Role of Tissue Plasminogen Activator Receptor LRP in Hippocampal Long-Term Potentiation (original) (raw)
Abstract
The low-density lipoprotein (LDL) receptor-related protein (LRP) is a multifunctional endocytic receptor that is expressed abundantly in neurons of the CNS. Both LRP and several of its ligands, including tissue plasminogen activator (tPA), apolipoprotein E/lipoproteins, α2-macroglobulin, and the β-amyloid precursor protein, have been implicated in various neuronal functions and in the pathogenesis of Alzheimer's disease. It has been reported that induction of tPA expression may contribute to activity-dependent synaptic plasticity in the hippocampus and cerebellum. In addition, long-term potentiation (LTP) is significantly decreased in mice lacking tPA. Here we demonstrate that tPA receptor LRP is abundantly expressed in hippocampal neurons and participates in hippocampal LTP. Perfusion of hippocampal slices with receptor-associated protein (RAP), an antagonist for ligand interactions with LRP, significantly reduced late-phase LTP (L-LTP). In addition, RAP also blocked the enhancing effect of synaptic potentiation by exogenous tPA in hippocampal slices prepared from tPA knock-out mice. Metabolic labeling and ligand binding analyses showed that both tPA and LRP are synthesized by hippocampal neurons and that LRP is the major cell surface receptor that binds tPA. Finally, we found that tPA binding to LRP in hippocampal neurons enhances the activity of cyclic AMP-dependent protein kinase, a key molecule that is known to be involved in L-LTP. Taken together, our results demonstrate that interactions between tPA and cell surface LRP are important for hippocampal L-LTP.
Keywords: LRP, tPA, LTP, PKA, Alzheimer's disease, hippocampus
The low-density lipoprotein (LDL) receptor-related protein (LRP) is a large multifunctional endocytic receptor that belongs to the LDL receptor gene family (Herz et al., 1988; Krieger and Herz, 1994; Bu, 1998). It is highly expressed in neurons of the CNS and binds and endocytoses more than 10 structurally and functionally distinct ligands, including apolipoprotein E (apoE)/lipoprotein, β-amyloid precursor protein (APP), and α2-macroglobulin (α2M) (Krieger and Herz, 1994). Recently, several lines of evidence have implicated LRP and LRP ligands in the pathogenesis of Alzheimer's disease (AD). A 39 kDa receptor-associated protein (RAP) functions as a molecular chaperone during the folding process of LRP and as a receptor antagonist to prevent premature ligand interaction with LRP during its trafficking within the secretory pathway (Bu and Schwartz, 1998). Recombinant RAP has been used extensively as an antagonist in the study of LRP biology (Bu, 1998).
One of the most significant clinical symptoms of AD is memory loss. Thus, understanding the physiological mechanism of age- and AD-related memory loss may potentially provide clues as to how this process can be intervened during aging and AD. Evi- dence from different studies demonstrates that the hippocampus and its related temporal lobe structures are important for explicit forms of memory (Squire and Zola-Morgan, 1991). Long-term potentiation (LTP) is one of the best models for investigating cellular and molecular mechanisms for memory formation and storage (Nicoll and Malenka, 1995). In the CA1 region of the hippocampus, LTP has two distinct phases: early-phase LTP (E-LTP) and late-phase LTP (L-LTP). One unique feature making L-LTP different from E-LTP is that it requires new protein synthesis, activity of cAMP-dependent protein kinase (PKA), and transcription (Frey et al., 1988, 1993, 1995; Huang and Kandel, 1994; Nguyen et al., 1994;Nguyen and Kandel, 1996; Qi et al., 1996) (for review, seeSchuman, 1997).
Tissue plasminogen activator (tPA) has been identified as one of the immediate early genes induced by neuronal activity including LTP (Qian et al., 1993). Under normal physiological conditions, tPA is widely expressed in the CNS (Qian et al., 1993; Bu et al., 1994; Seeds et al., 1995; Ware et al., 1995; Hayden and Seeds, 1996). However, the level of tPA is finely regulated by its inhibitor and cell surface receptor LRP (Bu et al., 1992a,b; Orth et al., 1992), and its synthesis is increased by activators of the cAMP-PKA pathway (Baranes et al., 1998). In the hippocampus, Qian et al. (1993) found that neuronal activity could induce the expression of mRNA of tPA in pyramidal neurons. More interestingly, L-LTP but not E-LTP is significantly decreased in mice lacking the gene encoding tPA (Carmeliet et al., 1994; Frey et al., 1996; Huang et al., 1996). These findings indicate that tPA plays an important role in L-LTP in the hippocampus. However, whether interaction between tPA and its cellular receptor LRP participates in L-LTP is unknown. Here, we present several lines of evidence indicating that interaction between tPA and LRP is important for hippocampal L-LTP.
MATERIALS AND METHODS
Materials. Human recombinant tPA was kindly provided by Genentech (South San Francisco, CA). tPA was initially dissolved in arginine phosphate buffer and dialyzed against PBS before use (Bu et al., 1992a). Recombinant rat RAP was produced as fusion protein with glutathione _S_-transferase (GST). The GST portion was cleaved off, and RAP was repurified via heparin-Sepharose column (Warshawsky et al., 1993). Polyclonal anti-human LRP [cross-reacts with mouse LRP; see Holtzman et al. (1995)], anti-rat RAP, and anti-human tPA [cross-reacts with mouse tPA; see Bu et al. (1992a)] antibodies have been described previously (Bu et al., 1992a,b, 1993, 1994, 1995;Holtzman et al., 1995).
Hippocampal slice preparation. For LTP recordings, transverse slices of hippocampus (400 μm thick) were prepared from wild-type (C57BL/6J, 8- to 12-weeks-old; The Jackson Laboratory, Bar Harbor, ME) or tPA knock-out mice (C57BL/6J, 8–12 weeks old; The Jackson Laboratory). The slices were maintained between 28°C and 30°C in a chamber where they were subfused with artificial CSF (ACSF) consisting of (in mm): 124 NaCl, 4.0 KCl, 2.0 CaCl2, 1.0 MgSO4, 1.0 Na2HPO4, 24.1 NaHCO3, 10 glucose, bubbled with 95% O2, 5% CO2. Slices were allowed to recover for at least 2 hr before experiments.
Electrophysiology. A bipolar tungsten stimulating electrode was placed to evoke postsynaptic responses. Extracellular field potentials were recorded with a glass microelectrode (3–12 MΩ, filled with ACSF). In some experiments, a second stimulating electrode was placed to serve as an independent control response in the same slice. The postsynaptic EPSPs were evoked at 0.02 Hz with a bipolar tungsten electrode placed at the CA3 region. The intensity of stimulation was decreased to evoke ∼1 mV EPSP. Paired-pulse facilitation was tested with different time intervals. The initial magnitude of the first EPSP was adjusted to 1.0 mV by changing the intensity of electrical stimulation. E-LTP was induced by one train tetanus consisting of one 100 Hz train for 1 sec at testing intensity (the intensity used for evoke baseline EPSP responses). Four-train tetanic stimulation, consisting of four 100 Hz trains for 1 sec at testing intensity with a 5 min interval between trains, was used to induce L-LTP. Field recordings of NMDA receptor-mediated EPSPs were performed in the presence of 10 μm CNQX and 100 μm Mg2+. Data are presented as a mean value ± SEM or percentage changes from control. Statistical comparisons are made with the use of either two-way or one-way ANOVAs (Newman–Keuls test for post hoc comparison). Student's test was applied for comparisons between paired groups. In all cases, p < 0.05 is considered significant.
Ligand binding assay. Freshly prepared mouse hippocampal slices (see above) were used for binding analysis of125I-tPA (5 nm) in the absence or presence of unlabeled tPA (500 nm) or RAP (500 nm) (Bu et al., 1992a, 1994). After 2 hr incubation at 4°C, slices were washed three times with cold buffer, and radioactivity associated with each slice was determined. The slices were then lysed with PBS containing 1% Triton X-100 for 1 hr at 4°C, and the protein concentration was determined. Each experiment was performed in triplicate with SEM given as error bars.
