Genetic Analysis on the Role of Integrin during Axon Guidance in Drosophila (original) (raw)
Abstract
Heterodimeric cell surface receptor integrin is widely expressed in the nervous system, but its specific role during axon development has not been directly tested in vivo. We show that the_Drosophila_ nervous system expresses low levels of positron-specific (PS) integrin subunits αPS1, αPS2, and βPS during embryonic axogenesis. Furthermore, certain subsets of neurons express higher levels of integrin mRNAs than do the rest. Null mutations in either the αPS1 or αPS2 subunit gene cause widespread axon pathfinding errors that can be rescued by supplying the wild-type integrin subunit to the mutant nervous system. In contrast, misexpressing either the αPS1 or αPS2 integrin subunit in all neurons leads to no obvious axon pathfinding errors. We propose that integrin does not itself serve as either a “clutch” constituting molecule or a specific growth cone “receptor,” as proposed previously, but rather as part of a molecular network that cooperatively guarantees accurate axon guidance.
Keywords: αPS1, αPS2, axon guidance, βPS, Drosophila, growth cone, inflated, integrin, multiple edematous wings, myospheroid, neuromuscular, pathfinding
Axon guidance relies on the activation of cell surface receptors that translate extrinsic cues into directed cytoskeletal rearrangement within a growth cone (Chiba and Keshishian, 1996; Goodman, 1996; Jay, 1996). Recent studies establish that many members of integrin, a cell adhesion/signaling molecule, are widely distributed in developing nervous systems (Schmidt et al., 1995;Varnum-Finney et al., 1995; Jones, 1996; Lallier et al., 1996; Martin et al., 1996; Shaw et al., 1996; Wu et al., 1996; Grotewiel et al., 1998). In vitro studies show that integrin is enriched at the tip of a growing axon, suggesting a role in axon guidance (Wu et al., 1996; Grabham and Goldberg, 1997; Takagi et al., 1998). One model proposes that integrin is an important component of the “clutch,” a molecular complex that is responsible for growth cone advancement by anchoring cytoplasmic actin filaments to the extracellular substrate (C. H. Lin et al., 1994; Schmidt et al., 1995). Another model predicts that integrin serves as a cell-specific recognition “receptor” that biases growth cone movement toward the ligand source (Kuhn et al., 1995, 1998). These ideas have not been tested_in vivo_.
We chose Drosophila as a model system for studying integrins because its nervous system consists of well-defined axon pathways (Chiba, 1998) and because it has a small set of integrin subunits similar in structure and function to those in vertebrates (MacKrell et al., 1988; Leptin et al., 1989; Wilcox, 1990; Brown, 1993, 1994; Yee and Hynes, 1993; Gotwals et al., 1994a,b; Brower et al., 1995; Bunch et al., 1998). A positron-specific (PS) αPS1 subunit (structurally similar to vertebrate laminin-responsive α3, α6, and α7 subunits) dimerizes with an βPS subunit (similar to the vertebrate β1 subunit) to form the laminin-binding PS1 integrin. An αPS2 subunit (similar to vertebrate RGD-dependent α5, α8, αv, and αIIb subunits) also dimerizes with the βPS subunit to form PS2 integrin that binds to molecules with the amino acid triplet RGD (e.g., tiggrin). These PS integrins serve as major cell adhesion molecules during embryogenesis, with PS1 integrin concentrated in ectodermal and endodermal tissues and PS2 integrin being most abundant in mesodermal tissues (Zusman et al., 1990; Bunch et al., 1992; Roote and Zusman, 1996). A role for PS integrins in embryonic neuronal differentiation has been speculated because βPS loss-of-function mutants fail to condense the CNS normally during early larvagenesis and primary neuronal culture from these mutants fail to develop normally on a laminin substrate (Donady and Seecof, 1972; Brown, 1994). The αPS3 subunit, a new member of the PS integrin family that dimerizes with a βPS subunit, has also been detected in the embryonic nervous system (Stark et al., 1997). Both laminin and tiggrin, known ligands for PS1 and PS2 integrins, respectively, are present along the embryonic axon pathways (Montell and Goodman, 1989; Fogerty et al., 1994). Despite all circumstantial evidence that they may be involved in neural development, expression patterns of PS integrins as well as their specific role during neuronal development have not yet been examined.
In this study, we show that PS integrins (αPS1, αPS2, and βPS subunits) are expressed in the embryonic nervous system, starting during the period of axogenesis, and we provide genetic evidence that neuronally expressed integrins are directly involved in axon development.
MATERIALS AND METHODS
Fly stocks. Null alleles for the αPS1 integrin subunit gene multiple edematous wings are_y_1_mew_M6 f_36a_p[ry+t7.2_:newFRT]18A/FM7c_ftz’-lacZ(a null allele) and y_1_mew_498_p[ry+t7.2_:newFRT]18A/FM7c_(a protein null allele); loss-of-function alleles for the αPS2 integrin subunit gene inflated are_g_2_if_K27E_f_36a_/FM7c_ftz’-lacZ (a null allele) and g_2_if_B2_f_36a/FM7c_ (a hypomorphic allele with an ∼10% protein expression level) (Brabant and Brower, 1993;Brower et al., 1995) (source, K. Stark, Massachusetts Institute of Technology, and Bloomington Fly Stock Center, Bloomington, IN). All of these mutations reach lethality by early larval stages. The genotypes of individual embryos examined were confirmed immunologically using the appropriate PS subunit antibodies, which reveal the presence or absence of particular PS subunits, and/or β-galactosidase antibodies, which detect the FM7c balancer chromosome that carries the marker transgene ftz′-lacZ (see Immunocytochemistry). For “neuron” rescue experiments, wild-type αPS2 gene was supplied to the nervous system in αPS2 null mutants (if_K27E) by the use of the GAL4 misexpression system (Brand et al., 1994) that combines a genomic neurotopic enhancer “_elav’-GAL4_III” (Sone et al., 1997) (source, E. Suzuki, University of Tokyo) to the GAL4-responsive transgene “_UAS-αPS2_wt” (Roote and Zusman, 1996) (source, D. Brower, University of Arizona):if_K27E/Y; UAS-αPS2_wt/elav’-GAL4_III. Misexpression of αPS1 and αPS2 was achieved by, respectively,GAL4_C155/+ (or Y); UAS-αPS1_wt_/+_ and_GAL4_C155_/+ (or Y); UAS-αPS2_wt_/+, in which_GAL4_C155, a GAL4 “enhancer trap” line (D. M. Lin et al., 1994) (source, C. Goodman, University of California at Berkeley), targets misexpression to all neurons similar to the “_elav’-GAL4_III” line.Canton S strain was used as a wild-type control. In addition, the βPS null mutant line (mys_xb87/Y) (Leptin et al., 1989) (source, K. Stark) was examined as a negative control for the immunocytochemistry data.
