Heart Failure Risk Stratification and Efficacy of Sodium-Glucose Cotransporter-2 Inhibitors in Patients with Type 2 Diabetes Mellitus (original) (raw)
. Author manuscript; available in PMC: 2020 Nov 5.
Abstract
Background:
Patients with type 2 diabetes mellitus (T2DM) are at increased risk of developing heart failure (HF). Sodium-glucose cotransporter-2 (SGLT2) inhibitors reduce the risk of hospitalization for HF (HHF) in patients with T2DM. We aimed to develop and validate a practical clinical risk score for HHF in patients with T2DM and assess whether this score can identify high-risk patients with T2DM who have the greatest reduction in risk for HHF with an SGLT2 inhibitor.
Methods:
We developed a clinical risk score for HHF in 8,212 patients with T2DM in the placebo arm of SAVOR-TIMI 53. Candidate variables were assessed using multivariable Cox regression, and independent clinical risk indicators achieving statistical significance of p<0.001 were included in the risk score. We externally validated the score in 8,578 patients with T2DM in the placebo arm of DECLARE-TIMI 58. The relative and absolute risk reductions in HHF with the SGLT2 inhibitor dapagliflozin were assessed by baseline HHF risk.
Results:
Five clinical variables were independent risk predictors of HHF: prior HF, history of atrial fibrillation, coronary artery disease, estimated glomerular filtration rate (eGFR), and urine albumin-to-creatinine ratio (UACR). A simple integer-based score (0–7 points) using these predictors identified a >20-fold gradient of HHF risk (p-trend <0.001) in both the derivation and validation cohorts, with c-indices of 0.81 and 0.78, respectively. Whereas relative risk reductions with dapagliflozin were similar for patients across the risk scores (25–34%), absolute risk reductions were greater in those at higher baseline risk (χ2 3.24; one-sided p-trend=0.04), with high-risk (2 points) and very high-risk patients (≥3 points) having 1.5% and 2.7% absolute reductions in Kaplan-Meier estimates of HHF risk at 4 years.
Conclusions:
Risk stratification using a novel clinical risk score for HHF in patients with T2DM identifies patients at higher risk for HHF who derive greater absolute benefit from SGLT2 inhibition.
Clinical Trial Registration:
URL: https://www.clinicaltrials.gov. Unique identifiers: and .
Keywords: Diabetes mellitus, heart failure, risk score, sodium-glucose cotransporter-2 inhibitors
Introduction
Type 2 diabetes mellitus (T2DM) and heart failure (HF) are highly prevalent diseases associated with substantial morbidity and mortality.1–4 Even after adjusting for the presence of coronary artery disease and its risk factors, T2DM remains an important independent risk factor for HF.5–7 Moreover, in contrast to the risks of myocardial infarction and stroke, the risk of HF in patients with T2DM persists even when traditional cardiovascular risk factors, such as smoking, hypertension, and LDL cholesterol, are well controlled.8 Sodium-glucose cotransporter-2 (SGLT2) inhibitors are a novel class of glucose-lowering therapies that have been shown to reduce the risk of hospitalization for HF (HHF) in patients with T2DM.9–12 The impact of SGLT2 inhibitors on HHF has highlighted the need for improved models of HF risk stratification in patients with T2DM, both to tailor individual treatment approaches and to inform future clinical trial design.
We therefore sought to identify independent clinical risk predictors of HHF in patients with T2DM and to assess whether these clinical characteristics identify high-risk patients with T2DM who have the greatest reduction in risk of HHF with an SGLT2 inhibitor. In the present analysis, we derive a practical, multivariable clinical risk score for HHF in patients with T2DM enrolled in the placebo arm of the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR)-Thrombolysis in Myocardial Infarction (TIMI) 53 trial13 and externally validate this score in placebo-treated patients in the Dapagliflozin Effect on CardiovascuLAR Events (DECLARE)-TIMI 58 trial. We then evaluate this risk stratification scheme as it relates to the clinical efficacy of the SGLT2 inhibitor dapagliflozin.
Methods
Study Population
The SAVOR-TIMI 53 and DECLARE-TIMI 58 trials were multinational, randomized, placebo-controlled trials enrolling patients with T2DM and either a history of established cardiovascular disease or multiple risk factors for cardiovascular disease. In SAVOR-TIMI 53, 16,492 patients (79% with established cardiovascular disease and 21% with multiple risk factors for cardiovascular disease) were randomized to treatment with either the dipeptidyl peptidase-4 (DPP-4) inhibitor saxagliptin (5 mg daily) or placebo and were followed for a median of 2.1 years. In DECLARE-TIMI 58, 17,160 patients (41% with established cardiovascular disease and 59% with multiple risk factors for cardiovascular disease) were randomized to treatment with either dapagliflozin (10 mg daily) or placebo and were followed for a median of 4.2 years. The ethics committees at participating centers approved the protocols for each trial. Written informed consent was obtained from all patients. We encourage parties interested in collaboration and data sharing to contact the corresponding author directly for further discussions.
