Active immunity and T-cell populations in pigs intraperitoneally inoculated with baculovirus-expressed transmissible gastroenteritis virus structural proteins (original) (raw)
Abstract
The intraperitoneal inoculation of pigs with baculovirus-expressed transmissible gastroenteritis virus (TGEV) structural proteins (S, N, M) in conjunction with thermolabile Escherichia coli mutant toxin (LT-R192G) in incomplete Freund’s adjuvant (IFA) was tested in an attempt to elicit active immunity to TGEV in gut-associated lymphoid tissues (GALT). Four groups of 63 (1–5-week-old) suckling, TGEV-seronegative pigs were used to assess the efficacy of the recombinant protein vaccine (group 3) in comparison with sham (group 1), commercial vaccine (group 2), and virulent TGEV Miller-strain-inoculated pigs (group 4). The TGEV-specific mucosal and systemic immune responses were measured after in vivo and in vitro stimulation with TGEV-antigens. The major T-cell subset distribution was analyzed in vivo and in vitro after stimulation of mononuclear cells with TGEV (from mesenteric lymph nodes of group 3 inoculated with TGEV-recombinant proteins). Induction of active immunity was assessed by challenge of pigs with virulent TGEV at 27 days of age. Baculovirus-expressed TGEV proteins coadministered with LT-R192G in IFA induced mesenteric lymph node immune responses associated with IgA-antibodies to TGEV and partial protection against TGEV-challenge. The high titers of serum IgG- and virus-neutralizing-antibodies to TGEV in group 3 pigs most likely reflected the dose of TGEV S-protein administered. At the day of TGEV-challenge, the in vitro stimulation of mononuclear cells from the mesenteric lymph nodes of group 3 pigs with inactivated TGEV resulted in an increase in double positive (CD4+CD8+), natural killer (CD2+CD4−CD8+dim) and cytotoxic (CD2+CD4−CD8+bright) T-cell phenotypes, accompanied by increased expression of interleukin-2 receptor and a decrease of the null (CD2−CD4−CD8−/SW6+) cell phenotype.
Keywords: Porcine coronavirus, Mucosal immunity, Lymphoid cells, Flow cytometry
1. Introduction
Transmissible gastroenteritis (TGE) is a devastating viral disease of pigs under 2 weeks of age, characterized by a mortality rate of up to 100%. Transmissible gastroenteritis virus (TGEV) remains enzootic in Europe, the USA and other countries with an intensive pork industry (Wesley et al., 1997, Paton et al., 1998). There are currently no effective TGEV vaccines which emphasizes the importance of developing new prevention and control methods for this pathogen.
In other species, the intraperitoneal administration (IP) of antigens elicited peritoneal B-cells which were able to repopulate other compartments of the common mucosal immune system including the lamina propria with IgA-antibody secreting cells (Wu and Russell, 1994). In preweaning and postweaning pigs the IP immunization with an Escherichia coli killed vaccine emulsified in an equal volume of Freund’s incomplete adjuvant reduced the mortality rate among vaccinated pigs by half that of unvaccinated controls (Husband and Seaman, 1979). Protective active immunity to TGEV in lactating sows is associated with stimulation of secretory IgA (SIgA) inductive sites in the GALT and the proposed trafficking of effector IgA antibody secreting cells to the intestine and mammary glands (Saif, 1996).
In our study, we examined the ability of the three major structural TGEV proteins (S = spike glycoprotein, N = nucleocapsid phosphoprotein and M = membrane glycoprotein) previously cloned and expressed in a baculovirus expression system to induce protective active immunity in pigs against challenge with TGEV Miller-strain in conjunction with the thermolabile E. coli mutant toxin (LT-R192G). The native TGEV S-glycoprotein (220 kDa) is the major structural TGEV protein capable of induction of virus-neutralizing (VN) antibodies and is responsible for virus attachment (Laude et al., 1993). For our experimental vaccine, the baculovirus-expressed TGEV S-recombinant (R2-2) which is truncated (150 kDa) but contains the four major antigenic sites (A, B, C, D) and induces VN-antibodies was used (Shoup et al., 1997, Park et al., 1998). In addition, a baculovirus-expressed N-phosphoprotein (47 kDa) and M-glycoprotein (29–36 kDa) were included because of their involvement in T-helper cell stimulation (Anton et al., 1995) and their effects on α-interferon production (Laude et al., 1993), respectively. Although several non-toxic LT-mutants and recombinants have been prepared with partial knockout of the ADP-ribosyltransferase activity (O’Neal et al., 1998, Giuliani et al., 1998), exploration of the mucosal adjuvanticity of such enzymatically inactive LT forms has not been adequately addressed in the context of their potential to stimulate IgA responses in farm animals.
In our study, in order to increase the immunogenicity of IP-delivered TGEV protein vaccines, two adjuvants were included: LT-R192G and IFA. Qualitative characteristics of B-cell responses, including the TGEV-specific B-memory cells from mucosal and systemic lymphoid tissues were examined for pigs inoculated IP with baculovirus-expressed TGEV proteins and compared with responses in pigs given a commercial TGEV vaccine, sham inocula or virulent TGEV. Also the changes in numbers of several T-cell phenotypes were measured at 27 days of age (day of challenge) by flow cytometry (CD4+CD8+ i.e., double positive T-cells, CD2+CD4−CD8+dim i.e. NK-cells, CD2+CD4−CD8+bright i.e. cytotoxic T-cells, CD2−CD4−CD8− i.e., null-cells and CD25+ i.e., memory T-cells) after the in vivo or in vitro stimulation of mononuclear cells from mesenteric lymph nodes of pigs inoculated with TGEV recombinant proteins. The TGEV-shedding in feces, TGE clinical symptoms and the presence of SIgA were measured after challenge of all pig-groups with virulent TGEV at 27 days of age.
2. Materials and methods
2.1. Inocula
A commercial killed TGEV vaccine licensed for IP use (TG-Emune with Imugen II, lot number 54023, Oxford Veterinary Laboratories, Worthington, MN) was purchased. Genes coding for TGEV S-, N-, and M-proteins (Shoup et al., 1997, Pulford and Britton, 1991a, Baudoux et al., 1995) were cloned and expressed in a baculovirus-expression system as described (Shoup et al., 1997). The amounts of TGEV-specific proteins contained within the baculovirus protein lysates were determined by the use of continuous-elution electrophoresis (Kyd et al., 1994). Briefly, this involved applying 3.5 mg of solubilized (total) lysate protein to a manifold-tube of the BioRad Mini Prep Cell (BioRad, Hercules, CA) containing a 6–10% separating and 4% stacking polyacrylamide gel based on the size of TGEV protein to be separated (S – 6%, N and M – 10%). Protein mixtures were fractionated based on their size at constant voltage (200 V) with a vacuum pump attached to collect the individual protein-fractions. The TGEV-specificity of individual fractions was tested by Western blot (Stott, 1989) using hyperimmune serum to TGEV Miller strain. The yield of TGEV S, N and M proteins was 0.15, 0.5 and 0.4 mg/ml, respectively, of the original (7 mg/ml) protein concentration which corresponded to 2, 7 and 6% of TGEV S, N and M proteins contained within the baculovirus lysates. The dose of TGEV recombinant proteins administered per individual pig was determined based on a dose-response pilot experiment with age-matched pigs and the S-protein, as the dose eliciting the highest TGEV-seroconversion measured by a VN-test (50 μg). Because of the high amount of TGEV-proteins needed for double inoculation of 16 group 3 pigs and relatively high expression levels (2–7%), the baculovirus protein lysates were IP-administered directly without the above semipurification step. The dose of other TGEV (N, M) and wild type baculovirus (ACNPV) proteins used was the same as that for the TGEV S-protein (50 μg). Prior to IP-administration, the TGEV-specificity of all baculovirus-expressed protein lysates was tested by Western blot (Stott, 1989) using hyperimmune serum to TGEV Miller-strain. As an adjuvant, the thermolabile E. coli mutant toxin LT-R192G (which has a glycine instead of an arginine at position 192 of the protein rendering it nontoxic at adjuvant-effective doses) was used (courtesy of Dr. J.D. Clements, Tulane University). The second dose-response pilot experiment was performed with the LT-R192G adjuvant in order to assess its potential toxicity. In our suckling conventional pigs, none of the LT-R192G doses administered IP, ranging from 1–25 μg/pig, resulted in diarrhea. The adjuvant dose used in our study was 10 μg/pig. The second adjuvant used was incomplete Freund’s adjuvant (IFA, Gibco, Grand Island, NY). IFA was mixed with an equal volume of TGEV-proteins containing the LT-R192G (2.5–5 ml of total volumes).