In situ hybridization. cDNAs for αPS1 (multiple edematous wings gene), αPS2 (inflated gene), and βPS (myospheroid gene) were subcloned into pBluescript to prepare digoxygenin-labeled antisense and sense (negative control) RNA probes (Bogaert et al., 1987; MacKrell et al., 1988; Wehrli et al., 1993) (source, D. Brower). Wild-type 9–18 hr whole embryos were processed for in situ hybridization and probed with RNA at concentrations of ∼1 ng/ml (βPS and αPS1) and ∼5 ng/ml (αPS2) for 2–3 hr (Broadus and Doe, 1995). The entire set of_in situ_ hybridization experiments was repeated independently three times to confirm reproducibility of the staining patterns.
Immunocytochemistry. Primary antibodies were as follows: mAb CF.6G11 (βPS subunit; 1:1000 dilution), mAb DK.1A4 (αPS1 subunit; 1:500 dilution), mAb CF.2C7 (αPS2 subunit; 1:500 dilution) (Wilcox et al., 1981; Brower et al., 1984) (source, D. Brower), mAb 1D4 (1:4 dilution) (Grenningloh et al., 1991) (source, C. Goodman), and anti-β-galactosidase (1:5000 dilution) (source, Promega, Madison, WI). Embryos were immunoprocessed as whole embryos or after fillet dissection on glass slides with “minipools” (Chiba et al., 1993). The mutant embryos were analyzed by double-immunolabeling with mAb 1D4 that stains CNS axon fascicles and motoneuron axons and an antibody against the respective integrin subunit and/or β-galactosidase (see Fly stocks). To preserve antigenicities for the PS subunits, we incubated the embryos with the primary antibodies before fixation. Analysis was based on abdominal A2–A7 segments of fillet-dissected preparations.
RESULTS
Embryonic neurons express PS integrins during axogenesis
We examined the expression pattern of αPS1, αPS2, and βPS subunits using in situ hybridization and immunocytochemistry in the embryonic nervous system (see Materials and Methods). All three PS subunit mRNAs are expressed widely in the nervous system (Fig.1). Their expression levels during hours 9–18 of embryogenesis are notably low compared with that in the other tissues that have been studied previously, such as muscles and apodemes (Fig. 1A_–_C, asterisks). αPS1 mRNA is detected widely in the CNS at a steady level during hours 13–18 (Fig. 1A). During this period, axogenesis occurs within the CNS as well as in the periphery. At the ventral midline, cells appear to accumulate slightly higher levels of αPS1 compared with most other cells in the CNS (Fig. 1A,open arrowheads). The αPS2 mRNA expression pattern differs somewhat from that of αPS1. Specific clusters of cells, one near the midline and a bilateral pair at mediolateral sites, express at relatively high levels of αPS2 in each segment of the CNS (Fig.1B, arrowheads). The αPS2 expression in the CNS peaks during hours 9–15 of embryogenesis. The βPS mRNA expression pattern partially overlaps with those of αPS1 and αPS2 mRNA in the CNS, with one prominent cluster of cells expressing relatively high levels at the ventral midline (Fig. 1C,open arrowheads). βPS mRNA expression in the CNS persists through hours 9–18. These in situ hybridization data suggest that the embryonic CNS expresses both PS1 (αPS1/βPS heterodimer) and PS2 (αPS2/βPS heterodimer) integrins during the period of axogenesis.
Fig. 1.
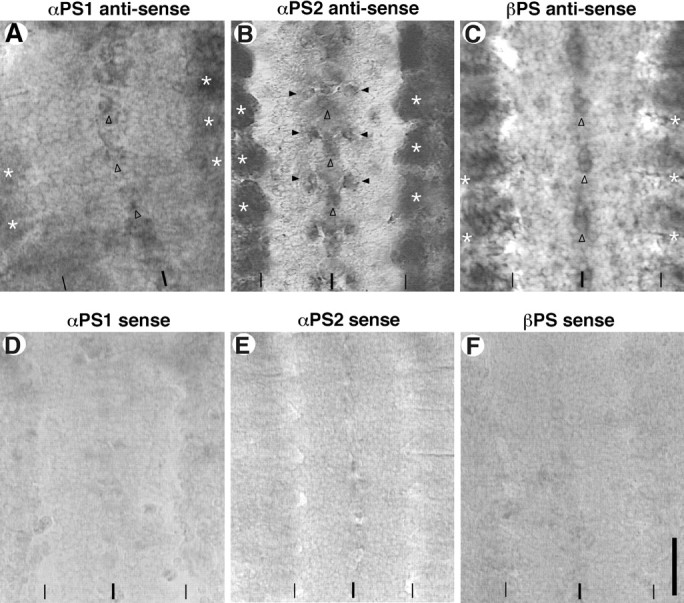
PS integrin mRNA in the wild-type_Drosophila_ embryonic CNS at hour 15. Embryos were probed with either antisense or sense (control) mRNA for the PS integrin subunits (see Materials and Methods). Panels show five abdominal segments of the CNS and surrounding mesoderm and/or ectodermal tissues in fillet-dissected preparations. All photos were taken at the focal planes slightly above the ventral surface of the CNS, except for (B) and (E) that are focused ∼10 μm below the dorsal surface of the CNS. The ventral midline and the lateral edges of the CNS are indicated by_thick_ and thin vertical lines, respectively, at the bottom of each_panel_. Anterior is to the top of each_panel_. A, αPS1 mRNA is expressed widely within the CNS, with slightly higher expression in a cluster of cells at the ventral midline (open arrowheads). Expression in the peripheral tissues (asterisks) is higher than that in the CNS. B, αPS2 mRNA is also widely expressed in the CNS. A midline cluster (open arrowheads) and bilaterally paired mediolateral clusters (closed arrowheads) of unidentified cells have noticeably high levels of expression. Muscles express αPS2 at very high levels (asterisks). C, βPS mRNA expression is also widespread. Relatively high levels of expression are seen in the CNS cells near the ventral midline (open arrowheads). Outside the CNS, both apodemes and muscles show high expression levels (asterisks). D–F, Sense mRNA for each of the three PS subunits served as negative controls for the in situ hybridization procedures. Scale bar: vertical line in F, 20 μm.
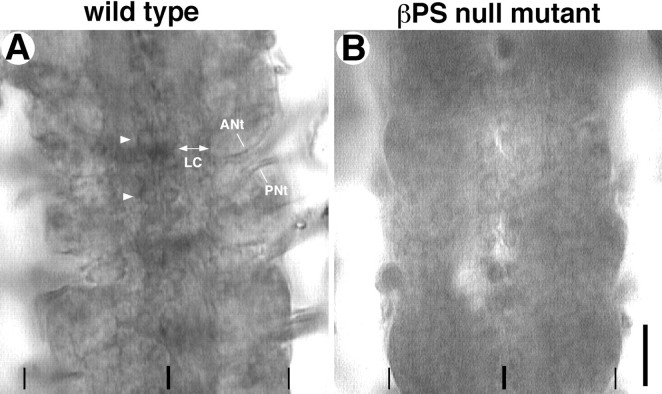
Consistent with the mRNA data, immunocytochemistry shows that the βPS protein subunit is unambiguously revealed on neuronal cell surfaces during hours 16–18 (Fig.2A). The major axon fascicles within the CNS, including the longitudinal connectives and the anterior and posterior nerve tracts (Fig. 2A), as well as the cell surfaces of at least some identified neurons, are labeled with the βPS antibodies (Fig. 2A,arrowheads). Unfortunately, both αPS1 and αPS2 subunits are below the threshold of detection with available antibodies. However, the detection of the βPS protein subunit on the neuronal cell surfaces suggests that the αPS subunits are likely forming functional heterodimers with the βPS subunit in those cells. These observations with in situ hybridization and immunocytochemistry have led us to conclude that, similar to vertebrate neurons, many Drosophila neurons express relatively low levels of integrin on the neuronal surface during axogenesis.
