Modern Plasma Fractionation (original) (raw)
Abstract
Protein products fractionated from human plasma are an essential class of therapeutics used, often as the only available option, in the prevention, management, and treatment of life-threatening conditions resulting from trauma, congenital deficiencies, immunologic disorders, or infections. Modern plasma product production technology remains largely based on the ethanol fractionation process, but much has evolved in the last few years to improve product purity, to enhance the recovery of immunoglobulin G, and to isolate new plasma proteins, such as α1-protease inhibitor, von Willebrand factor, and protein C. Because of the human origin of the starting material and the pooling of 10 000 to 50 000 donations required for industrial processing, the major risk associated to plasma products is the transmission of blood-borne infectious agents. A complete set of measures—and, most particularly, the use of dedicated viral inactivation and removal treatments—has been implemented throughout the production chain of fractionated plasma products over the last 20 years to ensure optimal safety, in particular, and not exclusively, against HIV, hepatitis B virus, and hepatitis C virus. In this review, we summarize the practices of the modern plasma fractionation industry from the collection of the raw plasma material to the industrial manufacture of fractionated products. We describe the quality requirements of plasma for fractionation and the various treatments applied for the inactivation and removal of blood-borne infectious agents and provide examples of methods used for the purification of the various classes of plasma protein therapies. We also highlight aspects of the good manufacturing practices and the regulatory environment that govern the whole chain of production. In a regulated and professional environment, fractionated plasma products manufactured by modern processes are certainly among the lowest-risk therapeutic biological products in use today.
COLLECTED HUMAN PLASMA may be used as a therapeutic product (known as “clinical plasma” or “fresh frozen plasma”) or as source material for the production of pharmaceutical fractionated products (also called “plasma products” or “plasma derivatives”). This complex biologic material contains hundreds of proteins covering a myriad of physiological functions. Many components still have undiscovered roles. The most abundant proteins, albumin and immunoglobulin (Ig) G, are present at about 35 and 10 g/L, respectively, representing about 80% of all plasma proteins. Less abundant proteins include the protease inhibitors, like _α_1-antitrypsin (AAT) (1.5 g/L) and antithrombin (AT) (300 mg/L), and the coagulation factors such as factor VIII (FVIII) (a few ng/L), which exhibit potent physiologic activity. Currently, about 20 different plasma protein therapeutics are used for treating life-threatening diseases or injuries associated to bleeding and thrombotic disorders, immunological diseases, infectious conditions, as well as tissue degenerating diseases, thus addressing the clinical needs of countless patients. An updated list of the major therapeutic applications of plasma protein products can be found elsewhere.1
This industrial process used to isolate therapeutic plasma proteins is known as “fractionation.” Over 23 to 28 million liters of human plasma are fractionated each year in the world, in batches of several thousand liters, in about 70 factories. Modern plasma fractionation combines manufacturing steps to isolate, in a sequential and integrated manner, the crude fractions that are further purified into individual therapeutic products. Validated dedicated steps inactivate and/or remove infectious agents potentially present in the starting plasma pool. This sophisticated industrial process is performed under highly hygienic conditions in licensed facilities (plasma fractionation plants) that are operated in compliance with good manufacturing practices and following quality assurance principles.
Over the years, plasma fractionation has evolved from a medical service activity mostly oriented toward the needs of local communities into a global manufacturing industry conforming to high regulatory standards. These strict requirements start from the collection of plasma for fractionation and include product manufacture and distribution steps. In this article, we review the most current practices encompassing the collection of plasma for fractionation, the core industrial plasma fractionation process, and the purification and pathogen reduction technologies of individual plasma products.
Production of Plasma for Fractionation
The practices used for the collection of plasma for fractionation have direct influence on the safety profile of protein products since individual donations contribute to large plasma pools used to manufacture therapeutic preparations intended for hundreds or even thousands of patients. It is therefore logical that the production of plasma is regarded as an integral part of the manufacture of modern fractionated products. Collection requirements of plasma for fractionation may differ from those relevant to fresh frozen plasma. In a regulated environment, plasma for fractionation is collected by licensed/registered blood establishments (blood centers and apheresis collection centers) that are inspected by the relevant National Regulatory Authorities (NRAs). Compelled by the same safety concerns, the plasma fractionators conduct audits to verify that the contractual plasma collection and quality and safety measures, agreed upon with the plasma supplier, are met. Areas of specific relevance include (a) procedures for donor screening and donation testing; (b) labeling, documentation, and traceability requirements; and (c) the handling of blood and plasma. Such information is part of the marketing license of plasma products and, in Europe, is assembled into a document called the “Plasma Master File.” Various requirements for the collection of plasma for fractionation have been described in various guides, eg, from the Pharmaceutical Inspection Convention and Pharmaceutical Inspection Cooperation Scheme (jointly referred to as PIC/S), US Food and Drug Administration (FDA) and in recent World Health Organization Recommendations.1 They are summarized below.
Donors Screening
Candidate donors are provided with educational materials and undergo a medical interview to establish the absence of risks or signs of infections and to prove compliance for a donation of plasma for fractionation (Table 1). Potential donors presenting a health hazard are asked to exclude themselves. Medical information of donors is acquired and archived. Continuous epidemiologic surveillance of the donor population is being required in some jurisdictions.2 It helps to establish the background level (prevalence and incidence) and trends of known infectious markers (eg, HIV 1 and 2 antibodies, hepatitis C virus [HCV] antibodies, and hepatitis B surface antigen [HBsAg]) in the population. This is also of interest for the early detection of emerging diseases, allowing early implementation of counter measures (such as more stringent donor screening processes or requirements for additional testing procedures).
Table 1.
Most Relevant Measures Taken to Prevent the Transmission of Plasma-Borne Infectious Agents by Fractionated Plasma Products
| Infectious agent | Blood establishment | Plasma fractionator | |||||
|---|---|---|---|---|---|---|---|
| Donor screening (exclusion) criteria | Individual serologic testing | Mini-pool NAT⁎ | Manufacturing pool testing† | Viral inactivation treatments | Removal by purification steps‡ | Removal by nanofiltration§ | |
| HIV I and II | Questionnaire | Anti-HIV 1 and 2 | Yes | Anti-HIV 1 and 2; HIV NAT | + | (+) | + |
| HBV | Questionnaire | HBsAg | Yes | HBsAg; HBV NAT | + | (+) | + |
| HCV | Questionnaire | Anti-HCV | Yes | Anti-HCV; HCV NAT | + | (+) | + |
| Hepatitis delta virus | (questionnaire) | ND | ND | ND | + | (+) | + |
| HAV | ND | ND | Yes | HAV NAT | ± | (+) | + |
| Hepatitis E virus | ND | ND | ND | ND | + | (+) | + |
| Hepatitis G virus | ND | ND | ND | ND | + | (+) | + |
| TT virus | ND | ND | ND | ND | + | (+) | + |
| B19 | ND | ND | Yes | B19 NAT | ± | (+) | + |
| WNV | ND | ND | ND | ND | + | (+) | + |
| vCJD | Questionnaire | ND | Not relevant | ND | Not relevant | (+)‖ | (+)‖ |
Donors eligible to donate plasma for fractionation are individuals who meet donation criteria (such as age and donation frequency), do not present risk factors of blood-born infectious agents, and comply with requirements defined by the plasma fractionator and the NRAs of the country of plasma collection and of use of the products. In most situations, eligibility of whole blood donors and apheresis donors overlap, apart from donation frequency which is higher for plasmapheresis donors. Eligibility criteria take into account scientific information about the risks of transmission of infectious agents by pooled plasma products (which may differ from those by blood components). Special criteria may exist for the collection of hyperimmune plasma (used to make hyperimmune IgG preparations), such as procedures for donors' immunization and minimal antibody titer.1
Collection Methods
Currently, about 35% of the plasma fractionated in the world is obtained by centrifugation of whole blood (“recovered” plasma), and 65% is obtained by apheresis. Blood/plasma collection, processing, and storage may affect plasma quality, as well as having an impact on the recovery of the most labile proteins such as FVIII. In particular, risks of activation of the coagulation, complement, and fibrinolytic systems, which may lead to generation of plasma proteases, should be avoided. To better preserve the integrity of recovered plasma and limit risks of activation of the coagulation cascade and of cellular components, (a) good mixing of the blood with the anticoagulant solution (a sodium citrate based solution1) should be ensured from the initiation till the end of the collection process; (b) the duration of the collection should not exceed 15 minutes; and (c) temperature variations of the blood should be avoided. A few hours after donation, whole blood is subjected to a centrifugation that separates the cellular elements (most specifically red cells) from plasma. The mean plasma volume obtained from one whole blood donation is about 220 mL but varies depending upon the volume of collected whole blood (most often 400-450 mL) and donor's hematocrit. Apheresis plasma (also called “source plasma”) is collected from donors through a process where blood is removed from the donor, anticoagulated (generally with a 4% sodium citrate solution),1 and immediately separated by physical means (centrifugation or filtration, or a combination of both) into components.3 At minimum, the red cells are returned to the donor while plasma is retained and collected in a container (bag or plastic bottle). The duration of a typical plasmapheresis procedure depends on the number of cycles (and, hence, the volume of plasma collected) and lasts generally from 35 to 70 minutes. Apheresis plasma volume may range from 450 to 880 mL, depending upon the country's regulations and collection protocol. Apheresis plasma can also be prepared as a by-product of plateletpheresis (“concurrent plasma”), a procedure used primarily for the collection of platelets.
