Chronic Divalproex Sodium to Attenuate Agitation and Clinical Progression of Alzheimer Disease (original) (raw)
. Author manuscript; available in PMC: 2020 Feb 6.
Abstract
Context:
Agitation and psychosis are common in Alzheimer disease and cause considerable morbidity. We attempted to delay or to prevent agitation and psychosis with the use of divalproex sodium (valproate).
Objective:
To determine whether treatment with valproate could delay or prevent emergence of agitation or psychosis.
Design, Setting, and Patients:
A multicenter, randomized, double-blind, placebo-controlled trial of flexible-dose valproate in 313 (of 513 screened) individuals with moderate Alzheimer disease who had not yet experienced agitation or psychosis. The study was conducted from November 1, 2005, through March 31, 2009, at 46 sites in the United States.
Intervention:
Participants were randomly assigned to valproate treatment at a target dose of 10 to 12 mg per kilogram of body weight per day or identical-appearing placebo for 24 months followed by a 2-month period of single-blind placebo treatment.
Main Outcome Measure:
Time to emergence of clinically significant agitation or psychosis.
Results:
A total of 122 participants (59 receiving valproate and 63 receiving placebo) completed 24 months of treatment while taking study medication; 42 (27 receiving valproate and 15 receiving placebo) reached 24 months having discontinued study medication; 150 reached month 26. There was no difference between groups in time to emergence of agitation or psychosis (Cox proportional hazard ratio, 0.96; _P_=.88). There was no difference between groups in change on any secondary outcome. The valproate group had higher rates of somnolence, gait disturbance, tremor, diarrhea, and weakness. Eighty-eight participants underwent magnetic resonance imaging scans at baseline and 12 months; the valproate group showed greater loss in hippocampal and whole-brain volume, accompanied by greater ventricular expansion (P<.001).
Conclusion:
Valproate treatment did not delay emergence of agitation or psychosis or slow cognitive or functional decline in patients with moderate Alzheimer disease and was associated with significant toxic effects.
Trial Registration:
clinicaltrials.gov Identifier: NCT00071721
ALZHEIMER DISEASE (AD) IS characterized by cognitive decline, impaired function, and neuropsychiatric signs and symptoms that are extremely common, with prevalence estimates of 60% to 80% and a lifetime risk of 90%.1–3 These signs and symptoms are associated with considerable morbidity, including earlier nursing home admission, more rapid progression of illness, exacerbation of functional and cognitive deficits, and increased caregiver distress. Neuropsychiatric features make caring for a person with dementia far more difficult4 and represent a major public health concern for which behavioral, psychological, and pharmacologic therapies have proven inadequate to date.5
The classification and prevalence of neuropsychiatric features vary with the methods used to study them, although patterns emerge.1,6 Agitation is particularly common and difficult to manage, the term referring to increased and distressing or disruptive psychomotor activity5 or “inappropriate verbal, vocal, or motor activity unexplained by apparent needs or confusion.”7(p712) Prior studies indicate that patients with mild to moderate AD without agitation have 1- and 2-year incidence rates ranging from 20% to 40% and from 50% to 60%, respectively.3,8–12 Once present, agitation tends to persist4,7,8,12–14 and commonly to co-occur with psychotic symptoms.15,16
Treatment of agitation and psychosis entails identification and reversal of physical, social, and environmental precipitants.5–17 Symptomatic pharmacotherapy is considered when these steps fail but is too often unsatisfactory. Reviews and meta-analyses indicate that antipsychotics may afford some relief from psychosis and/or agitation, but the adverse effect burden is problematic, and mortality may be increased in individuals with dementia.17 Physicians often use other agents instead, including anticonvulsants, but evidence for this practice is equivocal.17
On the basis of the high incidence and public health significance of agitation and psychosis in AD and the limited effectiveness of symptomatic treatment, the Alzheimer’s Disease Cooperative Study (ADCS) concluded that pharmacologic prevention of behavioral disturbance might be more effective than treatment of existing symptoms. We raised the question of whether therapy could delay, attenuate, or prevent the emergence of agitation and/or psychosis. Another rationale for our study was to demonstrate the feasibility of a trial design for prevention of behavioral disturbance in dementia.
At the time, divalproex sodium (valproate) had shown evidence of symptomatic benefit in small placebo-controlled trials, although insufficient to define clinical practice.5,18,19 Moreover, there are potential neuroprotective actions of valproate, some specific to the pathobiology of AD, which contributed to our decision to use it. These include evidence of reduced neuronal injury20; reduced apoptosis due to activation of bcl-2; reduced hyperphosphorylation of tau proteins and thus possibly reduced neurofibrillary tangle development; and increased cell survival by activation of a number of cellular signaling pathways, at concentrations in the range used for treatment of mania or epilepsy.21–25
This trial examined whether prophylactic therapy with well-tolerated doses of valproate could delay or prevent the emergence of psychiatric signs and symptoms in patients with AD who were not yet behaviorally symptomatic.26 In view of its possible neuroprotective potential, this trial was designed also to address whether treatment would attenuate cognitive and functional decline.
METHODS
PARTICIPANTS
The trial was conducted from November 1, 2005, through March 31, 2009, at 46 US sites. We enrolled 313 patients fulfilling criteria for probable or possible AD according to the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer Disease and Related Disorders Association. Main inclusion criteria were age greater than 54 years, weight greater than 39 kg, community residence, Mini-Mental State Examination (MMSE) score of 12 to 20, and absence of agitation or psychosis since the illness onset, defined by ratings of less than 1 on Neuropsychiatric Inventory (NPI)1 items assessing delusions, hallucinations, and agitation/aggression. Informed consent was obtained from an authorized representative and assent was obtained from the patient, the nature of the assent varying according to state and local institutional policies. The study was reviewed and approved by each site’s institutional review board.
INTERVENTIONS
This was a prospective, randomized, placebo-controlled, parallel-arm, flexible-dose trial in which participants were assigned to double-blind treatment for 2 years, followed by a 2-month single-blind placebo treatment period. Participants reaching end point or stopping therapy before 24 months had a treatment termination visit and were offered follow-up through month 24. Participants were assigned to 1 of 2 treatment groups in permuted blocks of 4, according to a randomization list created and maintained by the ADCS Data Core.
A target dose of 10 to 12 mg per kilogram of body weight per day of valproate was selected on the basis of tolerability in patients with dementia and a pilot study in outpatients with AD.27 The trial used a 125-mg enteric-coated extended-release divalproex sodium formulation or identical-appearing placebo manufactured and provided by Abbott Laboratories. Participants received 1 tablet daily by mouth for 1 week; weekly titration continued until a dose of 10 to12 mg per kilogram per day was achieved. However, dose reduction was permitted if warranted clinically; the target dose could be resumed if appropriate. Adherence was quantified with medication administration records; adherence of 80% was required.
