Destruction and Creation of Spatial Tuning by Disinhibition: GABAA Blockade of Prefrontal Cortical Neurons Engaged by Working Memory (original) (raw)
Abstract
Local circuit neurons in the dorsolateral prefrontal cortex (dPFC) of monkeys have been implicated in the cellular basis of working memory. To further elucidate the role of inhibition in spatial tuning, we iontophoresed bicuculline methiodide (BMI) onto functionally characterized neurons in the dPFC of monkeys performing an oculomotor delayed response task. This GABAA blockade revealed that both putative interneurons and pyramidal cells possess significant inhibitory tone in the awake, behaving monkey. In addition, BMI application primarily resulted in the loss of previously extant spatial tuning in both cell types through reduction of both isodirectional and cross-directional inhibition. This tuning loss occurred in both the sensorimotor and mnemonic phases of the task, although the delay activity of prefrontal neurons appeared to be particularly affected. Finally, application of BMI also created significant spatial tuning in a sizable minority of units that were untuned in the control condition. Visual field analysis of such tuning suggests that it is likely caused by the unmasking of normally suppressed spatially tuned excitatory input. These findings provide the first direct evidence of directional inhibitory modulation of pyramidal cell and interneuron firing in both the mnemonic and sensorimotor phases of the working memory process, and they implicate a further role for GABAergic interneurons in the construction of spatial tuning in prefrontal cortex.
Keywords: primate, inhibition, prefrontal cortex, interneurons, spatial tuning, bicuculline, working memory, fast spiking
The role of excitatory and inhibitory elements in the construction of spatially selective activity has been studied pharmacologically at the single-unit level in several sensory cortical areas. In the primary visual cortex (V1), both broadening of orientation tuning (Sillito, 1984; Sato et al., 1996) and reduction of directional selectivity (Murthy and Humphrey, 1999) have been observed with the application of bicuculline methiodide (BMI), a GABAA receptor antagonist. Microiontophoresis of GABA has been shown to reduce orientation selectivity of the response of cells recorded ∼500 μm away (Eysel et al., 1990). In primary somatosensory cortex, application of BMI was found to result in an increase of the size of receptive fields (Alloway et al., 1989; Alloway and Burton, 1991). Finally, in the rat barrel cortex, application of BMI and GABA resulted in reduced and increased spatial selectivity, respectively, of both putative GABAergic and spiny stellate neurons (Kyriazi et al., 1996). Overall, these results suggest that GABAA-mediated inhibition plays an important role in the generation of spatial selectivity in the primary sensory areas of cortex.
Spatially selective activity in the dorsolateral prefrontal cortex (dPFC) takes the form of the directionally selective neuronal responses during the mnemonic and sensorimotor periods of an oculomotor delayed response (ODR) task, a test of spatial working memory (Funahashi et al., 1989, 1990, 1991; Chafee and Goldman-Rakic, 1998; Rao et al., 1999). Such directionally selective mnemonic activity is thought to represent the neural substrate of spatial working memory (Funahashi et al., 1989). However, little is known about the precise role of inhibitory mechanisms in the regulation of such activity, although several studies have suggested that inhibition is important to working memory function. Injection of bicuculline into the dPFC of monkeys has been shown to disrupt the performance of a delayed response task (Sawaguchi et al., 1988, 1989). Two studies using single-unit recording in the dPFC of awake, behaving monkeys have demonstrated the relationships of spatial tuning between putative pyramidal cells (RS) and interneurons (FS). Wilson et al. (1994) demonstrated inverted patterns of activity between nearby (i.e., <400 μm) FS and RS units while monkeys engaged in various spatial, sensory-guided tasks, leading to the hypothesis that cross-directional inhibition may be important for spatial tuning in the monkey dPFC (Goldman-Rakic, 1995b). In a recent paper (Rao et al., 1999), we examined the tuning patterns of simultaneously recorded FS–RS pairs of monkeys performing the same eight-target ODR task considered in this paper. Members of such pairs were found to exhibit tuning properties that were very similar to each other, providing evidence that isodirectional inhibition between closely adjacent units may play an important role in spatial tuning of cortical neurons.
To elucidate the role of inhibition in the cellular circuits underlying spatial working memory, we microiontophoretically applied BMI onto functionally characterized prefrontal neurons in monkeys performing an ODR task. By pharmacologically disconnecting a significant fraction of the GABAA-mediated inhibitory input, we were able to determine the degree to which a neuron's spatial tuning is dependent on such input. This enabled us to characterize further the directional nature of the inhibition in the prefrontal cortex.
MATERIALS AND METHODS
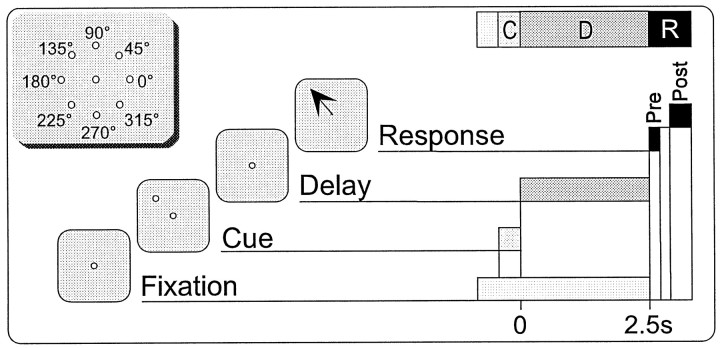
Experimental methods. Extracellular recordings were made in the left dPFC of two rhesus monkeys (Macaca mulata), in accordance with the Yale University Animal Care and Use Committee, as recently detailed in Rao et al. (1999). The animals were trained in the spatial ODR task shown in Figure 1. The monkey commenced each trial of the task by fixating within 2° of a central stimulus for 0.5 sec. The monkey then continued to fixate while one of eight peripheral stimuli (45° separation in circumference, 13° eccentricity) was illuminated for 0.5 sec. This was followed by a delay period of 2.5 or 3.0 sec, during which the monkey maintained fixation. At the end of this time, the central stimulus was extinguished, and the monkey had to make a saccade to within both 0.5 sec and 2_°_ of the position of the previously shown peripheral stimulus to be rewarded with fruit juice. The peripheral cues were presented in a semi-random order across trials such that, during the delay period, the monkey had to remember the cue position shown within the present trial to make the correct response. The monkey's eye position was monitored using a scleral implant/field-coil system (CNC-Engineering, Seattle, WA), and the ODR task was generated by a TEMPO system (Reflective Computing, St. Louis, MO).
Fig. 1.
The eight-target oculomotor delayed-response (ODR) task. Schematic of the ODR task showing the temporal relationship of the Cue, Delay, Pre, and Post epochs. Note that the latter two represent the presaccadic and postsaccadic components, respectively, of the response phase of the task. A 2.5 sec delay is shown, although both 2.5 and 3.0 sec delays were used. Inset, Cue locations for the eight-target ODR task. All cues were located at a 13° eccentricity.
The dura was punctured using a 25-gauge guide tube, and the electrode was advanced into the brain using a Narishige (Tokyo, Japan) MD-2 digital microdrive. The iontophoretic electrode itself was fabricated from seven-barrel glass (Friedrich and Dimmock, Millville, NJ) and 20 or 33 μm carbon fiber (ESLI, San Diego, CA) on a custom electrode puller. Two of the barrels of the microelectrode were filled with BMI at 1–2 mm concentrations, pH 4.0, and a NeuroPhore BH-2 iontophoretic system from Medical Systems Corporation (Greenvale, NY) was used to control drug delivery. The remaining two pairs of barrels on the microelectrode were filled with other drugs (generally dopaminergic or serotonergic agents) that varied on a monthly basis (Williams et al., 1997, 1998a,b). The drugs remained in the drug barrels for no more than 4 hr and remained protected from light during this time. The stability of BMI, in particular, was assured even toward the end of the experiment because its iontophoresis always resulted in increased unit activity. BMI was ejected at currents that typically varied from 15 to 25 nA (total range 10–250 nA), and a cyclical retaining current was used with currents ranging from 2 to 5 nA. The ejection current was typically started low and carefully titrated upward toward a stable value that resulted in a noticable increase in activity in the units being recorded from. Such careful titration was necessary to avoid localized epileptiform (bursting) activity, which manifested as rhythmic, large amplitude, polymorphic waveforms associated with a distinctive sound on the audio monitor. Epileptiform activity developed in <5% of the experiments performed, and in all such cases, recording was abandoned.