Both recovered and apheresis plasmas are suitable for the manufacture of the whole range of fractionated plasma products. The mean content in coagulation factors, more particularly FVIII, is lower in recovered than in apheresis plasma because of (a) longer processing time before freezing (whole blood must be further processed to separate cellular components and plasma), (b) higher ratio of anticoagulant and, possibly, (c) the higher level of cellular contamination that may release proteolytic enzymes affecting the stability of coagulation factors. Apheresis plasma contains less IgG when collected from frequent donors. Protein content and quality of fractionated proteins is apparently not affected by the apheresis system used, although residual cell content differ based upon the type and configuration of the cell separation device.4 Plasma from membrane apheresis procedures, as does recovered plasma prepared from whole blood leukoreduced on positively charged filters, may contain more activated complement component 3 and 5 (C3a, C5a) anaphylatoxins,5, 6, 7 but the impact on the quality or yield of fractionated products is unknown.
Testing of Pathogenic Agents
Various infectious agents have been identified as potential contaminants of human blood.8 Bacteria, parasites, and intracellular viruses are not transmitted by plasma products because they are destroyed by freeze-thaw steps or removed by the 0.2- to 1-_μ_m filtration steps used during the processing of fractionated products. Pathogenic plasma-borne viruses include HIV, HCV, hepatitis B virus (HBV), West Nile virus (WNV), hepatitis A virus (HAV) and parvovirus B19 (B19). The various complementary safety nets in place during the production chain of fractionated products, from donor selection to industrial product extraction, to optimize safety against these agents are summarized in Table 1. The importance of viral testing on the safety of plasma products has been reviewed.1, 9, 10 The extent of viral testing of plasma for fractionation takes into account the ability of validated fractionation processes to eliminate viral risks.11 Some testing is performed by blood establishments, other by plasma fractionators (Table 1). Individual plasma donations must be negative for anti-HIV 1 and 2, anti-HCV, and HBsAg. Genomic assays of plasma minipools for nonenveloped HAV and B19 may be performed.11, 12 Relevance of testing for the absence of HIV P24Ag or WNV nucleic acid testing (NAT), which may be justified for the safety of non–virally inactivated blood components, is arguable for plasma for fractionation subjected to robust viral reduction steps of enveloped viruses. The industrial manufacturing pool (usually the cryo-poor plasma that is the first homogeneous pooled plasma fraction) is also tested to confirm the absence of serologic and/or genomic viral markers of HIV, HBV, HCV, HAV, and B19. In spite of the most rigorous donor screening and donation testing, infectious viruses may still be present in plasma fractionation pools. Therefore, the viral inactivation-removal steps that have been deliberately introduced during plasma products manufacture—and that are described below—play a most critical role in ensuring safety. Altogether, these overlapping tests should ensure that the viral load of the manufacturing pool is both minimal and significantly below the viral reduction capacity of the manufacturing processes used.
Processing, Freezing, Storage, and Transportation of Plasma
Preserving FVIII during blood/plasma collection and preparation is important for most fractionators preparing coagulation factor concentrates. Apheresis plasma can generally be frozen quickly, ensuring optimal FVIII preservation. By contrast, whole blood has to be centrifuged to separate the various components. When blood is cooled to 4°C after collection, plasma should be separated and frozen within 6 to 8 hours to preserve FVIII, but when cooled rapidly at constant 20°C using devices like butanediol plates, coagulation factors are stable for up to 18 to 24 hours. Plasma frozen within 72 hours is suitable for IgG and albumin production.1 After separation from cellular elements, plasma for fractionation should be frozen rapidly below −20°C, −25°C, or −30°C, depending upon local regulations.13 Plasma used to manufacture only albumin and IgG may be frozen below −20 °C within 72 hours of collection.13 In the US Code of Federal Regulations, apheresis plasma should be stored at −20°C or colder immediately after collection. Rapid plasma freezing, to ensure rapid ice front velocity and core temperature of −20°C, preserves FVIII and appears more important than the actual freezing temperature itself.14 Plasma for fractionation is stored at less than −20 °C, or colder, typically for several months or more. Storage temperature should be as constant as possible, including the transportation to the fractionation facilities. Cross-continent or intercontinent shipment of plasma for fractionation is frequent.
Industrial Processing of Plasma
Production Flow Chart
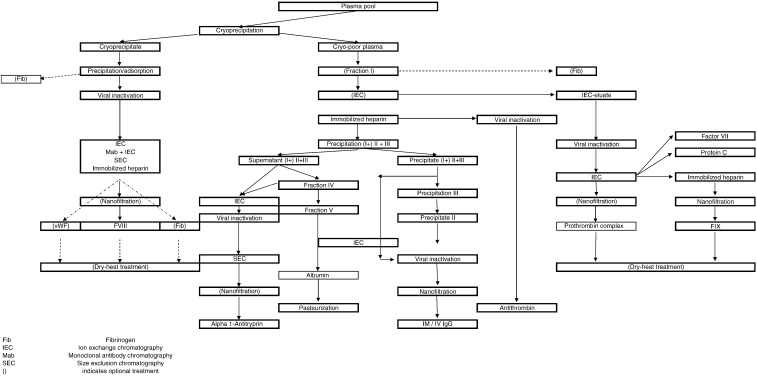
Production steps taking place at fractionation plants are summarized in Figure 1, and typical plasma protein downstream methods are presented in Table 2. Physical compliance with shipping requirements is verified at delivery of the plasma (packaging; labeling; shipping temperature as recorded, eg, on data loggers; availability of samples for additional testing, etc). Plasma is immediately quarantined in a walk-in freezer. Documentation is verified (such as signed certificate of origin and of control of the plasma; collection date, shipping container number, virology and immunohematology screening data; test kits used and batch number) and reconciled with actual delivery. Additional testing, such as mini-pool NAT (eg, HCV, HIV, HBV, HAV, and/or B19) and/or statistical determination of FVIII and protein content by sacrificial sampling of individual plasma donations may be done. Only individual plasma donations that comply with quality and testing requirements are used for fractionation. Before thawing, donations may be “conditioned” at controlled temperature for a few hours to facilitate opening of the plasma packs. Various methods, compatible with hygienic processing to limit bioburden, have been developed for pack opening. Frozen plasmas are expelled from the plastic containers and pooled for cryoprecipitation and further manufacturing steps, as described below. Intermediate fractions generated during production may be stored for subsequent pooling and processing. Purified sterile-filtered products are aseptically dispensed into final containers (glass vials or bottles). Albumin bottles undergo terminal pasteurization. Many products, but albumin and some IgG preparations, are freeze-dried, typically for a duration of 3 to 6 days, depending upon physicochemical characteristics and filled volumes. Batches are quarantined while quality controls and checks of production files take place. Batches meeting specifications are labeled and packaged and subsequently boxed and shipped for distribution. In a few countries, product batches may be released by regulatory authorities. The production cycle of fractionated products takes a few weeks to several months.
Fig 1.
Plasma fractionation flow-chart.
Table 2.