A caregiver training program was offered to all families on the basis of previous data showing that this intervention decreased communication difficulty and increased knowledge about AD, to reduce variance in behavioral outcomes relating to variable patient-caregiver interaction.28,29
CONCOMITANT THERAPY
Participants could receive stable doses of cholinesterase inhibitors and memantine. The following drugs were excluded: psychotropics (except for nontricyclic antidepressants for treatment of depressive symptoms and short-acting benzodiazepines up to thrice weekly for sleep), narcotic analgesics, antiparkinsonian medications, drugs with significant central anticholinergic or antihistaminergic effects, and other anticonvulsants.
OBJECTIVES/OUTCOMES
The primary outcome was an end point defined as the participant’s achievement of a score of at least3 on 1 or more NPI items assessing delusions, hallucinations, and agitation/aggression persisting for 2 weeks, and the study physician’s judgment that the new agitation and/or psychosis was clinically significant on the basis of an evaluation to rule out situational disturbances or delirium; or completion of study.
Behavioral outcomes were assessed at screening, baseline, and every 3 months until month 24, then at month 26; other outcomes, except the ADCS Clinical Global Impression of Change (CGIC) and the Quality of Life–AD, were assessed every 6 months; the ADCS-CGIC was assessed at 12 and 24 months and the Quality of Life–AD at baseline and last visit. There was telephone contact 6 weeks after each visit to establish whether end point had been reached.
Safety, tolerability, vital signs (weight, systolic and diastolic blood pressure, and temperature), adherence, and concomitant medications were recorded quarterly and at week 6. We developed an instrument assessing daytime drowsiness and sleeping; higher scores denote worse sedation. The ADCS Data and Safety Monitoring Board monitored the trial.
COGNITIVE MEASURES
The cognitive subscale30 of the AD Assessment Scale assesses cognition; the range is 0 to 70, and higher scores denote worse performance. The MMSE evaluates cognition; the range is 0 to 30, and lower scores denote worse performance.
CLINICAL/FUNCTIONAL MEASURES
The NPI1 assesses neuropsychiatric features of AD on the basis of an interview with a study partner, evaluating the frequency and severity of 12 neuropsychiatric features during the preceding 4 weeks. Frequency assessments range from 1 to 4, and severity assessments range from 1 to 3; the score is the product of severity and frequency.
The Cohen-Mansfield agitation inventory7 rates the frequencies of agitation during the preceding 2 weeks on a 7-point scale from 0 to 6. The ADCS–Activities of Daily Living31 assesses functional performance during the preceding 4 weeks; lower scores denote worse performance. The Clinical Dementia Rating32 is a global measure of severity of dementia, used either as a summary score called the sum of boxes or as a global rating ranging from 0 to 3. The ADCS-CGIC33 provides a categorical measure of efficacy. A physician rates the participant on a 7-point scale; higher scores denote worsening. The Quality of Life–AD34 scale provides a 13-item appraisal of quality of life; lower scores denote worsening.
VOLUMETRIC MAGNETIC RESONANCE IMAGING
A subset of participants had magnetic resonance imaging (MRI) scans at baseline and 12 months to explore the effects of valproate vs placebo on whole-brain volume, ventricular volume, and hippocampal volume. Nineteen of 46 clinical sites participated in the MRI substudy; 94 participants completed both baseline and 12-month scans. Semiautomated segmentation was used to extract bilateral hippocampal volumes (Medtronic Surgical Navigation Technologies, a division of Medtronic, Inc, Louisville, Colorado). Longitudinal change in whole-brain volume and ventricular size was assessed using a boundary shift integral technique.35
SAMPLE SIZE
We assumed incidence rates for agitation or psychosis of 25% in year 1 and 50% in year 2; a 33% reduction was deemed clinically relevant and realistic. We predicted that rates of dropout, mortality, and unavailability for follow-up would be similar to those of the 2-year ADCS selegiline/vitamin E study conducted in similar outpatients, in which the dropout rate was 6% for the primary outcome measure, obtainable by telephone.36 Assuming 20% unavailability for follow-up and an α (type I error) of .05, a statistical power of 84% could be achieved with 300 subjects using a survival analysis calculation based on the log rank statistic.37
STATISTICAL ANALYSIS
The primary analysis was a survival analysis using the Cox proportional hazards model, comparing time to end point in the drug and placebo groups and adjusting for potential confounding variables: age, sex, and baseline MMSE, total NPI, Clinical Dementia Rating sum of boxes, total Cohen-Mansfield agitation inventory, and ADCS–Activities of Daily Living. The assessment consisted of a univariate 2-sample test comparing baseline means between treatment groups and a bivariate measure of association between the baseline means and the time to end point. If for any variable the first test, assessing the equivalence of the baseline distributions, was significant at α=.10, and the second test, measuring association with response, was significant at α=.15, then the variable was included as a covariate in the Cox proportional hazards model; the same approach was used to select covariates for generalized estimating equation (GEE) analysis and analysis of covariance. The primary analysis was a modified intent-to-treat analysis including all participants with at least 1 postbaseline assessment. Kaplan-Meier curves were used to estimate and to examine survival rate of conversion by treatment.
Secondary variables were changes in total NPI score, Cohen-Mansfield agitation inventory total score, AD Assessment Scale–cognitive subscale performance, ADCS–Activities of Daily Living scale score, Clinical Dementia Rating sum of boxes, ADCSCGIC score, MMSE score, and Quality of Life–AD score. Emergence of agitation and/or psychosis during the end of study wash-out was analyzed using NPI and Cohen-Mansfield agitation inventory data. Drug-placebo differences in measures of brain volume by MRI were assessed. Continuous measures were analyzed using analysis of covariance and the GEE model, time-oriented variables with Cox proportional hazards regression, and binary incidence values with logistic regression. Changes in scales based on ordinal data, including the global Clinical Dementia Rating and ADCS-CGIC, were analyzed using ordinal logistic regression. Paired comparisons using t tests, analysis of covariance, and GEE were conducted in an exploratory fashion, without adjustment for multiple comparisons, to explore group differences at individual time points.
