Phase II Single-Arm Trial of Cladribine and Low-Dose Cytarabine Alternating with Decitabine as Frontline Therapy for Older Patients with Acute Myeloid Leukemia (original) (raw)
. Author manuscript; available in PMC: 2019 Oct 3.
Abstract
Background
Frontline therapy for older and/or unfit patients with acute myeloid leukemia (AML) remains unsatisfactory. We studied a new lower-intensity regimen of cladribine combined with low-dose cytarabine (LDAC) alternating with decitabine (DAC) aimed to improve outcomes in this population Based on our prior experience, we hypothesized that this combination would be safe and more active than current approaches with hypomethylating agents.
Methods
A cohort of 118 patients with a median age of 69 years (range, 49–85), 52 (44%) of whom were 70 years or older, were treated with cladribine plus LDAC (for two 28-day cycles) alternating with DAC (two 28-day cycles) for up to 18 cycles. The primary outcome measure was disease-free survival (DFS).
Findings
Of 118 patients, 48 (41%) had adverse karyotype, 20 (17%) had therapy-related AML, 11 (9%) had treated-secondary AML, and 20 (17%) had TP53 mutations. Sixty-nine patients (58%) achieved a complete remission (CR), and an additional 11 (9%) patients had CR with incomplete count recovery (CRi) for a CR/CRi rate of 68%. The median duration of response was 14.7 months. The median overall survival (OS) was 13.8 months overall, and 16.2 months in responders. The median DFS was 10.8 months. Among patients with normal karyotype, the CR/CRi rate was 84% with a median OS of 19.9 months. Exploratory analyses identified high response rates and improved outcomes in subsets of patients with mutations in NPM1, FLT3, DNMT3a, and RUNX1 treated on study, but adverse outcomes in patients with TP53 mutations. The regimen was well tolerated with 4- and 8-week mortality rates of 1% and 7%, respectively. The most common grade ≥ 3 non-hematologic adverse events were infection, hyperbilirubinemia, rash, and nausea.
Interpretation
The combination of cladribine and LDAC alternating with decitabine appears to be a safe and highly effective regimen for the treatment of older and/or unfit patients with newly diagnosed AML. Further confirmatory testing of this regimen is warranted and could provide a newer, more effective option for lower intensity therapy in this population..
Keywords: Elderly AML, maintenance, low intensity
INTRODUCTION
Acute myeloid leukemia (AML) is the most common acute leukemia diagnosed in adults, with an annual incidence of approximately 22,000 cases in the United States in 2017.1 The median age at diagnosis is 68 years, with the majority of patients being older than 65 years.1 Despite these demographics, most of the improvements in outcomes for patients with AML over the past decade have been primarily in younger patients. Dose-intensification of anthracyclines and cytarabine during induction therapy have led to higher response rates and improved overall survival, but may not be feasible or tolerated in older patients who make up the majority of cases.2,3 In addition to higher rates of comorbidities and organ dysfunction, AML in older patients is associated with adverse prognostic features, lower rates of complete remission, shorter remission durations, and higher early mortality than in younger patients with de novo disease.4–6
Developing lower-intensity regimens with less toxicity and good efficacy has been a priority for the treatment of older and unfit adults with AML. The current established options for such patients include hypomethylating agents (HMAs) like 5-azacytidine (AZA) or decitabine (DAC). While HMAs are well tolerated and have been associated with improved outcomes compared with supportive care, they remain unsatisfactory relative to standard approaches in younger and fit patients. Randomized trials in patients with AML have demonstrated rates of complete remission (CR) and CR with incomplete recovery of counts (CRi) of 18–28% with median overall survivals (OS) ranging from 8 to 10 months with HMAs.7,8
To improve on these outcomes, we previously studied a regimen of clofarabine + low-dose cytarabine (LDAC) cycles alternating with DAC in older patients (median age 68 years) with newly diagnosed AML.9 This regimen was designed to exploit the synergy between clofarabine and cytarabine to improve response rates and provide a low-intensity, prolonged regimen to maintain responses and improve OS. The program demonstrated a CR/CR with incomplete recovery of platelets (CRp) rate of 67%, a median overall survival of 11.1 months overall and a median survival of 18.5 months in responders.9 However, clofarabine was associated with clinically significant rates of prolonged myelosuppression, as well as kidney and liver injury. Furthermore, the relative cost of clofarabine made it less accessible for use in off-label indications. Cladribine, a purine nucleoside with single-agent and combination activity in AML, was also shown to induce DNA hypomethylation by its inhibition of S-adenosylhomocysteine (SAH) hydrolase – a mechanism distinct from traditional DNA methyltransferase inhibitors.10,11 Partnering this agent in a protocol that alternates with a traditional HMA such as DAC could be complementary - overcoming potential resistance mechanisms, optimizing hypomethylation, and translating into clinical benefit.
Additional studies have demonstrated the benefit of adding cladribine to standard induction in patients with AML.12,13 Cladribine is known to have single-agent activity in AML, is well tolerated, and has been shown to synergize with cytarabine.14–16 A recent randomized study demonstrated that cladribine, but not fludarabine, added to standard cytarabine and anthracycline (7+3), improved OS compared with 7+3 alone in patients with newly diagnosed AML.12 Based on these data, we investigated the combination of cladribine plus LDAC alternating with DAC in newly-diagnosed older or unfit patients with AML. We hypothesized that this regimen would similarly produce higher response rates and improved survival compared to current lower intensity approaches. Different from the prior regimen, we also made modifications to the schedule to allow more frequent cycling between cycles of cladribine/LDAC and DAC giving each for 2 cycles on a rotating basis. Herein are our results from the phase II trial investigating this approach.