Typical Plasma Protein Downstream Purification Methods
| Method | Description | Separation principle | Application |
|---|---|---|---|
| Cryoprecipitation | Thawing of whole plasma; at +1°C to +4°C | Differential solubility at cold positive temperature | Precipitation of FVIII, VWF, and fibrinogen |
| Ethanol precipitation | Successive precipitation steps of cryo-poor plasma by Ethanol (10%-40%), under precise conditions of pH (ca 7.4-4.5), temperature (−3 to −6°C), protein concentration, and ionic strength | Differential solubility in ethanol at cold negative temperature | Precipitation of fibrinogen, IgG, albumin, AAT, etc |
| Removal of precipitates by centrifugation or depth-filtration | |||
| Ion exchange chromatography | Binding of proteins on a solid support usually packed in a column. Can also be done as a batch process, eg, DEAE, QAE, CM… | Electric charge binding. Elution by increasing salt content or changing pH | Most coagulation factors, protease inhibitors, and anticoagulants |
| Affinity chromatography | Binding of proteins on a solid support most usually packed in a column; ligands include heparin, metals, and gelatin | Specific affinity ligand proteins. Elution usually by increasing salt content | AT, VWF, FIX, etc |
| Immunoaffinity | Binding of proteins on a solid support packed in a column. Ligands include murine monoclonal antibodies | Specific affinity antibodies-proteins. Elution usually by increasing salt content | FVIII, FIX, protein C |
| Size-exclusion chromatography | Injection of proteins on a solid support packed in a column | Separation based on differential molecular mass | AAT, FVIII |
| Ultrafiltration | Selective fractionation process on membranes of defined pore size that concentrates protein and removes low-molecular-weight solutes and salts | Separation based on differential molecular mass | All products |
| Microfiltration | Low-pressure cross-flow membrane process for separating colloidal and suspended particles in the range of 0.2 to 10 _μ_m | All products |
Core Fractionation Technology
Current core fractionation technology largely relies on a backbone process encompassing cryoprecipitation and cold ethanol precipitation steps, as developed in the 1940s by Cohn et al15 in the United States, or modified by Kistler and Nitschman16 in Europe. This process involves successive processing steps at defined ethanol concentrations, associated with shifts in pH, temperature, and osmolality that result in selective precipitation of proteins, most notably IgG and albumin. Precipitates are separated by centrifugation or filtration. In the last few years, the complexity of the fractionation process has increased by (a) the introduction of chromatography to isolate new proteins from existing fractions such as cryoprecipitate, cryo-poor plasma, and Cohn fractions; (b) the integration of chromatography to the ethanol fractionation process to increase IgG recovery; and (c) the implementation of dedicated viral inactivation or removal steps. Chromatography was introduced in the 1960s; however, its application developed mostly in the mid/late 1980s. Anion-exchange chromatography and affinity chromatography are frequently used to capture proteins at physiological pH and ionic strength, therefore best preserving functional activity.17, 18 Immobilized heparin and monoclonal antibodies are common affinity chromatography ligands. Chromatography is used for 4 specific goals: (a) improvement of products purity, (b) extraction of trace labile proteins, (c) optimization of protein recovery, and (d) removal of viral inactivation agents.
Figure 2illustrates a typical industrial fractionation scheme of standard plasma. Plasma packs (typically for a batch of 2000-4000 L) are opened under hygienic conditions, and plasma is expelled from the containers and thawed at 1°C to 4°C. Cryoprecipitate is isolated using refrigerated continuous centrifuges, recovered from the centrifugation bowls and frozen at −30°C or colder for storage until further pooling and processing. The cryo-poor plasma is immediately processed for primary chromatographic capture of labile coagulation factors (such as the factor IX [FIX] complex and its components) and protease inhibitors (such as AT and C1 esterase inhibitor [C1-inh]). The prepurified intermediates may be stored frozen until further processing. The coagulation factors/anticoagulant-depleted plasma undergoes sequential ethanol precipitation steps. This leads to successive precipitations of fibrinogen, IgG and albumin fractions, and intermediates for extraction of other therapeutic proteins, such as AAT (fraction IV-1), or IgM (fraction III). Depth filtration is preferred to centrifugation to separate precipitates and improve protein recovery. The fractionation of hyperimmune plasma (eg, anti-rhesus) is usually performed on small plasma batch sizes, increasingly using full chromatographic processes to optimize the recovery of IgG.
Fig 2.
Typical fractionation scheme.
Core Viral Reduction Methods
No documented transmission of HIV, HBV, or HCV by products subjected to dedicated viral inactivation treatments has been recorded since the end of the 1980s.19 Viral reduction treatments include inactivation steps (where viruses are “killed”) and removal steps (where viruses and proteins partition into distinct fractions). Use of one, or preferably two, distinct dedicated viral reduction treatments is the current “gold standard” for all plasma products. The first treatment is performed primarily to inactivate the most pathogenic viruses (HIV, HBV, and HCV), whereas the second reduction step targets nonenveloped viruses but also contributes to added safety against all agents. Most viral reduction treatments are integrated with the protein fractionation process (“in-process” treatments), but some currently based on heat inactivation procedures are applied on products filled in their final container (terminal treatment). Fractionators are required by regulatory authorities to conduct down-scale experimental validation studies using relevant model viruses to establish the efficacy and robustness of viral reduction procedures. The robustness of the viral reduction procedures in place is exemplified by the absence of transmission of WNV, an emerging virus.20 Similarly, the lipid-enveloped severe acute respiratory syndrome coronavirus has been shown to be inactivated by core viral inactivation treatments of plasma products.21, 22 It is also likely, but not proven by validation experiments yet, that avian flu and simian foamy viruses, which also have a lipid envelope, would be inactivated by current processes in place if present in plasma. Details on viral validation procedures can be found elsewhere.23, 24 The major characteristics of current viral reduction treatments are summarized in Table 3 9, 23 and discussed briefly here.
Table 3.
Viral Reduction Treatments of Licensed Plasma Products
| Treatment | Products | Target viruses in-process treatment | Comments |
|---|---|---|---|
| In-process treatment | |||
| Solvent-detergent | Coagulation factors (eg, FVIII, prothrombin complex, FIX, VWF, fibrinogen) | E | No, or limited, protein denaturation |
| AT | The SD agents are removed by subsequent protein purification steps | ||
| IgG | |||
| Fibrin sealants | |||
| Pasteurization | Coagulation factors (eg, FVIII, fibrinogen) | E | Protein stabilizers may limit viral inactivation |
| IgG | Most NE | B19 is heat resistant | |
| AAT | 10% to 30% loss of functional activity of coagulation factors | ||
| AT | |||
| Vapor heat | Coagulation factors (eg, FVIII) | E | |
| C1-inh | Most NE | As pasteurization | |
| Fibrin sealants | |||
| Low pH (pH 4) treatment | IgG | E | Most other plasma proteins loose functional activity at low pH |
| Caprylic acid treatment (<pH 5.5) | IgG and IgM | pH 4 sensitive NE | Most other plasma proteins loose functional activity at low pH |
| E | |||
| Nanofiltration | E | ||
| Coagulation factors (eg, FIX, FXI, FVIII, VWF) | NE | Viral removal by size-exclusion mechanism depends upon virus size and shape, and nanofilter porosity | |
| IgG | |||
| AAT | |||
| AT | |||
| Fibrin sealant | |||
| Terminal treatment | |||
| Pasteurization | Albumin | E | Only for a product withstanding liquid heat-treatment in the presence of small amount of stabilizers |
| NE | |||
| Dry heat | Coagulation factors (eg, FVIII, FIX, prothrombin complex, FXI) | Some E | Inactivation of heat-resistant viruses depends on temperature and duration |
| Some NE | Hardly inactivates B19 | ||
| 10% to 20% loss of functional activity of coagulation factors |
In-process viral inactivation treatments
The solvent-detergent (SD) treatment, developed in the mid 1980s,25 remains the most frequent core viral inactivation procedure of plasma products. Protein solutions are incubated for 4 to 6 hours at 24°C to 37°C in the presence of 0.3% to 1% Tri-_n_-butyl phosphate (TnBP) and 1% Tween-80 or Triton X-100. Typically, lipid enveloped viruses are inactivated in a matter of minutes, and the functional activity of even the most labile plasma proteins—with the possible exception of some serine prolease inhibitors—is well preserved, but nonenveloped viruses (less pathogenic in most individuals) are not inactivated. The SD agents are removed down to a level of a few parts per million usually by chromatographic adsorption or specific precipitation of proteins, or selective adsorption on hydrophobic chromatographic support.
Pasteurization, another common viral inactivation procedure, is a heat treatment of protein solutions for 10 hours at 60°C, a treatment that denatures viral proteins and inhibits virus replication.26 Pasteurization can inactivate both enveloped and nonenveloped viruses, but stabilizers, needed to limit loss of protein functionality, may decrease the rate and extent of viral inactivation.27 Stabilizers may be removed by ultrafiltration, protein precipitation, or chromatography. Vapor heat has also been used by one company; extent of virus inactivation is influenced by the temperature, duration, and pressure during treatment. Risk of neoantigen formation, which can enhance protein immunogenicity, should be considered when using heat-based inactivation processes.
