Dissociation in Effects of Lesions of the Nucleus Accumbens Core and Shell on Appetitive Pavlovian Approach Behavior and the Potentiation of Conditioned Reinforcement and Locomotor Activity byd-Amphetamine (original) (raw)
Abstract
Dopamine release within the nucleus accumbens (NAcc) has been associated with both the rewarding and locomotor-stimulant effects of abused drugs. The functions of the NAcc core and shell were investigated in mediating amphetamine-potentiated conditioned reinforcement and locomotion. Rats were initially trained to associate a neutral stimulus (Pavlovian CS) with food reinforcement (US). After excitotoxic lesions that selectively destroyed either the NAcc core or shell, animals underwent additional CS–US training sessions and then were tested for the acquisition of a new instrumental response that produced the CS acting as a conditioned reinforcer (CR). Animals were infused intra-NAcc with d-amphetamine (0, 1, 3, 10, or 20 μg) before each session. Shell lesions affected neither Pavlovian nor instrumental conditioning but completely abolished the potentiative effect of intra-NAcc amphetamine on responding with CR. Core-lesioned animals were impaired during the Pavlovian retraining sessions but showed no deficit in the acquisition of responding with CR. However, the selectivity in stimulant-induced potentiation of the CR lever was reduced, as intra-NAcc amphetamine infusions dose-dependently increased responding on both the CR lever and a nonreinforced (control) lever. Shell lesions produced hypoactivity and attenuated amphetamine-induced activity. In contrast, core lesions resulted in hyperactivity and enhanced the locomotor-stimulating effect of amphetamine. These results indicate a functional dissociation of subregions of the NAcc; the shell is a critical site for stimulant effects underlying the enhancement of responding with CR and locomotion after intra-NAcc injections of amphetamine, whereas the core is implicated in mechanisms underlying the expression of CS–US associations.
Keywords: ventral striatum, reward, dopamine, psychomotor stimulant, associative learning, drugs of abuse
The mesolimbic dopamine (DA) system that projects from the ventral tegmental area (VTA) to the nucleus accumbens (NAcc) has been implicated in the rewarding properties of intracranial self-stimulation, drugs of abuse, and natural reinforcers (for review, see Wise and Bozarth, 1987; Robbins and Everitt, 1996). Although there is general agreement that the dopaminergic innervation of the NAcc contributes to reinforcement processes, the functions of this system remain unclear. It is unlikely that mesolimbic DA simply mediates primary reinforcement because lesions of this system or infusions of DA receptor antagonists into the NAcc do not affect consummatory responses to food, water, or sex (Koob et al., 1978a;Everitt, 1990; Robbins and Everitt, 1992).
Conversely, there is psychopharmacological, neurochemical, and electrophysiological evidence that DA release in the NAcc is associated with anticipatory responses to reinforcing stimuli (Everitt, 1990;Phillips et al., 1991; Schultz et al., 1992; Williams et al., 1993), indicating that DA modulates reinforcement signals at the level of the NAcc (Robbins and Everitt, 1992). In this way, DA release in the NAcc may enhance behavioral responses during both Pavlovian and instrumental conditioning by potentiating approach responses to conditioned stimuli and increasing the control over instrumental behavior of stimuli associated with reinforcement (conditioned reinforcers; Everitt and Robbins, 1992; Robbins and Everitt, 1992).
The acquisition of responding with conditioned reinforcement (CR) provides a powerful means of investigating the contribution of Pavlovian conditioning to reinforcement-related instrumental behavior. Conditioned reinforcement is the process whereby a previously conditioned stimulus (CS) acts as the reinforcer for an instrumental action (Mackintosh, 1974). Responding for a conditioned reinforcer is potentiated by systemic (Hill, 1970) or intra-NAcc (Taylor and Robbins, 1984) administration ofd-amphetamine, having behavioral, anatomical, and neurochemical specificity (Cador et al., 1989; Taylor and Robbins, 1984, 1986). Of particular relevance for this study is that these effects have been shown to depend critically on DA receptor activation within the NAcc (Taylor and Robbins, 1986; Wolterink et al., 1993). Thus, use of this procedure allows the control over behavior by a conditioned reinforcer and how this control is modified by increasing dopamine transmission in the nucleus accumbens to be measured, rather than the ability of an appetitive conditioned stimulus to affect new learning per se.
In addition to its role in reinforcement processes, the NAcc has been implicated in spontaneous and psychomotor stimulant-potentiated locomotion. Systemic or intra-NAcc infusions of DA receptor agonists increase spontaneous activity (Kelly et al., 1975; Kelley et al., 1989;Swerdlow and Koob, 1989). This increased activity is blocked by DA-depleting lesions of the VTA or by previous intra-NAcc administration of DA receptor antagonists (Kelly et al., 1975;Wolterink et al., 1993). Dopamine depletion from the NAcc or VTA generally produces hypoactivity (Koob et al., 1978b), whereas cell body lesions of the entire NAcc produce a significant increase in spontaneous locomotor activity (Kelly and Roberts, 1983; Kafetzopoulos, 1986; Everitt et al., 1991).
The NAcc is a heterogeneous structure (Graybiel and Ragsdale, 1978;Jongen-Relo et al., 1993). It can be separated anatomically into core and shell subdivisions (Zaborszky et al., 1985; Voorn et al., 1989) situated in the dorsolateral and ventromedial regions of the nucleus, respectively, that can be dissociated immunocytochemically (Graybiel and Ragsdale, 1978; Voorn et al., 1994) and on the basis of their differential patterns of connectivity (Groenewegen et al., 1987;Berendse and Groenewegen, 1990; Zahm and Heimer, 1990, 1993; Hurley et al., 1991; Berendse et al., 1992; Wright et al., 1996).
The distinct pattern of core and shell output targets, with the core projecting to pallidal structures and the shell, in addition, projecting to more limbic domains, such as the lateral hypothalamus (Zahm and Heimer, 1990), suggests that the two regions may mediate different behavioral processes. Recently, functional dissociations of the NAcc shell and core have been provided using both excitotoxic lesions and excitatory amino acid modulation of the two NAcc subregions selectively (Maldonado-Irizarry and Kelley, 1995; Kelley et al., 1997). By using excitotoxic lesions that selectively destroy neurons in either the NAcc core or shell, we have investigated the functions of these two subregions in the processes underlying CR and its potentiation by stimulant drugs and also in mediating the locomotor stimulant effects of d-amphetamine.
MATERIALS AND METHODS
Subjects
Ninety-five male Lister hooded rats (Olac, Bicester, UK) were housed in pairs in a temperature-controlled (21°C) room on a 12 hr light/dark cycle. After recovery from surgery, animals were placed on a restricted feeding schedule and maintained at ∼85% of their free-feeding weight. Water was available ad libitum in the home cages. All animals used in these studies were treated in accordance with the United Kingdom 1986 Animals (Scientific Procedures) Act (project license PPL 80/00668).
Surgery
Animals in the locomotor activity study received bilateral lesions of the NAcc core or shell before any behavioral testing. Animals in the CR study received lesions after the Pavlovian CR training sessions. Before surgery, rats were anesthetized with Avertin [2,2,2-tribromoethanol, 2-methylbutan-2-ol, Dulbecco “A” PBS tablets, and ddH20 in tertiary amyl alcohol (Sigma, Poole, UK); 10 ml/kg body weight] and secured in a Kopf stereotaxic instrument with the incisor bar set at −3.3 mm. Fifteen animals for the CR study and eighteen animals for the activity study received NAcc core lesions, induced by infusing 0.5 μl of quinolinic acid (0.09m) over 3 min at the following site: anteroposterior (AP), +1.2 mm; lateral (L), ±1.8 mm; and dorsoventral (DV), −7.1 mm from dura. The pipette was removed 2 min after the infusion. Fourteen animals for the CR study and twelve animals for the activity study received NAcc shell lesions that were induced by infusing ibotenic acid (0.06 m) at three different sites. The coordinates, infusion volume, infusion time, and diffusion time for each injection were as follows: (1) AP, +1.6 mm; L, ± 1.1 mm; DV, −6.4 mm; 0.1 μl; 1 min; 2 min; (2) AP, +1.6 mm; L, ±1.1 mm; DV, −6.9 mm; 0.1 μl; 1 min; 1 min; (3) AP, +1.6 mm; L, ±1.1 mm; DV, −7.9 mm; 0.2 μl; 2 min; 1 min. Twelve rats (six core and six shell) for the CR study and twenty-four (12 core and 12 shell) for the activity study received NAcc sham lesions induced by infusing vehicle using the coordinates and infusion parameters described above.
All neurotoxin injections were made through a single burr hole using either a 1 or 5 μl SGE (Baton Rouge, LA) syringe (26 gauge code, 1BR-OC-7/0.47) with a custom-made glass micropipette attached to the end. Initially, pipettes (Intracel Ltd.) measured 1.2 mm external diameter and 0.69 mm internal diameter by 10 cm in length and were pulled using a Stoelting App-1 all-purpose Puller, model 52500, giving a final tip diameter of 50–100 μm and a length of 12 mm. Micropipettes were attached to the syringe using Araldite epoxy resin (CIBA) to ensure an airtight seal. All animals were given injections of glucose–saline (5–10 ml, i.p.) after surgery to aid recovery. There was no significant animal loss in the first week after surgery.