At each recording depth, we generally tested 8–12 trials of the task at each of the eight cue locations in a drug-free control condition. Upon establishing the stability of the units' waveforms and activity at the site, this control condition was followed by a similar number of trials during drug administration. These conditions were typically followed by both post-drug and recovery conditions, with the former representing a “wash out” period for the drug. This pattern—control, drug, post-drug, and recovery—was repeated up to three times at a given site. Initially, we determined that neurons generally recovered very poorly to bicuculline administration, presumably because of the long half-life of the drug. Hence, BMI was usually the sole drug tested at a site. Moreover, in those situations when multiple drugs were tested at one site, BMI was always tested last.
Neuronal data were acquired by a micro1401/Spike2 system (Cambridge Electronic Design, Cambridge, UK), which can sort up to eight units at a single site (we have successfully isolated as many as five) based on a waveform-matching algorithm. Units were categorized as being FS primarily on the basis of their short spike-base width (<0.90 msec), characteristic sound, relatively low amplitude, and relatively high firing rates (Rao et al., 1999). Finally, although some RS neurons could be tracked for several hundred micrometers, FS cells were rarely tracked for >20 μm, presumably reflecting the large principal apical dendrites of the former and the smaller soma size and dendritic field of the latter.
Analysis. For purposes of tuning analysis, each trial in the ODR was divided into four epochs—Cue, Delay, Pre, and Post—and the average spike rate across each epoch within a single trial was used in subsequent analysis (Fig. 1). The Cue epoch lasts for 0.5 sec and corresponds to the stimulus presentation phase of the task. Delay lasts for 2.5 or 3.0 sec and reflects the mnemonic component of the task. The presaccadic response epoch, Pre, starts immediately after the Delay epoch and lasts 0.25 sec. As the name implies, the bulk of the neuronal activity during the Pre epoch is associated with saccade initiation (Funahashi et al., 1989). By definition, a successful saccade is completed by the time the postsaccadic epoch, Post, begins: 0.5 sec after the end of Delay and lasting for 0.5 sec. The animal receives its reward 0.50–0.55 sec after Delay at the onset of the Post epoch. A given unit can show spatial tuning in none, any, or all of these epochs (Funahashi et al., 1989, 1990, 1991; Rao et al., 1999).
The directionality of a unit's response in each of the four epochs was assessed statistically using a vector algorithm technique that provides a value for the overall strength and direction of tuning as detailed inRao et al. (1999). An important step of this algorithm is the computation of the overall vector of a unit's firing within a condition. The magnitude of such a vector can result either from bursts of firing in a few trials in the untuned case or from a pattern of repeatable, spatially specific activity that is maintained throughout the majority of trials within the condition, a pattern that corresponds to our definition of spatial tuning. To discriminate between these tuned and untuned cases, we first calculate n individual “loop” vectors, each derived from the firing rates at the eight target directions for the _n_th trial. We then assess the contribution (using the scalar product) that the individual loop vectors made to the overall vector. The values of these scalar products vary between −1 and 1 (because of normalization). In the untuned case, the scalar products will tend to cluster around zero. However, as a unit's firing becomes more directional, the values of these scalar products will increase, equaling 1 in the ideal case in which a neuron only fires for targets located at one direction. Hence to statistically assess tuning, the scalar products are compared with thresholds using a nonparametric test (Wilcoxon signed-rank test, p < 0.05). The most basic level of tuning for our purposes corresponds to the case in which most of the loop vectors are within 90_°_of the overall vector. In this situation, the values of the dot products will be statistically greater than a threshold of 0. If the unit fails this test, it is declared untuned and assigned a tuning factor (TF) of zero. However, if the scalar product values are statistically greater than zero, the values are then compared with increasing thresholds at 0.05 increments up to a value of 0.5, providing TFs ranging from 1 to 10. Obviously, increasing values of the TF correspond to increasingly significant spatial tuning.
The direction of tuning of a unit is indicated by the algorithm as a tuning angle, θ, that varies between 0 and 360° and is calculated by computing the median angle of the individual loop vectors. [This choice of estimators for the overall direction of a unit's response was directed by the “sturdiness” against outliers that the circular median estimator possesses (Fisher, 1993). Conversely, outliers can have a profound effect on the angle of the overall vector, an otherwise natural choice as an estimator for this parameter.] Because of the vector nature of the analysis, the tuning angle generally does not necessarily coincide exactly with any of the eight target locations. The index (target location) closest to the overall tuning angle of a given neuron was defined as that unit's “preferred” index. The preferred index and the two indices immediately adjacent were defined as being the “isodirectional” indices. The three indices 180° distant to the three isodirectional indices were defined as the “cross-directional” indices.
The two particular changes in tuning occurring between the control and bicuculline conditions that were of interest to us were the creation and loss of statistically significant spatial tuning. We defined an increase in TF from a value of zero in the control condition to a value of 1 or more in the bicuculline condition as corresponding to the creation of tuning. Conversely, a decrease in TF from a value of 1 or more in the control to zero in the drug condition signified destruction or loss of tuning. In those units in which tuning in a given epoch in the control was destroyed by BMI application, the tuning angle, isodirectional indices, and cross-directional indices were all determined in the control condition. However, in the case of application of BMI creating a unit's tuning in a given epoch, these parameters had to be calculated in the drug condition.
The vector algorithm was implemented as a c++ program, which takes as input intermediate files created by a Spike2 script and creates an output file that is then imported directly into a database created in Filemaker from Claris (Santa Clara, CA). Other analyses and graphs were generated using Statview (Abacus Concepts, Berkeley, CA) and Deltagraph (DeltaPoint, Monterey, CA), respectively.
RESULTS
A total of 86 neurons were tested iontophoretically with BMI. Of these, 66 units were classified as RS units and 20 were classified as FS neurons. The application of BMI resulted in a statistically significant increase in overall activity in ∼70% of FS and RS neurons tested, as assessed by comparing the average spike rates across the trials of the control and BMI conditions using a one-way factorial ANOVA (p < 0.05). The magnitude of such activity increases varied widely, from ∼20% to more than fivefold, and no apparent differences between the FS and RS cells were noted. An example of an FS–RS pair (recorded simultaneously at the same site) onto which BMI was iontophoresed is shown in Figure2A. Note the severalfold increase in the mean firing rate of both neurons with the application of bicuculline. In four RS neurons, we observed a paradoxical reduction in activity with BMI administration; this may have been caused by aberrant waveform morphology or depolarization block during BMI administration. In all such cases, any previously extant spatial tuning was lost.
Fig. 2.