Fig. 2.
βPS integrin protein subunit in the embryonic CNS at hour 18. Fillet-dissected embryos were processed for βPS immunocytochemistry (see Materials and Methods). Panels_show four abdominal CNS segments. A, In a wild-type embryo (FM7c/Y), the βPS protein subunit is detected in the major axon tracts that include the pair of longitudinal connectives (LC) as well as the anterior nerve tract (ANt) and the posterior nerve tract (PNt). The latter two axon tracts contain motoneuron axons. In addition, surfaces of many neuronal cell bodies, including those of the pair of RP3 motoneurons at this focus (arrowheads), accumulate low levels of the βPS protein subunit. B, βPS null embryo (mys_xb87/Y), which has been dissected and immunoprocessed in the same minipool as the wild-type control, shows only the nonspecific background staining and serves as a negative control. Scale bar, 20 μm.
Loss of integrin disrupts axon guidance
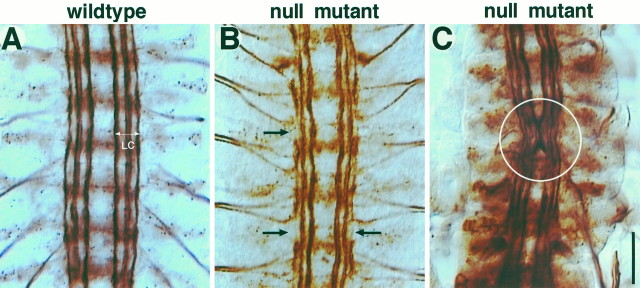
To analyze the role of the PS integrins in developing axons, we examined mutant embryos lacking the functional gene for either the αPS1 or αPS2 subunit. In these mutants, unlike in the βPS null mutants that exhibit grossly abnormal muscle development (Leptin et al., 1989), muscles develop apparently normally up to hour 18 through the major period of embryonic axogenesis. There is little sign that neuronal cell bodies have migrated to incorrect positions, in addition to the fact that the entire nervous system develops relatively normally. On closer examinations, however, both αPS1 and αPS2 loss-of-function mutant alleles exhibit similarly widespread and variable axon guidance defects (Fig.3B,C). In the CNS, the longitudinal connective normally contains three prominent axon fascicles that are easily visualized by mAb 1D4 (Fig.3A). Axons in the mutants appear somewhat wiggly (Fig.3C, circle) or partially disconnected (Fig.3B, arrows). The results are summarized in Figure4.
Fig. 3.
CNS axon defects in loss-of-function mutants at hour 18. Whole embryos were processed for mAb 1D4 immunocytochemistry (see Materials and Methods). Panels show five abdominal CNS segments in fillet-dissected embryos. A, In wild type (Canton S), each of the bilaterally paired longitudinal connectives (LC) contains three discrete axon fascicles that show little crossover among themselves.B, C, Axon fascicles are disorganized in the CNS. The two most common defects are “gaps” in the longitudinal fascicles (B, arrows) and “wiggles” within the axon fascicles (C, circle). With the level of analysis, it is difficult to resolve whether the gaps represent axons stalling or axons changing the fascicles. See Figure 4for summary. Scale bar, 20 μm.
Fig. 4.
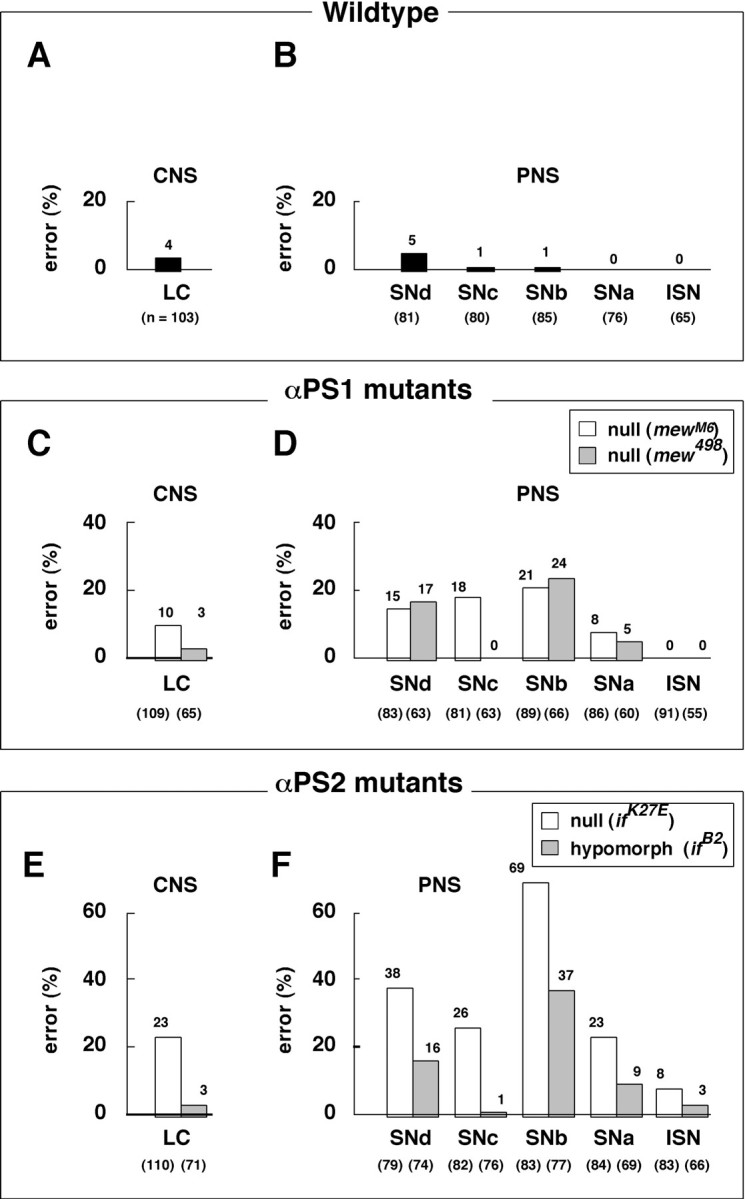
Summary of axon defects in loss-of-function mutants. Data are based on the hour 18 embryos after immunoprocessing with mAb 1D4 and fillet dissection. CNS axon fascicles were analyzed only in the longitudinal connectives (LC). Various classes of defects, such as the gaps and wiggles shown in Figure 3,B and C, are included as axon “errors.” In the periphery, all five groups of motoneuron axons (ISN, SNa, SNb,SNc, and SNd) were examined. In each group, axon defects are collectively summarized as errors. Examples of various peripheral nervous system (PNS) axon defects are shown below (see Fig. 5G–L). Both the error rates and sample sizes (the numbers of abdominal hemisegments scored; numbers_in parentheses below each bar) are indicated in the charts. The data for the αPS1 mutants are based on 10 mew_M8 and eight_mew_498 embryos, and the penetrance of the axon errors either in the CNS or PNS in each allele is ∼90%. Similarly, the data for the αPS2 mutants are based on nine_mew_M8 and eight_mew_498 embryos, and the penetrance of the axon errors either in the CNS or PNS ranges between 78 and 100%. There is no obvious correlation between the CNS and PNS segments in which axon defects occur. Taken together, these observations support the idea that the loss of integrin leads to widespread and stochastic axon guidance errors throughout the nervous system. A,B, Wild type (Canton S) shows very low axon error rates (0–5%) in the CNS (A) as well as in the periphery (PNS) (B). The data are based on eight embryos. C, D, αPS1 null mutants (mew_M6/Y and_mew_498/Y_) both show low-to-medium rates (0–24%) of axon errors in the CNS (C) and PNS (D).E, F, The αPS2 null mutant allele (if_K27E/Y_) exhibits medium-to-high rates (8–69%) of axon errors in the CNS (E) and PNS (F). The hypomorphic allele (if_B2/Y_) shows similar defects at slightly reduced rates (1–37%).
