Effect of Linagliptin vs Glimepiride on Major Adverse Cardiovascular Outcomes in Patients With Type 2 Diabetes: The CAROLINA Randomized Clinical Trial (original) (raw)
Key Points
Question
What is the effect of linagliptin compared with glimepiride on major cardiovascular events in patients with relatively early type 2 diabetes and elevated cardiovascular risk?
Findings
In this randomized noninferiority clinical trial that included 6033 participants followed up for a median of 6.3 years, the use of linagliptin compared with glimepiride added to usual care resulted in rates of the composite outcome (cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) of 11.8% vs 12.0%. The upper limit of the 95.47% CI of the hazard ratio was 1.14, which met the noninferiority criterion of a hazard ratio of less than 1.3.
Meaning
Compared with glimepiride, the use of linagliptin demonstrated noninferiority with regard to the risk of major cardiovascular events over a median of 6.3 years in patients with relatively early type 2 diabetes and elevated cardiovascular risk.
Abstract
Importance
Type 2 diabetes is associated with increased cardiovascular risk. In placebo-controlled cardiovascular safety trials, the dipeptidyl peptidase-4 inhibitor linagliptin demonstrated noninferiority, but it has not been tested against an active comparator.
Objective
This trial assessed cardiovascular outcomes of linagliptin vs glimepiride (sulfonylurea) in patients with relatively early type 2 diabetes and risk factors for or established atherosclerotic cardiovascular disease.
Design, Setting, and Participants
Randomized, double-blind, active-controlled, noninferiority trial, with participant screening from November 2010 to December 2012, conducted at 607 hospital and primary care sites in 43 countries involving 6042 participants. Adults with type 2 diabetes, glycated hemoglobin of 6.5% to 8.5%, and elevated cardiovascular risk were eligible for inclusion. Elevated cardiovascular risk was defined as documented atherosclerotic cardiovascular disease, multiple cardiovascular risk factors, aged at least 70 years, and evidence of microvascular complications. Follow-up ended in August 2018.
Interventions
Patients were randomized to receive 5 mg of linagliptin once daily (n = 3023) or 1 to 4 mg of glimepiride once daily (n = 3010) in addition to usual care. Investigators were encouraged to intensify glycemic treatment, primarily by adding or adjusting metformin, α-glucosidase inhibitors, thiazolidinediones, or insulin, according to clinical need.
Main Outcomes and Measures
The primary outcome was time to first occurrence of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke with the aim to establish noninferiority of linagliptin vs glimepiride, defined by the upper limit of the 2-sided 95.47% CI for the hazard ratio (HR) of linagliptin relative to glimepiride of less than 1.3.
Results
Of 6042 participants randomized, 6033 (mean age, 64.0 years; 2414 [39.9%] women; mean glycated hemoglobin, 7.2%; median duration of diabetes, 6.3 years; 42% with macrovascular disease; 59% had undergone metformin monotherapy) were treated and analyzed. The median duration of follow-up was 6.3 years. The primary outcome occurred in 356 of 3023 participants (11.8%) in the linagliptin group and 362 of 3010 (12.0%) in the glimepiride group (HR, 0.98 [95.47% CI, 0.84-1.14]; P < .001 for noninferiority), meeting the noninferiority criterion but not superiority (P = .76). Adverse events occurred in 2822 participants (93.4%) in the linagliptin group and 2856 (94.9%) in the glimepiride group, with 15 participants (0.5%) in the linagliptin group vs 16 (0.5%) in the glimepiride group with adjudicated-confirmed acute pancreatitis. At least 1 episode of hypoglycemic adverse events occurred in 320 (10.6%) participants in the linagliptin group and 1132 (37.7%) in the glimepiride group (HR, 0.23 [95% CI, 0.21-0.26]).
Conclusions and Relevance
Among adults with relatively early type 2 diabetes and elevated cardiovascular risk, the use of linagliptin compared with glimepiride over a median 6.3 years resulted in a noninferior risk of a composite cardiovascular outcome.
Trial Registration
ClinicalTrials.gov Identifier: NCT01243424
This randomized noninferiority clinical trial examines the effect of treatment with the dipeptidyl peptidase-4 inhibitor linagliptin vs the commonly used sulfonylurea glimepiride on cardiovascular safety in patients with type 2 diabetes and cardiovascular risk factors or established atherosclerotic cardiovascular disease.
Introduction
When choosing medications to manage type 2 diabetes, cardiovascular safety, glucose-lowering potency, hypoglycemia risk, effect on body weight, and cost are important considerations.1,2,3 Most guidelines state that metformin should be first-line therapy followed by various options for second-line treatment if sufficient glycemic control is not achieved after metformin monotherapy.1,2,3 Sulfonylureas and dipeptidyl peptidase-4 (DPP-4) inhibitors are the most commonly used second-line glucose-lowering treatments in many countries.4 Sulfonylureas are used mainly based on their low cost, well-established glucose-lowering action, and a long-standing experience in clinical practice. However, sulfonylureas are associated with increased risk of hypoglycemia1,3,5,6,7 and modest weight gain.1,5 In addition, there is an ongoing controversy regarding their long-term cardiovascular safety, based on early data from the University Group Diabetes Program in the 1960s8 and multiple observational and smaller studies indicating conflicting results.9,10
Linagliptin is a selective, once-daily, DPP-4 inhibitor approved for glycemic management of type 2 diabetes, with low risk of hypoglycemia and weight neutrality.11 To date, no head-to-head trial has compared the long-term effect of these agents on cardiovascular morbidity and mortality or glucose-lowering efficacy in patients with type 2 diabetes.
The Cardiovascular Outcome Study of Linagliptin vs Glimepiride in Type 2 Diabetes (CAROLINA) examined the effect of treatment with the DPP-4 inhibitor linagliptin vs the commonly used sulfonylurea glimepiride on cardiovascular safety in patients with relatively early type 2 diabetes and cardiovascular risk factors or established atherosclerotic cardiovascular disease using a noninferiority design.
Methods
The study protocol was approved by the institutional review board or independent ethics committee from each site, and all patients provided written informed consent; the trial protocol is available is Supplement 1 and the statistical analysis plan in Supplement 2.
The trial was conducted in accordance with the principles of the Declaration of Helsinki and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonisation and was approved by local authorities.
Trial Oversight
An independent, unmasked data monitoring committee regularly reviewed trial data. Investigator-reported cardiovascular outcome events, deaths, pancreatitis, and pancreatic cancer were prospectively captured and centrally adjudicated by clinical events committees masked to treatment assignment.
Trial Design
The trial design has been previously published.12 In brief, this was a multicenter, randomized, double-blind, active-controlled clinical trial conducted at 607 centers across 43 countries, aimed to continue until at least 631 participants had an adjudication-confirmed primary outcome event.
