumulated T cells at earlier and later post-traumatic stages is currently under investigation.
Table 1.
Numbers of proliferating spleen cells from rats subjected to spinal cord contusion
| Days after contusion | No antigen | MBP | OVA | ConA |
|---|---|---|---|---|
| 3 | 1198 ± 150 | 1121 ± 175 | 1181 ± 85 | 11461 ± 3216 |
| 7 | 1208 ± 151 | 1308 ± 146 | 1098 ± 66 | 9308 ± 3694 |
| 14 | 1109 ± 142 | 1350 ± 59 | 1164 ± 21 | 8994 ± 617 |
Fig. 4.
Expression of GATA-3 and IL-10, but not of IFNγ, by lymphocytes isolated from contused spinal cords. Lymphocytes were isolated from spinal cords 7 d after spinal cord contusion (SC-L). RNA extracted from the isolated cells was subjected to RT−PCR. RNA extracted from a T cell line directed to MBP [known to express both pro-inflammatory and anti-inflammatory cytokines (Moalem et al., 2000b)] was used as control. The lymphocytes recovered from contused spinal cords expressed mostly IL-10 and hardly any IFNγ.
Lack of endogenous protective autoimmunity in adult rats subjected to thymectomy at birth
To verify that the endogenous protective autoimmune response to injury is part of the body's own maintenance mechanism, we used adult SPD rats devoid of mature T cells as a result of having undergone thymectomy at birth. On reaching adulthood (2 months), all of the thymectomized rats underwent either spinal cord contusion or a sham operation, and 1 week later all were subjected to a unilateral crush lesion of the optic nerve. Nonthymectomized rats of the same age were subjected to similar optic nerve lesions and used as controls. As shown in Figure 5, counting of labeled RGCs after the injury showed that the number of spared neurons in the thymectomized rats (mean per square millimeter ± SEM, 73 ± 20; n = 10) was significantly smaller than in the nonthymectomized controls (179 ± 25; n = 9,p < 0.01, t test). The number of surviving neurons after an optic nerve injury that was preceded by spinal contusion (94 ± 14; n = 8) was also significantly lower in the thymectomized rats than in the rats with an intact thymus (p < 0.05, t test). No significant differences in the numbers of surviving RGCs were found between the two groups of thymectomized rats, whether or not the optic nerve injury was preceded by spinal contusion. Thus, the beneficial effect of previous contusion, which was observed in rats with an intact thymus, could not be detected in rats lacking mature T cells. These results substantiate our contention that the systemic response evoked by the spinal cord injury and found to be beneficial after subsequent injury to the optic nerve is mediated by T cells. They further suggest that damaged CNS nerves can indeed benefit from the injury-evoked T cell response but that this response in its natural form does not fully counteract the injury-induced degeneration and requires boosting. Our recent results seem to rule out the possibility that loss of the beneficial systemic response after the thymectomy is caused by nonimmune mechanisms; in rat strains that inherently lack a beneficial response to CNS injury, loss of RGCs in rats devoid of T cells was not higher than in nonthymectomized rats (J. Kipnis, E. Yoles, E. Hauben, I. Shaked, and M. Schwartz, unpublished observations).
Fig. 5.
Adult rats subjected to thymectomy at birth recover poorly from CNS injury. Thymectomized rats (n = 18) were subjected to a partial crush injury of their optic nerves. One week before the optic nerve crush, 8 of these rats underwent spinal cord contusion (precontused ON crush; thymectomized) and 10 underwent a sham operation (ON crush; thymectomized). Two weeks after optic nerve injury the surviving neurons were labeled, and 5 d later the retinas were excised and their RGCs counted. Normal rats (n = 9) were subjected to optic nerve crush only (ON crush). The number of RGCs per square millimeter in each animal is shown. Thymectomy had a significant effect on RGC survival, both in the absence of previous contusion (p < 0.003) and in the precontused group (p < 0.01; t test).