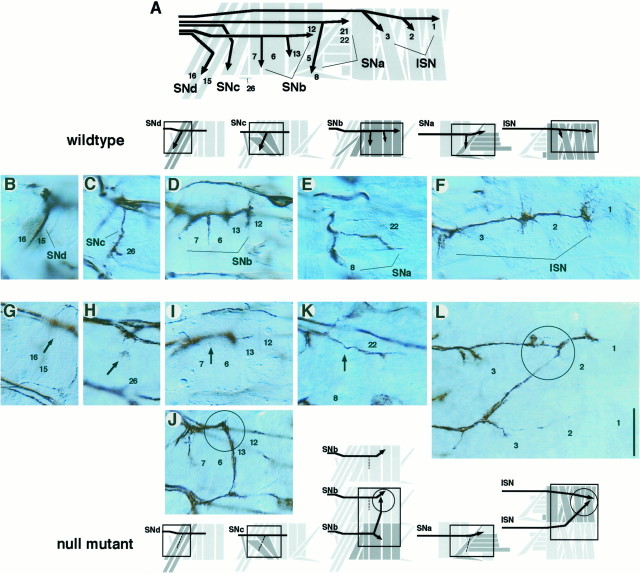
In the PNS, one can visualize specific groups of motoneuron axons with higher cellular resolution than is possible in the CNS. Null mutations in αPS1 and αPS2 subunit genes both result in similarly widespread axon pathfinding defects for all five known motoneuron groups, despite apparently normal muscle development (Fig.5G–L). The axon defects observed can be interpreted as a consequence of failing to turn at choice points and/or invading into neighboring muscle fields (Fig. 5,arrows, circles). These defects are most frequently detected in the SNb group (Fig.4D,F). It is important to note that the axons in these loss-of-function mutants can extend as far as in wild type and sometimes up to 30 μm beyond their normal stopping points (Fig. 5J, circle). This suggests that integrin is unlikely to serve simply as a clutch-constituting molecule, on which growth cones depend for the adequate traction needed to extend forward. Another point is that each axon group selects a range of alternative pathways without obvious preferences. It is therefore likely that the loss of integrin leads to losses in the responsiveness of an axon to a large array, rather than a small specific set, of guidance cues. Finally, in general, loss of PS2 integrin (αPS2 null mutation) leads to higher axon guidance errors than does loss of PS1 integrin (αPS1 null mutation). Future analysis is needed to determine specific contributions of these two forms of integrin. On the basis of the current data, we suggest that both the laminin-binding PS1 and RGD-dependent PS2 integrins are necessary for accurate axon guidance.
Fig. 5.
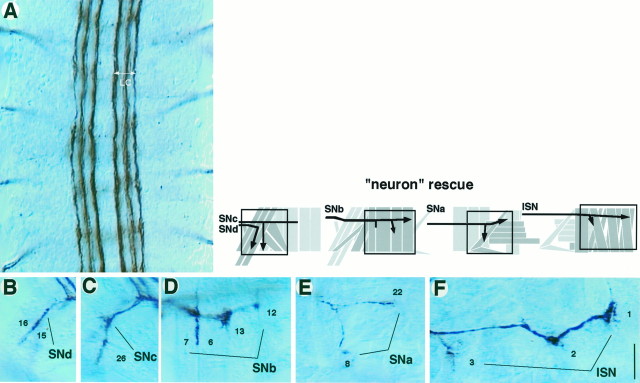
PNS axon defects in loss-of-function mutants at hour 18. Embryos were processed for mAb 1D4 immunocytochemistry and fillet-dissected. B–L show parts of the motoneuron axon pathways in a right PNS hemisegment. Boxes in the_schematics above_ and below B–J_approximately correspond to the areas shown in the_panels. A, Each hemisegment contains 30 uniquely identified muscles and is innervated by five groups (fascicles) of motoneuron axons: SNd,SNc, SNb, SNa, and_ISN_. The muscles that are targeted by each motoneuron group are labeled with identification numbers (13 of 30). B–F, In wild type (Canton S),SNd targets the ventral- (proximal) most muscles including 15 and 16 (B). _SNc_targets the next ventral-most muscles including 26 (C). SNb branches into several subfascicles and innervates ventrolateral muscles including 6, 7, 12, and 13 (D). The SNa motoneuron group extends toward the lateral muscles and bifurcates into two subfascicles; one subfascicle reaches transverse muscles 21, 22, and others, whereas another turns posteriorly to innervate 5 and 8 (E). ISN extends farthest and targets the dorsal- (distal) most muscles such as 1, 2, and 3 (F). G–L, In the null mutant embryos, the PNS axon fascicles exhibit various defects. The types of axon pathfinding defects seen for the αPS1 and αPS2 null mutants are very similar. SNd and SNc are sometimes missing (G, H,arrows). Axons that would normally reach this muscle region may be either stalling or bypassing. _SNb_frequently extends beyond the normal stopping point and invades into the neighboring segment (J, circle) or fails to form sub-branches at muscles 6 and 7 (I,arrow). SNa occasionally misses its posteriorly directed sub-branch and fails to innervate muscles 5 and 8 (K, arrow). ISN sometimes fails to obey segmental boundaries and merges with the ISN from adjacent segments (L, circle). See Figure4 for data summary. Scale bar, 20 μm.