Clinical Endpoint
The primary outcome for this analysis was hospitalization for heart failure (HHF), which was adjudicated centrally by the TIMI Clinical Events Committee (CEC) using established definitions in both the SAVOR-TIMI 53 and DECLARE-TIMI 58 trials. In both trials, HHF was defined as an event meeting all of the following criteria: (1) admission to the hospital for at least 12 (SAVOR-TIMI 53) or 24 hours (DECLARE-TIMI 58); (2) objective evidence of new or worsening HF (e.g., orthopnea, jugular venous distension, pulmonary basilar crackles, etc.); and (3) intensification of HF therapy (e.g., initiation of intravenous diuretics or inotropes). In the DECLARE-TIMI 58 trial, the definition of HHF also required a primary diagnosis of HF and new or worsening symptoms that were attributed to HF on presentation.
Candidate Risk Indicators
We selected 25 candidate risk indicators for this analysis, based on their prevalence, clinical relevance, and the ability to readily obtain the variables from the medical records of typical patients with T2DM. Selected risk indicators included age, sex, race, body-mass index (BMI), duration of T2DM, glycated hemoglobin (HbA1c), baseline insulin use, history of diabetic retinopathy, history of diabetic nephropathy, estimated glomerular filtration rate (eGFR), urine albumin-to-creatinine ratio (UACR), established coronary artery disease (CAD), previous myocardial infarction (MI), established peripheral artery disease (PAD), previous ischemic stroke, pre-existing HF, history of atrial fibrillation, previous percutaneous coronary intervention (PCI), previous coronary artery bypass grafting (CABG), dyslipidemia, hypertension, current smoking, heart rate (HR), systolic blood pressure (SBP), and diastolic blood pressure (DBP).
Statistical Analysis
In the derivation cohort (i.e., 8212 placebo-treated patients in SAVOR-TIMI 53), we evaluated the univariable associations between candidate risk indicators and the clinical outcome of HHF using a Cox proportional hazards model with the risk indicator as the independent variable (Table 1). Age, BMI, duration of T2DM, HbA1c, eGFR, UACR, HR, SBP, and DBP were modeled as continuous variables. All risk indicators achieving a significance level of p<0.10 on the univariable screen were included in a multivariable model.
Table 1.
Univariable risk of hospitalization for heart failure by baseline characteristics in the derivation cohort.
| Variable | Derivation Cohort (n=8212), % | Unadjusted Hazard Ratio(95% CI) | P-value |
|---|---|---|---|
| Demographics | |||
| Age, median (IQR), years | 65 (60 – 71) | 1.46 (1.28 – 1.68)* | <0.001 |
| Female sex | 32.7 | 0.77 (0.57 – 1.05) | 0.10 |
| White race | 75.1 | 1.16 (0.84 – 1.60) | 0.37 |
| Body-mass index, median (IQR), kg/m2 | 30 (27 – 35) | 1.16 (1.02 – 1.32)* | 0.023 |
| Diabetes History | |||
| Duration of type 2 diabetes, median (IQR), years | 10.3 (5.3 – 16.6) | 1.31 (1.16 – 1.47)* | <0.001 |
| Glycated hemoglobin, median (IQR), % | 7.6 (6.9 – 8.7) | 1.07 (0.94 – 1.22)* | 0.32 |
| Baseline insulin use | 41.0 | 2.18 (1.66 – 2.87) | <0.001 |
| Diabetic retinopathy | 12.3 | 1.35 (0.94 – 1.95) | 0.11 |
| Diabetic nephropathy | 17.6 | 2.35 (1.77 – 3.13) | <0.001 |
| Estimated glomerular filtration rate (eGFR), median (IQR), ml/min/1.73 m2 | 75.7 (59.2 – 90.3) | 0.50 (0.44 – 0.57)* | <0.001 |
| Urine albumin-to-creatinine ratio (UACR), median (IQR), mg/g | 17 (6 – 70) | 1.21 (1.15 – 1.28)* | <0.001 |
| Other Comorbidities | |||
| Coronary artery disease | 62.4 | 3.00 (2.13 – 4.24) | <0.001 |
| Prior myocardial infarction | 37.6 | 1.92 (1.47 – 2.52) | <0.001 |
| Peripheral artery disease | 12.2 | 1.29 (0.88 – 1.89) | 0.19 |
| Ischemic stroke | 12.7 | 1.14 (0.77 – 1.69) | 0.51 |
| Prior heart failure | 12.8 | 6.27 (4.79 – 8.21) | <0.001 |
| Atrial fibrillation | 7.4 | 3.78 (2.74 – 5.23) | <0.001 |
| Percutaneous coronary intervention | 25.0 | 1.33 (0.99 – 1.79) | 0.06 |
| Coronary artery bypass grafting | 23.5 | 2.25 (1.71 – 2.96) | <0.001 |
| Dyslipidemia | 71.2 | 1.44 (1.04 – 1.98) | 0.027 |
| Hypertension | 82.4 | 1.10 (0.77 – 1.58) | 0.61 |
| Current smoker | 14.0 | 1.01 (0.68 – 1.48) | 0.98 |
| Vital Signs | |||
| Heart rate, median (IQR), bpm | 70 (63 – 78) | 1.04 (0.91 – 1.18)* | 0.61 |
| Systolic blood pressure, median (IQR), mmHg | 137 (125 – 147) | 0.77 (0.67 – 0.88)* | <0.001 |
| Diastolic blood pressure, median (IQR), mmHg | 80 (71 – 85) | 0.66 (0.58 – 0.75)* | <0.001 |
We selected five independent clinical risk indicators of HHF achieving statistical significance at a stringent threshold of p<0.001 using an Akaike information criterion (AIC) criterion for model building based on consistency of forward and backward selection procedures. Additional statistical methods are detailed in the Supplemental Methods. Each of the five independent clinical risk indicators was assigned an integer weight proportional to the regression coefficient to create the TIMI Risk Score for Heart Failure in Diabetes (TRS-HFDM). Based on the distribution of integer risk scores within the derivation cohort, simple risk categories were defined to approximate quartiles of risk in the derivation cohort: 0 points (low risk), 1 point (intermediate risk), 2 points (high risk), and ≥3 points (very high risk).