Survival rate of retinal ganglion cells after axonal injury is increased in mice overexpressing a T cell receptor for myelin basic protein
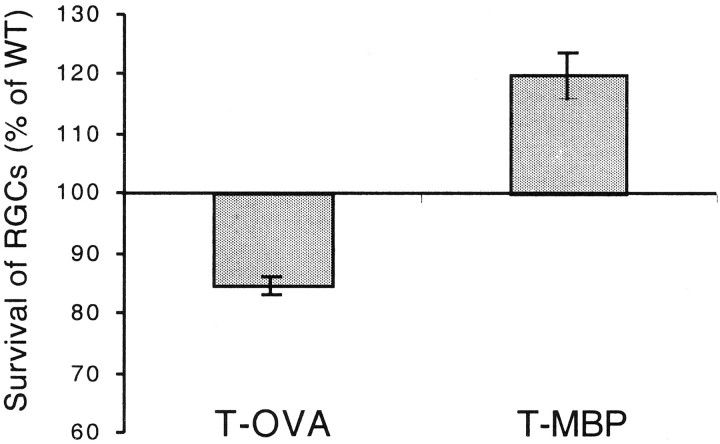
To further substantiate the physiological character of the beneficial T cell-mediated effect specific to self-antigens such as MBP, we assessed the survival rate of RGCs after crush injury to the optic nerves of transgenic mice overexpressing a T cell receptor for MBP. All RGCs in both optic nerves of wild-type and transgenic mice were stereotactically prelabeled. After 3 d the left optic nerve of each mouse was severely crushed, and the retinas on both sides were excised 1 week later. The contralateral nerves were left undamaged until the mice were killed, which is when their retinas were excised and used as controls. No differences in the total numbers of RGCs in the retinas of normal (undamaged) nerves were found between the wild-type and transgenic mice (t test, p = 0.7). One week after crush injury, the number of surviving RGCs per square millimeter in the transgenic mice was significantly higher than in the wild type (1573 ± 71, n = 10 compared with 1314 ± 53, n = 10; mean ± SE). The difference is statistically significant (t test,p < 0.009) (Fig. 6). Interestingly, in transgenic mice overexpressing a T cell receptor for OVA, and thus presumably lacking T cells specific to other antigens, the number of surviving RGCs per square millimeters after optic nerve crush injury (1367 ± 47, n = 9) was significantly lower (p < 0.0003) than in the wild type (1617 ± 25, n = 10). It should also be noted that the genetic backgrounds of the transgenic mice selected for overexpression of the T cell receptor for MBP and OVA were different (B10.PL and BALB/c, respectively). B10.PL is a mouse strain that shows a low recovery rate after optic nerve injury and is amenable to immunological boosting, whereas BALB/c mice are characterized by a high survival rate after optic nerve injury (Kipnis, Yoles, Hauben, Shaked, and Schwartz, unpublished observations). This difference was borne out by the present results: the RGC survival rate after optic nerve injury was 1617 ± 25 (mean per square millimeter ± SEM) in the wild-type BALB/c mice and 1314 ± 53 (mean per square millimeters ± SEM) in the B10.PL mice. These findings suggest that, compared with the wild type, mice expressing abnormally large numbers of T cell receptors for MBP are better endowed with an endogenous mechanism for coping with injury-induced stress. In contrast, mice lacking the ability to respond to any antigen (including self-antigens), like the transgenic mice overexpressing T cell receptors to OVA (Fig. 6), are defective with respect to their endogenous immune mechanism for coping with the stress imposed by injury.
Fig. 6.
Survival of retinal ganglion cells after optic nerve crush in transgenic mice overexpressing a receptor for anti-MBP T cells or anti-OVA T cells. All RGCs of both eyes of transgenic mice overexpressing a T cell receptor for MBP or a T cell receptor for OVA and of the corresponding wild-type mice were labeled with a stereotactically injected dye. Three days later the mice were subjected to a unilateral crush injury. After 1 week the retinas were excised and whole mounted, and their labeled (surviving) RGCs were counted. The mean numbers of RGCs ± SEM in each group of transgenic mice are expressed here as percentages of the matched wild type. Survival of the injured mice overexpressing a T cell receptor for MBP (n = 10) was significantly higher than in the corresponding wild type (n = 10;p < 0.009), whereas in mice overexpressing a T cell receptor for OVA (n = 9) survival was lower than in the corresponding wild type (n = 10;p < 0.0003; t test).
DISCUSSION
The results of this study suggest that beneficial T cell-dependent immunity is a physiological response to CNS trauma and that it partially counteracts the trauma-induced damage.