Study design and participants
This single-arm open-label, prospective phase II study (NCT01515527) was approved by the Institutional Review Board of MD Anderson Cancer Center. All patients provided written informed consent according to institutional guidelines. The study was conducted in concordance with the declaration of Helsinki.
Patients ≥ 60 years of age, adequate organ function, and ECOG performance status ≤ 2, with previously untreated AML (non-M3) and high-risk (≥ 10% marrow blasts or ≥ IPSS intermediate-2 risk) myelodysplastic syndrome (MDS) were eligible for enrollment. Patients < 60 years of age deemed unfit for intensive chemotherapy were also eligible Pregnant patients, those with inadequate organ function including an ejection fraction < 40%, and those with prior exposure to cladribine or decitabine were excluded. (Further details in Appendix page 1).
Procedures
Patients were sequentially enrolled. There was no randomization as part of the protocol. All enrolled patients were treated and evaluable. The treatment plan consisted of an alternating schedule of 2 cycles (28 days each) of cladribine + LDAC (cycle A) alternating with 2 cycles of decitabine (cycle B) for up to 18 cycles (Appendix page 3). Induction therapy (cycle 1) consisted of cladribine 5 mg/m2 intravenously (IV) over 1 – 2 hours on Days 1 to 5 and cytarabine 20 mg subcutaneously (SQ) twice daily (BID) on days 1 – 10. Patients could receive a 2nd cycle of induction chemotherapy if complete remission (CR) was not achieved after Cycle 1. Patients achieving a remission moved onto consolidation therapy, which consisted of cladribine 5 mg/m2 IV over 1 – 2 hours on Days 1 to 3 and cytarabine 20 mg SQ BID on days 1 – 10 (cycle A), alternating with decitabine 20 mg/m2 IV on days 1 – 5 (cycle B) as described above. Patients not achieving a CR, but deriving benefit from therapy could continue on protocol. Those not achieving a CR by 4 cycles were recorded as nonresponders. Cycles were given every 4 to 7 weeks depending on blood count recovery (absolute neutrophil count [ANC] ≥ 1 × 109/L and platelet count ≥ 50 × 109/L) and resolution of non-hematologic toxicities to ≤ grade 1. Dose interruptions and dose modifications were implemented for grade 3 or higher non-hematologic toxicity and missed doses were not made up. Further details on the treatment regimen can be found in the Appendix, page 1.
Outcomes
The primary outcome of this phase II trial was to assess efficacy, by evaluating complete response (CR) and disease-free survival (DFS). CR was defined as normalization of peripheral blood and bone marrow (BM) with < 5% blasts, a peripheral absolute neutrophil count (ANC) ≥ 1 × 109/L, and a platelet count ≥ 100 × 109/L. CRp was defined the same as CR, but without recovery of platelet count ≥ 100 × 109/L. CRi was defined the same as CR, but without recovery of either platelet count or neutrophil count to the above levels.Disease-free survival (DFS) was defined as the time interval from achieving CR or CRp until the date of first objective documentation of disease-relapse or death, whichever occurred first. Patients who were alive and relapse free at the last follow-up date were censored at that time. Overall survival (OS) was defined as the time interval from the date of treatment start until the date of death due to any cause. Patients who were alive at the last follow-up date were censored at that time.
Secondary outcomes included assessing overall survival (OS), overall response, and toxicity.
Exploratory outcomes
Minimal residual testing was performed on remission bone marrow samples, when available, using multi-parameter flow cytometry testing using a 4 tube 8-color panel to detect myeloblasts with aberrant immunophenotype as previously described.17 Conventional cytogenetic testing was performed on baseline bone marrow samples using standard methods. Molecular testing was performed on baseline bone marrow samples using a customized next generation whole exome sequencing assay as previously described.18 A separate multiplex fluorescent-based PCR analysis for internal tandem duplications (ITD) and kinase domain (D835) mutations in FLT3 was performed as previously described (further details on ancillary studies can be found in appendix page 2).19
Statistical Analysis
The study was continuously monitored for the primary endpoint, DFS using the method of Thall, Wooten, and Tannir.20 The trial was conducted using the Clinical Trial Conduct (CTC) website maintained by the Department of Biostatistics at MDACC. In addition, the toxicity was continuously monitored (defined as clinically significant grade 3 or greater non-hematologic toxicity possibly related to the study drugs and not responding to optimal management) that occurred during the first 2 cycles of treatment using the Bayesian approach of Thall, Simon, Estey.21 Multc Lean Desktop (version 2.0.0) was used to generate the stopping boundaries and the OC table and a beta(300,700) prior was used to approximate the target toxicity rate of 30%.
Patient characteristics were summarized using frequency (percentage) for categorical variables and median (range) for continuous variables. The probabilities of DFS and OS were estimated using the method of Kaplan and Meier. Log-rank test was used to compare DFS and OS between subgroups of patients.22 Safety data were summarized by AE category, severity and frequency. SAS (version 9.4) and Splus (version 8.2) were used for the data analyses.
