Cardiovascular complications in COVID-19 (original) (raw)
Abstract
Background
The coronavirus disease of 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). While systemic inflammation and pulmonary complications can result in significant morbidity and mortality, cardiovascular complications may also occur.
Objective
This brief report evaluates cardiovascular complications in the setting of COVID-19 infection.
Discussion
The current COVID-19 pandemic has resulted in over one million infected worldwide and thousands of death. The virus binds and enters through angiotensin-converting enzyme 2 (ACE2). COVID-19 can result in systemic inflammation, multiorgan dysfunction, and critical illness. The cardiovascular system is also affected, with complications including myocardial injury, myocarditis, acute myocardial infarction, heart failure, dysrhythmias, and venous thromboembolic events. Current therapies for COVID-19 may interact with cardiovascular medications.
Conclusions
Emergency clinicians should be aware of these cardiovascular complications when evaluating and managing the patient with COVID-19.
Keywords: COVID-19, Infectious disease, Cardiovascular, Dysrhythmia, Acute myocardial infarction, Heart failure, Myocarditis, Troponin
1. Introduction
The coronavirus disease of 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) first appeared in Wuhan, China [[1], [2], [3]]. It was officially declared a pandemic by the World Health Organization in March 2020 [1,3]. As of April 11, 2020, the COVID-19 pandemic has resulted in over 490,000 cases and 18,500 deaths in the United States, with all 50 states affected [2]. Over one million people are now infected worldwide [[1], [2], [3], [4]]. While much of the focus has been on the pulmonary complications, it is important for emergency clinicians to be aware of the cardiovascular complications, which can be a significant contributor to the mortality associated with this disease [[4], [5], [6], [7], [8], [9]]. This brief report will provide a focused overview of cardiovascular complications associated with COVID-19, including myocardial injury and myocarditis, acute myocardial infarction (AMI), heart failure, dysrhythmias, and venous thromboembolic events (VTE).
2. Methods
Authors searched PubMed and Google Scholar for articles using the keywords “COVID-19”, “SARS-CoV-2”, “heart”, “cardiac”, “cardiovascular”, “myocardial injury”, “myocarditis”, “acute myocardial infarction”, “acute coronary syndrome”, “dysrhythmia”, “arrhythmia”, “heart failure”, “venous thromboembolism”, “coagulable”. Authors included case reports, retrospective studies, prospective studies, systematic reviews and meta-analyses, clinical guidelines, and narrative reviews focusing on COVID-19 and cardiovascular effects and complications. Preprinted articles were also included. The literature search was restricted to studies published in English. Emergency physicians with experience in critical appraisal of the literature reviewed all of the articles and decided which studies to include for the review by consensus, with a focus on emergency medicine-relevant articles. A total of 45 articles were selected for inclusion.
3. Discussion
3.1. Pathophysiology and clinical features
SARS-CoV-2 is an enveloped, non-segmented, single-stranded, positive-sense RNA virus [2,[5], [6], [7], [8], [9]]. Angiotensin-converting enzyme 2 (ACE2) is a protein found on the surface of lung alveolar epithelial cells and enterocytes of the small intestine, which has been proposed as the entry site for SARS-CoV-2 [10]. ACE2 breaks down angiotensin II, a pro-inflammatory factor in the lung. Inhibition of ACE2 may be another factor in lung injury, as well as the cause of the systemic inflammation with cytokine release that can result in acute respiratory distress syndrome (ARDS) and multiorgan dysfunction [[11], [12], [13]]. Disruption in immune system regulation, increased metabolic demand, and procoagulant activity likely account for some of the increased risk of adverse outcomes in those with COVID-19-related cardiovascular disease (CVD) [8,9,14]. Specifically, systemic inflammation can destabilize vascular plaques, while the viral illness increases cytokine activity, increasing cardiac demand, similar to influenza [15,16]. Recent research, however, has suggested that the virus may also cause direct damage to the heart utilizing ACE2 receptors located within cardiac tissue [17].
The prevalence of CVD in COVID-19 patients is unclear, but preexisting CVD may be associated with a more severe COVID-19 infection [[4], [5], [6],18,19]. A meta-analysis of 1527 patients with COVID-19 found that the prevalence of hypertension was 17.1% and cardiac disease was 16.4%, and that these patients were more likely to require critical care [18]. Another study of 44,672 patients with COVID-19 found that a history of CVD was associated with a nearly five-fold increase in the case fatality rate when compared with patients without CVD (10.5% vs. 2.3%) [5]. Other studies suggest similar findings with increased risk of mortality in patients with prior CVD [[5], [6], [7], [8], [9],19].