As shown here, spinal cord injury in the rat evokes a systemic T cell-mediated neuroprotective response, which can be transferred by suitably activated splenocytes into rats with a new spinal injury. In adult rats subjected to thymectomy at birth, recovery from optic nerve injury was worse than the recovery in nonthymectomized rats. It is possible that the same autoimmune T cells (“encephalitogenic” T cells) may be both beneficial for neuronal survival and destructive of myelin, and that what really determines which of these effects will be manifested is the presence or absence of regulatory T cells. Accordingly, the two activities might be mediated by autoimmune T cells that are affected differently by the regulatory T cells common to both activities, or alternatively the presence of these regulatory T cells might make the two effects of the autoimmune T cells mutually exclusive (Nagaoka et al., 2000). The fact that encephalitogenic T cells can be neuroprotective was also recently documented by Hammarberg and his colleagues (2000), who demonstrated that the effects of harmful T cell-derived cytokines such as TNF-α and INF-γ can be curbed by the production of potent neurotrophic factors.
The finding of neuroprotection in transgenic mice overexpressing a T cell receptor for MBP but not in mice overexpressing a T cell receptor for OVA further substantiates the notion that antigenic specificity is critical for the observed neuroprotection. Activated anti-MBP T cells are known to cause EAE in rats (Ben-Nun et al., 1981; Ota et al., 1990;Bell and Steinman, 1991; Martin, 1997), but the endogenous anti-MBP T cells did not cause disease in the transgenic mice used in this study. The tight control of autoimmune T cells to avoid disease development in these transgenic mice has been attributed to the regulatory CD4(+) T cells that they express, albeit in small amounts (Goverman et al., 1993; Chen et al., 1994; Chen et al., 1996; Brabb et al., 1997;Olivares-Villagomez et al., 1998; Van de Keere and Tonegawa, 1998). EAE does occur in crosses of these mice with recombinant-activating gene-1 knock-out mice, in which no regulatory T cells are expressed. It is possible, for example, that regulatory cells in transgenic mice overexpressing a T cell receptor to MBP, although preventing the spontaneous development of disease, contribute to or at least do not prevent the occurrence of spontaneous post-traumatic neuroprotection. Whatever the role of the regulatory T cells in neuroprotection, our results imply that the ratio of encephalitogenic T cells to regulatory T cells might critically affect the autoimmune outcome**_,_**because the beneficial effect of MBP-activated splenocytes transferred from spinally contused rats diminished with increasing dosage of the transferred cells (data not shown).
In previous studies from our laboratory during which we used the crush-injured rat optic nerve and the contused rat spinal cord as models (Moalem et al., 1999a,b; Hauben et al., 2000a,b), recovery from partial injury in the CNS was shown to be promoted by the passive transfer of T cells directed against myelin-associated antigens. Similar recovery was obtained with T cells directed against encephalitogenic epitopes or cryptic epitopes of these antigens (Moalem et al., 1999a). Those findings suggested that autoimmune T cells may have a beneficial effect in the context of CNS injury. It was not clear, however, whether the observed autoimmune neuroprotection represents a physiological response to injury or was the result of an exogenous application of autoimmune T cells. The present work demonstrates that the T cell-mediated neuroprotection is likely to be a purposeful physiological response. It further demonstrates that this response reaches a peak within 7 d and is maintained for an additional 10 d, after which it disappears. The fact that the recovery from optic nerve injury was worse in thymectomized rats than in rats with an intact thymus suggests that the latter group derives at least some benefit from an endogenous T cell-related immunity, which evidently is amenable to therapeutic boosting. In rat strains that are inherently incapable of sustaining a beneficial response to injury, the absence of mature T cells (caused by thymectomy) did not worsen their recovery (Kipnis, Yoles, Hauben, Shaked, and Schwartz, unpublished observations). Other recent studies have also indicated that adaptive immunity plays a role in regulating the survival of neurons after nerve injury. Thus, for example, the neuronal loss after facial nerve injury was greater in mice deficient in functional T and B cells than in their wild-type controls (Serpe et al., 1999).
In a recent study from our laboratory, expression of the costimulatory molecule B7.2 in rats was found to be increased after spinal contusion and was further increased after passive transfer of autoimmune T cells that led to neuroprotection (Butovsky et al., 2001). In view of the recently discovered close relationship between Th2 expression and expression of the B7.2 receptor CD28 (Rodriguez-Palmero et al., 1999), it is possible that the T cells that were isolated from the contused spinal cord 1 week after contusion and express features reminiscent of Th2 cells, represent one of the T cell populations participating in the multicellular process of neuroprotection. These populations might include both encephalitogenic T cells and regulatory T cells, and the presence of the one without the other may not be sufficient for neuroprotection (Schwartz and Kipnis, 2001).
