COVID-19 pathophysiology: A review (original) (raw)
Abstract
In December 2019, a novel coronavirus, now named as SARS-CoV-2, caused a series of acute atypical respiratory diseases in Wuhan, Hubei Province, China. The disease caused by this virus was termed COVID-19. The virus is transmittable between humans and has caused pandemic worldwide. The number of death tolls continues to rise and a large number of countries have been forced to do social distancing and lockdown. Lack of targeted therapy continues to be a problem. Epidemiological studies showed that elder patients were more susceptible to severe diseases, while children tend to have milder symptoms. Here we reviewed the current knowledge about this disease and considered the potential explanation of the different symptomatology between children and adults.
Highlights
- •
In general, children are less susceptible to severe CODIV-19 than elder patients. - •
Severe COVID-19 adult patients showed higher proinflammatory markers with the presence of pathological T cells. - •
The dissection of immune response to COVID-19 in children is necessary given limited data on this topic.
1. Introduction
In December 2019, a series of acute atypical respiratory disease occurred in Wuhan, China. This rapidly spread from Wuhan to other areas. It was soon discovered that a novel coronavirus was responsible. The novel coronavirus was named as the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2, 2019-nCoV) due to its high homology (~80%) to SARS-CoV, which caused acute respiratory distress syndrome (ARDS) and high mortality during 2002–2003 [1]. The outbreak of SARS-CoV-2 was considered to have originally started via a zoonotic transmission associated with the seafood market in Wuhan, China. Later it was recognized that human to human transmission played a major role in the subsequent outbreak [2]. The disease caused by this virus was called Coronavirus disease 19 (COVID-19) and a pandemic was declared by the World Health Organization (WHO). COVID-19 has been impacting a large number of people worldwide, being reported in approximately 200 countries and territories [3,4]. As of April 7th, 2020, around 1,400,000 cases worldwide have been reported according to the Center for Systems Science and Engineering (CSSE) at John Hopkins University [5].
SARS-CoV-2 virus primarily affects the respiratory system, although other organ systems are also involved. Lower respiratory tract infection related symptoms including fever, dry cough and dyspnea were reported in the initial case series from Wuhan, China [6]. In addition, headache, dizziness, generalized weakness, vomiting and diarrhea were observed [7]. It is now widely recognized that respiratory symptoms of COVID-19 are extremely heterogeneous, ranging from minimal symptoms to significant hypoxia with ARDS. In the report from Wuhan mentioned above, the time between the onset of symptoms and the development of ARDS was as short as 9 days, suggesting that the respiratory symptoms could progress rapidly [6]. This disease could be also fatal. A growing number of patients with severe diseases have continued to succumb worldwide. Epidemiological studies have shown that mortalities are higher in elder population [8] and the incidence is much lower in children [9,10]. Current medical management is largely supportive with no targeted therapy available. Several drugs including lopinavir-ritonavir, remdesivir, hydroxychloroquine, and azithromycin have been tested in clinical trials [8,11,12], but none of them have been proven to be a definite therapy yet. More therapies are being tested in clinical trials. A large number of countries have implemented social distancing and lockdown to mitigate further spread of the virus. Here we will review our current knowledge of COVID-19 and consider the underlying mechanism to explain the heterogeneous symptomatology, particularly focusing on the difference between children and adult patients.
2. Epidemiological data of COVID-19
A large number of studies so far are reports based on experiences in China. At the beginning of the outbreak, COVID-19 cases were mostly observed among elderly people [13]. As the outbreak continued, the number of cases among people aged 65 years and older increased further, but also some increase among children (< 18 years) was observed. The number of male patients was higher initially, but no significant gender difference was observed as case number increased. The mean incubation period was 5.2 days. The combined case-fatality rate was 2.3% [14,15]. The risk factors of in-hospital death were studied using the data of two hospitals in Wuhan. Older age, higher sequential organ failure assessment (SOFA) score and d-dimer >1 μg/mL on admission were shown to be risk factors in the multi-variable analysis [8]. In the univariable analysis, the presence of coronary artery disease, diabetes and hypertension was also considered to be risk factors. The study of 85 fatal COVID-19 patients with median age of 65 years in Wuhan showed that the majority of patients died from multi-organ failure as respiratory failure, shock, and ARDS were seen in 94%, 81%, and 74% of cases, respectively [16]. As in line with the high prevalence of multi-organ failure, high d-dimer levels, fibrinogen and prolonged thrombin time were seen in severe diseases [17].
Following the outbreak in China, SARS-CoV-2 has spread worldwide. As of early April 2020, the reported number of COVID-19 patients is highest in the U.S., followed by Spain, Italy, Germany, France and China. Italy was significantly affected after the outbreak of China. Fatality rate was also higher in elder population as in Chinese series. The report from Italy showed the case-fatality rate of 7.2% [15,18], which was three times as high as the one in China. Although the case-fatality rate of patients aged 70 years or older was higher in Italy, it was very similar between age 0 and 69 years in both countries. As 23% of Italian was aged 65 years or older, the high case-fatality in Italy was somewhat explained by the demographic characteristics. The data from US and other countries is available in the number of resources [5,19]. We expect to learn experiences more from individual countries in the forthcoming future.