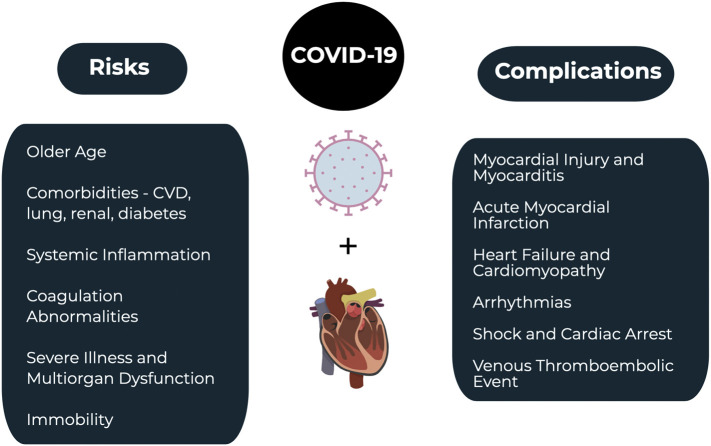
Severe or critical cases account for less than 20% of patients with COVID-19 [[5], [6], [7], [8], [9],[19], [20], [21], [22], [23]]. Patients with critical illness may present with pneumonia, ARDS, multiorgan dysfunction, and hemodynamic instability, as well as several cardiovascular complications [[5], [6], [7], [8], [9],[19], [20], [21], [22], [23]]. Cardiogenic shock is the most severe cardiac complication and may occur in those with critical illness [[5], [6], [7], [8], [9],19]. Fig. 1summarizes the relationship between COVID-19 and cardiac complications.
Fig. 1.
COVID-19 and the cardiovascular system.
3.2. Cardiovascular complications associated with COVID-19 infection
3.2.1. Myocardial injury and myocarditis
Prior viral illnesses, including Middle East respiratory syndrome coronavirus (MERS-CoV), have been associated with myocardial injury and myocarditis with troponin elevation, thought to be due to increased cardiac physiologic stress, hypoxia, or direct myocardial injury [4,[24], [25], [26], [27], [28], [29], [30], [31], [32], [33]]. One of the first reports of myocardial injury associated with SARS-CoV-2 was a study of 41 patients diagnosed with COVID-19 in Wuhan, China, wherein 5 patients (12%) had a high-sensitivity troponin I above the threshold of 28 pg/mL [7]. Subsequent studies have found that myocardial injury with an elevated troponin level may occur in 7–17% of patients hospitalized with COVID-19 and 22–31% of those admitted to the intensive care unit (ICU) [[7], [8], [9]]. Myocarditis has also been identified with high viral loads and mononuclear infiltrates identified on autopsy of some patients with COVID-19 [[26], [27], [28]]. In fact, one study suggested that up to 7% of COVID-19 related deaths were due to myocarditis [6].
Acute myocarditis presents across a variable range of clinical severity and is a significant diagnostic challenge in the COVID-19 era. Patients with COVID-19 can present with chest pain, dyspnea, dysrhythmia, and acute left ventricular dysfunction [[5], [6], [7], [8], [9]]. In patients with myocarditis and myocardial injury, serum troponin values will be abnormal. The electrocardiogram (ECG) can demonstrate a range of findings, in some cases mimicking acute coronary syndrome (ACS). The ECG abnormalities result from myocardial inflammation and include non-specific ST segment-T wave abnormalities, T wave inversion, and PR segment and ST segment deviations (depression and elevation). Echocardiography and consultation with cardiology, if either are available, is encouraged, as differentiating myocarditis and ACS is difficult. Echocardiographic evaluation is more likely to demonstrate a focal wall motion abnormality with active, significant ACS while severe forms of COVID-19-related myocarditis will show either no wall motion defects or global wall motion dysfunction [4,32]. ECG and echocardiographic abnormalities in the setting of COVID-19 are markers of illness severity and are correlated with worse outcomes [4,29,30]. Moreover, troponin elevations in patients with COVID-19 infection have been directly associated with an increased risk of adverse outcome in those patients with severe infection, including mortality [25,29,30].
3.2.2. Acute myocardial infarction
Severe systemic inflammation increases the risk of atherosclerotic plaque disruption and AMI [15,[30], [31], [32], [33]]. A 2018 study found that influenza and other select viral illnesses were associated with an increased risk of AMI within the first 7 days of disease diagnosis, with an incidence ratio of 6.1 for influenza and 2.8 for other viruses [15]. Another study of patients hospitalized for community-acquired pneumonia found an increased risk of active CVD that remained present for several years after hospitalization [31]. Due to extensive inflammation and hypercoagulability, the risk of AMI is likely present in patients with COVID-19 [4,32].
