Key Role of 5-HT1B Receptors in the Regulation of Paradoxical Sleep as Evidenced in 5-HT1B Knock-Out Mice (original) (raw)
Abstract
The involvement of 5-HT1B receptors in the regulation of vigilance states was assessed by investigating the spontaneous sleep–waking cycles and the effects of 5-HT receptor ligands on sleep in knock-out (5-HT1B−/−) mice that do not express this receptor type. Both 5-HT1B−/− and wild-type 129/Sv mice exhibited a clear-cut diurnal sleep–wakefulness rhythm, but knock-out animals were characterized by higher amounts of paradoxical sleep and lower amounts of slow-wave sleep during the light phase and by a lack of paradoxical sleep rebound after deprivation. In wild-type mice, the 5-HT1B agonists CP 94253 (1–10 mg/kg, i.p.) and RU 24969 (0.25–2.0 mg/kg, i.p.) induced a dose-dependent reduction of paradoxical sleep during the 2–6 hr after injection, whereas the 5-HT1B/1D antagonist GR 127935 (0.1–1.0 mg/kg, i.p.) enhanced paradoxical sleep. In addition, pretreatment with GR 127935, but not with the 5-HT1A antagonist WAY 100635, prevented the effects of both 5-HT1B agonists. In contrast, none of the 5-HT1B receptor ligands, at the same doses as those used in wild-type mice, had any effect on sleep in 5-HT1B−/− mutants. Finally, the 5-HT1Aagonist 8-OH-DPAT (0.2–1.2 mg/kg, s.c.) induced in both strains a reduction in the amount of paradoxical sleep. Altogether, these data indicate that 5-HT1B receptors participate in the regulation of paradoxical sleep in the mouse.
Keywords: serotonin, 5-HT1B receptor, paradoxical sleep, knock-out, mice
The idea that serotonin [5-hydroxytryptamine (5-HT)] is involved in the regulation of sleep–wakefulness cycles was proposed several decades ago (Koella et al., 1968; Jouvet, 1969) and has been further supported recently by using new means of investigations (Cespuglio et al., 1990; Portas and McCarley, 1994). The respective roles of various classes of central 5-HT receptors in this regulation have been investigated primarily by pharmacological means. Notably, it has been reported that 5-HT1A receptors are involved in the regulation of paradoxical sleep (PS) and wakefulness (W) (de Saint Hilaire-Kafi et al., 1987; Dzoljic et al., 1992; Tissier et al., 1993; Portas et al., 1996; Thakkar et al., 1998) and that 5-HT2A receptors participate in the control of slow-wave sleep (SWS) (Idzikowski et al., 1986; Dugovic et al., 1989).
Despite the development of numerous ligands in the past 15 years, it was not possible to investigate specifically the involvement of 5-HT1B receptors in the regulation of sleep–wakefulness cycles because of the paucity of selective agonists and antagonists able to cross the blood–brain barrier. Nevertheless, a few studies led to the suggestion that 5-HT1B receptor stimulation might exert a negative influence on PS (Dugovic et al., 1989; Dzoljic et al., 1992; Bjorvatn and Ursin, 1994).
Gene targeting is another means that allows a selective approach to study the role of a specific receptor in sleep regulations. To date, several groups have reported behavioral modifications in transgenic mutants (Montkowski et al., 1995; Sollars et al., 1996; Zhang et al., 1996; Tobler et al., 1997), notably the 5-HT1Breceptor gene knock-out (5-HT1B−/−) mutant mice (Saudou et al., 1994; Crabbe et al., 1996; Dulawa et al., 1997; Rocha et al., 1997).
The 5-HT1B receptor is located on both presynaptic serotoninergic terminals (Boschert et al., 1994), where it modulates 5-HT release (Engel et al., 1986), and nonserotoninergic terminals, where it modulates the release of, notably, acetylcholine (ACh) (Maura and Raiteri, 1986) and GABA (Stanford and Lacey, 1996). Interestingly, the latter two are involved in sleep–waking regulations (Gillin et al., 1985) at mesopontine tegmental (McCarley and Massaquoi, 1992) and basal forebrain (Cape and Jones, 1998) levels and in the dorsal raphe (Nitz and Siegel, 1997a), the locus ceruleus (Nitz and Siegel, 1997b), and the hypothalamic preoptic (Mendelson, 1998) nuclei.
The aim of the present study was to investigate the role of 5-HT1B receptors in sleep and wakefulness in mice. For this purpose, the spontaneous sleep–waking cycles and the recovery after selective paradoxical sleep deprivation were examined in 5-HT1B−/− mutants (Saudou et al., 1994) compared with wild-type 129/Sv mice. In addition, we analyzed in both strains the effects of treatments with 5-HT1B receptor ligands on the vigilance states. Studies were also performed with 5-HT1Areceptor ligands, whose well characterized effects on sleep and wakefulness in rodents (Dzoljic et al., 1992; Tissier et al., 1993) were used as a reference.
MATERIALS AND METHODS
All the procedures involving animals and their care were conducted in conformity with the institutional guidelines, which are in compliance with national and international laws and policies [Council Directive 87–848, October 19, 1987, from Ministère de l’agriculture et de la forêt, Service vétérinaire de la santé et de la protection animale, Permissions 0299 (to M.H.) and 0315 (to J.A.)].
Surgery
Wild-type (5-HT1B+/+) and 5-HT1B−/− mice, both with a pure 129/Sv genetic background (Ramboz et al., 1996), were used. At 2 months of age, when body weight was similar in both groups (range, 24–30 gm), animals were implanted under sodium pentobarbital anesthesia (70–75 mg/kg, i.p.) with the standard set of electrodes (made of enameled nichrome wire, 150 μm in diameter) for polygraphic sleep monitoring (Tissier et al., 1993). In brief, EEG electrodes were inserted through the skull onto the dura over the right cortex (2 mm lateral and 4 mm posterior to the bregma) and over the cerebellum (at midline, 2 mm posterior to lambda) (Tobler et al., 1997), electro-oculogram electrodes were positioned subcutaneously on each side of the orbit, and EMG electrodes were inserted into the neck muscles. All electrodes were anchored to the skull with superbond and acrylic cement (Limoge-Lendais et al., 1994) and soldered to a mini-connector also embedded in cement. After completion of surgery, animals were housed in individual cages (20 × 20 × 30 cm) and maintained under standard laboratory conditions: 12 hr light/dark cycle (light on at 7:00 A.M.), food and water available ad libitum, and 24 ± 1°C ambient temperature. The animals were allowed 7–10 d to recover, during which they were habituated to the recording conditions.
PS deprivation
Animals were placed for 12 hr, starting at the beginning of either the dark or the light period, on platforms (control conditions: 7.5 cm in diameter, 3 cm high; deprivation conditions: 3.5 cm in diameter, 4 cm high) surrounded by water (2 cm deep) (Pokk et al., 1996) at an ambient temperature of 25°C, with access to food and water ad libitum. At the end of this period, mice were returned to their home cage for 12 hr for recovery, the latter period thus occurring during the light or the dark period, respectively. Each mouse underwent the paired control and deprivation procedure (separated by at least 4 d), first during the dark period and second (at least 10 d later) during the light period.
Pharmacological procedures
Drugs were dissolved in 0.1 ml of saline, except CP 94253 [3-(1,2, 5,6-tetrahydro-4-pyridyl)-5-propoxypyrrolo[3,2-b]pyridine], which was dissolved in warm distilled water. All injections were performed at 9:30–10:00 A.M. WAY 100635 [_N_-[2-[4-(2-methoxyphenyl)-1-piperazinyl] ethyl]-_N_-(2-pyridinyl)cyclohexane carboxamide], GR 127935 [2′-methyl-4′-(5-methyl-[1,2,4] oxadiazol-3-yl)-biphenyl-4-carboxylic acid [4-methoxy-3-(4-methyl-piperazine-1-yl)-phenyl]amide], RU24969 [5-methoxy-3-(1,2,3,6-tetrahydro-4-pyridinyl)-1H-indole], and CP 94253 were injected intraperitoneally, and 8-OH-DPAT [8-hydroxy-2-(di-_n_-propylamino)-tetralin] was injected subcutaneously. A 15 min interval separated the two injections when animals were treated with an antagonist and then an agonist. For baseline data, mice were injected intraperitoneally or subcutaneously with the vehicle only, as appropriate. In each case, a delay of at least 48 hr separated two successive pharmacological tests to allow complete washout of drugs (Frances and Monier,1991; Koe et al., 1992; Pauwels, 1997).
