Use of Epidermal Growth Factor Receptor Inhibitor Erlotinib to Treat Palmoplantar Keratoderma in Patients With Olmsted Syndrome Caused by TRPV3 Mutations (original) (raw)
This case series investigates whether epidermal growth factor receptor transactivation with the inhibitor erlotinib could be used to treat palmoplantar keratoderma in patients with Olmsted syndrome caused by TRPV3 mutations.
Key Points
Question
Can epidermal growth factor receptor transactivation be targeted with erlotinib, an epidermal growth factor receptor inhibitor, to treat palmoplantar keratoderma in patients with Olmsted syndrome caused by transient receptor potential vanilloid 3 (TRPV3) mutations?
Findings
In this case report, 3 patients with severe and disabling Olmsted syndrome caused by TRPV3 mutations experienced remission of their palmoplantar keratoderma within less than 3 months of initiating therapy with erlotinib hydrochloride. Hyperkeratosis and pain disappeared, and remission was sustained with ongoing treatment without major adverse effects.
Meaning
This study’s findings suggest that erlotinib targets the molecular pathogenesis of Olmsted syndrome caused by TRPV3 mutations and may be an effective treatment for painful hyperkeratosis of this genetic disorder.
Abstract
Importance
Olmsted syndrome is a genodermatosis characterized by painful and mutilating palmoplantar keratoderma (PPK) that progresses from infancy onward and lacks an effective treatment. It is most often caused by mutations in the transient receptor potential vanilloid 3 (TRPV3) gene. In animal models and keratinocyte cell lines, TRPV3 signaling leads to epidermal growth factor receptor (EGFR) transactivation.
Objective
To examine the possibility of blocking EGFR transactivation with the inhibitor erlotinib hydrochloride to treat PPK in patients with Olmsted syndrome due to TRPV3 mutations.
Design, Setting, and Participants
In this case series, 3 patients from 2 unrelated families who had _TRPV3_-mutation–associated PPK were treated with erlotinib from May 5, 2018, through May 13, 2019.
Main Outcomes and Measures
Clinical follow-up included evaluation of PPK progression, pain and interventions for pain, as well as erlotinib dose adjustment based on treatment effect, plasma levels, and tolerance.
Results
The 3 patients (2 brothers aged 15 and 17 years and a 13-year-old girl) had severe palmoplantar hyperkeratosis, intolerable pain with erythromelalgia, severe growth delay, anorexia, and insomnia, which had been progressing since infancy despite numerous therapies. Two patients were confined to wheelchairs owing to intense pain and joint restrictions because of hyperkeratosis. All patients experienced depression and did not engage in social activities. Within 3 months of initiating therapy with erlotinib, hyperkeratosis and pain disappeared. All patients were able to touch the ground with their feet, wear shoes, and walk. Anorexia and insomnia remitted and paralleled improved growth. In addition, the patients resumed social activities. These improvements were sustained across 12 months of treatment and follow-up. The doses of erlotinib used were lower than those used in oncology, and only mild to moderate adverse effects were noted.
Conclusions and Relevance
The findings of this study report improvement of PPK in patients with Olmsted syndrome caused by TRPV3 mutations when treated with erlotinib. Targeting EGFR transactivation with erlotinib therapy may result in clinical remission in an orphan disease that lacks an effective intervention.