Role of Funding Source
The study was funded in part by the NIH Cancer Center Support Grant P30 CA016672 and Award Number P01 CA049639. The funding source had no role in the study design, collection/analysis of data, or writing of the report. There was no other funding sponsor. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
RESULTS
Patient Characteristics
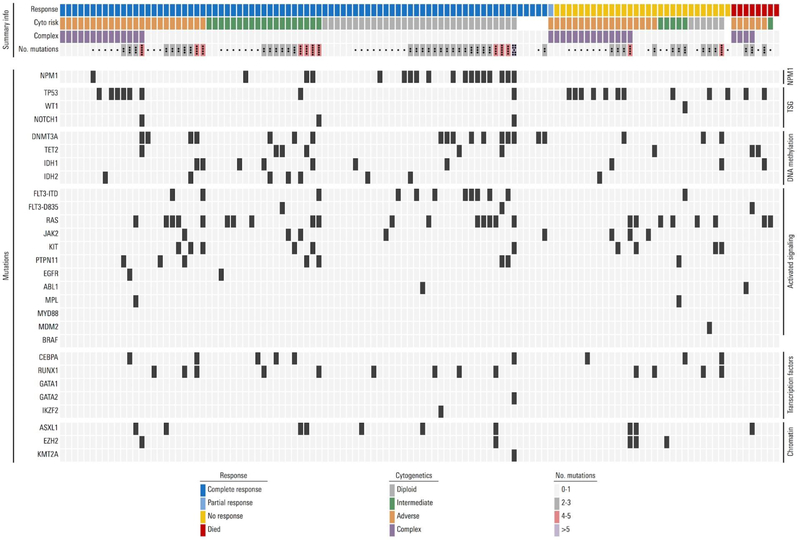
Between 2012 and 2017, a total of 118 patients were treated. Their median age was 69 years (range, 49–85); 52 (44%) patients were age 70 years or older and 2 (2%) patients under age 60 years were treated on study, who were deemed unfit for intensive chemotherapy. The median follow-up time on study was 13.6 months. Baseline patient and disease characteristics are summarized in Table 1. The median bone marrow blast count was 32%; 41% of patients had an adverse risk karyotype (by ELN), and 27% had a complex karyotype, defined as ≥ 3 chromosomal abnormalities. Twenty (17%) patients had received prior chemotherapy and/or radiation for a non-myeloid malignancy (therapy-related AML, t-AML), 30 (25%) had known prior MDS or MPN (secondary AML, s-AML), and 18 (15%) patients had prior treatment for MDS or MPN prior to being enrolled (treated-secondary AML, ts-AML23). All patients underwent molecular testing at baseline using a customized next generation sequencing panel including known AML-related mutations (Table 1). The most commonly detected were mutations in RAS (21%), TP53 (17%), DNMT3a (17%), NPM1 (15%), and FLT3-ITD (10%). The mutational landscape of patients treated on protocol and their relation to karyotype and response is depicted in Figure 1.
Table 1.
Baseline Patient Characteristics
| Characteristic | N (%) or median [Range] |
|---|---|
| Age | 69 [49* – 85 ] |
| ≥ 70 years | 52 (44) |
| Secondary AML | 30 (25) |
| Treated Secondary AML | 18 (15) |
| Marrow Blast % | 32 [3 – 95] |
| Peripheral Blood Blast % | 8 [0 – 88] |
| WBC [x 109/L] | 3.1 [0.5 – 72.3] |
| Platelets [x 109/L] | 38 [4 – 772] |
| Serum creatinine | 0.88 [0.36 – 1.94] |
| Total bilirubin | 0.6 [0.2 – 2] |
| LDH | 765 [301 – 8425] |
| Cytogenetics | |
| Diploid, -Y | 38 (32) |
| Adverse | 48 (41) |
| Intermediate | 24 (21) |
| Insuff. Metaphases/ ND | 7 (6) |
| Mutations | |
| RAS (KRAS=5, NRAS=20) | 25 (21) |
| TP53 | 20 (17) |
| DNMT3a | 20 (17) |
| NPM1 | 18 (15) |
| FLT3-ITD | 12 (10) |
| IDH1 | 10 (8) |
| RUNX1 | 10 (8) |
| CEBPA | 9 (8) |
| ASXL1 | 9 (8) |
| IDH2 | 8 (7) |
| PTPN11 | 7 (6) |
| JAK2 | 6 (5) |
| KIT | 4 (3) |
| TET2 | 4 (3) |
| FLT3-D835 | 3 (3) |
| EZH2 | 3 (3) |
| ATM | 1 (1) |
| EGFR | 1 (1) |
| GATA2 | 1 (1) |
| MLL | 1 (1) |
| MPL | 1 (1) |
| NOTCH1 | 1 (1) |
| WT1 | 1 (1) |
Figure 1. Mutational, Karyotypic Landscape and Response to Therapy in patients with AML.
Each column represents an individual patient treated on study. The top row indicates the response to therapy with cladribine and low-dose cytarabine alternating with DAC. The next rows indicate cytogenetic (cyto) risk category, the presence or absence of complex karyotype ( ≥ 3 abnormalities), the number of mutations present at diagnosis, and the presence or absence of each mutation listed.
Efficacy
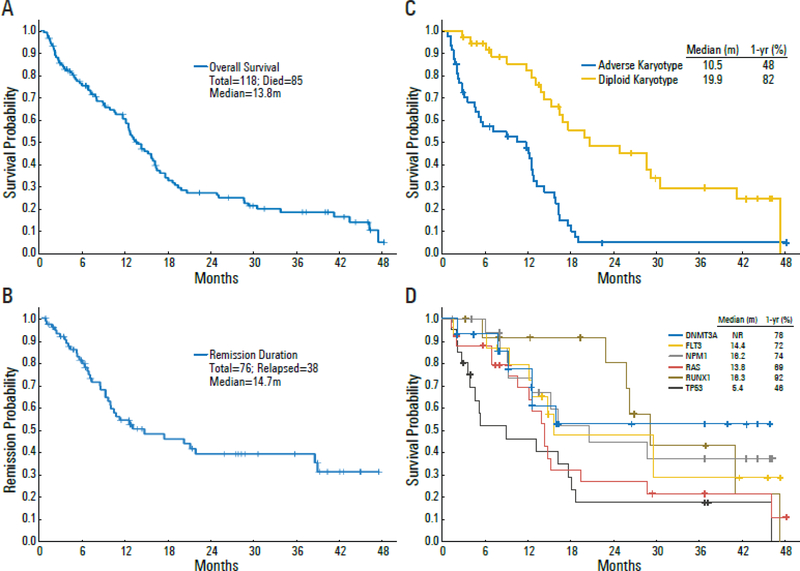
All patients were evaluable for response, summarized in Table 2. Sixty-nine patients (58%) achieved CR, and 11 patients (9%) achieved CRi (7 of which had a CRp) for an overall CR/CRi rate of 68%. Patients needed a median of 1 (range, 1 – 4) cycle to achieve a response, although delayed responses were seen with continued therapy in some patients. The cumulative rate of CR/CRi was 48% after cycle 1, 62% after cycle 2, 65% after cycle 3, and 68% after 4 cycles. Among patients who achieved a CR/CRp, the median remission duration was 14.7 months (Figure 2B). The median OS for the entire group was 13.8 months, with one and two year OS rates of 64% and 28%, respectively (Figure 2A). Among responders, the median OS was 16.2 months; the one and two year OS rates were 72% and 36%, respectively. The median DFS was 10.8 months (Appendix page 12).