Trial Participants
Adults with type 2 diabetes, glycated hemoglobin (HbA1c) level of 6.5% to 8.5%, and high cardiovascular risk were eligible for inclusion. Participants naive to sulfonylurea or glinide therapy had to have a HbA1c level of 6.5% to 8.5%, while participants who were currently treated with a sulfonylurea or glinide as monotherapy or in a dual combination with metformin or α-glucosidase inhibitor (who also were eligible for the trial) had to have an HbA1c level of 6.5% to 7.5%. The sulfonylurea or glinide were discontinued at randomization. High cardiovascular risk was defined as (1) established atherosclerotic cardiovascular disease (documented ischemic heart disease, cerebrovascular disease, or peripheral artery disease), (2) multiple risk factors (at least 2 of the following: type 2 diabetes duration >10 years, systolic blood pressure >140 mm Hg [or receiving at least 1 blood pressure–lowering treatment], current smoker, low-density lipoprotein cholesterol ≥135 mg/dL [3.5 mmol/L], or receiving lipid-lowering treatment), (3) age at least 70 years, and (4) evidence of microvascular complications (impaired kidney function [estimated glomerular filtration rate of 30-59 mL/min/1.73 m2], urine albumin/creatinine ratio ≥30 μg/mg, or proliferative retinopathy). Insulin therapy or previous exposure to DPP-4 inhibitors, glucagonlike peptide-1 receptor agonists, or thiazolidinediones were exclusion criteria, as was New York Heart Association class III to IV heart failure (eAppendix 3 and 4 in Supplement 3).
Information on race and ethnicity was captured by investigators based on self-classification by trial participants as reported in the electronic case record form (fixed categories) following written informed consent. This information was collected to allow for subgroup analysis, given some previous reports about potential heterogeneity of effects of sulfonylureas and incretin-based therapies on different genetic background,13,14 and as required by regulatory bodies.15
Trial Procedures
Participants were randomized 1:1 using an interactive telephone- and web-based system in a block size of 4 to receive 5 mg of once-daily oral linagliptin or 1 to 4 mg of once-daily glimepiride (Figure 1). Treatment assignment was determined by a computer-generated random sequence with stratification by center. Glimepiride was started at 1 mg/d and uptitrated to a potential maximum dose of 4 mg/d every 4 weeks during the first 16 weeks. After the first 16 weeks, participants returned for follow-up study visits every 16 weeks until the end of the study. A final follow-up visit was scheduled 30 days after treatment cessation. Investigators were encouraged to monitor and use additional medication for glycemic control per local guidelines, particularly if HbA1c was greater than 7.5% after the end of the titration phase. Recommended strategies were adjustments of background therapy or addition of pioglitazone, metformin, α-glucosidase inhibitor, or basal insulin. Investigators were also encouraged to manage all other cardiovascular risk factors in accordance with applicable guidelines and current standards of care. Participants who prematurely discontinued the study medication were followed up for ascertainment of cardiovascular events, mortality, adverse events, and other end points. Attempts were made to collect vital status and outcome event information on every randomized individual at study completion, in compliance with local law and regulations.
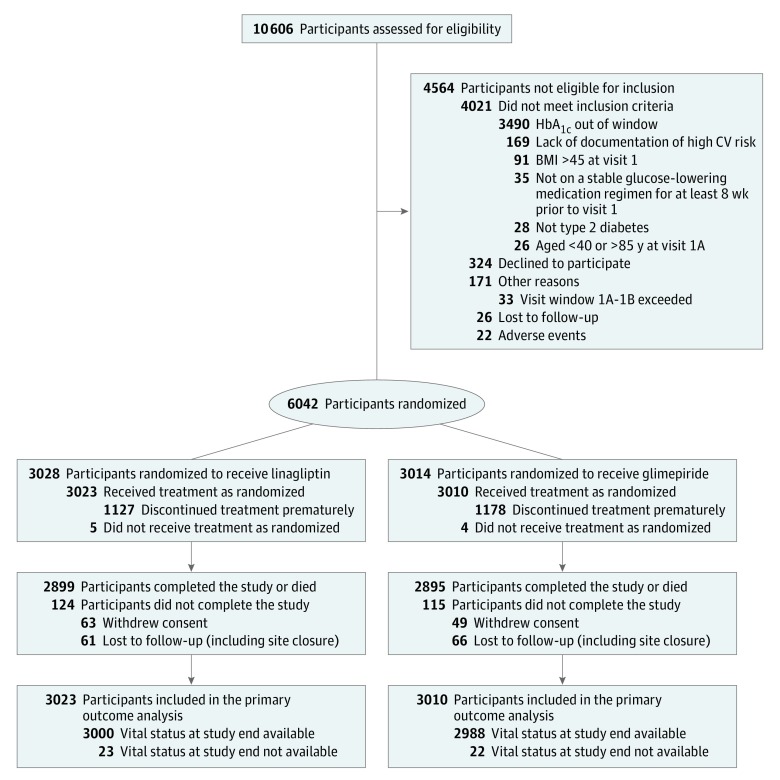
Figure 1. Enrollment, Randomization, and Follow-up of Participants in a Study of the Effect of Linagliptin vs Glimepiride on Cardiovascular Outcomes in Patients With Type 2 Diabetes.
There were 19 participants (9 in the linagliptin group and 10 in the glimepiride group) identified to have been enrolled and treated at multiple sites. For these participants, treatment group allocation according to first randomization was used and only objective data (eg, selected baseline characteristics, serious adverse events, and trigger events sent for adjudication) were included in the analyses. Patients could meet more than 1 exclusion criteria. BMI indicates body mass index; CV, cardiovascular; HbA1c, glycated hemoglobin.
Trial Outcomes
The primary end point was time to first occurrence of cardiovascular death, nonfatal myocardial infarction (MI), or nonfatal stroke (3-point major cardiovascular event [3P-MACE] composite). The original protocol included hospitalization for unstable angina in the primary end point (4-point major cardiovascular event [4P-MACE] composite); however, this was changed by a protocol amendment in April 2016, based on emerging evidence that a primary end point definition of 3P-MACE was preferred by regulators and consistent with other outcome trials of glucose-lowering therapies.16,17 The steering committee and sponsor remained blinded to all trial data prior to database lock. Time to first occurrence of 4P-MACE was hierarchically evaluated as the first of the prespecified key secondary end points, followed by analyses of the proportion of patients receiving treatment and maintaining HbA1c of less than or equal to 7.0% at the final follow-up visit who (1) were without the need for rescue medication, did not have any moderate/severe hypoglycemic episodes, and did not have greater than 2% weight gain or (2) were without the need for rescue medication and did not have greater than 2% weight gain between the end of titration and final visit.
