Microdeletion/microduplication of proximal 15q11.2 between BP1 and BP2: a susceptibility region for neurological dysfunction including developmental and language delay (original) (raw)
. Author manuscript; available in PMC: 2019 Oct 25.
Published in final edited form as: Hum Genet. 2011 Feb 27;130(4):517–528. doi: 10.1007/s00439-011-0970-4
Abstract
The proximal long arm of chromosome 15 has segmental duplications located at breakpoints BP1–BP5 that mediate the generation of NAHR-related microdeletions and microduplications. The classical Prader-Willi/Angelman syndrome deletion is flanked by either of the proximal BP1 or BP2 breakpoints and the distal BP3 breakpoint. The larger Type I deletions are flanked by BP1 and BP3 in both Prader-Willi and Angelman syndrome subjects. Those with this deletion are reported to have a more severe phenotype than individuals with either Type II deletions (BP2–BP3) or uniparental disomy 15. The BP1–BP2 region spans approximately 500 kb and contains four evolutionarily conserved genes that are not imprinted. Reports of mutations or disturbed expression of these genes appear to impact behavioral and neurological function in affected individuals. Recently, reports of deletions and duplications flanked by BP1 and BP2 suggest an association with speech and motor delays, behavioral problems, seizures, and autism. We present a large cohort of subjects with copy number alteration of BP1 to BP2 with common phenotypic features. These include autism, developmental delay, motor and language delays, and behavioral problems, which were present in both cytogenetic groups. Parental studies demonstrated phenotypically normal carriers in several instances, and mildly affected carriers in others, complicating phenotypic association and/or causality. Possible explanations for these results include reduced penetrance, altered gene dosage on a particular genetic background, or a susceptibility region as reported for other areas of the genome implicated in autism and behavior disturbances.
Introduction
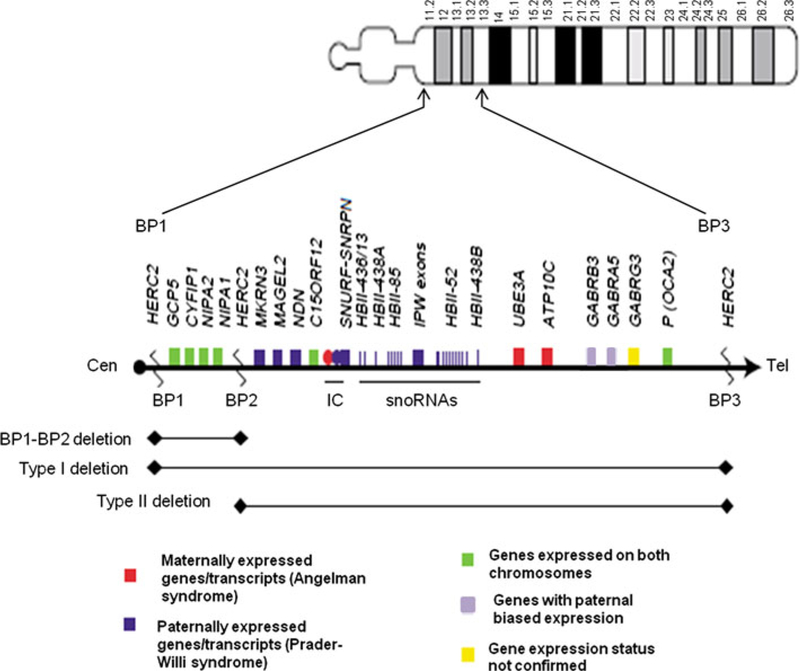
The proximal long arm of chromosome 15 contains a cluster of low copy repeats (LCRs) (Fig. 1), located at breakpoints BP1–BP5. These mediate various deletions and duplications via non-allelic homologous recombination (Locke et al. 2004; Makoff and Flomen 2007; Pujana et al. 2002). Deletion of the proximal q arm of chromosome 15 is best known for causing either Prader-Willi or Angelman syndromes (PWS/AS), depending on the parent of origin. The classic PWS/AS deletion is flanked by either of the proximal BP1 or BP2 and the more distal BP3 (Fig. 1). Individuals with Prader-Willi syndrome with Type I deletions (BP1–BP3) have been reported with a more severe phenotype than individuals with either Type II deletions (BP2–BP3) or uniparental disomy 15 (Butler et al. 2004; Hartley et al. 2005). However, other studies comparing the deletion types of PWS have not found the same results (Milner et al. 2005). However, two reports of Type I deletions in individuals with Angelman syndrome have shown more severe phenotypes compared with those having Type II deletions (Sahoo et al. 2007; Varela et al. 2004). The BP1–BP2 region spans approximately 500 kb and contains four evolutionarily conserved genes (NIPA1, NIPA2, CYFIP1, and TUBGCP5) that are not imprinted (Chai et al. 2003).
Fig. 1.
Chromosome 15 ideogram with proximal 15q expanded. BP1–BP2 region as compared with the larger BP1/BP2–BP3 Prader-Willi/Angelman syndrome critical regions
Three of these genes are implicated in central nervous system development and/or function. For example, NIPA1 is associated with spastic paraplegia (Goytain et al. 2007; Rainier et al. 2003) and the related NIPA2 gene recently identified as a magnesium transporter is widely expressed in the central nervous system (Goytain et al. 2008). The CYFIP1 gene codes a protein that is present in synaptosomal extracts and interacts with FMRP, the protein product of the FMR1 gene, which is responsible for fragile X syndrome. (Napoli et al. 2008) TUBGCP5 is a member of the cytoskeleton tubulin complex in cells. Quantitative expression data of each of the genes in the BP1–BP2 region have been analyzed in lymphoblastoid cell lines established from PWS subjects and compared with their behavioral and cognitive data. Significant correlations were noted specifically with decreased NIPA2 expression further suggesting a role for these genes in neurodevelopment and behavior (Bittel et al. 2006). Reports of disturbed expression via copy number changes of these genes appear to impact on behavioral and neurological function in affected individuals (Bittel et al. 2006; van der Zwaag et al. 2010), but larger studies with normal controls are needed.
Three reports have now been published of individuals with either deletion or duplication of the BP1–BP2 region (Doornbos et al. 2009; Murthy et al. 2007; van der Zwaag et al. 2010). While Murthy et al. (2007) reported a single consanguineous family with a BP1–BP2 deletion in whom a phenotype could be ascertained in all carriers, Doornbos et al. (2009) found phenotypically normal parental carriers in seven of their nine subjects with deletions, and also reported mildly dysmorphic features as part of the phenotypic spectrum. Recently, van der Zwaag et al. (2010) reported three individuals in a seven-member family with duplication of the BP1–BP2 region. The affected individuals all exhibited autistic features, and there were no phenotypically normal carriers in this family.
Herein, we report a cohort of 146 unrelated individuals referred for microarray analysis who have either deletions or duplications of the BP1–BP2 region collected from July 2008 to July 2010. The total number of subjects referred for microarray analysis during this time interval was approximately 17,000. The estimated total percentage was 0.86 for subjects having a copy number alteration of the BP1–BP2 region. Clinical information was available for 56 individuals with a deletion and 49 with duplication, and each group was compared with previously published reports. Common phenotypes noted in both the deletion and duplication subjects included developmental, motor, and speech delays, and neurological and/or behavior issues, specifically autism/ASD, ADD/ADHD, and tantrums.
Methods
Cytogenomic microarray analyses
Although different microarray platforms were used in the study of our cohort subjects, the SNP microarray analysis was used with the majority of subjects. Specifically, the Affymetrix v.6.0 platform was used by LabCorp. The gene chip contains over 900,000 SNP probes and 900,000 non-polymorphic copy number probes with a median spacing of 0.7 kb. Briefly, 500 ng of total genomic DNA extracted from peripheral blood lymphocytes was digested with _Nsp_I and _Sty_I and then ligated to _Nsp_I or _Sty_I adaptors, respectively, and amplified using a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA). PCR products were purified using AMPure beads (Agencourt Biosciences, Beverly, MA) and quantified using NanoDrop 8000 (Thermo Fisher, Wilmington, DE). Purified DNA was fragmented then biotin labeled and hybridized to the Affymetrix 6.0 GeneChip (Affymetrix, Santa Clara, CA). Data were analyzed using the Affymetrix Genotyping Console Browser v.3.01.