The treatment of AMI is controversial in COVID-19 patients. In patients diagnosed with an ST elevation myocardial infarction (STEMI) and COVID-19, the American College of Cardiology (ACC) states that while fibrinolysis may be considered in those with "low risk STEMI", defined by inferior STEMI with no right ventricular involvement or lateral AMI without hemodynamic compromise, percutaneous coronary intervention (PCI) is more commonly performed at most institutions and remains the treatment of choice [32]. If PCI is pursued, staff should don appropriate personal protective equipment (PPE), and a full decontamination of the catheterization laboratory should be performed following the procedure. For suspected COVID-19 in the setting of NSTEMI, diagnostic testing prior to catheterization is recommended; the ACC note that, in properly selected patients with confirmed COVID-19, conservative therapy may be sufficient. Patients who are hemodynamically unstable in the setting of NSTEMI should be managed similarly to those with STEMI [32].
3.2.3. Acute heart failure and cardiomyopathy
Acute heart failure can be the primary presenting manifestation of COVID-19 infection. One study found that acute heart failure may be present in 23% of patients in their initial presentation for COVID-19, with cardiomyopathy occurring in 33% of patients [8]. Another study found that heart failure was present in 24% of patients and was associated with an increased risk of mortality [33]. Among those with heart failure, nearly half did not have a known history of hypertension or CVD [33]. It is currently unknown if heart failure is due to new cardiomyopathy versus an exacerbation of previously undiagnosed heart failure [34]. It is important to be conscious of this potential cardiac dysfunction when administering intravenous fluids and avoid overaggressive fluid replacement. Importantly, right heart failure may also occur, particularly among those with ARDS and acute lung injury [4,19].
3.2.4. Dysrhythmias
Palpitations may be a presenting symptom in over 7% of patients with COVID-19 [26]. A range of dysrhythmias have been encountered in patients with COVID-19 infection. Most frequently, sinus tachycardia is seen in such patients, resulting from multiple, simultaneous causes (hypoperfusion, fever, hypoxia, anxiety, etc) [4]. One study found that dysrhythmias were present in 17% of hospitalized and 44% of ICU patients with COVID-19 [9]. Dysrhythmias may occur in the setting of viral illness due to hypoxia, inflammatory stress, and abnormal metabolism [4]. If dysrhythmias are associated with an elevation in serum troponin, the clinician should consider myocardial injury, acute myocarditis, and ACS in the differential diagnosis [4].
3.2.5. Venous thromboembolic event
Patients with COVID-19 are also at an increased risk of VTEs [35,36]. Systemic inflammation, abnormal coagulation status, multiorgan dysfunction, and critical illness are all potential contributing factors to the increased risk of VTE [7,8,[35], [36], [37], [38]]. Studies suggest significant coagulation pathway abnormalities in patients with COVID-19, including elevated D-dimer [7,8,[35], [36], [37], [38]]. One study of 25 patients with COVID-19 pneumonia found that an elevated D-dimer was present in all patients with a median of 6.06 micrograms/ml, with 10 patients having a pulmonary embolism (PE) diagnosed on computed tomography pulmonary angiography (CTPA) [37]. Patients with confirmed PE on CTPA demonstrated a median D-dimer level of 11.07 micrograms/ml [37]. D-dimer levels greater than 1 μg/mL were associated with an increased risk of death during hospitalization (odds ratio 18.4) in COVID-19-infected patients [8]. One study suggests anticoagulation, mainly with low molecular weight heparin, may be associated with reduced mortality in severe COVID-19 infections or those with D-dimer greater than six times the upper limit of normal [39].
3.2.6. Medication interactions
Many of the newly studied medications interact extensively with other cardiovascular drugs, including antihypertensives, antiarrhythmics, anticoagulants, antiplatelets, and statins [4]. Current medications under study include antivirals (e.g., remdesivir, ribavirin, lopinavir/ritonavir, favipiravir), antimalarials (e.g., chloroquine, hydroxychloroquine), azithromycin, corticosteroids, and biologics (tocilizumab) [4,[40], [41], [42]]. Lopinavir/ritonavir may cause QT and PR prolongation, particularly in those with baseline QT prolongation or in those taking medications that may cause QT prolongation [43]. These medications can also affect anticoagulant medications, antiplatelet agents, and statins [43]. Chloroquine and hydroxychloroquine affect the intracellular pH, which can result in electrolyte abnormalities, cardiotoxicity, and prolonged QT intervals; they may also interact with antiarrhythmic agents [44,45]. Methylprednisolone can cause electrolyte derangements, fluid retention, and hypertension [28]. A summary of the mechanism of action and effect of these medications is located in Table 1.