Axon guidance defects are rescued by supplying the wild-type integrin
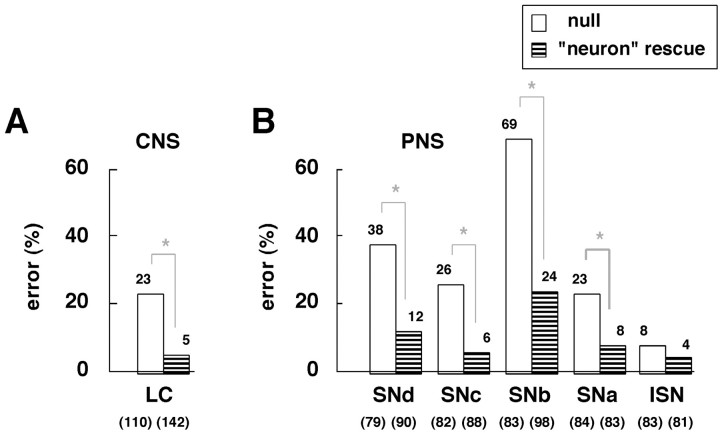
To test the direct role of integrin in axon development further, we attempted to rescue the axon defects by using a genomic neurotopic enhancer to drive expression of wild-type integrin gene in the nervous system of the null mutant (see Materials and Methods). We chose to focus on the αPS2 null mutants because they showed more severe PNS defects than did the αPS1 null mutants (Fig.6). The overall expression levels of the αPS2 protein subunit in this neuron rescue experiment were low and hardly detectable via immunocytochemistry, approximately mimicking endogenous expression in the CNS (data not shown). Consistent with a direct role for integrin, guidance defects are primarily rescued in both the CNS longitudinal connectives and the peripheral motor pathways (Figs. 6, 7). This result suggests that axons rely on their own integrin while responding accurately to local guidance cues.
Fig. 6.
A, B, The axon defects in αPS2 null mutants (if_K27E/Y_) can be partially rescued by supplying wild-type αPS2 gene to the nervous system in the mutant background (striped bars;if_K27E/Y; UAS-αPS2_wt_/elav’-GAL4_III; see Materials and Methods). In this neuron rescue experiment, the rates of axon defects in both the CNS (A) and PNS (B) revert toward those of wild type, yielding error rates significantly lower than those in αPS2 null mutants (asterisks; p < 0.001 by Chi2_t_ test). (See Fig. 7 for examples.) The parental lines used for the neuron rescue experiment were either wild-type–like (_UAS-αPS2_wt and_elav’-GAL4_III) or indistinguishable from the αPS2 null mutants (if_K27E/Y; UAS-αPS2_wt) in their axon fascicle organizations.
Fig. 7.
Neuron rescue experiment. Axon fascicle organizations in the αPS2 null mutant embryos (hour 18) after neuron rescue (see Materials and Methods) were visualized using mAb 1D4.A, Four abdominal CNS segments in a fillet-dissected embryo are shown. The longitudinal connectives (LC) revert to virtually wild type and exhibit three discrete fascicles (compare with Fig. 3A). B–F, In the PNS, axon fascicle organizations are very similar to that in wild type (compare with Fig. 5B–F). Both_SNd_ (B) and SNc(C) innervations are present at high frequencies.SNb axons show much-reduced error rates (D) compared with those in the αPS2 null mutants (compare with Fig. 5I, J).SNa increases its rate of forming the posteriorly directed sub-branch that reaches muscles 5 and 8 (E). ISN obeys the segment boundaries and looks very similar to wild type (F). See Figure 6, A and_B_, for data summary. Scale bar, 20 μm.
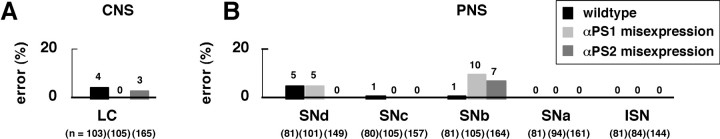
Integrin misexpression results in little growth cone guidance errors
How does neuronal integrin facilitate accurate axon guidance? A simple model is that integrin itself serves as a receptor for specific guidance cues, in much the same way as do a number of cell surface receptors (see Discussion). To test this idea, we expressed integrin ubiquitously in the wild-type CNS so that it would be present in neurons that normally express little or no integrin. We predicted that, if integrin serves as a receptor for specific growth cone guidance cues, many neurons with normally low or no integrin expression would alter their axon pathways. Contrary to our prediction, we found that axons exhibit little pathfinding error in either the CNS or periphery when either the αPS1 or αPS2 gene is misexpressed ubiquitously in the nervous system (Fig. 8). These results, as well as the results from the loss-of-function analysis described above, suggest that integrin is not likely to serve as a specific guidance receptor in vivo.
Fig. 8.
Summary of axon defects in gain-of-function experiments. Data are based on the 18 hr embryos (see Materials and Methods) that were immunoprocessed (mAb 1D4) and fillet-dissected, similar to those in Figures 4 and 6. A,B, Low-level neurotopic misexpression of either the αPS1 or αPS2 gene results in no apparent axon defects in the CNS (A) or PNS (B).
DISCUSSION
In this study, we have shown that integrin (αPS1, αPS2, and βPS subunits) is present in the developing _Drosophila_nervous system and have provided evidence that neuronal integrin is required for accurate axon guidance in vivo.
Neuronal integrin guides axons
Integrin, one of the best-studied classes of cell adhesion/signaling molecules in animals, has been shown to link intercellular signaling systems to a number of intracellular signaling pathways (Parsons, 1996; Schlaepfer and Hunter, 1998). It is expressed dynamically in a variety of cells during development wherein extensive intercellular communication is required. Evidence of potential roles of integrin during axon development is available from _in vitro_experiments (Schmidt et al., 1995; Varnum-Finney et al., 1995;Felsenfeld et al., 1996; Shaw et al., 1996; Wu et al., 1996; Grabham and Goldberg, 1997). However, recent mouse knock-out experiments fail to demonstrate the specific in vivo role of integrin during axon development, primarily because of the large number of integrin isoforms and their widespread expression patterns (Hynes, 1996). Therefore, to date, an in vivo demonstration of the roles of integrin in axon guidance has not been available.
Our genetic analysis on the Drosophila embryonic nervous system provides two lines of evidence that neuronal integrin is essential for accurate axon guidance. First, in the loss-of-function mutants for the αPS1 or αPS2 subunit gene, axons in the CNS and the periphery both exhibit widespread guidance errors (see Fig. 4 for summary). In the PNS, with its relatively simple cellular landscape, the miswired axons are seen frequently to extend beyond normal target regions (Fig. 5J,L). Therefore, although lack of integrin does not reduce general motility of growth cones, the accuracy of their guidance is diminished. Second, genetic rescue reverts the axonal defects toward the wild type (Figs. 6, 7). The fact that this is achieved by resupplying low levels of the wild-type integrin gene specifically to the nervous system of otherwise null mutant embryos strongly suggests that integrin is directly involved in the axon guidance.