This is the first demonstration, confirmed by four independent experimental approaches, that a T cell-dependent autoimmunity is evoked for the benefit of the individual. This finding may explain the frequently described presence of T cells directed to self-antigens (such as MBP) in healthy individuals, which in turn leads to the speculation that autoimmune T cells may in some cases be harmless or even useful (Cohen, 1992; Matzinger, 1994). On the basis of the present study, it seems reasonable to suggest that the traumatic event acts as a stress signal to the immune system, with the purpose of helping the damaged nerve cope with the threat of progressive degeneration, a potentially devastating event in the CNS. The spontaneous response, although beneficial if tightly regulated, may nevertheless be insufficiently effective, possibly because of the immune-privileged character of the CNS (Cohen and Schwartz, 1999; Schwartz et al., 1999a). These results support our contention that autoimmune protection, being the body's own physiological (although inadequate) response to injury, is worth boosting for therapeutic purposes.
Footnotes
The work was supported by Proneuron Ltd., Industrial Park, Ness-Ziona, Israel. M.S. holds the Maurice and Ilse Katz Professorial Chair in Neuroimmunology. I.R.C. is the incumbent of the Mauerberger Chair in Immunology. We thank S. Smith for editing this manuscript. We thank Dr. D. Teitelbaum for the gift of MBP, G. Avisar for animal maintenance, and R. Margalit for performing thymectomies on the rats.
E.Y. and E.H. contributed equally to the work.
Correspondence should be addressed to Dr. Michal Schwartz, Department of Neurobiology, The Weizmann Institute of Science, 76100 Rehovot, Israel. E-mail: michal.schwartz@weizmann.ac.il.
REFERENCES
- 1.Agrawal SK, Fehlings MG. Mechanisms of secondary injury to spinal cord axons in vitro: role of Na+, Na(+)-K(+)-ATPase, the Na(+) exchanger, and the Na(+)-Ca(2+) exchanger. J Neurosci. 1996;16:545–552. doi: 10.1523/JNEUROSCI.16-02-00545.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguayo AJ, Rasminsky M, Bray GM, Carbonetto S, McKerracher L, Villegas-Perez MP, Vidal-Sanz M, Carter DA. Degenerative and regenerative responses of injured neurons in the central nervous system of adult mammals. Philos Trans R Soc Lond B Biol Sci. 1991;331:337–343. doi: 10.1098/rstb.1991.0025. [DOI] [PubMed] [Google Scholar]
- 3.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 4.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 5.Beattie MS, Bresnahan JC, Komon J, Tovar CA, Van Meter M, Anderson DK, Faden AI, Hsu CY, Noble LJ, Salzman S, Young S. Endogenous repair after spinal cord contusion injuries in the rat. Exp Neurol. 1997;148:453–463. doi: 10.1006/exnr.1997.6695. [DOI] [PubMed] [Google Scholar]
- 6.Behrmann DL, Bresnahan JC, Beattie MS. Modeling of acute spinal cord injury in the rat: neuroprotection and enhanced recovery with methylprednisolone, U-74006F and YM-14673. Exp Neurol. 1994;126:61–75. doi: 10.1006/exnr.1994.1042. [DOI] [PubMed] [Google Scholar]
- 7.Bell RB, Steinman L. Trimolecular interactions in experimental autoimmune demyelinating disease and prospects for immunotherapy. Semin Immunol. 1991;3:237–245. [PubMed] [Google Scholar]
- 8.Ben-Nun A, Cohen IR. Experimental autoimmune encephalomyelitis (EAE) mediated by T cell lines: process of selection of lines and characterization of the cells. J Immunol. 1982;129:303–308. [PubMed] [Google Scholar]
- 9.Ben-Nun A, Wekerle H, Cohen IR. The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur J Immunol. 1981;11:195–199. doi: 10.1002/eji.1830110307. [DOI] [PubMed] [Google Scholar]
- 10.Bethea JR, Nagashima H, Acosta MC, Briceno C, Gomez F, Marcillo AE, Loor K, Green J, Dietrich WD. Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery after traumatic spinal cord injury in rats. J Neurotrauma. 1999;16:851–863. doi: 10.1089/neu.1999.16.851. [DOI] [PubMed] [Google Scholar]