Array comparative genomic hybridization (array CGH) was performed at the University of Alabama at Birmingham (UAB) for 12 individuals (8 deletions, 4 duplications), using a high-resolution oligo-array, which combines targeted and genome-wide coverage. This is a custom-designed array with >44,000 oligos (probes), which is based on the 4×44k ISCA (International Standard Cytogenomic Array) Consortium design, and manufactured by Agilent Technologies (Santa Clara, CA) (Baldwin et al. 2008). Mean backbone spacing is approximately 75 kb between probes. Genomic DNA was extracted from peripheral blood of patients and controls. DNA labeling, array hybridization, washing, and scanning were performed following the manufacturer’s protocol. The arrays were scanned using the Axon 4000B scanner (Molecular Devices, Sunnyvale, CA). The scanned arrays were analyzed using the Feature Extraction (version 9.5.1.1) and DNA Analytics (version 5.0) software (Agilent Technologies) in order to determine copy number losses and gains. Both deletions and duplication were confirmed by metaphase and interphase FISH analyses using the RP11–25C1 probe.
Last, the 105K oligo HD Scan from CMDX (Irvine, CA) was used for a single deletion individual and for parental follow-up studies. FISH analysis with probes targeting the region delineated by the oligonucleotide array CGH is routinely used for confirmation.
Fluorescence in situ hybridization and chromosome analysis
Fluorescence in situ hybridization (FISH) was performed for confirmation of cytogenomic microarray analyses (CMA) results using standard protocols. BAC clone RP11–1081C20 was used by LabCorp. This probe maps to linear position 20,302,217–20,521,542 Mb on UCSC Genome Browser hg18. BAC clone RP11–25C1 was used by the UAB, and maps to 20,523,633–20,665,052 Mb. Both probes are within the BP1–BP2 region.
G-banded chromosome analysis was performed on cultured blood lymphocytes using standard protocols at approximately the 500-band level. Note, not all individuals had chromosome analysis performed.
Control variants from Toronto Database of Genomic Variants
Variants used from Database of Genomic Variants (DGV) included the following: deletion variants #: 49473, 29969, 53882, and 49474; duplication variants #: 49472, 34529, and 8787. Variants 49472 (2 individuals), 49473 (4 individuals), and 49474 (2 individuals) are all reported in the study by Shaikh et al. (2009), and all were found in 2,026 Children’s Hospital of Philadelphia (CHOP) control individuals. The unique individuals included in this study may be viewed at (http://cnv.chop.edu). This study also included individuals with larger deletions and duplications of the BP1–BP2 region extending more proximally or distally who were not included in the calculation. Variant 29969 (2 individuals) is a deletion using 485 Human Genome Diversity Panel (HGDP) controls (Jakobsson et al. 2008). Variant 53882 (9 individuals) is a deletion using 1,064 HGDP controls (one individual is redundant between 53882 and 29969) and 790 National Institute of Neurological Disorders and Strokes (NINDS) controls (Itsara et al. 2009). Variant 34529 (5 individuals) is a duplication using 1,190 Ontario controls (Zogopoulos et al. 2007). Variant 8787 (1 individual) is a duplication using 506 German controls and 270 HapMap controls (Pinto et al. 2007).
Totals for duplication controls were summed from 2,026 CHOP, 1,190 Ontario, 776 German+HapMap (n = 3,992). Totals for deletion controls were summed from 2,026 CHOP, 484 HGDP, 1,063 HGDP, and 790 NINDS (n = 4,363). The total for both groups was summed from 2,026 CHOP, 1,190 Ontario, 776 German+HapMap, 484 HGDP, 1,063 HGDP, 790 NINDS (n = 6,329).
Results
We performed a case-only retrospective analysis of individuals referred for cytogenomic microarray analysis to various laboratories who were found to have deletion or duplication of BP1–BP2 on proximal 15q. Among approximately 17,000 subjects referred for microarray analysis from July 2008 to July 2010, 69 subjects (0.41% of total) were deleted for the BP1–BP2 region and 77 (0.45% of total) were duplicated, representing 0.86% of the total individuals referred for microarray analysis. The DGV lists several reports of copy number variants (CNVs) in this region, both deletion and duplication, but individuals in those studies were not examined for neurological/psychiatric behavioral phenotypes and were only adults (http://projects.tcag.ca/variation/). Most individuals in our study were under the age of 18 years, and many had neurological indications for testing. Of the deletion subjects, 44 were males and 25 were females, with ages ranging from <1 month to 17 years (mean 4.7 years, median 2.5 years). Of the duplication subjects, 54 were males, and 23 were females, ranging in age from <1 month to 48 years of age (mean 7.9 years, median 4.8 years). Two subjects had four copies of the BP1–BP2 region: one had an isodicentric chromosome 15 with a distal breakpoint at BP2 referred for autism, and one individual had a triplication of the region on a single homologue, referred for cerebral palsy and seizures. A third subject demonstrated a monocentric marker that consisted of 15pter->q11.2 to BP2, resulting in three copies of the region. All other duplication subjects had tandem duplication on a single homologue. Two individuals with duplication were phenotypically normal parents ascertained due to chromosomal abnormalities found in their children. In one, the affected child did not have the BP1–BP2 duplication but had a different alteration while the mildly affected parent had both alterations, and a phenotype more consistent with the copy number change found in the proband. Tables 1 and 2 outline the numbers and percentages of deletion and duplication subjects, respectively, studied, with the following features described below.
Table 1.
Comparison of deletion subjects
| Phenotype | Murthy et al. (2007) | Doornbos et al. (2009) | de Kovel et al. (2010) | Presentcases (%) | Totalevaluated(%) |
|---|---|---|---|---|---|
| Dysmorphic features | 1/2 | 6/9 | N/A | 27/56 (48) | 34/67 (51) |
| Developmental delay (general) | 2/2 | 7/8 | N/A | 33/56 (59) | 42/66 (64) |
| Motor delay | 1/2 | 8/8 | N/A | 20/56 (36) | 29/66 (44) |
| Speech delaya | 2/2 | 8/8 | N/A | 44/49 (90) | 54/59 (92) |
| Autism/autistic features/Aspergera | 1/2 | 4/8 | N/A | 14/49 (29) | 19/59 (32) |
| Major behavior problems | |||||
| ADD/ADHDa | 2/2 | 3/8 | N/A | 16/49 (33) | 21/59 (36) |
| OCD,ODD, self-injury, tantrums, etc.a | 0/2 | 4/8 | N/A | 16/49 (33) | 20/59 (34) |
| Other neurological problems (insomnia, abnormal brain MRI or EEG, etc.) | 0/2 | 0/9 | N/A | 20/56 (36) | 20/67 (30) |
| Total behavior/neurological problems | 2/2 | 8/9 | N/A | 35/56 (63) | 45/67 (67) |
| Intellectual disabilitya | 2/2 | 4/8 | N/A | 11/49 (22) | 17/59 (29) |
| Hypotonia | 1/2 | 1/9 | N/A | 11/56 (20) | 13/67 (19) |
| Ataxia/balance/dyspraxia issuesa | 1/2 | 4/8 | N/A | 15/49 (31) | 20/59 (34) |
| Seizures | 0/2 | 2/9 | 8/23b | 14/56 (25) | 24/90 (27) |
| De novo alteration | 2/9 | N/A | 1 (of 21 f/u) | ||
| 2nd alteration present | Proband from consanguineous union | 3/9 | N/A | 17/69 (25) | 20/78 (26) |
Table 2.