From the beginning of this outbreak, the percentage of children within the total COVID-19 patients was small. According to the data of the Chinese Center for Disease Control and Prevention (China CDC) from February 2020, children younger than 10 years of age and within the age of 11–19 years occupied 1% each of the total cases [14]. Considering this age group represents 20% of the total population, this may indicate less prevalence of COVID-19 in pediatric population. However, this may be underestimation of actual incidence in pediatric population if less tests were undertaken in children due to less symptoms. One confounding factor is that schools in China were closed for most of the epidemic due to the Chinese New Year holidays, which might have contributed to less exposure among children. In the report of 2134 pediatric patients with COVID-19 from the China CDC, 4.4%, 50.9%, 38.8%, and 5.9% of patients were diagnosed as asymptomatic, mild, moderate, or severe, respectively [20]. The definition of asymptomatic, mild, moderate, severe and critical is summarized in Table 1. In contrast, 18.5% of adult patients had severe diseases [20]. Infants were most vulnerable to severe type of infection; the proportion of severe and critical cases was 10.6%, 7.3%, 4.2%, 4.1% and 3.0% for the age group of <1, 1–5, 6–10, 11–15 and ≥16 years, respectively. The case-fatality rate of age group 0–9 and 10–19 was 0% each. In Italy, COVID-19 patients of age 8–18 years occupied only 1.2% [18]. The case-fatality rate of age group 0–9 and 10–19 was 0% and 0.2%, respectively, which was similar to Chinese experience. In the data from the Korean CDC on late March, 6.3% of all cases tested positive for COVID-19 were children under 19 years of age [21]. On April 6, 2020, the US CDC released the study of 2572 COVID-19 cases among children younger than 18 years [22]. Of all reported cases in the US, this occupied only 1.7% of the total cases, even though this age group makes up 22% of US population. Overall, the data suggested that children were less symptomatic than adults as in Chinese reports. Among the children for whom complete information was available, only 73% developed fever, cough, or shortness of breath. That's compared to 93% of adults reported in the same time frame, between the ages of 18 and 64 years. The estimated hospitalization rate for children aged 1 to 17 was 14% at most [22]. In contrast, infant accounted for the highest percentage of hospitalization (15–62%), which was again similar to the data from Chinese CDC. Despite the overall favorable outcome for pediatric population, a number of deaths have been reported in US and other countries, and further information needs to be obtained.
Table 1.
Classification of COVID-19 patients.
| Asymptomatic | COVID nucleic acid test positive. Without any clinical symptoms and signs and the chest imaging is normal |
|---|---|
| Mild | Symptoms of acute upper respiratory tract infection (fever, fatigue, myalgia, cough, sore throat, runny nose, sneezing) or digestive symptoms (nausea, vomiting, abdominal pain, diarrhea) |
| Moderate | Pneumonia (frequent fever, cough) with no obvious hypoxemia, chest CT with lesions. |
| Severe | Pneumonia with hypoxemia (SpO2 < 92%) |
| Critical | Acute respiratory distress syndrome (ARDS), may have shock, encephalopathy, myocardial injury, heart failure, coagulation dysfunction and acute kidney injury. |
Regarding the severity of COVID-19, there is a growing interest in the relationship between the severity of disease and gender. Although the Chinese series showed equal number of cases between males and females, the data suggested that more men than women suffered from severe disease and died [23,24]. The data from other countries demonstrated similar results [25]. Adverse outcomes of COVID-19 were associated with comorbidities, including hypertension, cardiovascular disease, and lung disease. These conditions are more prevalent in men and linked to smoking and drinking alcohol [25]. Sex-based immunological differences were pointed out as another potential explanation [13]. In addition, the study to examine factors influencing the adoption of protective behaviors, specifically within the context of pandemics, found that women were about 50% more likely to practice non-pharmaceutical behaviors, such as hand washing, face mask use and avoiding crowds compared to men [26], which may be in part responsible.
3. Mechanism of SARS-CoV-2 invasion into host cells
Coronaviruses are enveloped, positive-sense, single-stranded RNA viruses of ~30 kb. They infect a wide variety of host species [27]. They are largely divided into four genera; α, β, γ, and δ based on their genomic structure. α and β coronaviruses infect only mammals [28]. Human coronaviruses such as 229E and NL63 are responsible for common cold and croup and belong to α coronavirus. In contrast, SARS-CoV, Middle East respiratory syndrome coronavirus (MERS-CoV) and SARS-CoV-2 are classified to β coronaviruses.