RESULTS
PARTICIPANTS
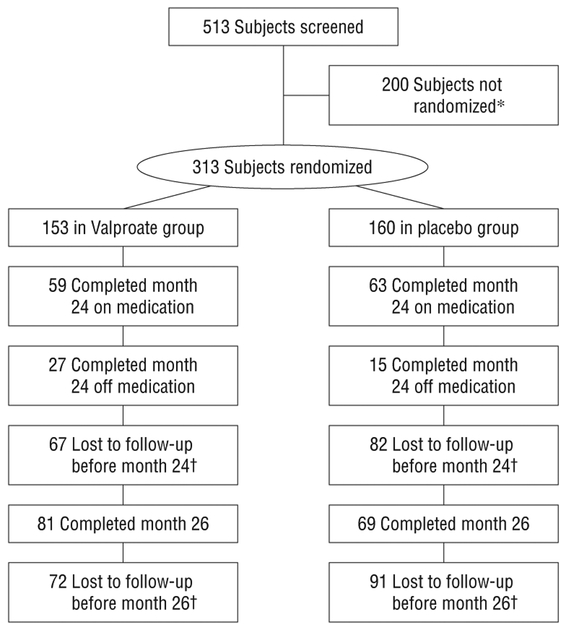
Five hundred thirteen patients were screened, and 313 were randomized to treatment (Figure 1). In the valproate group, 61.4% discontinued treatment prematurely, and in the placebo group, 60.6% did so; reasons are shown in Figure 1. Demographic and clinical characteristics are shown in Table 1. Use of medications of interest at baseline in placebo and valproate groups, respectively, were cholinesterase inhibitors (91.3% vs 94.1%), memantine (67.5% vs 62.1%), antidepressants (38.1% vs 36.6%), and antianxiety medications (0.6% vs 2.0%). Use of these medications did not differ between the 2 groups during the trial.
Figure 1.
Disposition of subjects. *Reasons for screen failures were as follows. Cognitive screen (Mini-Mental State Examination) out of range: 110; behavioral screen (Neuropsychiatric Inventory) out of range: 49; withdrawn consent/miscellaneous: 41; medically unstable: 16; excluded medication: 15; abnormal laboratory test result: 13; excluded comorbid diagnosis: 6; age out of range: 5. †Reasons for treatment discontinuation (as indicated by study site personnel) were as follows (number receiving placebo, number receiving valproate, respectively): lost to follow-up/death: 17, 13; concern about placebo condition: 8, 4; protocol violation: 2, 0; concern about length of protocol: 4, 4; concern about potential adverse effects of study medication: 12, 25; end point reached: 23, 23; investigator judgment: 8, 6; frequency of assessments: 3, 4; study partner unwilling or unable to participate: 12, 19; concern about safety risk: 4, 7; nonadherence: 7, 3; participant chose alternative treatment: 9, 4; other: 28, 30. (Note: a subject might have >1 reason for discontinuation.)
Table 1.
Baseline Demographic and Clinical Characteristics
| Characteristic | Placebo(n=160) | Valproate(n = 153) |
|---|---|---|
| Age, mean (SD), y | 76.6 (7.4) | 74.9 (8.2) |
| Years of education, mean (SD) | 13.6 (3.5) | 14.0 (3.0) |
| Years since AD onset, mean (SD) | 5.0 (2.5) | 4.6 (2.4) |
| Female sex, No. (%) | 101 (63.1) | 83 (54.2) |
| Race, No. (%) | ||
| Unknown | 1 (0.6) | 0 |
| Asian | 3 (1.9) | 2 (1.3) |
| Black | 8 (5.0) | 8 (5.2) |
| White | 148 (92.5) | 141 (92.2) |
| >1 Race | 0 | 2 (1.3) |
| Apolipoprotein E4, No. (%) | ||
| No | 39/136 (28.7) | 40/132 (30.3) |
| Yes | 97/136 (71.3) | 92/132 (69.7) |
Because the incidence of end points was lower than anticipated, the Data and Safety Monitoring Board performed an unplanned futility analysis in December 2007 and recommended that the trial be continued as planned.
TREATMENT
Overall adherence averaged more than 86.0%. The mean (SD) serum valproate level at month 12 was 42.7 (20.7) μg/mL; the mean modal dose was 250 mg/d.
EFFICACY
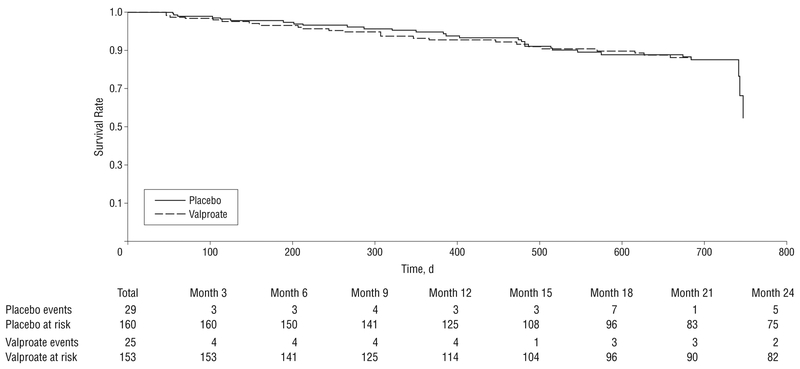
Time to reach end point did not differ by treatment arm on the basis of the Cox proportional hazards model (Figure 2) (hazard ratio, 0.96; _P_=.88) or by Kaplan-Meier curves. Twenty-nine participants receiving placebo reached end point compared with 25 receiving valproate. Table 2 summarizes mean scores over time for all secondary outcomes, showing absence of drug-placebo differences in other behavioral measures as well as cognitive, functional, and global outcomes using both GEE analysis and analysis of covariance, except for the sedation scale (see the Safety and Tolerability subsection).
Figure 2.
Survival to end point using Cox proportional hazards model. The hazard ratio was 0.96 (_P_=.88).
Table 2.