Introduction
Olmsted syndrome (OS) is a heterogeneous genodermatosis characterized by painful and debilitating, inflammatory palmoplantar keratoderma (PPK), pseudoainhum, curved thickened nails, and periorificial hyperkeratosis. No effective treatment is available, and patients’ quality of life is considerably altered. Two genes are implicated in OS: TRPV3 (OMIM 614594), which encodes transient receptor potential vanilloid 3 (TRPV3),1,2,3 a temperature-sensitive transient receptor potential cation channel that is highly expressed in keratinocytes4,5,6; and less frequently, MBTPS2 (OMIM 300294), which encodes membrane-bound transcription factor protease, site 2.2 The mechanism linking these mutated genes to the disease phenotype has not been fully elucidated.6
Cheng et al7 showed that TRPV3 activation is associated with epidermal growth factor receptor (EGFR) signaling through a process of transactivation,8,9 involving activation of the membrane protease ADAM17, which cleaves the membrane precursor of transforming growth factor α (TGFA), an EGFR ligand. Cheng et al observed that TRPV3 knockout mice had curled whiskers and a perm hair phenotype reminiscent of TGFA and EGFR hypomorphic waved_-_1 and waved_-_2 mutants.10 These authors also showed that EGFR activation lowers the TRPV3 activation threshold. Complementary to these data, genetic studies on mice indicated that EGFR plays an essential role in keratinocyte proliferation and differentiation as well as, more generally, skin homeostasis.3,10
Because functional studies of several dominant TRPV3 mutations have demonstrated a gain of function,1,3,11 we hypothesized that the signs and symptoms of OS could result from TRPV3-induced EGFR transactivation. We proposed administering erlotinib hydrochloride, an EGFR inhibitor, to treat 3 patients12,13 who were debilitated by progressive OS due to TRPV3 mutations.
Methods
In this case series of a prospective intervention that was not registered as a clinical trial, erlotinib therapy was started at an initial dosage of 70 mg/m2/d according to its use in treating other diseases. The dosage was adjusted based on tolerability and weight gain. Clinical outcomes were evaluated, including blood concentrations of erlotinib at the following times: monthly during the first 6 months and every 2 months thereafter. The institutional review board of the reference center for genodermatoses, the MAGEC-Necker Hospital, Paris, approved the study. Written informed consent was obtained from the patients’ parents prior to starting therapy.
Results
In this case series, 3 patients (2 brothers aged 15 and 17 years and a 13-year-old girl from 2 unrelated families) with _TRPV3_-mutation–associated PPK, were treated with erlotinib from May 5, 2018, through May 13, 2019.
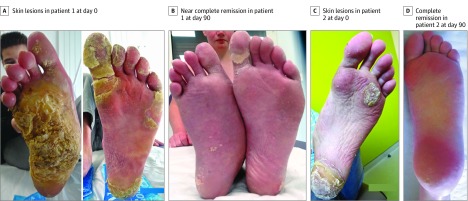
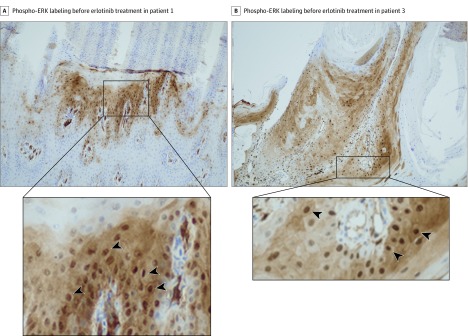
Patients 1 and 2 were brothers (Figure 1) and had compound heterozygous mutations p.Gly568Cys and p.Gln216_Gly262del in TRPV3.12 Patient 1 (Figure 1A), aged 17 years and profoundly depressed, had significant focal PPK with erythromelalgia symptoms and progressive right ankle deformity. He was confined to a wheelchair and placed his feet continuously on an ice pack. His growth and puberty were hindered because of this condition: weight, 48 kg (z score, −2.3); height, 1.54 m (z score, −2.9); and Tanner stage 3. Treatments with topical keratolytics, oral retinoids, and rapamycin were not effective. Administration of prednisone, 2 mg/kg/d, improved the patient’s pain and keratoderma, although these conditions relapsed at 1 mg/kg/d. His pain was constantly rated 7 to 10 on a 10-point visual analog scale, and it was resistant to opioids. He also had insomnia, loss of autonomy, and profound depression. A plantar skin biopsy showed strong phospho–extracellular signal-regulated kinase (ERK) expression by epidermal cells (Figure 2A and eMethods in the Supplement), which was not seen in control skin (eFigure in the Supplement), indicating activation of the ERK/mitogen-activated protein kinase (MAPK) pathway. Erlotinib therapy was initiated at 100 mg/d and increased to 125 mg/d on day 30. Pain reduced within days, with near-complete remission of the patient’s keratoderma by day 90 (Figure 1B). At 12 months, he was no longer depressed, he had no pain (0 on a 10-point visual analog scale), and pain treatment was discontinued. The patient was able to walk, wear shoes, and return to school. His weight was 53 kg (z score, −1.8), and height, 1.60 m (z score, −2.2). Tanner stage progressed to stage 5. Adverse effects included a mild acneiform eruption and moderate diffuse hair loss. Erlotinib blood concentrations varied between 558 ng/mL and 842 ng/mL.