The life cycle of the virus with the host consists of the following 5 steps: attachment, penetration, biosynthesis, maturation and release. Once viruses bind to host receptors (attachment), they enter host cells through endocytosis or membrane fusion (penetration). Once viral contents are released inside the host cells, viral RNA enters the nucleus for replication. Viral mRNA is used to make viral proteins (biosynthesis). Then, new viral particles are made (maturation) and released. Coronaviruses consist of four structural proteins; Spike (S), membrane (M), envelop (E) and nucleocapsid (N) [29]. Spike is composed of a transmembrane trimetric glycoprotein protruding from the viral surface, which determines the diversity of coronaviruses and host tropism. Spike comprises two functional subunits; S1 subunit is responsible for binding to the host cell receptor and S2 subunit is for the fusion of the viral and cellular membranes. Angiotensin converting enzyme 2 (ACE2) was identified as a functional receptor for SARS-CoV [30]. Structural and functional analysis showed that the spike for SARS-CoV-2 also bound to ACE2 [[31], [32], [33]]. ACE2 expression was high in lung, heart, ileum, kidney and bladder [34]. In lung, ACE2 was highly expressed on lung epithelial cells. Whether or not SARS-CoV-2 binds to an additional target needs further investigation. Following the binding of SARS-CoV-2 to the host protein, the spike protein undergoes protease cleavage. A two-step sequential protease cleavage to activate spike protein of SARS-CoV and MERS-CoV was proposed as a model, consisting of cleavage at the S1/S2 cleavage site for priming and a cleavage for activation at the S′2 site, a position adjacent to a fusion peptide within the S2 subunit [[35], [36], [37]]. After the cleavage at the S1/S2 cleavage site, S1 and S2 subunits remain non-covalently bound and the distal S1 subunit contributes to the stabilization of the membrane-anchored S2 subunit at the prefusion state [32]. Subsequent cleavage at the S′2 site presumably activates the spike for membrane fusion via irreversible, conformational changes. The coronavirus spike is unusual among viruses because a range of different proteases can cleave and activate it [38]. The characteristics unique to SARS-CoV-2 among coronaviruses is the existence of furin cleavage site (“RPPA” sequence) at the S1/S2 site. The S1/S2 site of SARS-CoV-2 was entirely subjected to cleavage during biosynthesis in a drastic contrast to SARS-CoV spike, which was incorporated into assembly without cleavage [32]. Although the S1/S2 site was also subjected to cleavage by other proteases such as transmembrane protease serine 2 (TMPRSS2) and cathepsin L [37,39], the ubiquitous expression of furin likely makes this virus very pathogenic.
4. Host response to SARS-CoV-2
The symptom of patients infected with SARS-CoV-2 ranges from minimal symptoms to severe respiratory failure with multiple organ failure. On Computerized tomography (CT) scan, the characteristic pulmonary ground glass opacification can be seen even in asymptomatic patients [40]. Because ACE2 is highly expressed on the apical side of lung epithelial cells in the alveolar space [41,42], this virus can likely enter and destroy them. This matches with the fact that the early lung injury was often seen in the distal airway. Epithelial cells, alveolar macrophages and dendritic cells (DCs) are three main components for innate immunity in the airway [43]. DCs reside underneath the epithelium. Macrophages are located at the apical side of the epithelium. DCs and macrophages serve as innate immune cells to fight against viruses till adaptive immunity is involved.
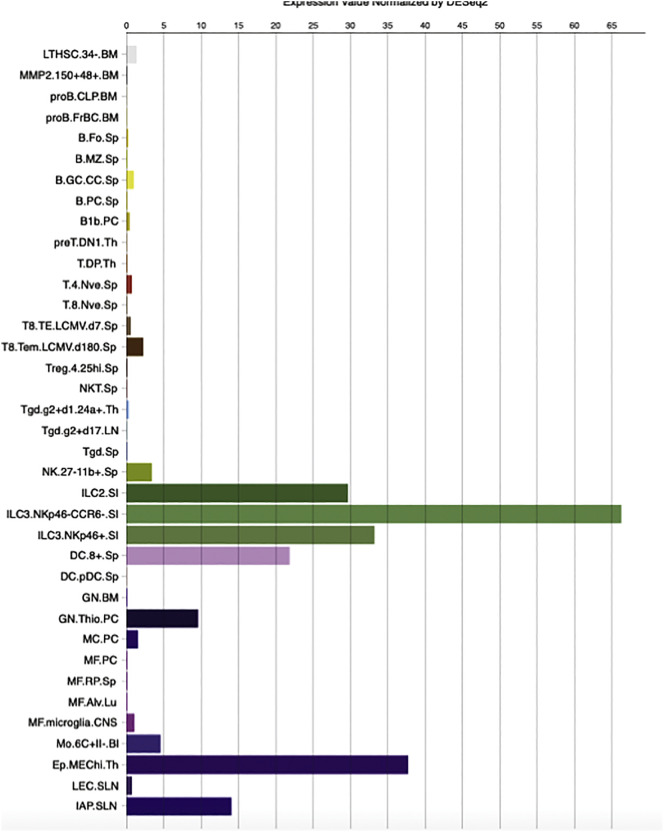
T cell mediated responses against coronaviruses have been previously reviewed [27]. T cell responses are initiated by antigen presentation via DCs and macrophages. How does SARS-CoV-2 enter APCs? DCs and macrophages can phagocytize apoptotic cells infected by virus [44]. For example, virus-infected apoptotic epithelial cells can be phagocytized by DCs and macrophages, which leads to antigen presentation to T cells. Or DCs and macrophages may be infected with virus primarily? Based on the Immunological Genome database (http://rstats.immgen.org), the expression of ACE2 on (splenic) dendritic cells and alveolar macrophages is present but limited (Fig. 1). Determining whether or not SARS-CoV-2 uses another protein to bind to APCs helps to answer this question. SARS-CoV can also bind to dendritic-cell specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) and DC-SIGN-related protein (DC-SIGNR, L-SIGN) in addition to ACE2 [[45], [46], [47]]. DC-SIGN is highly expressed on dendritic cells and macrophages. Another target for SARS-CoV-2, if any, can help the virus to directly infect DCs and alveolar macrophages. This needs future research. These antigen presenting cells move to the draining lymph nodes to present viral antigens to T cells. CD4+ and CD8+ T cells play a critical role. CD4+ T cells activate B cells to promote the production of virus-specific antibody, while CD8+ T cells can kill viral infected cells.