2.2. Pig vaccinations and challenge
Four groups of TGEV-seronegative conventional (Large White Landrace) pigs (n = 63) were inoculated IP at 7–9 and 15–17 days of age as follows. Group 1 (n = 29) consisted of four subgroups of negative control pigs inoculated as follows: 11 pigs were inoculated with wild type baculovirus (ACNPV) protein (50 μg/pig) in IFA; six pigs were inoculated with sterile phosphate saline buffer (PBS, pH 7.3) in IFA; six pigs were inoculated with LT-R192G (10 μg/pig) in PBS (pH 7.3) and six pigs were not inoculated. Group 2 (n = 12) pigs were inoculated with a commercial killed TGEV-vaccine licensed for IP use, according to the manufacturer’s instructions (Oxford Veterinary Laboratories) at the above time points. Group 3 (n = 16) pigs were inoculated at both 7–9 and 15–17 days of age with TGEV S, N and M proteins (50 μg of each/pig) together with LT-R192G (10 μg/pig) in IFA. Group 4 (n = 6) pigs served as positive controls and were oronasally infected at 11 days of age with 1 × 105 plaque forming unit (PFU) of virulent TGEV Miller strain. All of the group 4 pigs become infected, exhibited severe diarrhea and shed the TGEV in feces as measured at 15 days of age. All pigs were weaned at 24 days of age and challenged oronasally with 1 × 106 PFU of virulent TGEV Miller-strain at 27 days of age (post-challenge day, PCD 0).
2.3. Clinical signs, collection of rectal swabs and TGEV-antigen detection in feces
Clinical signs of TGE (0–5 scale) including dehydration, anorexia and lethargy were recorded daily between PCD 0 and 10. Clinical scores were as follows: 5 = moribund or dead; 4 = very weak, still able to stand; 3 = marked dehydration; 2 = moderate dehydration; 1 = mild dehydration; 0 = normal. Diarrhea scores were recorded as follows: 4 = watery, 3 = semi-liquid, 2 = mucoid, 1 = pasty, 0 = normal. The value for the clinical score average was calculated as the average of the clinical and diarrhea scores for each group as described (Sestak et al., 1996). Pigs were rectally swabbed at 2-day intervals up to PCD 12 and the presence of TGEV-antigens was measured by double-antibody sandwich ELISA as described (Sestak et al., 1996). The ELISA cut-off absorbance was calculated based on the mean ELISA absorbance of a population of known negative samples plus three times its standard deviation.
2.4. Serum samples and serum antibody assays
At the time of the first (7–9 days of age) and second (15–17 days of age) IP-inoculations, at PCD 0 (27 days of age) and PCD 6–7, the pigs were bled and serum was harvested and stored as described (Sestak et al., 1996). To detect TGEV-specific IgG and IgA antibodies in the serum of inoculated pigs, a monoclonal antibody-capture ELISA was used, as described (Park et al., 1998). The ELISA cut-off absorbance was established based on a population of negative sera as the ELISA mean absorbance plus three times the standard deviation. To detect the corresponding VN-antibodies, a plaque-reduction test was performed as described (Welch and Saif, 1988). The VN titer was expressed as the reciprocal of the highest serum dilution that neutralized cytopathic effects after 48 h at 37°C.
2.5. Enzyme-linked immunospot assay (ELISPOT)
At PCD 0 and PCD 6–7, ileum lamina propria (I), mesenteric lymph nodes (MLN) and peripheral blood (PBL) lymphoid tissues were collected from 3–6 pigs of each group (except group 4 where only two pigs were available at each time point due to severe TGE prior to PCD 0 associated with 33% mortality) and the mononuclear cells (MNCs) were isolated as described (VanCott et al., 1994, Yuan et al., 1996). Briefly, ELISPOT was performed by using a coating antigen of acetone-fixed TGEV Miller-strain infected swine testis cell monolayers grown in 96-well cell culture plates. For the test, 100 μl of a MNC suspension (I, MLN, PBL) was added to each duplicate well at 3 cell concentrations (5 × 105, 5 × 104, 5 × 103 MNC/well) and plates were incubated for 6 h at 37°C in a 5% CO2 atmosphere. After incubation, MNCs were removed by washing the plates six times in 20 mM Tris–HCL (pH 8.0) containing 0.15 M NaCl and 0.1% (v/v) Tween 20. Next, 100 μl of biotinylated MAB to porcine IgA or IgG (MAB 6D11 and 3H7, respectively) (Paul et al., 1989) in concentrations of 1 and 0.4 μg/ml, respectively, were added to each well for 2 h at 20–22°C. The dark-blue spots resulting from reactions between the TGEV-antigens and the antibodies secreted by the MNC were developed, using horseradish peroxidase-conjugated streptavidin (Streptavidin POD conjugate; Boehringer Mannheim, Indianapolis, IN) and tetramethylbenzidine chromogenic substrate (TMB; Kirkegaard & Perry Laboratories, Gaithersburg, MD). Numbers of antibody secreting cells (ASC) were counted as described by VanCott et al. (1994). The ELISPOT was performed for MNCs isolated on the day of euthanasia (PCD 0 and 6–7), which reflected the in vivo immune responses, or after 4–5 days of incubation with 28 μg/ml of inactivated TGEV-Miller strain (VanCott et al., 1994) which reflected the in vitro responses of memory B-cells (MLN, PBL only). In vitro memory responses for ileal lymphoid tissues were not evaluated due to difficulties with maintenance of sterility in these cultures. The ELISPOT counts of ASC restimulated in vitro by TGEV antigens indicated the presence of TGEV-specific IgA or IgG memory B-cells in MLNs and PBLs of the vaccinated/infected pigs.