Polygraphic recording
For the study of spontaneous sleep–waking cycles, each animal was recorded during 48 hr, beginning at 7:00 P.M., i.e., at the onset of the dark period. For PS deprivation experiments, mice were recorded during 24 consecutive hours, beginning at 7:00 P.M. for the first paired series and at 7:00 A.M. for the second one. For pharmacological studies, sleep–wakefulness parameters were recorded during the 8 hr after injections, i.e., from 10:00 A.M. to 6:00 P.M.
Data analysis and statistics
Polygraphic recordings were scored manually every 30 sec epoch, using the criteria validated for mice (Valatx and Bugat, 1974). Data were fed into a computer according to a method described previously (Tissier et al., 1993).
Spontaneous sleep–waking cycles. For each animal, the amounts of vigilance states were calculated over 3 hr periods throughout 48 hr and were averaged for the light and the dark phases. The mean ± SEM of these amounts (expressed in minutes) for each strain of mice was then used for calculating the ANOVA for the factor genotype. In case of significance (p < 0.05), the F test was followed by Student’s _t_test for mean comparisons.
PS deprivation. For each animal, the sleep amounts during the small platform condition and the following recovery period were compared with those during the large platform condition and the corresponding control recovery period, and expressed as percent of respective baseline. Two PS latencies were defined: one as the time interval between the beginning of the recovery phase and the first PS episode (PS latency) and the other as the time interval between the first SWS episode and the first PS one (intrasleep PS latency). Paired_t_ tests were performed to assess statistical significance of the data.
Pharmacological experiments. The effects of each dose of a given compound on each state of vigilance were analyzed for every 2 hr period after injection and expressed in minutes as mean ± SEM. The PS latency was defined as the time interval between the end of injection and the onset of the first PS episode. For a given treatment, each animal was referred to its own baseline represented by the data obtained after injection of vehicle. Statistical analyses were performed using ANOVA for the factor treatment, and in case of significance (p < 0.05), the F test was followed by Student’s t test (paired samples) for mean comparisons.
Chemicals
RU 24969 (0.25–5.0 mg/kg, i.p.) was obtained from Roussel-Uclaf (Romainville, France); WAY 100635 (0.05–1.0 mg/kg, i.p.) was from Wyeth Research (Princeton, NJ); 8-OH-DPAT (0.2–1.2 mg/kg, s.c.) was obtained from Research Biochemicals (Natick, MA); CP 94253 (1.0–10.0 mg/kg, i.p.) was from Pfizer Central Research (Groton, CT); and GR 127935 (0.1–1.0 mg/kg, i.p.) was from Glaxo-Wellcome (Ware, UK).
RESULTS
Previous studies have shown that 5-HT1B−/− mice develop normally, have no histologically detectable defects of the CNS, and do not exhibit obvious behavioral impairments (Ramboz et al., 1996). In the present study, we confirmed that 5-HT1B−/− mice had similar body weight as the wild-type mice and no apparent behavioral alterations.
Spontaneous sleep–wakefulness cycles
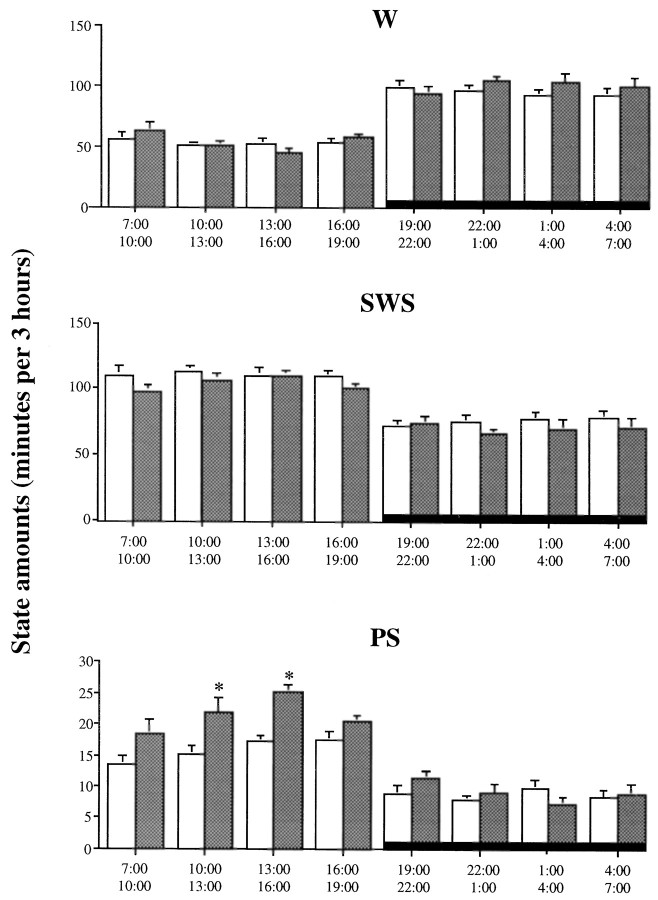
All mice exhibited a clear-cut diurnal sleep–waking rhythm, with larger amounts of sleep during the light period than during the dark one. Indeed, they spent ∼70% of the time asleep during the light phase (70.6 ± 0.8 and 69.8 ± 1.6% in seven 5-HT1B+/+ and eight 5-HT1B−/− mice, respectively) compared with ∼45% in the dark one (46.8 ± 1.8 and 44.0 ± 2.8%, respectively). However, the 5-HT1B−/− mice differed significantly (p < 0.05) from the wild-type mice by a greater amount of PS (11.9 ± 0.7% of total time compared with 8.9 ± 0.3% in the 5-HT1B+/+ group), at the expense of SWS (58.0 ± 1.3 and 61.8 ± 1.0%, respectively) during the 12 hr of the light phase (Table 1). No significant differences were found between the two groups during the dark phase.
Table 1.
Amounts of wakefulness (W), slow-wave sleep (SWS), and paradoxical sleep (PS) in 5-HT1B+/+ and 5-HT1B−/−mice
| State amounts (minutes) | ||||
|---|---|---|---|---|
| Genotype | Period | W | SWS | PS |
| 5-HT1B+/+ (n = 7) | light | 211.8 ± 5.5 | 444.8 ± 7.0 | 63.8 ± 2.3 |
| dark | 382.9 ± 13.2 | 302.5 ± 13.4 | 34.8 ± 1.9 | |
| 5-HT1B−/− (n = 8) | light | 216.8 ± 11.2 | 417.5 ± 9.3* | 86.1 ± 5.2* |
| dark | 403.2 ± 20.2 | 280.8 ± 20.0 | 36.4 ± 3.2 |
The analysis per 3 hr period indicates that the major difference between the two groups was a peak of PS in the middle of the light phase in mutant mice but not in 5-HT1B+/+ animals (Fig.1).
Fig. 1.
Diurnal variations of wakefulness (W), slow-wave sleep (SWS), and paradoxical sleep (PS) during 12 hr light/dark cycle (light on from 7:00 A.M. to 7:00 P.M.) in 5-HT1B+/+ (open bars) and 5-HT1B−/− (filled bars) mice. Data are expressed as min/3 hr (mean ± SEM of 7 and 8 animals, respectively). *p < 0.05, significantly different from the 5-HT1B+/+ group; Student’s _t_test.
Paradoxical sleep deprivation
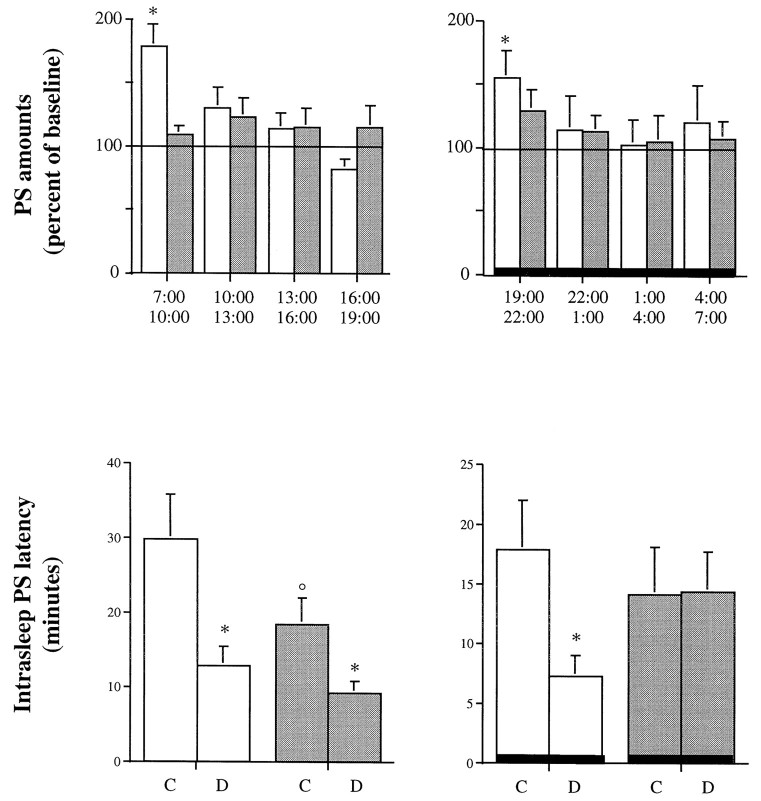
Only two mice (one in each strain) fell from the platform into the water during the deprivation protocol and were excluded from the analysis. During the deprivation periods (small platform), mice of both groups (n = 5–7) exhibited the same amounts of SWS but only 20–30% of PS (data not shown) compared with those observed under control conditions (large platform). Then, the amounts of PS in the wild-type group were significantly enhanced during the first 3 hr of the recovery period after PS deprivation for either the dark or the light phase (12 hr); in addition, the intrasleep PS latency (but not the PS latency) was reduced in wild-type mice (Fig.2). In contrast, in the 5-HT1B knock-out group, no significant increase in PS was observed for the recovery period (except for a trend after deprivation performed during the light phase), and the intrasleep PS latency was significantly reduced only after the deprivation performed during the dark phase (Fig. 2).