Fig. 1.
ACE expression on mouse immune cells.
ACE2 expression was examined in Immgen (http://rstats.immgen.org/Skyline/skyline.html).
BM, bone marrow; Sp, Spleen; PC, peritoneal cavity; Th, thymus; LN, lymph node; SI, small intestine; Lu, lung; CNS, central nervous system; SLN, subcutaneous lymph node.
Immunological studies were mainly reported in severe COVID-19 patients. Patients with severe diseases showed lymphopenia, particularly the reduction in peripheral blood T cells [48,49]. Patients with severe diseases were reported to have increased plasma concentrations of proinflammatory cytokines, including interleukin (IL)-6, IL-10, granulocyte-colony stimulating factor (G-CSF), monocyte chemoattractant protein 1 (MCP1), macrophage inflammatory protein (MIP)1α, and tumor necrosis factor (TNF)-α [6,48,49]. The more severe conditions patients were in, the higher their IL-6 levels were. CD4+ and CD8+ T cells were activated in those patients as suggested by higher expression of CD69, CD38 and CD44. Higher percentage of checkpoint receptor Tm3+PD-1+ subsets in CD4+ and CD8+ T cells showed that T cells were also exhausted. NK group 2 member A (NKG2A), another marker for exhaustion was elevated on CD8+ T cells [3]. Exhaustion of T cells could have led to the progression of the disease. Another interesting finding was that aberrant pathogenic CD4+ T cells with co-expressing interferon (IFN)-γ and granulocyte-macrophage colony-stimulating factor (GM-CSF) were seen in COVID-19 patients with severe disease [48]. GM-CSF production from T cells has been previously reported as a response to virus infection. GM-CSF can help to differentiate innate immune cells and augment T cell function, but it can initiate tissue damage at excess [50,51]. GM-CSF+IFN-γ+ CD4+ T cells were previously seen upon strong T cell receptor (TCR) responses in experimental autoimmune encephalomyelitis (EAE) models, where CD8+ T cells expressing GM-CSF were found at higher percentage and secreted IL-6. It is worth mentioning that these immunological studies were exclusively reported from adult patients. Immunological responses in pediatric population needs to be examined.
The study of SARS-CoV showed that virus infected lung epithelial cells produced IL-8 in addition to IL-6 [43]. IL-8 is a well-known chemoattractant for neutrophils and T cells. Infiltration of a large number of inflammatory cells were observed in the lungs from severe COVID-19 patients [52,53], and these cells presumably consist of a constellation of innate immune cells and adaptive immune cells. Among innate immune cells, we expect the majority to be neutrophils. Neutrophils can act as double-edged sword as neutrophils can induce lung injury [[54], [55], [56]]. The majority of the observed infiltrating adaptive immune cells were likely T cells, considering that the significant reduction in circulating T cells was reported. CD8+ T cells are primary cytotoxic T cells. Severe patients also showed pathological cytotoxic T cells derived from CD4+ T cells [57]. These cytotoxic T cells can kill virus but also contribute to lung injury [58]. Circulating monocytes respond to GM-CSF released by these pathological T cells. CD14+CD16+ inflammatory monocyte subsets, which seldom exist in healthy controls and were also found at significantly higher percentage in COVID-19 patients. These inflammatory CD14+CD16+ monocytes had high expression of IL-6, which likely accelerated the progression of systemic inflammatory response.
An interesting note is that ACE2 was significantly expressed on innate lymphoid cells (ILC)2 and ILC3 (Fig. 1). NK cells are a member of ILC1, which constitute a large portion of ILCs in the lung (~95%). ILC2 and ILC3 work for mucous homeostasis. So far there is a very limited study of ILC2 and ILC3 in coronavirus infection.