2.6. Flow cytometry (FCM)
Lymphoid cells isolated at PCD 0 from MLN of group 3 pigs (n = 4) were used for FCM analysis in order to measure T-cell phenotype ratios before (in vivo) and after in vitro stimulation of these cells with inactivated TGEV. Differences in distribution of T-cell phenotypes in vivo compared to in vitro immune responses in a single-color (CD25 and SWC6) or tri-color (CD2CD4CD8) setup were analyzed by the use of an indirect staining method. The MABs used as a primary reagent to stain the porcine lymphoid cell surface molecules were CD2, CD4, CD8, interleukin-2 receptor (IL-2-R/CD25) (VMRD, Pullman, WA: MSA4, 74-12-4, PT36B and PGBL25A, respectively) and porcine null cells (SWC6) (Serotec, Raleigh, NC: MCA1448). The secondary reagents were anti-mouse-IgG2a-Fluorescein isothiocyanate (FITC), anti-mouse-IgG2b-Tri-Color (TC), anti-mouse-IgG1-R-Phycoerytherin (PE) (Caltag, San Francisco, CA) and anti-rat-IgG-FITC (Caltag, San Francisco, CA). Isotype controls were mouse IgG2a, mouse IgG2b, mouse IgG1 and rat IgG (Caltag, San Francisco, CA). The MNCs were resuspended in buffer (PAB) composed of PBS, pH 7.3 containing 2 g/l of sodium azide and 2 g/l of bovine albumin at a concentration of 1 × 107 MNC per ml. Indirect staining for FCM was performed in U-bottom 96-well plates (Dynex, Chantilly, VA) where 100 μl of MAB diluted in PAB was applied, followed by 1 × 106 of MNC (100 μl). The MNC were incubated with MABs at 37oC for 30–60 min. After incubation with primary reagent, the MNC were centrifuged in 96-well plates at 435 × g for 5 min, supernatants were aspirated, cell pellets resuspended in 100 μl of PAB buffer and washed 2 more times to completely remove the unbound primary reagent. After three washes, the secondary, labeled reagents (100 μl per well) were added to 100 μl of MNC suspension and incubated at 37oC for 30–60 min. After staining the MNC with secondary reagents, three washes were performed as described above and MNC were transferred to the labeled 12 mm × 75 mm glass tubes (Fisher, Itasca, IL) containing 100 μl of 2% paraformaldehyde in PBS, pH 7.3, followed immediately by thorough mixing. Each tube was capped with parafilm and refrigerated in the dark, on a shaker (40 rpm/min) for up to 1 month, then tested by FCM. Each set of samples contained the unstained MNC controls, secondary antibody only-stained controls and isotype controls in order to assess the cut-off between the stained and unstained cell populations. Each FCM-measurement included approximately 100,000 events (Coulter Epics Elite; Coulter Corporation, Miami, FL).
2.7. Statistical analysis
The Mann Whitney nonparametric test was used to evaluate the differences (p < 0.05) between each group in TGEV VN-titer, TGEV IgG- and IgA-ELISA-titer at PCD 0, the ELISPOT for TGEV IgG- and IgA-ASC numbers at PCD 0 and 6–7, and in vivo versus in vitro T-cell numbers as detected in group 3 MLN tissues at PCD 0.
3. Results
3.1. Nature of IP-administered TGEV-proteins
The TGEV-specificity of the baculovirus-expressed proteins was confirmed by Western blot prior to their IP-inoculation into pigs (Fig. 1). The S, N and M-protein expression levels in baculovirus (2, 7 and 6%, respectively) were in accord with published data on the efficiency of the baculovirus expression system (Luckow and Summers, 1988).
Fig. 1.
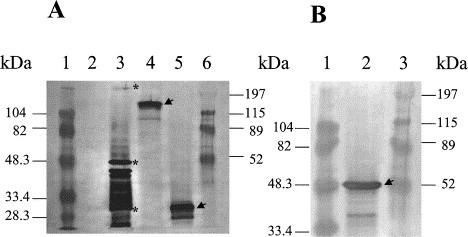
Three baculovirus-expressed TGEV major structural proteins (spike – S, nucleocapsid – N, membrane – M) were tested by Western blot and TGEV Miller-strain hyperimmune serum to confirm their TGEV-specificity prior to IP-administration to group 3 pigs. A: 1 – BioRad low range protein standard; 2 – protein lysate containing the wild type baculovirus ACNPV; 3 – protein lysate containing the purified TGEV Miller-strain: * indicates three major structural proteins in order S = 220 kDa, N = 47 kDa, M = 28–31 kDa; 4 – protein lysate containing baculovirus-expressed TGEV S-protein (clone R2-2 was truncated to 150 kDa); 5 – protein lysate containing the baculovirus-expressed TGEV M-protein; 6 – BioRad high range protein standard. B: 1 – BioRad low range protein standard; 2 – protein lysate containing the baculovirus-expressed TGEV N-protein; 3 – BioRad high range protein standard.
3.2. Serum antibody responses
Serum antibody responses were detected by monoclonal antibody-capture ELISA and VN tests (Table 1). At the day of the second IP-inoculation (PCD-10) no detectable TGEV IgG-antibodies (<20) or VN-antibodies (<4) were found in any of the groups (Table 1). Only TGEV IgG-antibodies and no TGEV IgA-antibodies were detected in the serum of the pigs except group 4 by PCD 0. At PCD 0 only the group inoculated with TGEV proteins (group 3) and group 4 infected at 11-days of age with virulent TGEV showed both IgG and VN antibody to TGEV (Table 1). No ELISA and very low or no VN-antibody titers were detected in group 2 previously inoculated with the TGEV commercial vaccine and the negative control group 1. The difference in geometric mean titers (GMTs) between groups 1 and 2 compared to groups 3 and 4 at PCD 0 was significant (_p_ < 0.05) as detected by both ELISA and VN tests (Table 1). At PCD 6–7 all groups seroconverted to TGEV with the highest ELISA IgG and VN antibodies in group 3. At PCD 0 group 3 had the highest ELISA IgG and VN antibodies among the four groups, but not significantly higher than group 4 at PCD 0, (_p_ > 0.05), reflecting the response elicited by the vaccine containing the baculovirus-expressed S-protein. No significant serum antibody differences were observed among the 4 subgroups of control group 1; therefore, the serum antibody and other results were presented as overall group 1 means.
Table 1.
Geometric mean antibody titers (GMT) against TGEV and 95% confidence (upper limit) interval in serum of the 4 groups of pigs as determined by monoclonal-antibody-sandwich ELISA (TGEV-IgG, IgA) and virus-neutralization test (TGEV-VN)a
| Geometric mean TGEV-specific ELISA/VN serum antibody titers, (95% confidence interval) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| PCD | Day of 2nd IP-inoculation (−10 PCD) | Day of TGEV-challenge (0 PCD) | Week after TGEV-challenge (6–7 PCD) | ||||||
| Group | IgA | IgG | VNT | IgA | IgG | VNT | IgA | IgG | VNT |
| 1 | <20 (0) | <20 (0) | <4 (0) | <20 (0) | <20 (0) | <4 (0) | <20 (0) | 23 (69) | 16 (36) |
| 2 | <20 (0) | <20 (0) | <4 (0) | <20 (0) | <20 (0) | 5 (26)b | <20 (0) | 581 (2023) | 1171 (9631) |
| 3 | <20 (0) | <20 (0) | <4 (0) | <20 (0) | 340 (3109)c | 569 (1264)c | <20 (0) | 2256 (16218) | 2263 (5480) |
| 4 | <20 (0) | <20 (0) | <4 (0) | 33 (83) | 237 (724)c | 240 (602)c | 157 (189) | 1138 (6420) | 930 (7803) |
3.3. TGEV antibody secreting cells (ASC)
By measuring the TGEV IgA- and IgG-ASCs from lymphoid tissues (Fig. 2) which are part of the mucosal immune system (I, MLN) compared to the systemic immune system (PBL), more consistent results were obtained with ASCs restimulated in vitro with inactivated TGEV (memory B-cells) than with ASC representing the in vivo antibody responses. In all groups tested at PCD 0 and 6–7 for I, MLN and PBL tissues, the TGEV IgG-ASCs were generally detected in equal or higher numbers (Fig. 2A–E) than IgA-ASCs. At PCD 0 few significant differences were detected for in vivo ASC numbers except for group 4 ileum in which low numbers of IgA and IgG ASC were detected in contrast to no ASC in ileum of groups 1–3 (Fig. 2A). For group 4 MLN (PCD 0 and 6–7) and groups 3 and 4 PBL (PCD 0), both IgA and IgG-ASC numbers were above the ASC numbers of groups 1 and 2 (p < 0.05, Fig. 2B and C). At PCD 6–7 in vivo IgG-ASC numbers in all 3 tissues (I, MLN, PBL) of groups 2–4 were greater than the group 1 IgG-ASC numbers. The IgG-ASC numbers in the MLN of groups 3 and 4 were greater than those for groups 1 and 2 (p < 0.05, Fig. 2A, B and C).
