Presynaptic Mechanism for Phorbol Ester-Induced Synaptic Potentiation (original) (raw)
Abstract
Phorbol ester facilitates transmitter release at a variety of synapses, and the phorbol ester-induced synaptic potentiation (PESP) is a model for presynaptic facilitation. To address the mechanism underlying PESP, we have made paired whole-cell recordings from the giant presynaptic terminal, the calyx of Held, and its postsynaptic target in the medial nucleus of the trapezoid body in rat brainstem slices. Phorbol ester potentiated EPSCs without affecting either presynaptic calcium currents or potassium currents. Protein kinase C inhibitors applied from outside or injected directly into the presynaptic terminal attenuated the PESP. Furthermore, presynaptic loading of a synthetic peptide with the sequence of the N-terminal domain of Doc2α interacting with Munc13–1 (Mid peptide) significantly attenuated PESP, whereas mutated Mid peptide had no effect. We conclude that the target of the presynaptic facilitatory effect of phorbol ester resides downstream of calcium influx and may involve both protein kinase C and Doc2α - Munc13–1 interaction.
Keywords: phorbol ester, synaptic facilitation, Doc2α, Munc13–1, protein kinase C, the calyx of Held, presynaptic recording
Phorbol ester enhances synaptic efficacy by increasing transmitter release at a variety of synapses (Malenka et al., 1986; Shapira et al., 1987). This presynaptic facilitatory effect of phorbol ester is thought to be mediated by protein kinase C (PKC) through (1) activation of calcium channels (Fossier et al., 1990; O’Dell and Alger, 1991; Parfitt and Madison, 1993; Swartz et al., 1993; Stea et al., 1995), (2) inhibition of potassium channels (Barban et al., 1985; Storm, 1987; Doerner et al., 1988; Hoffman and Johnston, 1998), or (3) activation of exocytotic machinery downstream of Ca2+ influx (Capogna et al., 1995; Redman et al., 1997). However, there is no direct evidence to indicate which, if any, of the above targets are involved in the phorbol ester-induced synaptic potentiation (PESP). Furthermore, an involvement of PKC in the PESP has been questioned recently at some synapses, where certain PKC inhibitors had no effect on the PESP (Redman et al., 1997). It has been reported that Munc13–1, a mammalian homolog of Caenorhabditis elegans unc13p, has a diacylglycerol (DAG) receptor similar in affinity to PKC and is localized in the plasma membrane near the release site (Betz et al., 1998). Munc13–1 interacts with the vesicular protein Doc2α in a DAG- or phorbol ester-dependent manner (Orita et al., 1997). A possible involvement of Munc13–1 in PESP has been suggested at the amphibian neuromuscular junction in cell culture, where over-expression of Munc13–1 augmented the PESP (Betz et al., 1998).
The calyx of Held in the rodent auditory brainstem is a giant glutamatergic nerve terminal of anterior ventral cochlear neuron forming synapse onto the somata of principal cells of medial nucleus of trapezoid body (MNTB) (Barnes-Davies and Forsythe, 1995). Because of its large size, it is possible to make direct whole-cell recordings from the nerve terminal (Forsythe, 1994; Borst et al., 1995; Takahashi et al., 1996) and also to load molecules directly into it through a patch pipette (Takahashi et al., 1998). Taking advantage of this preparation, we have studied the mechanism underlying PESP. Our results indicate that neither calcium nor potassium conductances are involved in the presynaptic effect of phorbol ester, suggesting an involvement of the mechanism downstream of Ca2+influx. By directly injecting the N-terminal peptide fragment of Doc2α or the PKC inhibitor peptide into the calyceal nerve terminal, we have demonstrated that the Doc2α-Munc13–1 interaction as well as the PKC activation may mediate the PESP.
MATERIALS AND METHODS
Preparation and solutions. Transverse slices of the superior olivary complex were prepared from 14- to 16-d-old Wistar rats killed by decapitation under halothane anesthesia. The MNTB neurons and calyces were viewed with a 60× (Olympus Optical, Tokyo, Japan) water immersion lens attached to an upright microscope (Axioskop; Zeiss). Each slice was superfused with artificial CSF (aCSF) containing (in mm): 120 NaCl, 2.5 KCl, 26 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, 10 glucose, 0.5 _myo_-inositol, 2 sodium pyruvate, and 0.5 ascorbic acid, pH 7.4, with 5% CO2 and 95% O2. For recording EPSCs, the aCSF contained routinely bicuculline methiodide (10 μm) and strychnine hydrochloride (0.5 μm) to block spontaneous inhibitory synaptic currents. Effect of phorbol ester on EPSCs was tested in the aCSF containing 1 mm[Ca2+] and 2 mm[Mg2+]. For recording presynaptic Ca2+ currents, 10 mmtetraethylammonium (TEA) chloride and 1 μmtetrodotoxin (TTX) were included in the aCSF. For recording presynaptic K+ current or spontaneous mEPSCs , 1 μm TTX was included in the aCSF. For recording presynaptic action potentials, presynaptic pipettes were filled with the solution containing (in mm): 97.5 potassium gluconate, 32.5 KCl, 10 HEPES, 0.2 EGTA, 1 MgCl2, 10 potassium glutamate, 2 ATP (Mg salt), 12 phosphocreatine, and 0.5 GTP, pH 7.4 adjusted with KOH. For recording presynaptic Ca2+ currents, potassium gluconate and KCl in the presynaptic pipette solution were replaced by 110 mm CsCl, 10 mm TEA chloride was added, and HEPES concentration was increased to 40 mm, pH 7.4 adjusted with CsOH. For postsynaptic recordings, pipette solution contained (in mm): 110 CsF, 30 CsCl, 10 HEPES, 5 EGTA, and 1 MgCl2. When the aCSF did not contain TTX,_N_-(2,6-diethylphenylcarbamoylmethyl)triethylammonium bromide (QX314; 5 mm) was included in the postsynaptic pipette solution to suppress action potential generation.