In additionto respiratory symptoms, thrombosis and pulmonary embolism have been observed in severe diseases. This is in line with the finding that elevated d-dimer and fibrinogen levels were observed in severe diseases. The function of the endothelium includes promotion of vasodilation, fibrinolysis, and anti-aggregation. Because endothelium plays a significant role in thrombotic regulation [59], hypercoagulable profiles seen in severe diseases likely indicate significant endothelial injury. Endothelial cells also express ACE2 [60,61]. Of note, the endothelial cells represent the one third of lung cells [62]. Microvascular permeability as a result of the endothelial injury can facilitate viral invasion.
5. Potential explanation for the difference between children and adults in COVID-19
Infants and young children are typically at high risk for admission to hospitals due to respiratory tract infection with viruses as respiratory syncytial virus and influenza virus. In contrast, pediatric COVID-19 patients have relatively milder symptoms in general compared to elder patients. The reason for this difference between children and adults remains elusive. Because the recent report suggested the correlation between the severity of COVID-19 and the amount of viral loads (or the duration of virus-shedding period) [63], children may have less virus loads even if they get COVID-19. In this line, a couple of hypotheses can be considered.
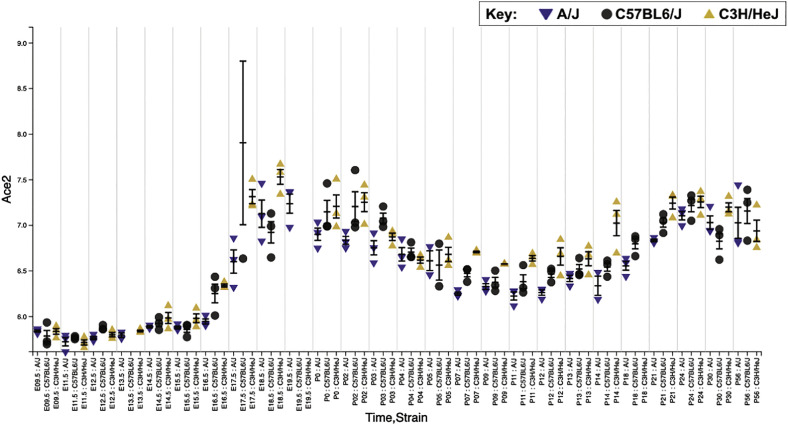
The first possibility is that the expression level of ACE2 may differ between adults and children. A previous study showed that ACE2 was more abundantly expressed on well-differentiated ciliated epithelial cells [42]. Human lung and epithelial cells continue to develop following the birth. ACE2 expression may be lower in pediatric population. From the lung gene expression analysis portal (https://research.cchmc.org/pbge/lunggens/mainportal.html), ACE2 expression in mice increased around at birth (Fig. 2). Its expression reduced till around P10, then increased. Because infants were susceptible to severe disease among children, this pattern may be in line with patients' clinical picture. In addition, gender may also affect ACE2 expression. ACE2 gene is located on the X-chromosome. Circulating ACE2 levels are higher in men than in women [64]. This may be in part responsible for the difference in severity and mortality between men and women both in the adult and the pediatric population [[22], [23], [24]].
Fig. 2.
Age-dependent ACE2 expression profiles in the mouse lung.
Using Lung Gene Expression Analysis Web Portal (https://research.cchmc.org/pbge/lunggens/mainportal.html), the expression of ACE2 was examined. This data was obtained from microarray experiments of three mice strains A/J mice, C57BL/6J mice and C3H/HeJ mice at different ages [72]. X axis showed age and mouse strain, and y axis showed ACE2 expression level. M.
The second possibility is that children have a qualitatively different response to the SARS-CoV-2 virus to adults. With ageing, continuous antigen stimulation and thymic involution lead to a shift in T cell subset distribution from naïve T cells to central memory T cells, effector T cells and effector memory T cells [65]. This process is accompanied by the loss of expression of co-stimulatory molecules such as CD27 and CD28, with increased susceptibility to infections [66]. Whether the appearance of pathological T cells in adult patients with severe COVID-19 diseases is due to the compensation for this fundamental ageing process or not is unclear. At the early stage after birth, CD4+ T cells are impaired in production of Th1 associated proinflammatory cytokines and skewed toward Th2 [67]. CD8+ T cells reduced expression of cytotoxic and inflammatory mediators. Less killing ability by T cells at early stage after birth may explain susceptibility to SARS-CoV-2 in infants. The study comparing aged and young macaques infected with SARS-CoV showed that aged macaques had more robust proinflammatory responses with worse lung pathology [68]. A similar result was reported using aged and young mice infected with SARS-CoV [69]. Severe COVID-19 infection is characterized by a massive proinflammatory response or cytokine storm that results in ARDS and multi-organ dysfunction (MODS). It has been also suggested that inflammatory responses in adults and children are much different [70]. Ageing is associated with increasing proinflammatory cytokines that govern neutrophil functions and have been correlated with the severity of ARDS. So far there is no animal model for SARS-CoV-2, but we expect to see a preclinical model in the future.