Other secondary cardiovascular end points included individual components of 3P-MACE and 4P-MACE and time to any confirmed adjudicated cardiovascular events (cardiovascular death, including fatal stroke and fatal MI; nonfatal MI; nonfatal stroke; hospitalization for unstable angina; transient ischemic attack; hospitalization for HF; hospitalization for coronary revascularization procedures). Secondary diabetes-related end points included change in laboratory parameters from baseline to final visit (eg, HbA1c, fasting plasma glucose, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides). In addition, we prespecified several tertiary cardiovascular end points (ie, occurrence of and time to first occurrence of each of the confirmed adjudicated end points), tertiary diabetes-related end points (eg, change of laboratory parameters from baseline to each planned week, hypoglycemia occurrence, change in weight and rescue medication use), and other end points (including noncardiovascular death and adverse events). All predefined outcomes and end point definitions are presented in Supplement 1, Supplement 3 (eAppendix 5), and Supplement 4.
Safety was assessed based on adverse events that occurred during treatment or within 7 days after the last dose of a study drug and coded using the Medical Dictionary for Drug Regulatory Activities version 21.0. Adverse events prespecified as being of special interest included hypersensitivity reactions, skin lesions, pancreatitis, pancreatic cancer, and hypoglycemia. Categories of hypoglycemia were analyzed as “any,” “moderate or severe,” “severe,” or “leading to hospitalization” (for definitions of each categorization, see eAppendix 5 in Supplement 3).
Statistical Analysis
The primary aim of the study was to evaluate whether linagliptin was noninferior to glimepiride for the time to 3P-MACE, defined by the upper limit of the multiplicity-adjusted 2-sided 95.47% CI for the hazard ratio (HR) of linagliptin relative to glimepiride of less than 1.3.15 This margin (ie, an upper limit of the 2-sided 95% CI <1.3) was deemed able to demonstrate a reassuring point estimate of overall cardiovascular risk between study groups in the context of a noninferiority assessment by the US Food and Drug Administration. A 5-step hierarchical testing strategy was prespecified, in which each subsequent test would be performed in case of significant prior results. If noninferiority was achieved for the primary outcome, the subsequent tests were (1) superiority test of 3P-MACE, (2) superiority test of 4P-MACE, (3) superiority test of the second key secondary end point (ie, proportion of patients receiving treatment and maintaining HbA1c ≤7.0% at the final visit who were without the need for rescue medication following the end of titration, did not have moderate/severe hypoglycemic episodes, and did not have >2% weight gain), and (4) superiority test of the third key secondary end point (ie, proportion of patients receiving treatment and maintaining HbA1c ≤7.0% at the final visit who were, from the end of titration, without the need for rescue medication and did not have >2% weight gain). Not adjusted for interim analyses, a total of 631 individuals with an adjudication-confirmed 3P-MACE would provide 90.9% power to demonstrate noninferiority (noninferiority margin, 1.3) of linagliptin vs glimepiride at the overall 1-sided α level of 2.5% assuming an HR of 1.0, and 80% power for superiority assuming an HR of 0.80. The 95.47% bound for the CI reflected an O’Brien-Fleming α-spending adjustment for the 2 interim analyses of the primary outcome,18 in addition to Bonferroni adjustment, to control for type I error for the change from 4P-MACE to 3P-MACE after the first interim analysis. The interim analyses were planned to be performed after 190 and 411 participants experienced a primary outcome event. Outcomes were analyzed in all randomized patients treated with at least 1 dose of the study drug (treated set) using the intention-to-treat principle. Patients were analyzed according to their randomized treatment group. Additional sensitivity analyses are described in eAppendix 6 in Supplement 3. Time-to-event outcomes were analyzed using a Cox proportional hazards model, with treatment assignment as a factor in the model. Proportional hazards assumptions were explored by plotting log(−log [survival function]) against the log of time × treatment group and checked for parallelism. Further, Schoenfeld residuals were plotted against time and log(time). For all Cox proportional hazards analyses, the proportional hazard assumption was met. Subgroup analyses included additional factors for subgroup and treatment by subgroup interaction.
In addition, Kaplan-Meier estimates are presented. Censoring was applied the day a participant was last known to be free of the specific outcome event. Because of declining numbers of participants at risk, Kaplan-Meier plots were truncated at 6.5 years after randomization. Logistic regression models with randomized treatment as the factor and χ2 tests were used to analyze noncardiovascular key secondary efficacy end points. For continuous parameters, the change from baseline over time was evaluated with a restricted maximum likelihood–based mixed-model repeated-measures approach (2-sided significance threshold P < .05; eAppendix 6 in Supplement 3). As prespecified, data were included up to the planned week that could theoretically be achieved by all patients. The prespecified approach for handling missing data are described in the statistical analysis plan (Supplement 2). The approach varied according to the statistical analysis employed (eg, censoring in Cox models and Kaplan-Meier plots for time-to-event analysis and mixed models for continuous variables). Specifically, we defined the censoring date for the time-to-event analysis as the last date a patient was known to be free of an end point event, including any start dates of adverse event/outcome events, onset dates of adjudicated-confirmed events, date of percutaneous coronary intervention/coronary artery bypass grafting, or date of trial completion (defined as the latest of date of the last clinic visit, telephone call, or contact if lost to follow-up). Except for the prespecified 5-step hierarchical testing strategy, there was no adjustment for multiple comparisons and, therefore, the results of subgroup analyses and other end points should be interpreted as exploratory. Safety assessments were conducted using descriptive statistics for adverse events, except for analyses of hypoglycemia, which was analyzed using a Cox proportional hazards model (2-sided P value threshold < .05). Analyses were conducted using SAS version 9.4 (SAS Institute).
Results
Trial Participants
Participants were screened from November 2010 through December 2012, with final follow-up on August 21, 2018. A total of 6042 participants were randomized, of whom 6033 received at least 1 dose of the study medication and were included in the primary outcome analysis (Figure 1).
Baseline clinical characteristics were well balanced between groups (Table 1), with 42% of all participants having prevalent atherosclerotic cardiovascular disease at the time of screening. Median (quartile [Q] 1, Q3) follow-up was 6.3 (5.9, 6.6) years in both the linagliptin and glimepiride groups. Median (Q1, Q3) study medication exposure was 5.9 years in the linagliptin group and 5.9 (3.4, 6.4) years in the glimepiride group (eAppendix 7 in Supplement 3). Cumulative participant-years of follow-up was 18 336 for the linagliptin group and 18 212 for the glimepiride group. Overall, 96.0% of participants completed the study, with 38.2% prematurely discontinuing the study drug (incidence rate per 100 years at risk of 7.6 in the linagliptin group and 8.0 in the glimepiride group). Vital status was available for 99.3% of participants at the end of the study (Figure 1).