Comparison of duplication subjects
| Phenotype | van der Zwaag et al. (2010) | Present cases(%) | Total evaluated (%) |
|---|---|---|---|
| Dysmorphic features | 0/3 | 22/49 (45) | 22/52 (42) |
| Developmental delay | 2/3 | 19/49 (39) | 21/52 (40) |
| Motor delay | 1/3 | 13/49 (27) | 14/52 (27) |
| Speech delaya | 1/3 | 22/44 (50) | 23/47 (49) |
| Autism/autistic features/Aspergera | 3/3 | 18/44 (41) | 20/47 (43) |
| Major behavior problemsa | |||
| ADD/ADHDa | 0/3 | 18/44 (41) | 18/47 (38) |
| ODD, self-injury, tantrums, etc.a | 1/3 | 10/44 (23) | 11/47 (23) |
| Other neurological problems (insomnia, personality issues, abnormal brain MRI or EEG, etc.) | 0/3 | 14/49 (29) | 14/52 (27) |
| Total behavior/neurological problems | 3/3 | 29/49 (59) | 32/52 (62) |
| Hypotonia | 0/3 | 7/49 (14) | 7/52 (13) |
| Ataxia/gait/balance/dyspraxia issuesa | 0/3 | 11/44 (25) | 11/47 (23) |
| Seizures | 0/3 | 6/49 (12) | 6/52 (12) |
| De novo alteration | N/A | 2b (of 23 f/u) | |
| Second alteration present | 0/3 | 16 (3 pts w/consanguinity) | 16/80 (20) |
Dysmorphology
While dysmorphic features were noted in almost half of our subjects, the lack of consistent physical abnormalities suggests that this region is not likely to be causative, either by duplication or deletion. Forty-nine of the 105 subjects for whom clinical information was available were found to have dysmorphic features, and of those, 14 individuals (29%) had secondary copy number changes observed by microarray analysis which may have contributed to the dysmorphic features seen in these individuals. Of those 14 individuals, only 1 had a parent with the same chromosomal abnormalities. The most common feature in these subjects was microcephaly, reported in 10 of 49 dysmorphic individuals.
Developmental delays/autism spectrum disorders
Of 56 deletion subjects with available clinical information, 7 were too young to develop speech (<1 year of age), and were not considered in the analysis for speech delay. Developmental delays were interpreted as delays in attainment of milestones and not specifically defined as a motor or language delay (Moeschler and Shevell 2006). Forty-four of the 49 (90%) individuals who were older than 1 year of age and had speech delays. 33 of 56 (59%) cases and 20 of 56 (36%) had developmental and motor delays, respectively. Taken together with the Murthy and Doornbos published data in 2009, developmental delay was noted for almost two-thirds of the total number of individuals with deletion. Speech delay was particularly prevalent, observed in almost every subject old enough to have developed speech at the time of testing.
Of our 49 subjects with 15q duplications for whom clinical information was obtained, 5 were younger than 1 year of age. Twenty-two of 44 (50%) individuals with duplications had speech delays, while 19 of 49 (39%) and 13 of 49 (27%) cases exhibited developmental and motor delays, respectively. One individual who was younger than 1 year of age was reported to have motor delay, and this subject did not have a secondary alteration. One of the individuals in the report by van der Zwaag et al. (2010) was initially diagnosed with pervasive developmental disorder prior to a diagnosis of ASD. Taken together, 21 of 52 (40%) of the total number of individuals with duplication exhibited developmental delay.
Using the Chi-square test, no significant difference was found between deletion and duplication subjects regarding developmental delay (p = 0.08), but taken together with previously reported subjects, statistical significance was reached (p = 0.02). Namely, individuals with deletion were significantly more likely to have developmental delays than were duplication subjects. The difference between the groups with regard to speech delay was significant in both our own cohort and the group as a whole (p < 0.0001), as speech delay was almost universal in individuals with the BP1–BP2 deletion. Regardless of the statistical significance of findings in deletion versus duplication subjects, these data must be couched with the caveat that all previous studies, as well as our own, have an ascertainment bias.
Of the deletion subjects with any type of delay, nine had secondary alterations, while three of the duplication subjects with delays had secondary alterations. Only one of the subjects inherited both alterations from the same parent for whom no clinical information was obtained. The exclusion of these subjects does not significantly alter the percentages of individuals with delays, and deletion subjects were still more likely than duplication subjects to have developmental and/or speech delay.
Recent literature suggests that very young children, as young as 13 months of age, can be diagnosed with ASD (Blackwell 2001; Chawarska et al. 2007; Shen et al. 2010) characterized by lack of social reciprocation and restricted, repetitive movements (Filipek et al. 1999). Of the 49 deletion subjects from our total cohort who were over 1 year of age, 14 (29%) were reported to have autism or autistic features. Autism was seen in approximately 30% of deletion subjects and 40% of duplication subjects from all studies. Eighteen of 44 (41%) duplication subjects from our cohort older than 1 year of age were reported to have ASD or autistic features. The difference in those individuals diagnosed with autism/ASD was not statistically significant for either our cohort or the total group (p = 0.26, and p = 0.25, respectively). One individual who was reported to have ASD inherited the deletion from a parent with a positive self and family history of dyslexia. The question remains as to whether the BP1–BP2 copy number alteration is enriched in autistic/ASD patients compared with unaffected individuals.
Neurological/behavior problems
Of 93 patients in our cohort for whom clinical information was available and who were over 1 year of age, 34 were reported with attention deficit disorder/attention deficit hyperactivity disorder (ADD/ADHD)—18 with duplication and 16 with deletion. The statistical difference between duplication subjects with ADD/ADHD and deletion subjects was not significant in our cohort (p = 0.13).
Of 102 patients from all studies who were over 1 year of age, 39 were reported with ADD/ADHD—all of the additional individuals had a deletion. Statistical significance was not reached in the total population by the addition of these five individuals (p = 0.06).
Forty-five of 67 (67%) deletion subjects from all studies for whom clinical information was available exhibited some sort of behavioral and/or neurological problem, such as tantrums, obsessive compulsive disorder, an abnormal brain MRI or EEG, or sleep problems. This is compared with 32 of 52 (62%) total subjects with duplications. Note that behavioral issues and “other neurological” are separate categories in Tables 1 and 2, and that some subjects may exhibit both. While a large percentage of both groups of subjects exhibited neurological/behavioral problems, no particular problem stood out. From our own cohort of 56 deletion patients with clinical information, 29 (52%) were reported to have behavioral/neurological problems, 6 of those individuals had secondary alterations, and 2 of those 6 had de novo secondary alterations. Of our 49 duplication subjects, 19 (39%) had behavioral/neurological problems, 3 of the 19 had secondary alterations, and 1 of those subjects inherited both alterations from the same parent for whom no clinical information was available. One parent with duplication was reported to have ADHD as a child, while two offspring who inherited the duplication were reported to have ASD. One parental carrier reported a family history of ADD/ADHD, but the proband was younger than 1 year of age and was referred for a congenital diaphragmatic hernia. This physical finding in the child may have been coincidental as it was not found in any other patient. Taken together, these data suggest that secondary alterations do not necessarily exacerbate a neurological phenotype.
Seizures
A recent report by de Kovel et al. (2010) implicated deletion of BP1–BP2 in susceptibility to idiopathic generalized epilepsy. Their cohort included 12 subjects with a deletion of 15q11.2 in 5 families. Not all of these families demonstrated the segregation of seizures with the 15q11.2 deletion, and in families in which seizures did segregate with deletion, not all of the individuals with the deletion were affected (Table 1). These results suggest that deletion of this region may be predisposing but not sufficient to cause seizures. For example, 30 of 142 (21%) individuals with a BP1–BP2 region copy number change (including individuals reported by de Kovel et al.) had a seizure disorder. Four individuals, all from our cohort, had an additional copy number change, one of which had a diagnosis of DiGeorge/VCF syndrome and confirmed 22q11.2 deletion. Only one of the four subjects in our cohort with a secondary alteration had parental follow-up studies; while the BP1–BP2 deletion was inherited, the secondary alteration was de novo. One duplication subject with seizures inherited the duplication from a phenotypically normal parent.