Figure 1. Evolution of Plantar Keratoderma in Patients 1 and 2.
Figure 2. Phospho–Extracellular Signal-Regulated Kinase (ERK) Labeling Before Erlotinib Treatment in Patients 1 and 3.
A, Strong nuclear staining (arrowheads) of numerous keratinocytes in the upper part of the hyperplastic epidermis below a thick parakeratosis area (original magnification ×100). B, Strong nuclear staining (arrowheads) of numerous keratinocytes in the hyperplastic epidermis (original magnification ×100).
Patient 2 (Figure 1C), aged 15 years, had less painful PPK, although his symptoms increased progressively until crutches were needed at age 14 years. Erlotinib therapy was started at 100 mg/d (height, 1.60 m [z score, −1.2]; weight, 39 kg [z score, −2.3]) and was reduced within 7 days to 50 mg/d because of abdominal pain and nausea that resolved completely. Pain medication was rapidly discontinued, and hyperkeratosis disappeared within 1 month (Figure 1D). Crutches were no longer necessary. Erlotinib doses were progressively adapted to the patient’s growth. His blood concentrations remained under 500 ng/mL. Twelve months later, his height was 1.65 m (z score, −1.1), and weight, 50 kg (z score, −1.2). Adverse effects included localized hair loss and superficial desquamation of pulp of the fingers and toes that disappeared with dose adjustment.
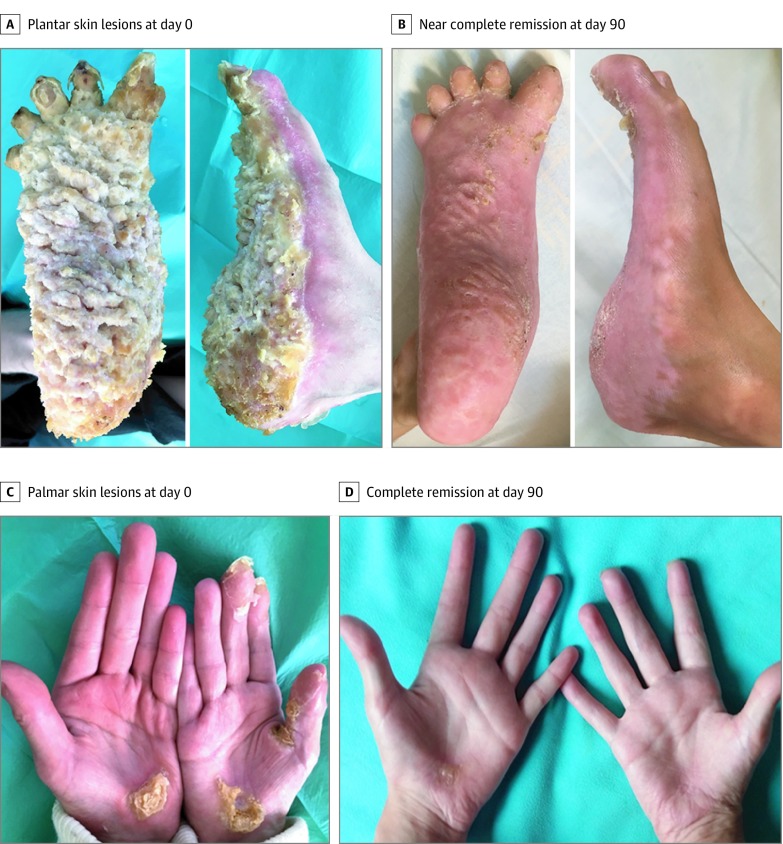
Patient 3 was a girl, aged 13 years, with a dominant heterozygous missense TRPV3 mutation p.Leu673Phe.13 She was deeply depressed, had manifestations of erythromelalgia and a considerably large plantar keratoderma (Figure 3A), predominantly on the right side, and had focal palmar keratoderma (Figure 3C). Her right leg was a little shorter than the left, with pseudoainhum of the right toes. Pain and joint restriction confined her to a wheelchair. Anorexia and insomnia severely hindered her growth and puberty as follows: weight, 22 kg (z score, −5.3); height, 1.28 m (z score, −4.1); and Tanner stage 1. Treatment with topical keratolytics, oral retinoids, topical and oral sirolimus (through 18 months), and oral corticosteroids was ineffective. Pain (5-10 on a 10-point visual analog scale) was only moderately improved by the use of high-dose opioids. A skin biopsy specimen showed strong phospho-ERK expression by epidermal cells (Figure 2B). Erlotinib therapy was started at 50 mg/d and then increased to 75 mg/d after 1 month. Pain significantly improved within 30 days. Hyperkeratosis disappeared within 90 days (Figure 3B and D). Pain medications were reduced and discontinued at 2 months. Depression, anorexia, and insomnia disappeared, and her puberty began at month 4. Within less than 6 months, the patient was running and wearing shoes. At 12 months, her weight was 33 kg (z score, −2.7) and height, 1.39 m (z score, −3.27) with a Tanner stage 4. The pseudoainhum had disappeared and the toes were distinct, with nails observed. Blood erlotinib concentration remained below 500 ng/mL. The only adverse effect was a mild diffuse alopecia. For all 3 patients, therapy was continued through 12 months of follow-up, and clinical remission of symptoms persisted.