The third possibility is that the simultaneous presence of other viruses in the mucosa lungs and airways, common in young children, can let SARS-CoV-2 virus compete with them and limit its growth [71]. At this point, we do not have study testing various viruses along with SARS-CoV-2 to determine this possibility.
Rather a combination of these possibilities may explain pediatric and adult COVID-19 phenotypes. Understanding why children in general are less susceptible to severe COVID-19 would help to design immunotherapy to eradicate this virus.
6. Conclusions
The pandemic by COVID-19 is a live issue affecting people worldwide. Without fundamental therapeutic interventions, current management is to reduce the virus spread and provide supportive care for diseased patients. There is an urgent need to develop targeted therapies. Understanding the difference in pediatric and adult responses to this virus may help to direct immune based therapeutics.
Financial support
This work was in part supported by CHMC Anesthesia Foundation (K.Y.), NIH R01GM118277 (K.Y.) and R21HD099194 (K.Y., S.K.)
Declaration of Competing Interest
None.
References
- 1.Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J., SW Group A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 2.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z. Early transmission dynamics in Wuhan, China, of Novel Coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., Xu Y., Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020 doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J., Litvinova M., Wang W., Wang Y., Deng X., Chen X., Li M., Zheng W., Yi L., Chen X., Wu Q., Liang Y., Wang X., Yang J., Sun K., Longini I.M., Jr., Halloran M.E., Wu P., Cowling B.J., Merler S., Viboud C., Vespignani A., Ajelli M., Yu H. Evolving epidemiology and transmission dynamics of coronavirus disease 2019 outside Hubei province, China: a descriptive and modelling study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.JHUoMCr center Journal. 2020 https://coronavirus.jhu.edu/map.html [Google Scholar]
- 6.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J., Fan Y., Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu X., Zhang L., Du H., Zhang J., Li Y.Y., Qu J., Zhang W., Wang Y., Bao S., Li Y., Wu C., Liu H., Liu D., Shao J., Peng X., Yang Y., Liu Z., Xiang Y., Zhang F., Silva R.M., Pinkerton K.E., Shen K., Xiao H., Xu S., Wong G.W.K., T Chinese Pediatric Novel Coronavirus Study SARS-CoV-2 infection in children. N Engl J Med. 2020 doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu H., Wu J., Hong L., Luo Y., Song Q., Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao B. A trial of Lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honore S., Colson P., Chabriere E., La Scola B., Rolain J.M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;105949 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Z., McGoogan J.M. Characteristics of and important lessons from the Coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 15.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 16.Du Y., Tu L., Zhu P., Mu M., Wang R., Yang P., Wang X., Hu C., Ping R., Hu P., Li T., Cao F., Chang C., Hu Q., Jin Y., Xu G. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational Study. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Y., Li T., Han M., Li X., Wu D., Xu Y., Zhu Y., Liu Y., Wang X., Wang L. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020 doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livingston E., Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. Journal. 2020 doi: 10.1001/jama.2020.4344. [DOI] [PubMed] [Google Scholar]
- 19.Tcfc Information. Journal.
- 20.Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z., Tong S. Epidemiological characteristics of 2143 Pediatric patients with 2019 coronavirus disease in China. Journal. 2020 doi: 10.1542/peds.2020-0702. [DOI] [Google Scholar]
- 21.Brodin P. Why is COVID-19 so mild in children? Journal. 2020 doi: 10.1111/apa.15271. [DOI] [PubMed] [Google Scholar]
- 22.CfDCa Prevention. Journal.