Animals in the CR study were also implanted bilaterally with stainless steel guide cannulae (22 gauge) 2 mm dorsal to the intended injection site in the NAcc (AP, +1.6 mm; ML, ±1.5 mm; DV, −4.1 mm) during the same surgery as that in which the lesions were made. Cannulae were fixed to the skull with dental cement, and stainless steel screws and were closed with stainless steel stylets. All animals were allowed to recover for 2 weeks before behavioral testing began.
Drugs and infusions
d-Amphetamine sulfate (Sigma) was dissolved in sterile PBS, pH 7.2, for intracerebral infusions and in sterile 0.9% saline for systemic injections. Intracerebral infusions were made through 29 gauge cannulae that extended 2 mm beyond the guide cannulae tips and were attached to an infusion pump (Harvard Apparatus) by polyethylene tubing. Infusions were in a volume of 0.5 μl per side over 60 sec with a 60 sec diffusion period. Before the first drug infusion, all animals were given a preliminary infusion of PBS and returned to their home cages so that any behavioral effects of tissue damage mechanically induced by the injection cannulae would occur before the test session.
Apparatus
Conditioned reinforcement training and testing took place in sound-attenuated operant chambers (26.5 × 22 × 20 cm) that were fitted with two retractable levers and a sucrose dipper situated between the levers (Med Associates). The operant chambers could be illuminated by a ceiling house light, and external noise was masked by ventilating fans mounted on the side of each box. Access to the dipper was allowed through a magazine (3.8 cm from the side and 5.5 cm from the grid floor) that could be illuminated with a tray light. The apparatus was controlled and data were collected by BBC or Acorn Archimedes microcomputers (Acorn Computers, Cambridge, UK) running the control languages Spider or Arachnid (Genes Cognition, Cambridge, UK).
Locomotor activity was tested in individual wire photocell cages (40 × 25 × 18 cm) that were transected by two parallel infrared photocell beams 6 cm from the cage ends and 1 cm from the cage floor. Beam breaks were registered in 10 min bins on-line by a BBC Master Series microcomputer equipped with a Spider extension (Genes Cognition). All testing was conducted in the dark phase of the light/dark cycle.
Behavioral procedures
Conditioned reinforcement. Forty-one animals underwent Pavlovian conditioning sessions in which the presentation of a CS (5 sec illumination of the tray light and house light off) preceded the US (5 sec elevation of the dipper filled with a 10% sucrose solution). The CS–US pairing was presented 30 times per session on a variable interval (VI), 60 sec schedule of reinforcement. All animals received 20 training sessions. The frequency and duration of magazine entries were detected by infrared beams that transected the entrance. The number of magazine entries during the VI, CS, and US periods was recorded, and a measure of discriminated approach was determined for each animal. Discriminated approach was calculated as the mean number of magazine entries during the CS period as a ratio of the mean number of magazine entries during the total trial period, excluding the duration of US presentation (CS + VI). Animals were given four retraining sessions when they had recovered from surgery to ensure a stable baseline of responding before the start of the test phase.
In Pavlovian to instrumental transfer test sessions, sucrose was not available, i.e., animals were tested in extinction. The two levers were introduced into the chambers, and depression of one lever (CR) resulted in the presentation of the CS (under a random ratio 2 schedule), whereas depression of the second lever had no programmed consequences (NCR). The ability of the CS to selectively increase responding on the CR lever provides a measure of the conditioned reinforcing properties of the CS (Mackintosh, 1974). The assignment of CR and NCR levers was counterbalanced within groups. Immediately before each of five tests, animals were infused intra-NAcc with d-amphetamine (0, 1, 3, 10, or 20 μg). All drug doses were administered in a Latin square design. The number of responses on each lever, as well as the number of magazine entries, were recorded during each 30 min test. All test sessions were separated by 48 hr. For statistical analyses, the responses on the CR and NCR lever were square root-transformed to maintain homogeneity of variance (Winer, 1971). Furthermore, the homogeneity of variance across groups in repeated-measures design ANOVAs was assessed by the Mauchly Sphericity test. When data sets significantly violated this requirement for a repeated-measures design ANOVA, the Greenhouse Geisser Epsilon correction parameter for degrees of freedom (Geisser and Greenhouse, 1959; Winer, 1971) was used to calculate a more conservative p value for each_F_ ratio. Finally, when appropriate, further analyses of three-way ANOVA interactions was undertaken using weighted mean-adjusted (pooled) Mean Square Error terms as described by Winer (1971).
Locomotor activity. Thirty-six animals were tested for locomotor responses to systemic injections ofd-amphetamine. They were given four 2 hr sessions in the activity cages to measure spontaneous locomotor activity and habituation to the cages. After this, all animals received 0, 0.5, 1.5, and 5.0 mg/kg of d-amphetamine (administered in ascending order of concentration) systemically (intraperitoneally), on separate days, and their activity levels were measured for 2 hr.
Histological assessment of lesions
At the completion of behavioral testing, animals were killed under deep barbiturate anesthesia and perfused intracardially with 0.9% saline followed by 4% paraformaldehyde (PFA). The brains were removed and post-fixed for 1 hr in PFA and then stored in a 20% w/v sucrose solution for 12–15 hr.
Coronal sections (40 μm) were cut through the forebrain on a freezing microtome. Every third section was stained for immunocytochemical analysis using antibodies raised against substance P and Calbindin 28 K. These sections were quenched for 5 min at room temperature in a solution of 10% methanol, 10% concentrated hydrogen peroxide, and 80% distilled water. After two 5 min washes in 0.05 mTris-buffered saline (TBS; pH 7.4), the sections were subjected to blocking with 1% goat serum (NGS) in TBS containing 0.2% Triton X-100 (TTBS) for 1 hr before being transferred without washing into a solution containing the primary antibody in the following dilution: monoclonal anti-Calbindin-D (mouse) antibody (Sigma) 1:500 and 1% NGS in TTBS; anti-substance P antibody 1:1000 with 1% NGS in TTBS. Sections were left overnight at room temperature on a shaker and then washed thoroughly in TBS. Sections were then incubated in goat anti-mouse (biotinylated) at a dilution of 1:200, or goat anti-rabbit (biotinylated) at a dilution of 1:50, with 1% NGS in TBS for 3 hr, then given three 5 min washes in TBS before a further 1 hr incubation with the avidin–biotin–peroxidase complex (ABC; Vector Laboratories, Burlingame, CA) at a dilution of 1:200 with 1% NGS in TBS (this solution was mixed 30 min before use). The sections were then washed once in TBS and twice in Tris nonsaline (TNS) before treatment with the chromogen diaminobenzidine (DAB): 10 mg/ml DAB and 0.67 μl/ml 30% hydrogen peroxide in TNS. Sections were incubated for ∼5 min until the required intensity of reaction was attained. After a final rinse in TBS, sections were mounted on gelatinized slides, allowed to dry overnight, and then dehydrated in ascending alcohols and coverslipped.
Alternate sections were mounted on gelatin-coated glass slides, then stained for Nissl substance using cresyl violet. The combined Nissl staining and immunocytochemistry allowed visualization of the NAcc core and shell regions. Calbindin is prevalent within the core subregion, whereas substance P immunoreactivity is relatively more intense in the shell (Voorn et al., 1989; Zahm and Brog, 1992). Thus, immunocytochemistry highlights the core and shell subregions during histological analysis, whereas staining with cresyl violet stain enables assessment of the extent and nature of excitotoxin-induced neuronal damage as well as gliosis-associated intracerebral infusions of quinolinic or ibotenic acids.
RESULTS
Histological assessment
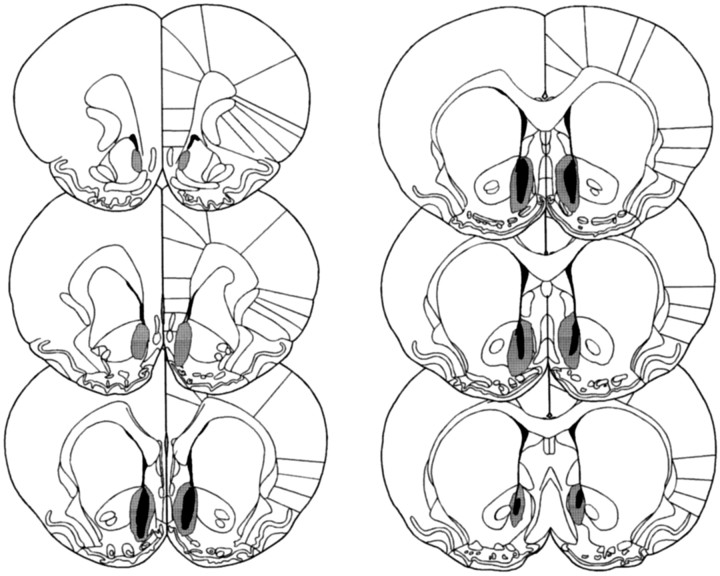
Figures 1 and2 show schematic representations of lesions of the NAcc core and shell, respectively, based on the stereotaxic atlas of the rat brain by Paxinos and Watson (1998). Delineation of the NAcc core and shell was also based on immunocytochemical and histological analyses of the striatum (Voorn et al., 1989; Zahm and Brog, 1992; Jongen-Relo et al., 1993, 1994; Heimer et al., 1995). NAcc shell lesions, resulting from infusions of ibotenic acid, destroyed all, or a great majority of, neurons in the caudal, mediodorsal shell (sometimes termed the septal pole), thus leaving the medial rostral pole and also the entire ventral and ventrolateral aspects of the shell intact (see Fig. 3 for photomicrograph of the lesion). Neuronal loss typically extended in an anteroposterior direction from +1.7 to +0.48 mm anterior to bregma and from the base of the lateral ventricle dorsally, to the ventral portions of the medial shell occasionally reaching the region of the olfactory tubercle. There was, in some cases, unilateral damage to the lateral septum or to the medial NAcc core. Animals with bilateral damage to any structures extraneous to the shell, including especially the medial core, were excluded from any further analysis. Finally, there was no evidence of damage to the ventral pallidum or the nucleus of the vertical limb of the diagonal band of Broca in any animals.