Data recording and analysis. Whole-cell patch-clamp recordings were made from MNTB principal neurons, presynaptic calyces, or simultaneously from both structures. EPSCs were evoked at 0.1 Hz throughout by extracellular stimulation of presynaptic axons using a bipolar platinum electrode positioned near the midline of a relatively thick slice (200 μm) or by presynaptic action potentials elicited directly by a whole-cell pipette in thin slices (150 μm). The resistance of patch pipette was 4–7 MΩ for presynaptic recordings and 2–4 MΩ for postsynaptic recordings. The series resistance of presynaptic recording was typically 10–20 MΩ and was compensated by 70–90% in voltage-clamp experiments. Current or potential recordings were made with a patch-clamp amplifier (Axopatch 200B; Axon Instruments, Foster City, CA). Records were low-pass-filtered at 2.5–20 kHz and digitized at 5–50 kHz by a CED 1401 interface (Cambridge Electronic Design). Presynaptic voltage-gated currents were leak-subtracted by using a scaled pulse divided by n (P/N) protocol (Forsythe et al., 1998; Takahashi et al., 1998). The magnitude of potentiation of EPSCs was evaluated from the mean amplitude of six consecutive events during 5–6 min after phorbol ester application divided by that of six events before application. Values in the text and figures are given as means ± SEM, and significance of difference was evaluated by one-way ANOVA or Kolmogorov–Smirnov test (for cumulative histograms) with 0.05 taken as the level of significance.
Drug application. Drugs were bath-applied by switching superfusates using solenoid valves. Peptides were injected into calyces through a superfusion tube directly installed in a presynaptic patch pipette. The superfusion tube was fabricated from an Eppendorf yellow tip heated and pulled to make an outer tip diameter of 50–70 μm. After back-filling the tube with pipette solutions containing synthetic peptides, it was inserted into a patch pipette with its tip 500–600 μm behind the tip of patch pipette. After obtaining control responses, the dialysis solution was delivered into presynaptic patch pipette with positive pressure manually applied through a syringe. When a fluorescence dye Lucifer yellow (0.05%) was injected by this method, fluorescence became detectable in a calyx within 1 min after injection and reached maximal intensity within 4 min. When FITC-conjugated albumin was injected, the whole calyx was stained within 5 min. The amino acid sequence of synthetic Mid peptide and mutated Mid peptide is IQEHMAINCPGPIRPIRQISDYFP and IYKDWAFNVCPGPIRPIRQISDYFP, respectively (Orita et al., 1997). PKC inhibitor peptide, PKCI (19–36), Mid peptide, or mutated Mid peptide was dissolved in pipette solution at 200 μm and loaded into calyces through the superfusion tube. Experiments were carried at room temperature (22–26°C).
RESULTS
Potentiation of EPSCs by phorbol esters
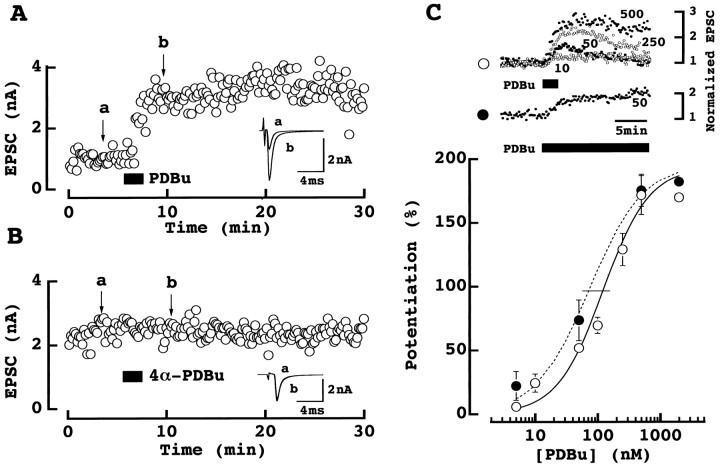
As illustrated in Figure1A, phorbol ester markedly potentiated the calyx-MNTB EPSCs as reported at other synapses (Malenka et al., 1986; Shapira et al., 1987). During bath-application of phorbol 1,2-dibutrate (PDBu; 0.5 μm for 2 min), EPSCs became larger reaching a maximal size within 5 min after application. The mean magnitude of this potentiation was 162 ± 37% (± SEM ; n = 6 cells, see also Fig.5A). The potentiation by PDBu at this concentration lasted for >20 min with no sign of decline. Other phorbol esters such as phorbol-12,13-diacetate or phorbol-12-myristate-13 acetate produced a similar potentiating effect (both at 0.5 μm;data not shown) but the inactive 4α-PDBu (0.5 μm) had no effect (−0.8 ± 9.9%;n = 7; Fig. 1B). The potentiating effect of PDBu on EPSCs was dose-dependent (Fig. 1C) with a maximum potentiation reached at around 0.5 μmand the 50% effective dose (EC50) being 121 nm with a 2 min application. Potentiation of EPSCs was transient when low doses of PDBu were applied for 2 min, but it was sustained when PDBu was continuously applied. The EC50 of continuously applied PDBu was 75 nm.