Primary hippocampal neuron cultures. Primary neurons from embryonic day 17–19 (E17–19) Swiss-Webster mouse embryos were prepared using the method described previously (Narita et al., 1997). In brief, hippocampal neurons were mechanically dissociated after treatment with trypsin (0.5 mg/ml, 15 min, 37°C). Cells were then plated onto poly-d-lysine (100 mg/ml)-coated six-well plates at a density of ∼200 neurons per millimeters squared and maintained in DMEM medium containing 5% horse serum, 5% fetal bovine serum, 2 mm glutamine, at 37°C in a humidified 5% CO2 incubator and used 24 hr after plating.
Ligand endocytosis assay. Hippocampal neurons were prepared and plated in 24-well plates. Binding of125I-tPA (5 nm) in the absence or presence of unlabeled RAP (500 nm) was performed at 4°C for 2 hr as described previously (Bu et al., 1992a, 1994). After washing at 4°C, some plates were warmed up to 37°C for the indicated time before returning to 4°C. RAP (500 nm) was included in the warm-up media to prevent rebinding of dissociated ligand. Cell monolayers were then treated with 0.25% (w/v) Pronase (Calbiochem, La Jolla, CA) in PBS for 30 min at 4°C to remove the remaining cell surface-bound ligands (Bu et al., 1992a). The solution that contained detached cells was then centrifuged at 4°C to pellet cells. Radioactivity associated with cell pellets represents internalized ligand, whereas radioactivity in the supernatant fraction represents surface-bound ligand.
Metabolic labeling and immunoprecipitation. Neurons were metabolically labeled with 200 mCi/ml [35S]methionine for 4 hr (Bu et al., 1992a). Both overlying media and cell lysates were then immunoprecipitated with either anti-tPA or anti-LRP antibody and analyzed via SDS-PAGE (7.5% acrylamide). Coimmunoprecipitation experiments of RAP and tPA were performed in the absence of detergent as described previously (Bu et al., 1995).
PKA assay. PKA activity was determined by using the PKA assay system from Life Technologies/BRL (Gaithersburg, MD). Hippocampal neurons were incubated with serum-free media without or with tPA (50 nm), or with tPA (50 nm) and RAP (500 nm) for 20 min at 37°C. After washing two times with ice-cold PBS, cell monolayers were scraped and homogenized in extraction buffer containing 1 mm3-isobutyl-1-methylxanthine and protease inhibitor cocktail (Complete, Boehringer Mannheim, Indianapolis, IN) (Goretzki and Mueller, 1998). After removal of cell debris via centrifugation, PKA activity was determined according to the manufacturer's instructions.
RESULTS
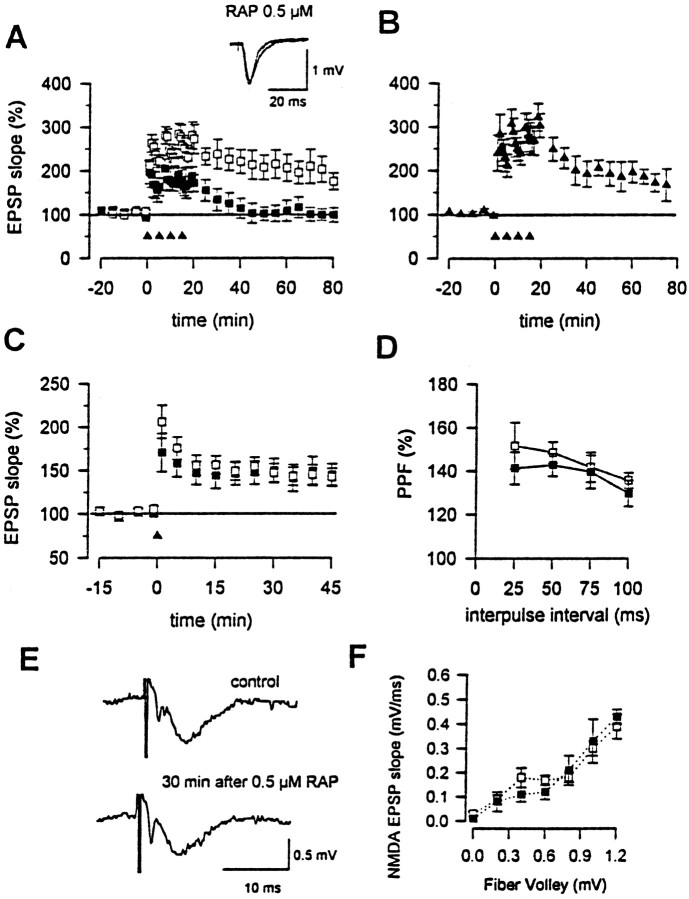
It has been reported that induction of tPA expression may contribute to activity-dependent synaptic plasticity in the hippocampus and cerebellum (Qian et al., 1993; Seeds et al., 1995). In addition, L-LTP is significantly decreased in mice lacking tPA (Huang et al., 1996). Although the mechanism of tPA's involvement in L-LTP is unknown, the importance of its interaction with cell surface receptors has been suspected. To test whether the interaction of tPA with its endocytic receptor LRP participates in L-LTP, we examined the potential effects of the LRP antagonist RAP on L-LTP. Hippocampal slices from mice were perfused with either buffer alone or buffer containing RAP, followed by induction of L-LTP. We found that application of RAP resulted in an inhibition of synaptic potentiation induced by a four-train tetanic stimulation (n = 7) (Fig.1A). The effect of RAP is dose-related, because perfusion with a lower concentration of RAP produced a smaller inhibition (n = 5) (Fig.1B). RAP itself did not significantly affect baseline EPSPs (n = 5) or potentiate EPSPs applied at 10 min after the delivery of four-train stimulation (n = 3). In some experiments, a second electrode was placed at the other side of the first electrode, and synaptic responses were tested before and 1 hr after the four-train stimulation. Synaptic responses at this second independent pathway were not significantly affected (_n_= 3).
Fig. 1.
LRP is important for hippocampal L-LTP.A, L-LTP is abolished by RAP pretreatment (0.5 μm, closed squares). L-LTP recorded with buffer alone is shown with open squares. Four-train stimulation is marked with arrowheads.Inset, Traces of EPSPs indicating that 40 min after the induction of L-LTP, hippocampal potentials with RAP treatment have returned to those of baseline. B, RAP at a lower concentration (50 nm) produces a smaller inhibition.C, RAP (0.5 μm, closed squares) does not affect E-LTP induced by one-train stimulation. E-LTP recorded with buffer alone is shown with open squares. D, Synaptic responses to a paired-pulse stimulation at different intervals (25, 50, 75, and 100 msec) are not affected by RAP (0.5 μm, closed squares).Open squares are recordings with buffer alone.E, RAP does not affect NMDA receptor-mediated EPSPs.F, The input-output curves of NMDA receptor-mediated EPSPs in control medium (open squares) and medium containing RAP (0.5 μm, closed squares).
In contrast to L-LTP, potentiation induced by one-train stimulation was not significantly affected by RAP perfusion (n = 5) (Fig. 1C), indicating that LRP is not required for E-LTP. In addition, neither presynaptic fiber volley (Fig. 1E) nor the paired-pulse facilitation was affected by RAP treatment (Fig.1D). Finally, NMDA receptor-mediated responses were not significantly affected by RAP (Fig.1E,F), indicating that RAP did not produce its effect through inhibition of NMDA receptors. The small changes of the fiber volley of responses in the recordings shown are mostly likely attributable to population responses recorded, because in these experiments the NMDA receptor-mediated currents were not affected, and the stimulation intensities were not changed over the entire recording period.