Fig. 2.
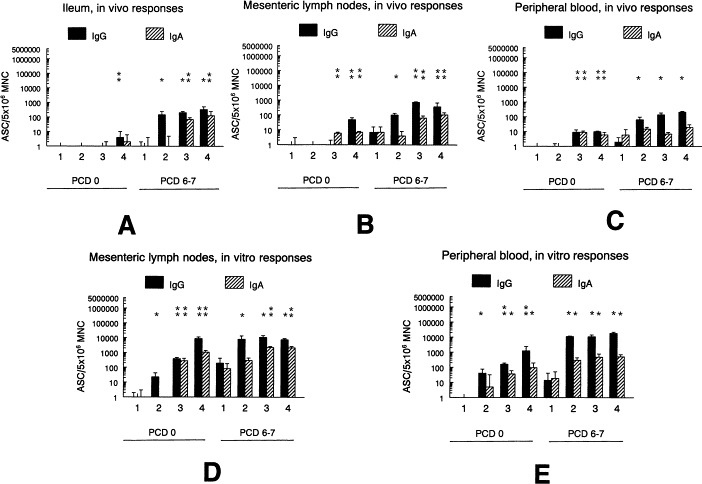
TGEV-specific IgG and IgA antibody secreting cells (ASC) as detected at PCD 0 and 6–7 from 2 to 8 pigs per time point of groups 1–4. A, B, C – in vivo ASC numbers for ileum lamina propria, mesenteric lymph node and peripheral blood mononuclear cells (MNC); D, E – in vitro memory ASC numbers (after 4–5 days of in vitro MNC stimulation with TGEV inactivated antigens) for mesenteric lymph node and peripheral blood lymphoid tissues. Differences in group ASC numbers were measured at PCD 0 and 6–7: * above the bars indicate significantly elevated (p < 0.05) numbers of ASC above the group 1 (negative controls) ASC numbers or ** above the group 1 and 2 (pigs inoculated with commercial TGEV-vaccine) ASC numbers.
After measuring the ASCs from in vitro stimulated (inactivated TGEV) MLN and PBL tissues, we found that at PCD 0 the IgG-ASCs were higher in groups 3 and 4 (p < 0.05) than in groups 1 and 2 (Fig. 2D and E) and IgA-ASCs were higher in MLN tissues of groups 3 and 4 (p < 0.05) than in groups 1 and 2 (Fig. 2D). After TGEV challenge (PCD 6–7), the vitro stimulated MNC from the MLN and PBL tissues showed no significant differences (IgG-ASC) among groups 2–4; however, the IgA-ASCs in MLN tissues of group 3 were significantly higher than groups 1 and 2 IgA-ASC numbers (p < 0.05), but not significantly different compared to group 4 (Fig. 2D). For the PBL tissues stimulated in vitro at PCD 6–7, there were no significant differences among groups 2–4 (all were significantly elevated above group 1) for IgA and IgG ASC numbers (Fig. 2E).
3.4. Flow cytometry analysis
From four pigs in group 3 (previously inoculated with the S, N and M TGEV structural proteins containing the LT-R192G toxin in IFA), MNC isolated from the MLN tissues were tested by flow cytometry after in vivo and in vitro (stimulation with inactivated TGEV) immune responses. By measuring the percentages of selected T-cell phenotypes: CD2+CD4+CD8−, CD2+CD4+CD8+, CD25+, CD2+CD4−CD8+bright, CD2+CD4−CD8+dim and CD2−CD4−CD8− (corresponding to T-helper, double positive, antigen-activated, cytotoxic T, NK and null cells, respectively), several differences in distributions of these phenotypes were observed after comparing in vivo and in vitro responses (Fig. 3A and B). After 4–5 days of stimulation of MNC with inactivated TGEV, a significant (p < 0.05) decrease in the CD2−CD4−CD8− phenotype and an increase in CD25+ (IL-2-R) expression occurred (Fig. 3). At the same time (PCD 0), increased numbers of CD2+CD4+CD8+, CD2+CD4−CD8+bright and CD2+CD4−CD8+dim cell phenotypes were observed; however these increases were not significant (Fig. 3). The MLN tissues collected from group 3 pigs (in vivo response) at PCD 0 were additionally tested for expression of exclusive porcine null cell marker (SW6) and 33+7% of MNC were positive (data not shown) compared to 44±9% of MNC positive for the CD2−CD4−CD8− phenotype.
Fig. 3.
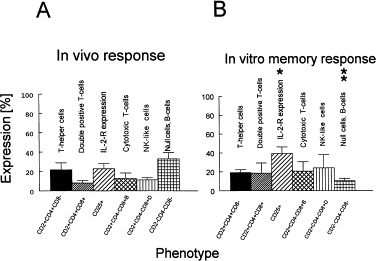
Percentages (%) of porcine T-cell phenotypes identified in mesenteric lymph node tissues of group 3 pigs at PCD 0. A – in vivo immune responses correspond to directly isolated, stained MNC tested in flow cytometry. B – in vitro memory immune responses correspond to MNC stimulated 4–5 days in vitro with inactivated TGEV and then stained and tested in flow cytometry: * indicates significantly elevated (p < 0.05) expression of IL-2-R as determined for in vitro memory response versus in vivo response or ** indicates significantly decreased (p < 0.05) numbers of CD2−CD4−CD8− phenotype as determined for in vitro memory response versus in vivo response.
3.5. TGE symptoms, TGEV shedding
Progression of clinical TGE-symptoms such as diarrhea, dehydration, anorexia, lethargy and shedding of TGEV antigen in feces of challenged pigs was measured from PCD 0 to PCD 9 in all groups (Fig. 4A and B), except for group 4 where due to 33% mortality associated with the first TGEV inoculation, lower numbers of pigs were available and TGE-symptoms were measured in this group only up to PCD 5. The most severe TGE-symptoms were observed in the TGEV seronegative control group 1 which did not possess any immunity to TGEV (Fig. 4A). Although the group 4 (positive controls previously inoculated with virulent TGEV) showed a high degree of active immunity to TGEV at PCD 0 associated with lack of TGEV shedding, this group continued to be stunted and still exhibited mild dehydration (result of initial TGEV infection at 11 days of age) compared to age-matched pigs (group 1–3). This impacted the group 4 TGE clinical scores measured after challenge at 27 days of age (Fig. 4A). Groups 2–3 (inoculated with commercial or recombinant TGE-vaccines) showed similar patterns of clinical signs which were completely ameliorated between PCD 6 and 9 (Fig. 4A). TGEV shedding in feces better reflected the degree of active local immunity to TGEV (as detected by ELISPOT in I- and MLN-lymphoid tissues). At PCD 1, no shedding of TGEV-antigen was detected in group 4 pigs, followed by 38% shedding in group 3 pigs, 86% shedding in group 2 pigs and 100% shedding in the negative control group 1 pigs (Fig. 4B). TGEV-shedding was curtailed in group 3 to 0% at PCD 6–7 and at PCD 8–9 in groups 1 and 2 (Fig. 4B).