Fig. 1.
Phorbol ester potentiated the calyx-MNTB EPSCs.A, PDBu (0.5 μm) bath-applied for 2 min (at a bar) potentiated the calyx-MNTB EPSCs evoked by extracellular stimulation. Six consecutive EPSCs before (a) and after (b) PDBu application are averaged and superimposed in inset. B, The inactive PDBu analog 4α-PDBu (0.5 μm) had no effect.a and b are as above. _C,Dose-dependent potentiation of EPSCs by PDBu (5–2000 nm).Top column shows time plots of EPSC amplitude. Data from 4–6 experiments at each dose (10–500 nm) are normalized to the mean EPSC amplitude before PDBu application. PDBu was applied for 2 min (○) or continuously (50 nm; ●). Bottom column shows dose–response curve of PDBu obtained by 2 min (○) or continuous (●) applications. The magnitude of EPSCs 5–6 min after PDBu application was measured. Data points and error bars represent means and SEMs derived from 3–6 cells. Curves are fitted to the data points according to the following equation: magnitude of potentiation (%) = [maximal potentiation]/[1 + (EC50/PDBu concentration)n], where maximal potentiation was 194 and 195% each for 2 min (○) and continuous (●) application. EC50 (indicated by a_horizontal bar) was 121 nm (○) and 75 nm (●), respectively. Hill coefficient was 1.2 (○) and 1.0 (●), respectively.
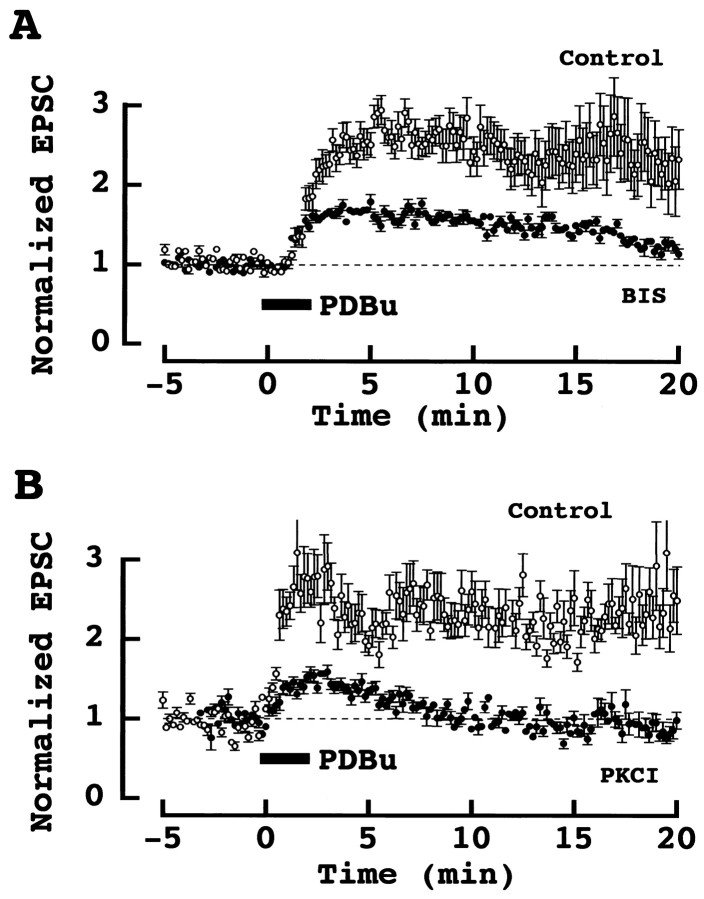
Fig. 5.
PKC inhibitors attenuated PESP. _A,_Effect of PDBu (0.5 μm) on EPSCs in the presence (●) and absence (○) of the PKC inhibitor BIS (1 μm). BIS was applied 5–10 min before PDBu. Mean amplitude and SEMs (error bars) derived from six (control) and five (BIS) cells are shown. EPSCs were evoked extracellularly. B, Effect of PDBu on EPSCs evoked by presynaptic action potentials in the presence (●) and absence (○) of the PKC inhibitor peptide (PKCI 19–36) in paired presynaptic and postsynaptic whole-cell recordings. PKCI had been injected into caclyceal presynaptic terminals 5–10 min before PDBu applications. Data derived from five cells each for control and PKCI-loaded calyces. Dashed lines indicate baselines derived from the mean amplitude of EPSCs before PDBu application in this and the next figure.
Effects of phorbol ester on quantal EPSCs
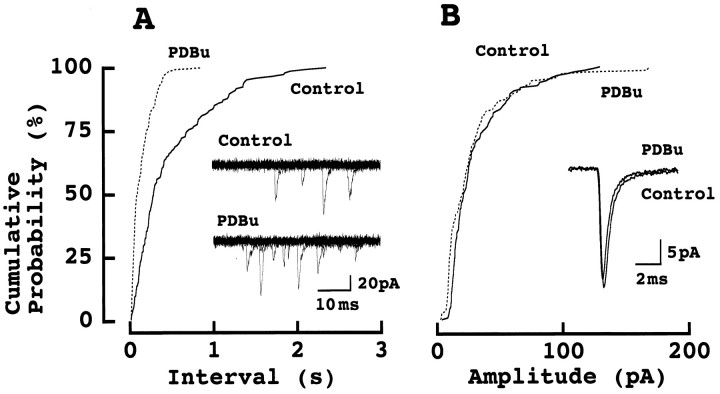
We subsequently examined the effect of PDBu on spontaneous miniature (m) EPSCs recorded in the presence of TTX (1 μm; Fig. 2). As shown in cumulative interval histograms, PDBu (0.5 μm) increased the mean frequency of mEPSCs (6.7 ± 3 Hz) on average by 2.1 ± 0.5-fold (n = 5; Fig. 2A). In contrast, neither the kinetics nor the amplitude of mEPSCs was significantly affected by PDBu (Fig. 2B). The mean amplitude of mEPSCs after PDBu application was 102 ± 2.9% (n = 6) of control before PDBu application, suggesting that this phorbol ester had no effect on postsynaptic glutamate receptor sensitivity. Thus, as reported at other synapses (Malenka et al., 1986; Shapira et al., 1987), the site of its action must be purely presynaptic.