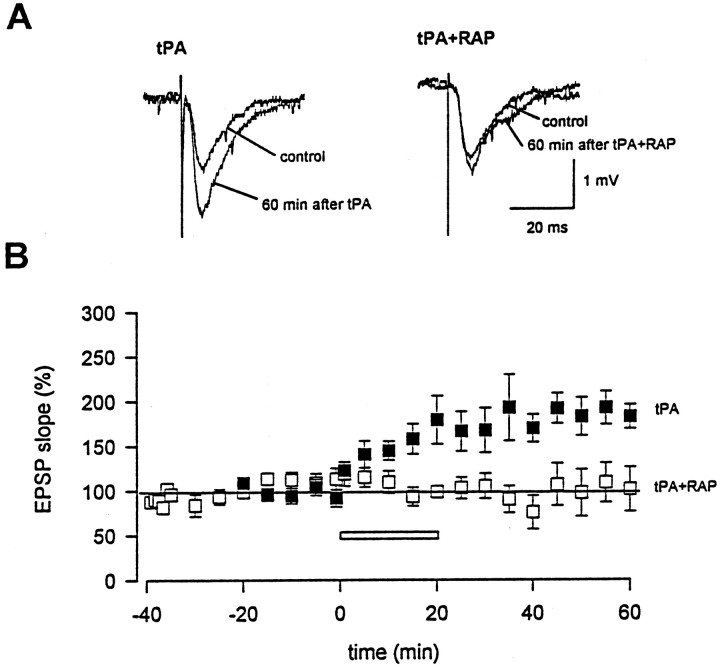
The inhibition of L-LTP by RAP mimics the abnormalities found in hippocampal slices of tPA knock-out mice (Huang et al., 1996), except that a complete disappearance of L-LTP in tPA knock-out mice requires 2 hr, whereas inhibition by RAP is seen within 40 min from the start of induction. Thus, although other ligands of LRP may also participate in L-LTP, we suspected that tPA was likely the major ligand whose interaction with LRP contributed to L-LTP. To directly assess whether interaction between tPA and LRP is important for L-LTP, we tested whether exogenous tPA can enhance synaptic potentiation in hippocampal slices of tPA knock-out mice and whether RAP could block this effect. Consistent with a previous report (Huang et al., 1996), synaptic potentiation induced by multiple tetanic stimulation was significantly decreased in tPA knock-out mice (n = 3). Application of tPA induced concentration-dependent potentiation. At a concentration of 10 nm, tPA produced a slight increase in potentiation, whereas at 50 nm, exogenous tPA induced significant potentiation (n = 6) (Fig.2). To examine whether the enhancement by tPA is mediated via its receptor LRP, we tested the effect of RAP. As seen in Figure 2, tPA-induced potentiation was completely blocked by pretreatment of slices with RAP (0.5 μm) for 30 min (n = 6). These results strongly suggest that tPA participates in L-LTP through its interaction with cell surface LRP. We also examined the potential effect of tPA on NMDA receptors. Application of tPA for an identical time period did not significantly affect NMDA receptor-mediated EPSCs (n = 3) (data not shown).
Fig. 2.
tPA-induced synaptic potentiation is blocked by RAP in tPA knock-out mice. A, Traces of EPSPs before and 60 min after 50 nm tPA application in the absence (left) or presence of 0.5 μm RAP (right). B, RAP pretreatment (0.5 μm, open squares) blocked synaptic potentiation induced by tPA. EPSPs in the absence of RAP pretreatment are indicated by filled squares. Application of 50 nm tPA is indicated by a bar.
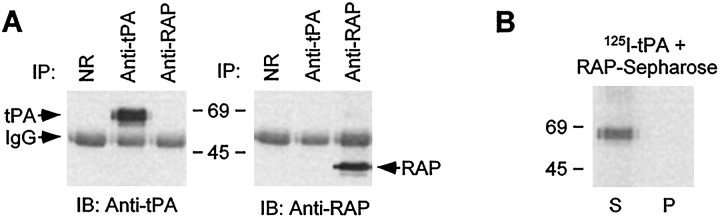
To exclude the possibility that RAP interacts directly with tPA and interferes with tPA function, we examined whether the two proteins interact with one another. First, we incubated RAP and tPA under conditions similar to those used in the electrophysiological experiments and immunoprecipitated the mixture with normal rabbit IgG, anti-tPA IgG, or anti-RAP IgG. The immunoprecipitated materials were then Western-blotted with either anti-tPA IgG or anti-RAP IgG (Fig.3A). As seen in the figure, anti-tPA antibody did not coimmunoprecipitate RAP in a mixture of tPA and RAP. Similarly anti-RAP antibody did not coimmunoprecipitate tPA. In the second experiment, we examined the ability of RAP-Sepharose to bind 125I-tPA. We have used this RAP-Sepharose previously to precipitate LRP and purify specific anti-RAP IgG (Bu et al., 1995). The result shows that RAP-Sepharose does not bind 125I-tPA (Fig.3B). These results clearly show that RAP does not interact with tPA. Thus, the results we obtained with RAP perfusion in our electrophysiological experiments are consistent with RAP antagonizing tPA's interaction with LRP.
Fig. 3.
RAP does not interact directly with tPA.A, RAP (1 μg) and tPA (1 μg) were incubated in ACSF for 1 hr at 30°C. The incubation mixture was then divided and incubated with normal rabbit IgG (NR), anti-tPA IgG, or anti-RAP IgG, followed with immunoprecipitation (IP) without detergent. The immunoprecipitated materials were then immunoblotted (IB) with either anti-tPA IgG or anti-RAP IgG. B, RAP-Sepharose was incubated with125I-tPA (5 nm) in ACSF for 1 hr at 30°C. After washing, both supernatant (S) and pellet (P) were analyzed via SDS-PAGE.
Both LRP and a mannose receptor have been shown to bind tPA (Otter et al., 1991; Bu et al., 1992b). In addition, several studies have described various tPA binding receptors on the cell surface of both endothelial and neuronal cells (Pittman et al., 1989; Verrall and Seeds, 1989; Hajjar et al., 1994; Fukao and Matsuo, 1998). However, only binding to LRP is inhibited by RAP (Bu et al., 1998). To determine which of these receptors plays a role in tPA binding to hippocampal neurons, we performed binding analysis of125I-tPA to hippocampal slices. The binding experiments were performed in the absence or presence of excess unlabeled tPA or RAP. As seen in Figure4A, RAP inhibited125I-tPA binding to hippocampal slices to the same extent as unlabeled tPA, suggesting that specific binding of tPA to hippocampal slices is mediated primarily via LRP.
Fig. 4.