- 23.Wenham C., Smith J., Morgan R., Gender and C-W Group COVID-19: the gendered impacts of the outbreak. Journal. 2020;395:846–848. doi: 10.1016/S0140-6736(20)30526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin J., Bai P., He W., Wu F., Liu W.F., Han D.M., Liu S., Yang J.K. Gender differences in patients with COVID-19: focus on severity and mortality. Journal. 2020 doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall K.S., Samari G., Garbers S., Casey S.E., Diallo D.D., Orcutt M., Moresky R.T., Martinez M.E., McGovern T. Centring sexual and reproductive health and justice in the global COVID-19 response. Journal. 2020;395:1175–1177. doi: 10.1016/S0140-6736(20)30801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moran K.R., Del Valle S.Y. A meta-analysis of the association between gender and protective behaviors in response to respiratory epidemics and pandemics. Journal. 2016;11 doi: 10.1371/journal.pone.0164541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Channappanavar R., Zhao J., Perlman S. T cell-mediated immune response to respiratory coronaviruses. Journal. 2014;59:118–128. doi: 10.1007/s12026-014-8534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabi F.A., Al Zoubi M.S., Kasasbeh G.A., Salameh D.M., Al-Nasser A.D. SARS-CoV-2 and Coronavirus disease 2019: what we know so far. Journal. 2020;9 doi: 10.3390/pathogens9030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosch B.J., van der Zee R., de Haan C.A., Rottier P.J. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. Journal. 2003;77:8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Journal. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y., Guo Y., Pan Y., Zhao Z.J. Structure analysis of the receptor binding of 2019-nCoV. Journal. 2020 doi: 10.1016/j.bbrc.2020.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Journal. 2020 doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Journal. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Journal. 2020 doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belouzard S., Chu V.C., Whittaker G.R. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Journal. 2009;106:5871–5876. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Millet J.K., Whittaker G.R. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Journal. 2014;111:15214–15219. doi: 10.1073/pnas.1407087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Journal. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belouzard S., Millet J.K., Licitra B.N., Whittaker G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Journal. 2012;4:1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Journal. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S., C China Medical Treatment Expert Group for Clinical characteristics of coronavirus disease 2019 in China. Journal. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. Journal. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jia H.P., Look D.C., Shi L., Hickey M., Pewe L., Netland J., Farzan M., Wohlford-Lenane C., Perlman S., McCray P.B., Jr. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. Journal. 2005;79:14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshikawa T., Hill T., Li K., Peters C.J., Tseng C.T. Severe acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells. Journal. 2009;83:3039–3048. doi: 10.1128/JVI.01792-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujimoto I., Pan J., Takizawa T., Nakanishi Y. Virus clearance through apoptosis-dependent phagocytosis of influenza A virus-infected cells by macrophages. Journal. 2000;74:3399–3403. doi: 10.1128/jvi.74.7.3399-3403.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeffers S.A., Tusell S.M., Gillim-Ross L., Hemmila E.M., Achenbach J.E., Babcock G.J., Thomas W.D., Jr., Thackray L.B., Young M.D., Mason R.J., Ambrosino D.M., Wentworth D.E., Demartini J.C., Holmes K.V. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Journal. 2004;101:15748–15753. doi: 10.1073/pnas.0403812101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marzi A., Gramberg T., Simmons G., Moller P., Rennekamp A.J., Krumbiegel M., Geier M., Eisemann J., Turza N., Saunier B., Steinkasserer A., Becker S., Bates P., Hofmann H., Pohlmann S. DC-SIGN and DC-SIGNR interact with the glycoprotein of Marburg virus and the S protein of severe acute respiratory syndrome coronavirus. Journal. 2004;78:12090–12095. doi: 10.1128/JVI.78.21.12090-12095.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Z.Y., Huang Y., Ganesh L., Leung K., Kong W.P., Schwartz O., Subbarao K., Nabel G.J. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. Journal. 2004;78:5642–5650. doi: 10.1128/JVI.78.11.5642-5650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou Y., Fu B., Zheng X., Wnag D., Zhao C., Qi Y., Sun R., Tian Z., Xu X., Wei H. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Journal. 2020 doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Journal. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang H., Wang S., Jiang T., Fan R., Zhang Z., Mu J., Li K., Wang Y., Jin L., Lin F., Xia J., Sun L., Xu B., Ji C., Chen J., Chang J., Tu B., Song B., Zhang C., Wang F.S., Xu R. High levels of circulating GM-CSF(+)CD4(+) T cells are predictive of poor outcomes in sepsis patients: a prospective cohort study. Journal. 2019;16:602–610. doi: 10.1038/s41423-018-0164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Croxford A.L., Lanzinger M., Hartmann F.J., Schreiner B., Mair F., Pelczar P., Clausen B.E., Jung S., Greter M., Becher B. The cytokine GM-CSF drives the inflammatory signature of CCR2+ monocytes and licenses autoimmunity. Journal. 2015;43:502–514. doi: 10.1016/j.immuni.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 52.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Journal. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.Y. Pulmonary pathology of Early-phase 2019 Novel Coronavirus (COVID-19) pneumonia in two patients with lung cancer. Journal. 2020 doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Young R.E., Thompson R.D., Larbi K.Y., La M., Roberts C.E., Shapiro S.D., Perretti M., Nourshargh S. Neutrophil elastase (NE)-deficient mice demonstrate a nonredundant role for NE in neutrophil migration, generation of proinflammatory mediators, and phagocytosis in response to zymosan particles in vivo. Journal. 2004;172:4493–4502. doi: 10.4049/jimmunol.172.7.4493. [DOI] [PubMed] [Google Scholar]