Table 1.
Medications and the cardiovascular system [4].
| Medication | Mechanism | Cardiovascular effects and medication interactions |
|---|---|---|
| Remdesivir | Nucleotide-analog inhibitor of RNA polymerases | - May cause hypotension, arrhythmias |
| Ribavirin | Inhibits RNA and DNA virus replication | - Interacts with anticoagulants- May cause severe hemolytic anemia |
| Lopinavir/Ritonavir | Lopinavir inhibits proteaseRitonavir inhibits CYP3A metabolism | - Interacts with anticoagulants, antiplatelets, statins, antiarrhythmics- May result in prolonged QTc, AV blocks, Torsades de pointes |
| Favipiravir | Inhibits RNA-dependent RNA polymerases | - Interacts with anticoagulants, statins, antiarrhythmics- May cause severe hemolytic anemia |
| Chloroquine and Hydroxychloroquine | Changes endosomal/organelle pH | - Interacts with antiarrhythmics- May cause direct myocardial toxicity; worsen cardiomyopathy; alter cardiac conduction; result in bundle branch block, AV block, ventricular arrhythmias, Torsades de pointes |
| Azithromycin | Interferes with protein synthesis, binds to 50s ribosome | - Interacts with anticoagulants, statins, antiarrhythmics, other QT prolonging agents- May result in dysrhythmias, prolonged QTc, Torsades de pointes |
| Interferon | Immune system activation | - May cause direct myocardial toxicity; worsen cardiomyopathy; alter cardiac conduction; cause hypotension or cardiac ischemia |
| Methylprednisolone | Reduces inflammation | - Interacts with anticoagulants- May cause fluid retention, hypertension, electrolyte changes |
| Tocilizumab | Inhibits IL-6 | - May increase medication metabolism such as statins- May cause hypertension |
4. Limitations
The current literature evaluating cardiovascular complications and effects associated with COVID-19 suffers from several limitations, including significant heterogeneity in patient selection, outcomes, comparators, and study design, as well as low numbers of included patients and high risk of bias. With the current pandemic, a significant amount of literature is published in preprint form, prior to completion of full peer review. Further data are needed concerning the discussed cardiovascular complications and COVID-19.
5. Conclusions
COVID-19 is associated with a number of cardiovascular complications, including myocardial injury and myocarditis, AMI, heart failure, dysrhythmias, and VTE. Some of the medications utilized to treat COVID-19 also have potential cardiac complications. It is important for the emergency clinicians to be aware of these complications when treating the COVID-19 patient.
Declaration of competing interest
None.
Acknowledgements
BL, WB, MG, and AK conceived the idea for this manuscript and contributed substantially to the writing and editing of the review. This manuscript did not utilize any grants, and it has not been presented in abstract form. This clinical review has not been published, it is not under consideration for publication elsewhere, its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and that, if accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright-holder. This review does not reflect the views or opinions of the U.S. government, Department of Defense, U.S. Army, U.S. Air Force, or SAUSHEC EM Residency Program.
Contributor Information
Brit Long, Email: brit.long@yahoo.com.
William J. Brady, Email: WB4Z@hscmail.mcc.virginia.edu.
References
- 1.World Health Organization WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. https://www.who.int/dg/speeches/detail/whodirector-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 Available online at.