- 11.Brabb T, Goldrath AW, von Dassow P, Paez A, Liggitt HD, Goverman J. Triggers of autoimmune disease in a murine TCR-transgenic model for multiple sclerosis. J Immunol. 1997;159:497–507. [PubMed] [Google Scholar]
- 12.Butovsky O, Hauben E, Schwartz M (2001) Morphological aspects of spinal cord autoimmune neuroprotection: colocalization of T cells with B7.2(CD86) and prevention of cyst formation. FASEB J, in press. [DOI] [PubMed]
- 13.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Inobe J, Kuchroo VK, Baron JL, Janeway CA, Jr, Weiner HL. Oral tolerance in myelin basic protein T-cell receptor transgenic mice: suppression of autoimmune encephalomyelitis and dose-dependent induction of regulatory cells. Proc Natl Acad Sci USA. 1996;93:388–391. doi: 10.1073/pnas.93.1.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen IR. The cognitive paradigm and the immunological homunculus. Immunol Today. 1992;13:490–494. doi: 10.1016/0167-5699(92)90024-2. [DOI] [PubMed] [Google Scholar]
- 16.Cohen IR, Schwartz M. Autoimmune maintenance and neuroprotection of the central nervous system. J Neuroimmunol. 1999;100:111–114. doi: 10.1016/s0165-5728(99)00190-3. [DOI] [PubMed] [Google Scholar]
- 17.Constantini S, Young W. The effects of methylprednisolone and the ganglioside GM1 on acute spinal cord injury in rats. J Neurosurg. 1994;80:97–111. doi: 10.3171/jns.1994.80.1.0097. [DOI] [PubMed] [Google Scholar]
- 18.Ehrhard PB, Erb P, Graumann U, Otten U. Expression of nerve growth factor and nerve growth factor receptor tyrosine kinase Trk in activated CD4-positive T cell clones. Proc Natl Acad Sci USA. 1993;90:10984–10988. doi: 10.1073/pnas.90.23.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher J, Yoles E, Levkovitch-Verbin H, Kay JF, Ben-Nun A, Schwartz M. Vaccination for neuroprotection in the mouse optic nerve: implications for optic neuropathies. J Neurosci. 2000;20:136–142. doi: 10.1523/JNEUROSCI.21-01-00136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goverman J, Woods A, Larson L, Weiner LP, Hood L, Zaller DM. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell. 1993;72:551–560. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]
- 21.Hammarberg H, Lidman O, Lundberg C, Eltayeb SY, Gielen AW, Muhallab S, Svenningsson A, Linda H, van der Meide PH, Cullheim S, Olsson T, Piehl F. Neuroprotection by encephalomyelitis: rescue of mechanically injured neurons and neurotrophin production by CNS-infiltrating T and natural killer cells. J Neurosci. 2000;20:5283–5291. doi: 10.1523/JNEUROSCI.20-14-05283.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hauben E, Nevo U, Yoles E, Moalem G, Agranov E, Mor F, Akselrod S, Neeman M, Cohen IR, Schwartz M. Autoimmune T cells as potential neuroprotective therapy for spinal cord injury. Lancet. 2000a;355:286–287. doi: 10.1016/s0140-6736(99)05140-5. [DOI] [PubMed] [Google Scholar]
- 23.Hauben E, Butovsky O, Nevo U, Yoles E, Moalem G, Agranov E, Mor F, Leibowitz-Amit R, Pevsner S, Akselrod S, Neeman M, Cohen IR, Schwartz M. Passive or active immunization with myelin basic protein promotes recovery from spinal cord contusion. J Neurosci. 2000b;20:6421–6430. doi: 10.1523/JNEUROSCI.20-17-06421.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirschberg DL, Schwartz M. Macrophage recruitment to acutely injured central nervous system is inhibited by a resident factor: a basis for an immune-brain barrier. J Neuroimmunol. 1995;61:89–96. doi: 10.1016/0165-5728(95)00087-i. [DOI] [PubMed] [Google Scholar]