- 55.Liu S., Su X., Pan P., Zhang L., Hu Y., Tan H., Wu D., Liu B., Li H., Li H., Li Y., Dai M., Li Y., Hu C., Tsung A. Neutrophil extracellular traps are indirectly triggered by lipopolysaccharide and contribute to acute lung injury. Journal. 2016;6:37252. doi: 10.1038/srep37252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koutsogiannaki S., Shimaoka M., Yuki K. The use of volatile anesthetics as sedatives for acute respiratory distress syndrome. Journal. 2019;6:27–38. doi: 10.31480/2330-4871/084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fang M., Siciliano N.A., Hersperger A.R., Roscoe F., Hu A., Ma X., Shamsedeen A.R., Eisenlohr L.C., Sigal L.J. Perforin-dependent CD4+ T-cell cytotoxicity contributes to control a murine poxvirus infection. Journal. 2012;109:9983–9988. doi: 10.1073/pnas.1202143109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Small B.A., Dressel S.A., Lawrence C.W., Drake D.R., 3rd, Stoler M.H., Enelow R.I., Braciale T.J. CD8(+) T cell-mediated injury in vivo progresses in the absence of effector T cells. Journal. 2001;194:1835–1846. doi: 10.1084/jem.194.12.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang M., Hao H., Leeper N.J., Zhu L., Early Career C. Thrombotic regulation from the endothelial cell perspectives. Journal. 2018;38:e90–e95. doi: 10.1161/ATVBAHA.118.310367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lovren F., Pan Y., Quan A., Teoh H., Wang G., Shukla P.C., Levitt K.S., Oudit G.Y., Al-Omran M., Stewart D.J., Slutsky A.S., Peterson M.D., Backx P.H., Penninger J.M., Verma S. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Journal. 2008;295:H1377–H1384. doi: 10.1152/ajpheart.00331.2008. [DOI] [PubMed] [Google Scholar]
- 61.Sluimer J.C., Gasc J.M., Hamming I., van Goor H., Michaud A., van den Akker L.H., Jutten B., Cleutjens J., Bijnens A.P., Corvol P., Daemen M.J., Heeneman S. Angiotensin-converting enzyme 2 (ACE2) expression and activity in human carotid atherosclerotic lesions. Journal. 2008;215:273–279. doi: 10.1002/path.2357. [DOI] [PubMed] [Google Scholar]
- 62.Zeng H., Pappas C., Belser J.A., Houser K.V., Zhong W., Wadford D.A., Stevens T., Balczon R., Katz J.M., Tumpey T.M. Human pulmonary microvascular endothelial cells support productive replication of highly pathogenic avian influenza viruses: possible involvement in the pathogenesis of human H5N1 virus infection. Journal. 2012;86:667–678. doi: 10.1128/JVI.06348-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Y., Yan L.M., Wan L., Xiang T.X., Le A., Liu J.M., Peiris M., Poon L.L.M., Zhang W. Viral dynamics in mild and severe cases of COVID-19. Journal. 2020 doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patel S.K., Velkoska E., Burrell L.M. Emerging markers in cardiovascular disease: where does angiotensin-converting enzyme 2 fit in? Journal. 2013;40:551–559. doi: 10.1111/1440-1681.12069. [DOI] [PubMed] [Google Scholar]
- 65.Saule P., Trauet J., Dutriez V., Lekeux V., Dessaint J.P., Labalette M. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Journal. 2006;127:274–281. doi: 10.1016/j.mad.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 66.Li M., Yao D., Zeng X., Kasakovski D., Zhang Y., Chen S., Zha X., Li Y., Xu L. Age related human T cell subset evolution and senescence. Journal. 2019;16:24. doi: 10.1186/s12979-019-0165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Connors T.J., Ravindranath T.M., Bickham K.L., Gordon C.L., Zhang F., Levin B., Baird J.S., Farber D.L. Airway CD8(+) T cells are associated with lung injury during infant viral respiratory tract infection. Journal. 2016;54:822–830. doi: 10.1165/rcmb.2015-0297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smits S.L., de Lang A., van den Brand J.M., Leijten L.M., Eijkemans M.J., van Amerongen G., Kuiken T., Andeweg A.C., Osterhaus A.D., Haagmans B.L. Exacerbated innate host response to SARS-CoV in aged non-human primates. Journal. 2010;6 doi: 10.1371/journal.ppat.1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roberts A., Deming D., Paddock C.D., Cheng A., Yount B., Vogel L., Herman B.D., Sheahan T., Heise M., Genrich G.L., Zaki S.R., Baric R., Subbarao K. A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. Journal. 2007;3 doi: 10.1371/journal.ppat.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wong H.R., Freishtat R.J., Monaco M., Odoms K., Shanley T.P. Leukocyte subset-derived genomewide expression profiles in pediatric septic shock. Journal. 2010;11:349–355. doi: 10.1097/PCC.0b013e3181c519b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nickbakhsh S., Mair C., Matthews L., Reeve R., Johnson P.C.D., Thorburn F., von Wissmann B., Reynolds A., McMenamin J., Gunson R.N., Murcia P.R. Virus-virus interactions impact the population dynamics of influenza and the common cold. Journal. 2019 doi: 10.1073/pnas.1911083116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beauchemin K.J., Wells J.M., Kho A.T., Philip V.M., Kamir D., Kohane I.S., Graber J.H., Bult C.J. Temporal dynamics of the developing lung transcriptome in three common inbred strains of laboratory mice reveals multiple stages of postnatal alveolar development. Journal. 2016;4 doi: 10.7717/peerj.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]