Distribution of Outcome Measures Over Timea
| Outcome | Baseline | 6 Months | 12 Months | 18 Months | 24 Months | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PLA | VAL | PLA | VAL | PLA | VAL | PLA | VAL | PLA | VAL | |
| NPI total score | 3.1 (2.6) | 2.6 (2.6) | 6.4 (7.7) | 6.6 (8.0) | 6.2 (7.9) | 5.9(7.6) | 8.8(10.1) | 7.1 (8.7) | 8.2 (9.8) | 8.3(10.8) |
| Delusions, NPI | ||||||||||
| No. of patients | 156 | 149 | 135 | 132 | 128 | 119 | 93 | 99 | 78 | 86 |
| Score | 0.032 (.18) | 0.060 (.24) | 0.447 (1.6) | 0.347 (1.0) | 0.276 (1.0) | 0.297 (.91) | 0.663 (1.7) | 0.398 (1.2) | 0.84 (2.1) | 0.593 (1.9) |
| Agitation, NPI | ||||||||||
| No. of patients | 156 | 149 | 132 | 124 | 127 | 118 | 92 | 98 | 75 | 86 |
| Score | 0.14 (.35) | 0.11 (.31) | 0.567 (1.3) | 0.411 (.98) | 0.59 1(1.6) | 0.37 3(1.0) | 0.815 (1.8) | 0.5 (1.5) | 0.88 (2.1) | 0.791 (2.0) |
| Hallucinations, NPI | ||||||||||
| No. of patients | 156 | 149 | 132 | 124 | 127 | 118 | 92 | 98 | 75 | 86 |
| Score | 0.02 (.14) | .007 (.08) | 0.091 (.47) | 0.234 (1.1) | 0.094 (0.57) | 0.144 (0.83) | 0.272 (1.08) | 0.276 (1.35) | 0.16 (.84) | 0.198 (1.0) |
| CMAI total | ||||||||||
| No. of patients | 156 | 149 | 132 | 124 | 127 | 118 | 92 | 98 | 75 | 86 |
| Score | 11.9 (10.4) | 10.9 (8.9) | 12.5 (12.2) | 11.6 (11.9) | 13.7 (12.5) | 12.0 (12.1) | 13.1 (11.4) | 11.5 (10.5) | 12.1 (10.9) | 10.6 (11.6) |
| ADAS-cog total | ||||||||||
| No. of patients | 154 | 148 | 135 | 131 | 117 | 111 | 91 | 98 | 83 | 91 |
| Score | 30.1 (9.8) | 29.4 (8.9) | 33.3 (11.3) | 33.9 (10.2) | 36.9 (12.9) | 37.9 (12.2) | 40.1 (13.0) | 39.5 (13.3) | 41.9 (14.4) | 42.3 (14.3) |
| MMSE total | ||||||||||
| No. of patients | 152 | 148 | 133 | 137 | 1117 | 112 | 89 | 96 | 76 | 86 |
| Score | 16.9 (2.9) | 16.9 (3.0) | 15.4 (5.) | 15.1 (4.6) | 14.3 (5.8) | 13.7 (5.2) | 12.8 (6.1) | 12.7 (5.2) | 11.7 (5.9) | 12.1 (6.0) |
| ADCS-ADL total | ||||||||||
| No. of patients | 156 | 149 | 140 | 137 | 118 | 111 | 90 | 94 | 76 | 82 |
| Score | 54.9 (13.0) | 56.9 (11.8 | 50.8 (14.2) | 50.2 (15.6) | 46.3 (16.3) | 43.4 (18.5) | 43.2 (16.4) | 38.5 (18.2) | 41 (14.81) | 35.1 (20.3) |
| CDR-SOB | ||||||||||
| No. of patients | 156 | 149 | 139 | 140 | 120 | 114 | 93 | 100 | 78 | 88 |
| Score | 7.5 (3.1) | 7.1 (2.7) | 8.7 (3.6) | 8.6 (3.1) | 9.9 (3.9) | 9.9 (3.7) | 10.8 (4.0) | 11.2 (4.0) | 11.5 (3.7) | 12 (4.1) |
| ADCS-CGIC | ||||||||||
| No. of patients | 156 | 149 | 138 | 137 | 119 | 111 | 90 | 96 | 74 | 85 |
| Score | 5.0 (0.9) | 5.1 (1.0) | 5.5 (1.1) | 5.7 (0.9) | ||||||
| Sedation scoreb | ||||||||||
| No. of patients | 110 | 107 | 78 | 86 | ||||||
| Score | 3.2 (2.7) | 3.3 (2.6) | 3.2 (3.2) | 3.8 (2.2) | 3.9 (3.3) | 4.5 (3.3) | 3.3 (3.3) | 4.9 (3.2) | 3.4 (3.1) | 4.9 (3.5) |
| Total No. | 156 | 149 | 136 | 131 | 116 | 110 | 93 | 99 | 78 | 86 |
There were drug-placebo differences favoring placebo at several time points, on the basis of analyses of change scores not adjusted for multiple comparisons. Specifically, mean (SD) changes on placebo vs drug (by t test analyses) were as follows: AD Assessment Scale–cognitive subscale at month 12 was 6.8 (7.8) vs 9.4 (8.2) (_P_=.01); the ADCS–Activities of Daily Living at month 6 was −4.5 (7.7) vs −7 (9.5) (_P_=.02), at month 12 was −10 (10.6) vs −14.4 (14.3) (_P_=.01), and at month 18 was −14.4 (12.1) vs −19.3 (15.6) (_P_=.02) (by GEE analysis); and the ADCS-CGIC score at month 12 was 5 (0.9) on placebo vs 5.1 (1.0) on drug (P<.05). Alzheimer Disease Assessment Scale–cognitive subscale scores were further analyzed by analysis of covariance with baseline score and age as covariates (because they were not balanced across groups and were associated with the outcome according to the criteria described previously); the difference in change scores at 12 months favoring placebo remained significant (_P_=.005). There was no difference between groups in change from baseline to month 24 in the total NPI and Cohen-Mansfield agitation inventory scores or the rate of nursing home placement.
SAFETY AND TOLERABILITY
One hundred fifty-one participants (94.4%) receiving placebo experienced at least 1 adverse event vs 145 (94.8%) receiving valproate (Table 3). Discontinuations due to adverse events occurred in 12 participants (7.5%) receiving placebo vs 25 (16.3%) receiving valproate. The adverse events more likely to occur with valproate included somnolence, gait disturbance, tremor, diarrhea, constipation, weakness, asthenia, and dyspnea; those more likely with placebo included agitation, anxiety, delusions, dizziness, hyperhydrosis, and rash. There were 105 (66.0%) serious adverse events in the placebo group vs 90 (57.0%) in the valproate group, including 12 deaths in the placebo group (7.5%) vs 9 (5.9%) in the valproate group; none were judged to be related to treatment. Sedation scores increased more over time for those receiving valproate vs placebo (treatment × time interaction on GEE modeling, _P_=.02) (Table 3).
Table 3.