Fig. 2.
Paradoxical sleep characteristics observed after a 12 hr PS deprivation performed during either the preceding dark period (left) or the preceding light period (right) in 5-HT1B+/+ (open bars) and 5-HT1B−/− (filled bars) mice. Top, PS amounts (mean ± SEM of 5 and 7 animals, respectively) are expressed as percent of the paired values obtained under control conditions (large platform).Bottom, Intrasleep PS latency observed at recovery is expressed as minutes (mean ± SEM) after control (C, large platform) or deprivation (D, small platform) conditions. *p < 0.05, significantly different from control conditions; paired Student’s_t_ test. °p < 0.05, significantly different from the 5-HT1B+/+ group; Student’s_t_ test.
Under control conditions in which mice were on the large platform, differences between the two groups were observed only during the light period. Thus, PS amounts were larger (+25.1%; p < 0.05; data not shown), and intrasleep PS latencies were smaller (−30.0%; p < 0.05) (Fig. 2) in 5-HT1B−/− mutants than in wild-type 5-HT1B+/+ mice.
Effects of 5-HT1B receptor ligands
Agonists
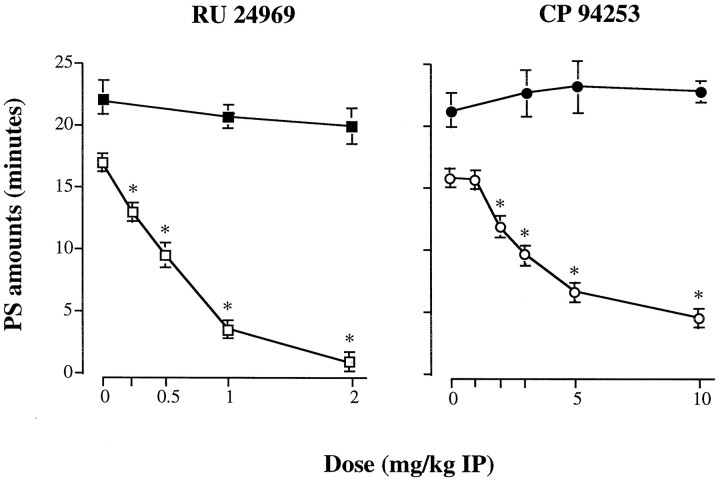
The 5-HT1A/1B agonist RU 24969 (Hoyer et al., 1994) (Figs. 3,4) and the selective 5-HT1Bagonist CP 94253 (Koe et al., 1992) (Table2, Fig. 4) induced a dose-related inhibition of PS during essentially the 2–6 hr after injection in wild-type mice (ANOVA; during the 0–4 hr period after treatment; RU 24969, F(4,31) = 78.68; p < 0.05; and CP 94253, F(5,30) = 39.5;p < 0.05) (Fig. 4). PS latency was significantly increased up to 257.8 ± 16.0 min (mean ± SEM;n = 8) with the highest dose of RU 24969 (2 mg/kg, i.p.), and 233.0 ± 14.8 min (mean ± SEM; _n_= 6) with that of CP 94253 (10 mg/kg, i.p.) compared with 45.3 ± 3.1 and 54.2 ± 5.3 min (p < 0.05; data not shown) after administration of the vehicle, respectively. In contrast, wakefulness and SWS were not modified, except at 1 and 2 mg/kg of RU 24969 (Fig. 3) and at 10 mg/kg of CP 94253 (Table 2) for which SWS was reduced during 2 and 4 hr, respectively, at the benefit of wakefulness. Finally, for both RU 24969 and CP 94253, no modification of sleep–waking cycles was observed during the 6–8 (Fig.3) and 4–8 (data not shown) hr periods after treatment, respectively.
Fig. 3.
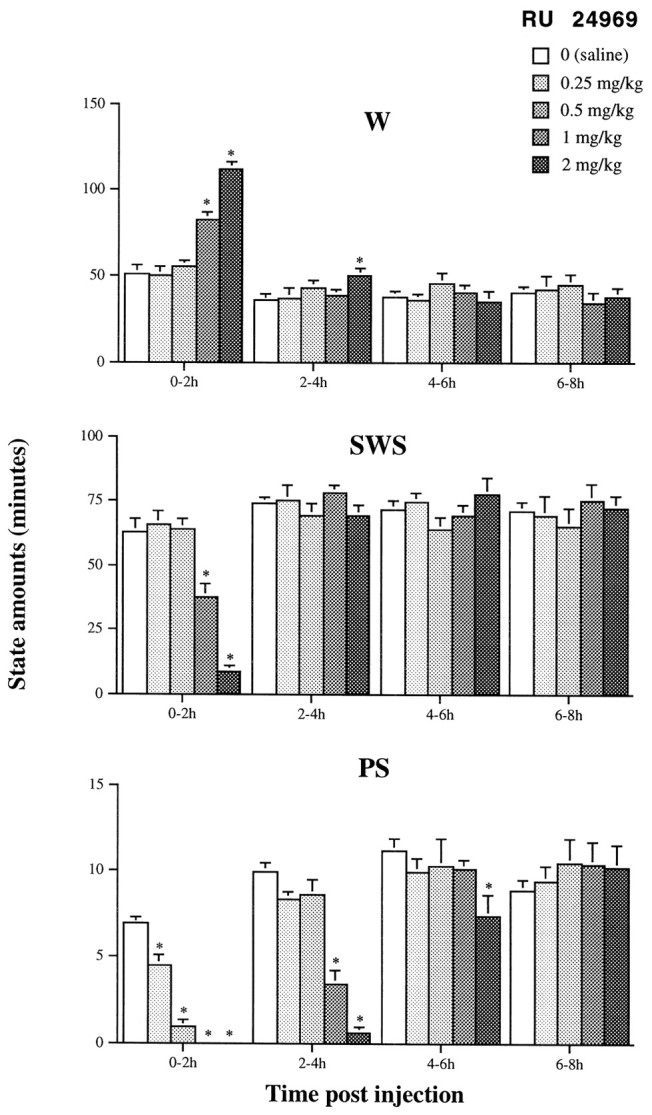
Effects of the 5-HT1A/1B agonist RU 24969 at various doses on sleep and wakefulness in 5-HT1B+/+ mice during the four successive 2 hr periods after injection. Results are expressed as min/2 hr (mean ± SEM of 8 animals; 6–8 tests for each dose). *p < 0.05, significantly different from baseline (open bars); paired Student’s t test.
Fig. 4.
Effects of RU 24969 (left) and CP 94253 (right) at various doses on PS in 5-HT1B+/+ (open symbols) and 5-HT1B−/− (filled symbols) mice during the 4 hr after injection in which an effect was observed. Results are expressed as minutes (mean ± SEM of 8 mice in each group for RU 24969 and 6 mice for CP 94253; 5–8 tests for each dose). *p < 0.05, significantly different from baseline (0 on abscissa); paired Student’s_t_ test. Complete set of data is available on request.
Table 2.