Table 1. Baseline Participant Characteristics in a Study of the Effect of Linagliptin vs Glimepiride on Cardiovascular Outcomes in Patients With Type 2 Diabetes.
| Characteristic | No. (%) | |
|---|---|---|
| Linagliptin (n = 3023) | Glimepiride (n = 3010) | |
| Age, mean (SD), y | 63.9 (9.5) | 64.2 (9.5) |
| Sex | ||
| Men | 1838 (60.8) | 1781 (59.2) |
| Women | 1185 (39.2) | 1229 (40.8) |
| Race | (n = 3014) | (n = 3000) |
| White | 2217 (73.6) | 2190 (73.0) |
| Asian | 531 (17.6) | 530 (17.7) |
| Black | 155 (5.1) | 169 (5.6) |
| American Indian/Alaska Native | 106 (3.5) | 108 (3.6) |
| Hawaiian/Pacific Islander | 5 (0.2) | 3 (0.1) |
| Ethnicity | (n = 3014) | (n = 3000) |
| Not Hispanic/Latino | 2495 (82.8) | 2487 (82.9) |
| Hispanic/Latino | 519 (17.2) | 513 (17.1) |
| Region | ||
| Europe | 1422 (47.0) | 1399 (46.5) |
| North America, New Zealand, or Australia | 618 (20.4) | 622 (20.7) |
| Asia | 465 (15.4) | 468 (15.5) |
| South America and Mexico | 454 (15.0) | 454 (15.1) |
| Africa (Tunisia and South Africa) | 64 (2.1) | 67 (2.2) |
| Smoking status | (n = 3014) | (n = 3000) |
| Neversmoker | 1356 (45.0) | 1442 (48.1) |
| Previous smoker | 1051 (34.9) | 977 (32.6) |
| Current smoker | 607 (20.1) | 581 (19.4) |
| Cardiovascular risk entry criteria | ||
| Vascular disease | 1051 (34.8) | 1038 (34.5) |
| Microvascular-related organ damage | 258 (8.5) | 254 (8.4) |
| Age ≥70 y | 566 (18.7) | 592 (19.7) |
| Multiple cardiovascular risk factors | 1132 (37.4) | 1111 (36.9) |
| Missing cardiovascular risk group category or all entries “no” | 16 (0.5) | 15 (0.5) |
| History of heart failure | (n = 3014) | (n = 3000) |
| Yes | 122 (4.1) | 149 (5.0) |
| No | 2892 (95.6) | 2851 (95.0) |
| Atherosclerotic cardiovascular disease | (n = 3014) | (n = 3000) |
| Any | 1272 (42.2) | 1250 (41.7) |
| Coronary artery disease | 968 (32.1) | 937 (31.2) |
| Cerebrovascular disease | 371 (12.3) | 356 (11.9) |
| Peripheral artery disease | 207 (6.9) | 200 (6.7) |
| History of hypertension | 3014 (100) | 3000 (100) |
| Yes | 2720 (90.2) | 2698 (89.6) |
| No | 294 (9.8) | 302 (10.1) |
| Microvascular disease | 3014 (100) | 3000 (1000) |
| Any | 847 (28.1) | 881 (29.4) |
| Diabetic neuropathy | 515 (17.1) | 495 (16.5) |
| Diabetic nephropathy | 352 (11.7) | 372 (12.4) |
| Diabetic retinopathy | 212 (7.0) | 236 (7.9) |
| eGFR (MDRD), mL/min/1.73 m2 | (n = 3011) | (n = 3000) |
| Mean (SD) | 76.5 (19.7) | 77.0 (19.8) |
| ≥90 | 693 (23.0) | 722 (24.1) |
| 60-89 | 1726 (57.3) | 1740 (58.0) |
| 30-59 | 576 (19.1) | 525 (17.5) |
| 15-29 | 13 (0.4) | 13 (0.4) |
| <15 | 3 (0.1) | 0 |
| UACR, mg/g | (n = 3007) | (n = 2988) |
| Median (Q1, Q3) | 9.7 (5.3, 31.8) | 9.7 (5.3, 30.1) |
| <30 | 2228 (74.1) | 2234 (74.8) |
| 30-300 | 645 (21.4) | 630 (21.1) |
| >300 | 134 (4.4) | 124 (4.1) |
| BMI, mean (SD) | 30.2 (5.2) (n = 3012) | 30.0 (5.1) (n = 2997) |
| Glycated hemoglobin, mean (SD), % | 7.2 (0.6) (n = 3013) | 7.2 (0.6) (n = 3000) |
| Fasting plasma glucose, mean (SD), mg/dL | 140 (31) (n = 3008) | 140 (30) (n = 2993) |
| Diabetes duration, median (Q1, Q3), y | 6.3 (3.0, 11.1) (n = 3001) | 6.2 (2.9, 10.9) (n = 2982) |
| Diabetes duration ≤5 y | (n = 3014) | (n = 3000) |
| Yes | 1224 (40.6) | 1212 (40.4) |
| No | 1790 (59.4) | 1788 (59.6) |
| Blood pressure | (n = 3014) | (n = 2998) |
| Systolic | 136 (16) | 136 (16) |
| Diastolic | 79 (10) | 79 (9) |
| Heart rate, mean (SD), beats/min | 71 (11) (n = 3014) | 71 (10) (n = 2998) |
| Total cholesterol, mean (SD), mg/dL | 177 (43) (n = 2893) | 177 (45) (n = 2866) |
| LDL cholesterol, mean (SD), mg/dL | 95 (35) (n = 2794) | 95 (36) (n = 2763) |
| HDL cholesterol, mean (SD), mg/dL | 48 (13) (n = 2889) | 49 (13) (n = 2854) |
| Triglycerides, median (Q1, Q3), mg/dL | 144 (106-200) (n = 2893) | 142 (105-196) (n = 2866) |
| Glucose-lowering therapy | (n = 3014) | (n = 3000) |
| Metformin | 2510 (83.3) | 2510 (83.7) |
| Sulfonylurea | 869 (28.8) | 846 (28.2) |
| α-Glucosidase inhibitor | 97 (3.2) | 92 (3.1) |
| Glinide | 28 (0.9) | 38 (1.3) |
| No. of glucose-lowering therapies | (n = 3014) | (n = 3000) |
| 0 | 274 (9.1) | 272 (9.1) |
| 1 | 1984 (65.8) | 1982 (66.1) |
| 2 | 736 (24.4) | 725 (24.2) |
| 3 | 20 (0.7) | 21 (0.7) |
| Blood pressure–lowering medications | (n = 3014) | (n = 3000) |
| ≥1 | 2662 (88.3) | 2682 (89.4) |
| ACE inhibitors | 1330 (44.1) | 1342 (44.7) |
| ARBs | 956 (31.7) | 928 (30.9) |
| β-Blockers | 1193 (39.6) | 1159 (38.6) |
| Calcium-channel antagonists | 891 (29.6) | 885 (29.5) |
| Diuretics | 1099 (36.5) | 1137 (37.9) |
| Select cardiovascular medications | (n = 3014) | (n = 3000) |
| Acetylsalicylic acid | 1410 (46.8) | 1413 (47.1) |
| Statins | 1913 (63.5) | 1987 (66.2) |
Primary End Point
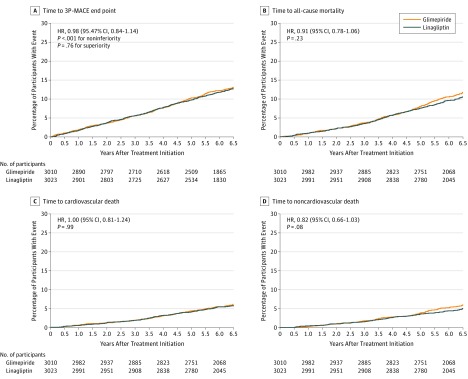
The primary 3P-MACE end point occurred in 356 of 3023 participants (11.8%) treated with linagliptin (2.1 per 100 person-years) and 362 of 3010 (12.0%) treated with glimepiride (2.1 per 100 person-years), meeting the criterion for noninferiority (HR, 0.98 [95.47% CI, 0.84-1.14], P <.001 for noninferiority; Table 2 and Figure 2A). The subsequent testing for superiority according to the prespecified testing procedure was not statistically significant (P = .76). Overall, the HR for 3P-MACE was consistent across prespecified subgroups (eAppendix 8 in Supplement 3).