Other
Other features often reported were gait or coordination problems, hypotonia, and intellectual disability. Approximately 30% of all individuals had ataxia or dyspraxia, while hypotonia and intellectual disability were seen less often: ataxia/coordination problems were reported for 15 of 49 deletions (31%) and 11 of 44 duplications (25%), and in 20 of 59 deletions (34%) and 11 of 47 (23%) duplications of subjects from all studies. Three individuals with deletion and one with duplication from our cohort had secondary alterations. One ataxic individual with a secondary copy number change inherited the BP1–BP2 deletion from a highly educated mother with unexplained psychiatric issues, but she did not have the second alteration. The other parent was not available for testing. None of the other deletion subjects with secondary alterations had parental follow-up studies. The individual with duplication was a father found by parental follow-up SNP array to have the BP1–BP2 duplication as the secondary alteration (not present in the child). His coordination issues were considered as part of a primary diagnosis of a spastic paraplegia (the other copy number alteration also found in the child). Interestingly, individuals with NIPA1 gene mutations have been reported with familial spastic paraplegia (Rainier et al. 2003). Six of the 15 ataxic subjects with the deletion and 4 of the 11 with duplications who have had parental follow-up, none have secondary alterations and all inherited the BP1–BP2 copy number change. Of these, four parents with deletion were reported to be phenotypically normal, while no clinical information was obtained for the other parents.
Hypotonia was observed in 11 of 56 deletion subjects (20%) in our cohort and 13 of 67 deletion subjects (19%) of all patients. From our cohort of 11 individuals, 1 individual had a secondary alteration, but was adopted so parents were not available for follow-up. One individual was found to have a confirmed de novo deletion, and five others who had parental follow-up studies inherited the deletion from phenotypically normal parents. In subjects with duplications, 7 of 49 individuals in our cohort (14%) were reported to have hypotonia. Of those, two individuals had secondary alterations, and one of those inherited both from the same parent for whom no clinical information was available. One individual had confirmed de novo duplication, and one other who had parental follow-up studies inherited the duplication from a phenotypically normal parent.
Intellectual disability was reported for 29% of deletion subjects, but not for any duplication subjects in both our cohort and the van der Zwaag report. Three individuals from our cohort with intellectual disability had secondary alterations but none have had parental follow-up studies.
Secondary alterations
Because SNP arrays are able to detect both consanguinity and uniparental disomy (UPD), those individuals have been included as having a secondary alteration to account for the possibility of a recessive mutation. Of 146 total individuals in our cohort with copy number changes of BP1–BP2, 33 individuals (22.6%) had secondary alterations—16 duplication subjects and 17 deletion subjects (see Supplementary Table). Two of those individuals demonstrated consanguinity by large (>10 Mb) contiguous regions of homozygosity on multiple chromosomes. One individual was suspected to have UPD2 by a large region (58 Mb) of homozygosity on a single chromosome. Of the remaining subjects with secondary alterations who have had parental follow-up testing (9 individuals), one parent had the same alteration at both loci. No clinical information was available regarding the phenotype of the parent in this instance. Three individuals exhibited biparental inheritance of each copy number change from phenotypically normal parents. Another subject inherited the BP1–BP2 deletion from a phenotypically normal parent while the secondary copy number change was found to be de novo. One subject inherited two copy number changes from a single parent for whom no clinical information was available, but this individual also had a large de novo deletion as a tertiary alteration. Interestingly, one individual who was found to have BP1–BP2 duplication was discovered by SNP array follow-up for a deletion ascertained in his child who did not have the 15q11.2 duplication. The clinical features of the parent were more consistent with the secondary deletion and were not reported to be as severe as those of the proband. This result suggests that in this individual, the duplication of BP1–BP2 did not contribute to the phenotype in the father such that he was ascertained instead of the child. The two consanguineous individuals have not been followed up, and there would not be a way to determine a recessive mutation in the genome by current array technologies. The individual in whom UPD2 was suspected was found to have biparental inheritance of this chromosome and may indicate distant relatedness of the parents. Such a large (58 Mb) region of homozygosity in distant relatives may harbor a recessive mutation that is not observed by array analysis.
Two individuals in the total cohort were found to have four copies of the BP1–BP2 region; one due to triplication of the region on a single homologue, and one due to an isodicentric marker. Chromosome 15 derived marker chromosomes that do not include the Prader-Willi/Angelman critical region have long been considered benign, although there have been reports of abnormal phenotypes associated with some cases (Cheng et al. 1994; Hou and Wang 1998). In the report by Hou and Wang, several affected individuals had markers that included the microsatellite D15S11, but were not positive for more distal probes (Hou and Wang 1998). The LCR that comprises BP2 extends from approximately 20,650,000–21,193,000 Mb, but D15S11 maps to a smaller LCR distal to BP2. It would be interesting to determine whether any _SNRPN_-negative isodicentric markers may be asymmetric and utilize this small repeat region in between BP2 and BP3. The one isodicentric 15 in this study was de novo and appeared by SNP array to be symmetrical with a distal breakpoint at BP2.
Parental follow-up studies
Of all 146 individuals with BP1–BP2 copy number change, 44 parental follow-up studies were performed, and only two individuals were found to have a confirmed de novo change (one deletion, one duplication), as did two individuals from the Doornbos report (Doornbos et al. 2009). The two individuals from our cohort had markedly different phenotypes. The subject with a deletion was an infant referred for elevated lactic acid and an overlapping fifth toe unilaterally. The subject with the duplication had global developmental delay and failure to thrive. This subject was nonverbal and not able to sit alone at 2 years of age. It seems unlikely that the BP1–BP2 copy number change in these individuals contributed significantly to their phenotypes, although this cannot be excluded. None of these four individuals had a secondary copy number alteration or structural chromosome abnormality. In addition, the parents of the individual in whom the isodicentric marker was found did not have the marker by karyotype analysis. Seven individuals were adopted and biological parents were not available. Four parents with deletions out of 11 for whom clinical and family histories were obtained were reported to have a positive family or self history of some of the features seen in the proband. One parent with duplication out of eight with clinical and family history provided reported a positive self history of ADHD in childhood.
Discussion
Regions of the genome that are found to be deleted or duplicated in an affected proband as well as unaffected parents or other carrier family members have been referred to as susceptibility loci or risk loci. As such, deletion or duplication of these regions may not be sufficient to cause a phenotype. Recent literature has hypothesized that for deletions of susceptibility regions, such as that at 16p12.1, secondary alterations may be necessary to cause a phenotype (Girirajan et al. 2010), whether by epistatic or additive effects. In their study, the authors demonstrated that approximately one-quarter (10/42) of individuals with deletion of 16p12.1 also had a secondary alteration. Two of those individuals inherited both alterations from the same parent, who were also affected (Girirajan et al. 2010). Deletion and duplication of this and other regions, such as 1q21.1 and 15q13.3 are known to result in variable phenotypes, ranging from normal to affected (Antonacci et al. 2010; Mefford et al. 2008; Sharp et al. 2008; van Bon et al. 2009). This variability of phenotypes presents a challenge in the interpretation of the significance of these types of CNVs, as well as a challenge in the counseling of families. Of all BP1–BP2 subjects, both in our cohort, as well as the previously reported patients, approximately 20% of individuals had secondary alterations. This total includes three consanguineous cases. Of the youngest subjects in our cohort (11 individuals ascertained before 1 year of age), only two had secondary alterations. We also had a single parent in whom a secondary alteration was discovered upon follow-up testing that was not found in the ascertained proband. While individuals with secondary alterations in this study often had a more severe phenotype, simply having a secondary alteration did not necessarily correlate with exacerbation of a phenotype. It may also be possible that a particular genetic background influences irrespective of whether a phenotype is ascertained or not, or in the case of infants, whether there are congenital abnormalities due to unrelated etiologies.