Adverse Events Reported in 5% or More of Either Treatment Group
| Adverse Event | No. (%) of Patients | ||
|---|---|---|---|
| Placebo(n=160) | Valproate(n=153) | Total(N=313) | |
| Abdominal discomfort | 13 (8.1) | 16 (10.5) | 29 (9.3) |
| Agitation | 56 (35.0) | 43 (28.1) | 99 (31.6) |
| Anxiety | 38 (23.8) | 24 (15.7) | 62 (19.8) |
| Apathy | 8 (5.0) | 9 (5.9) | 17 (5.4) |
| Arthralgia | 12 (7.5) | 21 (13.7) | 33 (10.5) |
| Asthenia | 35 (21.9) | 48 (31.4) | 83 (26.5) |
| Back pain | 19 (11.9) | 21 (13.7) | 40 (12.8) |
| Chest pain | 9 (5.6) | 10 (6.5) | 19 (6.1) |
| Confusional state | 79 (49.4) | 78 (51.0) | 157 (50.2) |
| Constipation | 13 (8.1) | 26 (17.0) | 39 (12.5) |
| Cough | 18 (11.3) | 21 (13.7) | 39 (12.5) |
| Crying | 22 (13.8) | 23 (15.0) | 45 (14.4) |
| Decreased appetite | 8 (5.0) | 8 (5.2) | 16 (5.1) |
| Delusion | 14 (8.8) | 10 (6.5) | 24 (7.7) |
| Depressed mood | 22 (13.8) | 34 (22.2) | 56 (17.9) |
| Depression | 10 (6.3) | 18 (11.8) | 28 (8.9) |
| Diarrhea | 19 (11.9) | 38 (24.8) | 57 (18.2) |
| Disturbance in attention | 39 (24.4) | 40 (26.1) | 79 (25.2) |
| Dizziness | 31 (19.4) | 25 (16.3) | 56 (17.9) |
| Dry mouth | 15 (9.4) | 16 (10.5) | 31 (9.9) |
| Dyspnea | 12 (7.5) | 20 (13.1) | 32 (10.2) |
| Fecal incontinence | 5 (3.1) | 8 (5.2) | 13 (4.2) |
| Fall | 51 (31.9) | 60 (39.2) | 111 (35.5) |
| Gait disturbance | 30 (18.8) | 51 (33.3) | 81 (25.9) |
| Hallucination | 5 (3.1) | 10 (6.5) | 15 (4.8) |
| Headache | 13 (8.1) | 16 (10.5) | 29 (9.3) |
| Hyperhidrosis | 10 (6.3) | 4 (2.6) | 14 (4.5) |
| Insomnia | 16 (10.0) | 20 (13.1) | 36 (11.5) |
| Joint swelling | 10 (6.3) | 14 (9.2) | 24 (7.7) |
| Muscular weakness | 14 (8.8) | 29 (19.0) | 43 (13.7) |
| Myalgia | 11 (6.9) | 11 (7.2) | 22 (7.0) |
| Naso pharyngitis | 10 (6.3) | 11 (7.2) | 21 (6.7) |
| Nausea | 10 (6.3) | 14 (9.2) | 24 (7.7) |
| Edema peripheral | 7 (4.4) | 10 (6.5) | 17 (5.4) |
| Pneumonia | 9 (5.6) | 7 (4.6) | 16 (5.1) |
| Pollakiuria | 17 (10.6) | 23 (15.0) | 40 (12.8) |
| Rash | 15 (9.4) | 7 (4.6) | 22 (7.0) |
| Restlessness | 31 (19.4) | 28 (18.3) | 59 (18.8) |
| Somnolence | 46 (28.8) | 65 (42.5) | 111 (35.5) |
| Syncope | 7 (4.4) | 8 (5.2) | 15 (4.8) |
| Tremor | 21 (13.1) | 44 (28.8) | 65 (20.8) |
| Urinary incontinence | 15 (9.4) | 16 (10.5) | 31 (9.9) |
| Urinary tract infection | 19 (11.9) | 19 (12.4) | 38 (12.1) |
| Vision blurred | 8 (5.0) | 10 (6.5) | 18 (5.8) |
| Vomiting | 7 (4.4) | 9 (5.9) | 16 (5.1) |
| Weight decreased | 11 (6.9) | 11 (7.2) | 22 (7.0) |
Vital signs and laboratory parameters did not differ significantly between groups at baseline. Participants receiving valproate experienced mild decreases in values for neutrophils, platelets, total and direct bilirubin, and albumin, along with slight increases in values for alanine aminotransferase and aspartate aminotransferase.
VOLUMETRIC MRI DATA
Eighty-eight individuals had MRI scans that passed quality control for hippocampal measures at both time points (45 taking placebo and 43 taking valproate), 66 for whole-brain measures (35 taking placeboand 31 taking valproate), and 71 for ventricular volumes (38 taking placebo-and 33 taking valproate). The valproate group showed greater mean (SD) rates of volume loss in hippocampal (−10.9%[7.3%] on the left and −12.4%[8.8%] on the right vs −5.6%[7.9%] and −6.3% [8.5%], respectively) and whole-brain volume (−3.5%[1.4%] vs −1.4%[1.1%]), accompanied by greater rates of ventricular expansion over 12 months (+24.5%[13.4%] vs +9.9%[5.7%]) (P<.001 for all comparisons). Regression models of clinical measures over 24 months revealed no group differences. However, in this MRI subset, mean (SD) MMSE scores showed a more rapid decline in the valproate group at month 12 (placebo=−2.0[4.3] and valproate=−3.9[4.0]; _P_=.04).
COMMENT
We undertook this trial to attempt secondary prevention of psychiatric signs and symptoms in individuals with AD who had not yet experienced agitation or psychosis. We found no treatment effect on time to end point, on any secondary behavioral measures, or on global clinical status. On the basis of laboratory evidence of possible neuroprotective potential, the trial was also designed to examine the effect of valproate on clinical measures of progression of dementia; no benefit was seen. Post hoc comparisons suggested possible worsening of some measures of cognition and of function at several time points. Tolerability and adverse events were consistent with prior reports of valproate use in this population and with prescribing information. The adverse event burden suggests that the maximally tolerated valproate dose was achieved in these elderly patients. Adherence was adequate, although blood levels of valproate achieved were relatively low.
We predicted an incidence of agitation or psychosis of approximately 50% by month 24 but observed an incidence of approximately 17%. We assumed a total treatment discontinuation rate of 36% and observed an actual rate of 60%. Although our assumptions proved incorrect, there was no evidence of the anticipated beneficial effects of valproate. One reason for this may be that NPI scores at baseline were 25% to 30% of those in recent clinical trials in outpatients with mild to moderate AD.38 The milder behavioral symptoms may have been due to selection and referral bias primarily from neurology research clinics, inclusion criteria that precluded those most likely to develop symptoms, and the fact that clinical trial participants tend to be healthy and well educated. Furthermore, the selection criteria may have excluded a proportion of patients who would have already become agitated in their mild to moderate stages of dementia, possibly accounting for the low incidence. The higher-than-expected discontinuation rate may have related to adverse events, intolerability of the medication over a long period, trial duration, availability of competing studies, and perceived lack of benefit. A recent 18-month trial of an experimental therapy with a very low adverse effect rate showed 40% discontinuation,38 consistent with our observations.
The blood levels of valproate may have been insufficient to engage the molecular targets of interest, especially glycogen synthase kinase-3 β, thus explaining the negative results. During the trial, new reports suggested that, in addition to laboratory evidence of engagement with the tau protein pathway, valproate may ameliorate amyloid dysregulation.39,40 This could be the case at higher concentrations than we achieved; however, higher doses would not have been tolerated in this sample.