Low pH incubation, usually at pH 4, at 30°C to 37°C for more than 20 hours, was introduced in the early 1980s to allow the intravenous infusion of IgG. This form of treatment was subsequently found to inactivate most lipid-enveloped viruses. Caprylic (octanoic) acid precipitation/incubation at pH below 6 is a recently introduced treatment of human IgG that can inactivate lipid-enveloped viruses.28, 29
Terminal viral inactivation treatments
In modern plasma fractionation, heat treatment of lyophilized products (dry heat) is used, due to limitations, mostly as a secondary viral inactivation step rather than the core inactivation treatment. The treatment is applied to some coagulation factor concentrates. Performed at 80°C for 72 hours or at 100°C for 30 minutes, generally in the presence of protein stabilizers, it provides added safety against HAV and other heat-sensitive viruses but may not be sufficient to exclude B19 transmission.23 Terminal (liquid) pasteurization at 60°C for 10 hours is the “gold standard” treatment of albumin preparations. The fatty acids, caprylate, and tryptophanate, which protect albumin from heat denaturation, are added at doses compatible with therapeutic use and, therefore, are not removed before product infusion.
Viral removal treatment
Nanofiltration is a specific viral filtration process applied to protein solutions using 15- to 75-nm multi-layers membranes, or equivalent systems, to remove viruses mostly by a sieving mechanism.30, 31 Introduced in the early to mid 1990s, it has reached wide acceptance as a robust viral removal step for essentially all products, apart from albumin. Nanofiltration is used to complement the core viral inactivation treatment and to provide enhanced safety against nonenveloped viruses or other resistant infectious agents. Virus removal can also incidentally take place during protein precipitation, chromatography, or filtration steps; these steps contribute to the lowering of virus load from the protein production stream; they are difficult to monitor; and therefore do not guarantee, as stand-alone procedures, sufficient safety margin.
Prion removal methods
Variant Creutzfeldt-Jakob disease (vCJD) can be transmitted by red blood cell concentrates, but to date, transmission has not been identified from plasma or plasma products. Because of its biological nature, the prion agent is thought to be resistant to current viral inactivation procedures used during plasma fractionation. The methods known to inactivate abnormal misfolded prion proteins associated with transmissible spongiform encephalopathies (PrPTSE) (such as oxidation, treatment with strong base, chaotropic agents, and extreme heat) destroy plasma proteins and, therefore, cannot be used. Still, modern processes of fractionated products would seem to ensure significant removal of PrPTSE, as suggested by experimental spiking studies.32 As scale-down and experimental transmissible spongiform encephalopathy (TSE) spiking models are developed, knowledge on the capacity of manufacturing processes of plasma proteins to remove prions is growing rapidly, although much uncertainty remains because the unknown biological nature of the human plasma associated infectious agent.33 Several processes for manufacturing FVIII, fibrinogen, von Willebrand factor (VWF), FIX, IgG, and albumin products have been shown to be capable of remaining prions in a consistent and reproducible manner.34, 35, 36 Generally, multistep fractionation processes are thought to be a contributing factor to PrPTSE elimination. Two to more than 5 log removals of spiked prions occur during standard protein purification steps such as precipitation with ethanol or polyethylene glycol (PEG), anion-exchange chromatography, and depth filtration.34, 35, 37 Mechanism of removal that may encompass an adsorption mechanism is still not fully understood and appears to be influenced by pH and the concentration of the precipitating agent. The partitioning process may reflect some prion aggregation because of prion hydrophobicity and insolubility. The removal capacity of nanofiltration membranes with pore sizes less than 75 or 35 nm has been extensively investigated,34, 38 and prion removal is likely due in part to molecular sieving. Extent of removal is related to the pore size of the filters and, presumably, to the aggregation state of PrPTSE. Removal is superior with membrane of pore sizes of 15 nm, compared to 35 nm. The nature and origin of the spiking agent used for PrPTSE clearance studies is of critical importance because various spikes differ in size and characteristics. Brain homogenate or brain-derived microsomal fractions from infected animals have usually been used as the source of PrPTSE spiking material. The most reliable and quantitative detection method of PrPTSE is based on animal bioassays, which require many animals and a time frame generally longer than 9 months. In vitro immunochemical methods, such as a Western blot assay, are being used, at least as a first marker of the presence of PrPTSE and associated infectivity, by detecting the protease-resistant fragment.33 Thus, the risk transmission of vCJD by human plasma products appears remote, but caution should prevail since the biochemical nature of the infectious agent in human blood is not known.
Manufacturing Processes of Plasma Protein Products
Technologies to extract coagulation factors, protease inhibitors, and IgG have evolved considerably in the last 20 years, leading to the development of products with improved safety and purity profiles. A description of major manufacturing techniques is given below.
Coagulation Factors
Factor VIII
Several generations of FVIII preparations have been developed since the mid-1980s, providing (a) safety from HIV and then HCV and HBV, (b) improved purity, and (c) enhanced safety from HAV and B19. Current development efforts focus on establishing prion removal.36 All currently licensed plasma-derived FVIII concentrates are purified from cryoprecipitate. In a typical process, cryoprecipitate is subjected to a combination of aluminium hydroxide adsorption and precipitation, or precipitation only (eg, using glycine), to reduce the level of trace vitamin K coagulation factors (as they may activate FVIII during the downstream purification steps) or load proteins such as fibrinogen. The purified cryoprecipitate extract usually undergoes viral inactivation typically by SD or pasteurization. Many processes include subsequent chromatography by anion exchange, monoclonal antibody affinity (using anti-FVIII or anti-VWF murine antibodies), or immobilized heparin affinity to remove protein contaminants (such as fibrinogen or fibronectin), most or part of the VWF, and the SD agents.39 Immunopurified FVIII eluate is further purified by chromatography to remove murine IgG ligands that may have leached. Prior to formulation and sterile filtration, some FVIII products are nanofiltered using membranes with a pore size of 35, 20, or even 15 nm if a partial dissociation of FVIII and high-molecular-weight VWF multimers is initiated. Alternatively, some freeze-dried preparations are subjected to heat treatment at 80°C or 100°C to inactivate nonenveloped viruses like HAV. Recovery of FVIII, as expressed per liter of plasma, is usually comprised between 100 and 200 IU (1 IU is defined as the physiological activity present in 1 mL of plasma). Factors lowering FVIII yield include cryoprecipitation (ca 30% loss), chromatographic purification (ca 20% to 30 %), and viral heat inactivation (15%-30%). Current FVIII concentrates have a specific activity between 10 and 250 IU/mg. Some products are formulated with human plasma–derived albumin, whereas others contain copurified VWF that helps stabilize FVIII. Purity of FVIII concentrates has not been convincingly demonstrated to enhance immunological safety. Long-term clinical experience indicates that the alleged reduced immunosuppressive effects of immunopurified preparations compared to lower-purity plasma-derived preparations, as claimed in the late 1990s, were probably unfounded. Processes used that, in part, influence residual VWF, may have an impact on the immunogenicity of plasma-derived FVIII products. Retrospective studies of previously untreated patients show that an SD-treated, nanofiltered, ion-exchange purified FVIII product containing VWF appears to be 2 times less likely to induce anti-FVIII inhibitors in hemophilia A patients than 2 full-length recombinant FVIII concentrates.40
von Willebrand factor
Because FVIII chromatographic purification removes all, or part, of VWF, FVIII products effective in treating Von Willebrand disease (VWD) are generally low-purity (“intermediate purity”) preparations prepared from cryoprecipitate by precipitation steps coextracting VWF and FVIII in a ratio of higher than 1. The low purity and the high protein content of these products technically restrict the choice of viral inactivation treatments to pasteurization or terminal dry heat at 80°C for 72 hours. One highly purified VWF concentrate, largely devoid of FVIII and, therefore, specific for VWD treatment, is prepared from cryoprecipitate by a 3-step chromatographic procedure (integrated with FVIII and fibrinogen purification processes) using 2 anion exchangers and immobilized gelatin polishing (to remove fibronectin).41 Viral reduction is by SD, 35-nm nanofiltration, and terminal dry heat at 80°C for 72 hours.42
Fibrinogen
There are 5 registered fibrinogen preparations available for treating a fibrinogenemia or hypofibrinogenemia.43 Traditional preparations are obtained by multiple precipitation steps of plasma or cryoprecipitate using ethanol and glycine, whereas other modern products are purified by chromatography. Viral reduction is achieved by SD treatment, often complemented by 35-nm nanofiltration or terminal dry heat treatment. Single-step pasteurization at 60°C for 20 hours is used for 1 product.