Table 2.
Responses and Early Mortality of patients with AML
| Response / Outcome | N | % |
|---|---|---|
| Evaluable for Response | 118 | 100 |
| CR | 69 | 58 |
| CRi | 11 | 9 |
| OR (CR + CRi) | 80 | 68 |
| No Response | 29 | 25 |
| Died ≤ 4 weeks | 1 | 1 |
| Median No. of cycles given (range) | 3 (1 – 18) | |
| Median No. of cycles to response (range) | 1 (1 – 4) |
Figure 2. Overall Survival and Remission Duration of patients in the study.
(A) Overall survival of total cohort. (B) Remission duration in patients achieving CR/CRi. (C) Overall survival of patient with diploid karyotype vs. adverse karyotype. (D) Overall survival in patient subsets by pretreatment mutation analysis. ‘m’: months; ‘NR’: not reached.
Two patients under age 60 years were treated on study, both who had extensive prior anthracycline exposure and one who had a myocardial infarction prior to starting therapy. Among the 116 patients age ≥ 60 years (median 69 years) the CR/CRi rate was 66%, with a median OS of 13.8 months, and median DFS of 10 months. Among patients ≥ 70 years (median 73 years), the CR/CRi rate was 71%, with median OS of 12.6 months and median DFS of 7.2 months (Appendix page 13).
Availability of baseline disease characteristics including karyotype and mutation status allowed determination of outcomes within specific genetic subgroups. These are summarized in Appendix page 14 and Figure 2. Among patients with a diploid karyotype, the CR/CRi rate was 84%, the median OS was 19.9 months and the one-year OS rate was 82% (Figure 2C). In contrast, response rate and OS were inferior in patients with adverse karyotypes: CR/CRi = 50%; median OS = 10.5 months; the same worse outcome was noted in patients with TP53 mutation: CR/CRi = 40%; median OS = 5.4 months.
Of the 80 patients who achieved a CR/CRi, 18 (23%) patients [median age 64 years (50 – 75)], went on to receive allogeneic stem cell transplant (SCT) while 62 [median age 71 years (49 – 95)] did not. There was no significant difference in survival between patients in remission who did or did not receive an allogeneic SCT (median OS: SCT 16.4 months vs. NO-SCT 15.9 months; P = 0.18). Of the patients who went for SCT, 9 (50%) had relapse with a median CR duration of 5.4 months from SCT, and 3 (17%) had non-relapse mortality.
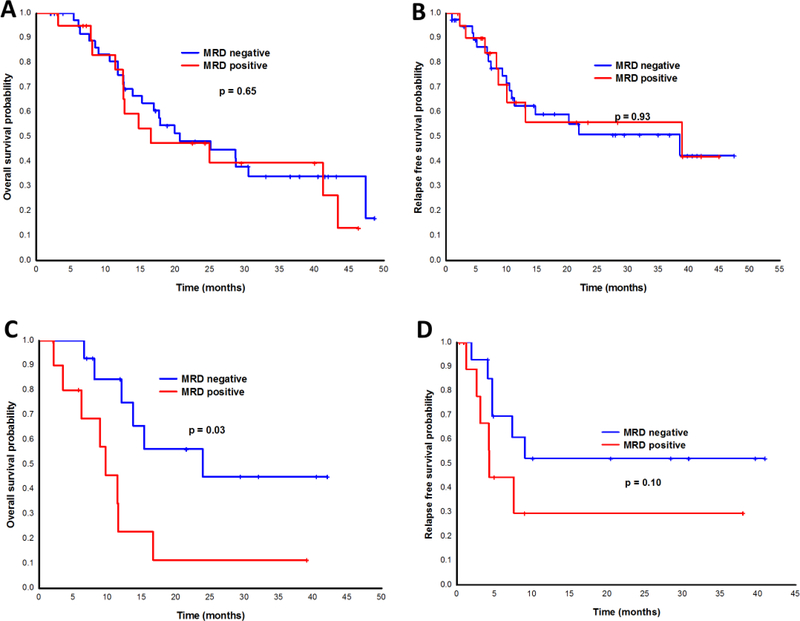
Minimal residual testing (MRD) by multiparameter flow cytometry was performed at the time of response assessment in 71 patients. MRD testing to determine the depth of response was done after cycle 1 (approximately Day 30) and after cycle 2 (approximately Day 60). Fifty-two patients had achieved a response (CR/CRi) by the Day 30 time point and had MRD testing available. Of these, 27 (52%) were MRD negative by flow cytometry. Twenty-six patients had a response at 60 days and available MRD testing. Of these, 13 (50%) patients were MRD negative. MRD positivity measured at 60 days, but not 30 days, was predictive for inferior OS and trended for inferior DFS (Figure 3).