We internally validated the risk score model using 1000 bootstrap samples. Within each bootstrap sample, we refitted the risk score model and compared the apparent performance in the bootstrap sample with the overall performance of the risk score model. In addition, we externally validated the integer risk score model in 8,578 patients from the placebo arm of DECLARE-TIMI 58.
Discrimination was assessed using Harrell’s c-index. Calibration was assessed graphically in the external validation cohort by comparing observed event rates with predicted risk, as well as by calculating the Nam-D’Agostino statistic.
We performed subgroup analyses to assess the performance of the risk score in patients: (1) with versus without a prior history of heart failure, and (2) with established atherosclerotic cardiovascular disease versus multiple risk factors for cardiovascular disease, calculating the risk score distribution and Harrell’s c-index in each group.
To test for a heterogeneous treatment effect of dapagliflozin according to baseline HHF risk (i.e., relative risk reduction), we used Cox proportional hazards regression modeling with a treatment (dapagliflozin vs. placebo)-by-risk score interaction term. To compare absolute differences in the treatment effect of dapagliflozin according to baseline HHF risk, we calculated the absolute risk reduction (ARR) by subtracting the Kaplan-Meier (KM) event rates for HHF at 4 years in patients treated with dapagliflozin from the KM event rates for HHF at 4 years in patients treated with placebo across all simple risk score categories. To assess the increasing trend in ARR in HHF with dapagliflozin by baseline HHF risk, we used a weighted least-squares model, regressing ARR on integer risk score bin. Since we hypothesized that ARR would increase with increasing risk score, we used a one-sided p-trend value for statistical hypothesis testing. We calculated the numbers needed to treat (NNT) to prevent one HHF at 4 years using the formula 1/ARR. All analyses were performed based on intention to treat with hematuria (randomization stratification factor in DECLARE-TIMI 58) as a covariate. As an exploratory analysis, we also tested for heterogeneity in the relative and absolute increase in risk of HHF with saxagliptin vs. placebo according to baseline HHF risk using the same approach.
All statistical analyses were performed in R version 3.5.3. All p-values are two-sided unless otherwise specified.
Results
Study Population
Baseline characteristics of the 8,212 patients with T2DM in the derivation cohort are summarized in Table 1. The median age was 65 years; 33% were women and 75% were white. The median duration of diabetes was 10 years, and the median HbA1c was 7.6%. A substantial proportion of patients had renal dysfunction as evidenced by either an eGFR <60 ml/min/1.73 m2 (26%) or UACR >30 mg/g (39%). Sixty-two percent of patients had established CAD, 7% had atrial fibrillation, and 13% had pre-existing HF. During a median follow-up of 2.1 years, 228 patients in the derivation cohort (2.8%) experienced at least one HHF event.
Baseline characteristics of the 8,578 patients with T2DM in the external validation cohort are shown in Supplemental Table 1. The median age was 65 years; 38% were women and 79% were white. The median duration of diabetes was 10 years, and the median HbA1c was 8.0%. Eight percent of patients had an eGFR <60 ml/min/1.73 m2 and 31% had a UACR >30 mg/g. Thirty-three percent of patients had established CAD, 7% had atrial fibrillation, and 10% had pre-existing HF. During a median follow-up of 4.2 years, 286 patients in the external validation cohort (3.3%) experienced at least one HHF event.
Development of a Novel Risk Score for Hospitalization for Heart Failure
The univariable associations between the 25 candidate baseline characteristics and risk of HHF are shown in Table 1. Of these, 17 were included in a multivariable risk model based on achieving a significance level of p<0.10 on the univariable screen. Five were then selected for inclusion in the final risk model based on achieving statistical significance at a stringent threshold of p<0.001. These variables were history of heart failure, history of atrial fibrillation, coronary artery disease, eGFR, and UACR (Supplemental Figure 1).