Effects of the 5-HT1B agonist CP 94253 at various doses on sleep and wakefulness in 5-HT1B+/+ and 5-HT1B−/− mice during the 4 hr after injection
| CP 94253 (mg/kg) | State amounts (minutes) | ||||
|---|---|---|---|---|---|
| 5-HT1B+/+ | 5-HT1B−/− | ||||
| 0–2 hr | 2–4 hr | 0–2 hr | 2–4 hr | ||
| W | 0 | 43.8 ± 4.8 | 30.0 ± 2.9 | 48.3 ± 2.7 | 40.5 ± 1.4 |
| 1 | 39.7 ± 2.8 | 36.0 ± 4.3 | |||
| 2 | 45.1 ± 1.9 | 31.5 ± 2.4 | |||
| 3 | 41.4 ± 3.2 | 41.8 ± 3.6 | 50.2 ± 4.6 | 35.0 ± 2.9 | |
| 5 | 48.6 ± 5.0 | 36.7 ± 3.5 | 44.9 ± 3.1 | 39.8 ± 2.6 | |
| 10 | 105.4 ± 4.5* | 56.2 ± 5.7* | 46.0 ± 1.9 | 35.1 ± 3.1 | |
| SWS | 0 | 69.8 ± 4.9 | 80.4 ± 3.2 | 62.8 ± 2.4 | 67.7 ± 2.1 |
| 1 | 73.8 ± 2.8 | 74.5 ± 4.3 | |||
| 2 | 71.6 ± 2.0 | 80.2 ± 2.9 | |||
| 3 | 77.0 ± 2.9 | 71.0 ± 4.0 | 61.8 ± 4.0 | 71.0 ± 1.7 | |
| 5 | 71.1 ± 4.9 | 78.3 ± 3.2 | 65.7 ± 3.3 | 66.9 ± 2.3 | |
| 10 | 14.6 ± 4.5* | 61.2 ± 6.0* | 65.4 ± 1.6 | 71.2 ± 2.8 | |
| PS | 0 | 6.4 ± 0.2 | 10.3 ± 0.6 | 8.8 ± 0.6 | 11.7 ± 0.6 |
| 1 | 6.5 ± 0.5 | 9.5 ± 1.0 | |||
| 2 | 3.1 ± 0.5* | 8.3 ± 0.7 | |||
| 3 | 1.7 ± 0.5* | 7.1 ± 0.8* | 8.1 ± 1.1 | 14.0 ± 1.6 | |
| 5 | 0.2 ± 0.2* | 4.9 ± 0.8* | 9.4 ± 0.7 | 13.2 ± 2.2 | |
| 10 | 0* | 2.6 ± 0.7* | 8.6 ± 0.6 | 13.8 ± 0.9 |
In the 5-HT1B−/− group, neither RU 24969 nor CP 94253, in the same dose ranges as those used in the 5-HT1B+/+ group, induced any significant alteration of sleep–wakefulness cycles (Fig.4, Table 2). However, at 3 and 5 mg/kg (data not shown), RU 24969 induced an inhibition of PS during 4 hr after injection (PS amounts of 8.9 ± 1.1 min; n = 8; and 0.3 ± 0.2 min;n = 5, respectively; compared with 22.0 ± 1.6 min after saline; p < 0.05). At 5 mg/kg, a consecutive PS rebound was observed during the 6–8 hr period after injection (PS amounts of 17.2 ± 1.6 min compared with 11.8 ± 1.8 min in saline-treated mice; mean ± SEM; n = 5;p < 0.05). Concomitantly, an increase in PS latency was observed in 5-HT1B−/− mice injected with 3 and 5 mg/kg RU 24969 (138.7 ± 11.1 min; n = 8; and 277.6 ± 23.6 min; n = 5, respectively; compared with 40.1 ± 3.2 min after saline; p < 0.05). The other states of vigilance were not affected, except wakefulness, which was significantly increased (+26 ± 8%; p < 0.05) during the first 2 hr after injection of 5 mg/kg RU 24969 (data not shown).
Antagonist
In 5-HT1B+/+ mice, the 5-HT1B/1Dantagonist GR 127935 (Pauwels, 1997), at the doses of 0.1, 0.5 (data not shown), and 1.0 (Fig. 5) mg/kg induced no modification of sleep–wakefulness during the first 2 hr period after treatment. Thereafter, a dose-dependent enhancement of PS amounts was observed (ANOVA; F(3,26) = 9.93;p < 0.05), in particular at 0.5 (data not shown) and 1.0 (Fig. 5) mg/kg. The other states of vigilance were not affected (data not shown).
Fig. 5.
Effects of the 5-HT1B/1D antagonist GR 127935 (hatched bars) on PS in 5-HT1B+/+ (left) and 5-HT1B−/− (right) mice during the four successive 2 hr periods after injection. Results are expressed as min/2 hr (mean ± SEM of 8 and 6 animals, respectively). *p < 0.05, significantly different from baseline (open bars); paired Student’s t test.
In 5-HT1B−/− mice, GR 127935 at 0.5 (data not shown) and 1.0 (Fig. 5) mg/kg had no effect on PS. However, an increase of SWS, at the expense of W, was observed for the first 2 hr after the administration of 0.5 mg/kg of this drug (data not shown).
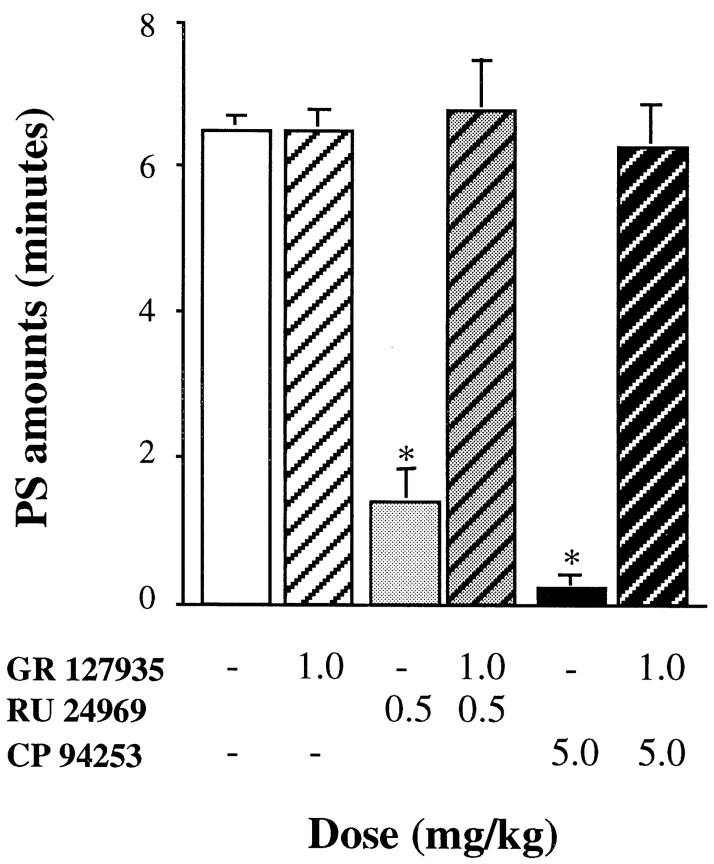
In 5-HT1B+/+ mice, the effects of both RU 24969 (0.5 mg/kg) and CP 94253 (5 mg/kg) on sleep–wakefulness cycles were prevented by pretreatment with GR 127935 at the dose of 1 mg/kg (Fig.6, Table3). In contrast, in 5-HT1B−/− mice, 1 mg/kg of GR 127935 (Table 3) did not prevent the effects of RU 24969 at the dose of 3 mg/kg (i.e., the dose inducing the same PS inhibition in 5-HT1B−/− mice as 0.5 mg/kg in wild-type mice) (Fig. 4).
Fig. 6.
Effects of the 5-HT1B/1D antagonist GR 127935 (hatched bars) on PS inhibition induced by RU 24969 (gray bars) or CP 94253 (black bars) in 5-HT1B+/+ mice during the 2 hr period after injection in which an effect was observed. Results are expressed as minutes (mean ± SEM of 8 animals; 8 and 6 tests for each treatment, respectively). *p < 0.05, significantly different from baseline (open bar); paired Student’s_t_ test. Complete set of data is available on request.
Table 3.