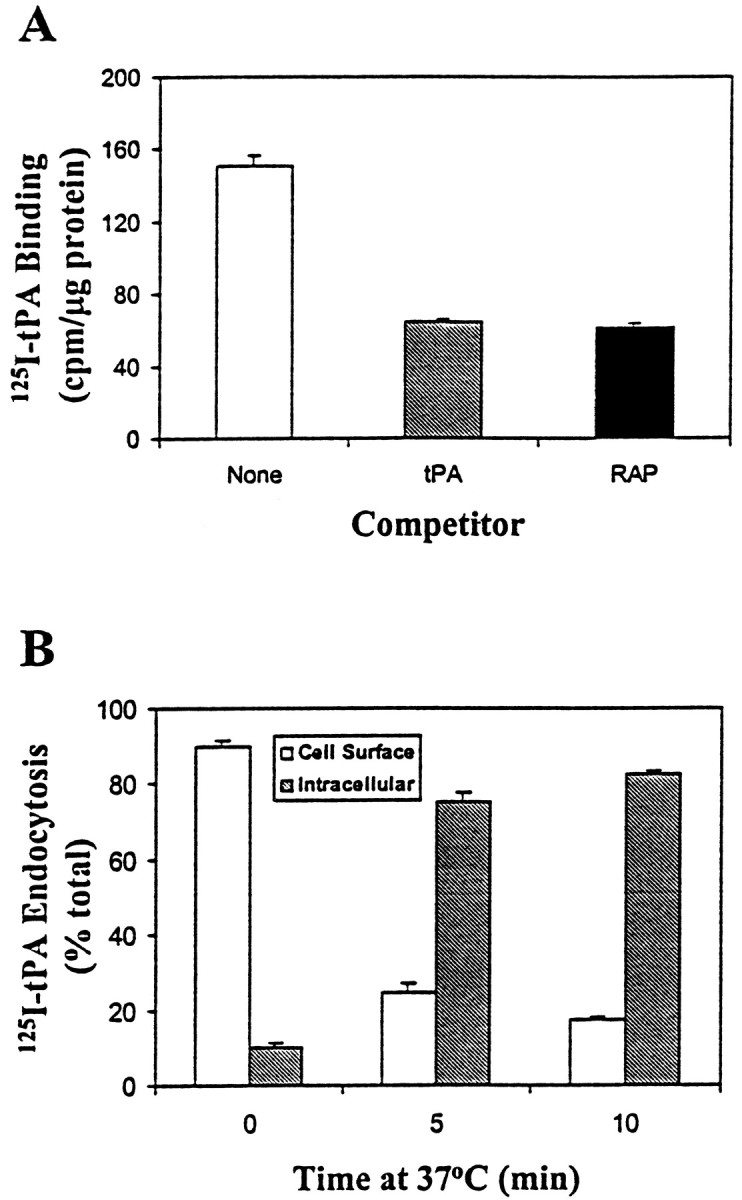
LRP is the major endocytic receptor for tPA expressed in hippocampal neurons. A, Fresh hippocampal slices are prepared and used for binding analysis of125I-tPA (5 nm) in the absence or presence of unlabeled tPA (500 nm) or RAP (500 nm). The experiment was performed in triplicate, with error bars representing SEM. RAP inhibits 125I-tPA binding to hippocampal slices to the same extent as unlabeled tPA, suggesting that binding of tPA to hippocampal slices is mediated entirely via LRP.B, Hippocampal neurons were prepared and plated in 24-well plates. Binding of 125I-tPA (5 nm) in the absence or presence of unlabeled RAP (500 nm) was performed at 4°C for 2 hr. Some plates were then warmed up to 37°C for either 5 or 10 min before returning to 4°C. Cell monolayers were then treated with 0.25% (w/v) Pronase for 30 min at 4°C to remove remaining cell surface-bound ligands. Radioactivity associated with cell pellets represents internalized ligand, whereas radioactivity in the supernatant fraction represents surface-bound ligand. Data represent LRP-mediated endocytosis after subtraction of those in the presence of excess RAP. The experiment was performed in triplicate, error bars representing SEM.
To examine whether LRP serves as an tPA endocytic receptor in neuronal cells similar to its role in hepatoma cells, we analyzed the kinetics of LRP-mediated tPA binding and uptake with primary cultured hippocampal neurons. First, we performed a saturation binding analysis of 125I-tPA on primary cultured neurons and found that there were ∼52,000 tPA-binding sites/neuron (data not shown) (Bu et al., 1992a, 1993). We then examined the kinetics of125I-tPA uptake by hippocampal neurons.125I-tPA (5 nm) was first allowed to bind to neurons at 4°C in the absence or presence of excess RAP. Some plates were then warmed up to 37°C for either 5 or 10 min, followed by analysis of cell surface and intracellular ligand distribution (Bu et al., 1992a, 1993). As shown in Figure4B, nearly 80% of cell surface-bound tPA had been endocytosed after 5 min of 37°C incubation. These kinetics of tPA internalization by LRP in neurons are similar to what we observed with hepatoma cells (Bu et al., 1992a, 1993) and suggest that LRP functions as an endocytic receptor for tPA in neurons.
To further examine the potential function of tPA and LRP, we analyzed the expression of tPA and LRP in hippocampal neurons. Primary cultured hippocampal neurons from E17 mouse embryos were metabolically labeled with [35S]cysteine for 4 hr. Both cell lysates and overlying media were then immunoprecipitated with either anti-tPA antibody or anti-LRP antibody, followed by analysis via SDS-PAGE. As seen in Figure5A, both tPA and LRP are detected in cell lysates, demonstrating that mouse hippocampal neurons synthesize both tPA and its receptor LRP. The immunoprecipitation condition (including 1% SDS) does not preserve any potential interaction between endogenous tPA and LRP, and thus potential interaction between these two proteins is not detected in this experiment. tPA is also detected in the overlying media, suggesting that tPA is actively secreted by these neurons and could potentially function extracellularly. We also detected complexes of tPA with its physiological inhibitor plasminogen activator inhibitor type-1 (PAI-1), which can either be produced by hippocampal neurons or be present as residual protein from serum used to culture neuronal cells. The abundant expression of LRP seen in these experiments is consistent with our previous studies on the distribution of LRP in the hippocampus, which showed strong immunoreactivity of LRP in hippocampal neurons and their processes (Holtzman and Fagan, 1998).
Fig. 5.
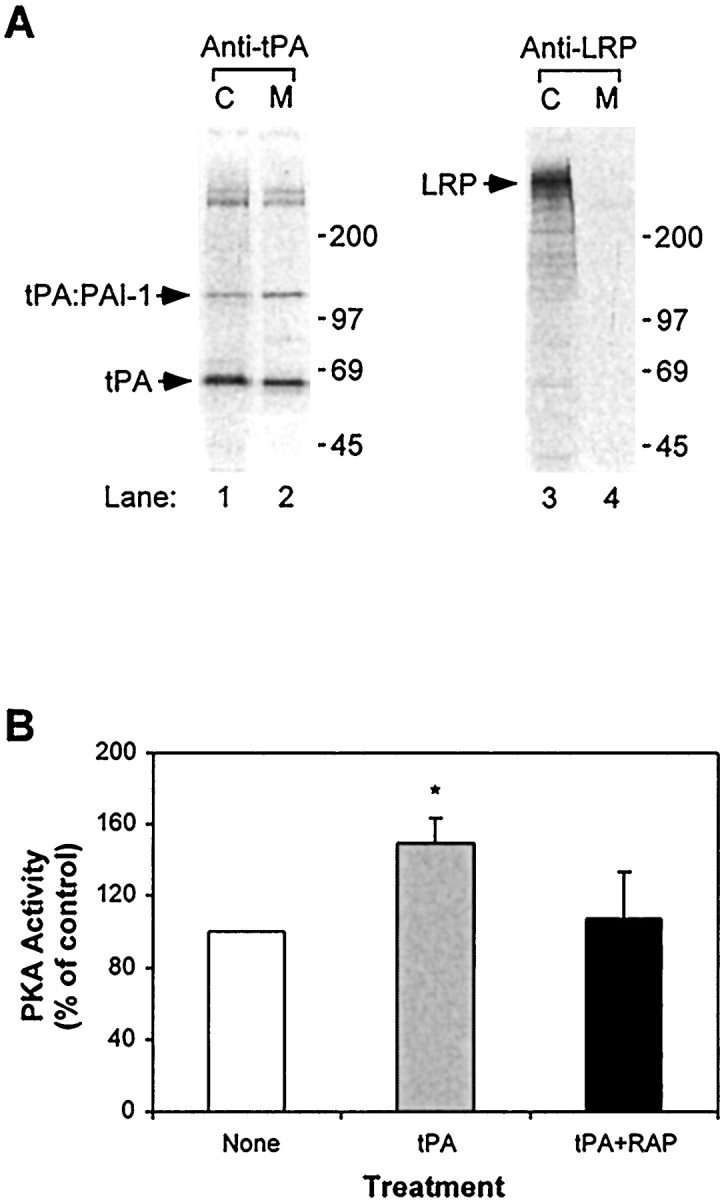
tPA and LRP expression in hippocampal neurons and activation of PKA. A, Primary cultured hippocampal neurons from E17 mouse embryos were metabolically labeled with [35S]cysteine for 4 hr. Both cell lysates (C) and overlying media (M) were immunoprecipitated with either anti-tPA antibody or anti-LRP antibody, followed by analysis via SDS-PAGE (7.5% acrylamide) and autoradiography. LRP is abundantly expressed in these neurons, whereas tPA is synthesized and secreted by these neurons. A band corresponding to the migration of tPA/PAI-1 complexes is also detected. The molecular size markers are given in kilodaltons. B, Binding of tPA to LRP enhances PKA activity. Primary hippocampal neurons were incubated without or with tPA (50 nm) or tPA (50 nm) and RAP (500 nm) at 37°C for 20 min. Cell lysates were then assayed for PKA activity. Results are averages of triplicate determinations, error bars representing SEM. *p < 0.05 compared with no treatment control.