Table 2. Primary End Point, Key Secondary Outcomes, and Other Secondary or Tertiary Cardiovascular End Points in a Study of the Effect of Linagliptin vs Glimepiride on Cardiovascular Outcomes in Patients With Type 2 Diabetes.
| Outcome | Linagliptin (n = 3023) | Glimepiride (n = 3010) | Incidence Rate/100 Patient-Years Difference, Linagliptin − Glimepiride (95% CI) | HRa/Odds Ratiob (95% CI) | ||
|---|---|---|---|---|---|---|
| No. (%) | Rate/100 Patient-Years | No. (%) | Rate/100 Patient-Years | |||
| Primary End Point | ||||||
| Cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke (3P-MACE) | 356 (11.8) | 2.1 | 362 (12.0) | 2.1 | 0.0 (−0.4 to 0.3) | 0.98 (0.84 to 1.14)a,c,d |
| Cardiovascular deathc | 129 (4.3) | 125 (4.2) | ||||
| Nonfatal myocardial infarction | 141 (4.7) | 138 (4.6) | ||||
| Nonfatal strokec | 86 (2.8) | 101 (3.4) | ||||
| Key Secondary End Points | ||||||
| Cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for unstable angina pectoris (4P-MACE) | 398 (13.2) | 2.3 | 401 (13.3) | 2.4 | 0.0 (−0.4 to 0.3) | 0.99 (0.86 to 1.14)a |
| Receiving treatment and maintaining HbA1c ≤7.0% at final visit [onwards from titration] without the need for rescue medication, without any moderate/severe hypoglycemic episodes, and without >2% weight gainc | 481 (16.0) | 305 (10.2) | 1.68 (1.44 to 1.96)b | |||
| Receiving treatment and maintaining HbA1c ≤7.0% at final visit [onwards from titration] without the need for rescue medication and without >2% weight gainc | 524 (17.4) | 422 (14.1) | 1.29 (1.12 to 1.48)b | |||
| Other Secondary or Tertiary Cardiovascular End Points | ||||||
| All-cause mortality | 308 (10.2) | 1.7 | 336 (11.2) | 1.8 | −0.2 (−0.4 to 0.1) | 0.91 (0.78 to 1.06)a |
| Cardiovascular mortality | 169 (5.6) | 0.9 | 168 (5.6) | 0.9 | 0.0 (−0.2 to 0.2) | 1.00 (0.81 to 1.24)a |
| Noncardiovascular mortality | 139 (4.6) | 0.8 | 168 (5.6) | 0.9 | −0.2 (−0.4 to 0.0) | 0.82 (0.66 to 1.03)a |
| Nonfatal myocardial infarction | 145 (4.8) | 0.8 | 142 (4.7) | 0.8 | 0.0 (−0.2 to 0.2) | 1.01 (0.80 to 1.28)a |
| Fatal or nonfatal myocardial infarction | 153 (5.1) | 0.9 | 148 (4.9) | 0.9 | 0.0 (−0.2 to 0.2) | 1.03 (0.82 to 1.29)a |
| Nonfatal stroke | 91 (3.0) | 0.5 | 104 (3.5) | 0.6 | −0.1 (−0.2 to 0.1) | 0.87 (0.66 to 1.15)a |
| Fatal or nonfatal stroke | 104 (3.4) | 0.6 | 120 (4.0) | 0.7 | −0.1 (−0.3 to 0.1) | 0.86 (0.66 to 1.12)a |
| Transient ischemic attack | 25 (0.8) | 0.1 | 33 (1.1) | 0.2 | 0.0 (−0.1 to 0.0) | 0.75 (0.45 to 1.26)a |
| Hospitalization for unstable angina | 60 (2.0) | 0.3 | 56 (1.9) | 0.3 | 0.0 (−0.1 to 0.1) | 1.07 (0.74 to 1.54)a |
| Coronary revascularization procedure | 202 (6.7) | 1.2 | 189 (6.3) | 1.1 | 0.1 (−0.2 to 0.3) | 1.06 (0.87 to 1.29)a |
| Hospitalization for heart failure | 112 (3.7) | 0.6 | 92 (3.1) | 0.5 | 0.1 (−0.1 to 0.3) | 1.21 (0.92 to 1.59)a |
| Investigator-reported heart failure eventse | 166 (5.5) | 1.0 | 155 (5.2) | 0.9 | 0.1 (−0.1 to 0.3) | 1.06 (0.85 to 1.32)a |
| Hospitalization for heart failure or cardiovascular death | 236 (7.8) | 1.3 | 234 (7.8) | 1.3 | 0.0 (−0.2 to 0.2) | 1.00 (0.84 to 1.20)a |
| Any adjudicated-confirmed cardiovascular eventf | 518 (17.1) | 3.1 | 535 (17.8) | 3.2 | −0.1 (−0.5 to 0.3) | 0.96 (0.85 to 1.09)a |
Figure 2. Time to Occurrence of End Points Based on Cox Regression Analyses in Patients Treated With at Least 1 Dose of the Study Drug.