Effects of the 5-HT1B/1D antagonist GR 127935, in association with the 5-HT1A/1B agonist RU 24969, on sleep and wakefulness in 5-HT1B+/+ and 5-HT1B−/− mice during the first 2 hr after injection
| State amounts (minutes) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 5-HT1B+/+ | 5-HT1B−/− | |||||||
| GR 127935 (mg/kg) | RU 24969 (mg/kg) | n | 0–2 hr | GR 127935 (mg/kg) | RU 24969 (mg/kg) | n | 0–2 hr | |
| W | 0 | 0 | 8 | 42.3 ± 3.9 | 0 | 0 | 6 | 51.9 ± 3.2 |
| — | 0.5 | 7 | 56.6 ± 1.6 | — | 3 | 6 | 52.8 ± 4.2 | |
| 1 | 0.5 | 8 | 39.2 ± 2.6 | 1 | 3 | 6 | 48.8 ± 7.8 | |
| SWS | 0 | 0 | 8 | 71.2 ± 3.9 | 0 | 0 | 6 | 60.0 ± 3.6 |
| — | 0.5 | 7 | 62.0 ± 2.0 | — | 3 | 6 | 66.3 ± 4.0 | |
| 1 | 0.5 | 8 | 74.0 ± 2.8 | 1 | 3 | 6 | 70.4 ± 7.4 | |
| PS | 0 | 0 | 8 | 6.5 ± 0.2 | 0 | 0 | 6 | 8.9 ± 0.7 |
| — | 0.5 | 7 | 1.4 ± 0.6* | — | 3 | 6 | 0.8 ± 0.4* | |
| 1 | 0.5 | 8 | 6.8 ± 0.7 | 1 | 3 | 6 | 0.8 ± 0.5* |
Effects of 5-HT1A receptor ligands
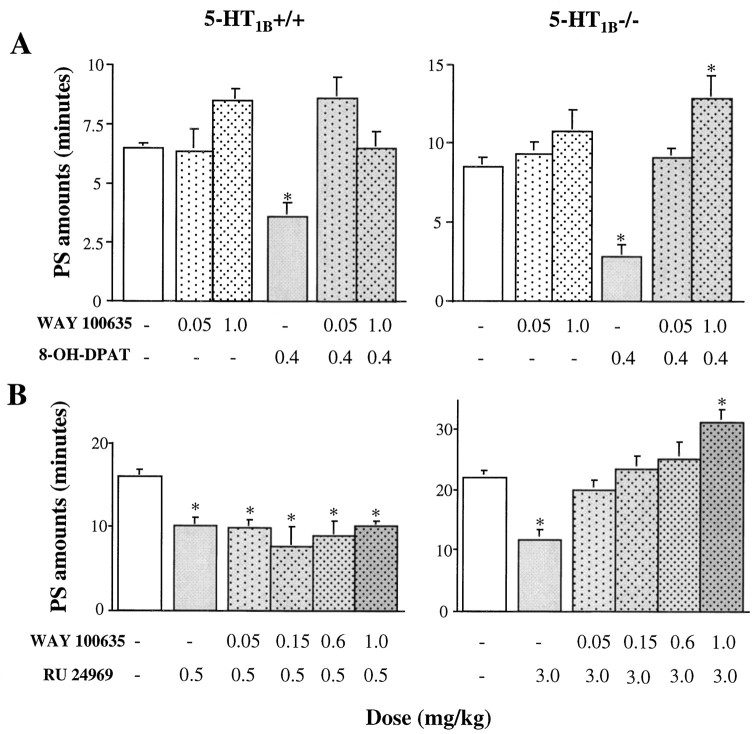
Agonist
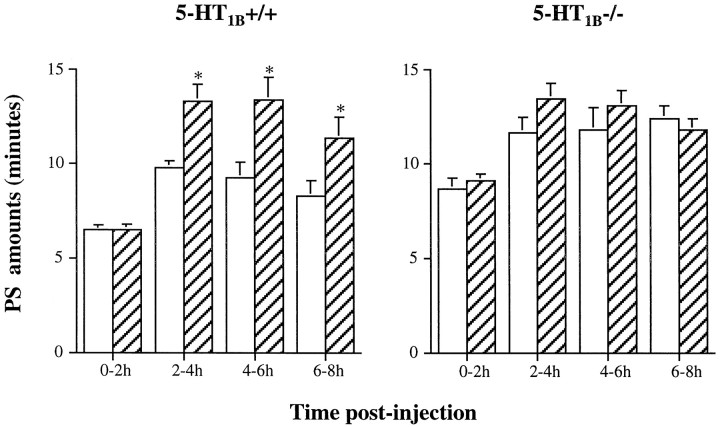
The 5-HT1A agonist 8-OH-DPAT (Hoyer et al., 1994) induced in all mice a dose-dependent inhibition of PS during the first 2 hr after injection (ANOVA; wild-type, F(4,27)= 33.93; p < 0.05; and knock-out,F(4,30) = 49.46; p < 0.05) (Table 4). This effect was significantly more pronounced in 5-HT1B−/− mice than in wild-type animals (p < 0.05; unpaired t test). In addition, in the 5-HT1B−/− group, a consecutive PS rebound was observed during the 4–6 hr after administration of 0.8 mg/kg 8-OH-DPAT, whereas such a rebound was observed at the dose of 1.2 mg/kg in the 5-HT1B+/+ group (Table 4). In both groups, 8-OH-DPAT also induced during the first 2 hr after injection an increase of W (ANOVA; 5-HT1B+/+ mice,F(4,27) = 14.08; p < 0.05; and 5-HT1B−/− mutants, F(4,30) = 14.89; p < 0.05), concomitant with a decrease of SWS (ANOVA; F(4,27) = 10.27; p < 0.05; and F(4,30) = 10.98; p < 0.05, respectively) (data not shown).
Table 4.
Effects of the 5-HT1A agonist 8-OH-DPAT at various doses on paradoxical sleep in 5-HT1B+/+ and 5-HT1B−/− mice during the 8 hr after injection
| 8-OH-DPAT (mg/kg) | PS amounts (minutes) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1B+/+ | 5-HT1B−/− | |||||||||
| n | 0–2 hr | 2–4 hr | 4–6 hr | 6–8 hr | n | 0–2 hr | 2–4 hr | 4–6 hr | 6–8 hr | |
| 0 | 8 | 6.6 ± 0.2 | 10.2 ± 0.5 | 9.8 ± 1.0 | 9.1 ± 0.7 | 8 | 8.6 ± 0.5 | 13.2 ± 1.5 | 12.2 ± 0.9 | 12.2 ± 0.8 |
| 0.2 | 7 | 5.6 ± 0.4 | 10.6 ± 1.2 | 10.9 ± 1.2 | 11.0 ± 0.8 | 6 | 5.2 ± 0.5* | 12.4 ± 1.2 | 12.3 ± 1.2 | 11.6 ± 0.5 |
| 0.4 | 7 | 3.6 ± 0.6* | 10.4 ± 1.0 | 11.1 ± 1.0 | 9.9 ± 1.2 | 8 | 2.8 ± 0.5* | 10.5 ± 0.9 | 11.1 ± 1.1 | 10.3 ± 0.4 |
| 0.8 | 8 | 2.7 ± 0.5* | 11.6 ± 1.4 | 11.7 ± 1.4 | 9.1 ± 0.7 | 7 | 0.9 ± 0.4* | 12.6 ± 1.0 | 16.5 ± 0.9* | 12.4 ± 1.8 |
| 1.2 | 8 | 0.3 ± 0.3* | 12.2 ± 0.6 | 15.7 ± 2.4* | 12.2 ± 0.5* | 6 | 0* | 9.0 ± 1.4 | 12.2 ± 1.0 | 10.4 ± 1.0 |
Antagonist
The 5-HT1A antagonist WAY 100635 (Fletcher et al., 1996), at the doses of 0.05–1.0 mg/kg, induced no significant modifications of sleep–waking cycles in any group of mice (data not shown). However, when WAY 100635 (0.05 and 1 mg/kg) was used as a pretreatment to 8-OH-DPAT (0.4 mg/kg), it prevented the effects of the latter 5-HT1A agonist on sleep–wakefulness cycles in both groups of mice (Fig. 7A).
Fig. 7.
A, Effects of the 5-HT1A antagonist WAY 100635 (dotted bars) on the 8-OH-DPAT-induced inhibition of PS in 5-HT1B+/+ (left) and 5-HT1B−/− (right) mice during the 2 hr period after injection in which an effect was observed. Results are expressed as percent of baseline (mean ± SEM of 7 animals in each group; 6–7 tests for each dose). B, Effects of the 5-HT1Aantagonist WAY 100635 (dotted bars) on the RU 24969-induced inhibition of PS in 5-HT1B+/+ (left) and 5-HT1B−/− (right) mice during the 4 hr after injection. Results are expressed as percentage of baseline (mean ± SEM of 7 and 9 animals, respectively; 4–5 tests for each dose). *p < 0.05, significantly different from baseline (open bars); paired Student’s t test. Complete set of data is available on request.