After modeling UACR and eGFR as categorical variables, the regression coefficients of pre-existing heart failure and UACR >300 mg/g (macroalbuminuria) were approximately double the magnitude of the other regression coefficients (which were all similar). Therefore, these variables were assigned weights of 2 points while the remaining variables were assigned weights of 1 point (Table 2).
Table 2.
The TIMI Risk Score for Heart Failure in Diabetes (TRS-HFDM) in the derivation cohort.
| Risk Indicator | Adjusted HR (95% CI) | P-value | Points |
|---|---|---|---|
| Prior heart failure | 4.22 (3.18 – 5.59) | <0.001 | 2 |
| Atrial fibrillation | 2.26 (1.62 – 3.14) | <0.001 | 1 |
| Coronary artery disease | 2.06 (1.45 – 2.93) | <0.001 | 1 |
| Estimated glomerular filtration rate (eGFR) | |||
| < 60 ml/min/1.73 m2 | 1.85 (1.40 – 2.46) | <0.001 | |
| Urine albumin-to-creatinine ratio (UACR) | |||
| >300 mg/g | 4.50 (3.18 – 6.36) | <0.001 | 2 |
| 30–300 mg/g | 2.08 (1.50 – 2.87) | <0.001 | 1 |
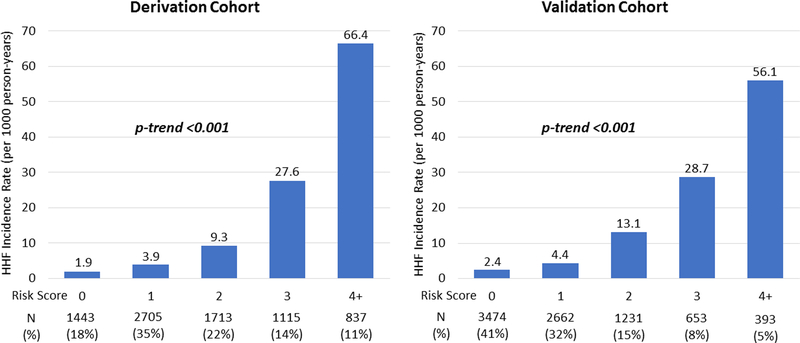
This simple integer-based scheme identified a >20-fold gradient of HHF risk in the derivation cohort (χ2W 819.4; p-trend <0.001) (Figure 1). Moreover, the integer-based score yielded a Harrell’s c-index of 0.81 (95% confidence interval [CI] 0.78–0.84), closely approximating the c-index of 0.82 (95% CI 0.79 – 0.84) for the non-integer five-variable risk model (Supplemental Table 2). The internal bootstrap validation yielded an optimism-corrected c-index of 0.81 (95% CI 0.78 – 0.83), suggesting minimal over-fitting of the model (Supplemental Table 2).
Figure 1. Incidence rate of hospitalization for heart failure by risk score in the derivation and validation cohorts.
The incidence rates of hospitalization for heart failure in the derivation and validation cohorts are shown. The integer-based scheme identified a >20-fold gradient of risk in both the derivation (χ2W 819.4; p-trend <0.001) and validation cohorts (χ2W 724.3; p-trend <0.001). HHF indicates hospitalization for heart failure.
In the external validation cohort, the integer-based score also demonstrated a strong graded relationship with HHF risk (Figure 1 and Supplemental Figure 2) and yielded a c-index of 0.78 (95% CI 0.75–0.81) (Supplemental Table 2). In addition, the integer score was well-calibrated in the external validation cohort, with observed KM HHF event rates at 4 years closely matching predicted KM event rates at 4 years. Furthermore, the Nam-D’Agostino statistic for calibration (nonsignificant p-values indicate adequate calibration) for the integer score at 4 years was 4.64 (p=0.20) (Supplemental Figure 3).
In the subgroup of patients with a prior history of heart failure in the derivation cohort (n=988), the median risk score was 4 (3–5), and the risk score range was 2 to 7. In patients with no prior history of heart failure (n=7561), the median risk score was 1 (1–2), and the risk score range was 0 to 5. There was a significant graded risk of HHF by risk score in each subgroup, and the Harrell’s c-indices were 0.70 (95% CI, 0.65–0.75) and 0.77 (95% CI, 0.72–0.81), respectively. Similarly, the risk score performed well both in patients with established cardiovascular disease (n=6221; Harrell’s c-index 0.80, 95% CI 0.76–0.83) and in patients with multiple risk factors for cardiovascular disease (n=1592; Harrell’s c-index 0.77, 95% CI 0.65–0.90) (Supplemental Table 3).
Although the risk score also identified gradients of risk for major adverse cardiovascular events (MACE) (i.e., cardiovascular death, myocardial infarction, or ischemic stroke) and non-cardiovascular death, the discrimination and fold increases in risk were substantially more modest for those outcomes than they were for HHF (Supplemental Figure 4).