A, Composite end point of cardiovascular death, first nonfatal myocardial infarction, or first nonfatal stroke (3-point major cardiovascular event [3P-MACE] outcome). Median (quartile [Q] 1, Q3) follow-up was 6.2 (5.8, 6.6) years in the linagliptin group and 6.2 (5.6, 6.5) years in the glimepiride group. The 95.47% CI for the primary end point was adjusted for multiplicity due to 2 interim analyses and change of the primary end point. B, Median (Q1, Q3) follow-up was 6.3 (5.9, 6.6) years in the linagliptin group and 6.3 (5.9, 6.6) years in the glimepiride group. C, Median (Q1, Q3) follow-up was 6.3 (5.9, 6.6) years in the linagliptin group and 6.3 (5.9, 6.6) years in the glimepiride group. D, Median (Q1, Q3) follow-up was 6.3 (5.9, 6.6) years in the linagliptin group and 6.3 (5.9, 6.6) years in the glimepiride group. 3P-MACE indicates 3-point major adverse cardiovascular event.
Key Secondary End Points
Because the result of the test for superiority was null, findings for the key secondary outcomes are presented descriptively. Post hoc analytic results can be found in eAppendix 9 and eTable 3 in Supplement 3. The secondary 4P-MACE outcome occurred in 398 of 3023 participants (13.2%) in the linagliptin group and 401 of 3010 (13.3%) in the glimepiride group (Table 2). The second key secondary end point of the proportion of patients receiving treatment and maintaining HbA1c less than or equal to7.0% at the final visit who were (following the end of titration) without the need for rescue medication, without any moderate/severe hypoglycemic episodes, and without greater than 2% weight gain occurred in 481 of 3023 participants (16.0%) in the linagliptin group and 305 of 3010 (10.2%) in the glimepiride group (Table 2; eAppendix 9 in Supplement 3). The third key secondary end point of the proportion of patients receiving treatment and maintaining HbA1c less than or equal to 7.0% at the final visit who were (following the end of titration) without the need for rescue medication and did not have greater than 2% weight gain occurred in 524 of 3023 participants (17.4%) in the linagliptin group and in 422 of 3010 (14.1%) in the glimepiride group (Table 2; eAppendix 9 Supplement 3).
Other Secondary and Tertiary Cardiovascular End Points
Death from any cause was not significantly different between participants in the linagliptin (308 of 3023 [10.2%]) and glimepiride (336 of 3010 [11.2%]) groups (HR, 0.91 [95% CI, 0.78-1.06]; Figure 2B), with an HR for cardiovascular death of 1.00 (95% CI, 0.81-1.24; Figure 2C) and an HR for noncardiovascular death of 0.82 (95% CI, 0.66-1.03; Figure 2D; eAppendix 9 in Supplement 3). The distribution of causes of noncardiovascular death in the linagliptin group (139 of 3023 participants [4.6%]) and the glimepiride group (168 of 3010 participants [5.6%]) is provided in eAppendix 10 in Supplement 3. Adjudication-confirmed hospitalizations for HF, alone or included in composite outcomes with cardiovascular mortality or investigator-reported HF events, were not significantly different between groups (Table 2; eAppendix 9 in Supplement 3).
Secondary and Tertiary Diabetes-Related and Other End Points
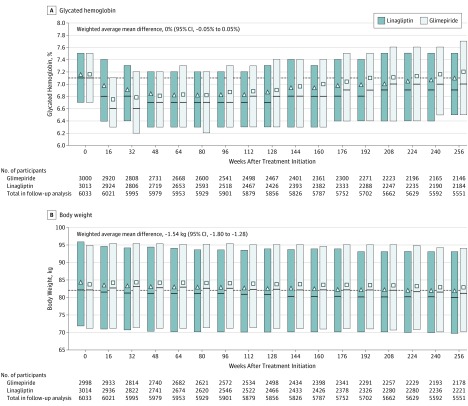
The mean (SD) dose of glimepiride over the trial duration was 2.9 (1.1) mg daily (eAppendix 11 in Supplement 3), with 49% of participants using the highest 4-mg dose at week 16 and 61% at week 256. Initially, the effect on adjusted mean change in HbA1c favored glimepiride over linagliptin, but overall there was no significant difference between the groups (weighted mean treatment difference in adjusted means until week 256, 0% [95% CI, −0.05% to 0.05%]; Figure 3A). Introduction of additional glucose-lowering therapies occurred in similar proportions across study groups, with a pattern of shorter time to introduction in the linagliptin group compared with the glimepiride group (eAppendix 12 in Supplement 3).
Figure 3. Glycated Hemoglobin (HbA1c) and Weight Over Time by Treatment Groups .
Weighted average mean difference for panels A and B based on mixed-model repeated measures, including treatment, week repeated within participants, week × treatment interaction, continuous baseline HbA1c and weight, and baseline HbA1c × week and weight × week interaction for patients who received at least 1 dose of a study drug and had a baseline and at least 1 postbaseline measurement. The squares and triangles indicate the unadjusted mean, the solid lines indicate the median (quartile [Q] 1, Q3), and the dashed lines indicate the median value at baseline. A, Median (Q1, Q3) follow-up was 6.1 (5.2, 6.4) years in the linagliptin group and 6.1 (4.8, 6.4) years in the glimepiride group. B, Median (Q1, Q3) follow-up was 6.1 (5.2, 6.5) years in the linagliptin group and 6.1 (4.9, 6.4) years in the glimepiride group.
Modest weight gain was observed in the glimepiride group early in the study and maintained thereafter, with a weighted mean between-group difference of −1.54 kg (95% CI, −1.80 to −1.28; Figure 3B). Fasting plasma glucose, blood pressure, and lipid levels over time were not significantly different between groups (eAppendix 13 and 14 in Supplement 3).
Frequencies of adverse events, serious adverse events, and adverse events leading to discontinuation of study medication were comparable between groups (Table 3). Overall, the number of participants with at least 1 hospitalization was 1245 (41.2%) in the linagliptin group and 1303 (43.3%) in the glimepiride group. There was no between-group imbalance in adjudication-confirmed pancreatitis or pancreatic cancer.