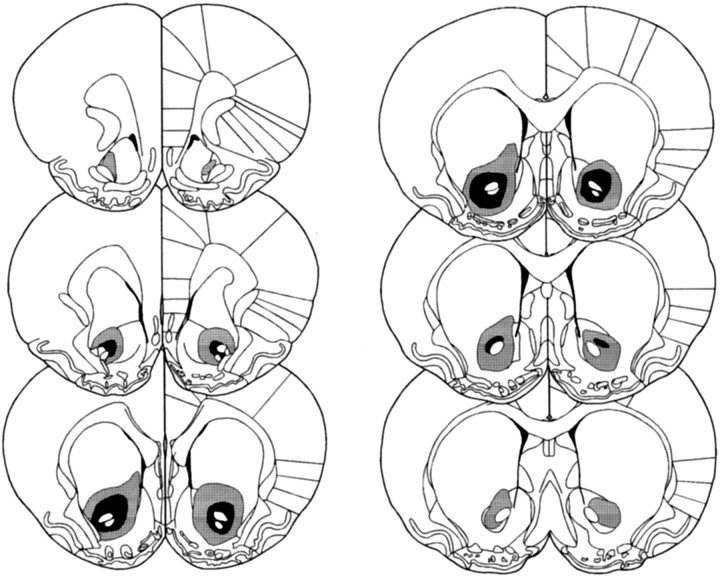
Fig. 1.
Schematic representation of excitotoxic lesions to the NAcc core. Shaded areas represent the smallest (black) and largest (gray) extent of neuronal damage in a single animal. Coronal sections are +2.7 mm anterior through +0.48 mm posterior to bregma (Paxinos and Watson, 1998).
Fig. 2.
Schematic representation of excitotoxic lesions to the NAcc shell. Shaded areas represent the smallest (black) and largest (gray) extent of neuronal damage in a single animal. Coronal sections are +2.7 mm anterior through +0.48 mm posterior to bregma (Paxinos and Watson, 1998).
Fig. 3.
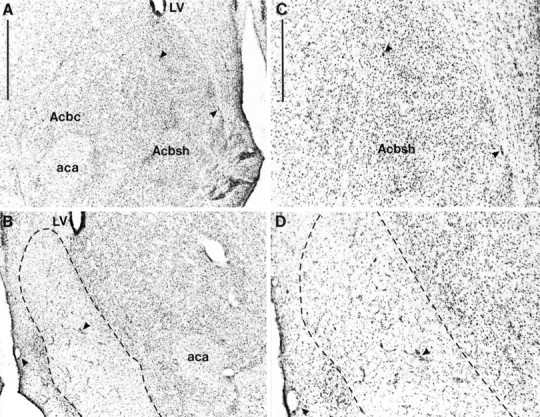
Photomicrographs showing cresyl violet-stained coronal sections through the nucleus accumbens (∼+1.2 mm from bregma). A, Sham lesion; B, nucleus accumbens shell lesion. C, High magnification of the sham lesion section shown in A; D, high magnification of the shell lesion section shown in B. The lesioned area is indicated by the dotted lines. Scale bars: A, 1 mm; C, 500 μm.Arrowheads show identical landmarks in _A_and B, and C and D.aca, Anterior commissure; Acbc, nucleus accumbens core; Acbsh, nucleus accumbens shell;LV, lateral ventricle.
Lesions of the NAcc core resulting from infusions of quinolinic acid encompassed most of the core subregion. Figure4 shows photomicrographs of a representative coronal section from an NAcc core lesion and a sham control stained with cresyl violet. Neuronal loss and associated gliosis extended, in an anteroposterior direction, rostrally from +2.5 to +0.5 mm anterior to bregma. Generally, the lesion did not extend ventrally or caudally into ventral pallidum or olfactory tubercle. Neuronal damage was often caused to ventral parts of the overlying caudate putamen, although this was usually unilateral in nature. Similarly, neuronal loss was occasionally seen in the lateral or ventrolateral shell. Animals with bilateral damage of this kind were excluded from further analysis of the behavioral data. Finally, animals with any damage to the medial and dorsomedial shell were excluded from the behavioral analysis.
Fig. 4.
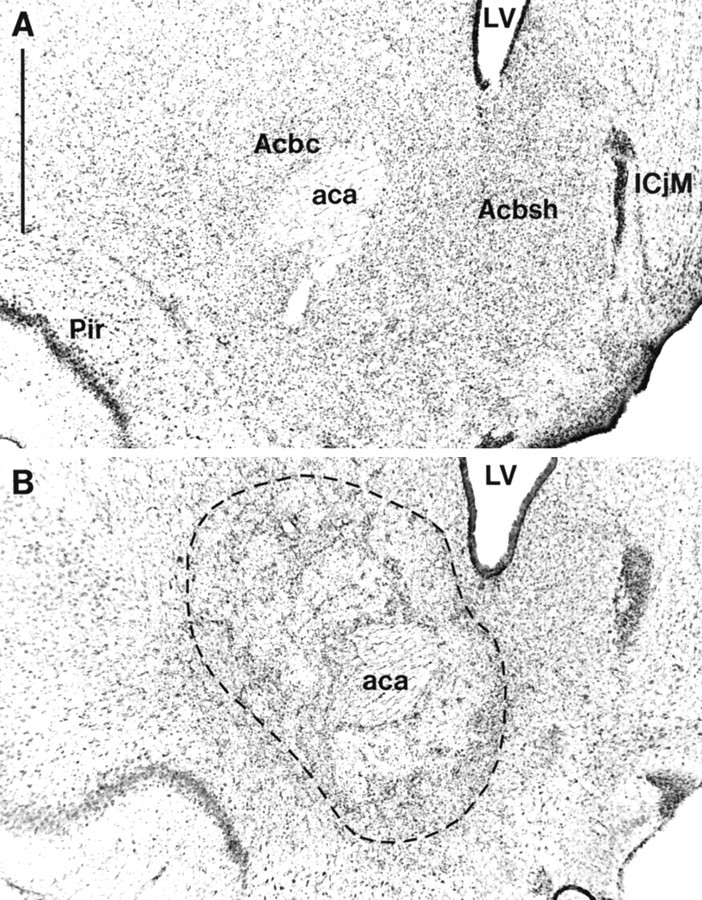
Photomicrographs showing cresyl violet-stained coronal sections through the nucleus accumbens (∼+1.2 mm from bregma). A, Sham lesion; B, nucleus accumbens core lesion. The lesioned area is indicated by the_dotted lines_. Scale bar: A, 1 mm.aca, Anterior commissure; Acbc, nucleus accumbens core; Acbsh, nucleus accumbens shell;ICjM, major islands of calleja; LV, lateral ventricle; Pir, piriform cortex.
During experimental testing, implanted cannulae became detached from the head mountings of some subjects. The data from these animals were not included in the subsequent statistical analyses (four core, two shell, and four sham animals). Similarly, the data from animals with lesions that were incomplete or extended beyond the target area, as revealed by histological examination, were also discarded (six core and 11 sham). Twenty-two animals remained in the CR study, seven shell-lesioned, seven core-lesioned, and eight sham-lesioned. Forty-six animals remained in the locomotor study, six shell-lesioned, 16 core-lesioned, and 24 sham-lesioned.
Conditioned reinforcement
Pavlovian conditioning
The effects of NAcc core and shell lesions on discriminated approach during CS–US training sessions are shown in Figure5. Discriminated approach was calculated as the number of approaches during the CS period as a ratio of approaches during the total CS and VI period. An increase in this ratio across sessions indicates that responses during the CS period are increasing relative to responses during the VI period. ANOVA of the four presurgical Pavlovian sessions (comparing the three experimental groups before surgery) showed that there were no differences in this measure of discriminated approach between the three groups either through a main effect of lesion (F(2,19)= 0.52; p = 0.6) or through a lesion × session interaction (F(6,57) = 0.33; p = 0.92). However, all animals demonstrated Pavlovian learning as expressed as an increase in conditioned responding over sessions (F(3,57) = 22.28; p = 0.0001).