Figure 3. Evolution of Palmoplantar Keratoderma (PPK) in Patient 3.
Discussion
This study’s findings report the disappearance of PPK and pain in 3 patients diagnosed with OS due to TRPV3 mutations within less than 3 months of starting erlotinib therapy. In addition, their anorexia and insomnia resolved, and their growth improved. Only mild adverse effects were observed, and the benefits persisted through 12 months of treatment and follow-up. To support these findings, a 31-year-old woman with disabling OS was treated with erlotinib, starting in 2009 and continuing for 2 years, in addition to administering oral retinoids and surgical parings to decrease keratinocyte proliferation. A partial but clear improvement in her painful PPK was observed.14 However, this patient was not genetically characterized.
This study’s therapeutic approach focused on the gene involved in the patients’ disease. The purpose of using an EGFR inhibitor was to break the vicious cycle of reciprocal TRPV3/EGFR activation initiated by constitutive activation of mutated TRPV3. The erlotinib-induced complete remission in the patients confirmed EGFR’s role as an important mediator of TRPV3 activity. This finding was in agreement with in vivo/in vitro experimental observations7 and in situ activation of the ERK/MAPK pathway observed in these patients. The rapid pain relief suggested that unidentified mediators secreted by abnormal keratinocytes triggered cutaneous nociceptive neuronal receptors.5 We anticipate that these patients will need to be maintained on erlotinib treatment at the lowest dose that keeps them in remission, as long as no considerable adverse effects are observed. Treatment resistance, as observed in oncology, is not expected because the biological properties of abnormal keratinocytes differ from those of malignant cells, usually resulting from an accumulation of mutations that endow the cells with invasive growth properties as well as DNA repair and apoptosis defects.15
Limitations
This study has limitations. Additional studies with more patients and other OS mutations are needed to confirm these results.
Conclusions
Erlotinib therapy, in lower doses than those used in oncology, may be an effective treatment for PPK in patients with OS caused by TRPV3 mutations. Targeting EGFR transactivation by drug repurposing leads to considerable remission in patients with a nonmalignant disease displaying abnormal keratinocytes proliferation. This therapy could be extended to other keratinization disorders with similar pathological mechanisms.