Fibrin sealants
Fibrin sealants (fibrin glues) comprise fibrinogen-rich and purified thrombin concentrates. When mixing the 2 components, a strong adhesive clot exhibiting hemostatic, sealing, and healing properties is formed almost instantaneously or within a few seconds, offering multiple topical surgical applications. Fibrinogen is prepared by precipitation methods from cryoprecipitate, or from the Cohn fraction I; the fraction may also contain fibronectin, VWF, or factor XIII (FXIII), which may confer other physiological functions.44 Fibrinogen fractions are virally inactivated by SD, pasteurization, vapor-heat treatment, and/or nanofiltration. The fibrinogen concentration is typically above 80 g/L and may be formulated in the presence of an antifibrinolytic agent.
Prothrombin complex
Prothrombin complex concentrate (PCC) is a mixture of vitamin K–dependent coagulation factors in which FIX, factor II, and factor X and proteins C and S have a low specific activity between 0.5 and 2 IU/mg.45 A few products contain also factor VII (FVII), but usually at levels lower than that of FIX. The manufacture is usually based on a 1960s method that involves diethylaminoethyl (DEAE) Sephadex or DEAE cellulose adsorption of cryo-poor plasma, but downstream ethanol fractions can also be used as starting materials.46 Anion exchangers coextract proteins sharing the presence of gamma-carboxyglutamic acid residues, whereas bulk plasma proteins, such as albumin and IgG or AT and AAT remain in the unbound fraction. Precipitation with tricalcium phosphate is used for one product. Viral reduction is most often achieved by SD (TnBP-Tween 80) treatment, complemented by 35- or 15-nm nanofiltration or by terminal dry heat. Pasteurization and vapor heat are applied to 2 products. Viral inactivation treatment by SD requires one subsequent ion-exchange chromatographic step for removal of the virus-inactivating agents. Recovery is in the range of 250 to 380 IU of FIX per liter of plasma.
Single FIX
High-purity FIX products were developed in late 1989,47 leading to reduced risks of thromboembolism, compared to PCC, in hemophilia B patients. FIX is isolated by chromatographic purification of the PCC using anion exchange combined with either immobilized heparin, metal chelate affinity, or monoclonal antibody. These processes yield FIX concentrates with a mean specific activity in the range of 100 to 150 IU/mg and a yield between 200 and 300 IU/L of plasma.
Factor VII
Three specific concentrates rich in FVII and with reduced amount of other vitamin K–dependent clotting factors are currently licensed to control bleeding in deficient patients. The manufacturing process includes ion-exchange chromatography or aluminium hydroxide adsorption, following a downstream procedure similar to that of PCC and FIX. Viral inactivation is achieved by SD treatment, vapor heat, or dry heat.
Factor XI
Two factor XI (FXI) concentrates are currently available for deficient patients. One, of low purity, is purified by chromatography of cryo-poor plasma on DEAE cellulose and immobilized heparin. After freeze-drying, the product is virally inactivated by dry heat. In the other product, FXI is captured by adsorptive filtration then highly purified by cation-exchange chromatography.48 This product undergoes dual viral reduction processing by SD and 15-nm nanofiltration.
Factor XIII
Factor XIII is a transglutaminase that catalyzes the final step in the coagulation cascade, cross-linking the loose fibrin polymer into a highly organized structure. The early generation of FXIII concentrates for the treatment of FXIII-deficient patients was extracted from placenta, but 2 plasma-derived products have subsequently been developed. One is purified from a cold-ethanol fraction from cryoprecipitate supernatant, purified by precipitation with sodium citrate and removal of fibrinogen by heating. The product is pasteurized in sorbitol solution, ultrafiltered to remove sorbitol, adsorbed with bentonite, and freeze-dried. The other product is obtained by precipitation steps and is also pasteurized. Factor XIII is also a component of some fibrin sealants. Although still subject to discussion, the presence of FXIII is claimed to contribute to fibrin _γ_-chain cross-linking and tensile strength, possibly improving the hemostatic effect.49
Activated Coagulation Factors
Human thrombin concentrates are available so far only as components of fibrin sealant. Thrombin is prepared by activation of the PCC, usually in the presence of calcium chloride, followed by viral inactivation treatment by SD, purification by cation-exchange chromatography, and viral removal by 15-nm nanofiltration. Final thrombin concentration is usually between 300 and 1000 IU/mL, but preparations with lower potency are available when a slower speed for clot formation of the sealant is preferred for some surgical applications requiring longer time for tissue gluing.
A procedure for large-scale production of a purified plasma-derived FVIIa has been developed in Japan.50 FVII is purified by anion exchange and immunoaffinity chromatography and converted to FVIIa by autoactivation on an anion-exchange resin and incubation in the presence of Ca2+ for 18 hours at 10°C. This preparation is virally reduced by nanofiltration and dry-heating and is intended for the treatment of hemophiliacs with antibodies against FVIII or FIX.
Anticoagulants and Protease Inhibitors
Antithrombin
Antithrombin concentrates were the first plasma products extracted by affinity chromatography. Production from cryo-poor plasma usually comprises ion exchange chromatography to remove the PCC components, followed by capture of AT on immobilized heparin. Viral inactivation is traditionally achieved by pasteurization in the presence of sodium citrate or a combination of sucrose and glycine, although SD treatment is used as well. Because heat treatment may partially denature AT, a second adsorption step on immobilized heparin can be used to remove altered molecules. Recovery is between 250 and 350 U/L of plasma. Fraction IV-1 is an alternative starting material, but yield is significantly lower.
_α_1-Antitrypsin (_α_1-protease inhibitor)
There are now several licensed AAT concentrates, including 3 in the United States. _α_1-Antitrypsin augmentation therapy is indicated for the treatment of patients with lung emphysema secondary to congenital AAT deficiency. Because AAT shares many physicochemical properties, in particular, molecular weight and isoelectric point, with albumin, it has been difficult to design production methods for AAT not affecting the existing production process for albumin. Most preparations are recovered from fraction IV1-4. Purification from this waste fraction is rather cumbersome and involves PEG precipitation and ion-exchange chromatography, considerably compromising recovery (∼0.2 g/L). The first preparations developed in the 1990s were virally inactivated by pasteurization, but dual viral reduction steps using SD and nanofiltration are also now used. Recent isoelectrofocusing studies have provided evidence indicating that new anodal AAT variants are present in at least one of the FDA-licensed product, suggesting alteration of some isoforms during purification. Loss of a C-terminal positive charged lysine, secondary to carboxypeptidase activity, was proposed as an explanation for these isoelectrofocusing migration shifts. Under circumstances when clinical demand for albumin decreases, extracting AAT from upstream fractions, such as the supernatant II+III,51 appears a logical trend, offering the possibility of a more effective production scheme, characterized by a recovery of 0.6 to 1 g/L.51
Protein C
There are 2 protein C concentrates manufactured in Europe and 1 in Japan. In one process, the PCC undergoes cascade purification on 3 ion exchangers,52 whereas in the other, immunoaffinity and affinity chromatographic processes are combined with ion exchange. Viral reduction is achieved by SD treatment, which can be combined with 15-nm nanofiltration or vapor-heat treatment.53
C1-esterase inhibitor
C1-esterase inhibitor concentrates are used for the treatment of acute phases of angioedema, primarily in the oropharyngeal region and gastrointestinal tract in patients with congenital or acquired C1-inh deficiency. There are 3 products licensed in Europe. Products are generally purified by chromatography from the cryo-poor plasma after extraction of the PCC and, potentially, AT. Viral inactivation is achieved by pasteurization, vapor heat, or SD, possibly combined with nanofiltration.
Albumin
Essentially all therapeutic albumin preparations are prepared by fractionation of cryo-poor (or PCC-poor and/or AT-poor and/or C1-inh-poor) plasma by ethanol fractionation. A critical upstream process step (precipitation II+III) separates the IgG fraction. To optimize recovery, the precipitates generated during the ethanol fractionation process are separated by depth filtration. Albumin recovery of 75% to 85% (25-28 g/L) and purity of 96% to 98% are typically obtained. Some processes combine ethanol fractionation with a polishing ion exchange chromatography, which generally improves product purity to ca 99%, whereas in one production method, albumin is purified mostly by anion exchange, cation-exchange, and size-exclusion chromatography. The adjustment of the concentration of the purified fraction, typically from 4% to 25%, is achieved by ultrafiltration. The standard viral inactivation method is pasteurization, which, according to most Pharmacopeias, should be performed in the final container rather than on the albumin batch before aseptic filling. Current mean albumin yield is 24 to 26 g/L of plasma.