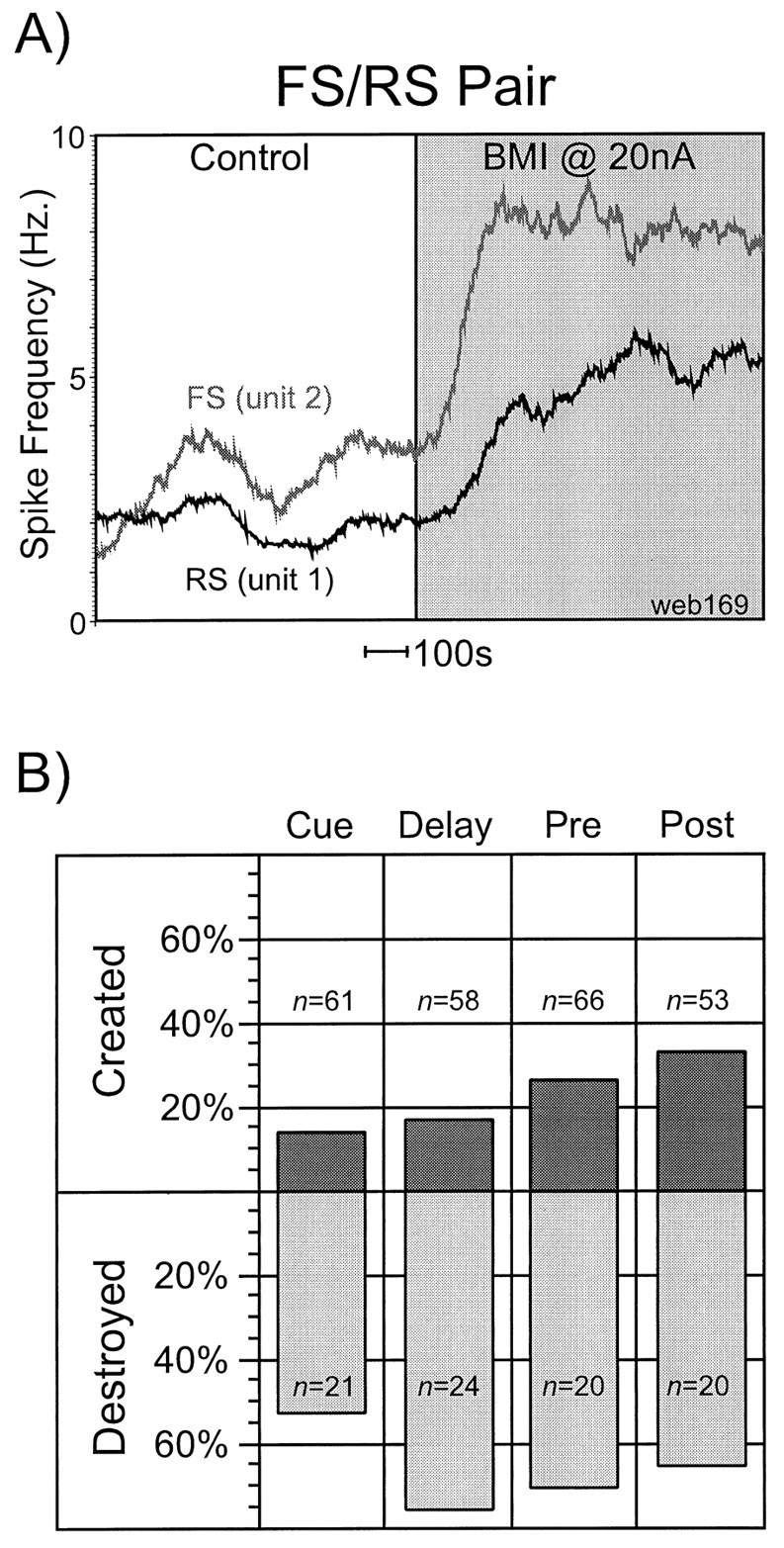
Effects of BMI application on activity and tuning of PFC neurons. A, An example of an FS–RS pair (recorded simultaneously at the same site) onto which BMI was iontophoresed at 20 nA. The vertical axis represents firing rate in Hertz, and the horizontal axis is time; a_time bar_ denoting 100 sec is indicated for scale. Note the severalfold increase in the mean firing rate of both neurons with the application of BMI. B, The results of the iontophoretic application of BMI on spatial tuning for the entire neuronal population (i.e., combined FS and RS populations). The_n_ values in the top and_bottom_ halves of the figure represent the number of units that were untuned or tuned, respectively, in each epoch in the control condition. The bar graphs represent the percentage of this number that developed (top) or lost (bottom) tuning with the application of BMI.Top, Application of BMI created spatial tuning in a considerable number of neurons that were untuned in the control condition, and the frequency of this phenomenon increased as the trial progressed from the Cue epoch to the _Post_epoch. Bottom, The predominant effect on previously extant tuning was the loss of this tuning; _Delay_appeared most susceptible to this effect.
BMI application onto tuned neurons
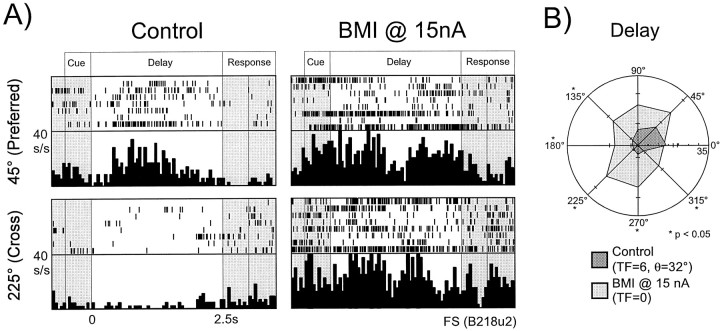
In the control condition, ∼70% of RS and FS neurons that were subsequently tested with bicuculline exhibited statistically significant spatial tuning (i.e., TF ≥1) in at least one of the four epochs. The results of the iontophoretic application of BMI on spatial tuning for the entire neuronal population (i.e., combined FS and RS populations) are shown in Figure 2B. The n_values in the top and bottom halves of the figure represent the number of units that were untuned or tuned, respectively, in each epoch in the control condition. The bar graphs themselves represent the percentage of this number in which tuning was created (top) or lost (bottom) with the application of BMI. From the bottom half of the figure, we can see that the predominant effect of BMI application on RS and FS neurons that were spatially tuned in the control condition was the loss of this tuning. Some variation was noted between epochs, with Delay period activity being the most susceptible to tuning destruction by the loss of GABAA-mediated inhibition, and Cue period activity being the least vulnerable. An example of an FS neuron that lost its Delay tuning with the application of BMI at 15 nA is shown in Figure 3 in rastergram and histogram format for the preferred index and a cross-directional index (A) and in polar-plot format for all directions (B). In the control condition, this unit displayed a highly statistically significant tuned response (TF = 6, θ = 32°) with a preferred index of 45°. From Figure3A,B, it is evident that in the control condition, activation during Delay was present for targets located at 0 and 45°, whereas suppression of activity occurred at the cross-directional indices (i.e., those located at 180, 225, and 270°). Application of BMI at 15 nA resulted in the destruction of tuning (TF = 0 in this condition), primarily as a result of increased activity at the cross-directional indices. In Figure3A, it is apparent that although activity at the 45° index was modestly increased with the application of BMI, activity at the 225° target location showed marked elevation. In Figure3B, statistically significant increases (Student's unpaired_t test; p < 0.05) in activity occurred at all directions, with the exceptions of the isodirectional indices (i.e., 0, 45, 90°).
Fig. 3.
Loss of Delay tuning in an FS neuron with BMI application. A, Rastergrams and histograms (bin width 50 msec) presented for the preferred index (45°) and a cross-directional index (225°) for an FS neuron. In the control condition (left), this unit shows marked activation during_Delay_ at the 45° index. However, the unit's response is relatively suppressed at the 225° location. With the application of BMI at 15 nA (right), Cue activity at both indices equalizes, and the unit's tuning in this epoch is lost.B, The data for the same unit as above presented in polar plot form. Means and standard error measurements for all eight indices are presented for both the Control and_BMI_ conditions. Statistically significant differences in activity (two-tailed Student's t test,p < 0.05) between the two conditions at each index are denoted with asterisks. In the control condition, the unit shows strong tuning (TF=6, θ = 32°) with preferential activation at the isodirectional indices (45, 0, and to a lesser degree 90°) and suppression at the cross-directional indices (180, 225, and 270°). With the application of BMI, activity at the isodirectional indices is not significantly increased. However, the activity at the cross-directional indices is markedly enhanced, reaching significance at three indices.
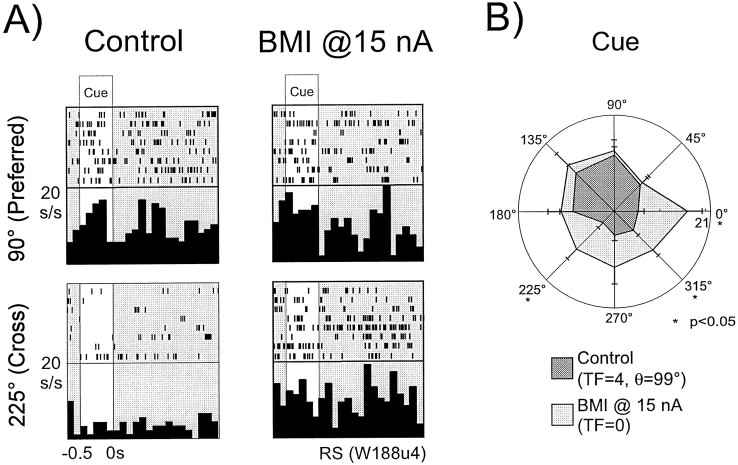
An example of an RS neuron that lost its Cue tuning with the application of BMI at 15 nA is shown in Figure4, again in both rastergram and histogram format (A) and polar plot format (B). This unit showed significant Cue tuning during the control condition (TF = 4, θ = 99°). Activation was primarily present for targets located at 90° (the preferred index) and 135°, whereas suppression was noted at the cross-directional indices (225, 270, and 315°). Application of BMI at 15 nA resulted in the destruction of this tuning once again as a result of increased activity in the cross-directional indices. In Figure4A, it is apparent that the activity for the cross-directional target location (225°) was markedly elevated over the control condition with the application of BMI, whereas activity at the preferred direction was relatively unchanged. In Figure4B, we see that statistically significant increases (Student's unpaired t test; p < 0.05) in activity occurred for targets located at 0, 225, and 315°.