Efficacy of Dapagliflozin by Baseline Risk of Hospitalization for Heart Failure
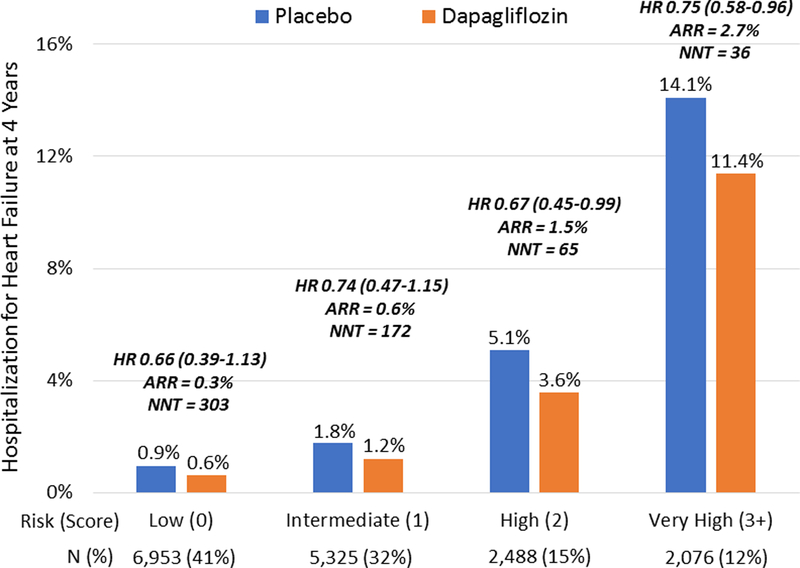
The efficacy of dapagliflozin vs. placebo was assessed in the 17,160 patients who were randomized in the DECLARE-TIMI 58 trial based on intention-to-treat. Risk categories, defined as low (0 points), intermediate (1 point), high (2 points), and very high (≥3 points), represented 41% (n=6,953), 32% (n=5,325), 15% (n=2,488), and 12% (n=2,076), of the analysis population, respectively. Using this simplified scheme, the strong gradient of HHF risk was consistent in placebo- and dapagliflozin-treated patients within this population (p-trend <0.001 for each).
Whereas relative risk reductions in HHF with dapagliflozin were similar across the risk score categories (25–34%; p-interaction = 0.95), absolute risk reductions were greater in those patients at higher baseline risk (χ2 3.24; one-sided p-trend = 0.04). Specifically, patients with low, intermediate, high, and very high risk of HHF had ARR of 0.3% (95% CI, −0.1% to 0.8%), 0.6% (95% CI, −0.1% to 1.3%), 1.5% (95% CI, −0.1% to 3.2%), and 2.7% (95% CI, 0.3% to 5.7%), translating into NNTs of 303, 172, 65, and 36 to prevent one HF hospitalization at 4 years, respectively (Figure 2).
Figure 2. Treatment effect of dapagliflozin by baseline risk of hospitalization for heart failure.
Whereas relative risk reductions were similar across risk score categories, absolute reductions in Kaplan-Meier estimates of hospitalization for heart failure (HHF) risk at 4 years (i.e., ARR) were greater in those at higher baseline risk (χ2 3.24; one-sided p-trend=0.04). ARR indicates absolute risk reduction; HR, hazard ratio; NNT, number needed to treat.
Safety of Saxagliptin by Baseline Risk of Hospitalization for Heart Failure
In the SAVOR-TIMI 53 trial (n=16,492), the low (0 points), intermediate (1 point), high (2 points), and very high (≥3 points) risk categories represented 18% (n=2,882), 35% (n=5,443), 22% (n=3,467), and 25% (n=3,894) of the analysis population, respectively. The strong gradient of HHF risk was consistent in both the placebo- and saxagliptin-treated patients within this population (p-trend <0.001 for each). In addition, there was also an increasing gradient of absolute risk differences for HHF by treatment arm (χ2 4.53; one-sided p-trend = 0.02) (Supplemental Figure 5).
Discussion
In this analysis, we developed and externally validated a novel, integer-based clinical risk score for predicting HHF in patients with T2DM. This simple risk score includes five routinely assessed clinical variables: history of heart failure, history of atrial fibrillation, coronary artery disease, eGFR, and UACR. The risk score had excellent discrimination, with a c-index of 0.78–0.81 and a >20-fold gradient of HHF risk in two large clinical trial cohorts of patients with T2DM. Notably, the risk score appeared to perform well both in patients with and without a prior history of heart failure. Whereas the relative risk reductions in HHF with the SGLT2 inhibitor dapagliflozin were similar for patients across the risk score categories, given the strong gradient of baseline risk, the score identified a strong gradient of increasing absolute reduction in HHF risk with dapagliflozin. The results of this analysis therefore offer a practical tool to assist clinicians with risk stratification, counseling, and therapeutic decision-making in patients with T2DM.