Figure 3. Outcomes by Minimal Residual Disease (MRD) Detection in patients with AML.
(A) Overall survival and (B) disease-free survival of patients by flow cytometry MRD detection at day 30. (C) Overall survival and (D) disease-free survival of patients by flow cytometry MRD detection at day 60.
Toxicity
Overall the regimen was well tolerated in this cohort of older AML patients. Myelosuppression was universal. Non-hematologic toxicities deemed at least possibly-related to study treatment are summarized in Table 3. Most toxicities were Grade 1 or 2 in severity, were self-limiting, and resolved after supportive care. The most common non-hematologic adverse events attributed to treatment were elevated bilirubin, rash, and nausea. Two patients developed grade 2/3 renal failure and one required hemodialysis. One patient (1%) died within the first 4-weeks, and 8 (7%) within the first 8-weeks on study. All patients who died within the first 8-weeks on therapy had not achieved a response in their leukemia. The patient who died within the first 4 weeks had refractory t-AML from treatment of metastatic endometrial cancer, who developed gram negative bacteremia, septic shock, and multi-system organ failure. There were 88 documented grade 3 or higher infectious events, 9 (10%) of which resulted in deaths on study. There were 4 clinically significant hemorrhagic events deemed unrelated to treatment, including 2 patients with CNS bleed (1 grade 5), 1 grade 4 gastrointestinal bleed, and 1 grade 3 vaginal bleed.
Table 3.
Non-Hematologic Adverse Events (At least possibly related) in patients with AML
| Adverse Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Total |
|---|---|---|---|---|---|---|
| Infection (Per occurrence) | 77 | 2 | 9 | 88 | ||
| Elevated total bilirubin | 13 | 11 | 2 | 26 | ||
| Rash | 12 | 1 | 13 | |||
| Nausea | 13 | 13 | ||||
| Diarrhea | 8 | 1 | 9 | |||
| AST/ALT Elevation | 6 | 1 | 7 | |||
| Elevated creatinine | 3 | 3 | 1 | 7 | ||
| Mucositis | 5 | 2 | 7 | |||
| Constipation | 6 | 6 | ||||
| Pruritis | 2 | 2 | 4 | |||
| Cardiac Arrythmia | 2 | 1 | 1 | 4 | ||
| Cardiac, Other (NSTEMI, CHF, Hypotension) | 4 | 4 | ||||
| Headache | 2 | 1 | 3 | |||
| Fatigue | 2 | 1 | 3 | |||
| Tumor Lysis Syndrome | 3 | 3 | ||||
| Dry Skin | 1 | 1 | 2 | |||
| Neuropathy | 1 | 1 | 2 | |||
| Musculoskeletal Pain | 1 | 1 | 2 | |||
| Edema | 1 | 1 | 2 | |||
| Anorexia | 1 | 1 | ||||
| Confusion | 1 | 1 | ||||
| Dizziness | 1 | 1 | ||||
| Hiccups | 1 | 1 | ||||
| Hand Foot Syndrome | 1 | 1 | ||||
| Tremor | 1 | 1 | ||||
| Vomiting | 1 | 1 |
Genetic Subgroups
In an exploratory fashion, we correlated response and outcome in genetically defined subgroups (Appendix, page 14). Among the individual molecular subgroups, the highest rates of CR/CRi were seen in patients with mutations in NPM1 (100%), FLT3-ITD and FLT3-D835 (87%), and DNMT3a (85%). These were also associated with relatively good survival, with one-year survival rates ranging from 72–78% (Figure 2D). NPM1, methylation and signaling gene mutations are frequently co-occurring in AML. When accounting for overlapping detection of mutations in these three genes, a total of 34/39 patients (87%) with a mutation detected in any combination of NPM1, FLT3 (FLT3-ITD and/or FLT-D835), and DNMT3A achieved CR/CRi. Among patients with NPM1 mutation and/or any DNA-methylation gene mutation, 37/45 patients (82%) achieved CR/CRi, and among non-_NPM1_-mutated patients with any DNMT3A or FLT3 mutation, 15/20 (75%) achieved CR/CRi. In contrast, the most difficult group were patients with _TP53_-mutated AML, who had a CR/CRi rate of 40% and one-year OS probability of 46%. Co-mutations may have a role in modifying risk profile among individual patients with multiple mutations, but progressively smaller subgroups limited meaningful analysis. The relationships between co-mutations, cytogenetics, and response are depicted in Figure 1.
DISCUSSION
The current lower-intensity approach to the treatment of older and unfit patients with AML remains unsatisfactory. With the aim to improve outcomes, we investigated a new therapeutic combination of cladribine and LDAC alternating with DAC in older patients with AML. This is, to our knowledge, the first report of our prospective phase II study with 118 patients, carefully annotated with pretreatment genetic testing and assessment of MRD by multiparameter flow cytometry. Their median age was 69 years, 44% of whom were over the age of 70 years. The CR/CRi rate was 68%, and the median overall survival was about 14 months. Over half the patients achieved MRD negativity at the time of CR. Unlike intensive chemotherapy, however, Day 60 rather than Day 30 MRD predicted for OS. In patients who achieved a response, the median OS was 16.2 months compared to 4.7 months in those who did not. Among patients with a diploid karyotype, the CR/CRi rate was 84%, and the median OS was 19.9 months. The regimen was very well tolerated in this patient population, with 4- and 8-week mortality rates of 1% and 7%, respectively.