Valproate appeared to reduce brain volumes after 12 months compared with placebo in these individuals. This result was not associated with cognitive or functional decline using our main clinical outcomes, although the MMSE data suggest greater cognitive decline in the valproate group. The finding is open to interpretation; however, there are reports of reversible brain atrophy and cognitive impairment in children and young adults,41–44 and the drug prescribing information states: “Reports have noted reversible cerebral atrophy and dementia associated with valproate therapy.” These reported effects of valproate on brain volumes of individuals with AD are not well understood; it is unclear if they may be due to direct drug effects, such as osmotic shifts or metabolic toxicity,41–45 or influences on AD pathology, and it is unknown whether these effects are reversible or clinically relevant.
The results of this trial, along with inconclusive results of preliminary trials as well as subsequent negative findings from a large trial,46 should discourage prophylactic or symptomatic use of valproate in dementia. Given the public health significance of the behavioral features of dementia and the limited safety and efficacy of available psychotropic agents, it is still appropriate to pursue the goal of secondary prevention of agitation and psychosis. Other agents may merit investigation with this type of trial design.
Acknowledgments
Clinical Monitors: Karen Croot, BA, Viviana Messick, BS, Alan Pamoleras, BA, Rebecca Ryan-Jones, PhD (University of California, San Diego); Gina Garcia-Camilo, MD, Mario Schittini, MD, MPH, Amer Malik, MD (Mount Sinai School of Medicine, Bronx, New York; Kris Gravanda Brugger, BA, Pamela A. Saunders, PhD (Georgetown University, Washington, DC); and Janet Kastelan, MA (New York University, New York).
Financial Disclosure: During the trial, Dr Tariot served as a consultant to Abbott Laboratories, and the Banner Alzheimer’s Institute received support as an investigative site in a multicenter Abbott-sponsored trial of a different antidementia agent. Dr Tariot also received grant or research support from AstraZeneca, Avid, Baxter, Elan Pharmaceuticals, GlaxoSmithKline, Johnson & Johnson, Eli Lilly, Medivation, Merck, Myriad, Pfizer, Takeda, and Wyeth; served as a consultant for or received consulting fees from AC Immune, Acadia, Adamas, Allergan, AstraZeneca, Avid, Baxter, Bristol-Myers Squibb, Eisai, Elan, Eli Lilly, Epix, Forest, GlaxoSmithKline, Medavante, Medivation, Merck, Myriad, Pfizer, Roche, sanofi-aventis, Schering-Plough, Toyama, Transition Therapeutics, Worldwide Clinical Trials, and Wyeth; and received stock options from Adamas and Medavante. Dr Schneider served as an editor on the Cochrane Collaborations Dementia and Cognitive Improvement Group, which oversees systematic reviews of drugs for cognitive impairment and dementia; received a grant from the Alzheimer’s Association for a registry for dementia and cognitive impairment trials; received grant or research support from AstraZeneca, Baxter, Elan Pharmaceuticals, Forest Laboratories, Johnson & Johnson, Eli Lilly, Myriad, Novartis, Pfizer, Takeda, and Wyeth; and served as a consultant for or received consulting fees from Abbott Laboratories, AC Immune, Accera, Allergan, Allon, Alzheimer Drug Discovery Foundation, AstraZeneca, Bristol-Myers Squibb, Elan, Eli Lilly, Exonhit, Forest, GlaxoSmithKline, Institute IPSEN, Johnson & Johnson, Lundbeck, Myriad, Medavante, Medivation, Merck, Novartis, Pfizer, Roche, sanofi-aventis, Servier, Schering-Plough, Schwabe, Teva, Toyama, Transition Therapeutics, Voyager, and Wyeth. Dr Cummings served as a consultant to Abbott Laboratories, Acadia, Accera, ADAMAS, Astellas, Avanir, Bristol-Myers Squibb, CoMentis, Eisai, Elan, EnVivo, Forest, GlaxoSmithKline, Janssen, Lilly, Lundbeck, Medivation, Merck, Merz, Myriad, Neuren, Neurokos, Novartis, Noven, Orion Pharmaceuticals, Pfizer, Prana, reMYND, Schering-Plough, Signum Bioscience, Sonexa, Takeda, Toyama, and Wyeth; owns stock in ADAMAS, Prana, Sonexa, and Neurokos; owns the copyright of the NPI; and has served as an expert consultant on risperidone and ropinerol. Dr Loy received an investigator-initiated grant from Abbott Laboratories during the trial. Dr Ismail served on speakers’ bureaus for Novartis, Pfizer, Merck, and Forest Pharmaceuticals, and received research support from the National Institute on Aging (NIA), National Institute of Mental Health, Bristol-Myers Squibb, Pfizer, and Merck. Dr Porsteinsson served on speakers’ bureaus for Abbott, AstraZeneca, Eisai, Forest, Janssen, Ortho-McNeil, and Pfizer; consulted to Abbott, Eisai, Elan, Janssen, Medivation, Pfizer, Takeda, Transition Therapeutics, and Toyama; and received research support from Abbott, AstraZeneca, Baxter, BMS, Eisai, Elan, Eli Lilly, Forest, Janssen, Medivation, Merck, Mitsubishi, Myriad Neurosciences, Neurochem, Ono Pharma, Pfizer, Sanofi, Takeda, Toyama, and Wyeth. Dr Weiner served as an editor for Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association; received research support from Merck and Avid; served as a consultant to Bayer Schering Pharma, Lilly, CoMentis, Neurochem, Sam and Rose Stein Institute for Research on Aging–University of California, San Diego, Eisai, Avid, Aegis, Genentech, Allergan, Bristol-Myers Squibb, Forest, Pfizer, McKinsey, Mitsubishi, Novartis, the National Institutes of Health (NIH), Department of Defense, and the Veterans Administration; received travel support from several nonprofit organizations; and received stock options from Synarc and Elan. Dr Jack received research support from the NIH, Pfizer, and Baxter; and consulted to GE, Lean, and Lilly. Dr Aisen served on a scientific advisory board for NeuroPhage; served as a consultant to Elan Corporation, Wyeth, Eisai Inc., Schering-Plough, Bristol-Myers Squibb, Eli Lilly, NeuroPhage, Merck, Roche, Amgen, Genentech, Abbott, Pfizer, Novartis, Bayer, Astellas, Dainippon, Biomarin, Solvay, Otsuka, Daiichi, and Medivation; received research support from Pfizer, Baxter, and the NIH (grants NIA U01-AG10483 [principal investigator], NIA U01-AG024904 [coordinating center director], NIA R01-AG030048 [principal investigator], and R01-AG16381 [Co-investigator]); and received stock options from Medivation and NeuroPhage.
Funding/Support: The NIA supported this trial (U01 AG 10483). Additional support was provided by a research grant and material support from Abbott Laboratories.