Fig. 2.
Phorbol ester increased the frequency of mEPSCs but had no effect on the amplitude of mEPSCs. _A,_Cumulative interval histograms of mEPSCs recorded from an MNTB principal cell under TTX. Each 200 events were sampled before (control) and 5 min after PDBu (0.5 μm) application. Ten consecutive records before and 6 min after PDBu application are superimposed in inset. B, Cumulative amplitude histogram of mEPSCs from the same cell. Superimposed records in inset are averaged EPSCs of 200 events each before and after PDBu application. No significant difference in the amplitude of mEPSCs between PDBu and control in Kolmogorov–Smirnov test.
Lack of phorbol ester effect on presynaptic calcium currents
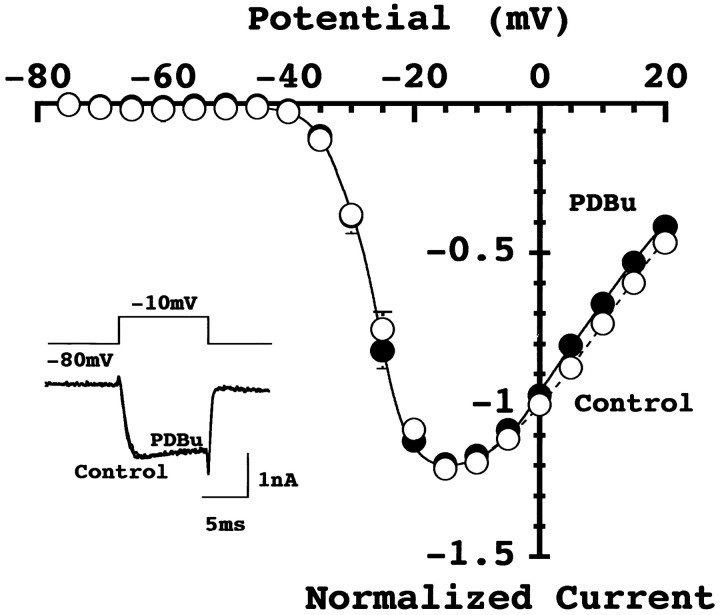
Previous studies on somatic or recombinant Ca2+ currents indicate that phorbol ester can enhance Ca2+ currents (Fossier et al., 1990; O’Dell and Alger, 1991; Parfitt et al., 1993; Stea et al., 1995). It was then speculated that a similar potentiation might occur at the presynaptic nerve terminals. We have directly tested this possibility by recording the presynaptic Ca2+ currents from the giant nerve terminal, the calyx of Held. The Ca2+currents at the calyx have been pharmacologically identified as P-type (Forsythe et al., 1998; Iwasaki and Takahashi, 1998), and they can be attenuated by agonists of metabotropic glutamate receptors (Takahashi et al., 1996) or GABABreceptors (Takahashi et al., 1998). As illustrated in Figure3, PDBu (0.5 μm) had no effect on presynaptic Ca2+ currents at all membrane potential examined, with the mean magnitude of Ca2+ currents at −10 mV being 98 ± 3% of control (n = 5). These results indicate that the PESP is not mediated by presynaptic Ca2+channels, at least at this mammalian brainstem synapse.
Fig. 3.
Phorbol ester had no effect on presynaptic calcium currents. Voltage-dependent Ca2+ currents were evoked in calyceal presynaptic terminals by a depolarizing pulse from −80 mV holding potential to −10 mV before and after PDBu application (0.5 μm, two traces superimposed in_inset_). The Ca2+ current–voltage relationships before (●) and 6–9 min after (○) PDBu application. Data points and error bars are means and SEMs of Ca2+ current amplitude from five calyces. The mean amplitude of Ca2+ currents at −10 mV was 928 ± 9.4 pA in control and 904 ± 7.3 pA after PDBu application (n = 5). Lines are drawn by eyes in this and the next figure.