Fig. 4.
Loss of Cue tuning in an RS neuron with BMI application. A, Rastergrams and histograms (bin width 50 msec) presented for the preferred index (90°) and a cross-directional index (225°) for an RS neuron. In the control condition (left), this unit shows strong activation in the Cue epoch at the 90 and 135° indices. However, the unit's response is relatively suppressed at the 225° location. With the application of BMI at 15 nA (right), Cue activity at both indices becomes equal, resulting in tuning loss. B, The data for the same unit as above presented in polar plot form. Means and standard error measurements for all eight indices are presented for both the control and BMI conditions. Statistically significant differences in activity (two-tailed Student's t test,p < 0.05) between the two conditions at each index are denoted with asterisks. In the control condition, the unit shows strong Cue tuning (_TF=4,_θ =99°) with strong activation at the isodirectional indices (90, 135, and to a lesser degree 45°) and suppression at the cross-directional indices (225, 270, and 315°). With the application of BMI, activity at the isodirectional indices is relatively unchanged. However, the activity at the cross-directional indices is markedly enhanced, reaching significance at both 225 and 315°.
An example of an FS neuron that loses its tuning in the postsaccadic epoch is shown in Figures 5_A_in polar plot format. In the control condition, the unit shows broad excitation for targets in the ipsilateral visual field with very little activation for targets in the contralateral visual field (TF = 1, θ = 177°, preferred index at 180°). However, application of BMI at 20 nA causes a statistically significant (p < 0.05) increase in activity at all target locations except 90°, resulting in a complete loss of spatial selectivity.
Fig. 5.
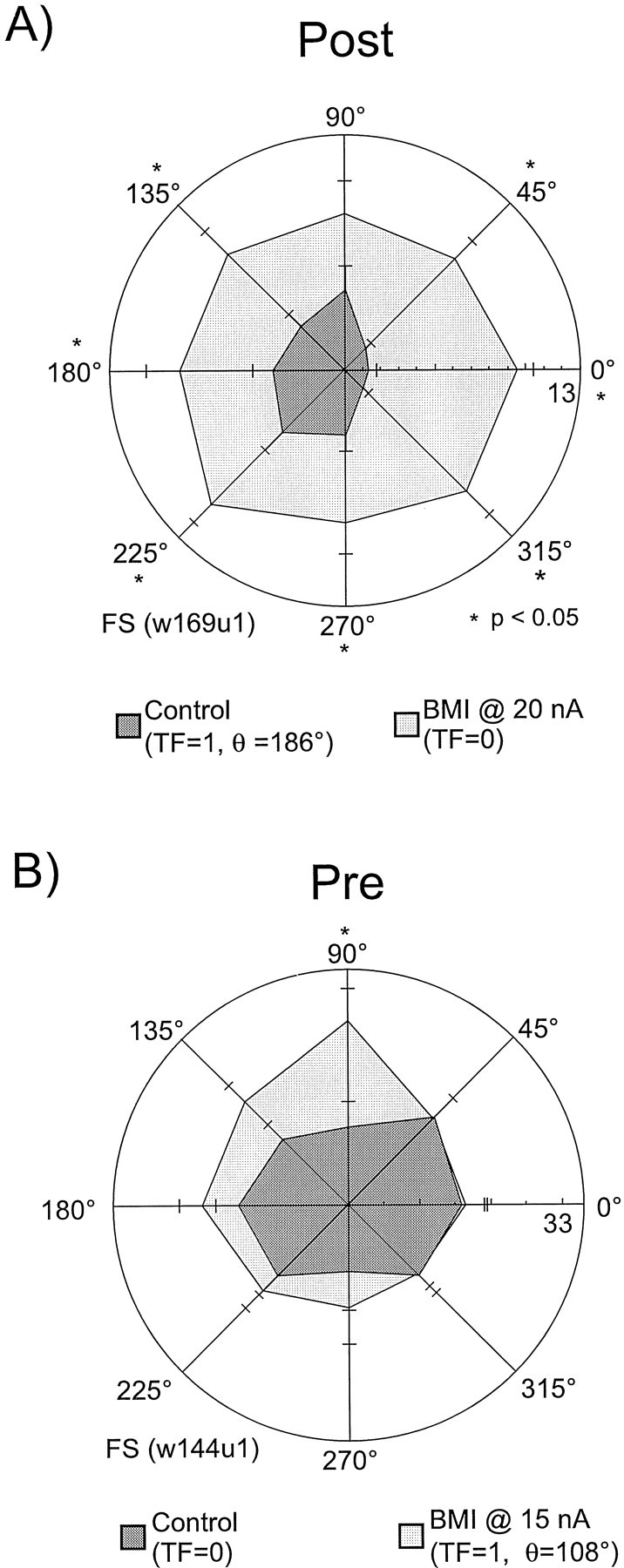
Tuning loss during Post and creation of Pre tuning in two different FS units with BMI application. A, Loss of Post tuning in an FS neuron with the application of BMI. In the control condition, this unit shows broad activation for all targets located in the ipsilateral visual field (_TF=1,θ =186°, preferred index = 180°). Application of BMI at 20 nA results in the complete loss of spatial selectivity in this unit's Post response. B, The effects of BMI application onto an untuned FS unit. In the control condition, this unit displayed very little spatial selectivity during the_Pre epoch. Application of BMI resulted in a significant increase in activity at only one cue location, 90°, the preferred direction of firing for this unit.
BMI application onto untuned neurons
Application of BMI created spatial tuning in a considerable number of neurons that were untuned in the control condition (Fig.2B, top). The frequency of this phenomenon increased as the trial progressed from the Cue to the Post epochs, with the latter displaying more than twice the percentage of tuning creation of the former. The effect of BMI application onto an untuned FS unit is shown in Figure 5B. In the control condition, this unit displayed very little spatial selectivity during the Pre epoch. Application of BMI resulted in a significant increase in activity at only one cue location, 90°, the preferred direction of firing for this unit in the drug condition.
In Figure 6 is shown an RS unit in which statistically significant Cue and Post tuning was created by BMI application. It is evident from Figures 6A(left),B, that this unit's activity in both of these epochs in the control condition was relatively slow and lacked spatial selectivity (TF = 0 for both epochs). However, application of BMI at 15 nA resulted in a spatially selective increase in activity during both of these epochs (Fig. 6A, right, B). Activity in Cue increased markedly for targets located at and around 0° with the application of BMI; however, activity for cues located in the ipsilateral visual field (135, 180, and 225°) was only minimally increased over the control condition. This pattern of activity increase resulted in a statistically significant tuning in this epoch in the BMI condition (TF = 2, θ =16°). In contrast, activity during Post increased primarily in the ipsilateral visual field with the application of BMI, again resulting in statistically significant tuning (TF = 1, θ = 211°, preferred index of 225°). Note the near inversion of tuning angles between the Cue and Post epochs in this unit. Such inversion is not an uncommon pattern of activation in the prefrontal cortex (Funahashi et al., 1989, 1991; Rao et al., 1999).
Fig. 6.
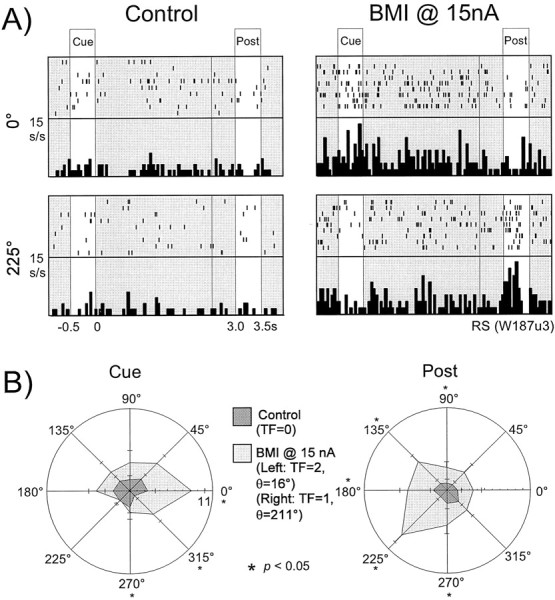
Creation of both Cue and Post tuning in an RS neuron with the application of BMI. A, Rastergram and histogram (bin width 50 msec) data presented for both the 0 and 225° directions for this RS neuron. In the control condition (left), this unit's activity in both the_Cue_ and Post epochs was relatively slow and lacked spatial selectivity (TF = 0 for both epochs). However, application of BMI at 15 nA resulted in a spatially selective increase in activity during both of these epochs (right). Activity in Cue increased markedly at 0° (contralateral visual field) with the application of BMI; however, activity for cues located at 225° was only minimally increased over the control condition. In contrast, activity during Post increased preferentially at 225° (ipsilateral visual field) with the application of BMI.B, The data for the same unit as above presented in polar plot form. Means and standard error measurements for all eight indices are presented for both control and BMI conditions. Statistically significant differences in activity (two-tailed Student's t test, p < 0.05) between the two conditions at each index are denoted with_asterisks_. In the control condition, the unit shows suppressed, nonspatially specific activity during both the_Cue_ (left) and Post(right) epochs (TF = 0 in both cases). However, application of BMI resulted in preferential activation of this unit during Cue at and around the 0° index, thus resulting in statistically significant tuning (_TF=2,θ_=18°). Conversely, activity increases during Post during the drug condition occurred primarily around the 225° location, resulting in a TF = 1 and a θ = 211°.