We have presented a large cohort of individuals with deletion or duplication of proximal 15q from BP1 to BP2 and compared those results with previously published literature. The phenotypic spectrum of both deletions and duplications appears to be primarily neurological and includes developmental and speech delays, and in many instances, other neurologic and/or behavior problems such as autism spectrum disorders or autistic features and ADD/ ADHD. A complicating issue with regard to early speech delay is the lack of knowledge that an individual might have as an adult regarding their own early development. It is plausible that speech delay may be outgrown once a person learns to speak. Possible explanations for such variable results may include reduced penetrance of the copy number change, altered gene dosage on a particular genetic background, or a susceptibility region as reported for other areas of the genome implicated in autism and behavior disturbances. Larger extended family studies showing effects in multiple family members are needed to better define the impact of altered gene dosage of the BP1– BP2 region on behavioral and neurological functions.
It is well documented that copy number variation within the human genome can influence phenotypes and predispose individuals to disease (Menten et al. 2006; Shaikh et al. 2009), although the mechanisms by which they may do so is currently unknown. The DGV lists several reports of CNVs in this region, both deletion and duplication. While control individuals are described as healthy, they were not examined with regard to neurological/psychiatric phenotypes. We examined the frequency of CNVs reported from studies utilizing only SNP analyses for just the 500 kb encompassing BP1–BP2 listed on DGV (see “Methods” for variants; http://projects.tcag.ca/variation/). Several variants excluded from consideration also included proximal material that is composed largely of repeat sequence. Based on these criteria, duplications were reported in 8/3,992 individuals (0.20%) and deletions in 16/4,363 individuals (0.37%) (Itsara et al. 2009; Jakobsson et al. 2008; Pinto et al. 2007; Shaikh et al. 2009; Zogopoulos et al. 2007). A total of 24/6,329 (0.38%) control subjects were found with a deletion or duplication (see “Methods” for variants). This is slightly less than half as frequent as found in our cohort (0.86% total, 0.45% duplication, 0.41% deletion), although we acknowledge ascertainment bias in our study. In a study of CNVs associated with schizophrenia and neuropsychiatric disorders, the BP1– BP2 deletion was also shown to be enriched relative to a large control population (0.55 vs. 0.19%, respectively (Stefansson et al. 2008). While this CNV was not significantly associated with schizophrenia, it was significantly associated with other unspecified psychiatric problems. With deletion frequencies that are similar between our present study and DGV control subjects, it is difficult to conclusively determine the overall phenotypic effects of copy number changes of this region. It is not clear why the frequency is lower in the control subjects of the Stefansson study. Therefore, it seems possible, and even likely, that in affected individuals there may exist a neuronal/neurological pathway defect in addition to the altered copy number of the genes in this region that has yet to be elucidated. Supporting this idea, we found that simply having a secondary alteration was not sufficient to elicit a phenotype. We hypothesize that an associated phenotype may depend on the context of additional alterations and whether they may be in a related physiological pathway. The influence of copy number variation, whether alone or combined with other genetic interactions, upon neurodevelopmental and neuropsychiatric phenotypes has been documented for several genomic regions and several disorders including epilepsy, schizophrenia, and autism (El-Hattab et al. 2010; Girirajan and Eichler 2010; Heinzen et al. 2010; Pinto et al. 2010; Stefansson et al. 2008; Walsh et al. 2008; Xu et al. 2009). Multiple copies of proximal 15q, and in particular, maternally derived _SNRPN_-positive isodicentric markers, have been associated with susceptibility to autism in several studies (Nurmi et al. 2003a, b; Philippe et al. 1999). We observed a single subject with a _SNRPN_-negative isodicentric marker who was autistic and a single subject with a triplication on a single homologue with cerebral palsy and seizures. If one considers having four copies of the BP1–BP2 region a secondary alteration over simply having three copies due to duplication, one might expect a higher frequency of individuals with a neurological phenotype in these cases. Larger studies examining the clinical features of subjects who have previously been found with proximal 15q derived markers that were negative for genes distal to BP2 are needed in order to determine whether these may contribute to an ASD phenotype.
Published reports have described behavioral differences between Type I (flanked by BP1 and BP3) and Type II (flanked by BP2 and BP3) deletion Prader-Willi and Angelman syndrome individuals, but these results are not consistently seen in all studies (Butler et al. 2004; Milner et al. 2005). The previously published literature regarding subjects deleted or duplicated only between BP1 and BP2 has been limited because of a small sample size and ascertainment bias. Although we did not study individuals with PWS/AS, based on the data presented in this study, it seems reasonable to suggest that there may be at least a predisposition to developmental delay, language delay, and behavior disturbances in Type I deletion subjects.
This is the largest study to date analyzing the possibility of a phenotype associated with a copy number change of the BP1–BP2 region on proximal 15q, even though we acknowledge ascertainment bias in our cohort. Many of the subjects in our cohort were referred for microarray analysis because of unexplained delays or other neurological presentations. The frequency of both deletion and duplication of BP1–BP2 taken together in this study is 0.86%. As such, it is one of the most variable regions in the genome, and clearly in many individuals (such as apparently unaffected carrier parents) it does not appear to be sufficient to cause a phenotype. While deletion of BP1–BP2 demonstrated significance over duplication with regard to speech delay and developmental delay in our cohort, it is noteworthy that both were equally common in our study. Although recurrent microduplications have been reported with milder phenotypes than the reciprocal deletions (Menten et al. 2006), it is possible that with a move towards a “genotypefirst” approach to genomic disorders that include more subtle neuropsychiatric phenotypes, duplications may be more frequent than previously thought. We hypothesize that BP1–BP2 copy number changes may increase susceptibility to neuropsychiatric/neurodevelopmental problems, and careful clinical evaluation of both probands and parents is warranted. Elucidation of the functions and cellular and molecular pathways of the gene products encoded by the genes within the BP1–BP2 region will aid in our understanding of the possible influences of this CNV on neurological processes and neurodevelopment. With continued research into the effects that CNVs have, we may begin to understand the variation in phenotypes for many of the susceptibility loci throughout the genome and including proximal 15q.
Supplementary Material
1
Acknowledgments
The authors would like to acknowledge the following genetic counselors for help obtaining clinical information: Jenny Shafer, Huong Cabral, Lori Carpenter, Amy Dexter, Chantal Kelly, Dagny Patton, Eddie Williams, and Courtney Yerxa. RDB would like to acknowledge Brian Williford, Joshua Kesler, and Sharon Griffin for their technical contributions performing the arrays and FISH for LabCorp.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00439-011-0970-4) contains supplementary material, which is available to authorized users.
Contributor Information
Rachel D. Burnside, Laboratory Corporation of America, 1904 Alexander Dr., Research Triangle Park, NC 27709, USA
Romela Pasion, Laboratory Corporation of America, 1904 Alexander Dr., Research Triangle Park, NC 27709, USA.
Fady M. Mikhail, Department of Genetics, University of Alabama at Birmingham, Birmingham, AL 35294, USA
Andrew J. Carroll, Department of Genetics, University of Alabama at Birmingham, Birmingham, AL 35294, USA
Nathaniel H. Robin, Department of Genetics, University of Alabama at Birmingham, Birmingham, AL 35294, USA
Erin L. Youngs, Departments of Psychiatry and Behavioral Sciences and Pediatrics, Kansas University, Kansas City, KS 66160, USA
Inder K. Gadi, Laboratory Corporation of America, 1904 Alexander Dr., Research Triangle Park, NC 27709, USA
Elizabeth Keitges, LabCorp/Dynacare, Seattle, WA 98122, USA.
Vikram L. Jaswaney, Laboratory Corporation of America, 1904 Alexander Dr., Research Triangle Park, NC 27709, USA
Peter R. Papenhausen, Laboratory Corporation of America, 1904 Alexander Dr., Research Triangle Park, NC 27709, USA
Venkateswara R. Potluri, LabCorp/Dynagene, Houston, TX 77054, USA
Hiba Risheg, LabCorp/Dynacare, Seattle, WA 98122, USA.