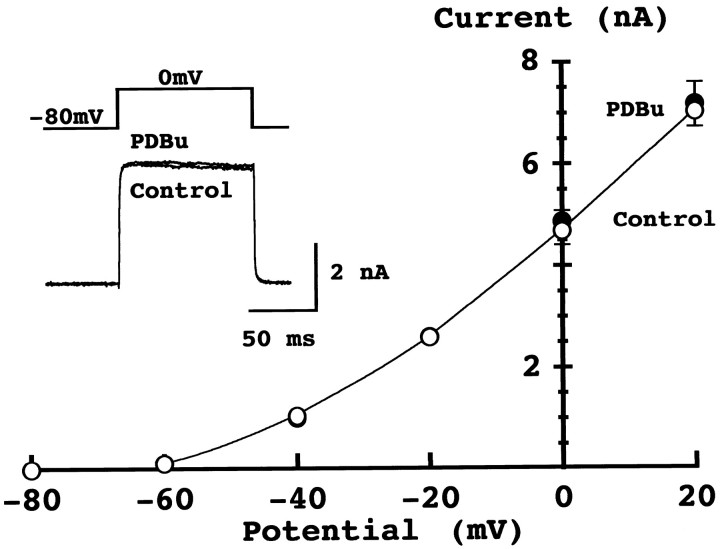
Presynaptic potassium currents are unaffected by phorbol ester
Voltage-gated potassium channel currents in hippocampal neurons are attenuated by phorbol esters (Barban et al., 1985; Storm, 1987;Doerner et al., 1988; Hoffman and Johnston, 1998). If phorbol ester attenuates presynaptic potassium channels, this would presumably lead to increased Ca2+ influx, thereby enhancing transmitter release. We have tested this possibility by recording presynaptic potassium currents. As shown in Figure4, PDBu (0.5 μm) had no effect on the presynaptic voltage-dependent potassium currents. The mean amplitude of potassium current at 0 mV was 4.7 ± 0.4 nA in control and 4.9 ± 0.5 nA after PDBu application (n = 5). It has been also reported that G-protein-coupled inward rectifying potassium (GIRK) conductance can be suppressed by PKC activation (Takano et al., 1995). Although any change in GIRK can be revealed as a change in holding current (Takahashi et al., 1998), PDBu had no effect on the holding current (98.9 ± 5.7%; n = 5), suggesting that GIRK is not involved in the PESP. These results indicate that neither the calcium conductance nor potassium conductance in the presynaptic terminal is involved in the PESP at this synapse. Therefore, the target of phorbol ester must be downstream of Ca2+ influx as has been suggested for secretory cells (Gillis et al., 1996).
Fig. 4.
Phorbol ester had no effect on presynaptic potassium currents. Voltage-dependent K+ currents recorded from the calyx of Held in the presence of TTX. Potassium currents were evoked by a depolarizing pulse from −80 mV holding potential to 0 mV before and after PDBu application (superimposed in_inset_). The K+ current–voltage relationships before (○) and 5–7 min after (●) PDBu application. Data points and error bars derived from five calyces each.
Involvement of PKC in phorbol ester-induced synaptic potentiation
To address whether PKC is involved in the effect of phorbol esters, we tested a number of PKC inhibitors on PESP. Bisindolylmaleimid (BIS; 1 μm), a competitive inhibitor for the ATP-binding site of PKC, partially but significantly attenuated the phorbol ester-induced synaptic facilitation (Fig.5A). Potentiation of EPSCs 5–6 min after application of PDBu (0.5 μm) was 64 ± 9.6% (n = 5) in the presence of BIS, whereas it was 162 ± 37% (n = 6) in control (see above). Calphostin C (0.5 μm), a competitive inhibitor for the phorbol ester-binding site of PKC, also significantly suppressed the PESP with the potentiation being 48 ± 24% (n = 3) in its presence (data not shown). A more specific tool to test an involvement of PKC is the PKC inhibitor peptide (PKCI; 19–36), which acts as a pseudosubstrate for PKC. We injected PKCI into the calyceal presynaptic nerve terminals during paired presynaptic and postsynaptic whole-cell recordings. EPSCs were evoked by presynaptic action potentials elicited in calyceal nerve terminals with a patch pipette (Takahashi et al., 1996; 1998). In control experiments, externally applied PDBu (0.5 μm) potentiated EPSCs (Fig. 5B) with a magnitude (138 ± 29%; n = 5) comparable to that observed for the extracellularly evoked EPSCs (no significant difference). When PKCI was injected into the calyx, the peptide by itself had no effect on EPSCs (data not shown), but PESP was significantly attenuated, with the magnitude of potentiation being only 27 ± 12% (n = 5; Fig. 5B). Taken together, these results suggest that PKC is involved in the phorbol ester-induced synaptic potentiation.
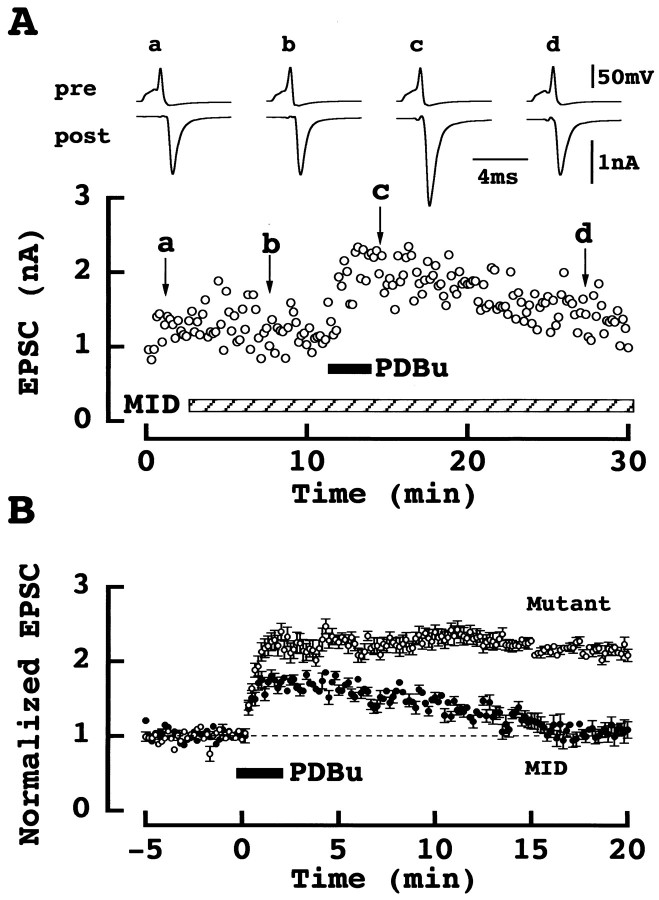
Involvement of Doc2α-Munc13–1 interaction in phorbol ester-induced synaptic potentiation
We next examined the possibility that the Doc 2α-Munc13–1 interaction (Orita et al., 1997) underlies the PESP. For this purpose, we injected into the calyx a synthetic peptide corresponding to the N-terminal domain of Doc 2α, which interacts with Munc13–1 (the Mid domain: amino acid residues 13–37, see Materials and Methods). This Mid peptide alone blocks Doc 2α-Munc13–1 interaction in vitro (Orita et al., 1997) and also blocks synaptic transmission when injected into presynaptic neurons in culture (Mochida et al., 1998). In contrast, at the calyx of Held synapse, Mid peptide had no appreciable effect on EPSCs (Fig.6A), with the amplitude of EPSCs remaining as 116 ± 11% (n = 7) 10 min after injection. However, in the presence of Mid, potentiation of EPSCs by PDBu was significantly attenuated in amplitude and no longer sustained (Fig. 6A). The magnitude of PESP 5 min after Mid application was 64 ± 6.2% (n = 7 vs 138 ± 29% in control, see above). As a control, we employed a mutated Mid peptide, which has no effect on Doc 2α-Munc13–1 interaction (Mochida et al., 1998). When the mutated Mid peptide was similarly loaded into calyces, PESP was not attenuated (124 ± 15%; n = 5; Fig. 6B).