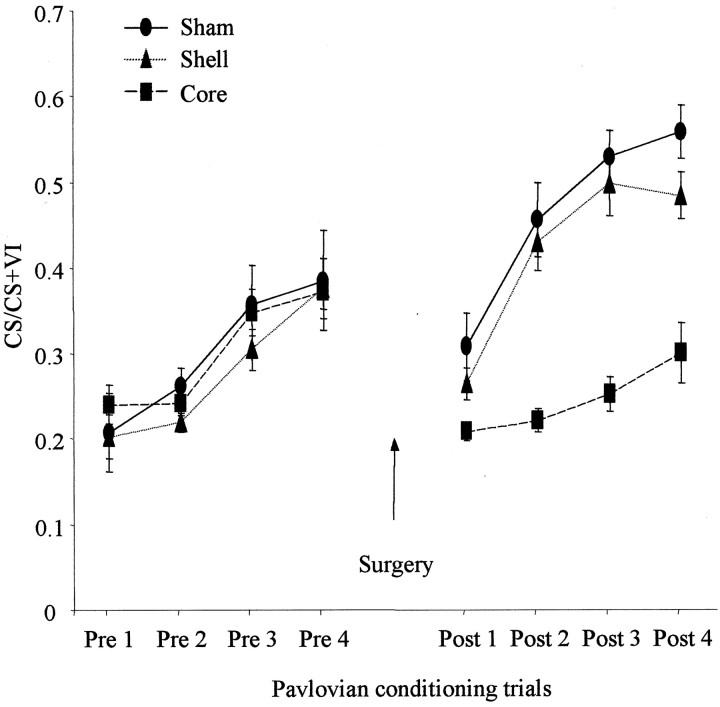
Fig. 5.
Effect of NAcc core and shell lesions on Pavlovian-discriminated approach. The mean ± SEM ratio of approach responses during the CS relative to the CS plus VI period is shown for four presurgical and four postsurgical sessions for core-, shell-, and sham-lesioned animals.
ANOVA comparing postsurgery discriminated approach revealed a significant lesion × surgery interaction (F(6,57) = 2.9; p < 0.05).Post hoc analysis of simple interactions demonstrated that animals with core lesions exhibited a reduced level of discriminated approach relative to shell- and sham-lesioned animals during the postsurgery conditioning sessions (p < 0.05). Furthermore, whereas shell- and sham-lesioned animals showed a significant increase in discriminated approach over the four trials (p < 0.05), core-lesioned animals showed no significant change in their approach behavior (p = 0.13).
Baseline activity (overall magazine entries during the session), as measured by the total frequency and duration of magazine entries, was not affected by either lesion (duration: lesion × session interaction, F(2,22) = 2.26, p = 0.13; main effect of lesion, F(2,22) = 3.04,p = 0.09; frequency: lesion × session interaction, F(2,22) = 0.13, p = 0.73; main effect of lesion, F(2,22) = 1.49;p = 0.25), demonstrating the specific discriminated nature of the core lesion deficit.
Acquisition of a new response with conditioned reinforcement
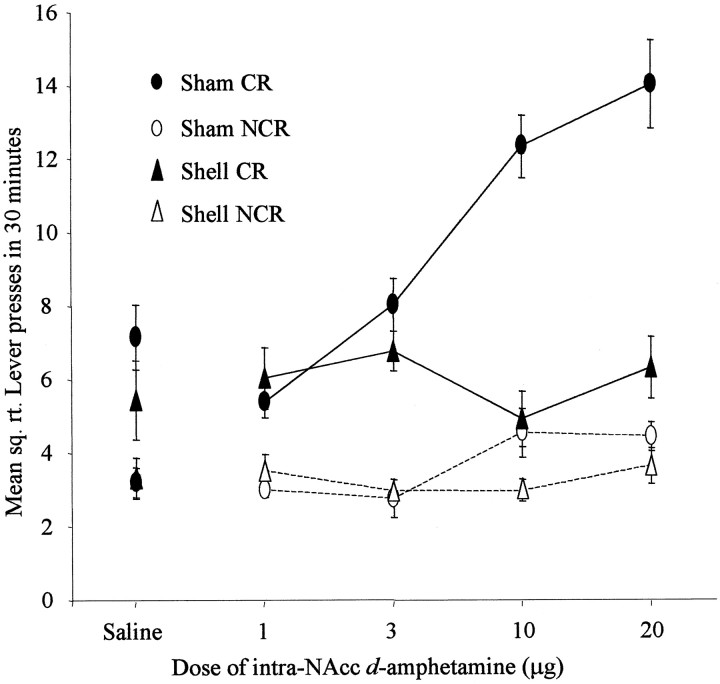
The effects of NAcc core and shell lesions on the acquisition of responding with CR are shown in Figures 6and 7. The actual mean number of responses over the 30 min testing periods made by sham-lesioned animals varied from 56.6 and 10.5 (for CR and NCR levers, respectively) after saline to 208 and 19.9 at the 20 μg d-amphetamine dose. Responses made by core-lesioned animals similarly ranged from 52.1 and 19.6 (CR and NCR after saline infusion) to 121.2 and 30.2 (after 20 μg d-amphetamine), whereas those for shell-lesioned animals ranged from 36.9 and 12.2 (CR and NCR, respectively) to 43.8 and 13.9 (after 20 μg d-amphetamine).
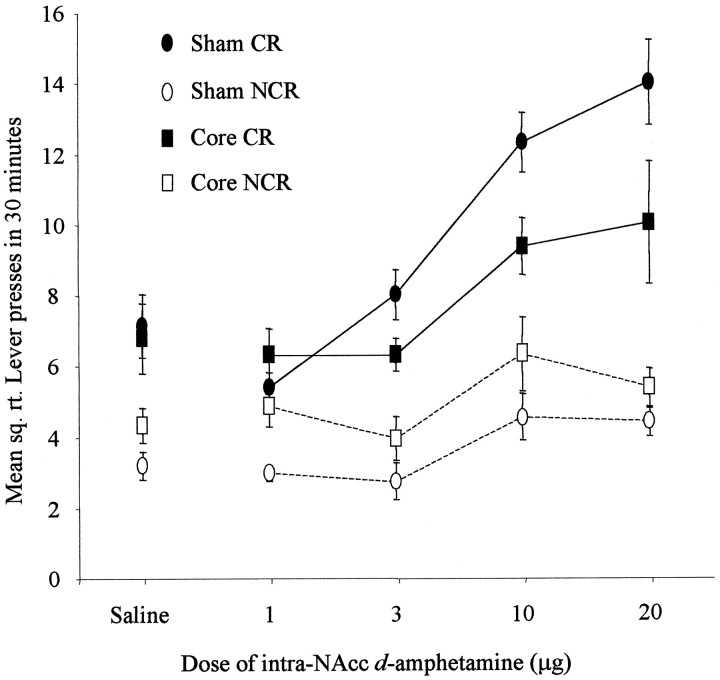
Fig. 6.
Effect of NAcc core lesions on the acquisition of responding with CR. Data points represent the mean square root ± SEM responses on the lever producing the conditioned reinforcer (CR) and the control lever (NCR) for sham- and core-lesioned animals after intra-NAcc injections ofd-amphetamine (0, 1, 3, 10, and 20 μg).
Fig. 7.
Effect of NAcc shell lesions on the acquisition of responding with CR. Data points represent the mean square root ± SEM responses on the lever producing the conditioned reinforcer (CR) and the control lever (NCR) for sham- and shell-lesioned animals after intra-NAcc injections ofd-amphetamine (0, 1, 3, 10, and 20 μg).
The data were square root-transformed to maintain homogeneity of variance. ANOVA comparing CR and NCR responses for each group acrossd-amphetamine doses revealed statistical significance for all interactions, including lesion × lever × dose (F(5,50) = 4.14; p < 0.005), lesion × lever (F(2,19) = 11.08;p < 0.005), lesion × dose (F(8,76) = 3.85; p < 0.005), and lever × dose (F(3,50) = 9.17;p < 0.0001). All three main effects were also statistically significant: lesion (F(2,19) = 8.41; p < 0.005), lever (F(1,19) = 137.55; p < 0.0001), and dose (F(4,76) = 9.85; p < 0.0001). Because of the three-way interaction, the nature of these effects was investigated further by post hoc analysis of simple interactions and simple main effects by studying the factors of lever and dose separately in each experimental group.
These post hoc analyses revealed that sham-lesioned animals made significantly more responses on the CR than the NCR lever and indicated that these animals further showed a dose-dependent increase in responding on the CR lever but not the NCR lever (lever × dose interaction, F(4,76) = 15.33; p< 0.05).
The analysis of core-lesioned animals produced significant main effects of lever (F(1,19) = 24.58; p < 0.05) and dose (F(4,19) = 3.72;p < 0.05), revealing that the stimulant effects of intra-NAcc d-amphetamine and the control over behavior by CR were intact. However, core-lesioned animals did not show a significant lever × dose interaction (F(4,76) = 2.06; p = ns), suggesting that the interaction of CR and its potentiation by amphetamine was absent. There was a dose-dependent increase in responding, but this was not selective for the CR lever.
Shell-lesioned animals were not impaired in responding with CR (main effect of lever, F(1,19) = 21.38;p < 0.05). However, the stimulant effects of intra-NAcc d-amphetamine were abolished after shell lesions (main effect of dose, F(4,19) = 0.39;p = ns; lever × dose interaction,F(4,76) = 0.84; p = ns).