These results can be viewed in the context of currently available therapies that are recommended for older or unfit patients with newly diagnosed AML. HMAs such as AZA or DAC were initially developed and approved for the treatment of myelodysplastic syndrome. Several single-arm and randomized studies have confirmed their safety and efficacy in AML and form the basis for current practice. Frontline single-arm studies of AZA in older patients with AML have reported overall response rates of 25–31% and median OS ranging from 7.7 to 9.4 months.24–26 For DAC, response rates have been reported in the range of 24–26%, with a median OS of 5.5 to 7.7 months.27,28 In a post hoc analysis of a randomized phase III trial of AZA versus conventional care regimens (CCR) in AML patients with marrow blasts of 20–30%, AZA resulted in a response rate of 18% and was associated with an improvement in OS.29 A subsequent phase III trial (AZA-001) specifically studied older patients with newly diagnosed AML with marrow blast ≥ 30% and WBC < 15. AZA was associated with a CR/CRi rate of 28% and a trend for improved OS compared to CCR (10.4 months vs. 6.5 months, HR=0.84; P=0.08).7 After a preplanned censoring for subsequent treatment, the survival benefit for AZA achieved statistical significance (12.1 vs. 6.9 months, HR = 0.75; P=0.01).7 In a similar phase III trial with DAC (DACO-016) for older patients with newly diagnosed AML, DAC produced a CR/CRi rate of 17.8% compared to 7.8% for the control arm, translating to an improved OS of 7.7 months versus 5 months (HR 0.85, P=0.11).8
Results of the current trial compare favorably to the experience with HMAs in AML and are comparable to results of intensive chemotherapy, with less toxicity and lower rates of early mortality. In the large Swedish registry study examining the role of intensive chemotherapy in older patients with AML, CR rates ranged from 41–65%, and varied significantly by age and performance status.30 The early mortality (30-day) rates ranged from 9% in patients aged 60–64 years to 26% for those over the age of 80, with even higher mortality rates in those with poor performance status.30 Overall survival among older patients ranged from 80–184 days overall and 189–385 days among patients selected for intensive chemotherapy, similar to but lower than OS among older subsets on this study. In a retrospective single-institution study comparing intensive chemotherapy to epigenetic therapy in older patients with AML (median age 71 years), we reported a CR/CRp rate of 47%, 8-week mortality of 18%, and a median OS of 6.7 months with intensive chemotherapy.31 With better supportive care, early death rate has improved over time, even with intensive chemotherapy.32
The cladribine/LDAC regimen was well tolerated. Myelosuppression, as expected, occurred in all patients. The most common non-hematologic toxicities were transient elevations in liver function tests and nausea. Two patients developed clinically significant renal failure – one of whom required hemodialysis. This compares favorably with our prior experience using clofarabine in a similar low-intensity protocol, which had a higher frequency of clinically relevant kidney and liver toxicity in patients with AML.9. All patients were hospitalized during the first cycle of therapy, and subsequent therapy was administered as an outpatient. During the outpatient cycles of cladribine and LDAC, the LDAC was self- or home-administered when feasible to help reduce the number of hospital visits. Eight patients (7%) died during the first 8-weeks on therapy, and all 8 had persistent or resistant disease at the time of death.
The recent discovery of recurrent somatic mutations, paired with existing knowledge of recurrent cytogenetic abnormalities has made it clear that AML is a heterogeneous disease and that pretreatment genetic abnormalities play an important role in prediction of response and prognostication. In our study, we observed notable variations in outcome in several subsets. In addition to diploid karyotype predicting for high response rates and good long term survival, we noted high response rates among patients with NPM1 mutation, DNMT3a mutation, and FLT3-ITD mutation. Survival was better than average for patients with mutations in NPM1, RUNX1, and DNMT3a relative to the entire cohort.. Conversely, mutation in TP53 was associated with adverse karyotype, lower response rates, and significantly inferior OS compared with those patients without a TP53 mutation. Of course, these mutations do not occur in isolation and co-mutations have been shown to modify outcomes.33 However, comparing ever-smaller subgroups would be difficult to analyze due to small numbers and is a limitation of this study. The landscape of co-mutations, cytogenetics, and outcome have been depicted further in Figure 2 and the Appendix for reference. While exploratory, we hope that these initial differential signals of activity will promote further investigation.
Recurrent mutations in AML have not only refined prognostication, but have also provided insight into the disease pathobiology, leading to the development of target-specific small molecular inhibitors that exploit this biology. FLT3 inhibitors (sorafenib, midostaurin, others) have demonstrated significant activity in patients with _FLT3_-mutated AML when combined with chemotherapy.34,35 Inhibitors of mutant IDH1 and IDH2 enzymes have demonstrated response rates of 30–40% in relapsed _IDH_-mutant AML.36,37 Venetoclax, a BCL-2 inhibitor, appears to have significant activity in combination with chemotherapy.38 Each of these classes of agents have been combined with HMAs for older patients with AML. A phase Ib study of venetoclax in combination with HMAs recently reported good tolerability and CR/CRi rates of 60–65% in older patients with AML.38 Similarly, a preliminary analysis of IDH1 or IDH2 inhibitors combined with HMAs in newly diagnosed patients with IDH mutated AML has reported an overall response rate of 50%.39 As we move into an era of target-specific therapy for AML, the optimal chemotherapy backbone must come into focus. While HMAs have been utilized as the de facto standard low-intensity therapy, our data suggest superior response rates, better long-term survival, and excellent tolerability in patients treated with cladribine plus LDAC relative to prior experience with HMAs.. Does the low intensity backbone for combination studies need to be re-examined? Larger, randomized trials are needed to answer this question.