Brooke Rush, Laboratory Corporation of America, 1904 Alexander Dr., Research Triangle Park, NC 27709, USA.
Janice L. Smith, LabCorp/Dynagene, Houston, TX 77054, USA
Stuart Schwartz, Laboratory Corporation of America, 1904 Alexander Dr., Research Triangle Park, NC 27709, USA.
James H. Tepperberg, Laboratory Corporation of America, 1904 Alexander Dr., Research Triangle Park, NC 27709, USA
Merlin G. Butler, Departments of Psychiatry and Behavioral Sciences and Pediatrics, Kansas University, Kansas City, KS 66160, USA
References
- Antonacci F, Kidd JM, Marques-Bonet T, Teague B, Ventura M, Girirajan S, Alkan C, Campbell CD, Vives L, Malig M, Rosenfeld JA, Ballif BC, Shaffer LG, Graves TA, Wilson RK, Schwartz DC, Eichler EE (2010) A large and complex structural polymorphism at 16p12.1 underlies microdeletion disease risk. Nat Genet 42:745–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin EL, Lee JY, Blake DM, Bunke BP, Alexander CR, Kogan AL, Ledbetter DH, Martin CL (2008) Enhanced detection of clinically relevant genomic imbalances using a targeted plus whole genome oligonucleotide microarray. Genet Med 10:415–429 [DOI] [PubMed] [Google Scholar]
- Bittel DC, Kibiryeva N, Butler MG (2006) Expression of 4 genes between chromosome 15 breakpoints 1 and 2 and behavioral outcomes in Prader-Willi syndrome. Pediatrics 118:e1276–e1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell J (2001) Clinical practice guideline: screening and diagnosing autism. J Am Acad Nurse Pract 13:534–536 [DOI] [PubMed] [Google Scholar]
- Butler MG, Bittel DC, Kibiryeva N, Talebizadeh Z, Thompson T (2004) Behavioral differences among subjects with Prader-Willi syndrome and type I or type II deletion and maternal disomy. Pediatrics 113:565–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai JH, Locke DP, Greally JM, Knoll JH, Ohta T, Dunai J, Yavor A, Eichler EE, Nicholls RD (2003) Identification of four highly conserved genes between breakpoint hotspots BP1 and BP2 of the Prader-Willi/Angelman syndromes deletion region that have undergone evolutionary transposition mediated by flanking duplicons. Am J Hum Genet 73:898–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarska K, Klin A, Paul R, Volkmar F (2007) Autism spectrum disorder in the second year: stability and change in syndrome expression. J Child Psychol Psychiatry 48:128–138 [DOI] [PubMed] [Google Scholar]
- Cheng SD, Spinner NB, Zackai EH, Knoll JH (1994) Cytogenetic and molecular characterization of inverted duplicated chromosomes 15 from 11 patients. Am J Hum Genet 55:753–759 [PMC free article] [PubMed] [Google Scholar]
- de Kovel CG, Trucks H, Helbig I, Mefford HC, Baker C, Leu C, Kluck C, Muhle H, von Spiczak S, Ostertag P, Obermeier T, Kleefuss-Lie AA, Hallmann K, Steffens M, Gaus V, Klein KM, Hamer HM, Rosenow F, Brilstra EH, Trenite DK, Swinkels ME, Weber YG, Unterberger I, Zimprich F, Urak L, Feucht M, Fuchs K, Moller RS, Hjalgrim H, De Jonghe P, Suls A, Ruckert IM, Wichmann HE, Franke A, Schreiber S, Nurnberg P, Elger CE, Lerche H, Stephani U, Koeleman BP, Lindhout D, Eichler EE, Sander T (2010) Recurrent microdeletions at 15q11.2 and 16p13.11 predispose to idiopathic generalized epilepsies. Brain 133:23–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doornbos M, Sikkema-Raddatz B, Ruijvenkamp CA, Dijkhuizen T, Bijlsma EK, Gijsbers AC, Hilhorst-Hofstee Y, Hordijk R, Verbruggen KT, Kerstjens-Frederikse WS, van Essen T, Kok K, van Silfhout AT, Breuning M, van Ravenswaaij-Arts CM (2009) Nine patients with a microdeletion 15q11.2 between breakpoints 1 and 2 of the Prader-Willi critical region, possibly associated with behavioural disturbances. Eur J Med Genet 52:108–115 [DOI] [PubMed] [Google Scholar]
- El-Hattab AW, Zhang F, Maxim R, Christensen KM, Ward JC, Hines-Dowell S, Scaglia F, Lupski JR, Cheung SW (2010) Deletion and duplication of 15q24: molecular mechanisms and potential modification by additional copy number variants. Genet Med 12:573–586 [DOI] [PubMed] [Google Scholar]
- Filipek PA, Accardo PJ, Baranek GT, Cook EH Jr, Dawson G, Gordon B, Gravel JS, Johnson CP, Kallen RJ, Levy SE, Minshew NJ, Ozonoff S, Prizant BM, Rapin I, Rogers SJ, Stone WL, Teplin S, Tuchman RF, Volkmar FR (1999) The screening and diagnosis of autistic spectrum disorders. J Autism Dev Disord 29:439–484 [DOI] [PubMed] [Google Scholar]
- Girirajan S, Eichler EE (2010) Phenotypic variability and genetic susceptibility to genomic disorders. Hum Mol Genet 19:R176–R187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girirajan S, Rosenfeld JA, Cooper GM, Antonacci F, Siswara P, Itsara A, Vives L, Walsh T, McCarthy SE, Baker C, Mefford HC, Kidd JM, Browning SR, Browning BL, Dickel DE, Levy DL, Ballif BC, Platky K, Farber DM, Gowans GC, Wetherbee JJ, Asamoah A, Weaver DD, Mark PR, Dickerson J, Garg BP, Ellingwood SA, Smith R, Banks VC, Smith W, McDonald MT, Hoo JJ, French BN, Hudson C, Johnson JP, Ozmore JR, Moeschler JB, Surti U, Escobar LF, El-Khechen D, Gorski JL, Kussmann J, Salbert B, Lacassie Y, Biser A, McDonald-McGinn DM, Zackai EH, Deardorff MA, Shaikh TH, Haan E, Friend KL, Fichera M, Romano C, Gecz J, DeLisi LE, Sebat J, King MC, Shaffer LG, Eichler EE (2010) A recurrent 16p12.1 microdeletion supports a two-hit model for severe developmental delay. Nat Genet 42:203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goytain A, Hines RM, El-Husseini A, Quamme GA (2007) NIPA1(SPG6), the basis for autosomal dominant form of hereditary spastic paraplegia, encodes a functional Mg2+ transporter. J Biol Chem 282:8060–8068 [DOI] [PubMed] [Google Scholar]
- Goytain A, Hines RM, Quamme GA (2008) Functional characterization of NIPA2, a selective Mg2+ transporter. Am J Physiol Cell Physiol 295:C944–C953 [DOI] [PubMed] [Google Scholar]
- Hartley SL, Maclean WE Jr, Butler MG, Zarcone J, Thompson T (2005) Maladaptive behaviors and risk factors among the genetic subtypes of Prader-Willi syndrome. Am J Med Genet A 136:140–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzen EL, Radtke RA, Urban TJ, Cavalleri GL, Depondt C, Need AC, Walley NM, Nicoletti P, Ge D, Catarino CB, Duncan JS, Kasperaviciute D, Tate SK, Caboclo LO, Sander JW, Clayton L, Linney KN, Shianna KV, Gumbs CE, Smith J, Cronin KD, Maia JM, Doherty CP, Pandolfo M, Leppert D, Middleton LT, Gibson RA, Johnson MR, Matthews PM, Hosford D, Kalviainen R, Eriksson K, Kantanen AM, Dorn T, Hansen J, Kramer G, Steinhoff BJ, Wieser HG, Zumsteg D, Ortega M, Wood NW, Huxley-Jones J, Mikati M, Gallentine WB, Husain AM, Buckley PG, Stallings RL, Podgoreanu MV, Delanty N, Sisodiya SM, Goldstein DB (2010) Rare deletions at 16p13.11 predispose to a diverse spectrum of sporadic epilepsy syndromes. Am J Hum Genet 86:707–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou JW, Wang TR (1998) Unusual features in children with inv dup(15) supernumerary marker: a study of genotype-phenotype correlation in Taiwan. Eur J Pediatr 157:122–127 [DOI] [PubMed] [Google Scholar]