Several studies have shown that cAMP and PKA play important roles in L-LTP (Qi et al., 1996; Abel et al., 1997). A recent study by Goretzki and Mueller (1998) has shown that binding of LRP with its ligand lactoferrin or uPA/PAI-1 complex in a human melanoma cell line caused a significant elevation of cAMP and PKA activity. In addition, tPA was found to induce endothelial cell proliferation by activating PKA (Welling et al., 1996). Thus, we examined whether tPA binding to LRP in hippocampal neurons also activates the cAMP/PKA pathway. As shown in Figure 5B, PKA activity was significantly increased when hippocampal neurons were incubated with exogenous tPA (50 nm). In addition, the activation of PKA activity by tPA was attenuated in the presence of excess RAP. These results suggest that the cAMP/PKA pathway is at least one of the intracellular events activated by tPA-LRP interactions.
DISCUSSION
In the current study, we present evidence showing that interaction of tPA with its cell surface receptor LRP appears to play an important role in hippocampal L-LTP. Perfusion of hippocampal slices with LRP antagonist RAP significantly and rapidly abolished hippocampal L-LTP. In addition, RAP also abolished the enhancing effects of exogenous tPA on synaptic transmission in hippocampal slices of tPA knock-out mice. We also present evidence that LRP is abundantly expressed in hippocampal neurons and is the major tPA receptor present on the cell surface. Finally, we demonstrated that tPA binding to LRP in hippocampal neurons increases PKA activity, an intracellular signaling pathway that is known to be involved in L-LTP.
tPA is a serine protease that catalyzes the conversion of plasminogen to plasmin and degrades certain components of extracellular matrix (Lijnen and Collen, 1991). tPA activity is regulated via both inactivation by its inhibitor PAI-1 and cellular clearance by cell surface LRP. A recent study has suggested that tPA inhibitors can block the function of tPA in L-LTP (Baranes et al., 1998). However, our current studies demonstrated that binding of tPA to its cell surface receptor LRP plays an important role in L-LTP. Thus, it is possible that L-LTP is maintained by both intracellular signaling events (e.g., Ca2+ influx, PKA activation, etc.) and extracellular structural changes (e.g., synaptic growth, synaptic connections). The former events (intracellular signaling) may be triggered by tPA binding to LRP, whereas tPA's protease activity may contribute to structural changes that associate with L-LTP and learning and memory (Baranes et al., 1998). Because binding of tPA to LRP does not require its protease activity (Bu et al., 1992a,b; Orth et al., 1994), it is possible that the receptor binding function and the protease activity of tPA have distinct roles in neuronal plasticity and other neuronal activity. In support of this hypothesis, a recent study has demonstrated a nonproteolytic function by tPA in neuroprotection (Kim et al., 1999). Alternatively, inhibitors of tPA protease activity such as PAI-1 may alter the way tPA interacts with LRP, which in turn blocks the signaling events induced by interaction of LRP with native tPA. Toward this possibility, it is interesting to note that studies byWillnow et al. (1994) and Orth et al. (1994) have shown that tPA and tPA/PAI-1 complexes bind to overlapping but not identical sites within the second domain of LRP.
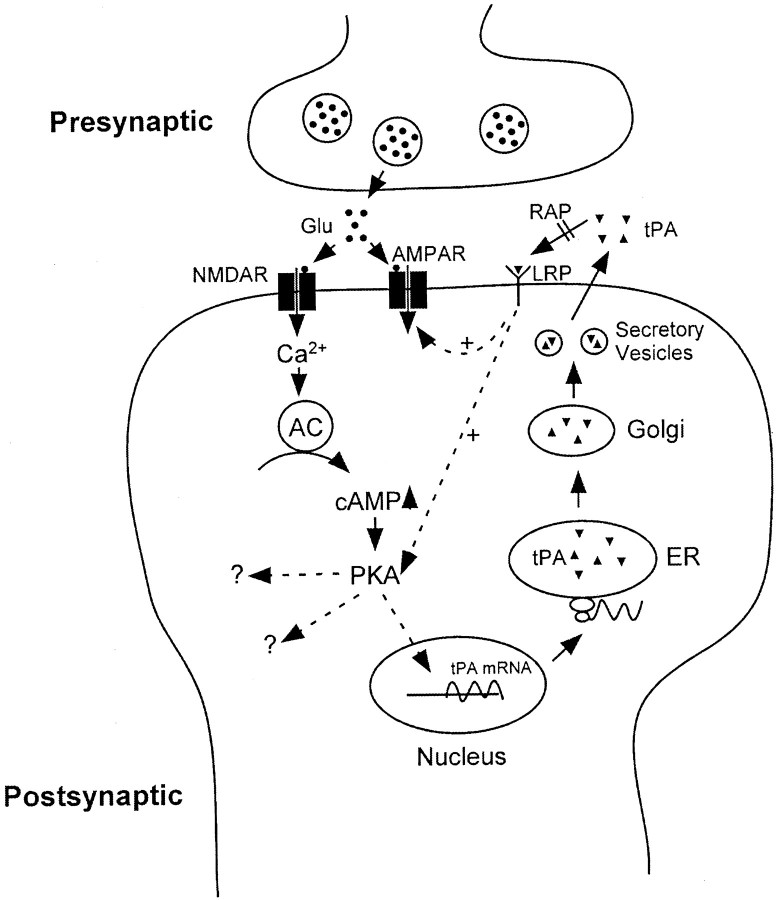
Several previous studies have suggested the presence of tPA binding protein on the cell surface that might serve as a docking receptor for tPA (Pittman et al., 1989; Verrall and Seeds, 1989; Hajjar et al., 1994; Fukao and Matsuo, 1998). However, our current study suggests that tPA is rapidly endocytosed by LRP after its binding to the receptor, suggesting that LRP likely functions as an endocytosis/signaling receptor as opposed to a docking receptor. This function of LRP is similar to those of neurotrophin receptors, which on binding of neurotrophin trigger both endocytosis and signal transduction. In the case of NGF binding to its receptor trkA, it is interesting to note that studies by Grimes et al. (1996) have shown that trkA continues to signal after endocytosis via “signaling endosomes,” as a means of retrograde signaling from distal axons to the neuronal cell body. Thus, the major events occurring after tPA interaction with LRP on neurons may include both endocytosis and signal transduction. The later event may link to intracellular signaling pathways involved in L-LTP. Taken together, we propose the following model regarding how the tPA/LRP pathway might participate in L-LTP in the CA1 region of the hippocampus (Fig.6).
Fig. 6.
Schematic model of tPA/LTP pathway in L-LTP. See Discussion.