- 25.Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, Kolbeck R, Hoppe E, Oropeza-Wekerle RL, Bartke I, Stadelmann C, Lassmann H, Wekerle H, Hohlfeld R. Activated human T cells, B cells and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazarov-Spiegler O, Solomon AS, Zeev-Brann AB, Hirschberg DL, Lavie V, Schwartz M. Transplantation of activated macrophages overcomes central nervous system regrowth failure. FASEB J. 1996;10:1296–1302. doi: 10.1096/fasebj.10.11.8836043. [DOI] [PubMed] [Google Scholar]
- 27.Lynch DR, Dawson TM. Secondary mechanisms in neuronal trauma. Curr Opin Neurol. 1994;7:510–516. doi: 10.1097/00019052-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Martin R. Immunological aspects of experimental allergic encephalomyelitis and multiple sclerosis and their application for new therapeutic strategies. J Neural Transm Suppl. 1997;49:53–67. doi: 10.1007/978-3-7091-6844-8_6. [DOI] [PubMed] [Google Scholar]
- 29.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 30.Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen IR, Schwartz M. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med. 1999a;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- 31.Moalem G, Monsonego A, Shani Y, Cohen IR, Schwartz M. Differential T cell response in central and peripheral nerve injury: connection with immune privilege. FASEB J. 1999b;13:1207–1217. doi: 10.1096/fasebj.13.10.1207. [DOI] [PubMed] [Google Scholar]
- 32.Moalem G, Yoles E, Leibowitz-Amit R, Muller-Gilor S, Mor F, Cohen IR, Schwartz M. Autoimmune T cells retard the loss of function in injured rat optic nerves. J Neuroimmunol. 2000a;106:189–197. doi: 10.1016/s0165-5728(00)00240-x. [DOI] [PubMed] [Google Scholar]
- 33.Moalem G, Gdalyahu A, Shani Y, Otten U, Lazarovici P, Cohen IR, Schwartz M. Production of neurotrophins by activated T cells: implications for neuroprotective autoimmunity. J Autoimmun. 2000b;15:331–345. doi: 10.1006/jaut.2000.0441. [DOI] [PubMed] [Google Scholar]
- 34.Nagaoka H, Yu W, Nussenzweig MC. Regulation of RAG expression in developing lymphocytes. Curr Opin Immunol. 2000;12:187–190. doi: 10.1016/s0952-7915(99)00070-9. [DOI] [PubMed] [Google Scholar]
- 35.Olivares-Villagomez D, Wang Y, Lafaille JJ. Regulatory CD4(+) T cells expressing endogenous T cell receptor chains protect myelin basic protein-specific transgenic mice from spontaneous autoimmune encephalomyelitis. J Exp Med. 1998;188:1883–1894. doi: 10.1084/jem.188.10.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ota K, Matsui M, Milford EL, Mackin GA, Weiner HL, Hafler DA. T cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990;346:183–187. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- 37.Peinado-Ramon P, Salvador M, Villegas-Perez MP, Vidal-Sanz M. Effects of axotomy and intraocular administration of NT-4, NT-3, and brain-derived neurotrophic factor on the survival of adult rat retinal ganglion cells. A quantitative in vivo study. Invest Ophthalmol Vis Sci. 1996;37:489–500. [PubMed] [Google Scholar]
- 38.Popovich PG, Stokes BT, Whitacre CC. Concept of autoimmunity following spinal cord injury: possible roles for T lymphocytes in the traumatized central nervous system. J Neurosci Res. 1996;45:349–363. doi: 10.1002/(SICI)1097-4547(19960815)45:4<349::AID-JNR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 39.Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 40.Popovich PG, Whitacre CC, Stokes BT. Is spinal cord injury an autoimmune disease? Neuroscientist. 1998;4:71–76. [Google Scholar]
- 41.Prewitt CM, Niesman IR, Kane CJ, Houle JD. Activated macrophage/microglial cells can promote the regeneration of sensory axons into the injured spinal cord. Exp Neurol. 1997;148:433–443. doi: 10.1006/exnr.1997.6694. [DOI] [PubMed] [Google Scholar]
- 42.Rabchevsky AG, Streit WJ. Grafting of cultured microglial cells into the lesioned spinal cord of adult rats enhances neurite outgrowth. J Neurosci Res. 1997;47:34–48. [PubMed] [Google Scholar]
- 43.Ransom BR, Stys PK, Waxman SG. The pathophysiology of anoxic injury in the central nervous system white matter. Stroke. 1990;21:52–57. [PubMed] [Google Scholar]