Fig. 6.
Mid peptide attenuated PESP. _A,_Synthetic Mid peptide (Mid) had no effect on EPSCs (bottom sample records) evoked by presynaptic action potentials (top records) in simultaneous presynaptic and postsynaptic whole-cell recording at a calyx-MNTB synapse. Sample records were averaged from six events before (a) and 5 min after (b) Mid application, and 2 min (c) and 18 min (d) after PDBu application. B, Effect of PDBu on EPSCs at calyces loaded with Mid (●) or mutated Mid (○). Data derived from seven cells for Mid and five cells for mutated Mid. The difference was significant between Mid and mutated Mid and also between Mid and control (Fig. 5B; ○), but not significant between the mutated Mid and control (Fig. 5B; ○; one-way ANOVA).
DISCUSSION
At the brainstem auditory synapse formed by the calyx of Held, we have studied the facilitatory effect of phorbol ester on synaptic transmission. As reported previously (Malenka et al., 1986; Shapira et al., 1987; but see Caroll et al., 1998), phorbol ester had no effect on the amplitude of spontaneous miniature EPSCs, confirming that the site of its action is predominantly presynaptic. Direct whole-cell recordings from the calyx of Held indicated that phorbol ester had no effect on presynaptic Ca2+ currents. It has been reported that phorbol ester has no effect on recombinant Ca2+ channels containing α1A subunit, but enhances Ca2+ channels containing α1B subunit (Stea et al., 1995). Since Ca2+ channels triggering transmitter release at the calyx of Held are predominantly P-type containing α1A subunits (Forsythe et al., 1998; Iwasaki and Takahashi, 1998), possible involvement of N (α1B) type Ca2+channels in PESP at other synapses cannot be excluded from the present study (but see Yawo, 1999). Our results also indicate that phorbol ester has no effect on presynaptic K+currents. Thus, the mechanism for PESP must reside at the downstream of Ca2+ influx as in secretory cells, where phorbol ester increases hormonal secretion without involving a change in intracellular Ca2+ concentration (Gillis et al., 1996).
In chromaffin cells (Gillis et al., 1996), retinal bipolar cells (Minami et al., 1998), and hippocampal synapses in culture (Stevens and Sullivan 1998), phorbol ester is postulated to increase the size of the releasable pool of synaptic vesicles by accelerating replenishment from a “reservoir pool” (but see Yawo, 1999). What then might be the molecular target of phorbol esters? Diacylglycerol (DAG) and phorbol esters bind to the regulatory C1-domain of PKC and anchor the enzyme to the plasma membrane, thereby stabilizing its active conformation (Newton, 1997). The phorbol ester-induced synaptic facilitation was attenuated by bath-application of PKC inhibitors BIS or calphostin C and also by the PKC inhibitory peptide directly injected into the calyx through whole-cell recording pipette. These results suggest that PKC is involved, at least in part, in the PESP. However, in spite of relatively high concentrations, the blocking effect of PKC inhibitors was incomplete, implying that there may be an additional mechanism mediating the effect of phorbol ester. While an involvement of PKC in the PESP has been suspected (Scholfield and Smith, 1989; Redman et al., 1997), it was recently reported that phorbol esters or DAG stimulates the vesicular protein Doc2α to interact with the plasma membrane-associated protein Munc13–1 (Orita et al., 1997). The N-terminal domain (Mid) of Doc2α is involved in this interaction. We have demonstrated that the synthetic Mid peptide introduced into the calyx of Held attenuates PESP. This effect appears specific since the mutated Mid peptide had no effect. Therefore, we conclude that the Doc2α-Munc13–1 interaction is also involved in the PESP. In line with our results, it has been reported that presynaptic over-expression of Munc13–1 enhanced phorbol ester-dependent synaptic potentiation at Xenopus neuromuscular junctions in culture (Betz et al., 1998).