Immunoglobulin G
Polyvalent IgG preparations, either for intramuscular or intravenous uses, are traditionally prepared from the fraction II that is obtained by stepwise fractionation of cryo-poor plasma using cold ethanol at concentrations up to 25%. Increasingly, IgG products are extracted from up-streamed ethanol precipitated fractions, such as supernatant III or precipitate II+III, to optimize recovery. Intermediate IgG fractions are subjected to ion-exchange chromatography, caprylic acid, or PEG precipitations to remove protein contaminants, proteolytic enzymes, and/or aggregates. Most current viral inactivation procedures are low pH incubation, pasteurization, or SD; the caprylic acid treatment, recently introduced in the manufacture of human IgG products, is also a robust viral inactivation process of IgG. Dedicated viral removal by 15- to 35-nm nanofiltration is commonly used to increase the safety against nonenveloped viruses, especially in a situation when the core viral inactivation treatments target only lipid-enveloped viruses. The IgG recovery has long been in the 2.7- to 3.2-g/L range when combining traditional ethanol fractionation processes and centrifugation. Depth-filtration and/or chromatographic purification from upstream fractions have improved the mean recovery to the 3.5- to 4.5-g/L range, or more. Total chromatographic procedures are increasingly used for the production of hyperimmune IgG products because such processes are amenable to the fractionation of smaller plasma volumes and can optimize recovery. The manufacturing process includes at least 2 dedicated viral reduction treatments.
Other Potential Plasma Protein Therapies or Indications
Other protein components have been fractionated at pilot-scale and subjected to experimental or human trials. Inter–_α_-trypsin inhibitor (ITI) is a Kunitz-type serine proteinase inhibitor. Its inhibitory capacity is carried by bikunin, a chondroitin 4-sulfate proteoglycan, which is covalently linked to its heavy chains H1 and H2, but can be released by proteolytic cleavage. An ITI concentrate has been obtained by fractionation of the prothrombin complex on an anion exchanger followed by immobilized heparin and viral inactivation by SD. ITI, as a reservoir of bikunin, may be involved in control of inflammatory processes. In a porcine model of endotoxin shock, ITI improved the hemodynamic, oxygenation, and coagulation parameters.54 Administration of ITI very early after the onset of sepsis or repeated injections at later time points (10 and 20 hours) maintains cardiovascular stability and significantly reduces mortality in a rat model.55
Transferrin is the major iron binding plasma protein which may prevent cytotoxic effects or predisposition to septic infection due to accumulated free non-transferrin-bound iron when normal iron use is hampered and/or apotransferrin production is decreased. A liquid apotransferrin concentrate has been obtained from Cohn fraction IV by 2 ion exchange chromatographic steps and ultrafiltration. Viral safety was ensured by SD treatment, nanofiltration, and PEG precipitation. The product had intact iron binding capacity, and maintained the bacterial growth inhibitory effect in serum. In hematological stem cell transplant patients, the product prevented the appearance of non-transferrin-bound iron.
Apolipoprotein A-I is the principal protein component of the plasma high-density lipoproteins. It prevents the accumulation of cholesterol-loaded macrophages which deposit on the arterial wall as foam cells. Apolipoprotein A-I inhibits hepatic lipase and lipoprotein lipase in vitro. A concentrate has been isolated by precipitation from Cohn Fraction III.56 Intravenous injections in men were well tolerated in an early clinical trial; clinical observations were consistent with combined inhibition of hepatic lipase and lipoprotein lipase activities. Possible clinical applications include the treatment of hypercholesterolemic patients and atherosclerosis. Recombination with lecithin forms a high-density lipoprotein complex that could help limit inflammation, endotoxin-induced activation of coagulation, and fibrinolysis in septic conditions.
Mannan-binding lectin (MBL) is a component of the innate (aspecific) immune system that can bind repetitive structures of mannan groups, such as those on the surface of micro-organisms, activating the complement system and leading to the destruction of a large variety of micro-organisms. The relatively frequent congenital deficiency of MBL is associated to recurrent infections, especially in infants when the specific immune system has not yet matured. An MBL concentrate has been produced from COHN fraction III.57
Plasmin is the major fibrinolytic enzyme in plasma. Encouraging results as a new fibrinolytic agent have been obtained in animal models where plasmin was applied directly to the clot through a catheter to treat peripheral arterial occlusion.
von Willebrand factor cleaving protease (VWF-CP, ADAMTS13) cleaves ultra-large multimers of VWF that enter the blood stream directly after biosynthesis by endothelial cells. If developed, a purified VWF-CP could be useful to treat, in place of plasma, patients with thrombotic thrombocytopenic purpura with congenital or acquired deficiency of VWF-CP.
Activated protein C can be of value for the treatment of sepsis, as demonstrated through the clinical use of a recombinant preparation. There is no therapeutic plasma-derived activated protein C products available yet for therapeutic use, although a process for a highly purified preparation, where cryo-poor plasma is purified by immunoaffinity and anion-exchange chromatographic steps, and SD viral inactivation has been described.58
There is also research needed to investigate alternative indications for currently available products. Potential clinical use of C1-inh, aimed at benefiting from its role as inhibitor or attenuator of the activation of complement and contact systems, include septicemia, myocardial infarction, capillary leak syndrome, pancreatitis, and organ transplantation. Intravenous use of a FXIII concentrate was found to increase epidermal growth factor and transforming growth factor–β, suggesting that it may accelerate wound healing of anastomotic leaks and nonhealing fistulas. Factor XIII was also found to have osteoinductive properties, suggesting use in bone tissue engineering.
Regulatory Aspects
A review on plasma fractionation should also cover the role played by national and international regulatory authorities. Over the last few years, assuring the safety of large-pool plasma products has posed formidable challenges to regulatory authorities and fractionators alike. The complexity of the field, encompassing the diversity in blood and plasma product types and manufacturing processes, made it difficult to enact balanced decisions toward ensuring both product safety and guarantee of supply. Since the 1980s, the agencies regulating the plasma fractionation industry have developed a comprehensive set of measures to ensure the viral safety of plasma products. Multiple layers of regulatory oversight of the plasma industry have been established to ensure overlapping safeguards against the risks of the transmission of blood-borne infectious agents. Several regulations, guidances, position statements have been issued by agencies like the US FDA and the European Medicine Evaluation Agency, which are updated as needed. Those cover important safety aspects required at all stages of the manufacturing chain, from activities at blood establishment preparing plasma for fractionation, extending to the manufacturing and distribution of plasma products. Regulatory oversight includes epidemiologic surveillance of the donor population; donor deferral policies and screening practices; mandatory donation testing; testing of manufacturing plasma pools; validation of viral reduction procedures and other production steps; as well as the assessment of product quality, safety, and efficacy for marketing authorization. Most of relevant information on plasma collection is assembled in Europe in the Plasma Master File, which allows establishing key levels of information regarding the quality and safety of the plasma raw material. Post marketing, reports on adverse reactions associated with plasma products should be transmitted to NRAs and could prompt emergency response procedures and product recalls.10 Some harmonization of the requirements for manufacture and supply of plasma products in the United States, Europe, and Japan is taking place under the auspices of the International Conference on Harmonisation, but much work remains to be done. Such measures, nonetheless, provide the framework through which modern plasma products now exhibit a very high level of quality, safety, and efficacy. However, the rigidity of the regulatory system has been an impediment to more significant technological evolution of the plasma fractionation process because process changes are currently associated to major regulatory work.
Conclusions
The Cohn plasma fractionation method initially designed to obtain albumin has, over the years, developed rather successfully into a well-established industrial procedure isolating a wide range of clinically useful products. Today, more than 20 different protein products, and more if one considers the variety of hyperimmune IgG preparations, can be extracted through large-scale fractionation of human plasma. The soundness and large-scale adaptability of the technology, on the one hand, and the rigidity of the current regulatory framework, on the other, explains why this technology remains the main core method in use at industrial scale, although it implies suboptimal yield for most proteins, apart from albumin. The technology has increased in complexity over the years, with the greatest progresses in purification being, without doubt, associated with the use of chromatographic methods that have made possible the development of new protein therapeutics and impressive improvements in product purity and quality. The fractionation scheme has also changed dramatically through the introduction of in-process viral reduction treatments, which have required the addition of downstream techniques such as chromatography and ultrafiltration.