Fig. 4.
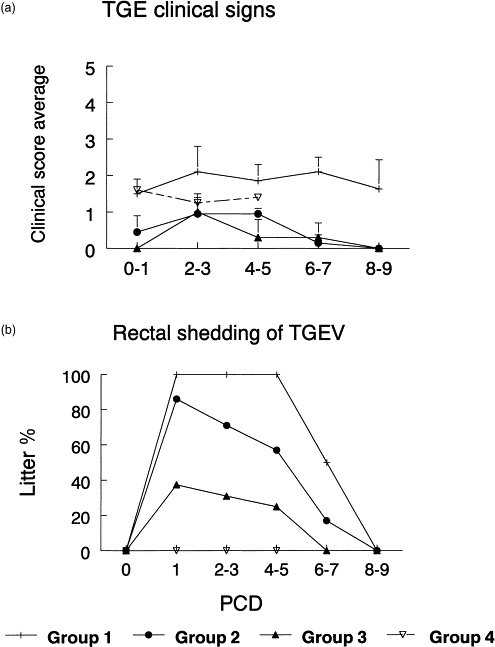
Progression of TGE clinical symptoms (A) and TGEV shedding in rectal swabs (B) as tested for the 4 groups of pigs (group 1 = negative controls; group 2 = pigs IP-immunized with TG-Emune commercial vaccine; group 3 = pigs IP-immunized with baculovirus-expressed TGEV S, M and N proteins in IFA containing the LT-toxin of E. coli; group 4 = pigs exposed (at 11 days of age) to virulent TGEV), after challenge exposure with virulent (1 × 106 PFU/pig) TGEV at 27 days of age (PCD 0). TGE symptoms were expressed as group mean + standard error of the mean; TGEV shedding as % of pigs shedding the virus in rectal swabs/total number of inoculated pigs within a group.
4. Discussion
During this decade emphasis has been on construction of TGEV protein subunit vaccines. Several systems were used to express the TGEV S, M, and N proteins such as E. coli, salmonella, adenovirus, vaccinia virus and baculovirus (Britton et al., 1987, Pulford and Britton, 1991b, Godet et al., 1991, Enjuanes et al., 1992, Tuboly et al., 1995, Torres et al., 1995, Torres et al., 1996, Smerdou et al., 1996, Shoup et al., 1997). Although some protective antibodies were induced in inoculated animals, the protection was only partial (Torres et al., 1995), not reported (Smerdou et al., 1996) or VN-antibodies were mostly IgG (Saif and Wesley, 1992, Shoup et al., 1997, Park et al., 1998). Moreover, TGEV-immunogens expressed in procaryotes were not glycosylated, soluble or did not induce VN-antibodies (Saif and Wesley, 1992). Human adenovirus vectors were reported to undergo an abortive replication in the porcine gut and loose the TGEV (S) inserts (Torres et al., 1996). The S protein expressed in the baculovirus expression system induced VN antibodies to TGEV in the serum of rats and pigs after parenteral application (Tuboly et al., 1995, Shoup et al., 1997). However, these serum VN-antibodies were not protective (Godet et al., 1991, Tuboly et al., 1995, Shoup et al., 1997). Only IgG antibodies to TGEV were detected in sow’s colostral and milk whey after a baculovirus-expressed S protein was administered with IFA intramammarily (IMM) and intramuscularly (IM) to TGEV seronegative pregnant sows (Shoup et al., 1997). Moreover, there was no significant impact on litter morbidity or mortality after TGEV challenge exposure of these litters (Shoup et al., 1997).
The use of additional adjuvants, immunomodulators and routes of administration remains to be explored to try to increase the immunogenicity of TGEV protein subunits. The adjuvant activity of bacterial toxins such as Vibrio cholerae toxin (CT) and heat-labile enterotoxin (LT) from enterotoxigenic E. coli has been reported using the mouse model (McGhee et al., 1992, Katz et al., 1997). Both CT and LT enhanced mucosal IgA responses, T-cell proliferation, IL-2 production and extended immunological memory (McGhee et al., 1992, Katz et al., 1997). The ability to target IgA inductive sites makes LT and CT ideal mucosal adjuvants (if the toxic effects can be suppressed) after coupling or coadministration with other antigens, even poor immunogens (Wu and Russell, 1994). Several non-toxic LT mutants and recombinants have been prepared with partial knockout of their ADP-ribosyltransferase activity (which is associated with enhanced cyclic AMP formation in the affected intestinal cells) (Giuliani et al., 1998). As a nontoxic antigen carrier system, the LT-B subunit has been coexpressed as a fusion protein with TGEV-S in a Samonella expression system and some TGEV neutralizing antibodies were induced in a mouse model after oral administration (Enjuanes and Van der Zeijst, 1995). However, further exploration of the mucosal adjuvanticity of enzymatically inactive forms of LT (mutants or recombinants) needs to be addressed in the context of its potential to stimulate IgA immune responses and protective immunity.
The IP route of immunization with CT conjugated to bacterial antigens has attracted attention recently in mouse models because peritoneal B-cells are able to repopulate other compartments of the common mucosal immune system, including the intestinal lamina propria with IgA-ASC (Wu and Russell, 1994). Moreover the feasibility of accessing the GALT via the serosal surface after IP administration of antigens was suggested in general for mucosal vaccine formulations (Husband, 1993). The IP immunization of preweaning and postweaning pigs with a killed E. coli vaccine reduced the mortality rate among vaccinated pigs by half that of unvaccinated controls (Husband and Seaman, 1979). Thus, our hypothesis was that IP inoculation of pigs with recombinant TGEV structural proteins combined with recombinant LT may be used to deliver these vaccines to GALT and elicit intestinal IgA antibodies and protection to TGEV.
Systemic immune responses (represented by serum IgG-antibodies to TGEV) in group 3 pigs reflected the immunogenic properties of the TGEV S-protein administered IP. IgA-antibodies to TGEV were not detected in the serum of any group at PCD 0 except low titers in group 4 pigs, reflecting use of a non-replicating recombinant protein vaccine or killed TGEV administered IP. Coadministration of TGEV-proteins with LT-R192G resulted in local production of IgA-ASC as detected by ELISPOT at PCD 0 in the MLN (but not ileum) of group 3 pigs. In our previous studies, administration of baculovirus-expressed S with or without M recombinant proteins (IM, IMM, IP) without LT-R192G resulted in only systemic (IgG) and very low local (IgA) immune responses to TGEV, or IgA was not measured (Shoup et al., 1997, Sestak et al., 1997, Park et al., 1998). In this study at PCD 0, the increase observed in both TGEV IgG- and IgA-ASCs after in vitro stimulation with inactivated TGEV-antigens (memory response) suggested that memory ASC for TGEV-specific IgA were present in groups 2–4 (MLN-tissues) at the time of challenge (PCD 0), but there was no or very little transudation of IgA into the serum as measured by ELISA (<20) after vaccination. These findings are consistent with those of others who reported serum IgA-antibodies to TGEV only during the convalescent (>PCD 7) but not during the acute (<PCD 7) stage of TGE (Kodoma et al., 1981). Other factors which most likely contributed to the lack of detectable serum IgA to TGEV in group 3 pigs prior to PCD 6–7 was the use of a nonreplicating protein vaccine and the possibility that even when IgA ASC were induced locally in GALT as demonstrated by ELISPOT, the numbers of IgA ASC were low and the efficiency of transudation of IgA into the serum was low. In previous studies, it was found that in suckling pigs passive immunity against TGEV-challenge was mostly associated with IgA (Saif and Wesley, 1992).