Population analyses
The mechanisms by which changes in tuning occurred with the application of BMI were examined at the population level to ascertain whether these changes show directional dependence. In particular, we examined the occurrence of isodirectional or cross-directional disinhibition for both destruction and creation of tuning. We first determined the percentage of units within a given population displaying statistically significant increases in activity (p < 0.05, unpaired Student's _t_test, control vs BMI conditions) at the indices located in the isodirectional and cross-directional indices. (Because there were three indices each within the isodirectional and cross-directional fields, a statistically significant change in activity at an individual index was counted as one-third for a given unit. Hence, a unit that showed significant increases in activity at all three cross-directional indices, for example, would count as one toward the total.) For the RS neurons, this analysis was performed on both an epoch-by-epoch basis and at the level of the entire population. For the FS neurons, however, only a population level analysis could be performed because of the relatively limited numbers of such neurons. The results are shown in Figure 7A. For both the RS and FS populations (Fig. 7A, right), there was a bias toward cross-directional disinhibition in units whose tuning was destroyed with BMI. On the other hand, a bias toward isodirectional disinhibition was present in units of both populations that developed tuning with the application of BMI. This general pattern was also apparent for the Cue, Delay, and Post epochs for RS neurons (Fig.7A, left). During the Delay epoch, a large percentage of units whose tuning was affected by BMI application showed statistically significant changes in activity in the isodirectional and cross-directional indices. Finally, we found no neurons that had lost tuning solely through isodirectional disinhibition.
Fig. 7.
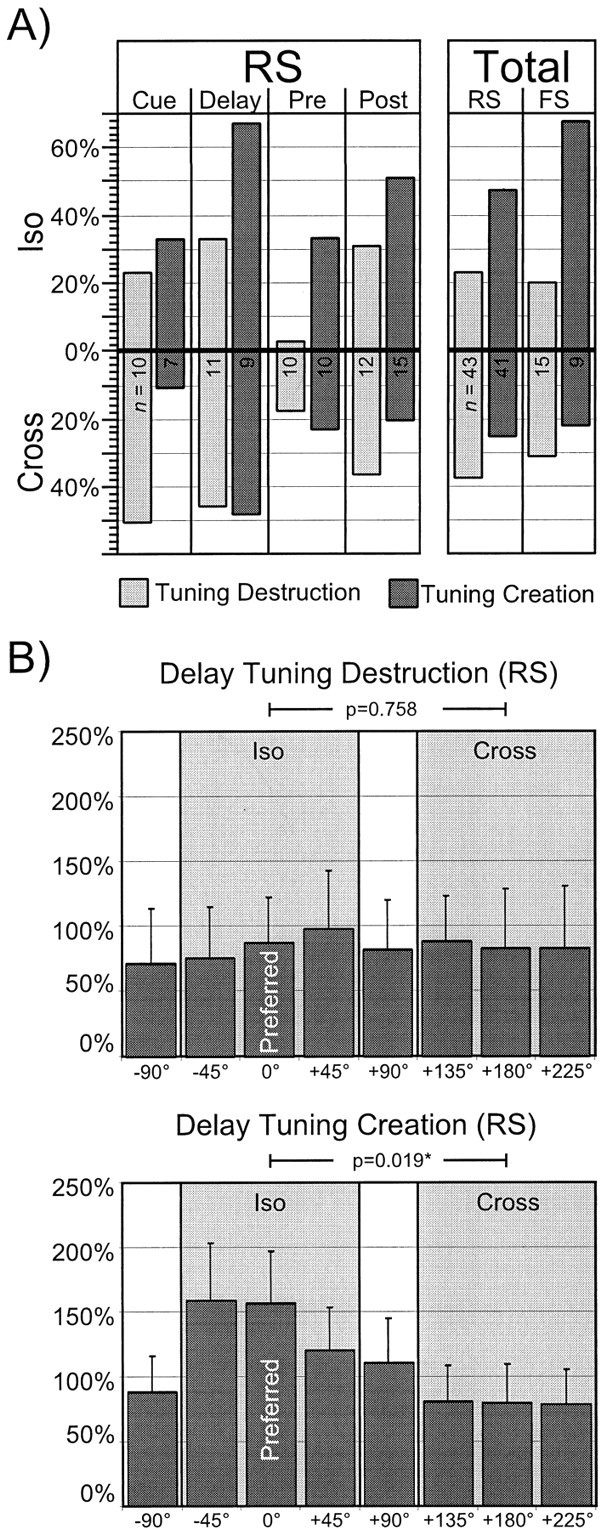
Isodirectional and cross-directional disinhibition in FS and RS populations. A, The percentage of units displaying statistically significant increases in activity (p < 0.05, unpaired Student's_t_ test, control vs BMI conditions) at the indices located in the isodirectional (top) and cross-directional (bottom) target locations for RS units by epoch (left) and for the RS and FS populations (right). As defined, there are three indices each within the isodirectional and cross-directional fields; hence, a statistically significant change in activity at an individual index is counted as one-third for a given unit. Within each epoch (left) or population (right), data for tuning destruction and creation are presented in the bar graphs on the_left_ and right, respectively. The_n_ values within each bar refer to the total number of units from which the proportions that the bar graphs represent were calculated. Left, In all epochs, there was a bias toward cross-directional disinhibition in units whose tuning was destroyed by BMI application, and a bias toward isodirectional disinhibition in units whose tuning was created by BMI application. Note that during Delay, a relatively large percentage of units whose tuning was affected by BMI application showed statistically significant changes in activity in the isodirectional and cross-directional directions.Right, For both the RS and FS populations, there was an overall bias toward cross-directional disinhibition in units whose tuning was destroyed with BMI and a bias toward isodirectional disinhibition in units that developed tuning with the application of BMI. B, Preferred index centered mean relative change plots for the RS units in Delay. The preferred index (normalized at 0°) is indicated, and all other indices are shown relative to this index. The_ordinate_ shows the percentage increase of the population at each index. The error bars denote the SEM (see Results for further details). Top, Tuning destruction. Activity increases in the isodirections were similar to those seen in the cross-directions. A factorial ANOVA comparing the combined data at all three isodirectional indices with the combined data at all three cross-directional indices was not significant. These results suggest that isodirectional and cross-directional disinhibition contributed relatively evenly to the loss of tuning in this population. Bottom, Tuning creation. A significant increase in activity at the combined isodirectional indices was found by ANOVA in this case (p = 0.019), suggesting that disinhibition at the isodirectional indices was more important than that occurring at the cross-directions for creation of tuning.
We further assessed the magnitude of activation at the isodirectional and cross-directional indices using a “mean- relative change plot” that was centered on the preferred index. This analysis was effected by first determining the relative change in activity at each index in the BMI condition as compared with the mean of the activity at all indices in the control condition for each unit. These values were then centered, in the current analysis, on the preferred direction for each unit. Finally, the mean and standard errors of the activity changes for each direction relative to the preferred direction were calculated across all units in a given population. The results for RS neurons in the delay epoch are shown in Figures 7B for tuning destruction (top) and creation (bottom). For the former, activity increases at all of the indices were relatively even. Not surprisingly, activity increases in the isodirections were not statistically different from those at the cross-directional indices. These results suggest that isodirectional and cross-directional disinhibition contributed relatively evenly to the tuning loss in this population. However, for those neurons whose tuning was created with BMI, a significant increase in activity at the isodirectional indices was found, confirming the results of Figure 7A(left). Note that the large variances found in the data presented in Figure 7B (top) and (bottom) were primarily a result of the wide range of activity increases that occurred with BMI application.