The change in protein drivers, with the prominent clinical role now played by IgG, and the requirements to increase protein recovery and optimize the fractionation process may crystallize the incentive for most fractionators to abandon relative technical conservatism and introduce significant technological changes in processing technology. The production of IgG from precipitate (I)+ II+III, already implemented by some manufacturers and, possibly, that of AAT from supernatant (I)+ II+III, are signs of a gradual evolution in the direction of total chromatographic processing. With so much gained, over the last few years, in the understanding of the key parameters building plasma product quality, safety, and efficacy, one can hope that the regulatory paths, in particular, with regard to clinical studies, to license known protein therapeutics prepared by improved, more efficient technology should be simplified. This would, in turn, contribute to an improved supply.
As the plasma fractionation industry has so far been targeting mostly proteins that were obvious candidates for replacement therapy, it is also hoped that it will invest more into developing new products because plasma remains a unique source of potential therapeutic proteins. Proactive research and development work should be encouraged to isolate and evaluate new therapies among the well characterized plasma proteins with still unknown function.
Finally, one should not forget that plasma protein therapies are expensive and largely inaccessible to the developing world. The new market drivers in rich countries are likely to diminish economical interest in the manufacture of FVIII that remain a major protein therapy in need in the developing world. The belief that decreased use of plasma-derived FVIII or FIX in developed economies (as hemophiliacs are switching to recombinant therapies) would increase the supply of products to developing countries may be not economically viable. Rather, this switch to recombinant products in rich countries can make poor countries unable to afford the increased cost of products no longer subsidized by the premium price paid in rich countries. It therefore remains important to develop affordable viral inactivation and processing technologies gradually allowing developing countries to make use of local plasma resources in a safe manner.59
References
- 1.WHO Recommendations for the production, quality control and regulation of plasma for fractionation. 2005. http://www.who.int/bloodproducts
- 2.CHMP: Guideline on epidemiological data on blood transmissible infections. For inclusion in the Guideline on the Scientific data requirements for a Plasma Master File. (EMEA/CPMP/BWP/3794/03). EMEA/CPMP/BWP/125/04, January 2005. http://www.emea.eu.int: European Medicine Agency, 2005.
- 3.Burgstaler E. Current instrumentation for apheresis. In: McLeod B.C., Price T.H., Weinstein R., editors. Apheresis: Principles and practice. ed 2. AABB Press; Bethesda, MD: 2003. pp. 95–130. [Google Scholar]
- 4.Burnouf T., Kappelsberger C., Frank K. Residual cell content in plasma from 3 centrifugal apheresis procedures. Transfusion. 2003;11:1522–1526. doi: 10.1046/j.1537-2995.2003.00543.x. [DOI] [PubMed] [Google Scholar]
- 5.Sonntag J., Emeis M., Vornwald A. Complement activation during plasma production depends on the apheresis technique. Transfus Med. 1998;8:205–208. doi: 10.1046/j.1365-3148.1998.00150.x. [DOI] [PubMed] [Google Scholar]
- 6.Burnouf T., Eber M., Kientz D. Assessment of complement activation during membrane-based plasmapheresis procedures. J Clin Apheresis. 2004;19:142–147. doi: 10.1002/jca.20019. [DOI] [PubMed] [Google Scholar]
- 7.Cardigan R., Sutherland J., Garwood M. The effect of leukocyte depletion on the quality of fresh-frozen plasma. Br J Haematol. 2001;114:233–240. doi: 10.1046/j.1365-2141.2001.02907.x. [DOI] [PubMed] [Google Scholar]
- 8.Dodd R.Y. Current safety of the blood supply in the United States. Int J Hematol. 2004;80:301–305. doi: 10.1532/ijh97.04123. [DOI] [PubMed] [Google Scholar]
- 9.Burnouf T., Radosevich M. Reducing the risk of infection from plasma products: Specific preventative strategies. Blood Rev. 2000;14:94–110. doi: 10.1054/blre.2000.0129. [DOI] [PubMed] [Google Scholar]
- 10.Farrugia A. World Federation of Hemophilia; Montreal: 2004. Guide for the assessment of clotting factor concentrates for the treatment of hemophilia; p. 55. www.wfh.org. [Google Scholar]
- 11.Farrugia A. Plasma for fractionation: Safety and quality issues. Haemophilia. 2004;10:334–340. doi: 10.1111/j.1365-2516.2004.00911.x. [DOI] [PubMed] [Google Scholar]
- 12.Willkommen H., Schmidt I., Lower J. Safety issues for plasma derivatives and benefit from NAT testing. Biologicals. 1999;27:325–331. doi: 10.1006/biol.1999.0227. [DOI] [PubMed] [Google Scholar]
- 13.Anonymous . European Pharmacopeia; Strasbourg: 2005. Monograph of human plasma for fractionation 01/2005:0853 corrected. [Google Scholar]
- 14.Swärd-Nilsson A.-M., Persson P.-O., Johnson U. Factors influencing Factor VIII activity in frozen plasma. Vox Sang. 2006;90:33–39. doi: 10.1111/j.1423-0410.2005.00715.x. [DOI] [PubMed] [Google Scholar]
- 15.Cohn E., Strong L., Hughes W. Preparation and properties of serum and plasma proteins. IV. A system for the separation into fractions of the protein and lipoprotein components of biological tissues and fluids. J Am Chem Soc. 1946;68:459–475. doi: 10.1021/ja01207a034. [DOI] [PubMed] [Google Scholar]
- 16.Kistler P., Nitschmann H. Large-scale production of human plasma fractions. Eight years experience with the alcohol fractionation procedure of Nitschmann, Kistler and Lergier. Vox Sang. 1962;7:414–424. doi: 10.1111/j.1423-0410.1962.tb03274.x. [DOI] [PubMed] [Google Scholar]
- 17.Burnouf T. Integration of chromatography with traditional plasma protein fractionation methods. Bioseparation. 1991;1:383–396. [Google Scholar]
- 18.Burnouf T., Radosevich M. Affinity chromatography in the industrial purification of plasma proteins for therapeutic use. J Biochem Biophys Methods. 2001;49:575–586. doi: 10.1016/s0165-022x(01)00221-4. [DOI] [PubMed] [Google Scholar]
- 19.Tabor E. The epidemiology of virus transmission by plasma derivatives: clinical studies verifying the lack of transmission of hepatitis B and C viruses and HIV type 1. Transfusion. 1999;39:1160–1168. doi: 10.1046/j.1537-2995.1999.39111160.x. [DOI] [PubMed] [Google Scholar]
- 20.Kreil T.R. West Nile virus: Recent experience with the model virus approach. Dev Biol (Basel) 2004;118:101–105. [PubMed] [Google Scholar]
- 21.Yunoki M., Urayama T., Yamamoto I. Heat sensitivity of a SARS-associated coronavirus introduced into plasma products. Vox Sang. 2004;87:302–303. doi: 10.1111/j.1423-0410.2004.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabenau H.F., Biesert L., Schmidt T. SARS-coronavirus (SARS-CoV) and the safety of a solvent/detergent (S/D) treated immunoglobulin preparation. Biologicals. 2005;33:95–99. doi: 10.1016/j.biologicals.2005.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO . Guidelines on viral inactivation and removal procedures intended to assure the viral safety of human blood plasma products. 2003. pp. 1–72. (Geneva, www.WHO.int) [Google Scholar]
- 24.CPMP . European Agency for the Evaluation of Medicinal Products (EMEA); London: 1996. Note for Guidance on Virus Validation Studies: the Design, Contribution and Interpretation of Studies Validating the Inactivation and Removal of Viruses (revised). CPMP/BWP/CPMP/5136/03. http://www.emea.eu.int. [Google Scholar]