Partial protection in our group 3 pigs (38% shed TGEV in feces at PCD 1 comparing to 100% in group 1, plus clinical TGE scores did not rise above the value of 1) most likely reflected the fact that a substantial number of TGEV IgA-precursor ASC were formed in this group based on inoculation with TGEV recombinant proteins and LT-R192G as detected by the in vitro ELISPOT at PCD 0 and 6–7. Only the group 4 pigs (inoculated with virulent TGEV prior to TGEV challenge) showed higher numbers of TGEV IgA-ASCs at PCD 0 (Fig. 2) in ileum and MLN tissues. In contrast even after in vitro stimulation with TGEV-antigens, no or very low numbers of TGEV-specific ASCs were detected at PCD 0 in group 2 (inoculated with commercial vaccine) reflecting the poor efficacy of the commercial TGEV vaccine used in our study in inducing IgA antibody responses. The highest numbers of TGEV IgA-ASCs among the 4 groups of pigs were found in group 4 pigs which did not shed TGEV at PCD 0–5. In this group however, the previous TGEV-inoculation (at 11-days of age) resulted in marked stunting of pigs (compared to age-matched non-infected pigs), mild dehydration and a 33% mortality rate by PCD 0 when all groups were challenged with TGEV. It is important to mention, however, that evaluation of TGE-symptoms after infection of 27-day-old-pigs in this study was not as informative an indicator as it can be in future studies when recombinant TGEV proteins might be administered orally in the form of capsules to pregnant sows and TGE-symptoms then evaluated in neonatal pigs.
The induction of cell-mediated immunity after TGEV infection has been investigated by macrophage migration inhibition (Frederick and Bohl, 1976), leukocyte migration inhibition (Woods, 1977), direct lymphocyte cytotoxicity (Shimizu and Shimizu, 1979), spontaneous cell-mediated cytotoxicity, antibody-dependent cell-mediated cytotoxicity (Cepica and Derbyshire, 1983), and antigen specific lymphocyte proliferation (Brim et al., 1995). Among all mammalian species studied, swine show the most diverse T-lymphocyte populations. The porcine T-cell populations are unique in that they contains several major subsets which do not exist or play only a minor role in other species. About 10–50% of porcine T-cells show the CD2−SW1+ phenotype and this subpopulation has been referred to as porcine null cells (Binns, 1994). Although the CD4 and CD8 antigens show a similar T-cell expression pattern compared to other mammals, i.e. T-helper cells and cytotoxic T-cells, respectively (Saalmuller and Bryant, 1994), two unusual (major) subpopulations of porcine T-cells have been identified based on coexpression of these two antigens: CD4+CD8+ and CD4−CD8−, i.e. double positive and double negative or null cells (Pescowitz et al., 1994, Saalmuller and Bryant, 1994). The exact function of these two enigmatic porcine T-cell subsets remains to be elucidated (Saalmuller et al., 1996) although the results from some studies suggest that porcine CD4+CD8+ T-cells exhibit properties of antigen experienced cells or memory T-cells (Zuckermann and Husmann, 1996). It was reported that the TGEV-N-phosphoprotein contains at least 3 T-helper cell epitopes suggesting the involvement of T-helper cells in TGEV antibody synthesis (Anton et al., 1995). In our study the impact of the coadministration of TGEV structural proteins with LT-R192G in IFA on the distribution of major T-cell subsets and expression of IL-2-R was measured at PCD 0 for MNC of MLN from the in vivo and in vitro (inactivated TGEV) stimulated cultures. The IL-2-R is expressed on the surface of antigen/mitogen activated, and not resting cells (Smith, 1988, Minami et al., 1993). In our study we found significantly increased IL-2-R expression on MNC from the MLN of group 3 pigs after their in vitro stimulation with inactivated TGEV antigens as measured at PCD 0. The significant increase of IL-2-R expression was accompanied by a significant decrease in the CD2−CD4−CD8− phenotype, suggesting the possibility of null cell transition into other, perhaps more functional cell types such as double-positive T-cells, NK-cells or cytotoxic T-cells. For group 3 the occurrence of double-positive, NK and cytotoxic T-cell phenotypes was increased for in vitro MLN cultures of MNC above the level corresponding to the in vivo responses but not significantly. Our data (increased occurrence of CD4+CD8+ and IL-2-R+ cells in vitro) are in agreement with investigators who suggested the involvement of T-helper cell epitopes in TGEV-antibody synthesis and that double-positive CD4+CD8+ T-cells show properties of memory T-cells (Anton et al., 1995, Zuckermann and Husmann, 1996). The positive identification of porcine CD2− null cells can be performed by detection of SWC6 antigen expression (Saalmuller and Bryant, 1994). In our group 3 pigs, 33 ± 7% of MNC from MLN were identified as SWC6+. Considering the percentages and the standard errors (CD2−CD4−CD8− phenotype: 44 ± 9% and SW6+ phenotype: 33 ± 7%), it is possible that the group 3 CD2−CD4−CD8− MNC population consisted of predominantly SWC6+ cells and the rest were other cell-types not expressing CD2, CD4 and CD8 antigens such as B-cells, etc. In the case of porcine CD8+ cells, two different levels of expression (high and low or bright and dim) had been previously described as corresponding to cytotoxic T (CD8+bright) and NK (CD8+dim) cells (Pescovitz et al., 1988, Saalmuller et al., 1994). In our study both cytotoxic T and NK cell phenotypes were identified based on bright and dim expression of the CD8 antigen. After in vitro stimulation of group 3 MNC from the MLN with inactivated TGEV antigens (PCD 0), an increased number of cytotoxic T and NK cell phenotypes suggested an involvement of these cells in immune responses after IP-inoculation of pigs with baculovirus-expressed TGEV structural proteins. This finding is in agreement with the findings of others who observed NK- and cytotoxic T-cell activity after TGEV-infection of neonatal pigs (Cepica and Derbyshire, 1984).
In our study baculovirus-expressed TGEV structural proteins (S, N and M) coadministered IP with E. coli mutant LT-toxin (LT-R192G) induced MLN immune responses associated with IgA-antibodies to TGEV coinciding with reduced TGEV shedding in the feces of challenged pigs. Moreover, our results suggest that TGEV subunit vaccines based on baculovirus recombinants administered IP with LT-R192G can also actively stimulate systemic immune responses. The in vitro immune response in group 3 pigs at PCD 0 compared to the in vivo responses was associated with a significant upregulation of IL-2-R, the decreased occurrence of CD2−CD4−CD8− (null cells) phenotype (p < 0.05) and the increased occurrence of CD4+CD8+ (double positive T-cells), CD2+CD4−CD8+dim (NK-cells) and CD2+CD4−CD8+bright (cytotoxic T-cells) phenotypes (_p_ > 0.05). Further improvements in the efficiency of this type of vaccine could be explored by the use of additional carrier systems such as Immuno-Stimulating-Complexes (ISCOMs), biodegradable microspheres, etc.