Supplement.
eMethods. Immunohistochemistry Method
eFigure. Controls for Phospho-ERK Labeling
References
- 1.Lin Z, Chen Q, Lee M, et al. Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am J Hum Genet. 2012;90(3):558-564. doi: 10.1016/j.ajhg.2012.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson NJ, Cole C, Milstone LM, et al. Expanding the phenotypic spectrum of Olmsted syndrome. J Invest Dermatol. 2015;135(11):2879-2883. doi: 10.1038/jid.2015.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He Y, Zeng K, Zhang X, et al. A gain-of-function mutation in TRPV3 causes focal palmoplantar keratoderma in a Chinese family. J Invest Dermatol. 2015;135(3):907-909. doi: 10.1038/jid.2014.429 [DOI] [PubMed] [Google Scholar]
- 4.Nilius B, Bíró T, Owsianik G. TRPV3: time to decipher a poorly understood family member! J Physiol. 2014;592(2):295-304. doi: 10.1113/jphysiol.2013.255968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo J, Hu H. Thermally activated TRPV3 channels. Curr Top Membr. 2014;74:325-364. doi: 10.1016/B978-0-12-800181-3.00012-9 [DOI] [PubMed] [Google Scholar]
- 6.Szöllősi AG, Vasas N, Angyal Á, et al. Activation of TRPV3 regulates inflammatory actions of human epidermal keratinocytes. J Invest Dermatol. 2018;138(2):365-374. doi: 10.1016/j.jid.2017.07.852 [DOI] [PubMed] [Google Scholar]
- 7.Cheng X, Jin J, Hu L, et al. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell. 2010;141(2):331-343. doi: 10.1016/j.cell.2010.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prenzel N, Zwick E, Daub H, et al. EGF receptor transactivation by G-protein–coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402(6764):884-888. doi: 10.1038/47260 [DOI] [PubMed] [Google Scholar]
- 9.Wetzker R, Böhmer FD. Transactivation joins multiple tracks to the ERK/MAPK cascade. Nat Rev Mol Cell Biol. 2003;4(8):651-657. doi: 10.1038/nrm1173 [DOI] [PubMed] [Google Scholar]
- 10.Schneider MR, Werner S, Paus R, Wolf E. Beyond wavy hairs: the epidermal growth factor receptor and its ligands in skin biology and pathology. Am J Pathol. 2008;173(1):14-24. doi: 10.2353/ajpath.2008.070942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang G, Wang K. The Ca2+-permeable cation transient receptor potential TRPV3 channel: an emerging pivotal target for itch and skin diseases. Mol Pharmacol. 2017;92(3):193-200. doi: 10.1124/mol.116.107946 [DOI] [PubMed] [Google Scholar]
- 12.Duchatelet S, Guibbal L, de Veer S, et al. Olmsted syndrome with erythromelalgia caused by recessive transient receptor potential vanilloid 3 mutations. Br J Dermatol. 2014;171(3):675-678. doi: 10.1111/bjd.12951 [DOI] [PubMed] [Google Scholar]
- 13.Duchatelet S, Pruvost S, de Veer S, et al. A new TRPV3 missense mutation in a patient with Olmsted syndrome and erythromelalgia. JAMA Dermatol. 2014;150(3):303-306. doi: 10.1001/jamadermatol.2013.8709 [DOI] [PubMed] [Google Scholar]
- 14.Kenner-Bell BM, Paller AS, Lacouture ME. Epidermal growth factor receptor inhibition with erlotinib for palmoplantar keratoderma. J Am Acad Dermatol. 2010;63(2):e58-e59. doi: 10.1016/j.jaad.2009.10.052 [DOI] [PubMed] [Google Scholar]
- 15.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement.
eMethods. Immunohistochemistry Method
eFigure. Controls for Phospho-ERK Labeling