- 2.Coronavirus Disease 2019 (COVID-19) Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html
- 3.World Health Organization Situation report. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200403-sitrep-74-covid-19-mp.pdf?sfvrsn=4e043d03_4
- 4.Driggin E., Madhavan M.V., Bikdeli B. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. Mar 18 2020 doi: 10.1016/j.jacc.2020.03.031. [pii: S0735-1097(20)34637-4, in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. Feb 24 2020 doi: 10.1001/jama.2020.2648. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020:1–3. doi: 10.1007/s00134-020-05991-x. [published online ahead of print, 2020 Mar 3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. Mar 28 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. Feb 7 2020 doi: 10.1001/jama.2020.1585. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. Jun 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. Mar 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ge X.Y., Li J.L., Yang X.L. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. Apr 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Libby P., Simon D.I. Inflammation and thrombosis: the clot thickens. Circulation. 2001;103:1718–1720. doi: 10.1161/01.cir.103.13.1718. [DOI] [PubMed] [Google Scholar]
- 15.Kwong J.C., Schwartz K.L., Campitelli M.A. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378:345–353. doi: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 16.Davis M.M., Taubert K., Benin A.L. Influenza vaccination as secondary prevention for cardiovascular disease: a science advisory from the American Heart Association/American College of Cardiology. J Am Coll Cardiol. 2006;48:1498–1502. doi: 10.1016/j.jacc.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. Mar 30 2020 doi: 10.1093/cvr/cvaa078. [pii: cvaa078, Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li B., Yang J., Zhao F. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. Mar 11 2020 doi: 10.1007/s00392-020-01626-9. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murthy S., Gomersall C.D., Fowler R.A. Care for critically ill patients with COVID-19. JAMA. Mar 11 2020 doi: 10.1001/jama.2020.3633. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. Feb 24 2020 doi: 10.1016/S2213-2600(20)30079-5. [pii: S2213-2600(20)30079-5, Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss P., Murdoch D.R. Clinical course and mortality risk of severe COVID-19. Lancet. 2020;395(10229):1014–1015. doi: 10.1016/S0140-6736(20)30633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Intensive Care National Audit & Research Centre. ICNARC report on COVID-19 in critical care. March 27, 2020. Accessed March 30, 2020.
- 24.Alhogbani T. Acute myocarditis associated with novel middle east respiratory syndrome coronavirus. Ann Saudi Med. 2016;36:78–80. doi: 10.5144/0256-4947.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen C., Zhou Y., Wang D.W. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. Mar 2020 doi: 10.1007/s00059-020-04909-z. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu K., Fang Y.Y., Deng Y. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) Feb 7 2020 doi: 10.1097/CM9.0000000000000744. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. Apr 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y., Yang Y., Zhang C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed]
- 30.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed]
- 31.Corrales-Medina V.F., Alvarez K.N., Weissfeld L.A. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015;313:264–274. doi: 10.1001/jama.2014.18229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welt F.G.P., Shah P.B., Aronow H.D., from the American College of Cardiology’s (ACC) Interventional Council and the Society of Cardiovascular Angiography and Intervention (SCAI) Catheterization laboratory considerations during the coronavirus (COVID-19) pandemic: from ACC’s Interventional Council and SCAI. JACC. 2020 doi: 10.1016/j.jacc.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. Mar 26 2020;368 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buzon J., Roignot O., Lemoine S. Takotsubo cardiomyopathy triggered by influenza A virus. Intern Med. 2015;54:2017–2019. doi: 10.2169/internalmedicine.54.3606. [DOI] [PubMed] [Google Scholar]
- 35.Xie Y., Wang X., Yang P., Zhang S. COVID-19 complicated by acute pulmonary embolism. Radiology: Cardiothoracic Imaging. 2020;2(2) doi: 10.1148/ryct.2020200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Danzi G.B., Loffi M., Galeazzi G., Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. Mar 30 2020 doi: 10.1093/eurheartj/ehaa254. [pii: ehaa254, Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J, Wang X, Zhang S, et al. Findings of acute pulmonary embolism in COVID-19 patients. Lancet Infectious Disease. [Available at SSRN: https://ssrn.com/abstract=3548771 or http://dx.doi.org/10.2139/ssrn.3548771\].
- 38.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. Apr 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang N., Bai H., Chen X. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. Mar 27 2020 doi: 10.1111/jth.14817. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chavez S., Long B., Koyfman A., Liang S. Coronavirus disease (COVID-19): a primer for emergency physicians. American Journal of Emergency Medicine. March 24, 2020 doi: 10.1016/j.ajem.2020.03.036. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elfiky A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248 doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chu C.M., Cheng V.C., Hung I.F. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.KALETRA(R) oral film coated tablets, oral solution, lopinavir ritonavir oral film coated tablets, oral solution. Product insert. AbbVie Inc. (per FDA); North Chicago, IL: 2013. [Google Scholar]
- 44.Page R.L., II, O’Bryant C.L., Cheng D. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134:e32–e69. doi: 10.1161/CIR.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 45.Tonnesmann E., Kandolf R., Lewalter T. Chloroquine cardiomyopathy - a review of the literature. Immunopharmacol Immunotoxicol. 2013;35:434–442. doi: 10.3109/08923973.2013.780078. [DOI] [PubMed] [Google Scholar]