Acknowledgements
The authors thank Dr. D.J. Jackwood for providing the TGEV S-clone produced by Dr. D.I. Shoup, Dr. H. Laude for providing the TGEV N and M-clones, Dr. J.D. Clements for providing the LT-R192G toxin and Center for Retrovirus Research Cytometry Laboratory for the help with flow cytometry. The assistance of Mrs. Kathy Gadfield with virus-neutralization tests and Mr. Bert Bishop with statistical analysis is greatly appreciated. This study was supported by state and federal funds appropriated to the Ohio Agricultural Research and Development Center, The Ohio State University.
References
- Anton I.M., Sune C., Meloen R.H., Borras-Cuesta F., Enjuanes L. A transmissible gastroenteritis coronavirus nucleoprotein epitope elicits T helper cells that collaborate in the in vitro antibody synthesis to the three major structural viral proteins. Virology. 1995;212:746–751. doi: 10.1006/viro.1995.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudoux P., Charley B., Laude H. Recombinant expression of the TGEV membrane glycoprotein M. Adv. Exp. Med. & Biol. 1995;380:305–310. doi: 10.1007/978-1-4615-1899-0_49. [DOI] [PubMed] [Google Scholar]
- Binns R.M. The Null/γδ TCR+ T cell family in the pig. Vet. Immunol. & Immunopathol. 43. 1994;1994:69–77. doi: 10.1016/0165-2427(94)90122-8. [DOI] [PubMed] [Google Scholar]
- Brim T.A., VanCott J.L., Lunney J.K., Saif L.J. Cellular immune responses of pigs after primary inoculation with porcine respiratory coronavirus or transmissible gastroenteritis virus and challenge with transmissible gastroenteritis virus. Vet. Immunol. Immunopathol. 1995;48:35–54. doi: 10.1016/0165-2427(94)05416-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton P., Garwes D.J., Page K., Walmsley J. Expression of porcine transmissible gastroenteritis virus genes in E. coli as beta-galactosidase chimeric proteins. Adv. Exp. Med. Biol. 1987;218:55–64. doi: 10.1007/978-1-4684-1280-2_7. [DOI] [PubMed] [Google Scholar]
- Cepica A., Derbyshire J.B. Antibody-dependent cell-mediated cytotoxicity and spontaneous cell-mediated cytotoxicity against cells infected with porcine transmissible gastroenteritis virus. Can. J. Comp. Med. 1983;47:298–303. [PMC free article] [PubMed] [Google Scholar]
- Cepica A., Derbyshire J.B. The effect of adoptive transfer of mononuclear leukocytes from an adult donor on spontaneous cell-mediated cytotoxicity and resistance to transmissible gastroenteritis in neonatal piglets. Can. J. Comp. Med. 1984;48:360–364. [PMC free article] [PubMed] [Google Scholar]
- Enjuanes L., Sune C., Gebauer F. Antigen selection and presentation to protect against transmissible gastroenteritis coronavirus. Vet. Microbiol. 1992;33(1-4):249–262. doi: 10.1016/0378-1135(92)90053-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjuanes, L., Van der Zeijst, B.A.M., 1995. Molecular basis of transmissible gastroenteritis virus epidemiology. In: Siddell, S.G. (Ed.), The Coronaviridae, Wurzburg, Germany, Plenum Press, New York, Ch. 16, pp. 337–376.
- Frederick G.T., Bohl E.H. Local and systemic cell-mediated immunity against transmissible gastroenteritis, and intestinal viral infection of swine. J. Immunol. 1976;116:1000–1004. [PubMed] [Google Scholar]
- Giuliani M.M., Del Giudice G., Giannelli V., Dougan G., Douce G., Rappuoli R., Pizza M. Mucosal adjuvanticity and immunogenicity of LTR72, a novel mutant of Escherichia coli heat-labile enterotoxin with partial knockout of ADP-ribosyltransferase activity. J. Exp. Med. 1998;187:1123–1132. doi: 10.1084/jem.187.7.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godet M., Rasschaert D., Laude H. Processing and antigenicity of entire and anchor-free spike glycoprotein S of coronavirus TGEV expressed by recombinant baculovirus. Virology. 1991;185:732–740. doi: 10.1016/0042-6822(91)90544-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husband A.J. Novel vaccination strategies for the control of mucosal infection. Vaccine. 1993;11:107–112. doi: 10.1016/0264-410x(93)90003-g. [DOI] [PubMed] [Google Scholar]
- Husband A.J., Seaman J.T. Vaccination of piglets against Escherichia coli enteritis. Austr. Vet. J. 1979;55:435–436. doi: 10.1111/j.1751-0813.1979.tb05601.x. [DOI] [PubMed] [Google Scholar]
- Katz J.M., Lu X., Young S.A., Galphin J.C. Adjuvant activity of the heat-labile enterotoxin from enterotoxigenic Escherichia coli for oral administration of inactivated influenza virus vaccine. J. Inf. Dis. 1997;175:352–363. doi: 10.1093/infdis/175.2.352. [DOI] [PubMed] [Google Scholar]
- Kodoma Y., Ogata M., Shimizu Y. Serum immunoglobulin A antibody response in swine infected with transmissible gastroenteritis virus, as determined by indirect immunoperoxidase antibody test. Am. J. Vet. Res. 1981;42:437–442. [PubMed] [Google Scholar]
- Kyd J.M., Taylor D., Cripps A.W. Conservation of immune responses to proteins isolated by preparative polyacrylamide gel electrophoresis from the outer membrane of nontypeable Haemophilus influezae. Infect. Immunol. 1994;62:5652–5658. doi: 10.1128/iai.62.12.5652-5658.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laude H., Van-Reeth K., Pensaert M.B. Porcine respiratory coronavirus: molecular features and virus – host interactions. Vet. Res. 1993;24:125–150. [PubMed] [Google Scholar]
- Luckow V.A., Summers M.D. Trends in the development of baculovirus expression vectors. Biotechnol. 1988;6:47–55. [Google Scholar]
- McGhee J.R., Mestecky J., Dertzbaugh M.T., Eldridge J.H., Hirasawa M., Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10:75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- Minami Y., Kono T., Miyazaki T., Taniguchi T. The IL-2 receptor complex: its structure, function and target genes. Annu. Rev. Immunol. 1993;11:245–267. doi: 10.1146/annurev.iy.11.040193.001333. [DOI] [PubMed] [Google Scholar]
- O’Neal C.M., Clements J.D., Estes M.K., Conner M.E. Rotavirus 2/6 virus-like particles administered intranasally with cholera toxin, Escherichia coli heat-labile toxin (LT), and LT-R192G induce protection from rotavirus challenge. J. Virol. 1998;72:3390–3393. doi: 10.1128/jvi.72.4.3390-3393.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Sestak K., Hodgins D.C., Shoup D.I., Ward L.A., Jackwood D.J., Saif L.J. Immune response of sows vaccinated with attenuated transmissible gastroenteritis virus (TGEV) and recombinant TGEV spike protein vaccine and protection of their suckling piglets against virulent TGEV challenge. Am. J. Vet. Res. 1998;59:1002–1008. [PubMed] [Google Scholar]
- Paton, D.J., Ibata, G., McGoldrick, A., Jones, T,O., Pritchard, G.C., 1998. Attempted isolation and characterization of recent British isolates of transmissible gastroenteritis, Proc 15th IPVS, Birmingham, England, 5–9 July, p. 391.