Respective contributions of 5-HT1B and 5-HT1A receptors to the effects of RU 24969 on sleep–wakefulness cycles
Because RU 24969 is a mixed 5-HT1A/1B agonist (Hoyer et al., 1994), we examined whether PS inhibition induced by large doses (3 and 5 mg/kg) of this ligand in 5-HT1B−/− mice could be caused by its action at 5-HT1A receptors. Thus, the 5-HT1A antagonist WAY 100635 was used as pretreatment to RU 24969 at doses equivalent for their effect on PS in the respective groups, i.e., 0.5 mg/kg in 5-HT1B+/+ mice (Fig. 3) and 3 mg/kg in 5-HT1B−/− mutants (Fig. 7B). WAY 100635 at doses of 0.15–1.0 mg/kg prevented totally the effect of RU 24969 in 5-HT1B−/− mice but not in wild-type animals (Fig. 7B). At the highest dose tested, 1 mg/kg, WAY 100635, in combination with either 8-OH-DPAT or RU 24969, produced a significant enhancement of PS in 5-HT1B−/− mice but not in wild-type animals (Fig.7A,B).
DISCUSSION
Knock-out mice lacking the 5-HT1B receptor (5-HT1B−/−) and the corresponding wild-type controls (5-HT1B+/+) exhibit similar diurnal sleep–waking cycles, with predominance of wakefulness during the dark period and sleep during the light one. These data are comparable with those reported previously in various strains of mice (Mitler et al., 1977;Kitahama and Valatx, 1980; Oliverio, 1980; Richardson et al., 1985;Tobler et al., 1997).
Interestingly, it was found here that 5-HT1B−/− mice exhibited during the light period significantly larger amounts of PS and smaller amounts of SWS than 5-HT1B+/+ animals and no PS rebound after selective PS deprivation. Whether such alterations of spontaneous sleep characteristics and homeostatic processes (Barbato and Wehr, 1998) are a direct consequence of the 5-HT1Breceptor gene disruption or are attributable to other factors is open to discussion.
Various adaptive mechanisms resulting from the absence of the 5-HT1B receptors might have occurred during development in 5-HT1B−/− mice. Indeed, in the latter mutants, the lack of expression of the 5-HT1B heteroreceptor might facilitate cholinergic (Maura and Raiteri, 1986) and GABAergic (Stanford and Lacey, 1996) neurotransmission and thus induce an enhancement of PS amounts (Gillin et al., 1985; McCarley and Massaquoi, 1992; Nitz and Siegel, 1997a,b; Cape and Jones, 1998). In addition, because 5-HT1B receptors are also autoreceptors on serotoninergic terminals (Engel et al., 1986; Boschert et al., 1994), their absence might also have an influence, in turn, on 5-HT1A receptors, which participate in the regulation of PS (de Saint Hilaire-Kafi et al., 1987; Dzoljic et al., 1992; Tissier et al., 1993; Portas et al., 1996; Thakkar et al., 1998).
With respect to the lack of PS rebound observed in the 5-HT1B−/− group after deprivation, it should be noted that, in contrast to another study (Gonzalez et al., 1996) in which the platforms were of smaller size than the ones used here, mice were not totally deprived of PS under our conditions. In fact, 5-HT1B−/− animals might exhibit a significant PS rebound after more drastic PS deprivation, but we purposely did not choose such an experimental design to minimize possible stress factors involved in this paradigm (Pokk et al., 1996). Still, after major PS deprivation for 12 hr, 5-HT1B+/+, but not 5-HT1B−/−, mice exhibited a significant PS rebound. The absence of 5-HT1B receptors, notably at the level of the locus ceruleus in which these receptors are expressed normally (Weissmann-Nanopoulos et al., 1985; Bobker and Williams, 1989; Clement et al., 1992), might account in part for this phenomenon. Indeed, lesion by_N_-(2-chloroethyl)-_N_-ethyl-2-gromobenzylamine of noradrenergic neurons in the locus ceruleus has been reported to suppress PS rebound in rats subjected to PS deprivation (Gonzalez et al., 1996). In contrast, rebound after pharmacologically induced PS inhibition persisted after such a lesion (Gonzalez et al., 1996), like that observed in 5-HT1B−/− mice after RU 24969 or 8-OH-DPAT treatment. This suggests that the lack of rebound after PS deprivation in 5-HT1B−/− mice is probably not because of some ceiling effect but rather of impairment of homeostatic regulation of PS. Finally, although the target areas for these phenomena have not yet been characterized, possible alterations in 5-HT, ACh, and GABA neurotransmission in 5-HT1B−/− mice might account for the differences in PS regulations between the two genotypes.
A second reason for the differences in spontaneous sleep and PS rebound between the two strains might be a difference in genetic background (Gerlai, 1996; Valatx and Bugat, 1974; Kitahama and Valatx, 1980;Tobler et al., 1997) rather than the specific 5-HT1Breceptor gene disruption. However, because backcrossing was performed with the strain that gave embryonic stem cells for homologous recombination, i.e., 129/Sv (Saudou et al., 1994), both 5-HT1B−/− and 5-HT1B+/+ mice have the same pure genetic background (Ramboz et al., 1996).
In fact, our pharmacological data provide strong support to the idea that the increased amounts of spontaneous PS in knock-out mice can be accounted for by the absence of 5-HT1B receptors. In particular, blockade of the latter by GR 127935 (Pauwels, 1997) induced an increase of PS amounts in 5-HT1B+/+, but not 5-HT1B−/−, mice (Fig. 5), so that the wild-type mice exhibited the same levels of PS as those occurring spontaneously in the 5-HT1B−/− strain.
In addition, stimulation of 5-HT1B receptors by the selective agonist CP 94253 (Koe et al., 1992) and the mixed 5-HT1A/1B agonist RU 24969 (Hoyer et al., 1994) induced a dose-dependent decrease of PS in 5-HT1B+/+ mice (Table 2, Fig. 4). That the inhibitory action of CP 94253 and RU 24969 on PS actually resulted from 5-HT1B receptor activation was confirmed by the fact that GR 127935 (Fig. 6), but not the 5-HT1A antagonist WAY 100635 (Fig. 7B), prevented this action and that, in 5-HT1B−/− mice, the same compounds in the same dose range altered neither sleep nor wakefulness. Previous studies in rats also supported the idea that 5-HT1B receptors are involved in a negative modulation of PS (Bjorvatn and Ursin, 1994; Monti et al., 1995).
At the largest doses of the 5-HT1B agonists used, both a decrease of SWS and an enhancement of W were observed in 5-HT1B+/+ mice, concomitantly with the PS reduction. These effects are similar to those reported in the rat (Dugovic et al., 1989;Dzoljic et al., 1992) and are probably not secondary to some hyperlocomotor activity triggered by this compound, notably because the doses of RU 24969 used here (0.25–2.0 mg/kg) were 10-fold lower than those required for the latter effect to occur in rodents (Green et al., 1984). However, if the action of 5-HT1B agonists on sleep and wakefulness can be accounted for by selective activation of 5-HT1B receptors, the effects of RU 24969 at higher doses (3 and 5 mg/kg) in 5-HT1B knock-out mice deserve some comments. These effects (reduction of PS and increase of W) could not be secondary to hyperlocomotion or ascribed to an action of RU 24969 at some residual 5-HT1B receptors, because they were not prevented by the 5-HT1B/1D antagonist GR 127935 (Table 3). Rather, the effects on PS of large doses of RU 24969 in 5-HT1B−/− mice would be attributable to the 5-HT1A component of this ligand. Indeed, RU 24969 binds to 5-HT1A receptors with an affinity only fivefold lower than to 5-HT1B receptors (Peroutka, 1986; Hoyer et al., 1994), and the use of large doses of this ligand in 5-HT1B−/− mice might activate 5-HT1A receptors sufficiently to induce a PS decrease, as expected of a 5-HT1A agonist (de Saint Hilaire-Kafi et al., 1987; Dzoljic et al., 1992; Tissier et al., 1993). In agreement with this interpretation, the effect of RU 24969 on PS in 5-HT1B−/− mice could be completely prevented by the selective 5-HT1A antagonist WAY 100635 (Fletcher et al., 1996).
In the absence of 5-HT1B receptors, 5-HT1Areceptors might have exhibited some adaptive changes in comparison with those in wild-type animals. However, in both groups of mice, the 5-HT1A agonist 8-OH-DPAT induced a dose-dependent reduction of PS during the 2–4 hr after injection (Table 4), associated with an increase of W at the expense of SWS at the largest doses used (data not shown). These effects, which are similar to those observed in the rat (de Saint Hilaire-Kafi et al., 1987), were probably not caused by 8-OH-DPAT-induced hypothermia (Goodwin et al., 1985). Indeed, body temperature monitoring after subcutaneous injection of 0.2–0.8 mg/kg 8-OH-DPAT showed that hypothermia was maximum (−1 and −3°C) 15–30 min after injection and disappeared within the following 15–30 min in both 5-HT1B−/− and wild-type mice (B. Boutrel and J. Adrien, unpublished observations). In contrast, the effects of 8-OH-DPAT on sleep persisted during 2–3 hr, well beyond the duration of drug-induced hypothermia. Interestingly, an increased reactivity of sleep to 8-OH-DPAT (Table 4) and WAY 100635 (Fig.7A,B) was observed in 5-HT1B−/− compared with wild-type mice. This would suggest that 5-HT1A receptors developed some functional supersensitivity in 5-HT1B−/− mice, but further studies are needed to directly address this question.