Neuronal integrin and its low expression levels
Previous studies on Drosophila PS integrins have focused mostly on their involvement during the development of non-neuronal tissues, including wing epithelia, muscle attachment sites, dorsal vessels, and gut (Leptin et al., 1989; Wilcox et al., 1989; Wilcox, 1990; Zusman et al., 1990; Brown, 1993; Gotwals et al., 1994b; Brower et al., 1995; Fernandes et al., 1996; Stark et al., 1997;Bunch et al., 1998). The primary function of integrin is thought to be that of cell adhesion in these nonmigratory cells. Much less is known about the roles of integrin in the nervous system, where it is expressed at relatively low levels. In vitro work with chick dorsal root ganglion neurons shows that protein kinase C-dependent cytoplasmic signaling is involved in the behavioral modification of growth cones that occurs after their contacting laminin, the major ligand for integrin (Kuhn et al., 1995). This suggests that in axons, with its relatively low expression levels, integrin may primarily serve as a mediator of cell signaling via its specific association with a variety of cytoplasmic proteins. In vivo analysis on the physiological functions of the cytoplasmic domains of the PS integrin subunits in Drosophila have just begun in a variety of tissues (Martin-Bermudo et al., 1997; Li et al., 1998). It would be interesting to extend such analysis and test the roles of the cytoplasmic domains of integrin in the context of axon guidance.
New models for the role of integrin in axon guidance
Although our genetic analysis demonstrates that neuronally expressed integrin is essential for accurate axon guidance, the results are inconsistent with some of the previously proposed models concerning the role of integrin in axon development. One model, known as the clutch model, stems from the in vitro observations that, when a growth cone advances, actin filaments inside filopodia are held down to the substrate (Lin and Forscher, 1995). When applied to integrin, this clutch model proposes that integrin, an integral membrane molecule, serves as a mechanical link between specific extracellular substrates and the cytoskeleton and thus provides the traction necessary for growth cone advancement. Support for this “integrin-as-clutch” model comes from an in vitro study in which integrin activation is suggested to lock integrin to actin filaments in filopodia (Felsenfeld et al., 1996). The model predicts that, when integrin is missing, growth cones will lose much of their traction and will reduce their forward motility. However, our in vivo analysis has revealed little reduction in growth cone motility as a result of genetic deletion of the integrin subunits. Instead, there is an increase in growth cone targeting errors. Thus, a simple clutch model does not seem to adequately explain the role of integrin in axon development.
In the alternative receptor model, neuronal integrin itself is proposed to serve a pivotal role in turning a growth cone by reacting to specifically localized extrinsic cues (Kuhn et al., 1995). This model predicts that, when integrin is lacking, growth cones that normally express integrin will select alternative pathways at the choice points where the ligands of integrin are normally present. Along the same line of logic, if integrin is misexpressed in growth cones that normally do not express it, this will alter the pathfinding of these growth cones in specific ways as well. Our observations suggest that this receptor model is not predictive of the role of integrin in axon guidance. It is noteworthy that, in vitro, integrin is clearly capable of exerting enough biases on a growth cone to turn it toward the source of specific ligands such as laminin (Kuhn et al., 1998). However, whether the natural ligands of integrin are distributed in specific patterns_in vivo_ is still not clear. Available evidence hints that laminin and tiggrin, the known ligands for the PS integrins in_Drosophila_, are both rather ubiquitously expressed along the entire substrate that the majority of neuronal axons grow over (Montell and Goodman, 1989; Fogerty et al., 1994). Therefore, we favor the view that, in vivo, integrin is unlikely to serve as a specific axon guidance receptor.
What then is the role of integrin in the axon guidance, because its absence does lead to widespread loss of guidance accuracy? In one simple model, integrin functions as an adhesion molecule that controls the speed of growth cone advancement. In this “speed control” model, low levels of integrin are necessary to prevent the axons from extending too fast and missing the chance to interact with guidance cues. In a more elaborate model, integrin serves as an interface that links a specific cue recognition at the surface of the growth cone to internal cytoplasmic and cytoskeletal signaling events. In this “interface” model, integrin cooperates with a number of other growth cone receptors, such as EphR family receptor tyrosine kinases, DCC-type netrin receptors, receptor-type phosphotyrosine phosphatases, Fasciclin III, Connectin, and other unknown receptors (Speicher et al., 1998; Treubert and Brummendorf, 1998). Activation of any of these specific growth cone receptors is coupled to integrin-mediated intracellular signaling systems. The model predicts that integrin and associated molecules are rapidly recruited to, and/or activated at, particular filopodia that have contacted a specific target or guidepost cell. It also anticipates that mutations that delete only one of the many different growth cone receptors that are interfaced by integrin do not always produce pronounced axon guidance errors. On the other hand, deleting integrin would cause widespread axon defects, as observed in this study. Integrin, with its extensive opportunity to work with a large array of molecules (Clark and Brugge, 1995; Schlaepfer and Hunter, 1998), seems to be in an ideal position to signal-amplify a local event of minimal amplitude and to generate a bias large enough to steer a growth cone with accuracy.
Footnotes
This work was supported by National Institutes of Health Grant NS35049, National Science Foundation Grant IBN-95-14531, and the Lucille P. Markey Charitable Trust (A.C.). We thank Dan Brower and Mike Graner (University of Arizona), Karen Stark (Massachusetts Institute of Technology), Corey Goodman (University of California at Berkeley), and Emiko Suzuki (University of Tokyo) for generous gifts of reagents. We also thank Hiroyuki Kose (National Institute of Genetics, Japan) and Yasumitsu Takagi (Tsukuba Research Consortium, Japan) for discussion and Anna Huttenlocher, Steven Kaufman, Xiaomao Zhu, and the current members of the Chiba Lab (University of Illinois) for comments on this manuscript.
Correspondence should be addressed to Dr. Akira Chiba, B605 Chemical and Life Science Laboratory, 601 South Goodwin Avenue, Urbana, IL 61801.