To help ascertain the etiology of tuning creation (i.e., whether this phenomenon resulted from the release of suppressed excitatory input or from more nonspecific disinhibition), we analyzed the visual field preference of tuning that was created in the RS neuronal population. These results are shown in Figure8A. The spatial tuning that became evident with the application of BMI showed a contralateral visual field bias during the Cue and Delay epochs (>55%). The Pre epoch was relatively unbiased. Finally, a strong ipsilateral visual bias was noted during the Post epoch (almost 75% ipsilateral vs ∼25% contralateral). These data are confirmed for the Delay and Post epochs (the epochs showing the most visual field bias) in the mean relative change plots centered on the 270° location in Figure8B (top) and (bottom), respectively. In Delay, activity increases at the contralateral locations were greater (although not statistically significantly) than those in the ipsilateral visual field. These results were in contrast to those obtained in Post, where increases in the ipsilateral indices were profoundly larger than those noted in the contralateral indices. However, these changes did not reach statistical significance. (The high variance present in this data were caused, again, by the wide variability in activity increases that occurred with BMI application. However, in these analyses, variability was further increased because of the fact that data in these graphs were centered about the 270° target location instead of the preferred direction for a given cell. At a population level, this resulted in the combining of the isodirectional data of one unit with the cross-directional data of another, data that were shown to have significantly different means in Fig. 7B.)
Fig. 8.
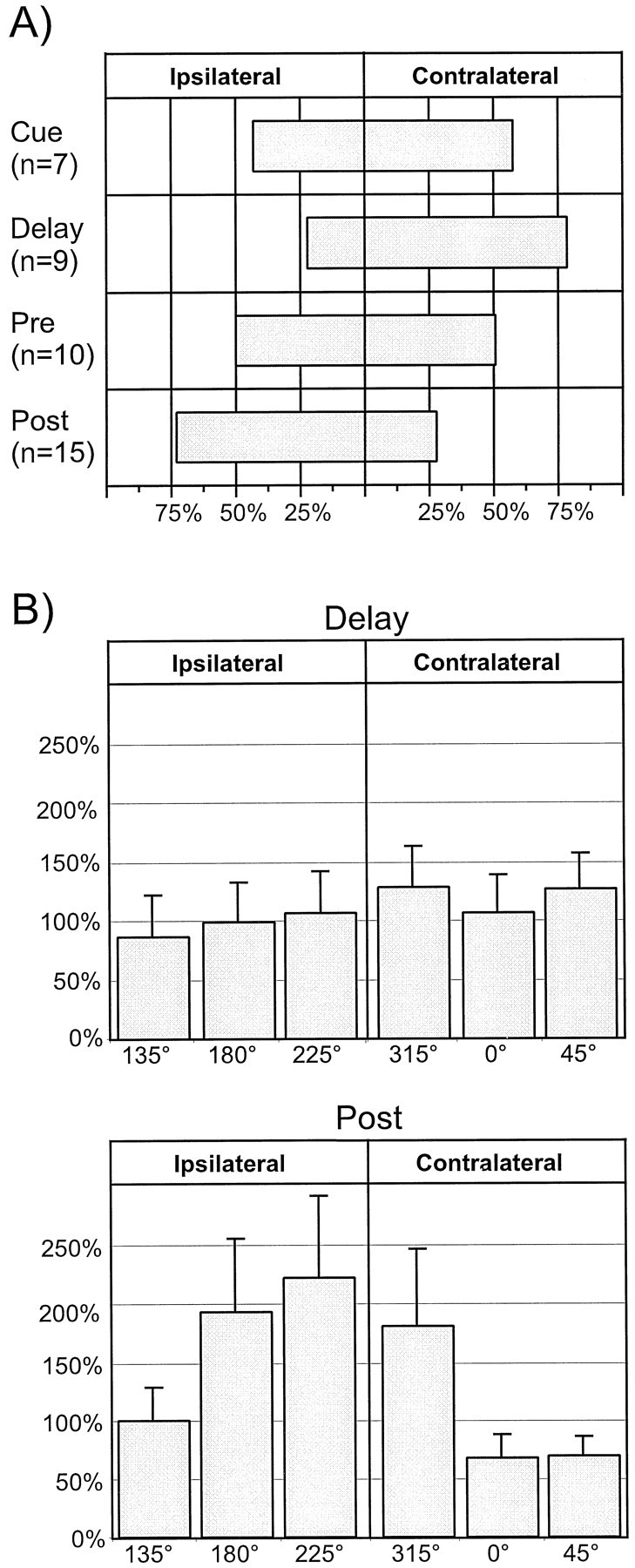
Visual field preference of tuning that was created in the RS neuronal population. A, Percentage of the total number of units (shown by n) that became tuned in each epoch that demonstrated ipsilateral or contralateral visual field tuning. The spatial tuning that became evident with the application of BMI showed a contralateral visual field bias during the_Cue_ (>55%) and Delay (>75%) epochs. The Pre epoch was relatively unbiased. Finally, a strong ipsilateral visual bias was noted during the Post epoch (almost 75% ipsilateral vs ∼25% contralateral). B, Visual field centered mean relative change plots for_Delay_ and Post data. The ordinate shows the percentage increase in activity of the population at each indicated target location, and the error bars denote the SEM (see Results for further details). Top, During Delay, this analysis reveals some increased disinhibition for indices located in the contralateral visual field (i.e., 315, 0, and 225°). No significant differences were found between these data by ANOVA.Bottom, In Post, increases in the ipsilateral indices (135, 180, 225°) were profoundly larger than those noted in the contralateral indices. Again, these changes did not reach statistical significance by ANOVA.
DISCUSSION
In the current work, we investigated the action of GABAA-mediated inhibition on single units in the primate dPFC involved in the eight-target ODR task. We found that both the FS and RS neurons receive significant GABAergic tone in vivo. The predominant effect of BMI application on spatially tuned FS and RS neurons was found to be tuning loss, mediated by a combination of isodirectional and cross-directional disinhibition. Mnemonic activity appeared somewhat more susceptible to tuning loss than did other epochs. Finally, we found that a subpopulation of previously untuned neurons became tuned with the application of BMI. The tuning thus created showed a contralateral visual field bias in Cue and became progressively more ipsilaterally biased as the task progressed.
Effects of BMI on overall activity
BMI application produced a general increase in the overall activity level of both FS and RS neurons. This result was anticipated for the RS population given the evidence on the connectivity between inhibitory interneurons and pyramidal neurons in prefrontal cortex (Williams et al., 1992; Jones, 1993; Gabbott and Bacon, 1996). However, BMI application was also associated with an increase in activity in a majority of the FS population as well. Several mechanisms may account for this effect on these putative inhibitory interneurons. The FS units that increased their activity may be directly disinhibited by BMI from other inhibitory interneurons. In fact, large basket cells have been shown to form a significant percentage of presumed inhibitory contacts onto the soma of other basket cells, and conversely, GABAergic contacts have been found on the soma of basket cells (Williams et al., 1992;Kisvarday et al., 1993). Furthermore, it has recently been shown that up to two-thirds of this input appears to originate from other parvalbumin-containing neurons (Gonchar and Burkhalter, 1999), in some cases resulting in reciprocal connectivity (Tamas et al., 1998). The FS neurons whose activity increased may also have received feed-forward disinhibition from excitatory neurons that were themselves disinhibited. Evidence for feed-forward excitation of local circuit inhibitory neurons from nearby pyramidal cells has been found from_in vitro_ dual intracellular recording studies (Thomson and Deuchars, 1997). Finally, presumed inhibitory autologous synapses (“autapses”) have been found to exist on a subpopulation of cortical inhibitory interneurons (Thomson et al., 1996; Tamas et al., 1997), and presynaptic GABAA receptors have also been demonstrated (Vautrin et al., 1994; Xi and Akasu, 1996). BMI application onto either arrangement should result in the disinhibition of the presynaptic GABAergic neuron. The mechanisms described above are not mutually exclusive to one another. In light of the heterogeneity of the FS population (Kawaguchi, 1993, 1995; Kawaguchi and Kubota, 1997), a combination of mechanisms is likely to be responsible for the degree of activation observed in this population.