In conclusion, the lack of effects of 5-HT1B receptor agonists on the vigilance states in 5-HT1B−/− mice demonstrated that PS inhibition by these ligands in wild-type mice actually resulted from the specific stimulation of 5-HT1Breceptors. Both the larger amounts of PS during the light phase in 5-HT1B−/− mice and the PS increase in response to 5-HT1B receptor blockade in wild-type mice support the idea that 5-HT1B receptors mediate a 5-HT-dependent tonic inhibitory control of PS under physiological conditions.
Footnotes
This research was supported by the Institut National de la Santéet de la Recherche Médicale, Direction des Recherches Etudes et Techniques Grant 95/142, and European Community Grant Biotech BIO 4 CT 96.0752. Benjamin Boutrel was a recipient of a Ministère de l’Education Nationale, de l’Enseignement Supérieur et de la Recherche fellowship during performance of this work. The generous gifts of drugs by Glaxo-Wellcome, Pfizer, Roussel-Uclaf, and Wyeth are gratefully acknowledged. We acknowledge the excellent secretarial assistance of Claude Sais.
Correspondence should be addressed to Benjamin Boutrel, Institut National de la Santé et de la Recherche Médicale U288, CHU Pitié-Salpêtrière, 91 Boulevard de l’Hôpital, 75634 Paris Cedex 13, France.
REFERENCES
- 1.Barbato G, Wehr TA. Homeostatic regulation of REM sleep in humans during extended sleep. Sleep. 1998;21:267–276. doi: 10.1093/sleep/21.3.267. [DOI] [PubMed] [Google Scholar]
- 2.Bjorvatn B, Ursin R. Effect of the selective 5-HT1B agonist, CGS 12066B, on the sleep/waking stages and EEG power spectrum in rats. J Sleep Res. 1994;3:97–105. doi: 10.1111/j.1365-2869.1994.tb00112.x. [DOI] [PubMed] [Google Scholar]
- 3.Bobker DH, Williams JT. Serotonin agonists inhibit synaptic potentials in the rat locus cerulus in vitro via 5-hydroxytryptamine1A and 5-hydroxytryptamine1B receptors. J Pharmacol Exp Ther. 1989;250:37–43. [PubMed] [Google Scholar]
- 4.Boschert U, Aït Amara D, Segu L, Hen R. The mouse 5-hydroxytryptamine1B receptor is localized predominantly on axon terminals. Neuroscience. 1994;58:167–182. doi: 10.1016/0306-4522(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 5.Cape EG, Jones BE. Differential modulation of high-frequency γ-electroencephalogram activity and sleep–wake state by noradrenaline and serotonin microinjections into the region of cholinergic basalis neurons. J Neurosci. 1998;18:2653–2666. doi: 10.1523/JNEUROSCI.18-07-02653.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cespuglio R, Houdouin F, Oulerich M, El Mansari M, Jouvet M. Axonal and somato-dendritic modalities of serotonin release: their involvement in sleep preparation, triggering and maintenance. J Sleep Res. 1990;1:150–156. doi: 10.1111/j.1365-2869.1992.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 7.Clement HW, Gemsa D, Wesemann W. Serotonin–norepinephrine interactions: a voltammetric study on the effect of serotonin receptor stimulation followed in the N. raphe dorsalis and the locus coeruleus of the rat. J Neural Transm Gen Sect. 1992;88:11–23. doi: 10.1007/BF01245033. [DOI] [PubMed] [Google Scholar]
- 8.Crabbe JC, Phillips TJ, Feller DJ, Hen R, Wenger CD, Lessov CN, Schafer GL. Elevated alcohol consumption in null mutant mice lacking 5-HT1B serotonin receptors. Nat Genet. 1996;14:98–101. doi: 10.1038/ng0996-98. [DOI] [PubMed] [Google Scholar]
- 9.de Saint Hilaire-Kafi S, Hjorth S, Gaillard J. Effects of 8-OH-DPAT on the sleep–waking cycle in the rat. In: Dourish CT, Ahlenius S, Hutson PH, editors. Brain 5-HT1A receptors. Horwood; Chichester, UK: 1987. pp. 135–139. [Google Scholar]
- 10.Dugovic C, Wauquier A, Leysen JE, Marannes R, Janssen PAJ. Functional role of 5-HT2 receptors in the regulation of sleep and wakefulness in the rat. Psychopharmacology. 1989;97:436–442. doi: 10.1007/BF00439544. [DOI] [PubMed] [Google Scholar]
- 11.Dulawa SC, Hen R, Scearce-Levie K, Geyer MA. Serotonin1B receptor modulation of startle reactivity, habituation, and prepulse inhibition in wild-type and serotonin1B knock out mice. Psychopharmacology. 1997;132:125–134. doi: 10.1007/s002130050328. [DOI] [PubMed] [Google Scholar]
- 12.Dzoljic MR, Ukponmwan OE, Saxena PR. 5-HT1-like receptor agonists enhance wakefulness. Neuropharmacology. 1992;31:623–633. doi: 10.1016/0028-3908(92)90140-k. [DOI] [PubMed] [Google Scholar]
- 13.Engel G, Göthert M, Hoyer D, Schlicker E, Hillenbrand K. Identity of inhibitory presynaptic 5-hydroxytyptamine (5-HT) autoreceptors in the rat brain cortex with 5-HT1B binding sites. Naunyn-Schmiedeberg’s Arch Pharmacol. 1986;332:1–7. doi: 10.1007/BF00633189. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher A, Forster EA, Bill DJ, Brown G, Cliffe IA, Hartley JE, Jones DE, Mc Lenachan A, Stanhope KJ, Critchley DJP, Childs KJ, Middlefell VC, Lanfumey L, Corradetti R, Laporte AM, Gozlan H, Hamon M, Dourish CT. Electrophysiological, biochemical, neurohormonal and behavioral studies with WAY-100635, a potent, selective, and silent 5-HT1A receptor antagonist. Behav Brain Res. 1996;73:337–353. doi: 10.1016/0166-4328(96)00118-0. [DOI] [PubMed] [Google Scholar]
- 15.Frances H, Monier C. Tolerance to the behavioural effect of serotoninergic (5-HT1B) agonists in the isolation-induced social behavioural deficit test. Neuropharmacology. 1991;30:623–627. doi: 10.1016/0028-3908(91)90082-m. [DOI] [PubMed] [Google Scholar]
- 16.Gerlai R. Gene-targeting studies of mammalian behavior: is the mutation or the background genotype? Trends Neurosci. 1996;19:177–181. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- 17.Gillin JC, Sitaram N, Janowsky D, Risch C, Huey L, Storch FS. Cholinergic mechanisms in REM sleep. In: Wauquier A, Gaillard JM, Monti J, Radulovacki M, editors. Sleep: neurotransmitters and neuromodulators. Raven; New York: 1985. pp. 153–164. [Google Scholar]
- 18.Gonzalez MM, Valatx JL, Debilly G. Role of the locus coeruleus in the sleep rebound following two different sleep deprivation methods in the rat. Brain Res. 1996;740:215–226. doi: 10.1016/s0006-8993(96)00871-2. [DOI] [PubMed] [Google Scholar]
- 19.Goodwin GM, De Souza RJ, Green AR. The pharmacology of the hypothermic response in mice to 8-hydroxy-(di-n-propylamino) tetralin (8-OH-DPAT): a model of presynaptic 5-HT1 function. Neuropharmacology. 1985;24:1187–1194. doi: 10.1016/0028-3908(85)90153-4. [DOI] [PubMed] [Google Scholar]
- 20.Green AR, Guy AP, Gardner CR. The behavioural effects of RU 24969, a suggested 5-HT1 receptor agonist, in rodents and the effect on the behaviour of treatment with antidepressants. Neuropharmacology. 1984;23:655–661. doi: 10.1016/0028-3908(84)90147-3. [DOI] [PubMed] [Google Scholar]
- 21.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PPA. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 22.Idzikowski C, Mills FJ, Glennard R. 5-Hydroxytryptamine-2 antagonist increases human slow wave sleep. Brain Res. 1986;378:164–168. doi: 10.1016/0006-8993(86)90299-4. [DOI] [PubMed] [Google Scholar]
- 23.Jouvet M. Biogenic amines and the states of sleep. Science. 1969;163:32–41. doi: 10.1126/science.163.3862.32. [DOI] [PubMed] [Google Scholar]