Apart from interaction with Doc2α, Munc13–1 can also interact with N-terminal of syntaxin 1, a SNARE protein thought to be involved in synaptic vesicle fusion (Betz et al., 1997). On the other hand, PKC can phosphorylate another SNARE protein, SNAP-25 (Fujita et al., 1996; Shimazaki et al., 1996), thereby stimulating catecholamine release from PC12 cells (Shimazaki et al., 1996). Furthermore, PKC can phosphporylate Munc-18, which interacts with SNARE proteins. Thus the SNARE protein may be a common effector downstream of PKC and Munc13–1 for the phorbol ester-induced synaptic potentiation.
Munc13–1 is a mammalian homolog of C. elegans unc-13p and, by analogy, is thought to contribute to vesicle docking and exocytosis. In support of this hypothesis, Mochida et al. (1998) showed that Mid peptide blocked synaptic transmission when injected into the presynaptic neuron in culture. However, this effect was not observed at the calyx of Held, where the peptide was directly injected into the nerve terminal through patch pipette perfusion. Since the blocking effect of Mid at cultured synapses appears slow and activity-dependent (Mochida et al., 1998), Munc13–1 might be involved in vesicular replenishing process rather than exocytotic process. It is also possible that exocytotic machineries are different between synapses.
Footnotes
This work was supported by the “Research for the Future” Program by The Japan Society for the Promotion of Sciences. We thank Drs. Masami Takahashi, Toshiya Manabe, Tetsuhiro Tsujimoto, and Brian Robertson for critically reading this manuscript.
Correspondence should be addressed to Tomoyuki Takahashi, Department of Neurophysiology, University of Tokyo Faculty of Medicine, Tokyo 113–0033, Japan.
REFERENCES
- 1.Barban JM, Snyder SH, Alger BE. Protein kinase C regulates ionic conductance in hippocampal pyramidal neurons: electrophysiological effects of phorbol esters. Proc Natl Acad Sci USA. 1985;82:2538–2542. doi: 10.1073/pnas.82.8.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes-Davies M, Forsyhe ID. Pre- and postsynaptic glutamate receptors at a giant excitatory synapse in rat auditory brainstem slices. J Physiol (Lond) 1995;488:387–406. doi: 10.1113/jphysiol.1995.sp020974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betz A, Okamoto M, Benseler F, Brose N. Direct interaction of the rat unc-13 homologue Munc 13–1 with the N terminus of syntaxin. J Biol Chem. 1997;272:2520–2526. doi: 10.1074/jbc.272.4.2520. [DOI] [PubMed] [Google Scholar]
- 4.Betz A, Ashery U, Rickmann M, Augustin I, Neher E, Sudhof T, Rettig J, Brose N. Munc13–1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron. 1998;21:123–136. doi: 10.1016/s0896-6273(00)80520-6. [DOI] [PubMed] [Google Scholar]
- 5.Borst JGG, Helmchen F, Sakmann B. Pre- and postsynaptic whole-cell recordings in the medial nucleus of the trapezoid body of the rat. J Physiol (Lond) 1995;489:825–840. doi: 10.1113/jphysiol.1995.sp021095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capogna M, Gahwiler BH, Thompson SM. Presynaptic enhancement of inhibitory synaptic transmission by protein kinases A and C in the rat hippocampus in vitro. J Neurosci. 1995;15:1249–1260. doi: 10.1523/JNEUROSCI.15-02-01249.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll RC, Nicoll RA, Malenka RC. Effects of PKA and PKC on miniature excitatory postsynaptic currents in CA1 pyramidal cells. J Neurophysiol. 1998;80:2797–2800. doi: 10.1152/jn.1998.80.5.2797. [DOI] [PubMed] [Google Scholar]
- 8.Doerner D, Pitler TA, Alger BE. Protein kinase C activators block specific calcium and potassium current components in isolated hippocampal neurons. J Neurosci. 1988;8:4069–4078. doi: 10.1523/JNEUROSCI.08-11-04069.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forsythe ID. Direct patch recording from identified presynaptic terminals mediating glutamatergic EPSCs in the rat CNS, in vitro. J Physiol (Lond) 1994;479:381–387. doi: 10.1113/jphysiol.1994.sp020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forsythe ID, Tsujimoto T, Barnes-Davies M, Cuttle MF, Takahashi T. Inactivation of presynaptic calcium current contributes to synaptic depression at a fast central synapse. Neuron. 1998;20:797–807. doi: 10.1016/s0896-6273(00)81017-x. [DOI] [PubMed] [Google Scholar]
- 11.Fossier P, Baux G, Tauc L. Activation of protein kinase C by presynaptic FLRFamide receptors facilitates transmitter release at an Aplysia cholinergic synapse. Neuron. 1990;5:479–486. doi: 10.1016/0896-6273(90)90087-v. [DOI] [PubMed] [Google Scholar]
- 12.Fujita Y, Sasaki T, Fukui K, Kotani H, Kimura T, Hata Y, Sudhof TC, Scheller RH, Takai Y. Phosphorylation of Munc-18/n-Sec1/rbSec1 by protein kinase C. J Biol Chem. 1996;271:7265–7268. doi: 10.1074/jbc.271.13.7265. [DOI] [PubMed] [Google Scholar]