Tuning loss
A major consequence of the disinhibition resulting from application of BMI was the loss of tuning in all task phases of both RS and FS neurons, a result that directly implicates GABAA-mediated inhibition in both the mnemonic and sensorimotor phases of the spatial working memory process. This tuning loss can occur by at least three mechanisms, the first of which would involve increases in activity for the isodirectional indices alone. Obviously, if a pharmacological manipulation increased activity only at the preferred direction, the tuning of the neuron would be improved (a point we will return to below). However, increases in activity at the adjacent target locations can cause a loss of tuning by reducing spatial selectivity. A second mechanism would be through increases in activity in a unit's cross-directional indices. This would tend to reduce the overall directionality of a unit's response. Finally, a third mechanism would involve combinations of these two mechanisms occurring simultaneously. In particular, spatial tuning could be lost if activity increased equally for all target locations, thus reducing a unit's signal-to-noise ratio and directional specificity. Examples were found of units that lost their spatial tuning via the second and third mechanisms described above. For example, both the FS unit in Figure 3 and the RS unit in Figure 4 lost their respective tuning in the drug condition and showed significant activity increases only in the cross-directional indices; activity in the isodirectional indices was not changed significantly. However, the FS unit in Figure 4B lost its tuning by increased activity at both the isodirectional and cross-directional fields. Overall, decreases in cross-directional inhibition, either alone or in conjunction with decreases in isodirectional inhibition, occurred in the majority of both RS and FS neurons that lost their tuning (Fig. 7). Moreover, we never observed a unit losing its spatial tuning solely through increases in activity in the isodirectional fields. Taken together, the results demonstrate that both isodirectional and cross-directional inhibitory mechanisms may play an important role in the generation of spatially tuned activity in the dPFC during a spatial working memory task.
Tuning creation
A novel finding of this work was the result that application of BMI could create statistically significant spatial tuning in a previously untuned dPFC neuron. As suggested above, this phenomenon was attributable to isolated increases in activity in one or two adjacent indices. We hypothesized that such tuning was a result of the “unmasking” of input that was suppressed in the control condition, a phenomenon that has been described in sensory cortical areas (Jacobs and Donoghue, 1991; Eysel et al., 1998). To test this hypothesis, we assessed the visual field biases of the tuning created with BMI application. If this tuning was the result of isodirectional disinhibition, we would expect the visual field tuning biases in each of the epochs to be in keeping with what is normally found without drug (Rao et al., 1999). This was, in fact, what we found at both the population and single unit levels (Figs. 8 and 6, respectively). Thus, isodirectional inhibition may be playing a role in both shaping spatial specificity and regulating the threshold of excitatory inputs, allowing only those of behaviorally appropriate intensity through.
As shown in Figure 2B, the percentage of previously untuned units that became tuned with the application of BMI increased as the task progressed, becoming maximal in the postsaccadic epoch. In context of the discussion above, this implies that isodirectional suppression of input itself becomes more prominent as the task progresses, possibly as a result of Post tuning being biased toward the ipsilateral visual field (Rao et al., 1999). The precise physiological significance of this finding requires further investigation.
Sources of isodirectional and cross-directional inhibition
In previous work, we demonstrated that closely adjacent neurons possessed tuning similar to one another, suggesting that such neurons may share common afferent input and thus may form a functional microcolumn, and we also suggested that isodirectional inhibition on a given unit may arise from nearby microcolumns (Rao et al., 1999). Our current findings also support the hypothesis that cross-directional inhibition is important for prefrontal working memory function (Goldman-Rakic, 1995b). A significant portion of such inhibition may originate from the contralateral hemisphere, because several studies have suggested that prefrontal working memory function is hemispherically lateralized. Microinjections of dopamine in the dPFC of monkeys performing the ODR task created mnemonic scotomas primarily in the contralateral visual field (Sawaguchi and Goldman-Rakic, 1994). It has also been shown that spatial tuning during the sensory, mnemonic, and early response phases of the task tends to show a predominantly contralateral visual field bias (Rao et al., 1999). Furthermore, input from the contralateral hemisphere has been shown to terminate ipsilaterally in a columnar manner (Goldman and Nauta, 1977), a result that may have important consequences for the topography of inhibition in the dPFC.
Role of inhibition in PFC activity
As we have shown, during the sensorimotor phases of the task, application of BMI primarily resulted in the loss of previously extant spatial tuning. Earlier studies have demonstrated that the dPFC connects primarily to higher order sensory and motor cortical areas (Goldman-Rakic, 1987) and thus processes highly abstracted information via its afferent and efferent connections. Hence, one could argue that_de novo_ creation of spatial selectivity during the sensorimotor task phases simply for low-level feature detection (as seen in V1) is redundant, suggesting other roles for the inhibitory mechanisms in the dPFC. One such role of inhibition in the dPFC may be attentional control, adjusting the focus of prefrontal cortical mechanisms to the task at hand. Thus, inhibition may implement part of the role that has been attributed to the “central executive” component of working memory (Baddeley, 1992; Goldman-Rakic, 1995a).
The data presented in Figure 2B suggest that mnemonic activity was the most susceptible to tuning loss by BMI application. It has been hypothesized that sustained delay period activity is likely to be at least partially dependent on activation of local or intracortical recurrent loops (Lisman et al., 1998; Lisman and Fallon, 1999). Such activity should be, by necessity, heavily regulated by inhibitory circuitry, a hypothesis that is consistent with models of neocortical recurrency (Douglas et al., 1995). Hence, such activity should be highly susceptible to the effects of BMI.
Conclusions
Previous studies of neurons in the dPFC of monkeys by Funahashi et al. (1989, 1990, 1991) demonstrated that the property of directional selectivity was not limited to the neurons of the sensory and motor areas. In the present study, we show that GABAA-mediated inhibition plays an important role at the cellular level in the processes underlying spatial working memory in the dPFC, improving spatial selectivity and possibly playing critical roles in the attentional control mechanisms of central executive function.
Footnotes
This work was supported by National Institute of Mental Health Grants P50 MH44866 and R37 MH3854 (P.S.G.-R.). Further support was provided by the Medical Scientist Training Program of the National Institutes of Health (S.G.R).
Correspondence should be addressed to Dr. Srinivas G. Rao, Section of Neurobiology, Yale University School of Medicine, P.O. Box 208001, New Haven, CT 06520-8001. E-mail:srinivas.rao@yale.edu.