- Itsara A, Cooper GM, Baker C, Girirajan S, Li J, Absher D, Krauss RM, Myers RM, Ridker PM, Chasman DI, Mefford H, Ying P, Nickerson DA, Eichler EE (2009) Population analysis of large copy number variants and hotspots of human genetic disease. Am J Hum Genet 84:148–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson M, Scholz SW, Scheet P, Gibbs JR, VanLiere JM, Fung HC, Szpiech ZA, Degnan JH, Wang K, Guerreiro R, Bras JM, Schymick JC, Hernandez DG, Traynor BJ, Simon-Sanchez J, Matarin M, Britton A, van de Leemput J, Rafferty I, Bucan M, Cann HM, Hardy JA, Rosenberg NA, Singleton AB (2008) Genotype, haplotype and copy-number variation in worldwide human populations. Nature 451:998–1003 [DOI] [PubMed] [Google Scholar]
- Locke DP, Segraves R, Nicholls RD, Schwartz S, Pinkel D, Albertson DG, Eichler EE (2004) BAC microarray analysis of 15q11–q13 rearrangements and the impact of segmental duplications. J Med Genet 41:175–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makoff AJ, Flomen RH (2007) Detailed analysis of 15q11–q14 sequence corrects errors and gaps in the public access sequence to fully reveal large segmental duplications at breakpoints for Prader-Willi, Angelman, and inv dup(15) syndromes. Genome Biol 8:R114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mefford HC, Sharp AJ, Baker C, Itsara A, Jiang Z, Buysse K, Huang S, Maloney VK, Crolla JA, Baralle D, Collins A, Mercer C, Norga K, de Ravel T, Devriendt K, Bongers EM, de Leeuw N, Reardon W, Gimelli S, Bena F, Hennekam RC, Male A, Gaunt L, Clayton-Smith J, Simonic I, Park SM, Mehta SG, Nik-Zainal S, Woods CG, Firth HV, Parkin G, Fichera M, Reitano S, Lo Giudice M, Li KE, Casuga I, Broomer A, Conrad B, Schwerzmann M, Raber L, Gallati S, Striano P, Coppola A, Tolmie JL, Tobias ES, Lilley C, Armengol L, Spysschaert Y, Verloo P, De Coene A, Goossens L, Mortier G, Speleman F, van Binsbergen E, Nelen MR, Hochstenbach R, Poot M, Gallagher L, Gill M, McClellan J, King MC, Regan R, Skinner C, Stevenson RE, Antonarakis SE, Chen C, Estivill X, Menten B, Gimelli G, Gribble S, Schwartz S, Sutcliffe JS, Walsh T, Knight SJ, Sebat J, Romano C, Schwartz CE, Veltman JA, de Vries BB, Vermeesch JR, Barber JC, Willatt L, Tassabehji M, Eichler EE (2008) Recurrent rearrangements of chromosome 1q21.1 and variable pediatric phenotypes. N Engl J Med 359:1685–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menten B, Maas N, Thienpont B, Buysse K, Vandesompele J, Melotte C, de Ravel T, Van Vooren S, Balikova I, Backx L, Janssens S, de Paepe A, De Moor B, Moreau Y, Marynen P, Fryns JP, Mortier G, Devriendt K, Speleman F, Vermeesch JR (2006) Emerging patterns of cryptic chromosomal imbalance in patients with idiopathic mental retardation and multiple congenital anomalies: a new series of 140 patients and review of published reports. J Med Genet 43:625–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner KM, Craig EE, Thompson RJ, Veltman MW, Thomas NS, Roberts S, Bellamy M, Curran SR, Sporikou CM, Bolton PF (2005) Prader-Willi syndrome: intellectual abilities and behavioural features by genetic subtype. J Child Psychol Psychiatry 46:1089–1096 [DOI] [PubMed] [Google Scholar]
- Moeschler JB, Shevell M (2006) Clinical genetic evaluation of the child with mental retardation or developmental delays. Pediatrics 117:2304–2316 [DOI] [PubMed] [Google Scholar]
- Murthy SK, Nygren AO, El Shakankiry HM, Schouten JP, Al Khayat AI, Ridha A, Al Ali MT (2007) Detection of a novel familial deletion of four genes between BP1 and BP2 of the Prader-Willi/Angelman syndrome critical region by oligo-array CGH in a child with neurological disorder and speech impairment. Cytogenet Genome Res 116:135–140 [DOI] [PubMed] [Google Scholar]
- Napoli I, Mercaldo V, Boyl PP, Eleuteri B, Zalfa F, De Rubeis S, Di Marino D, Mohr E, Massimi M, Falconi M, Witke W, Costa-Mattioli M, Sonenberg N, Achsel T, Bagni C (2008) The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E-BP. Cell 134:1042–1054 [DOI] [PubMed] [Google Scholar]
- Nurmi EL, Amin T, Olson LM, Jacobs MM, McCauley JL, Lam AY, Organ EL, Folstein SE, Haines JL, Sutcliffe JS (2003a) Dense linkage disequilibrium mapping in the 15q11–q13 maternal expression domain yields evidence for association in autism. Mol Psychiatry 8: 624–34, 570 [DOI] [PubMed] [Google Scholar]
- Nurmi EL, Dowd M, Tadevosyan-Leyfer O, Haines JL, Folstein SE, Sutcliffe JS (2003b) Exploratory subsetting of autism families based on savant skills improves evidence of genetic linkage to 15q11–q13. J Am Acad Child Adolesc Psychiatry 42:856–863 [DOI] [PubMed] [Google Scholar]
- Philippe A, Martinez M, Guilloud-Bataille M, Gillberg C, Rastam M, Sponheim E, Coleman M, Zappella M, Aschauer H, Van Maldergem L, Penet C, Feingold J, Brice A, Leboyer M (1999) Genome-wide scan for autism susceptibility genes. Paris Autism Research International Sibpair Study. Hum Mol Genet 8:805–812 [DOI] [PubMed] [Google Scholar]
- Pinto D, Marshall C, Feuk L, Scherer SW (2007) Copy-number variation in control population cohorts. Hum Mol Genet 16 Spec No. 2: R168–73 [DOI] [PubMed] [Google Scholar]
- Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, Regan R, Conroy J, Magalhaes TR, Correia C, Abrahams BS, Almeida J, Bacchelli E, Bader GD, Bailey AJ, Baird G, Battaglia A, Berney T, Bolshakova N, Bolte S, Bolton PF, Bourgeron T, Brennan S, Brian J, Bryson SE, Carson AR, Casallo G, Casey J, Chung BH, Cochrane L, Corsello C, Crawford EL, Crossett A, Cytrynbaum C, Dawson G, de Jonge M, Delorme R, Drmic I, Duketis E, Duque F, Estes A, Farrar P, Fernandez BA, Folstein SE, Fombonne E, Freitag CM, Gilbert J, Gillberg C, Glessner JT, Goldberg J, Green A, Green J, Guter SJ, Hakonarson H, Heron EA, Hill M, Holt R, Howe JL, Hughes G, Hus V, Igliozzi R, Kim C, Klauck SM, Kolevzon A, Korvatska O, Kustanovich V, Lajonchere CM, Lamb JA, Laskawiec M, Leboyer M, Le Couteur A, Leventhal BL, Lionel AC, Liu XQ, Lord C, Lotspeich L, Lund SC, Maestrini E, Mahoney W, Mantoulan C, Marshall CR, McConachie H, McDougle CJ, McGrath J, McMahon WM, Merikangas A, Migita O, Minshew NJ, Mirza GK, Munson J, Nelson SF, Noakes C, Noor A, Nygren G, Oliveira G, Papanikolaou K, Parr JR, Parrini B, Paton T, Pickles A, Pilorge M et al. (2010) Functional impact of global rare copy number variation in autism spectrum disorders. Nature 466:368–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujana MA, Nadal M, Guitart M, Armengol L, Gratacos M, Estivill X (2002) Human chromosome 15q11–q14 regions of rearrangements contain clusters of LCR15 duplicons. Eur J Hum Genet 10:26–35 [DOI] [PubMed] [Google Scholar]