Role of the Sponsors: The study design was approved by an oversight committee of the NIA. Representatives of the NIA participated in steering committee meetings of the ADCS during the trial. The NIA was not otherwise involved in the design and conduct of the trial; in the collection, management, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript. Abbott Laboratories did not participate in the design of the study, analysis or interpretation of the data, or preparation of the manuscript.
Additional Contributions: We acknowledge the valuable contribution of Husseini Manji, MD.
REFERENCES
- 1.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. [DOI] [PubMed] [Google Scholar]
- 2.Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JC. Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry. 2000;157(5):708–714. [DOI] [PubMed] [Google Scholar]
- 3.Paulsen JS, Salmon DP, Thal LJ, Romero R, Weisstein-Jenkins C, Galasko D, Hofstetter CR, Thomas R, Grant I, Jeste DV. Incidence of and risk factors for hallucinations and delusions in patients with probable Alzheimer’s disease. Neurology. 2000;54:1965–1971. [DOI] [PubMed] [Google Scholar]
- 4.Cohen-Mansfield J, Werner P. Longitudinal changes in behavioral problems in old age: a study in an adult day care population. J Gerontol A Biol Sci Med Sci. 1998;53(1):M65–M71. [DOI] [PubMed] [Google Scholar]
- 5.Rabins PV, Blacker D, Rovner BW, Rummans T, Schneider LS, Tariot PN, Blass DM, McIntyre JS, Charles SC, Anzia DJ, Cook IA, Finnerty MT, Johnson BR, Nininger JE, Schneidman B, Summergrad P, Woods SM, Berger J, Cross CD, Brandt HA, Margolis PM, Shemo JP, Blinder BJ, Duncan DL, Barnovitz MA, Carino AJ, Freyberg ZZ, Gray SH, Tonnu T, Kunkle R, Albert AB, Craig TJ, Regier DA, Fochtmann LJ; American Psychiatric Association Practice Guidelines Work Group on Alzheimer’s Disease and Other Dementias. Practice guidelines for the treatment of patients with dementia and other dementias, 2nd ed. Am J Psychiatry. 2007;164(12)(suppl):5–56. [PubMed] [Google Scholar]
- 6.Tariot PN, Mack JL, Patterson MB, Edland SD, Weiner MF, Fillenbaum G, Blazina L, Teri L, Rubin E, Mortimer JA, Stern Y; the CERAD Behavioral Pathology Committee. The Behavior Rating Scale for Dementia of the Consortium to Establish a Registry for Alzheimer’s Disease. Am J Psychiatry. 1995;152:1349–1357. [DOI] [PubMed] [Google Scholar]
- 7.Cohen-Mansfield J, Billig N. Agitated behaviors in the elderly, I: a conceptual review. J Am Geriatr Soc. 1986;34(10):711–721. [DOI] [PubMed] [Google Scholar]
- 8.Levy ML, Cummings JL, Fairbanks LA, Bravi D, Calvani M, Carta A. Longitudinal assessment of symptoms of depression, agitation, and psychosis in 181 patients with Alzheimer’s disease. Am J Psychiatry. 1996;153(11):1438–1443. [DOI] [PubMed] [Google Scholar]
- 9.Morris JC, Cyrus PA, Orazem J, Mas J, Bieber F, Ruzicka BB, Gulanski B. Metrifonate benefits cognitive, behavioral, and global function in patients with Alzheimer’s disease. Neurology. 1998;50(5):1222–1230. [DOI] [PubMed] [Google Scholar]
- 10.Raskind MA, Cyrus PA, Ruzicka BB, Gulanski BI; Metrifonate Study Group. The effects of metrifonate on the cognitive, behavioral, and functional performance of Alzheimer’s disease patients. J Clin Psychiatry. 1999;60(5):318–325. [DOI] [PubMed] [Google Scholar]
- 11.Hope T, Keene J, Fairburn CG, Jacoby R, McShane R. Natural history of behavioural changes and psychiatric symptoms in Alzheimer’s disease: a longitudinal study. Br J Psychiatry. 1999;174:39–44. [DOI] [PubMed] [Google Scholar]
- 12.McShane R, Keene J, Fairburn C, Jacoby R, Hope T. Psychiatric symptoms in patients with dementia predict the later development of behavioural abnormalities. Psychol Med. 1998;28(5):1119–1127. [DOI] [PubMed] [Google Scholar]
- 13.Devanand DP, Jacobs DM, Tang MX, Del Castillo-Castaneda C, Sano M, Marder K, Bell K, Bylsma FW, Brandt J, Albert M, Stern Y. The course of psychopatho-logic features in mild to moderate Alzheimer disease. Arch Gen Psychiatry. 1997; 54(3):257–263. [DOI] [PubMed] [Google Scholar]
- 14.McCarty HJ, Roth DL, Goode KT, Owen JE, Harrell L, Donovan K, Haley WE. Longitudinal course of behavioral problems during Alzheimer’s disease: linear versus curvilinear patterns of decline. J Gerontol A Biol Sci Med Sci. 2000; 55(4):M200–M206. [DOI] [PubMed] [Google Scholar]
- 15.Aarsland D, Cummings JL, Yenner G, Miller B. Relationship of aggressive behavior to other neuropsychiatric symptoms in patients with Alzheimer’s disease. Am J Psychiatry. 1996;153(2):243–247. [DOI] [PubMed] [Google Scholar]
- 16.Gilley DW, Wilson RS, Beckett LA, Evans DA. Psychotic symptoms and physically aggressive behavior in Alzheimer’s disease. J Am Geriatr Soc. 1997;45 (9):1074–1079. [DOI] [PubMed] [Google Scholar]
- 17.Jeste DV, Blazer D, Casey D, Meeks T, Salzman C, Schneider L, Tariot PN, Yaffe K. ACNP white paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porsteinsson AP, Tariot PN, Erb R, Cox C, Smith E, Jakimovich L, Noviasky J, Kowalski N, Holt CJ, Irvine C. Placebo-controlled study of divalproex sodium for agitation in dementia. Am J Geriatr Psychiatry. 2001;9(1):58–66. [PubMed] [Google Scholar]
- 19.Tariot PN, Schneider L, Mintzer J, Cutler A, Cunningham M, Thomas J, Somerville K. Safety and tolerability of divalproex sodium for the treatment of signs and symptoms of mania in elderly patients with dementia: results of a double-blind, placebo-controlled trial. Curr Ther Res Clin Exp. 2001;62(1):51–67. [Google Scholar]
- 20.Mark RJ, Ashford JW, Goodman Y, Mattson MP. Anticonvulsants attenuate amyloid [H9252]-peptide neurotoxicity, Ca2+ deregulation, and cytoskeletal pathology. Neurobiol Aging. 1995;16(2):187–198. [DOI] [PubMed] [Google Scholar]
- 21.Chen G, Zeng WZ, Yuan PX, Huang LD, Jiang YM, Zhao ZH, Manji HK. The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS. J Neurochem. 1999;72(2):879–882. [DOI] [PubMed] [Google Scholar]