In summary, sham-lesioned animals responded more on the CR lever at all doses of d-amphetamine, and this response was selectively potentiated in a dose-dependent manner. Core-lesioned animals were not significantly impaired in the acquisition of a new response with CR. Moreover, intra-NAcc infusions of d-amphetamine produced a significant stimulation of responding. However, the stimulus control over responding on the CR and NCR levers after intra-NAccd-amphetamine was impaired in these animals. Shell-lesioned animals were completely unimpaired in the acquisition of a new response with CR, but these lesions abolished the potentiative effect of intra-NAcc d-amphetamine. Thus, the CS successfully acted as a conditioned reinforcer for operant lever pressing in all groups. d-amphetamine selectively potentiated responding with CR in sham-lesioned animals only, whereas there was less control over this behavior by the conditioned reinforcer after NAcc core lesions and a complete abolition of the potentiative effects of d-amphetamine in animals with NAcc shell lesions.
Discriminated approach to the magazine during CR testing
ANOVAs were used to compare the duration and frequency of magazine entries during CR testing. Under control vehicle treatment there was a significant main effect of lesion on duration of magazine entries (F(2,21) = 4.31; p < 0.05) caused by the fact that core-lesioned animals spent significantly more time at the magazine than either sham- or shell-lesioned animals. Statistical analysis of the effect of d-amphetamine on the duration of magazine approaches revealed a significant lesion × dose interaction (F(8,84) = 8.15;p < 0.0001) as the duration of approaches was reduced in both the core- and sham-lesioned animals equivalently and dose-dependently, whereas the shell-lesioned group showed a dose-dependent increase in approach duration.
Analysis of the frequency of magazine entries revealed no significant group differences after saline injections (F(2,21) = 2.73; p = 0.09). Comparisons of the effect of d-amphetamine on the frequency of magazine approaches showed no significant lesion × dose interaction (F(3,32) = 1.95; p = 0.14) or main effect of dose (F(2,32) = 0.8;p = 0.43). The significant main effect of lesion (F(2,21) = 5.16; p < 0.05) was caused by a reduced frequency of approaches made by shell-lesioned animals across doses of d-amphetamine relative to both sham- and core-lesioned animals.
In summary, core-lesioned animals showed an increase in the duration of magazine approach under saline treatment. Furthermore, compared with both sham- and core-lesioned animals, animals with NAcc shell lesions showed a general reduction in the frequency of magazine entries and a dose-dependent increase in the duration of magazine entry afterd-amphetamine injections, perhaps reflecting an overall reduction in locomotor activity by these animals.
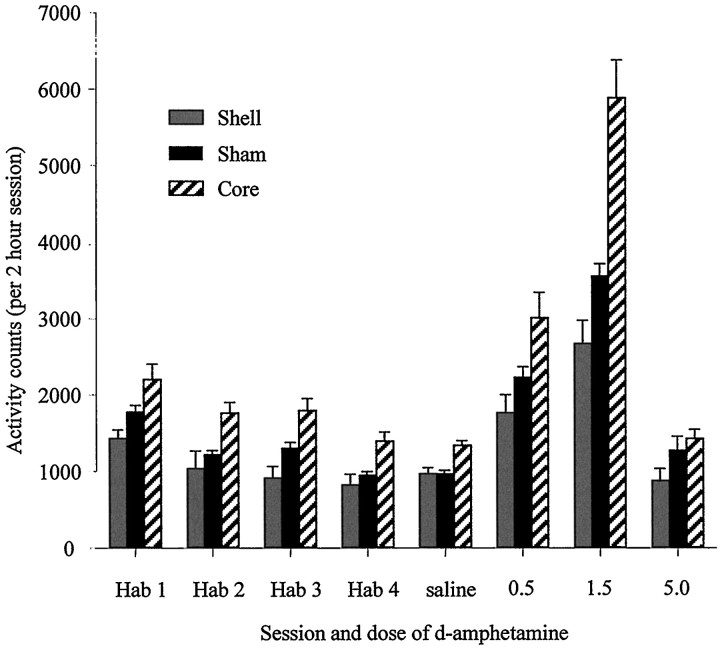
Locomotor activity
Figure 8 shows the activity levels of shell-, core-, and sham-lesioned animals during habituation sessions and after each drug injection. ANOVA comparing locomotor scores of the three groups across habituation sessions revealed no lesion × session interaction (F(4,93) = 0.59;p = 0.69). Activity levels of all three groups declined over the four habituation sessions (main effect of session,F(2,93) = 39.03; p < 0.001), but there was also a significant main effect of lesion (F(2,42) = 8.97; p = 0.001).Post hoc tests revealed that this was caused by the increased activity of core-lesioned animals compared with both sham- and shell-lesioned animals.
Fig. 8.
Effect of NAcc core and shell lesions on spontaneous and d-amphetamine-induced locomotion.Vertical bars represent the mean ± SEM photocell beam breaks for each group during habituation sessions (Hab) and after systemic injections of d-amphetamine (saline, 0.5, 1.5, 5.0 mg/kg).
A one-way ANOVA comparing activity levels after saline injections revealed a significant effect of lesion (F(2,43)= 6.81; p = 0.003), and post hoc tests indicated that the core-lesioned group’s activity was significantly higher than that of shell- and sham-lesioned groups (which were not different from one another). Because of this baseline difference, a multivariate analysis of covariance was undertaken to compare activity levels after the three systemic drug injections, using the activity levels after saline injections as the covariates. This analysis revealed that activity levels after injections of 0.5 and 1.5 mg/kgd-amphetamine differed significantly from saline (F(1,41) = 17.31; p < 0.001 and_F_(1,41) = 14.20; p = 0.001, respectively), but not after the 5.0 mg/kg d-amphetamine injection (F(1,41) = 0.62; p = 0.44). Thus, activity was significantly elevated in all groups after 0.5 and 1.5 mg/kg injections but not after 5.0 mg/kg. There was also a lesion × drug interaction (F(2,41) = 7.11;p = 0.002) at the 1.5 mg/kg dose. _Post hoc_analysis of this interaction revealed that the activity scores of the core-lesioned rats were significantly higher than both shell- and sham-lesioned groups, whereas the shell-lesioned group showed significantly lower locomotor activity scores than shams. A repeated-measures ANOVA using the percentage change from baseline activity levels corroborated the results from the multivariate analysis of covariance.
Ratings of stereotypy were taken (ranging from 0 to 8; see Mittleman et al., 1991) at 30, 60, 90, and 120 min during the final (5.0 mg/kg) test session. For example, a score of 0 was given for inactivity (of behavior), 2 for continuous locomotor activity, 4 for continuous stereotypy over a wide area, and 6 for pronounced, continuous stereotypy in a restricted area. Average stereotypy scores for each group over the 2 hr session are shown in Table1. Because of the nature of these data, nonparametric tests were used to analyze stereotypy ratings. Thus, a Kruskal–Wallis one-way ANOVA assessed group differences at each of the four time points. There were no significant group differences at any time point (critical value for χ2 at p < 0.05 = 5.99; calculated values were 5.22 at 30 min, 0.19 at 60 min, 1.09 at 90 min, and 0.18 at 120 min). Thus, whereas all animals demonstrated stereotypy after a systemic injection of 5.0 mg/kgd-amphetamine, there were no differences between groups.
Table 1.
Effect of NAcc core and shell lesions on stereotypy
| Group | 30 | 60 | 90 | 120 |
|---|---|---|---|---|
| Sham | 4.33 | 4.66 | 4.29 | 3.33 |
| Core | 3.67 | 4.68 | 4.37 | 3.37 |
| Shell | 4.16 | 4.83 | 4.66 | 3.5 |
In summary, animals with lesions of the core were more active than their sham-lesioned controls during a test of spontaneous locomotor activity. Furthermore, their locomotor response to systemicd-amphetamine was significantly enhanced relative to sham controls. Thus, lesions of the core produced an enhancement of the locomotor potentiative effects of d-amphetamine. In contrast, rats with lesions of the shell were less active than sham-lesioned controls during the measurement of spontaneous locomotor activity, and the potentiative effects of systemicd-amphetamine were significantly attenuated in these animals (at the 1.5 mg/kg dose).
DISCUSSION
To investigate the possibly dissociable functions of the NAcc core and shell, we have developed excitotoxic amino acid-induced lesions that selectively destroy these two regions and investigated their effects on two fundamental effects of psychomotor stimulant drugs; the potentiation of conditioned reinforcement and locomotor hyperactivity, as well as appetitive Pavlovian behavior and the acquisition of instrumental responding with CR. Lesions of the NAcc shell completely abolished the potentiation of instrumental behavior with CR after intra-NAcc infusions of d-amphetamine. Shell lesions also produced locomotor hypoactivity and attenuatedd-amphetamine-induced increases in locomotor activity. By contrast, in core-lesioned animals, intra-NAccd-amphetamine infusions dose-dependently increased responding on CR and NCR (control) levers, thus demonstrating intact stimulant potentiation combined with a reduction of stimulus control. Core lesions also produced locomotor hyperactivity and enhanced the locomotor-stimulating effect of systemicdamphetamine. NAcc shell lesions affected neither Pavlovian conditioning nor CR as assessed in the acquisition of a new response procedure. In contrast, NAcc core lesions impaired discriminated approach to a Pavlovian-conditioned stimulus but did not affect the acquisition of a new instrumental response with CR.
These findings indicate basic interactions between the dopamine-dependent effect of stimulants such as amphetamine and associative information that we have shown to be dependent on limbic afferents to the NAcc (Cador et al., 1989). They also indicate that different aspects of the learned control over appetitive behaviors are mediated by distinct regions within the NAcc, as revealed here by demonstrating a double dissociation between the effects of lesions of the NAcc shell and core on responses to amphetamine and associative learning mechanisms.