- Rainier S, Chai JH, Tokarz D, Nicholls RD, Fink JK (2003) NIPA1 gene mutations cause autosomal dominant hereditary spastic paraplegia (SPG6). Am J Hum Genet 73:967–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo T, Bacino CA, German JR, Shaw CA, Bird LM, Kimonis V, Anselm I, Waisbren S, Beaudet AL, Peters SU (2007) Identification of novel deletions of 15q11q13 in Angelman syndrome by array-CGH: molecular characterization and genotype-phenotype correlations. Eur J Hum Genet 15:943–949 [DOI] [PubMed] [Google Scholar]
- Shaikh TH, Gai X, Perin JC, Glessner JT, Xie H, Murphy K, O’Hara R, Casalunovo T, Conlin LK, D’Arcy M, Frackelton EC, Geiger EA, Haldeman-Englert C, Imielinski M, Kim CE, Medne L, Annaiah K, Bradfield JP, Dabaghyan E, Eckert A, Onyiah CC, Ostapenko S, Otieno FG, Santa E, Shaner JL, Skraban R, Smith RM, Elia J, Goldmuntz E, Spinner NB, Zackai EH, Chiavacci RM, Grundmeier R, Rappaport EF, Grant SF, White PS, Hakonarson H (2009) High-resolution mapping and analysis of copy number variations in the human genome: a data resource for clinical and research applications. Genome Res 19:1682–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp AJ, Mefford HC, Li K, Baker C, Skinner C, Stevenson RE, Schroer RJ, Novara F, De Gregori M, Ciccone R, Broomer A, Casuga I, Wang Y, Xiao C, Barbacioru C, Gimelli G, Bernardina BD, Torniero C, Giorda R, Regan R, Murday V, Mansour S, Fichera M, Castiglia L, Failla P, Ventura M, Jiang Z, Cooper GM, Knight SJ, Romano C, Zuffardi O, Chen C, Schwartz CE, Eichler EE (2008) A recurrent 15q13.3 microdeletion syndrome associated with mental retardation and seizures. Nat Genet 40:322–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Dies KA, Holm IA, Bridgemohan C, Sobeih MM, Caronna EB, Miller KJ, Frazier JA, Silverstein I, Picker J, Weissman L, Raffalli P, Jeste S, Demmer LA, Peters HK, Brewster SJ, Kowalczyk SJ, Rosen-Sheidley B, McGowan C, Duda AW 3rd, Lincoln SA, Lowe KR, Schonwald A, Robbins M, Hisama F, Wolff R, Becker R, Nasir R, Urion DK, Milunsky JM, Rappaport L, Gusella JF, Walsh CA, Wu BL, Miller DT (2010) Clinical genetic testing for patients with autism spectrum disorders. Pediatrics 125:e727–e735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Rujescu D, Cichon S, Pietilainen OP, Ingason A, Steinberg S, Fossdal R, Sigurdsson E, Sigmundsson T, BuizerVoskamp JE, Hansen T, Jakobsen KD, Muglia P, Francks C, Matthews PM, Gylfason A, Halldorsson BV, Gudbjartsson D, Thorgeirsson TE, Sigurdsson A, Jonasdottir A, Jonasdottir A, Bjornsson A, Mattiasdottir S, Blondal T, Haraldsson M, Magnusdottir BB, Giegling I, Moller HJ, Hartmann A, Shianna KV, Ge D, Need AC, Crombie C, Fraser G, Walker N, Lonnqvist J, Suvisaari J, Tuulio-Henriksson A, Paunio T, Toulopoulou T, Bramon E, Di Forti M, Murray R, Ruggeri M, Vassos E, Tosato S, Walshe M, Li T, Vasilescu C, Muhleisen TW, Wang AG, Ullum H, Djurovic S, Melle I, Olesen J, Kiemeney LA, Franke B, Sabatti C, Freimer NB, Gulcher JR, Thorsteinsdottir U, Kong A, Andreassen OA, Ophoff RA, Georgi A, Rietschel M, Werge T, Petursson H, Goldstein DB, Nothen MM, Peltonen L, Collier DA, St Clair D, Stefansson K (2008) Large recurrent microdeletions associated with schizophrenia. Nature 455:232–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bon BW, Mefford HC, Menten B, Koolen DA, Sharp AJ, Nillesen WM, Innis JW, de Ravel TJ, Mercer CL, Fichera M, Stewart H, Connell LE, Ounap K, Lachlan K, Castle B, Van der Aa N, van Ravenswaaij C, Nobrega MA, Serra-Juhe C, Simonic I, de Leeuw N, Pfundt R, Bongers EM, Baker C, Finnemore P, Huang S, Maloney VK, Crolla JA, van Kalmthout M, Elia M, Vandeweyer G, Fryns JP, Janssens S, Foulds N, Reitano S, Smith K, Parkel S, Loeys B, Woods CG, Oostra A, Speleman F, Pereira AC, Kurg A, Willatt L, Knight SJ, Vermeesch JR, Romano C, Barber JC, Mortier G, Perez-Jurado LA, Kooy F, Brunner HG, Eichler EE, Kleefstra T, de Vries BB (2009) Further delineation of the 15q13 microdeletion and duplication syndromes: a clinical spectrum varying from non-pathogenic to a severe outcome. J Med Genet 46:511–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zwaag B, Staal WG, Hochstenbach R, Poot M, Spierenburg HA, de Jonge MV, Verbeek NE, van ‘t Slot R, van Es MA, Staal FJ, Freitag CM, Buizer-Voskamp JE, Nelen MR, van den Berg LH, van Amstel HK, van Engeland H, Burbach JP (2010) A co-segregating microduplication of chromosome 15q11.2 pinpoints two risk genes for autism spectrum disorder. Am J Med Genet B Neuropsychiatr Genet 153B:960–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela MC, Kok F, Otto PA, Koiffmann CP (2004) Phenotypic variability in Angelman syndrome: comparison among different deletion classes and between deletion and UPD subjects. Eur J Hum Genet 12:987–992 [DOI] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, Nord AS, Kusenda M, Malhotra D, Bhandari A, Stray SM, Rippey CF, Roccanova P, Makarov V, Lakshmi B, Findling RL, Sikich L, Stromberg T, Merriman B, Gogtay N, Butler P, Eckstrand K, Noory L, Gochman P, Long R, Chen Z, Davis S, Baker C, Eichler EE, Meltzer PS, Nelson SF, Singleton AB, Lee MK, Rapoport JL, King MC, Sebat J (2008) Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science 320:539–543 [DOI] [PubMed] [Google Scholar]
- Xu B, Woodroffe A, Rodriguez-Murillo L, Roos JL, van Rensburg EJ, Abecasis GR, Gogos JA, Karayiorgou M (2009) Elucidating the genetic architecture of familial schizophrenia using rare copy number variant and linkage scans. Proc Natl Acad Sci USA 106:16746–16751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zogopoulos G, Ha KC, Naqib F, Moore S, Kim H, Montpetit A, Robidoux F, Laflamme P, Cotterchio M, Greenwood C, Scherer SW, Zanke B, Hudson TJ, Bader GD, Gallinger S (2007) Germline DNA copy number variation frequencies in a large North American population. Hum Genet 122:345–353 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1