- 24.Kitahama K, Valatx JL. Instrumental and pharmacological paradoxical sleep deprivation in mice: strain differences. Neuropharmacology. 1980;19:529–535. doi: 10.1016/0028-3908(80)90022-2. [DOI] [PubMed] [Google Scholar]
- 25.Koe KB, Nielsen JA, Macor JE, Heym J. Biochemical and behavioural studies of the 5-HT1B receptor agonist, CP-94,253. Drug Dev Res. 1992;26:241–250. [Google Scholar]
- 26.Koella W, Feldstein A, Czicman J. The effect of para-chlorophenylalanine on the sleep of cats. Electroencephalogr Clin Neurophysiol. 1968;25:481–490. doi: 10.1016/0013-4694(68)90158-2. [DOI] [PubMed] [Google Scholar]
- 27.Limoge-Lendais I, Robert C, Degrange M, Goldberg M, Stinus L, Limoge A. Study on superbond adhesion to the skull for chronic electrode implantation in the rat. Neurosci Protocols. 1994;70:1–11. [Google Scholar]
- 28.Maura G, Raiteri M. Cholinergic terminals in rat hippocampus possess 5-HT1B receptors mediating inhibition of acetylcholine release. Eur J Pharmacol. 1986;129:333–337. doi: 10.1016/0014-2999(86)90443-7. [DOI] [PubMed] [Google Scholar]
- 29.McCarley RW, Massaquoi SG. Neurobiological structure of the revised limit cycle reciprocal interaction model of REM cycle control. J Sleep Res. 1992;1:132–137. doi: 10.1111/j.1365-2869.1992.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 30.Mendelson WB. Effects of parenterally administered triazolam on sleep in rats with lesions of the preoptic area. Pharmacol Biochem Behav. 1998;61:81–86. doi: 10.1016/s0091-3057(98)00080-x. [DOI] [PubMed] [Google Scholar]
- 31.Mitler MM, Lund R, Sokolove PG, Pittendrigh CS, Dement WC. Sleep and activity rhythms in mice: a description of circadian patterns and unexpected disruptions in sleep. Brain Res. 1977;131:129–145. doi: 10.1016/0006-8993(77)90033-6. [DOI] [PubMed] [Google Scholar]
- 32.Monti JM, Monti D, Jantos H, Ponzoni A. Effects of selective activation of the 5-HT1B receptor with CP-94,253 on sleep and wakefulness in the rat. Neuropharmacology. 1995;34:1647–1651. doi: 10.1016/0028-3908(95)00112-3. [DOI] [PubMed] [Google Scholar]
- 33.Montkowski A, Barden N, Wotjak C, Stec I, Ganster J, Meaney M, Engelmann M, Reul J, Landgraf R, Holsboer F. Long-term antidepressant treatment reduces behavioural deficits in transgenic mice with impaired glucocorticoid receptor function. J Neuroendocrinol. 1995;7:841–845. doi: 10.1111/j.1365-2826.1995.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 34.Nitz D, Siegel J. GABA release in the dorsal raphe nucleus: role in the control of REM sleep. Am J Physiol. 1997a;42:R451–R455. doi: 10.1152/ajpregu.1997.273.1.R451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nitz D, Siegel J. GABA release in the locus coeruleus as a function of sleep/wake state. Neuroscience. 1997b;78:795–801. doi: 10.1016/s0306-4522(96)00549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliverio A. Sleep and activity rhythm in mice. Waking Sleeping. 1980;4:155–166. [PubMed] [Google Scholar]
- 37.Pauwels PJ. 5-HT1B/D receptor antagonists. Gen Pharmacol. 1997;29:293–303. doi: 10.1016/s0306-3623(96)00460-0. [DOI] [PubMed] [Google Scholar]
- 38.Peroutka SJ. Pharmacological differentiation and characterization of 5-HT1A, 5-HT1B, 5-HT1C binding sites in rat frontal cortex. J Neurochem. 1986;47:529–540. doi: 10.1111/j.1471-4159.1986.tb04532.x. [DOI] [PubMed] [Google Scholar]
- 39.Pokk P, Liljequist S, Zharkovsky A. Ro 15–4513 potentiates, instead of antagonizes, ethanol-induced sleep in mice exposed to small platform stress. Eur J Pharmacol. 1996;317:15–20. doi: 10.1016/s0014-2999(96)90061-8. [DOI] [PubMed] [Google Scholar]
- 40.Portas CM, McCarley RW. Behavioural state-related changes of extracellular serotonin concentration in the dorsal raphe nucleus: a microdialysis study in the freely moving cat. Brain Res. 1994;648:306–312. doi: 10.1016/0006-8993(94)91132-0. [DOI] [PubMed] [Google Scholar]
- 41.Portas CM, Thakkar M, Rainnie D, McCarley RW. Microdialysis perfusion of 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) in the dorsal raphe nucleus decreases serotonin release and increases rapid eye movement sleep in the freely moving cat. J Neurosci. 1996;16:2820–2828. doi: 10.1523/JNEUROSCI.16-08-02820.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramboz S, Saudou F, Aït Amara D, Belzung C, Segu L, Misslin R, Buhot MC, Hen R. 5-HT1B receptor knock out: behavioural consequences. Behav Brain Res. 1996;73:305–312. doi: 10.1016/0166-4328(96)00119-2. [DOI] [PubMed] [Google Scholar]
- 43.Richardson GS, Moore-Ede MC, Czeisler CA, Dement WC. Circadian rhythms of sleep and wakefulness in mice: analysis using long-term automated recording of sleep. Am J Physiol. 1985;248:R320–R329. doi: 10.1152/ajpregu.1985.248.3.R320. [DOI] [PubMed] [Google Scholar]
- 44.Rocha B, Ator R, Emmett-Oglesby M, Hen R. Intravenous cocaine self-administration in mice lacking 5-HT1B receptors. Pharmacol Biochem Behav. 1997;57:407–412. doi: 10.1016/s0091-3057(96)00444-3. [DOI] [PubMed] [Google Scholar]
- 45.Saudou F, Aït Amara D, Dierich A, LeMeur M, Ramboz S, Segu L, Buhot MC, Hen R. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science. 1994;265:1875–1878. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- 46.Sollars P, Ryan A, Ogilvie M, Pickard G. Altered circadian rhythmicity in the Wocko mouse, a hyperactive transgenic mutant. NeuroReport. 1996;7:1245–1248. doi: 10.1097/00001756-199605170-00004. [DOI] [PubMed] [Google Scholar]
- 47.Stanford IM, Lacey MG. Differential actions of serotonin, mediated by 5-HT1B and 5-HT2C receptors, on GABA-mediated synaptic input to rat substantia nigra pars reticulata neurons in vitro. J Neurosci. 1996;16:7566–7573. doi: 10.1523/JNEUROSCI.16-23-07566.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thakkar MM, Strecker RE, McCarley RW. Behavioral state control through differential serotonergic inhibition in the mesopontine cholinergic nuclei: a simultaneous unit recording and microdialysis study. J Neurosci. 1998;18:5490–5497. doi: 10.1523/JNEUROSCI.18-14-05490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tissier MH, Lainey E, Fattaccini CM, Hamon M, Adrien J. Effects of ipsapirone, a 5-HT1A agonist, on sleep/wakefulness cycles: probable post-synaptic action. J Sleep Res. 1993;2:103–109. doi: 10.1111/j.1365-2869.1993.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 50.Tobler I, Deboer T, Fischer M. Sleep and sleep regulation in normal and prion protein-deficient mice. J Neurosci. 1997;17:1869–1879. doi: 10.1523/JNEUROSCI.17-05-01869.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valatx JL, Bugat R. Facteurs génétiques dans le déterminisone du cycle veille-sommeil chez la seuris. Brain Res. 1974;69:315–330. doi: 10.1016/0006-8993(74)90009-2. [DOI] [PubMed] [Google Scholar]
- 52.Weissmann-Nanopoulos D, Mach E, Magre J, Demassey Y, Pujol JF. Evidence for the localization of 5-HT1A binding sites on serotonin containing neurons in the raphe dorsalis and raphe centralis nuclei of the rat brain. Neurochem Int. 1985;7:1061–1072. doi: 10.1016/0197-0186(85)90156-1. [DOI] [PubMed] [Google Scholar]
- 53.Zhang J, Obal F, Fang J, Collins B, Krueger J. Non-rapid eye movement sleep is suppressed in transgenic mice with deficiency in the somatotropic system. Neurosci Lett. 1996;220:97–100. doi: 10.1016/s0304-3940(96)13232-8. [DOI] [PubMed] [Google Scholar]