Application of the Risk Score in the Treatment of Patients with Type 2 Diabetes Mellitus
It is well established that HF is both a frequent and prognostically important cardiovascular complication of T2DM.5–7 Data from both observational studies and clinical trial cohorts suggest that the development of HF in patients with T2DM is associated with anywhere from a 4- to 10-fold increase in mortality risk.14, 15 Moreover, in contrast to the risk of myocardial infarction, the risk of HF in patients with T2DM persists even when other traditional cardiovascular risk factors, such as hypertension and hyperlipidemia, are well-controlled.8 Several mechanisms have been suggested to explain the relationship between T2DM and HF, but the pathophysiology and underlying molecular mechanisms remain incompletely understood.16, 17
Further complicating the relationship between T2DM and HF is the fact that several glucose-lowering therapies, most notably the thiazolidinediones,18, 19 have been shown to increase the risk of developing HF. Due to concerns about the cardiovascular safety of new glucose-lowering therapies, the United States Food and Drug Administration (FDA) and European Medicines Agency (EMA) mandated that all new diabetes therapies undergo rigorous evaluation in large-scale post-marketing cardiovascular outcomes trials.20 Over the last decade, the results of these trials have dramatically shifted the focus of diabetes care from just reducing HbA1c to also improving cardiovascular and renal outcomes.
Notably, members of two new drug classes—SGLT2 inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists—have been shown to reduce the risk of MACE, defined as the composite of myocardial infarction, stroke, and cardiovascular death, in patients with established atherosclerotic cardiovascular disease.21 In addition, SGLT2 inhibitors have a particularly robust effect on reducing the risk of HHF in patients with T2DM.21 The mechanisms by which each of these drug classes achieve their cardiovascular benefits remains unclear; however, multiple mechanistic studies are seeking to address this question. Regardless, the distinct profile of cardiovascular benefit for each of these drug classes highlights the need for improved models of risk stratification to optimally treat patients with T2DM.
SGLT2 inhibitors safely reduce HbA1c without causing hypoglycemia, lower SBP, and augment weight loss, and are therefore an effective therapy for all patients with T2DM. Moreover, SGLT2 inhibitors also prevent the progression of kidney disease, reduce the risk of MACE in patients with established atherosclerotic cardiovascular disease, and reduce the risk of HHF regardless of baseline atherosclerotic risk category or prior heart failure status.12 Our analysis confirms that the relative risk reduction in HHF is consistent regardless of baseline risk. However, by using a simple, validated, clinical risk score for HHF, clinicians can better characterize the risk profile of their patients and identify those patients who have the greatest absolute reduction in risk of HHF from treatment with SGLT2 inhibitors.
Beyond the immediate clinical application for identifying risk of HHF and absolute risk reduction in HHF with SGLT2 inhibition in patients with T2DM, our risk score may also be used as an aid in the design of future clinical trials. Given the results of cardiovascular outcomes trials of glucose-lowering therapies to date, HHF has emerged as an important endpoint in such trials. Thus, our risk score could serve as a stratification tool to select patients with T2DM who may have the greatest potential risk and/or benefit from future therapeutic interventions.
Limitations
There are several limitations to this analysis. First, the derivation cohort database did not include details of the HF history or echocardiographic data, including indicators of HHF risk such as left ventricular [LV] ejection fraction and diastolic dysfunction.22 As such, these variables were not included as candidate risk variables in the derivation of our risk score. Although these variables may have theoretically enhanced the prognostic performance of the risk model, the objective of this analysis was to develop and validate a clinical risk score for HHF using patient characteristics that can be readily obtained from the medical record of a typical patient with T2DM. Second, because creatinine clearance (CrCl) <60 mL/min was an exclusion criterion in the DECLARE-TIMI 58 trial, the range of diabetic renal disease among patients enrolled in that trial was more restricted and thus the proportion of patients with higher risk scores was lower in the validation cohort than in the derivation cohort. Third, our risk score was derived and validated in two clinical trial cohorts, which may influence the generalizability of these results. Future validation studies in non-clinical trial populations will be important in assessing the performance of the score in a broad population of patients with T2DM. Finally, despite the great scientific interest in the role of serum biomarkers and genetic variants in HF risk stratification of patients with T2DM, these variables were also excluded from the risk model in order to prioritize parsimony and practicality. Future analyses may seek to evaluate whether biomarkers and genetic variants add to clinical variables in identifying high-risk patients with T2DM who have the greatest reduction in risk of HHF with an SGLT2 inhibitor.
Conclusions
Improved models of heart failure risk stratification are needed for patients with T2DM. We developed and externally validated a novel and practical clinical risk score for prediction of HHF in patients with T2DM that includes prior heart failure, history of atrial fibrillation, coronary artery disease, eGFR, and UACR using two large clinical trial cohorts. This simple clinical risk prediction tool has excellent discrimination, is well-calibrated, and can help clinicians counsel patients about their risk of HHF and identify those patients at higher risk for HHF who have a greater absolute reduction in HHF risk with SGLT2 inhibitors.
Supplementary Material
Supplemental Publication Material
Clinical Perspective.
What is new?
- We developed and externally validated the TIMI Risk Score for Heart Failure in Diabetes (TRS-HFDM), a novel, integer-based clinical risk score for predicting hospitalization for heart failure (HHF) in patients with type 2 diabetes mellitus (T2DM) that includes prior heart failure, history of atrial fibrillation, coronary artery disease, estimated glomerular filtration rate (eGFR), and urine albumin-to-creatinine ratio (UACR).