REFERENCES
- 1.Alloway KD, Burton H. Differential effects of GABA and bicuculline on rapidly- and slowly-adapting neurons in primary somatosensory cortex of primates. Exp Brain Res. 1991;85:598–610. doi: 10.1007/BF00231744. [DOI] [PubMed] [Google Scholar]
- 2.Alloway KD, Rosenthal P, Burton H. Quantitative measurements of receptive field changes during antagonism of GABAergic transmission in primary somatosensory cortex of cats. Exp Brain Res. 1989;78:514–532. doi: 10.1007/BF00230239. [DOI] [PubMed] [Google Scholar]
- 3.Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- 4.Chafee MV, Goldman-Rakic PS. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J Neurophysiol. 1998;79:2919–2940. doi: 10.1152/jn.1998.79.6.2919. [DOI] [PubMed] [Google Scholar]
- 5.Douglas RJ, Koch C, Mahowald M, Martin KAC, Suarez HH. Recurrent excitation in neocortical circuits. Science. 1995;269:981–985. doi: 10.1126/science.7638624. [DOI] [PubMed] [Google Scholar]
- 6.Eysel UT, Crook JM, Machemer HF. GABA-induced remote inactivation reveals cross-orientation inhibition in the cat striate cortex. Exp Brain Res. 1990;80:626–630. doi: 10.1007/BF00228003. [DOI] [PubMed] [Google Scholar]
- 7.Eysel UT, Shevelev IA, Lazareva NA, Sharaev GA. Orientation tuning and receptive field structure in cat striate neurons during local blockade of intracortical inhibition. Neuroscience. 1998;84:25–36. doi: 10.1016/s0306-4522(97)00378-3. [DOI] [PubMed] [Google Scholar]
- 8.Fisher NI. Statistical analysis of circular data. Cambridge UP; Cambridge: 1993. [Google Scholar]
- 9.Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 10.Funahashi S, Bruce CJ, Goldman-Rakic PS. Visuospatial coding in primate prefrontal neurons revealed by oculomotor paradigms. J Neurophysiol. 1990;63:814–831. doi: 10.1152/jn.1990.63.4.814. [DOI] [PubMed] [Google Scholar]
- 11.Funahashi S, Bruce CJ, Goldman-Rakic PS. Neuronal activity related to saccadic eye movements in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1991;65:1464–1483. doi: 10.1152/jn.1991.65.6.1464. [DOI] [PubMed] [Google Scholar]
- 12.Gabbott PL, Bacon SJ. Local circuit neurons in the medial prefrontal cortex (areas 24a,b,c, 25 and 32) in the monkey: I. Cell morphology and morphometrics. J Comp Neurol. 1996;364:567–608. doi: 10.1002/(SICI)1096-9861(19960122)364:4<567::AID-CNE1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 13.Goldman PS, Nauta WJ. Columnar distribution of cortico-cortical fibers in the frontal association, limbic, and motor cortex of the developing rhesus monkey. Brain Res. 1977;122:393–413. doi: 10.1016/0006-8993(77)90453-x. [DOI] [PubMed] [Google Scholar]
- 14.Goldman-Rakic PS. Circuitry of the frontal association cortex and its relevance to dementia. Arch Gerontol Geriat. 1987;6:299–309. doi: 10.1016/0167-4943(87)90029-x. [DOI] [PubMed] [Google Scholar]
- 15.Goldman-Rakic PS. Architecture of the prefrontal cortex and the central executive. Ann NY Acad Sci. 1995a;769:71–83. doi: 10.1111/j.1749-6632.1995.tb38132.x. [DOI] [PubMed] [Google Scholar]
- 16.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995b;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 17.Gonchar Y, Burkhalter A. Differential subcellular localization of forward and feedback interareal inputs to parvalbumin expressing GABAergic neurons in rat visual cortex. J Comp Neurol. 1999;406:346–360. doi: 10.1002/(sici)1096-9861(19990412)406:3<346::aid-cne4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs KM, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991;251:944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- 19.Jones EG. GABAergic neurons and their role in cortical plasticity in primates. Cereb Cortex. 1993;3:361–372. doi: 10.1093/cercor/3.5.361-a. [DOI] [PubMed] [Google Scholar]
- 20.Kawaguchi Y. Groupings of nonpyramidal and pyramidal cells with specific physiological and morphological characteristics in rat frontal cortex. J Neurophysiol. 1993;69:416–431. doi: 10.1152/jn.1993.69.2.416. [DOI] [PubMed] [Google Scholar]
- 21.Kawaguchi Y. Physiological subgroups of nonpyramidal cells with specific morphological characteristics in layer II/III of rat frontal cortex. J Neurosci. 1995;15:2638–2655. doi: 10.1523/JNEUROSCI.15-04-02638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- 23.Kisvarday ZF, Beaulieu C, Eysel UT. Network of GABAergic large basket cells in cat visual cortex (area 18): implication for lateral disinhibition. J Comp Neurol. 1993;327:398–415. doi: 10.1002/cne.903270307. [DOI] [PubMed] [Google Scholar]
- 24.Kyriazi HT, Carvell GE, Brumberg JC, Simons DJ. Quantitative effects of GABA and bicuculline methiodide on receptive field properties of neurons in real and simulated whisker barrels. J Neurophysiol. 1996;75:547–560. doi: 10.1152/jn.1996.75.2.547. [DOI] [PubMed] [Google Scholar]
- 25.Lisman JE, Fallon JR. What maintains memories? Science. 1999;283:339–340. doi: 10.1126/science.283.5400.339. [DOI] [PubMed] [Google Scholar]
- 26.Lisman JE, Fellous JM, Wang XJ. A role for NMDA-receptor channels in working memory. Nat Neurosci. 1998;1:273–275. doi: 10.1038/1086. [DOI] [PubMed] [Google Scholar]
- 27.Murthy A, Humphrey AL. Inhibitory contributions to spatiotemporal receptive-field structure and direction selectivity in simple cells of cat area 17. J Neurophysiol. 1999;81:1212–1224. doi: 10.1152/jn.1999.81.3.1212. [DOI] [PubMed] [Google Scholar]
- 28.Rao SG, Williams GV, Goldman-Rakic PS. Isodirectional tuning of adjacent interneurons and pyramidal cells during working memory: evidence for microcolumnar organization in PFC. J Neurophysiol. 1999;81:1903–1916. doi: 10.1152/jn.1999.81.4.1903. [DOI] [PubMed] [Google Scholar]
- 29.Sato H, Katsuyama N, Tamura H, Hata Y, Tsumoto T. Mechanisms underlying orientation selectivity of neurons in the primary visual cortex of the macaque. J Physiol (Lond) 1996;494:757–771. doi: 10.1113/jphysiol.1996.sp021530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawaguchi T, Goldman-Rakic PS. The role of D1-dopamine receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. J Neurophysiol. 1994;71:515–528. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- 31.Sawaguchi T, Matsumura M, Kubota K. Delayed response deficit in monkeys by locally disturbed prefrontal neuronal activity by bicuculline. Behav Brain Res. 1988;31:193–198. doi: 10.1016/0166-4328(88)90023-x. [DOI] [PubMed] [Google Scholar]
- 32.Sawaguchi T, Matsumura M, Kubota K. Delayed response deficits produced by local injection of bicuculline into the dorsolateral prefrontal cortex in Japanese macaque monkeys. Exp Brain Res. 1989;75:457–469. doi: 10.1007/BF00249897. [DOI] [PubMed] [Google Scholar]
- 33.Sillito AM. Functional considerations of the operation of GABAergic inhibitory processes in the visual cortex. In: Jones EG, Peters A, editors. Cerebral cortex, Ed 2. Plenum; New York: 1984. pp. 91–117. [Google Scholar]
- 34.Tamas G, Buhl EH, Somogyi P. Massive autaptic self-innervation of GABAergic neurons in cat visual cortex. J Neurosci. 1997;17:6352–6364. doi: 10.1523/JNEUROSCI.17-16-06352.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamas G, Somogyi P, Buhl EH. Differentially interconnected networks of GABAergic interneurons in the visual cortex of the cat. J Neurosci. 1998;18:4255–4270. doi: 10.1523/JNEUROSCI.18-11-04255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomson AM, Deuchars J. Synaptic interactions in neocortical local circuits: dual intracellular recordings in vitro. Cereb Cortex. 1997;7:510–522. doi: 10.1093/cercor/7.6.510. [DOI] [PubMed] [Google Scholar]
- 37.Thomson AM, West DC, Hahn J, Deuchars J. Single axon IPSPs elicited in pyramidal cells by three classes of interneurones in slices of rat neocortex. J Physiol (Lond) 1996;496:81–102. doi: 10.1113/jphysiol.1996.sp021667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vautrin J, Schaffner AE, Barker JL. Fast presynaptic GABAA receptor-mediated Cl− conductance in cultured rat hippocampal neurones. J Physiol (Lond) 1994;479:53–63. doi: 10.1113/jphysiol.1994.sp020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams GV, Rao SG, Goldman-Rakic PS. Attenuation of memory fields in primate prefrontal cortical pyramidal cells and interneurons by selective 5-HT2 antagonists. Soc Neurosci Abstr. 1997;23:627.7. [Google Scholar]
- 40.Williams GV, Rao SG, Goldman-Rakic PS. Serotonin2 receptor modulation of mnemonic coding in prefrontal cortex. Biol Psychiatry. 1998a;43:7S. [Google Scholar]
- 41.Williams GV, Rao SG, Leung H-C, Goldman-Rakic PS. Effects of dopamine D4 antagonists on mnemonic activity of neurons in primate prefrontal cortex. Biol Psychiatry. 1998b;43:41–42S. [Google Scholar]
- 42.Williams SM, Goldman-Rakic PS, Leranth C. The synaptology of parvalbumin-immunoreactive neurons in the primate prefrontal cortex. J Comp Neurol. 1992;320:353–369. doi: 10.1002/cne.903200307. [DOI] [PubMed] [Google Scholar]
- 43.Wilson FS, O'Scalaidhe SP, Goldman-Rakic PS. Functional synergism between putative gamma-aminobutyrate-containing neurons and pyramidal neurons in prefrontal cortex. Proc Natl Acad Sci USA. 1994;91:4009–4013. doi: 10.1073/pnas.91.9.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xi ZX, Akasu T. Presynaptic GABAA receptors in vertebrate synapses. Kurume Med J. 1996;43:115–122. doi: 10.2739/kurumemedj.43.115. [DOI] [PubMed] [Google Scholar]