- 13.Gillis KD, Mossner R, Neher E. Protein kinase C enhances exocytosis from chromaffin cells by increasing the size of the readily releasable pool of secretory granules. Neuron. 1996;16:1209–1220. doi: 10.1016/s0896-6273(00)80147-6. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman DA, Johnston D. Downregulation of transient K+ channels in dendrites of hippocampal CA1 pyramidal neurons by activation of PKA and PKC. J Neurosci. 1998;18:3521–3528. doi: 10.1523/JNEUROSCI.18-10-03521.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwasaki S, Takahashi T. Developmental changes in calcium channel types mediating synaptic transmission in rat auditory brainstem. J Physiol (Lond) 1998;509:419–423. doi: 10.1111/j.1469-7793.1998.419bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malenka RC, Madison DV, Nicoll RA. Potentiation of synaptic transmission in the hippocampus by phorbol esters. Nature. 1986;321:175–177. doi: 10.1038/321175a0. [DOI] [PubMed] [Google Scholar]
- 17.Minami N, Berglund K, Sakaba T, Kohmoto H, Tachibana M. Potentiation of transmitter release by protein kinase C in goldfish retinal bipolar cells. J Physiol (Lond) 1998;512:219–225. doi: 10.1111/j.1469-7793.1998.219bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mochida S, Orita S, Sakaguchi G, Sasaki T, Takai Y. Role of the Doc2α- Munc13–1 interaction in the neurotransmitter release process. Proc Natl Acad Sci USA. 1998;95:11418–11422. doi: 10.1073/pnas.95.19.11418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newton AC. Regulation of protein kinase C. Curr Opin Cell Biol. 1997;9:161–167. doi: 10.1016/s0955-0674(97)80058-0. [DOI] [PubMed] [Google Scholar]
- 20.O’Dell T J, Alger BE. Single calcium channels in rat and guinea-pig hippocampal neurons. J Physiol (Lond) 1991;436:739–767. doi: 10.1113/jphysiol.1991.sp018577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orita S, Naito A, Sakaguchi G, Maeda M, Igarashi H, Sasaki T, Takai Y. Physical and functional interactions of Doc2 and Munc13 in Ca2+ -dependent exocytotic machinery. J Biol Chem. 1997;272:16081–16084. doi: 10.1074/jbc.272.26.16081. [DOI] [PubMed] [Google Scholar]
- 22.Parfitt KD, Madison DV. Phorbol esters enhance synaptic transmission by a presynaptic, calcium-dependent mechanism in rat hippocampus. J Physiol (Lond) 1993;471:245–268. doi: 10.1113/jphysiol.1993.sp019900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redman RS, Searl TJ, Hirsh JK, Silinsky EM. Opposing effects of phorbol esters on transmitter release and calcium currents at frog motor nerve endings. J Physiol (Lond) 1997;501:41–48. doi: 10.1111/j.1469-7793.1997.041bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scholfield CN, Smith AJ. A phorbol diester-induced enhancement of synaptic transmission in olfactory cortex. Br J Pharmacol. 1989;98:1344–1350. doi: 10.1111/j.1476-5381.1989.tb12683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shapira R, Silberberg SD, Ginsburg S, Rahamimoff R. Activation of protein kinase C augments evoked transmitter release. Nature. 1987;325:58–60. doi: 10.1038/325058a0. [DOI] [PubMed] [Google Scholar]
- 26.Shimazaki Y, Nishiki T, Omori A, Sekiguchi M, Kamata Y, Kozaki S, Takahashi M. Phosphorylation of 25-kDa synaptosome-associated protein. Possible involvement in protein kinase C-mediated regulation of neurotransmitter release. J Biol Chem. 1996;271:14548–14553. doi: 10.1074/jbc.271.24.14548. [DOI] [PubMed] [Google Scholar]
- 27.Stea A, Soong TW, Snutch TP. Determinants of PKC-dependent modulation of a family of neuronal calcium channels. Neuron. 1995;15:929–940. doi: 10.1016/0896-6273(95)90183-3. [DOI] [PubMed] [Google Scholar]
- 28.Stevens CF, Sullivan JM. Regulation of the readily releasable vesicle pool by protein kinase C. Neuron. 1998;21:885–893. doi: 10.1016/s0896-6273(00)80603-0. [DOI] [PubMed] [Google Scholar]
- 29.Storm JF. Phorbol ester broaden the action potential in CA1 hippocampal pyramidal cells. Neurosci Lett. 1987;75:71–74. doi: 10.1016/0304-3940(87)90077-2. [DOI] [PubMed] [Google Scholar]
- 30.Swartz KJ, Merritt A, Bean BP, Lovinger DM. Protein kinase C modulates glutamate receptor inhibition of Ca2+ channels and synaptic transmission. Nature. 1993;361:165–168. doi: 10.1038/361165a0. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi T, Forsythe ID, Tsujimoto T, Barnes-Davies M, Onodera K. Presynaptic calcium current modulation by a metabotropic glutamate receptor. Science. 1996;274:594–597. doi: 10.1126/science.274.5287.594. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi T, Kajikawa Y, Tsujimoto T. G-protein-coupled modulation of presynaptic calcium currents and transmitter release by a GABAB receptor. J Neurosci. 1998;18:3138–3146. doi: 10.1523/JNEUROSCI.18-09-03138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takano K, Stanfield PR, Nakajima S, Nakajima Y. Protein kinase C-mediated inhibition of an inward rectifier potassium channel by substance P in nucleus basalis neurons. Neuron. 1995;14:999–1008. doi: 10.1016/0896-6273(95)90338-0. [DOI] [PubMed] [Google Scholar]
- 34.Yawo H. Protein kinase C potentiates transmitter release from the chick ciliary presynaptic terminal by increasing the exocytotic fusion probability. J Physiol (Lond) 1999;515:169–180. doi: 10.1111/j.1469-7793.1999.169ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]