Effects of NAcc shell and core lesions on responses to amphetamine
NAcc shell lesions completely abolished the potentiative effects of intra-NAcc d-amphetamine on the control over behavior by a conditioned reinforcer. Shell lesions also resulted in hypoactivity during the habituation sessions to locomotor activity cages and attenuated the stimulant effect of systemic d-amphetamine. These results suggest that a major property of stimulant drugs to potentiate both the impact of motivationally relevant environmental cues on instrumental behavior and locomotor activity rely critically on the integrity of the NAcc shell.
These findings are consistent with reports of changes in DA transmission selectively within the NAcc shell (relative to the NAcc core or the dorsal striatum), in response to intravenous infusions of several drugs of abuse (Pontieri et al., 1995, 1996; Carlezon and Wise, 1996; Tanda et al., 1997) and selective increases in energy metabolism as measured by 2-deoxyglucose autoradiography in the NAcc shell produced by such drugs (Pontieri et al., 1994; Orzi et al., 1996). Highly palatable, preferred foods also increase DA selectively in the NAcc shell (Tanda et al., 1994) as do Pavlovian CSs paired with food (Phillips et al., 1993; Wilson et al., 1995). Such observations (Robbins and Everitt, 1992) have led authors to suggest a role for NAcc DA in incentive motivation (Phillips and Fibiger, 1987) and reward (Wise and Bozarth, 1987) and more specifically, a role for DA in the shell in the attribution of incentive properties to CSs (DiChiara, 1998), with drugs of abuse usurping this process, thereby producing abnormal “incentive learning”. Although the NAcc shell is clearly implicated in aspects of responding to both drug-related and natural reinforcers, the present findings of intact Pavlovian and instrumental conditioning in NAcc shell-lesioned animals suggest that, rather than an associative or incentive motivational role, a key function of the dopaminergic innervation of the NAcc shell is to potentiate ongoing instrumental responding in the presence of motivationally significant stimuli.
Lesions of the central nucleus of the amygdala (CeA) also block the potentiative effects of intra-NAcc d-amphetamine on responding with CR (Burns et al., 1993; Robledo et al., 1996). Although the CeA does not project directly to the NAcc, it may influence striatal DA function via its projections to midbrain DA neurons (Simon et al., 1979; Wallace et al., 1992; Han et al., 1997). Alternatively, the NAcc shell and CeA may be functionally related as part of the continuum known as the extended amygdala (Heimer et al., 1991; Alheid et al., 1995). Dopamine-depleting lesions of the amygdala (Simon et al., 1988), as well as infusions of D1 receptor antagonists (Hurd et al., 1997) both profoundly alter dopamine concentration or release in the NAcc, indicating the tight functional relationship between these components of the extended amygdala.
Lesions of the ventral subiculum block the potentiative effects ofd-amphetamine on locomotor activity and abolish the effects of intra-NAcc d-amphetamine without affecting the impact of the CR on instrumental performance (Burns et al., 1993), much like the shell lesions in the present study. This similarity in the functional effects of NAcc shell and ventral subiculum lesions is significant in the context of the strong preferential glutamatergic projection from the ventral subiculum to that part of the NAcc shell (septal pole) that was lesioned here (Fig. 2). Thus, information reaching the NAcc concerned with the nature and direction of behavior, which depends on the integrity of the basolateral amygdala (BLA) (Cador et al., 1989; Burns et al., 1993), presumably via its projections to both the NAcc core and shell (Groenewegen et al., 1987) may be “gain-amplified” by dopamine transmission in the shell in a way that is critically dependent on the integrity of its glutamatergic inputs arising from the ventral subiculum (Burns et al., 1993; Blaha et al., 1997; Brudzynski and Gibson, 1997).
Although NAcc core lesions did not affect the acquisition of responding with CR under control (saline) conditions, the interaction between intra-NAcc d-amphetamine and responding with CR appears to depend on the integrity of the NAcc core. It is of interest, therefore, that animals with lesions of the BLA show similar, although greater, impairments in the same task, including a loss of control over responding for the CR under control conditions (Cador et al., 1989;Burns et al., 1993). Similarities in the effects of NAcc and BLA manipulations have been reported previously (Everitt et al., 1989,1991; Everitt and Robbins, 1992) and have led us to suggest that the integrity of the BLA is critical for stimulus–reward information to gain influence over voluntary behavior (Everitt et al., 1991; Burns et al., 1993; Robbins and Everitt, 1996). Thus, limbic corticostriatal circuits involving the BLA and NAcc core may be essential for the influence of associative stimulus–reward information on goal-directed action. The effects of NAcc shell lesions to abolish the potentiative effects of intra-NAcc d-amphetamine, whereas NAcc core lesions disrupt discriminative control after intra-NAcc infusions of damphetamine are reminiscent of models of striatal function based on separate striatal zones being responsible for behavioral “choice” and “vigour” (Kelly and Moore, 1976;Robbins and Everitt, 1982; Koshikawa et al., 1996). Such models can now be refined both behaviorally and neuroanatomically on the basis of the functional dissociations revealed in the present and related studies (Balleine and Killcross, 1994; Kelley et al., 1997) and the neuronal interactions known to occur in shell and core compartments of the striatum determined by the pattern of termination of discrete limbic cortical afferents (Pennartz et al., 1994).
NAcc core lesions also resulted in increased spontaneous locomotor activity and an enhanced response to systemicdamphetamine. This may reflect motoric response disinhibition after core lesions resulting from reductions in inhibitory striatal GABAergic outflow to several striatal output target structures directly concerned with the control of locomotor activity (Alexander and Crutcher, 1990) that are also further susceptible to the effects of DA release within remaining parts (shell) of the NAcc. Similar mechanisms may underlie the significant increase in responding on the NCR control lever during the conditioned reinforcement procedure.
Effects of NAcc shell and core lesions on associative learning mechanisms
Animals with lesions of the NAcc shell were not impaired in Pavlovian conditioning as assessed by their discriminated approach to a Pavlovian CS+, or in the acquisition of responding with CR. Intra-NAcc infusions of d-amphetamine also dose-dependently increased magazine approach duration, relative to NAcc core- and sham-operated groups, supporting further the selective effects ofd-amphetamine in the NAcc core on the mechanisms subserving discriminated approach. Thus, the NAcc shell does not appear to be significantly involved in associative learning mechanisms assessed in this study. Lesions of the NAcc core, by contrast, retarded the expression of the CS–US association such that animals in this group showed a decrease in levels of discriminated approach relative to prelesion performance. It may seem paradoxical that core-lesioned animals who showed a deficit in discriminated approach subsequently acquired responding with CR. However, Kelley et al. (1997) reported similar effects with blockade of NMDA receptors in the NAcc. Furthermore, we have demonstrated previously that disruptions in the formation of a CS–US association do not necessarily produce deficits in the acquisition of a new response for the Pavlovian-paired stimulus (Olmstead et al., 1998) and vice versa (Burns et al., 1993). Our results confirm, therefore, that the neural mechanisms through which conditioned reinforcers control instrumental behavior are dissociable neurally from processes that mediate the expression of a CS–US relationship through Pavlovian approach behavior (Cador et al., 1989;Burns et al., 1993).
Implications for the functions of the nucleus accumbens
There are two major findings in this study: (1) the NAcc shell mediates both the potentiation by d-amphetamine of the control over instrumental responding by conditioned reinforcers and of locomotor activity; (2) the NAcc core is involved in the conditioned preparatory aspects of Pavlovian associative learning and may also modulate the associative control over instrumental responding after intra-NAcc d-amphetamine. These results, therefore, indicate that there is functional specificity within the NAcc and its associated circuitries. The potentiative effects on behavior ofd-amphetamine are critically dependent on the NAcc shell, whereas the expression or potentiation of Pavlovian conditioned responses generated by the presentation of incentive stimuli (Rescorla and Solomon, 1967; Dickinson and Balleine, 1994) depends on the integrity of the NAcc core. Furthermore, control over goal-directed actions and incentive motivation per se may depend on functional interactions between limbic cortical structures, including the BLA, cingulate, and prefrontal cortex, which may interface with different striatal zones (Cador et al., 1989;McAlonan et al., 1993; Bussey et al., 1997; Floresco et al., 1997). Thus, different limbic corticostriatal afferents passing through the NAcc within ventral striatopallidal circuits may be involved in qualitatively different functional processes but may undergo similar modulation at the level of the NAcc.
Footnotes
This work was supported by a Medical Research Council Programme Grant (G9537855) to B.J.E., T.W.R., and A. Dickinson. J.A.P. was supported by a Biotechnology and Biological Sciences Research Council research studentship and an Oon Khye Beng Ch’hia Tsio Scholarship.
Correspondence should be addressed to Barry J. Everitt, Department of Experimental Psychology, University of Cambridge, Downing Street, Cambridge, UK CB2 3EB.