- 22.Chen G, Huang LD, Jiang YM, Manji HK. The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. J Neurochem. 1999;72(3): 1327–1330. [DOI] [PubMed] [Google Scholar]
- 23.Imahori K, Uchida T. Physiology and pathology of tau protein kinases in relation to Alzheimer’s disease. J Biochem. 1997;121(2):179–188. [PubMed] [Google Scholar]
- 24.Bennett GD, Wlodarczyk B, Calvin JA, Craig JC, Finnell RH. Valproic acid–induced alterations in growth and neurotrophic factor gene expression in murine embryos [published correction appears in Reprod Toxicol. 2000;14(2)181]. Reprod Toxicol. 2000;14(1):1–11. [DOI] [PubMed] [Google Scholar]
- 25.Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001;276(39):36734–36741. [DOI] [PubMed] [Google Scholar]
- 26.Tariot PN, Loy R, Ryan JM, Porsteinsson A, Ismail S. Mood stabilizers in Alzheimer’s disease: symptomatic and neuroprotective rationales. Adv Drug Deliv Rev. 2002;54(12):1567–1577. [DOI] [PubMed] [Google Scholar]
- 27.Profenno LA, Jakimovich L, Holt C, Porsteinsson A, Tariot PN. A randomized, double-blind, placebo-controlled pilot trial of safety and tolerability of two doses of divalproex sodium in outpatients with probable Alzheimer’s disease. Curr Alzheimer Res. 2005;2(5):553–558. [DOI] [PubMed] [Google Scholar]
- 28.Ripich DN. Functional communication with AD patients: a caregiver training program. Alzheimer Dis Assoc Disord. 1994;8(suppl 3):95–109. [PubMed] [Google Scholar]
- 29.Ripich D, Ziol E, Fritsch T, Durand EJ. Training Alzheimer’s disease caregivers for successful communication. Clin Gerontol. 1999;21:37–53. [Google Scholar]
- 30.Mohs RC, Knopman D, Petersen RC, Ferris SH, Ernesto C, Grundman M, Sano M, Bieliauskas L, Geldmacher D, Clark C, Thal LJ. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer’s Disease Assessment Scale that broaden its scope: The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S13–S21. [PubMed] [Google Scholar]
- 31.Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, Ferris S. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease: the Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S33–S39. [PubMed] [Google Scholar]
- 32.Berg L Clinical Dementia Rating (CDR). Psychopharmacol Bull. 1988;24(4):637–639. [PubMed] [Google Scholar]
- 33.Schneider LS, Olin JT, Doody RS, Clark CM, Morris JC, Reisberg B, Schmitt FA, Grundman M, Thomas RG, Ferris SH. Validity and reliability of the Alzheimer’s Disease Cooperative Study–Clinical Global Impression of Change: the Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S22–S32. [DOI] [PubMed] [Google Scholar]
- 34.Logsdon RG, Gibbons LE, McCurry SM, Teri L. Quality of life in Alzheimer’s disease: patient and caregiver reports. J Ment Health Aging. 1999;5:21–32. [Google Scholar]
- 35.Gunter JL, Shiung MM, Manduca A, Jack CR Jr. Methodological considerations for measuring rates of brain atrophy. J Magn Reson Imaging. 2003;18(1): 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grndman M, Woodbury P, Growdon J, Cotman CW, Pfeiffer E, Schneider LS, Thal LJ; for the Alzheimer’s Disease Cooperative Study. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease: the Alzheimer’s Disease Cooperative Study. N Engl J Med. 1997;336(17):1216–1222. [DOI] [PubMed] [Google Scholar]
- 37.Lachin JM, Foulkes MA. Evaluation of sample size and power for analyses of survival with allowance for nonuniform patient entry, losses to follow-up, noncompliance, and stratification. Biometrics. 1986;42(3):507–519. [PubMed] [Google Scholar]
- 38.Green RC, Schneider LS, Amato DA, Beelen AP, Wilcock G, Swabb EA, Zavitz KH; Tarenflurbil Phase 3 Study Group. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA. 2009;302(23):2557–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su Y, Ryder J, Li B, Wu X, Fox N, Solenberg P, Brune K, Paul S, Zhou Y, Liu F, Ni B. Lithium, a common drug for bipolar disorder treatment, regulates amyloid-β precursor protein processing. Biochemistry. 2004;43(22):6899–6908. [DOI] [PubMed] [Google Scholar]
- 40.Qing H, He G, Ly PT, Fox CJ, Staufenbiel M, Cai F, Zhang Z, Wei S, Sun X, Chen CH, Zhou W, Wang K, Song W. Valproic acid inhibits Aβ production, neuritic plaque formation, and behavioral deficits in Alzheimer’s disease mouse models. J Exp Med. 2008;205(12):2781–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLachlan RS. Pseudoatrophy of the brain with valproic acid monotherapy. Can J Neurol Sci. 1987;14(3):294–296. [DOI] [PubMed] [Google Scholar]
- 42.Guerrini R, Belmonte A, Canapicchi R, Casalini C, Perucca E. Reversible pseudoatrophy of the brain and mental deterioration associated with valproate treatment. Epilepsia. 1998;39(1):27–32. [DOI] [PubMed] [Google Scholar]
- 43.Yamanouchi H, Ota T, Imataka G, Nakagawa E, Eguchi M. Reversible altered consciousness with brain atrophy caused by valproic acid. Pediatr Neurol. 2003; 28(5):382–384. [DOI] [PubMed] [Google Scholar]
- 44.Galimberti CA, Diegoli M, Sartori I, Uggetti C, Brega A, Tartara A, Arbustini E. Brain pseudoatrophy and mental regression on valproate and a mitochondrial DNA mutation. Neurology. 2006;67(9):1715–1717. [DOI] [PubMed] [Google Scholar]
- 45.Garcia M, Huppertz J, Ziyeh S, Buechert M, Schumacher M, Mader I. Valproate-induced changes in brain volume in patients with epilepsy: assessment with H-MRS. Epilepsia. 2009;50(3):486–492. [DOI] [PubMed] [Google Scholar]
- 46.Tariot PN, Raman R, Jakimovich L, Schneider L, Porsteinsson A, Thomas R, Mint-zer J, Brenner R, Schafer K, Thal L; Alzheimer’s Disease Cooperative Study; Valproate Nursing Home Study Group. Divalproex sodium in nursing home residents with possible or probable Alzheimer Disease complicated by agitation: a randomized, controlled trial. Am J Geriatr Psychiatry. 2005;13(11):942–949. [DOI] [PubMed] [Google Scholar]