Table 3. Adverse Events of Participants in a Study of the Effect of Linagliptin vs Glimepiride on Cardiovascular Outcomes in Patients With Type 2 Diabetes.
| Adverse Eventsa | Linagliptin (n = 3023) | Glimepiride (n = 3010) | ||
|---|---|---|---|---|
| No. (%) | Rate/100 Patient-Years | No. (%) | Rate/100 Patient-Years | |
| Any adverse eventsb | 2821 (93.6) | 121.9 | 2855 (95.2) | 144.5 |
| Serious adverse events | 1403 (46.4) | 12.8 | 1448 (48.1) | 13.5 |
| Adverse events leading to study medication discontinuationb | 414 (13.7) | 2.8 | 448 (14.9) | 3.1 |
| Any hospitalization | 1245 (41.2) | 9.2 | 1303 (43.3) | 9.8 |
| Hypersensitivity reactionsc | 404 (13.4) | 3.0 | 346 (11.5) | 2.6 |
| Angioedema events with concomitant ACE inhibitor/ARB use at baselined | 42 (1.9) | 0.4 | 41 (1.9) | 0.4 |
| Pemphigoidb | 5 (0.2) | <0.1 | 0 | 0.0 |
| Skin lesionsb | 9 (0.3) | <0.1 | 4 (0.1) | <0.1 |
| Adjudication-confirmed acute pancreatitis | 15 (0.5) | 0.1 | 16e (0.5) | 0.1 |
| Adjudication-confirmed chronic pancreatitis | 3 (0.1) | <0.1 | 0 (0.0) | 0.0 |
| All cancers | 280 (9.3) | 1.6 | 303 (10.1) | 1.7 |
| Colorectal cancer | 32 (1.1) | 0.2 | 30 (1.0) | 0.2 |
| Adjudication-confirmed pancreatic cancer | 16 (0.5) | 0.1 | 24 (0.8) | 0.1 |
| Gastric cancer | 9 (0.3) | 0.1 | 5 (0.2) | <0.1 |
| Thyroid cancer | 1 (<0.1) | <0.1 | 3 (0.1) | <0.1 |
| Hypoglycemic adverse eventsb | ||||
| ≥1 Investigator-reported episode of hypoglycemia | 320 (10.6) | 2.3 | 1132 (37.7) | 11.1 |
| ≥1 Investigator-reported episode of symptomatic hypoglycemia with plasma glucose ≤70 mg/dL or severe hypoglycemia | 195 (6.5) | 1.4 | 927 (30.9) | 8.4 |
| ≥1 Investigator-reported episode of severe hypoglycemiaf | 10 (0.3) | 0.1 | 65 (2.2) | 0.5 |
| ≥1 Episode of hospitalized hypoglycemia | 2 (0.1) | <0.1 | 27 (0.9) | 0.2 |
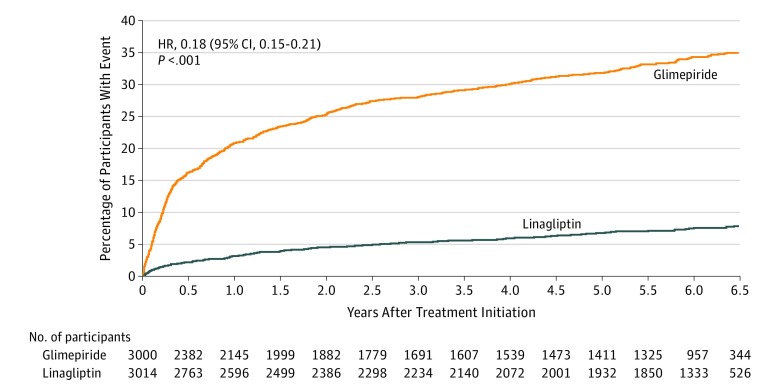
Incidence of hypoglycemic events was lower in the linagliptin group than in the glimepiride group across all predefined hypoglycemia severity categories (Table 3). Rates of investigator-reported hypoglycemia were 2.3 events per 100 participant-years in the linagliptin group and 11.1 per 100 participant-years in the glimepiride group (incidence rate difference, −8.7 [95% CI, −9.4 to −8.0]; HR, 0.23 [95% CI, 0.21-0.26]; P < .001); rates of moderate or severe hypoglycemic events were 1.4 per 100 participant-years in the linagliptin group and 8.4 per 100 participant-years in the glimepiride group (incidence rate difference, −7.0 [95% CI, −7.6 to −6.5]; HR, 0.18 [95% CI, 0.15-0.21]; P < .001; Figure 4). Rates of severe hypoglycemic events were 0.07 per 100 participant-years in the linagliptin group and 0.45 per 100 participant-years in the glimepiride group (incidence rate difference, −0.4 [95% CI, −0.5 to −0.3]; HR, 0.15 [95% CI, 0.08-0.29]; P < .001; Table 3), and hospitalization due to hypoglycemia rates were 0.01 per 100 patient-years in the linagliptin group vs 0.18 per 100 patient-years in the glimepiride group (incidence rate difference, −0.2 [95% CI, −0.2 to −0.1]; HR, 0.07 [95% CI, 0.02-0.31]; P < .001; Table 3). Hypoglycemia risk was increased across the entire dose range for the glimepiride group (eAppendix 15 in Supplement 3). A consistently lower hypoglycemia risk was observed in the linagliptin group than in the glimepiride group across all subgroups analyzed (eAppendix 16 in Supplement 3).
Figure 4. Moderate or Severe Hypoglycemia Over Time by Treatment Groups.
Median (quartile 1, quartile 3) follow-up was 5.9 (2.8, 6.5) years in the linagliptin group and 4.3 (0.8, 6.2) years in the glimepiride group. Moderate or severe hypoglycemia was defined as time to the first occurrence of symptomatic investigator-defined hypoglycemic adverse event with plasma glucose ≤70 mg/dL or a severe hypoglycemic adverse event. Analysis based on hypoglycemic adverse events occurring between first study drug intake until 7 days after receiving the study drug for the final time. Severe hypoglycemia was defined as an event requiring the assistance of another person to actively administer carbohydrate, glucagon, or other resuscitative actions. Hazard ratio (HR) for hypoglycemia derived by Cox regression model analyses in patients treated with ≥1 dose of the study drug.
Discussion
In this long-term, multicenter, double-blind, randomized, active comparator trial of individuals with relatively early type 2 diabetes at elevated cardiovascular risk, linagliptin was noninferior to glimepiride for the combined 3P-MACE end point.
Currently, 4 large cardiovascular outcome trials have established the cardiovascular safety of DPP-4 inhibitors vs placebo in patients with type 2 diabetes at a high cardiovascular risk,20,21,22,23 including the Cardiovascular and Renal Microvascular Outcome Study with Linagliptin (CARMELINA).23 In 2009, when the current trial was designed, sulfonylureas were the most commonly used second-line glucose-lowering agents after metformin, followed by DPP-4 inhibitors, but no head-to-head cardiovascular outcome trial existed for those 2 classes of medications. The current study demonstrates noninferior cardiovascular safety effects for linagliptin vs glimepiride when used predominantly as a second-line glucose-lowering treatment option after metformin.
The current study reaffirms clinical recommendations to choose an oral agent after metformin based on proven cardiovascular benefit,1,2 which none of the agents studied provide. However, when additional glucose-lowering therapy is required, a DPP-4 inhibitor, such as linagliptin, is an option with a low risk of hypoglycemia and weight gain.