- 25.Horowitz B., Wiebe M.E., Lippin A. Inactivation of viruses in labile blood derivatives. I. Disruption of lipid-enveloped viruses by tri(n-butyl)phosphate detergent combinations. Transfusion. 1985;25:516–522. doi: 10.1046/j.1537-2995.1985.25686071422.x. [DOI] [PubMed] [Google Scholar]
- 26.Nowak T., Niedrig M., Bernhardt D. Inactivation of HIV, HBV, HCV related viruses and other viruses in human plasma derivatives by pasteurisation. Dev Biol Stand. 1993;81:169–176. [PubMed] [Google Scholar]
- 27.Ng P.K., Dobkin M.B. Pasteurization of antihemophilic factor and model virus inactivation studies. Thromb Res. 1985;39:439–447. doi: 10.1016/0049-3848(85)90167-7. [DOI] [PubMed] [Google Scholar]
- 28.Dichtelmuller H., Rudnick D., Kloft M. Inactivation of lipid enveloped viruses by octanoic acid treatment of immunoglobulin solution. Biologicals. 2002;30:135–142. doi: 10.1006/biol.2002.0332. [DOI] [PubMed] [Google Scholar]
- 29.Korneyeva M., Hotta J., Lebing W. Enveloped virus inactivation by caprylate: A robust alternative to solvent-detergent treatment in plasma derived intermediates. Biologicals. 2002;30:153–162. doi: 10.1006/biol.2002.0334. [DOI] [PubMed] [Google Scholar]
- 30.Burnouf T., Radosevich M. Nanofiltration of plasma-derived biopharmaceutical products. Haemophilia. 2003;9:24–37. doi: 10.1046/j.1365-2516.2003.00701.x. [DOI] [PubMed] [Google Scholar]
- 31.Burnouf T., Radosevich M., Goubran H.A. Place of nanofiltration for assuring viral safety of biologicals. Curr Nanosci. 2005;1:189–201. [Google Scholar]
- 32.Foster P.R., Welch A.G., McLean C. Studies on the removal of abnormal prion protein by processes used in the manufacture of human plasma products. Vox Sang. 2000;78:86–95. doi: 10.1159/000031156. [DOI] [PubMed] [Google Scholar]
- 33.WHO WHO guidelines on tissue infectivity distribution in transmissible spongiform encephalopathies. 2006. http://www.who.int/bloodproducts
- 34.Flan B., Aubin J.T. Evaluation de l'efficacité des procédés de purification des proteins plasmatiques à éliminer les agents transmissibles non conventionnels. Virologie. 2005;9:S45–S56. [Google Scholar]
- 35.Foster P.R. Removal of TSE agents from blood products. Vox Sang. 2004;87:7–10. doi: 10.1111/j.1741-6892.2004.00444.x. (suppl 2) [DOI] [PubMed] [Google Scholar]
- 36.Foster P.R., Griffin B.D., Bienek C. Distribution of a bovine spongiform encephalopathy-derived agent over ion-exchange chromatography used in the preparation of concentrates of fibrinogen and factor VIII. Vox Sang. 2004;86:92–99. doi: 10.1111/j.0042-9007.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- 37.Burdick M.D., Pifat D.Y., Petteway S.R., Jr Clearance of prions during plasma protein manufacture. Transfus Med Rev. 2006;20:57–62. doi: 10.1016/j.tmrv.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Tateishi J., Kitamoto T., Mohri S. Scrapie removal using Planova virus removal filters. Biologicals. 2001;29:17–25. doi: 10.1006/biol.2001.0269. [DOI] [PubMed] [Google Scholar]
- 39.Burnouf T., Burnouf-Radosevich M., Huart J.J. A highly purified factor VIII:c concentrate prepared from cryoprecipitate by ion-exchange chromatography. Vox Sang. 1991;60:8–15. doi: 10.1111/j.1423-0410.1991.tb00864.x. [DOI] [PubMed] [Google Scholar]
- 40.Goudemand J., Rothschild C., Demiguel V. Influence of the type of factor VIII concentrate on the incidence of factor VIII inhibitors in previously untreated patients with severe hemophilia A. Blood. 2006;107:46–51. doi: 10.1182/blood-2005-04-1371. [DOI] [PubMed] [Google Scholar]
- 41.Burnouf-Radosevich M., Burnouf T. Chromatographic preparation of a therapeutic highly purified von Willebrand factor concentrate from human cryoprecipitate. Vox Sang. 1992;62:1–11. doi: 10.1111/j.1423-0410.1992.tb01159.x. [DOI] [PubMed] [Google Scholar]
- 42.Mazurier C., Poulle M., Samor B. In vitro study of a triple-secured von Willebrand factor concentrate. Vox Sang. 2004;86:100–104. doi: 10.1111/j.0042-9007.2004.00398.x. [DOI] [PubMed] [Google Scholar]
- 43.Kasper C.K., Costa e Silva M. Registry of clotting factor concentrates. World Federation of Hemophilia; Montréal: 2005. pp. 1–13. [Google Scholar]
- 44.Radosevich M., Goubran H.A., Burnouf T. Fibrin sealant: Scientific rationale, production methods, properties, and current clinical use. Vox Sang. 1997;72:133–143. doi: 10.1046/j.1423-0410.1997.7230133.x. [DOI] [PubMed] [Google Scholar]
- 45.Pejaudier L., Kichenin-Martin V., Boffa M.C. Appraisal of the protein composition of prothrombin complex concentrates of different origins. Vox Sang. 1987;52:1–9. doi: 10.1111/j.1423-0410.1987.tb02979.x. [DOI] [PubMed] [Google Scholar]
- 46.Burnouf T. Chromatography in plasma fractionation: benefits and future trends. J Chromatogr B Biomed Appl. 1995;664:3–15. doi: 10.1016/0378-4347(94)00532-a. [DOI] [PubMed] [Google Scholar]
- 47.Burnouf T., Michalski C., Goudemand M. Properties of a highly purified human plasma factor IX:c therapeutic concentrate prepared by conventional chromatography. Vox Sang. 1989;57:225–232. doi: 10.1111/j.1423-0410.1989.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 48.Burnouf-Radosevich M., Burnouf T. A therapeutic, highly purified factor XI concentrate from human plasma. Transfusion. 1992;32:861–867. doi: 10.1046/j.1537-2995.1992.32993110761.x. [DOI] [PubMed] [Google Scholar]
- 49.Dickneite G., Metzner H.J., Kroez M. The importance of factor XIII as a component of fibrin sealants. J Surg Res. 2002;107:186–195. doi: 10.1006/jsre.2002.6495. [DOI] [PubMed] [Google Scholar]
- 50.Tomokiyo K., Yano H., Imamura M. Large-scale production and properties of human plasma-derived activated Factor VII concentrate. Vox Sang. 2003;84:54–64. doi: 10.1046/j.1423-0410.2003.00247.x. [DOI] [PubMed] [Google Scholar]
- 51.Burnouf T., Constans J., Clerc A. Biochemical and biological properties of an alpha 1-antitrypsin concentrate. Vox Sang. 1987;52:291–297. doi: 10.1111/j.1423-0410.1987.tb04895.x. [DOI] [PubMed] [Google Scholar]
- 52.Radosevich M., Zhou F.L., Huart J.J. Chromatographic purification and properties of a therapeutic human protein C concentrate. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;790:199–207. doi: 10.1016/s1570-0232(03)00091-6. [DOI] [PubMed] [Google Scholar]
- 53.Kasper C.K., Brooker M. ed 7. World Federation of Hemophilia; Montreal: 2006. Registry of clotting factor concentrates; pp. 1–14. [Google Scholar]
- 54.Jourdain M., Carrette O., Tournoys A. Effects of inter-alpha-inhibitor in experimental endotoxic shock and disseminated intravascular coagulation. Am J Respir Crit Care Med. 1997;156:1825–1833. doi: 10.1164/ajrccm.156.6.9611100. [DOI] [PubMed] [Google Scholar]
- 55.Wu R., Cui X., Lim Y.P. Delayed administration of human inter-alpha inhibitor proteins reduces mortality in sepsis. Crit Care Med. 2004;32:1747–1752. doi: 10.1097/01.ccm.0000132903.14121.0e. [DOI] [PubMed] [Google Scholar]
- 56.Peitsch M.C., Kress A., Lerch P.G. A purification method for apolipoprotein A-I and A-II. Anal Biochem. 1989;178:301–305. doi: 10.1016/0003-2697(89)90642-8. [DOI] [PubMed] [Google Scholar]
- 57.Kilpatrick D.C. Isolation of human mannan binding lectin, serum amyloid P component and related factors from Cohn fraction III. Transfus Med. 1997;7:289–294. doi: 10.1046/j.1365-3148.1997.d01-40.x. [DOI] [PubMed] [Google Scholar]
- 58.Orthner C.L., Ralston A.H., Gee D. Large-scale production and properties of immunoaffinity-purified human activated protein C concentrate. Vox Sang. 1995;69:309–318. doi: 10.1111/j.1423-0410.1995.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 59.Burnouf T., Goubran H.A., Radosevich M. A minipool process for solvent-detergent treatment of cryoprecipitate at blood centres using a disposable bag system. Vox Sang. 2006;91:56–62. doi: 10.1111/j.1423-0410.2006.00772.x. [DOI] [PubMed] [Google Scholar]