During the induction of LTP, postsynaptic depolarization induced by tetanic stimulation removes Mg2+blockade of NMDA receptors at postsynaptic cells. Ca2+ influx through the NMDA receptor channels activates a series of postsynaptic signaling pathways, including action on adenylyl cyclase and production of a second messenger cAMP. cAMP then activates PKA, which in turn activates various intracellular pathways and immediate early genes, including that of tPA. As a result, the synthesis of tPA in the ER is increased. After trafficking through the secretory pathway, tPA is secreted into extracellular space where it either functions in extracellular matrix as a protease in synaptic plasticity (Baranes et al., 1998) or binds to its cell surface receptor LRP (which can be antagonized by RAP) and activates cellular processes that enhance synaptic potentiation, as demonstrated in the current study. The later process may be a result of intracellular signaling events (e.g., further activation of PKA) on tPA-LRP interaction and/or regulation of the AMPA receptor. Potential signaling functions of LRP have been suggested from various observations. First, several studies have shown that apoE3, a ligand of LRP, increases neurite extension via LRP (Bellosta et al., 1995;Holtzman et al., 1995; Narita et al., 1997). Second, our previous studies have shown that negative feedback regulation of tPA gene expression in colon fibroblasts is mediated via tPA interaction with cell surface LRP (Hardy et al., 1997). Third, recent studies have shown that the LRP tail interacts with a heterotrimeric GTP-binding protein (Goretzki and Mueller, 1998) and two cytoplasmic adaptor proteins, FE65 and mammalian Disabled (Trommsdorff et al., 1998).
Gene-disruption studies by Herz et al. (1992) have concluded that LRP is essential for early mouse embryonic development. Embryos homozygous for LRP deficiency do not survive long enough to allow examination of LRP's role during neuronal development, although some developmental neuronal delay was observed in homozygous LRP knock-out embryos (Herz et al., 1993). Recent studies have shown that functional cell surface LRP is required for normal hippocampal neuronal process development and growth in vitro (Narita et al., 1997). In addition, a recent study using a genetic approach demonstrated that two members of the LDL receptor family, the very low density lipoprotein receptor and apoE receptor 2, play important roles in neuronal migration and hippocampal development (Trommsdorff et al., 1999). Thus, it is possible that tPA-LRP interaction is essential during neuronal development, similar to its role in L-LTP as described in the current study.
Additionally, genetic and other evidence suggest that at least three LRP ligands—apoE/lipoprotein, APP, and α2M—are likely to play important roles in the pathogenesis of AD. First, LRP is the major neuronal receptor for apoE/lipoproteins (Bellosta et al., 1995; Holtzman et al., 1995; Fagan et al., 1996), and the ε4 allele of apoE is a strong genetic risk factor for AD (Corder et al., 1993; Strittmatter et al., 1993). Second, immunoreactivity for several LRP ligands (e.g., apoE, α2M, and tissue factor pathway inhibitor), as well as LRP itself, has been found in senile plaques (Abraham et al., 1988; Rebeck et al., 1995; Hollister et al., 1996). Third, LRP has been shown to mediate the endocytosis of both secreted and transmembrane forms of APP (Kounnas et al., 1995; Knauer et al., 1996), suggesting that LRP may play a role in the metabolism of APP and in some way modify the generation of amyloid peptides. Fourth, several recent studies (Kang et al., 1997; Lendon et al., 1997; Hollenbach et al., 1998) have reported a possible association between polymorphisms within the LRP gene and AD. Finally, studies by Blacker et al. (1998) and Liao et al. (1998) identified a genetic association between polymorphisms within α2M and risk of AD. In sum, it is possible that regulation of LRP expression in CNS neurons can directly impact the catabolism and functions of its ligands, such that decreased uptake and signaling or increased extracellular accumulation of these ligands, or both, lead to the neuropathological changes associated with AD. These results indicate that LRP and its interactions with some of its ligands may play an important role in neuronal function and in the pathogenesis of neurological diseases. Disruption of these interactions may lead to neuronal impairment, including some of those seen in AD. Our current results provide the first evidence that an interaction between tPA and LRP plays an important role in L-LTP, a form of synaptic plasticity that is believed to contribute to learning and memory (Hawkins et al., 1993; Moser et al., 1998). Our biochemical and cellular analyses as well as previous work (Qian et al., 1993; Seeds et al., 1995; Huang et al., 1996) confirm the presence of these molecular components within hippocampal neurons, indicating that these molecules are likely to participate in synaptic potentiation in vivo.
Although our analyses with apoE–knock-out mice did not show any abnormality in L-LTP (data not shown), it is interesting to note that mice expressing the C terminus of APP (Nalbantoglu et al., 1997) or APP695SWE (Chapman et al., 1999) had impaired learning and decreased LTP. In addition, a recent study has found impaired synaptic transmission in the hippocampus of mice overexpressing a mutant form of APP (717V-F), which preceded amyloid deposition by several months (Hsia et al., 1999). These results together with our present study suggest that cellular components (e.g., LRP, tPA, APP) that are important for synaptic transmission could be disrupted independent of amyloid plaque formation during aging and AD.
Footnotes
This work was supported by National Institutes of Health (NIH) Grant NS37525 and a Faculty Scholar Award from Alzheimer's Association to G.B., NIH Grant AG13956 to D.H., and a grant from the Alzheimer's Disease Research Center at Washington University School of Medicine to M.Z. We thank Masaaki Narita for preparation of primary hippocampal neurons and Alan Schwartz for critical reading and comments on this manuscript.
Correspondence should be addressed to Dr. Guojun Bu, Department of Pediatrics, Washington University School of Medicine, CB 8116, One Children's Place, St. Louis, MO 63110. E-mail:bu@kids.wustl.edu.
REFERENCES
- 1.Abel T, Nguyen PV, Barad M, Deuel TAS, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- 2.Abraham CR, Selkoe DJ, Potter H. Immunochemical identification of the serine protease inhibitor alpha 1-antichymotrypsin in the brain amyloid deposits of Alzheimer's disease. Cell. 1988;52:487–501. doi: 10.1016/0092-8674(88)90462-x. [DOI] [PubMed] [Google Scholar]
- 3.Baranes D, Lederfein D, Huang Y-Y, Chen M, Baily CH, Kandel ER. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron. 1998;21:813–825. doi: 10.1016/s0896-6273(00)80597-8. [DOI] [PubMed] [Google Scholar]
- 4.Bellosta S, Nathan BP, Orth M, Dong LM, Mahley RW, Pitas RE. Stable expression and secretion of apolipoproteins E3 and E4 in mouse neuroblastoma cells produces differential effects on neurite outgrowth. J Biol Chem. 1995;270:27063–27071. doi: 10.1074/jbc.270.45.27063. [DOI] [PubMed] [Google Scholar]
- 5.Blacker D, Wilcox MA, Laird NM, Rodes L, Horvath SM, Go RCP, Perry R, Watson B, Bassett SS, McInnis MG, Albert MS, Hyman BT, Tanzi RE. Alpha-2 macroglobulin is genetically associated with Alzheimer's disease. Nat Genet. 1998;19:357–360. doi: 10.1038/1243. [DOI] [PubMed] [Google Scholar]
- 6.Bu G. Receptor-associated protein: a specialized chaperone and antagonist for members of the LDL receptor gene family. Curr Opin Lipidol. 1998;9:149–155. doi: 10.1097/00041433-199804000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Bu G, Schwartz AL. RAP, a novel type of ER chaperone. Trends Cell Biol. 1998;8:272–276. doi: 10.1016/s0962-8924(98)01283-5. [DOI] [PubMed] [Google Scholar]