REFERENCES
- 1.Bogaert T, Brown N, Wilcox M. The Drosophila PS2 antigen is an invertebrate integrin that, like the fibronectin receptor, becomes localized to muscle attachments. Cell. 1987;51:929–940. doi: 10.1016/0092-8674(87)90580-0. [DOI] [PubMed] [Google Scholar]
- 2.Brabant MC, Brower DL. PS2 integrin requirements in Drosophila embryo and wing morphogenesis. Dev Biol. 1993;157:49–59. doi: 10.1006/dbio.1993.1111. [DOI] [PubMed] [Google Scholar]
- 3.Brand A, Manoukian AS, Perrimon N. Ectopic expression in Drosophila. In: Goldstein LSB, Fyrberg EA, editors. Methods in cell biology. Academic; San Diego: 1994. pp. 683–696. [DOI] [PubMed] [Google Scholar]
- 4.Broadus J, Doe CQ. Evolution of neuroblast identity: seven-up and prospero expression reveal homologous and divergent neuroblast fates in Drosophila and Schistocerca. Development. 1995;121:3989–3996. doi: 10.1242/dev.121.12.3989. [DOI] [PubMed] [Google Scholar]
- 5.Brower D, Wilcox M, Piovant M, Smith RJ, Reger LA. Related cell-surface antigens expressed with positional specificity in Drosophila imaginal discs. Proc Natl Acad Sci USA. 1984;81:7485–7489. doi: 10.1073/pnas.81.23.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brower DL, Bunch TA, Mukai L, Adamson TE, Wehrli M, Lam S, Friedlander E, Roote CE, Zusman S. Nonequivalent requirements for PS1 and PS2 integrin at cell attachments in Drosophila: genetic analysis of the αPS1 integrin subunit. Development. 1995;121:1311–1320. doi: 10.1242/dev.121.5.1311. [DOI] [PubMed] [Google Scholar]
- 7.Brown NH. Integrins hold Drosophila together. BioEssays. 1993;15:383–390. doi: 10.1002/bies.950150604. [DOI] [PubMed] [Google Scholar]
- 8.Brown NH. Null mutations in the αPS2 and βPS integrin genes have distinct phenotypes. Development. 1994;120:1221–1231. doi: 10.1242/dev.120.5.1221. [DOI] [PubMed] [Google Scholar]
- 9.Bunch TA, Salatino R, Engelsgjerd MC, Mudai L, West RF, Brower DL. Characterization of mutant alleles of myospheroid, the gene encoding the β subunit of the Drosophila PS integrin. Genetics. 1992;132:519–528. doi: 10.1093/genetics/132.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bunch TA, Graner MW, Fessler LI, Fessler JH, Schneider KD, Kerschen A, Choy LP, Burgess BW, Brower DL. The PS2 integrin ligand tiggrin is required for proper muscle function in Drosophila. Development. 1998;125:1679–1689. doi: 10.1242/dev.125.9.1679. [DOI] [PubMed] [Google Scholar]
- 11.Chiba A. Early development of Drosophila neuromuscular junction: a model for studying neuronetwork development. Neuromuscular junctions in Drosophila Budnik V, Gramotes S. 1998. Academic; San Diego, in press. [Google Scholar]
- 12.Chiba A, Keshishian H. Neuronal pathfinding and recognition: roles of cell adhesion molecules. Dev Biol. 1996;180:424–432. doi: 10.1006/dbio.1996.0316. [DOI] [PubMed] [Google Scholar]
- 13.Chiba A, Hing H, Cash S, Keshishian H. Growth cone choices of Drosophila motoneurons in response to muscle fiber mismatch. J Neurosci. 1993;13:714–732. doi: 10.1523/JNEUROSCI.13-02-00714.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 15.Donady JJ, Seecof RL. Effect of the gene lethal(1) myospheroid on Drosophila embryonic cells in vitro. In Vitro. 1972;8:7–12. doi: 10.1007/BF02617937. [DOI] [PubMed] [Google Scholar]
- 16.Felsenfeld DP, Choquet D, Sheetz M. Ligand binding regulates the directed movement of β1 integrins on fibroblasts. Nature. 1996;383:438–440. doi: 10.1038/383438a0. [DOI] [PubMed] [Google Scholar]
- 17.Fernandes JJ, Celniker SE, Vijay Raghavan K. Development of the indirect flight muscle attachment sites in Drosophila: role of the PS integrins and the stripe gene. Dev Biol. 1996;176:166–184. doi: 10.1006/dbio.1996.0125. [DOI] [PubMed] [Google Scholar]
- 18.Fogerty FJ, Fessler LI, Bunch TA, Yaron Y, Parker CG, Nelson RE, Brower DL, Gullberg D, Fessler JH. Tiggrin, a novel Drosophila extracellular matrix protein that functions as a ligand for Drosophila αPS2 βPS integrins. Development. 1994;120:1747–1758. doi: 10.1242/dev.120.7.1747. [DOI] [PubMed] [Google Scholar]
- 19.Goodman CS. Mechanisms and molecules that control growth cone guidance. Annu Rev Neurosci. 1996;19:341–377. doi: 10.1146/annurev.ne.19.030196.002013. [DOI] [PubMed] [Google Scholar]
- 20.Gotwals PJ, Fessler LI, Wehrli M, Hynes RO. Drosophila PS1 integrin is a laminin receptor and differs in ligand specificity from PS2. Proc Natl Acad Sci USA. 1994a;91:11447–11451. doi: 10.1073/pnas.91.24.11447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gotwals PJ, Paine-Saunders SE, Stark KA, Hynes RO. Drosophila integrins and their ligands. Curr Opin Cell Biol. 1994b;6:734–739. doi: 10.1016/0955-0674(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 22.Grabham PW, Goldberg DJ. Nerve growth factor stimulates the accumulation of β1 integrin at the tips of filopodia in the growth cones of sympathetic neurons. J Neurosci. 1997;17:5455–5465. doi: 10.1523/JNEUROSCI.17-14-05455.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grenningloh G, Rehm EJ, Goodman CS. Genetic analysis of growth cone guidance in Drosophila: fasciclin II functions as a neuronal recognition molecule. Cell. 1991;67:45–57. doi: 10.1016/0092-8674(91)90571-f. [DOI] [PubMed] [Google Scholar]
- 24.Grotewiel MS, Beck CDO, Wu KH, Zhu XR, Davis RL. Integrin-mediated short-term memory in Drosophila. Nature. 1998;391:455–460. doi: 10.1038/35079. [DOI] [PubMed] [Google Scholar]
- 25.Hynes RO. Targeted mutations in cell adhesion genes: what have we learned from them? Dev Biol. 1996;180:402–412. doi: 10.1006/dbio.1996.0314. [DOI] [PubMed] [Google Scholar]
- 26.Jay D. Molecular mechanisms of directed growth cone motility. Perspect Dev Neurobiol. 1996;4:137–145. [PubMed] [Google Scholar]
- 27.Jones LS. Integrins: possible functions in the adult CNS. Trends Neurosci. 1996;19:68–72. doi: 10.1016/0166-2236(96)89623-8. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn TB, Schmidt MF, Kater SB. Laminin and fibronectin guideposts signal sustained but opposite effects to passing growth cones. Neuron. 1995;14:275–285. doi: 10.1016/0896-6273(95)90285-6. [DOI] [PubMed] [Google Scholar]
- 29.Kuhn TB, Williams CV, Dou P, Kater SB. Laminin directs growth cone navigation via two temporally and functionally distinct calcium signals. J Neurosci. 1998;18:184–194. doi: 10.1523/JNEUROSCI.18-01-00184.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lallier TE, Whittaker CA, DeSimone DW. Integrin α6 expression is required for early nervous system development in Xenopus laevis. Development. 1996;122:2539–2554. doi: 10.1242/dev.122.8.2539. [DOI] [PubMed] [Google Scholar]