- 44.Rapalino O, Lazarov-Spiegler O, Agranov E, Velan GJ, Fraidakis M, Yoles E, Solomon A, Gepstein R, Katz A, Belkin M, Hadani M, Schwartz M. Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nat Med. 1998;4:814–821. doi: 10.1038/nm0798-814. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez-Palmero M, Hara T, Thumbs A, Hunig T. Triggering of T cell proliferation through CD28 induces GATA-3 and promotes T helper type 2 differentiation in vitro and in vivo. Eur J Immunol. 1999;29:3914–3924. doi: 10.1002/(SICI)1521-4141(199912)29:12<3914::AID-IMMU3914>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz M. Autoimmune involvement in CNS trauma is beneficial if well controlled. Prog Brain Res. 2000;128:259–263. doi: 10.1016/S0079-6123(00)28023-0. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz M, Kipnis J (2001) Protective autoimmunity: regulation and prospects for vaccination after brain and spinal cord injuries. Trends Mol Med, in press. [DOI] [PubMed]
- 48.Schwartz M, Yoles E. Optic nerve degeneration and potential neuroprotection: implications for glaucoma. Eur J Ophthalmol. 1999;9:S9–11. doi: 10.1177/112067219900901S07. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz M, Moalem G, Leibowitz-Amit R, Cohen IR. Innate and adaptive immune responses can be beneficial for CNS repair. Trends Neurosci. 1999a;22:295–299. doi: 10.1016/s0166-2236(99)01405-8. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz M, Cohen IR, Lazarov-Spiegler O, Moalem G, Yoles E. The remedy may lie in ourselves: prospects for immune cell therapy in central nervous system protection and repair. J Mol Med. 1999b;77:713–717. doi: 10.1007/s001099900047. [DOI] [PubMed] [Google Scholar]
- 51.Serpe CJ, Kohm AP, Huppenbauer CB, Sanders VM, Jones KJ. Exacerbation of facial motoneuron loss after facial nerve transection in severe combined immunodeficient (SCID) mice. J Neurosci 19 1999. RC7 (1–5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van de Keere F, Tonegawa S. CD4(+) T cells prevent spontaneous experimental autoimmune encephalomyelitis in anti-myelin basic protein T cell receptor transgenic mice. J Exp Med. 1998;188:1875–1882. doi: 10.1084/jem.188.10.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Villegas-Perez MP, Vidal-Sanz M, Rasminsky M, Bray GM, Aguayo AJ. Rapid and protracted phases of retinal ganglion cell loss follow axotomy in the optic nerve of adult rats. J Neurobiol. 1993;24:23–36. doi: 10.1002/neu.480240103. [DOI] [PubMed] [Google Scholar]
- 54.Waxman SG. Aminopyridines and the treatment of spinal cord injury. J Neurotrauma. 1993;10:19–24. doi: 10.1089/neu.1993.10.19. [DOI] [PubMed] [Google Scholar]
- 55.Yoles E, Schwartz M. N-methyl-d-aspartate-receptor antagonist protects neurons from secondary degeneration after partial optic nerve crush. J Neurotrauma. 1997;14:665–675. doi: 10.1089/neu.1997.14.665. [DOI] [PubMed] [Google Scholar]
- 56.Yoles E, Schwartz M. Degeneration of spared axons following partial white matter lesion: implications for optic nerve neuropathies. Exp Neurol. 1998a;153:1–7. doi: 10.1006/exnr.1998.6811. [DOI] [PubMed] [Google Scholar]
- 57.Yoles E, Schwartz M. Elevation of intraocular glutamate in rats with partial lesion of the optic nerve. Arch Ophthalmol. 1998b;116:906–910. doi: 10.1001/archopht.116.7.906. [DOI] [PubMed] [Google Scholar]
- 58.Yoles E, Zalish M, Lavie V, Duvdevani R, Ben Bassat S, Schwartz M. GM1 reduces injury-induced metabolic deficits and degeneration in the rat optic nerve. Invest Ophthalmol Vis Sci. 1992;33:3586–3591. [PubMed] [Google Scholar]
- 59.Yoles E, Belkin M, Schwartz M. HU-211, a nonpsychotropic cannabinoid, produces short- and long-term neuroprotection after optic nerve axotomy. J Neurotrauma. 1996;13:49–57. doi: 10.1089/neu.1996.13.49. [DOI] [PubMed] [Google Scholar]