- 8.Bu G, Morton PA, Schwartz AL. Identification and partial characterization by chemical cross-linking of a binding protein for tissue-type plasminogen activator (t-PA) on rat hepatoma cells. A plasminogen activator inhibitor type 1-independent t-PA receptor. J Biol Chem. 1992a;267:15595–15602. [PubMed] [Google Scholar]
- 9.Bu G, Williams S, Strickland DK, Schwartz AL. Low density lipoprotein receptor-related protein/α2- macroglobulin receptor is an hepatic receptor for tissue-type plasminogen activator. Proc Natl Acad Sci USA. 1992b;89:7427–7431. doi: 10.1073/pnas.89.16.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bu G, Maksymovitch EA, Schwartz AL. Receptor-mediated endocytosis of tissue-type plasminogen activator by low density lipoprotein receptor-related protein on human hepatoma HepG2 cells. J Biol Chem. 1993;268:13002–13009. [PubMed] [Google Scholar]
- 11.Bu G, Maksymovitch EA, Nerbonne JM, Schwartz AL. Expression and function of the low density lipoprotein receptor-related protein (LRP) in mammalian central neurons. J Biol Chem. 1994;269:18521–18528. [PubMed] [Google Scholar]
- 12.Bu G, Geuze HJ, Strous GJ, Schwartz AL. 39 kDa receptor-associated protein is an ER resident protein and molecular chaperone for LDL receptor-related protein. EMBO J. 1995;14:2269–2280. doi: 10.1002/j.1460-2075.1995.tb07221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmeliet P, Schoonjans L, Kiecken L, Ream B, Degen J, Bronson R, DeVos R, Van den Oord JJ, Collen D, Mulligan RC. Physiological consequences of loss of plasminogen activator gene function in mice. Nature. 1994;368:419–424. doi: 10.1038/368419a0. [DOI] [PubMed] [Google Scholar]
- 14.Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TVP, Hyman BT, Younkin SG, Hsiao KK. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- 15.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 16.Fagan AM, Bu G, Sun Y, Daugherty A, Holtzman DM. Apolipoprotein E-containing high density lipoprotein promotes neurite outgrowth and is a ligand for the low density lipoprotein receptor-related protein. J Biol Chem. 1996;271:30121–30125. doi: 10.1074/jbc.271.47.30121. [DOI] [PubMed] [Google Scholar]
- 17.Frey U, Krug M, Reymann KG, Matthies H. Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Res. 1988;452:57–65. doi: 10.1016/0006-8993(88)90008-x. [DOI] [PubMed] [Google Scholar]
- 18.Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- 19.Frey U, Frey S, Schollmeier F, Krug M. Influence of actinomycin D, a RNA-synthesis inhibitor, on long-term potentiation in rat hippocampal neurons in vivo and in vitro. J Physiol (Lond) 1995;490:703–711. doi: 10.1113/jphysiol.1996.sp021179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frey U, Muller M, Kuhl D. A different form of long-lasting potentiation revealed in tissue plasminogen activator mutant mice. J Neurosci. 1996;16:2057–2063. doi: 10.1523/JNEUROSCI.16-06-02057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukao H, Matsuo O. Analysis of tissue-type plasminogen activator receptor (t-PAR) in human endothelial cells. Semin Thromb Hemost. 1998;24:269–273. doi: 10.1055/s-2007-995853. [DOI] [PubMed] [Google Scholar]
- 22.Golden JP, Rana JZ, Davis J, Zahm DS, Jacquin MF. Organization of the proximal, orbital segment of the infraorbital nerve at multiple intervals after axotomy at birth: a quantitative electron microscopic study in rat. J Comp Neurol. 1993;338:159–174. doi: 10.1002/cne.903380203. [DOI] [PubMed] [Google Scholar]
- 23.Goretzki L, Mueller BM. Low-density-lipoprotein-receptor-related protein (LRP) interacts with a GTP-binding protein. Biochem J. 1998;336:381–386. doi: 10.1042/bj3360381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grimes ML, Zhou J, Beattie EC, Yuen EC, Hall DE, Valletta JS, Topp KS, LaVail JH, Bunnett NW, Mobley WC. Endocytosis of activated TrkA: evidence that nerve growth factor induces formation of signaling endosomes. J Neurosci. 1996;16:7950–7964. doi: 10.1523/JNEUROSCI.16-24-07950.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hajjar KA, Jacovina AT, Chacko J. An endothelial cell receptor for plasminogen/tissue plasminogen activator. J Biol Chem. 1994;269:21191–21197. [PubMed] [Google Scholar]
- 26.Hardy MM, Feder J, Wolfe RA, Bu G. Low density lipoprotein receptor-related protein modulates the expression of tissue-type plasminogen activator in human colon fibroblasts. J Biol Chem. 1997;272:6812–6817. doi: 10.1074/jbc.272.10.6812. [DOI] [PubMed] [Google Scholar]
- 27.Hawkins RD, Kandel ER, Siegelbaum SA. Learning to modulate transmitter release: themes and variations in synaptic plasticity. Annu Rev Neurosci. 1993;16:625–665. doi: 10.1146/annurev.ne.16.030193.003205. [DOI] [PubMed] [Google Scholar]
- 28.Hayden SM, Seeds NW. Modulated expression of plasminogen activator system components in cultured cells from dissociated mouse dorsal root ganglia. J Neurosci. 1996;16:2307–2317. doi: 10.1523/JNEUROSCI.16-07-02307.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herz J, Hamann U, Rogne S, Myklebost O, Gausepohl H, Stanley KK. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 1988;7:4119–4127. doi: 10.1002/j.1460-2075.1988.tb03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herz J, Clouthier DE, Hammer RE. LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell. 1992;71:411–421. doi: 10.1016/0092-8674(92)90511-a. [DOI] [PubMed] [Google Scholar]
- 31.Herz J, Couthier DE, Hammer RE. Correction: LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell. 1993;73:428. doi: 10.1016/0092-8674(93)90130-i. [DOI] [PubMed] [Google Scholar]
- 32.Hollenbach E, Ackermann S, Hyman BT, Rebeck GW. Confirmation of an association between a polymorphism in exon 3 of the low-density lipoprotein receptor-related protein gene and Alzheimer's disease. Neurology. 1998;50:1905–1907. doi: 10.1212/wnl.50.6.1905. [DOI] [PubMed] [Google Scholar]
- 33.Hollister RD, Kisiel W, Hyman BT. Immunohistochemical localization of tissue factor pathway inhibitor-1 (TFPI-1), a Kunitz protease inhibitor, in Alzheimer's disease. Brain Res. 1996;728:13–19. [PubMed] [Google Scholar]
- 34.Holtzman DM, Pitas RE, Kilbridge J, Nathan B, Mahley RW, Bu G, Schwartz AL. LRP mediates apolipoprotein E-dependent neurite outgrowth in CNS-derived neuronal cells. Proc Natl Acad Sci USA. 1995;92:9480–9484. doi: 10.1073/pnas.92.21.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holtzman DM, Fagan AM. Potential role of apoE in structural plasticity in the nervous system: implications for disorders of the central nervous system. Trends Cardiovasc Med. 1998;8:250–255. doi: 10.1016/s1050-1738(98)00017-6. [DOI] [PubMed] [Google Scholar]
- 36.Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll RA, Mucke L. Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Proc Natl Acad Sci USA. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang YY, Kandel ER. Recruitment of long-lasting and protein kinase A-dependent long-term potentiation in the CA1 region of hippocampus requires repeated tetanization. Learning Memory. 1994;1:74–82. [PubMed] [Google Scholar]
- 38.Huang YY, Bach ME, Lipp HP, Zhuo M, Wolfer DP, Hawkins RD, Schoonjans L, Kandel ER, Godfraind JM, Mulligan R, Collen D, Carmeliet P. Mice lacking the gene encoding tissue-type plasminogen activator show a selective inference with late-phase long-term potentiation in both Schaffer collateral and mossy fiber pathways. Proc Natl Acad Sci USA. 1996;93:8699–8704. doi: 10.1073/pnas.93.16.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang DE, Saitoh T, Chen X, Xia Y, Masliah E, Hansen LA, Thomas RG, Thal LJ, Katzman R. Genetic association of the low-density lipoprotein receptor-related protein gene (LRP), an apolipoprotein E receptor, with late-onset Alzheimer's disease. Neurology. 1997;49:56–61. doi: 10.1212/wnl.49.1.56. [DOI] [PubMed] [Google Scholar]