- 31.Leptin M, Bogaert T, Lehmann R, Wilcox M. The function of PS integrins during Drosophila embryogenesis. Cell. 1989;56:401–408. doi: 10.1016/0092-8674(89)90243-2. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Graner MW, Williams EL, Roote CE, Bunch TA, Zusman S. Requirements for the cytoplasmic domain of the αPS1, αPS2, βPS integrin subunits during Drosophila development. Development. 1998;125:701–711. doi: 10.1242/dev.125.4.701. [DOI] [PubMed] [Google Scholar]
- 33.Lin C-H, Forscher P. Growth cone advance is inversely proportional to retrograde F-actin flow. Neuron. 1995;14:763–771. doi: 10.1016/0896-6273(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 34.Lin CH, Thompson CA, Forscher P. Cytoskeletal reorganization underlying growth cone motility. Curr Opin Neurobiol. 1994;4:640–647. doi: 10.1016/0959-4388(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 35.Lin DM, Fetter RD, Kopczynski C, Grenningloh G, Goodman CS. Genetic analysis of fasciclin II in Drosophila: defasciculation, refasciculation, and altered fasciculation. Neuron. 1994;13:1055–1069. doi: 10.1016/0896-6273(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 36.MacKrell AJ, Blumberg B, Hynes SR, Fessler JH. The lethal myospheroid gene of Drosophila encodes a membrane protein homologous to vertebrate integrin beta subunits. Proc Natl Acad Sci USA. 1988;85:2633–2637. doi: 10.1073/pnas.85.8.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin PT, Kaufman SJ, Kramer RH, Sanes JR. Synaptic integrins in developing, adult, and mutant muscle: selective association of α1, α7, and α7β integrins in the neuromuscular junction. Dev Biol. 1996;174:125–139. doi: 10.1006/dbio.1996.0057. [DOI] [PubMed] [Google Scholar]
- 38.Martin-Bermudo MD, Dunin-Borkowski OM, Brown NH. Specificity of PS integrin function during embryogenesis resides in the α subunit extracellular domain. EMBO J. 1997;16:4184–4193. doi: 10.1093/emboj/16.14.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montell DJ, Goodman CS. Drosophila laminin: sequence of β2 subunit and expression of all three subunits during embryogenesis. J Cell Biol. 1989;109:2441–2453. doi: 10.1083/jcb.109.5.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parsons JT. Integrin-mediated signaling: regulation by protein tyrosine kinases and small GTP-binding proteins. Curr Opin Cell Biol. 1996;8:146–152. doi: 10.1016/s0955-0674(96)80059-7. [DOI] [PubMed] [Google Scholar]
- 41.Roote CE, Zusman S. Alternatively spliced forms of the Drosophila αPS2 subunit of integrin are sufficient for viability and can replace the function of the αPS1 subunit of integrin in the retina. Development. 1996;122:1985–1994. doi: 10.1242/dev.122.6.1985. [DOI] [PubMed] [Google Scholar]
- 42.Schlaepfer DD, Hunter T. Integrin signalling and tyrosine phosphorylation: just the FAKs? Trends Cell Biol. 1998;8:151–157. doi: 10.1016/s0962-8924(97)01172-0. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt CE, Dai J, Lauffenburger DA, Sheetz MP, Horwitz AF. Integrin-cytoskeletal interactions in the neuronal growth cones. J Neurosci. 1995;15:3400–3407. doi: 10.1523/JNEUROSCI.15-05-03400.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaw CE, Milner R, Compston AS, French-Constant C. Analysis of integrin expression on oligodendrocytes during axo-glial interaction by using rat-mouse xenoculture. J Neurosci. 1996;16:1163–1172. doi: 10.1523/JNEUROSCI.16-03-01163.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sone M, Hoshino M, Suzuki E, Kuroda S, Kaibuchi K, Nakagoshi H, Saigo K, Nabeshima Y, Hama C. Still life, a protein in synaptic terminals of Drosophila homologous to GDP-GTP exchangers. Science. 1997;275:543–547. doi: 10.1126/science.275.5299.543. [DOI] [PubMed] [Google Scholar]
- 46.Speicher S, Garcia-Alonso L, Carmena A, Martin-Bermudo M, de la Escalera S, Jimenez F. Neurotactin functions in concert with other identified CAMs in growth cone guidance in Drosophila. Neuron. 1998;20:221–233. doi: 10.1016/s0896-6273(00)80451-1. [DOI] [PubMed] [Google Scholar]
- 47.Stark KA, Yee GH, Roote CE, Williams EL, Zusman S, Hynes RO. A novel α integrin subunit associates with βPS and functions in tissue morphogenesis and movement during Drosophila development. Development. 1997;124:4583–4594. doi: 10.1242/dev.124.22.4583. [DOI] [PubMed] [Google Scholar]
- 48.Takagi Y, Ui-Tei K, Miyake T, Hirohashi S. Laminin-dependent integrin clustering with tyrosine-phosphorylated molecules in a Drosophila neuronal cell line. Neurosci Lett. 1998;244:149–152. doi: 10.1016/s0304-3940(98)00145-1. [DOI] [PubMed] [Google Scholar]
- 49.Treubert U, Brummendorf T. Functional cooperation of β1-integrins and members of the Ig superfamily in neurite outgrowth induction. J Neurosci. 1998;18:1795–1805. doi: 10.1523/JNEUROSCI.18-05-01795.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Varnum-Finney B, Venstrom K, Muller U, Kypta R, Backus C, Chiquet M, Reicharrdt LF. The integrin receptor α8β1 mediates interactions of embryonic chick motor and sensory neurons with Tenascin-C. Neuron. 1995;14:1213–1222. doi: 10.1016/0896-6273(95)90268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wehrli M, DiAntonio A, Fearnley I, Smith R, Wilcox M. Cloning and characterization of αPS1, a novel Drosophila melanogaster integrin. Mech Dev. 1993;43:21–36. doi: 10.1016/0925-4773(93)90020-x. [DOI] [PubMed] [Google Scholar]
- 52.Wilcox M. Genetic analysis of the Drosophila PS integrins. Cell Differ Dev. 1990;32:391–400. doi: 10.1016/0922-3371(90)90055-2. [DOI] [PubMed] [Google Scholar]
- 53.Wilcox M, Brower DL, Smith RJ. A position-specific cell surface antigen in the Drosophila wing imaginal disc. Cell. 1981;25:159–164. doi: 10.1016/0092-8674(81)90240-3. [DOI] [PubMed] [Google Scholar]
- 54.Wilcox M, DiAntonio A, Leptin M. The function of PS integrins in Drosophila wing morphogenesis. Development. 1989;107:891–897. doi: 10.1242/dev.107.4.891. [DOI] [PubMed] [Google Scholar]
- 55.Wu DY, Wang LC, Mason CA, Goldberg DJ. Association of β1 integrin with phosphotyrosine in growth cone filopodia. J Neurosci. 1996;16:1470–1478. doi: 10.1523/JNEUROSCI.16-04-01470.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yee GH, Hynes RO. A novel, tissue-specific integrin subunit, βv, expressed in the midgut of Drosophila melanogaster. Development. 1993;118:845–858. doi: 10.1242/dev.118.3.845. [DOI] [PubMed] [Google Scholar]
- 57.Zusman S, Patel-King RS, French-Constant C, Hynes RO. Requirements for integrins during Drosophila development. Development. 1990;108:391–402. doi: 10.1242/dev.108.3.391. [DOI] [PubMed] [Google Scholar]