- The risk score had excellent discrimination in two large clincial trial cohorts, was well-calibrated, and identified a strong gradient of increasing absolute reduction in risk of HHF with the sodium-glucose cotransporter-2 (SGLT2) inhibitor dapagliflozin.
What are the clinical implications?
- This analysis confirms that the relative risk reduction in HHF with SGLT2 inhibitors is consistent regardless of baseline heart failure risk.
- However, by using TRS-HFDM, a simple, validated clinical risk score for HHF, clinicians can better educate patients about their risk of HHF and identify those patients who have a greater absolute reduction in HHF risk with SGLT2 inhibitors.
Sources of Funding
D.D.B. is supported by a T32 postdoctoral training grant from the National Heart, Lung, and Blood Institute (T32 HL007604). The SAVOR-TIMI 53 and DECLARE-TIMI 58 studies were supported by AstraZeneca.
Disclosures
D.D.B. has nothing to disclose. S.D.W. reports grants from AstraZeneca, Bristol-Myers Squibb, Sanofi Aventis, and Amgen; grants and personal fees from Arena, Daiichi Sankyo, Eisai, Eli Lilly, and Janssen; grants and consulting fees from Merck (additionally his spouse is employed by Merck); and personal fees from Aegerion, Allergan, AngelMed, Boehringer Ingelheim, Boston Clinical Research Institute, Icon Clinical, Lexicon, St Jude Medical, Xoma, Servier, AstraZeneca, and Bristol-Myers Squibb. B.M.S. has received research grants from AstraZeneca, Eisai, Novartis, and Merck; has received consulting fees from AstraZeneca, Biogen Idec, Boehringer Ingelheim, Covance, Dr. Reddy’s Laboratories, Eisai, Elsevier Practice Update Cardiology, GlaxoSmithKline, Lexicon, Merck, Novo Nordisk, Sanofi, and St. Jude’s Medical; and has equity in Health [at] Scale. Y.G. supports grant support from Novartis. O.M. reports grants and personal fees from AstraZeneca, Bristol-Myers Squibb, and Novo Nordisk and personal fees from Eli Lilly, Sanofi, Merck Sharp & Dohme, Boehringer Ingelheim, Johnson & Johnson, and Novartis. S.A.M. reports grant support from Abbott Laboratories, Amgen, AstraZeneca, Critical Diagnostics, Daiichi-Sankyo, Eisai, Genzyme, Gilead, GlaxoSmithKline, Intarcia, Janssen Research and Development, The Medicines Company, MedImmune, Merck, Novartis, Poxel, Pfizer, Roche Diagnostics, and Takeda, and has received honorarium from Amgen. D.L.B. discloses the following relationships - Advisory Board: Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Medtelligence/ReachMD (CME steering committees), Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Fractyl, Merck, Novo Nordisk, PLx Pharma, Takeda. L.A.L. reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Novo Nordisk, and Sanofi; personal fees from Merck and Servier; and grants from GlaxoSmithKline. D.K.M. reports personal fees from AstraZeneca, Boehringer Ingelheim, Janssen Research and Development, Sanofi US, Merck Sharp & Dohme, Lilly USA, Novo Nordisk, GlaxoSmithKline, Lexicon, Eisai, Esperion, Metavant, Pfizer, and Applied Therapeutics. J.P.H.W. reports grants, personal fees and consultancy fees (paid to his institution) from AstraZeneca, personal fees and consultancy fees (paid to his institution) from Boehringer Ingelheim, personal fees and consultancy fees (paid to his institution) from Lilly, grants, personal fees and consultancy fees (paid to his institution) from Novo Nordisk, personal fees and consultancy fees (paid to his institution) from Janssen, personal fees and consultancy fees (paid to his institution) from Napp, personal fees and consultancy fees (paid to his institution) Mundipharma, personal fees, consultancy fees (paid to his institution) from Sanofi, grants, personal fees and consultancy fees (paid to his institution) from Takeda, consultancy fees (paid to his institution) from Wilmington Healthcare, outside the submitted work. P.J., P.A.J., and A.M.L. are employees of AstraZeneca. I.R. reports personal fees from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Concenter BioPharma and Silkim, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, Orgenesis, Pfizer, Sanofi, SmartZyme Innovation, Panaxia, FuturRx, Insuline Medical, Medial EarlySign, CameraEyes, Exscopia, Dermal Biomics, Johnson & Johnson, Novartis, Teva, GlucoMe, and DarioHealth. E.B. reports research grants through his institution from AstraZeneca, Daiichi Sankyo, GlaxoSmithKline, Merck, and Novartis; personal fees for consultancy from Cardurion, MyoKardia, Sanofi, and Verve; fees for lectures from Medscape; uncompensated consultancies and lectures from Merck, Novartis, and The Medicines Company. M.S.S. reports institutional research grants to the TIMI Study Group at Brigham and Women’s Hospital from Amgen, AstraZeneca, Bayer, Daiichi-Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen Research and Development, Medicines Company, MedImmune, Merck, Novartis, Poxel, Pfizer, Quark Pharmaceuticals and Takeda; consulting fees from Amgen, Anthos Therapeutics, AstraZeneca, Bristol-Myers Squibb, CVS Caremark, DalCor, Dyrnamix, Esperion, IFM Therapeutics, Intarcia, Ionis, Janssen Research and Development, Medicines Company, MedImmune, Merck, Novartis; and is a is a member of the TIMI Study Group, which has also received research grant support through Brigham and Women’s Hospital from: Abbott, Aralez, BRAHMS, Roche, and Zora Biosciences.