- Paul P., Mengeling W.L., Malstrom C.E., van Deusen R.A. Production and characterization of monoclonal antibodies to porcine immunoglobulin gamma, alpha, and light chains. Am. J. Vet. Res. 1989;50:471–475. [PubMed] [Google Scholar]
- Pescovitz M.D., Lowman M.A., Sachs D.H. Expression of T-cell associated antigens by porcine natural killer cells. Immunology. 1988;65:267–271. [PMC free article] [PubMed] [Google Scholar]
- Pescowitz M.D., Sakopoulos A.G., Gaddy J.A., Husmann R.J., Zuckerman F.A. Porcine peripheral blood CD4+/CD8+ dual expressing T-cells. Vet. Immunol. Immunopathol. 1994;43:53–62. doi: 10.1016/0165-2427(94)90120-1. [DOI] [PubMed] [Google Scholar]
- Pulford D.J., Britton P. Intracellular processing of the porcine coronavirus transmissible gastroenteritis virus spike protein expressed by recombinant vaccinia virus. Virology. 1991;182:765–773. doi: 10.1016/0042-6822(91)90617-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulford D.J., Britton P. Expression and cellular localisation of porcine transmissible gastroenteritis virus N and M proteins by recombinant vaccinia viruses. Virus Res. 1991;18(2-3):203–217. doi: 10.1016/0168-1702(91)90019-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmuller A., Bryant J. Characteristics of porcine T lymphocytes and T-cell lines. Vet. Immunol. Immunopathol. 1994;43:45–52. doi: 10.1016/0165-2427(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Saalmuller A., Aasted B., Canals A., Dominguez J., Goldman T., Lunney J.K., Maurer S., Pescovitz M.D., Pospisil R., Salmon H., Trebichavsky I., Valpotic I., Vizcaino J.S., Zuckermann F. Analyses of mAb reactive with porcine CD8. Vet. Immunol. Immunopathol. 1994;43:249–254. doi: 10.1016/0165-2427(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Saalmuller A., Denham S., Haverson K., Davis B., Dominguez J., Pescowitz M.D., Stokes C.C., Zuckermann F., Lunney J.K. The second international swine CD workshop. Vet. Immunol. Immunopathol. 1996;54:155–158. doi: 10.1016/s0165-2427(96)05675-9. [DOI] [PubMed] [Google Scholar]
- Saif, L.J., Wesley, R.D., 1992. Transmissible gastroenteritis. In: Leman, A.D., Straw, B.E., Mengeling, W.L., D’Allaire, S., Taylor, D.J. (Eds.), Diseases of Swine, 7th ed., Iowa State University Press, Ames, IA, pp. 362–386.
- Saif L.J. Mucosal immunity: an overview and studies of enteric and respiratory coronavirus infections in a swine model of enteric disease. Vet. Immunol. Immunopathol. 1996;54:163–169. doi: 10.1016/S0165-2427(96)05702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestak K., Lanza I., Park S.K., Weilnau P.A., Saif L.J. Contribution of passive immunity to porcine respiratory coronavirus to protection against transmissible gastroenteritis virus challenge exposure in suckling pigs. Am. J. Vet. Res. 1996;57:664–671. [PubMed] [Google Scholar]
- Sestak, K., Lewis, P.A., Gillespie, A., Saif, L.J., 1997. Immune responses in pigs intraperitoneally (IP) inoculated with baculovirus-expressed transmissible gastroenteritis virus S and M proteins. Proc 78th Ann Meeting of the Conf. Research Workers in Anim. Dis., Chicago, Il, 10–11 November, abstract 133.
- Shimizu M., Shimizu Y. Demonstration of cytotoxic lymphocytes to virus-infected target cells in pigs inoculated with transmissible gastroenteritis virus. Am. J. Vet. Res. 1979;40:208–213. [PubMed] [Google Scholar]
- Shoup D.I., Jackwood D.J., Saif L.J. Active and passive immune responses to transmissible gastroenteritis virus (TGEV) in swine inoculated with recombinant baculovirus-expressed TGEV spike glycoprotein vaccines. Am. J. Vet. Res. 1997;58:242–250. [PubMed] [Google Scholar]
- Smerdou C., Urniza A., Curtis III R., Enjuanes L. Characterization of transmissible gastroenteritis coronavirus S protein expression products in avirulent S. typhimurium Δcya Δcrp: persistance, stability and immune response in swine. Vet. Microbiol. 1996;48:87–100. doi: 10.1016/0378-1135(95)00141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.A. Interleukin-2: inception, impact, impact and implications. Science. 1988;240:1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Stott D.I. Immunoblotting and dot blotting. J. Immunol. Methods. 1989;119:153–187. doi: 10.1016/0022-1759(89)90394-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres J.M., Sanchez C., Sune C., Smerdou C., Prevec L., Graham F., Enjuanes L. Induction of antibodies protecting against transmissible gastroenteritis coronavirus (TGEV) by recombinant adenovirus expressing TGEV spike protein. Virology. 1995;213:503–516. doi: 10.1006/viro.1995.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres J.M., Covadonga A., Ortega A., Mittal S., Graham F., Enjuanes L. Tropism of human adenovirus type 5-based vectors in swine and their ability to protect against transmissible gastroenteritis coronavirus. J. Virol. 1996;70:3770–3780. doi: 10.1128/jvi.70.6.3770-3780.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuboly T., Nagy E., Derbyshire B. Passive protection of piglets by recombinant baculovirus induced transmissible gastroenteritis virus specific antibodies. Can. J. Vet. Res. 1995;59:70–72. [PMC free article] [PubMed] [Google Scholar]
- VanCott J.L., Brim T.A., Lunney J.K., Saif L.J. Contribution of antibody-secreting cells induced in mucosal lymphoid tissues of pigs inoculated with respiratory or enteric strains of coronavirus to immunity against enteric coronavirus challenge. J. Immunol. 1994;152:3980–3990. [PubMed] [Google Scholar]
- Welch S.K., Saif L.J. Monoclonal antibodies to a virulent strain of transmissible gastroenteritis virus: comparison of reactivity with virulent and attenuated virus. Arch. Virol. 1988;101:221–235. doi: 10.1007/BF01311003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley R.D., Woods R.D., McKean J.D., Senn M.K., Elazhary Y. Prevalence of coronavirus antibodies in Iowa swine. Can. J. Vet. Res. 1997;61:305–308. [PMC free article] [PubMed] [Google Scholar]
- Woods R.D. Leukocyte migration-inhibition procedure for transmissible gastroenteritis viral antigens. Am. J. Vet. Res. 1977;38:1267–1269. [PubMed] [Google Scholar]
- Wu H.-Y., Russell M.W. Comparison of systemic and mucosal priming for mucosal immune responses to a bacterial protein antigen given with or coupled to cholera toxin (CT) B subunit, and effects of pre-existing anti-CT immunity. Vaccine. 1994;12:215–222. doi: 10.1016/0264-410x(94)90197-x. [DOI] [PubMed] [Google Scholar]
- Yuan L., Ward L.A., Rosen B.I., To T.L., Saif L.J. Systemic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J. Virol. 1996;70:3075–3083. doi: 10.1128/jvi.70.5.3075-3083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckermann F.A., Husmann R.J. Functional and phenotypic analysis of porcine peripheral blood CD4/CD8 double-positive T cells. Immunology. 1996;87:500–512. [PMC free article] [PubMed] [Google Scholar]