This underscores to an important limitation of this study. This is a single-arm study with comparator data limited to historical and published experience. Inherent in this is the difficulty in comparing patients across studies and the risk of selection bias. However, to try and minimize selection bias, the study was positioned as the priority study for newly diagnosed, older patients with AML, with the goal of enrolling consecutive AML patients that met screening criteria. Inclusion criteria were not overly stringent, to allow broad enrollment of patients, including those with recent or concurrent non-AML malignancy and ts-AML. Patients with favorable karyotype AML were not treated on the current trial and offered different, more intensive approaches. While initial therapy for AML is important in determining longer term outcome, subsequent therapy at the time relapse also plays a role. Since most of our patients were referred to us from outside, limited information was available about subsequent treatments in patients who relapsed. We did, however, have information on 19 patients who were refractory to therapy on protocol and decided to continue treatment at our center; 10 (54%) patients received HMA-based therapy with 3 (43%) responses, 7 (37%) received intensive chemotherapy with 3 (43%) responses, and 2 (11%) received investigational therapy with 1 response (50%). The median OS of non-responding patients was 4.7 months.
In summary, the prolonged, lower-intensity regimen of cladribine plus LDAC alternating with DAC was well tolerated and highly effective in an older or unfit population of patients with AML. Response to therapy was associated with longer survival. Pretreatment genomic testing identified subsets of patients with distinct clinical outcomes. Further studies are needed to confirm the activity of this regimen compared to HMAs and its role in lower intensity combination therapy.
Supplementary Material
1
Research in Context.
Evidence before this study
A systematic review was not performed prior to initiating the trial. However, the literature was reviewed with PubMed for studies from 2000 to present reporting the outcomes in older patients with newly diagnosed acute myeloid leukemia (AML). Several retrospective studies and prospective clinical trials indicated that outcomes for older patients have been poor, associated with low response rates to standard chemotherapy and high early mortality. Recent single-arm and randomized trials studying lower intensity approaches such as low-dose cytarabine or hypomethylating agents (HMAs) in older AML report remission rates in the range of 18–28% and median overall survivals of 8–10 months. Based on evidence of synergy with cladribine and cytarabine in AML, we designed a lower-intensity approach to study this combination for newly diagnosed older patients. We hypothesized that this combination would produce higher remission rates, better DFS, and better OS compared to the historical experience with HMAs in this population.
Added value of this study
In this single-arm, phase II trial, we studied the combination of cladribine and low-dose cytarabine alternating with decitabine as low intensity therapy for older patients with newly diagnosed AML. To our knowledge, this is the first report of this combination of cladribine and low-dose cytrarabine tested. in older patients (aged ≥ 60 years).with AML.The regimen produced high response rates and improved survival outcomes compared with currently established lower-intensity therapies. The regimen was highly tolerable with 4- and 8-week mortality rates of 1% and 7%, respectively. Moreover, pretreatment cytogenetic and molecular characterization allowed exploratory analyses of remission rates and survival in distinct subsets of patients.
Implications of all the available evidence
Our findings indicated that the combination of cladribine and low-dose cytarabine alternating with decitabine is a highly active and well tolerated regimen for older patients with AML. Confirmation of these data in a randomized phase III trial might potentially supplant hypomethylating agents as the de facto standard lower- intensity backbone for AML, providing more effective therapy and improved outcomes.
ACKNOWLEDGEMENTS
The study has been supported in part by the MD Anderson Cancer Center Support Grant P30 CA016672 and Award Number P01 CA049639. None of the authors declare any relevant conflict of interest related to this work.
Funding
The study has been supported in part by the NIH Cancer Center Support Grant P30 CA016672 and Award Number P01 CA049639.
The study has been supported in part by the MD Anderson Cancer Center Support Grant P30 CA016672 and Award Number P01 CA049639
The study has been presented in part during annual meetings of the American Society of Clinical Oncology (ASCO 2014) and the European Hematology Association (EHA 2016)
REFERENCES
- 1.Surveillance Epidemiology and End Results Program of the National Cancer Institute. SEER Cancer Stat Facts: Acute Myeloid Leukemia (AML). 2018.
- 2.Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. The New England journal of medicine 2009; 361(13): 1249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willemze R, Suciu S, Meloni G, et al. High-dose cytarabine in induction treatment improves the outcome of adult patients younger than age 46 years with acute myeloid leukemia: results of the EORTC-GIMEMA AML-12 trial. J Clin Oncol 2014; 32(3): 219–28. [DOI] [PubMed] [Google Scholar]
- 4.Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017; 129(4): 424–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kantarjian H, O’Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer 2006; 106(5): 1090–8. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian H, Ravandi F, O’Brien S, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood 2010; 116(22): 4422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015; 126(3): 291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol 2012; 30(21): 2670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kadia TM, Faderl S, Ravandi F, et al. Final results of a phase 2 trial of clofarabine and low-dose cytarabine alternating with decitabine in older patients with newly diagnosed acute myeloid leukemia. Cancer 2015; 121(14): 2375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spurgeon S, Yu M, Phillips JD, Epner EM. Cladribine: not just another purine analogue? Expert opinion on investigational drugs 2009; 18(8): 1169–81. [DOI] [PubMed] [Google Scholar]