REFERENCES
- 1.Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- 2.Alheid GF, de Olmos JS, Beltramino CA. Amygdala and extended amygdala. In: Paxinos G, editor. The rat nervous system, Vol 1, forebrain and midbrain, Ed 2. Academic; Sydney: 1995. pp. 495–578. [Google Scholar]
- 3.Balleine B, Killcross S. Effects of ibotenic acid lesions of the nucleus accumbens on instrumental action. Behav Brain Res. 1994;65:181–193. doi: 10.1016/0166-4328(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 4.Berendse HW, Groenewegen HJ. Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. J Comp Neurol. 1990;299:187–228. doi: 10.1002/cne.902990206. [DOI] [PubMed] [Google Scholar]
- 5.Berendse HW, Galisdegraaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- 6.Blaha CD, Yang CR, Floresco SB, Barr AM, Phillips AG. Stimulation of the ventral subiculum of the hippocampus evokes glutamate receptor-mediated changes in dopamine efflux in the rat nucleus accumbens. Eur J Neurosci. 1997;9:902–911. doi: 10.1111/j.1460-9568.1997.tb01441.x. [DOI] [PubMed] [Google Scholar]
- 7.Brudzynski SM, Gibson CJ. Release of dopamine in the nucleus accumbens caused by stimulation of the subiculum in freely moving rats. Brain Res Bull. 1997;42:303–308. doi: 10.1016/s0361-9230(96)00290-0. [DOI] [PubMed] [Google Scholar]
- 8.Burns LH, Robbins TW, Everitt BJ. Differential effects of excitotoxic lesions of the basolateral amygdala, ventral subiculum and medial prefrontal cortex on responding with conditioned reinforcement and locomotor activity potentiated by intra-accumbens infusions of d-amphetamine. Behav Brain Res. 1993;55:167–183. doi: 10.1016/0166-4328(93)90113-5. [DOI] [PubMed] [Google Scholar]
- 9.Bussey TJ, Muir JL, Everitt BJ, Robbins TW. Triple dissociation of anterior cingulate, posterior cingulate, and medial frontal cortices on visual discrimination tasks using a touchscreen testing procedure for the rat. Behav Neurosci. 1997;111:920–936. doi: 10.1037//0735-7044.111.5.920. [DOI] [PubMed] [Google Scholar]
- 10.Cador M, Robbins TW, Everitt BJ. Involvement of the amygdala in stimulus reward associations: interaction with the ventral striatum. Neuroscience. 1989;30:77–86. doi: 10.1016/0306-4522(89)90354-0. [DOI] [PubMed] [Google Scholar]
- 11.Carlezon WA, Wise RA. Rewarding actions of phencyclidine and related drugs in nucleus accumbens shell and frontal cortex. J Neurosci. 1996;16:3112–3122. doi: 10.1523/JNEUROSCI.16-09-03112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiChiara G. A motivational learning hypothesis of the role of mesolimbic dopamine in compulsive drug use. J Psychopharmacol. 1998;12:54–67. doi: 10.1177/026988119801200108. [DOI] [PubMed] [Google Scholar]
- 13.Dickinson A, Balleine B. Motivational control of goal-directed action. Anim Learn Behav. 1994;22:1–18. [Google Scholar]
- 14.Everitt BJ. Sexual motivation: a neural and behavioral analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev. 1990;14:217–232. doi: 10.1016/s0149-7634(05)80222-2. [DOI] [PubMed] [Google Scholar]
- 15.Everitt BJ, Robbins TW. Amygdala-ventral striatal interactions and reward-related processes. In: Aggleton JP, editor. The amygdala. Wiley-Liss; New York: 1992. pp. 401–430. [Google Scholar]
- 16.Everitt BJ, Cador M, Robbins TW. Interactions between the amygdala and ventral striatum in stimulus reward associations: studies using a 2nd-order schedule of sexual reinforcement. Neuroscience. 1989;30:63–75. doi: 10.1016/0306-4522(89)90353-9. [DOI] [PubMed] [Google Scholar]
- 17.Everitt BJ, Morris KA, O’Brien A, Robbins TW. The basolateral amygdala ventral striatal system and conditioned place preference: further evidence of limbic striatal interactions underlying reward-related processes. Neuroscience. 1991;42:1–18. doi: 10.1016/0306-4522(91)90145-e. [DOI] [PubMed] [Google Scholar]
- 18.Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graybiel AM, Ragsdale CW. Histochemically distinct compartments in the striatum of human, monkey and cat demonstrated by acetylcholinesterase staining. Proc Natl Acad Sci USA. 1978;75:5723–5726. doi: 10.1073/pnas.75.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groenewegen HJ, Vermeulen-Van der Zee E, Kortschot AT, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat: a study using anterograde transport of phaseolus-vulgaris leucoagglutinin. Neuroscience. 1987;23:103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- 21.Han JS, McMahan RW, Holland P, Gallagher M. The role of an amygdalo-nigrostriatal pathway in associative learning. J Neurosci. 1997;17:3913–3919. doi: 10.1523/JNEUROSCI.17-10-03913.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heimer L, Zahm DS, Alheid GF. Basal ganglia. In: Paxinos G, editor. The rat nervous system, Ed 2. Academic; Sydney: 1995. pp. 579–628. [Google Scholar]
- 23.Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- 24.Hill RT. Facilitation of conditioned reinforcement as a mechanism of psychomotor stimulation. In: Costa E, Garratini S, editors. Amphetamine and related compounds. Raven; New York: 1970. pp. 781–795. [Google Scholar]
- 25.Hurd YL, McGregor A, Ponten M. In vivo amygdala dopamine levels modulate cocaine self-administration behaviour in the rat: D1 dopamine receptor involvement. Eur J Neurosci. 1997;9:2541–2548. doi: 10.1111/j.1460-9568.1997.tb01683.x. [DOI] [PubMed] [Google Scholar]
- 26.Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- 27.Jongen-Relo AL, Groenewegen HJ, Voorn P. Evidence for a multicompartmental histochemical organization of the nucleus accumbens in the rat. J Comp Neurol. 1993;337:267–276. doi: 10.1002/cne.903370207. [DOI] [PubMed] [Google Scholar]
- 28.Jongen-Relo AL, Voorn P, Groenewegen HJ. Immunohistochemical characterization of the shell and core territories of the nucleus accumbens in the rat. Eur J Neurosci. 1994;6:1255–1264. doi: 10.1111/j.1460-9568.1994.tb00315.x. [DOI] [PubMed] [Google Scholar]
- 29.Kafetzopoulos E. Effects of amphetamine and apomorphine on locomotor activity after kainic acid lesion of the nucleus accumbens septi in the rat. Psychopharmacology. 1986;88:271–274. doi: 10.1007/BF00180823. [DOI] [PubMed] [Google Scholar]
- 30.Kelley AE, Gauthier AM, Lang CG. Amphetamine microinjections into distinct striatal subregions cause dissociable effects on motor and ingestive behavior. Behav Brain Res. 1989;35:27–39. doi: 10.1016/s0166-4328(89)80005-1. [DOI] [PubMed] [Google Scholar]
- 31.Kelley AE, Smith-Roe SL, Holahan MR. Response-reinforcement learning is dependent on N-methyl-d-aspartate receptor activation in the nucleus accumbens core. Proc Natl Acad Sci USA. 1997;94:12174–12179. doi: 10.1073/pnas.94.22.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly PH, Moore KE. Mesolimbic dopaminergic neurones in the rotational model of nigrostriatal function. Nature. 1976;263:695–696. doi: 10.1038/263695a0. [DOI] [PubMed] [Google Scholar]
- 33.Kelly PH, Roberts DCS. Effects of amphetamine and apomorphine on locomotor activity after 6-OHDA and electrolytic lesions of the nucleus accumbens septi. Pharmacol Biochem Behav. 1983;19:137–143. doi: 10.1016/0091-3057(83)90322-2. [DOI] [PubMed] [Google Scholar]
- 34.Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- 35.Koob GF, Fray PJ, Iversen SD. Self-stimulation at the lateral hypothalamus and locus coeruleus after specific unilateral lesions of the dopamine system. Brain Res. 1978a;146:123–140. doi: 10.1016/0006-8993(78)90222-6. [DOI] [PubMed] [Google Scholar]
- 36.Koob GF, Riley SJ, Smith SC, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi and olfactory tubercle on feeding, locomotor activity, and amphetamine anorexia in the rat. J Comp Physiol Psych. 1978b;92:917–927. doi: 10.1037/h0077542. [DOI] [PubMed] [Google Scholar]
- 37.Koshikawa N, Kitamura M, Kobayashi M, Cools AR. Contralateral turning elicited by unilateral stimulation of dopamine D-1 and D-2 receptors in the nucleus accumbens of rats is due to stimulation of these receptors in the shell, but not the core, of this nucleus. Psychopharmacology. 1996;126:185–190. doi: 10.1007/BF02246447. [DOI] [PubMed] [Google Scholar]
- 38.Mackintosh NJ. The psychology of animal learning. Academic; London: 1974. [Google Scholar]
- 39.Maldonado-Irizarry CS, Kelley AE. excitotoxic lesions of the core and shell subregions of the nucleus accumbens differentially disrupt body weight regulation and motor activity in rat. Brain Res Bull. 1995;38:551–559. doi: 10.1016/0361-9230(95)02030-2. [DOI] [PubMed] [Google Scholar]