Glossary
Non
standard Abbreviations and Acronyms
ARR
absolute risk reduction
CEC
Clinical Events Committee
CrCl
creatinine clearance
DECLARE
Dapagliflozin Effect on CardiovascuLAR Events
DPP-4
dipeptidyl peptidase-4
GLP1
glucagon-like peptide 1
HHF
hospitalization for heart failure
MACE
major adverse cardiovascular events
SAVOR
Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus
SGLT2
sodium-glucose cotransporter-2
TIMI
Thrombolysis in Myocardial Infarction
TRS-HFDM
TIMI Risk Score for Heart Failure in Diabetes
UACR
urine albumin-to-creatinine ratio
References
- 1.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U and Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–49. [DOI] [PubMed] [Google Scholar]
- 2.Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, Jarolim P, Udell JA, Mosenzon O, Im K, Umez-Eronini AA, Pollack PS, Hirshberg B, Frederich R, Lewis BS, McGuire DK, Davidson J, Steg PG, Bhatt DL, Committee S-TS and Investigators*. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130:1579–1588. [DOI] [PubMed] [Google Scholar]
- 3.Cavender MA, Steg PG, Smith SC Jr., Eagle K, Ohman EM, Goto S, Kuder J, Im K, Wilson PW, Bhatt DL and Investigators RR. Impact of Diabetes Mellitus on Hospitalization for Heart Failure, Cardiovascular Events, and Death: Outcomes at 4 Years From the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Circulation. 2015;132:923–931. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS, American Heart Association Council on E, Prevention Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 5.Boudina S and Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. [DOI] [PubMed] [Google Scholar]
- 6.McMurray JJ, Gerstein HC, Holman RR and Pfeffer MA. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol. 2014;2:843–851. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB, Solomon SD, Granger CB, Swedberg K, Yusuf S, Pfeffer MA, McMurray JJ and Investigators C. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:1377–1385. [DOI] [PubMed] [Google Scholar]
- 8.Rawshani A, Rawshani A, Franzen S, Sattar N, Eliasson B, Svensson AM, Zethelius B, Miftaraj M, McGuire DK, Rosengren A and Gudbjornsdottir S. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2018;379:633–644. [DOI] [PubMed] [Google Scholar]
- 9.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE and Investigators E-RO. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 10.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR and Group CPC. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644–657. [DOI] [PubMed] [Google Scholar]
- 11.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS and Investigators D-T. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380:347–357. [DOI] [PubMed] [Google Scholar]
- 12.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH and Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. [DOI] [PubMed] [Google Scholar]
- 13.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I, Committee S-TS and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. [DOI] [PubMed] [Google Scholar]
- 14.Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL and Goff DC, Jr. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27:699–703. [DOI] [PubMed] [Google Scholar]
- 15.Carr AA, Kowey PR, Devereux RB, Brenner BM, Dahlof B, Ibsen H, Lindholm LH, Lyle PA, Snapinn SM, Zhang Z, Edelman JM and Shahinfar S. Hospitalizations for new heart failure among subjects with diabetes mellitus in the RENAAL and LIFE studies. Am J Cardiol. 2005;96:1530–1536. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues B, Cam MC and McNeill JH. Myocardial substrate metabolism: implications for diabetic cardiomyopathy. J Mol Cell Cardiol. 1995;27:169–179. [DOI] [PubMed] [Google Scholar]
- 17.Bahrami H, Bluemke DA, Kronmal R, Bertoni AG, Lloyd-Jones DM, Shahar E, Szklo M and Lima JA. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;51:1775–1783. [DOI] [PubMed] [Google Scholar]
- 18.Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM and Krumholz HM. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation. 2005;111:583–590. [DOI] [PubMed] [Google Scholar]
- 19.Lago RM, Singh PP and Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. 2007;370:1129–1136. [DOI] [PubMed] [Google Scholar]
- 20.Scirica BM. The Safety of Dipeptidyl Peptidase 4 Inhibitors and the Risk for Heart Failure. JAMA Cardiol. 2016;1:123–125. [DOI] [PubMed] [Google Scholar]
- 21.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH and Sabatine MS. Comparison of the Effects of Glucagon-Like Peptide Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors for Prevention of Major Adverse Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus. Circulation. 2019;139:2022–2031. [DOI] [PubMed] [Google Scholar]
- 22.From AM, Scott CG and Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J Am Coll Cardiol. 2010;55:300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Publication Material