Limitations
This study has several limitations. First, because the trial recruited participants with relatively early type 2 diabetes and insulin treatment was an exclusion criterion, the results may not necessarily be applicable to patients with more advanced disease. While there was no statistically significant heterogeneity in the effects on the 3P-MACE outcome in subgroups based on diabetes duration or cardiovascular risk at baseline, the study may have been underpowered to test for interactions. Second, inherent for many long-term trials is the early termination of study medication, which could have influenced the results. However, medication exposure was comparable between study groups, and annualized discontinuation rates are in line with most of the contemporary cardiovascular outcome trials of glucose-lowering therapies, all of which were of shorter duration.17,18,20,21,24 Furthermore, analyses limited to events that were occurring while patients were receiving study medication yielded results consistent with the primary analysis.
Conclusions
Among adults with relatively early type 2 diabetes and elevated cardiovascular risk, the use of linagliptin compared with glimepiride over a median of 6.3 years resulted in a noninferior risk of a composite cardiovascular outcome.
Supplement 1.
Clinical trial protocol
Supplement 2.
Statistical analysis plan
Supplement 4.
List of predefined endpoints beyond those listed in the trial statistical analysis plan
Supplement 5.
Data sharing statement
References
- 1.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018: a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669-2701. doi: 10.2337/dci18-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Das SR, Everett BM, Birtcher KK, et al. 2018 ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018;72(24):3200-3223. doi: 10.1016/j.jacc.2018.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guidelines on Second- and Third-Line Medicines and Type of Insulin for the Control of Blood Glucose Levels in Non-Pregnant Adults With Diabetes Mellitus. Geneva, Switzerland: World Health Organization; 2018 . http://apps.who.int/iris/bitstream/handle/10665/272433/9789241550284-eng.pdf?ua=1. [PubMed]
- 4.Montvida O, Shaw J, Atherton JJ, Stringer F, Paul SK. Long-term trends in antidiabetes drug usage in the U.S.: real-world evidence in patients newly diagnosed with type 2 diabetes. Diabetes Care. 2018;41(1):69-78. doi: 10.2337/dc17-1414 [DOI] [PubMed] [Google Scholar]
- 5.Foroutan N, Muratov S, Levine M. Safety and efficacy of dipeptidyl peptidase-4 inhibitors vs sulfonylurea in metformin-based combination therapy for type 2 diabetes mellitus: systematic review and meta-analysis. Clin Invest Med. 2016;39(2):E48-E62. doi: 10.25011/cim.v39i2.26481 [DOI] [PubMed] [Google Scholar]
- 6.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002-2012. doi: 10.1056/NEJMsa1103053 [DOI] [PubMed] [Google Scholar]
- 7.Heaton PC, Desai VC, Kelton CM, Rajpathak SN. Sulfonylurea use and the risk of hospital readmission in patients with type 2 diabetes. BMC Endocr Disord. 2016;16:4. doi: 10.1186/s12902-016-0084-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meinert CL, Knatterud GL, Prout TE, Klimt CR. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes: II: mortality results. Diabetes. 1970;19(suppl):789-830. [PubMed] [Google Scholar]
- 9.Bain S, Druyts E, Balijepalli C, et al. Cardiovascular events and all-cause mortality associated with sulphonylureas compared with other antihyperglycaemic drugs: a Bayesian meta-analysis of survival data. Diabetes Obes Metab. 2017;19(3):329-335. doi: 10.1111/dom.12821 [DOI] [PubMed] [Google Scholar]
- 10.Schramm TK, Gislason GH, Vaag A, et al. Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur Heart J. 2011;32(15):1900-1908. doi: 10.1093/eurheartj/ehr077 [DOI] [PubMed] [Google Scholar]
- 11.Tradjenta (linagliptin) tablets prescribing information. Boehringer Ingelheim Pharmaceuticals website. http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing+Information/PIs/Tradjenta/Tradjenta.pdf. Accessed June 25, 2019.
- 12.Marx N, Rosenstock J, Kahn SE, et al. design and baseline characteristics of the cardiovascular outcome trial of linagliptin versus glimepiride in type 2 diabetes (CAROLINA). Diab Vasc Dis Res. 2015;12(3):164-174. doi: 10.1177/1479164115570301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loganadan NK, Huri HZ, Vethakkan SR, Hussein Z. Genetic markers predicting sulphonylurea treatment outcomes in type 2 diabetes patients: current evidence and challenges for clinical implementation. Pharmacogenomics J. 2016;16(3):209-219. doi: 10.1038/tpj.2015.95 [DOI] [PubMed] [Google Scholar]
- 14.Zimdahl H, Ittrich C, Graefe-Mody U, et al. Influence of TCF7L2 gene variants on the therapeutic response to the dipeptidylpeptidase-4 inhibitor linagliptin. Diabetologia. 2014;57(9):1869-1875. doi: 10.1007/s00125-014-3276-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Department of Health and Human Services . Diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. US Food and Drug Administration website. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071627.pdf. Published December 2018. Accessed June 25, 2019.
- 16.Marx N, McGuire DK, Perkovic V, et al. Composite primary end points in cardiovascular outcomes trials involving type 2 diabetes patients: should unstable angina be included in the primary end point? Diabetes Care. 2017;40(9):1144-1151. doi: 10.2337/dc17-0068 [DOI] [PubMed] [Google Scholar]
- 17.Center for Drug Evaluation and Research . Meeting expectations to exclude a CV risk margin of 1.3. In Application number: 204042Orig1s000 summary review, page 20. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204042Orig1s000SumR.pdf. Accessed June 25, 2019.
- 18.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549-556. doi: 10.2307/2530245 [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247-254. [DOI] [PubMed] [Google Scholar]
- 20.Scirica BM, Bhatt DL, Braunwald E, et al. ; SAVOR-TIMI 53 Steering Committee and Investigators . Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317-1326. doi: 10.1056/NEJMoa1307684 [DOI] [PubMed] [Google Scholar]
- 21.White WB, Cannon CP, Heller SR, et al. ; EXAMINE Investigators . Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327-1335. doi: 10.1056/NEJMoa1305889 [DOI] [PubMed] [Google Scholar]
- 22.Green JB, Bethel MA, Armstrong PW, et al. ; TECOS Study Group . Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232-242. doi: 10.1056/NEJMoa1501352 [DOI] [PubMed] [Google Scholar]
- 23.Rosenstock J, Perkovic V, Johansen OE, et al. ; CARMELINA Investigators . Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321(1):69-79. doi: 10.1001/jama.2018.18269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGuire DK, Marx N, Johansen OE, Inzucchi SE, Rosenstock J, George JT. FDA guidance on antihyperglyacemic therapies for type 2 diabetes: one decade later. Diabetes Obes Metab. 2019;21(5):1073-1078. doi: 10.1111/dom.13645 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement 1.
Clinical trial protocol
Supplement 2.
Statistical analysis plan
Supplement 4.
List of predefined endpoints beyond those listed in the trial statistical analysis plan
Supplement 5.
Data sharing statement