- 40.Kim Y-H, Park J-H, Hong SH, Koh JY. Nonproteolytic neuroprotection by human recombinant tissue plasminogen activator. Science. 1999;284:647–650. doi: 10.1126/science.284.5414.647. [DOI] [PubMed] [Google Scholar]
- 41.Knauer MF, Orlando RA, Glabe CG. Cell surface APP751 forms complexes with protease nexin 2 ligands and is internalized via the low density lipoprotein receptor-related protein (LRP). Brain Res. 1996;740:6–14. doi: 10.1016/s0006-8993(96)00711-1. [DOI] [PubMed] [Google Scholar]
- 42.Kounnas MZ, Moir RD, Rebeck GW, Bush AI, Argraves WS, Tanzi RE, Hyman BT, Strickland DK. LDL receptor-related protein, a multifunctional apoE receptor, binds secreted β-amyloid precursor protein and mediates its degradation. Cell. 1995;82:331–340. doi: 10.1016/0092-8674(95)90320-8. [DOI] [PubMed] [Google Scholar]
- 43.Krieger M, Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP). Annu Rev Biochem. 1994;63:601–637. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- 44.Lendon CL, Talbot CJ, Craddock NJ, Han SW, Wragg M, Morris JC, Goate AM. Genetic association studies between dementia of the Alzheimer's type and three receptors for apolipoprotein E in a Caucasian population. Neurosci Lett. 1997;222:187–190. doi: 10.1016/s0304-3940(97)13381-x. [DOI] [PubMed] [Google Scholar]
- 45.Liao A, Nitsch RM, Greenberg SM, Finckh U, Blacker D, Albert M, Rebeck GW, Gomezisla T, Clatworthy A, Binetti G, Hock C, Muellerthomsen T, Mann U, Zuchowski K, Beisiegel U, Staehelin H, Growdon JH, Tanzi RE, Hyman BT. Genetic association of an alpha-2-macroglobulin (Val1000Ile) polymorphism and Alzheimer's disease. Hum Mol Genet. 1998;7:1953–1956. doi: 10.1093/hmg/7.12.1953. [DOI] [PubMed] [Google Scholar]
- 46.Lijnen HR, Collen D. Strategies for the improvement of thrombolytic agents. Thromb Haemost. 1991;66:88–110. [PubMed] [Google Scholar]
- 47.Moser EI, Krobert KA, Moser MB, Morris RG. Impaired spatial learning after saturation of long-term potentiation. Science. 1998;281:2038–2042. doi: 10.1126/science.281.5385.2038. [DOI] [PubMed] [Google Scholar]
- 48.Nalbantoglu J, Tirado-Santiago G, Lahsaini A, Poirier J, Goncalves O, Verge G, Momoli F, Welner SA, Massicotte G, Julien JP, Shapiro ML. Impaired learning and LTP in mice expressing the carboxy terminus of the Alzheimer amyloid precursor protein. Nature. 1997;387:500–505. doi: 10.1038/387500a0. [DOI] [PubMed] [Google Scholar]
- 49.Narita M, Bu G, Holtzman DM, Schwartz AL. The low density lipoprotein receptor-related protein (LRP), a multifunctional ApoE receptor, modulates hippocampal neurite development. J Neurochem. 1997;68:587–595. doi: 10.1046/j.1471-4159.1997.68020587.x. [DOI] [PubMed] [Google Scholar]
- 50.Nguyen PV, Kandel ER. A macromolecular synthesis-dependent late phase long-term potentiation requiring cAMP in the medial perforant pathway of rat hippocampal slices. J Neurosci. 1996;16:3189–3198. doi: 10.1523/JNEUROSCI.16-10-03189.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nguyen PV, Abel T, Kandel ER. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- 52.Nicoll RA, Malenka RC. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature. 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- 53.Orth K, Madison EL, Gething MJ, Sambrook JF, Herz J. Complexes of tissue-type plasminogen activator and its serpin inhibitor plasminogen-activator inhibitor type 1 are internalized by means of the low density lipoprotein receptor-related protein/α2-macroglobulin receptor. Proc Natl Acad Sci USA. 1992;89:7422–7426. doi: 10.1073/pnas.89.16.7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orth K, Willnow T, Herz J, Gething MJ, Sambrook J. Low density lipoprotein receptor-related protein is necessary for the internalization of both tissue-type plasminogen activator-inhibitor complexes and free tissue-type plasminogen activator. J Biol Chem. 1994;269:21117–21122. [PubMed] [Google Scholar]
- 55.Otter M, Barrett-Bergshoeff MM, Rijken DC. Binding of tissue-type plasminogen activator by the mannose receptor. J Biol Chem. 1991;266:13931–13935. [PubMed] [Google Scholar]
- 56.Pittman RN, Ivins JK, Buettner HM. Neuronal plasminogen activators: cell surface binding sites and involvement in neuronal outgrowth. J Neurosci. 1989;9:4269–4286. doi: 10.1523/JNEUROSCI.09-12-04269.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qi M, Zhuo M, Skalhegg BS, Brandon EP, Kandel ER, McKnight GS, Idzerda RL. Impaired hippocampal plasticity in mice lacking the Cβ1 catalytic subunit of cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1996;93:1571–1576. doi: 10.1073/pnas.93.4.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qian Z, Gilbert ME, Colicos MA, Kandel ER, Kuhl D. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature. 1993;361:453–457. doi: 10.1038/361453a0. [DOI] [PubMed] [Google Scholar]
- 59.Rebeck GW, Harr SD, Strickland DK, Hyman BT. Multiple, diverse senile plaque-associated proteins are ligands for an apolipoprotein E receptor, α2-macroglobulin receptor/low density lipoprotein receptor-related protein. Ann Neurol. 1995;37:211–217. doi: 10.1002/ana.410370212. [DOI] [PubMed] [Google Scholar]
- 60.Schuman EM. Synapse specificity and long-term information storage. Neuron. 1997;18:339–342. doi: 10.1016/s0896-6273(00)81234-9. [DOI] [PubMed] [Google Scholar]
- 61.Seeds NW, Williams BL, Bickford PC. Tissue plasminogen activator induction in Purkinje neurons after cerebellar motor learning. Science. 1995;270:992–994. doi: 10.1126/science.270.5244.1992. [DOI] [PubMed] [Google Scholar]
- 62.Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 63.Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer's disease. Proc Natl Acad Sci USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun YL, Wu S, Bu G, Onifade MK, Patel SN, Ladu MJ, Fagan AM, Holtzman DM. Glial fibrillary acidic protein-apolipoprotein E (apoE) transgenic mice: astrocyte-specific expression and differing biological effects of astrocyte-secreted apoE3 and apoE4 lipoproteins. J Neurosci. 1998;18:3261–3272. doi: 10.1523/JNEUROSCI.18-09-03261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trommsdorff M, Borg JP, Margolis B, Herz J. Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J Biol Chem. 1998;273:33556–33560. doi: 10.1074/jbc.273.50.33556. [DOI] [PubMed] [Google Scholar]
- 66.Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J. Reeler/disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and apoE receptor 2. Cell. 1999;9:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- 67.Verrall S, Seeds NW. Characterization of 125I-tissue plasminogen activator binding to cerebellar granule neurons. J Cell Biol. 1989;109:265–271. doi: 10.1083/jcb.109.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ware JH, DiBenedetto AJ, Pittman RN. Localization of tissue plasminogen activator mRNA in adult rat brain. Brain Res. 1995;37:275–281. doi: 10.1016/0361-9230(95)00008-3. [DOI] [PubMed] [Google Scholar]
- 69.Warshawsky I, Bu G, Schwartz AL. Identification of domains on the 39-kDa protein that inhibit the binding of ligands to the low density lipoprotein receptor-related protein. J Biol Chem. 1993;268:22046–22054. [PubMed] [Google Scholar]
- 70.Welling TH, Huber TS, Messina LM, Stanley JC. Tissue plasminogen activator increases canine endothelial cell proliferation rate through a plasmin-independent, receptor-mediated mechanism. J Surg Res. 1996;66:36–42. doi: 10.1006/jsre.1996.0369. [DOI] [PubMed] [Google Scholar]
- 71.Willnow TE, Orth K, Herz J. Molecular dissection of ligand binding sites on the low density lipoprotein receptor-related protein. J Biol Chem. 1994;269:15827–15832. [PubMed] [Google Scholar]