- 40.McAlonan GM, Robbins TW, Everitt BJ. Effects of medial dorsal thalamic and ventral pallidal lesions on the acquisition of a conditioned place preference: further evidence for the involvement of the ventral striatopallidal system in reward-related processes. Neuroscience. 1993;52:605–620. doi: 10.1016/0306-4522(93)90410-h. [DOI] [PubMed] [Google Scholar]
- 41.Mittleman G, Jones GH, Robbins TW. Sensitization of amphetamine-stereotypy reduces plasma-corticosterone: implications for stereotypy as a coping response. Behav Neural Biol. 1991;56:170–182. doi: 10.1016/0163-1047(91)90584-d. [DOI] [PubMed] [Google Scholar]
- 42.Olmstead MC, Robbins TW, Everitt BJ. Basal forebrain cholinergic lesions enhance conditioned approach responses to stimuli predictive of food. Behav Neurosci. 1998;112:611–629. doi: 10.1037//0735-7044.112.3.611. [DOI] [PubMed] [Google Scholar]
- 43.Orzi F, Passarelli F, Lariccia M, Digrezia R, Pontieri FE. Intravenous morphine increases glucose utilization in the shell of the rat nucleus accumbens. Eur J Pharmacol. 1996;302:49–51. doi: 10.1016/0014-2999(96)00128-8. [DOI] [PubMed] [Google Scholar]
- 44.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- 45.Pennartz CMA, Da Silva FHL, Groenewegen HJ. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioral, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 46.Phillips AG, Fibiger HC. Anatomical and neurochemical substrates of drug reward determined by the conditioned place preference technique. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. Springer; New York: 1987. pp. 275–290. [Google Scholar]
- 47.Phillips G, Willner P, Sampson D, Nunn J, Muscat R. Time-dependent, schedule-dependent, and reinforcer-dependent effects of pimozide and amphetamine. Psychopharmacology. 1991;104:125–131. doi: 10.1007/BF02244566. [DOI] [PubMed] [Google Scholar]
- 48.Phillips AG, Atkinson LJ, Blackburn JR, Blaha CD. Increased extracellular dopamine in the nucleus accumbens of the rat elicited by a conditional stimulus for food: an electrochemical study. Can J Physiol Pharmacol. 1993;71:387–393. doi: 10.1139/y93-059. [DOI] [PubMed] [Google Scholar]
- 49.Pontieri FE, Colangelo V, Lariccia M, Pozzilli C, Passarelli F, Orzi F. Psychostimulant drugs increase glucose utilization in the shell of the rat nucleus accumbens. NeuroReport. 1994;5:2561–2564. doi: 10.1097/00001756-199412000-00039. [DOI] [PubMed] [Google Scholar]
- 50.Pontieri FE, Tanda G, DiChiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the shell as compared with the core of the rat nucleus accumbens. Proc Natl Acad Sci USA. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pontieri FE, Tanda G, Orzi F, DiChiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- 52.Rescorla RA, Solomon RL. Two-process learning theory: relationships between Pavlovian conditioning and instrumental learning. Psychol Rev. 1967;74:151–182. doi: 10.1037/h0024475. [DOI] [PubMed] [Google Scholar]
- 53.Robbins TW, Everitt BJ. Functional studies of the central catecholamines. Int Rev Neurobiol. 1982;23:303–365. doi: 10.1016/s0074-7742(08)60628-5. [DOI] [PubMed] [Google Scholar]
- 54.Robbins TW, Everitt BJ. Functions of dopamine in the dorsal and ventral striatum. Semin Neurosci. 1992;4:119–127. [Google Scholar]
- 55.Robbins TW, Everitt BJ. Neurobehavioral mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- 56.Robledo P, Robbins TW, Everitt BJ. Effects of excitotoxic lesions of the central amygdaloid nucleus on the potentiation of reward-related stimuli by intra-accumbens amphetamine. Behav Neurosci. 1996;110:981–990. doi: 10.1037//0735-7044.110.5.981. [DOI] [PubMed] [Google Scholar]
- 57.Schultz W, Apicella P, Scarnati E, Ljungberg T. Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci. 1992;12:4595–4610. doi: 10.1523/JNEUROSCI.12-12-04595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simon H, Le Moal M, Calas A. Efferents and afferents of the ventral tegmental-A10 region studied after local injection of (3h) leucine and horseradish peroxidase. Brain Res. 1979;178:77–86. doi: 10.1016/0006-8993(79)90085-4. [DOI] [PubMed] [Google Scholar]
- 59.Simon H, Taghzouti K, Gozlan H, Studler JM, Louilot A, Herve D, Glowinski J, Tassin JP, Le Moal M. Lesion of dopaminergic terminals in the amygdala produces enhanced locomotor response to d-amphetamine and opposite changes in dopaminergic activity in prefrontal cortex and nucleus accumbens. Brain Res. 1988;447:335–340. doi: 10.1016/0006-8993(88)91136-5. [DOI] [PubMed] [Google Scholar]
- 60.Swerdlow NR, Koob GF. Norepinephrine stimulates behavioral activation in rats following depletion of nucleus accumbens dopamine. Pharmacol Biochem Behav. 1989;33:595–599. doi: 10.1016/0091-3057(89)90394-8. [DOI] [PubMed] [Google Scholar]
- 61.Tanda G, Carboni E, Frau R, DiChiara G. Increase of extracellular dopamine in the prefrontal cortex: a trait of drugs with antidepressant potential. Psychopharmacology. 1994;115:285–288. doi: 10.1007/BF02244785. [DOI] [PubMed] [Google Scholar]
- 62.Tanda G, Pontieri FE, DiChiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu 1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- 63.Taylor JR, Robbins TW. Enhanced behavioral control by conditioned reinforcers following microinjections of D-amphetamine into the nucleus accumbens. Psychopharmacology. 1984;84:405–412. doi: 10.1007/BF00555222. [DOI] [PubMed] [Google Scholar]
- 64.Taylor JR, Robbins TW. 6-hydroxydopamine lesions of the nucleus accumbens, but not of the caudate-nucleus, attenuate enhanced responding with reward-related stimuli produced by intra-accumbens d-amphetamine. Psychopharmacology. 1986;90:390–397. doi: 10.1007/BF00179197. [DOI] [PubMed] [Google Scholar]
- 65.Voorn P, Gerfen CR, Groenewegen HJ. Compartmental organization of the ventral striatum of the rat: immunohistochemical distribution of enkephalin, substance-p, dopamine, and calcium-binding protein. J Comp Neurol. 1989;289:189–201. doi: 10.1002/cne.902890202. [DOI] [PubMed] [Google Scholar]
- 66.Voorn P, Brady LS, Schotte A, Berendse HW, Richfield EK. Evidence for two neurochemical divisions in the human nucleus accumbens. Eur J Neurosci. 1994;6:1913–1916. doi: 10.1111/j.1460-9568.1994.tb00582.x. [DOI] [PubMed] [Google Scholar]
- 67.Wallace DM, Magnuson DJ, Gray TS. Organisation of amygdaloid projections to brainstem dopaminergic, noradrenergic and adrenergic cell groups in the rat. Brain Res Bull. 1992;28:447–454. doi: 10.1016/0361-9230(92)90046-z. [DOI] [PubMed] [Google Scholar]
- 68.Williams GV, Rolls ET, Leonard CM, Stern C. Neuronal re-sponses in the ventral striatum of the behaving macaque. Behav Brain Res. 1993;55:243–252. doi: 10.1016/0166-4328(93)90120-f. [DOI] [PubMed] [Google Scholar]
- 69.Wilson C, Nomikos GG, Collu M, Fibiger HC. Dopaminergic correlates of motivated behavior: importance of drive. J Neurosci. 1995;15:5169–5178. doi: 10.1523/JNEUROSCI.15-07-05169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Winer BJ. Statistical principles in experimental design. McGraw-Hill; New York: 1971. [Google Scholar]
- 71.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- 72.Wolterink G, Phillips G, Cador M, Donselaar-Wolterink I, Robbins TW, Everitt BJ. Relative roles of ventral striatal d(1) and d(2) dopamine-receptors in responding with conditioned reinforcement. Psychopharmacology. 1993;110:355–364. doi: 10.1007/BF02251293. [DOI] [PubMed] [Google Scholar]
- 73.Wright CI, Beijer AVJ, Groenewegen HJ. Basal amygdaloid complex afferents to the rat nucleus accumbens are compartmentally organized. J Neurosci. 1996;16:1877–1893. doi: 10.1523/JNEUROSCI.16-05-01877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zaborszky L, Alheid GF, Beinfeld MC, Eiden LE, Heimer L, Palkovits M. Cholecystokinin innervation of the ventral striatum: a morphological and radioimmunological study. Neuroscience. 1985;14:427. doi: 10.1016/0306-4522(85)90302-1. [DOI] [PubMed] [Google Scholar]
- 75.Zahm DS, Brog JS. On the significance of subterritories in the accumbens part of the rat ventral striatum. Neuroscience. 1992;50:751–767. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]
- 76.Zahm DS, Heimer L. 2 transpallidal pathways originating in the rat nucleus accumbens. J Comp Neurol. 1990;302:437–446. doi: 10.1002/cne.903020302. [DOI] [PubMed] [Google Scholar]
- 77.Zahm DS, Heimer L. Specificity in the efferent projections of the nucleus accumbens in the rat: comparison of the rostral pole projection patterns with those of the core and shell. J Comp Neurol. 1993;327:220–232. doi: 10.1002/cne.903270205. [DOI] [PubMed] [Google Scholar]