- 11.Wyczechowska D, Fabianowska-Majewska K. The effects of cladribine and fludarabine on DNA methylation in K562 cells. Biochemical pharmacology 2003; 65(2): 219–25. [DOI] [PubMed] [Google Scholar]
- 12.Holowiecki J, Grosicki S, Giebel S, et al. Cladribine, but not fludarabine, added to daunorubicin and cytarabine during induction prolongs survival of patients with acute myeloid leukemia: a multicenter, randomized phase III study. J Clin Oncol 2012; 30(20): 2441–8. [DOI] [PubMed] [Google Scholar]
- 13.Holowiecki J, Grosicki S, Robak T, et al. Addition of cladribine to daunorubicin and cytarabine increases complete remission rate after a single course of induction treatment in acute myeloid leukemia. Multicenter, phase III study. Leukemia 2004; 18(5): 989–97. [DOI] [PubMed] [Google Scholar]
- 14.Gandhi V, Estey E, Keating MJ, Chucrallah A, Plunkett W. Chlorodeoxyadenosine and arabinosylcytosine in patients with acute myelogenous leukemia: pharmacokinetic, pharmacodynamic, and molecular interactions. Blood 1996; 87(1): 256–64. [PubMed] [Google Scholar]
- 15.Kornblau SM, Gandhi V, Andreeff HM, et al. Clinical and laboratory studies of 2-chlorodeoxyadenosine +/− cytosine arabinoside for relapsed or refractory acute myelogenous leukemia in adults. Leukemia 1996; 10(10): 1563–9. [PubMed] [Google Scholar]
- 16.Santana VM, Mirro J Jr., Kearns C, Schell MJ, Crom W, Blakley RL. 2-Chlorodeoxyadenosine produces a high rate of complete hematologic remission in relapsed acute myeloid leukemia. J Clin Oncol 1992; 10(3): 364–70. [DOI] [PubMed] [Google Scholar]
- 17.Xu J, Jorgensen JL, Wang SA. How Do We Use Multicolor Flow Cytometry to Detect Minimal Residual Disease in Acute Myeloid Leukemia? Clinics in laboratory medicine 2017; 37(4): 787–802. [DOI] [PubMed] [Google Scholar]
- 18.Luthra R, Patel KP, Reddy NG, et al. Next-generation sequencing-based multigene mutational screening for acute myeloid leukemia using MiSeq: applicability for diagnostics and disease monitoring. Haematologica 2014; 99(3): 465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin P, Jones D, Medeiros LJ, Chen W, Vega-Vazquez F, Luthra R. Activating FLT3 mutations are detectable in chronic and blast phase of chronic myeloproliferative disorders other than chronic myeloid leukemia. American journal of clinical pathology 2006; 126(4): 530–3. [DOI] [PubMed] [Google Scholar]
- 20.Thall PF, Wooten LH, Tannir NM. Monitoring event times in early phase clinical trials: some practical issues. Clinical trials 2005; 2(6): 467–78. [DOI] [PubMed] [Google Scholar]
- 21.Thall PF, Simon RM, Estey EH. Bayesian sequential monitoring designs for single-arm clinical trials with multiple outcomes. Statistics in medicine 1995; 14(4): 357–79. [DOI] [PubMed] [Google Scholar]
- 22.Mantel N Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer chemotherapy reports 1966; 50(3): 163–70. [PubMed] [Google Scholar]
- 23.Boddu P, Kantarjian HM, Garcia-Manero G, et al. Treated secondary acute myeloid leukemia: a distinct high-risk subset of AML with adverse prognosis. Blood advances 2017; 1(17): 1312–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Ali HK, Jaekel N, Junghanss C, et al. Azacitidine in patients with acute myeloid leukemia medically unfit for or resistant to chemotherapy: a multicenter phase I/II study. Leuk Lymphoma 2012; 53(1): 110–7. [DOI] [PubMed] [Google Scholar]
- 25.Maurillo L, Venditti A, Spagnoli A, et al. Azacitidine for the treatment of patients with acute myeloid leukemia: report of 82 patients enrolled in an Italian Compassionate Program. Cancer 2012; 118(4): 1014–22. [DOI] [PubMed] [Google Scholar]
- 26.Thepot S, Itzykson R, Seegers V, et al. Azacitidine in untreated acute myeloid leukemia: a report on 149 patients. Am J Hematol 2014; 89(4): 410–6. [DOI] [PubMed] [Google Scholar]
- 27.Cashen AF, Schiller GJ, O’Donnell MR, DiPersio JF. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol 2010; 28(4): 556–61. [DOI] [PubMed] [Google Scholar]
- 28.Lubbert M, Ruter BH, Claus R, et al. A multicenter phase II trial of decitabine as first-line treatment for older patients with acute myeloid leukemia judged unfit for induction chemotherapy. Haematologica 2012; 97(3): 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol 2010; 28(4): 562–9. [DOI] [PubMed] [Google Scholar]
- 30.Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood 2009; 113(18): 4179–87. [DOI] [PubMed] [Google Scholar]
- 31.Quintas-Cardama A, Ravandi F, Liu-Dumlao T, et al. Epigenetic therapy is associated with similar survival compared with intensive chemotherapy in older patients with newly diagnosed acute myeloid leukemia. Blood 2012; 120(24): 4840–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Othus M, Kantarjian H, Petersdorf S, et al. Declining rates of treatment-related mortality in patients with newly diagnosed AML given ‘intense’ induction regimens: a report from SWOG and MD Anderson. Leukemia 2014; 28(2): 289–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. The New England journal of medicine 2016; 374(23): 2209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ravandi F, Cortes JE, Jones D, et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol 2010; 28(11): 1856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. The New England journal of medicine 2017; 377(5): 454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 2017; 130(6): 722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DiNardo CD, Stein EM, de Botton S, et al. Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. The New England journal of medicine 2018; 378(25): 2386–98. [DOI] [PubMed] [Google Scholar]
- 38.DiNardo CD, Pratz KW, Letai A, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. The Lancet Oncology 2018; 19(2): 216–28. [DOI] [PubMed] [Google Scholar]
- 39.DiNardo CD, Stein AS, Fathi AT, et al. Mutant Isocitrate Dehydrogenase (mIDH) Inhibitors, Enasidenib or Ivosidenib, in Combination with Azacitidine (AZA): Preliminary Results of a Phase 1b/2 Study in Patients with Newly Diagnosed Acute Myeloid Leukemia (AML). Blood 2017; 130(Suppl 1): 639- [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1