Safety and immunogenicity of a modified vaccinia virus Ankara vector vaccine candidate for Middle East respiratory syndrome: an open-label, phase 1 trial (original) (raw)
Abstract
Background
The Middle East respiratory syndrome coronavirus (MERS-CoV) causes a respiratory disease with a case fatality rate of up to 35%. Given its potential to cause a public health emergency and the absence of efficacious drugs or vaccines, MERS is one of the WHO priority diseases warranting urgent research and development of countermeasures. We aimed to assess safety and tolerability of an anti-MERS-CoV modified vaccinia virus Ankara (MVA)-based vaccine candidate that expresses the MERS-CoV spike glycoprotein, MVA-MERS-S, in healthy adults.
Methods
This open-label, phase 1 trial was done at the University Medical Center Hamburg-Eppendorf (Hamburg, Germany). Participants were healthy men and women aged 18–55 years with no clinically significant health problems as determined during medical history and physical examination, a body-mass index of 18·5–30·0 kg/m2 and weight of more than 50 kg at screening, and a negative pregnancy test for women. A key exclusion criterion was a previous MVA vaccination. For the prime immunisation, participants received doses of 1 × 107 plaque-forming unit (PFU; low-dose group) or 1 × 108 PFU (high-dose group) MVA-MERS-S intramuscularly. A second identical dose was administered intramuscularly as a booster immunisation 28 days after first injection. As a control group for immunogenicity analyses, blood samples were drawn at identical study timepoints from six healthy adults, who did not receive any injections. The primary objectives of the study were safety and tolerability of the two dosage levels and reactogenicity after administration. Immunogenicity was assessed as a secondary endpoint by ELISA and neutralisation tests. T-cell immunity was evaluated by interferon-γ-linked enzyme-linked immune absorbent spot assay. All participants who were vaccinated at least once were included in the safety analysis. Immunogenicity was analysed in the participants who completed 6 months of follow-up. This trial is registered with ClinicalTrials.gov, NCT03615911, and EudraCT, 2014-003195-23
Findings
From Dec 17, 2017, to June 5, 2018, 26 participants (14 in the low-dose group and 12 in the high-dose group) were enrolled and received the first dose of the vaccine according to their group allocation. Of these, 23 participants (12 in the low-dose group and 11 in the high-dose group) received a second dose of MVA-MERS-S according to their group allocation after a 28-day interval and completed follow-up. Homologous prime–boost immunisation with MVA-MERS-S revealed a benign safety profile with only transient mild-to-moderate reactogenicity. Participants had no severe or serious adverse events. 67 vaccine-related adverse events were reported in ten (71%) of 14 participants in the low-dose group, and 111 were reported in ten (83%) of 12 participants in the high-dose group. Solicited local reactions were the most common adverse events: pain was observed in 17 (65%; seven in the low-dose group vs ten in the high-dose group) participants, swelling in ten (38%; two vs eight) participants, and induration in ten (38%; one vs nine) participants. Headaches (observed in seven participants in the low-dose group vs nine in the high-dose group) and fatigue or malaise (ten vs seven participants) were the most common solicited systemic adverse events. All adverse events resolved swiftly (within 1–3 days) and without sequelae. Following booster immunisation, nine (75%) of 12 participants in the low-dose group and 11 (100%) participants in the high-dose group showed seroconversion using a MERS-CoV S1 ELISA at any timepoint during the study. Binding antibody titres correlated with MERS-CoV-specific neutralising antibodies (Spearman's correlation r=0·86 [95% CI 0·6960–0·9427], p=0·0001). MERS-CoV spike-specific T-cell responses were detected in ten (83%) of 12 immunised participants in the low-dose group and ten (91%) of 11 immunised participants in the high-dose group.
Interpretation
Vaccination with MVA-MERS-S had a favourable safety profile without serious or severe adverse events. Homologous prime–boost immunisation induced humoral and cell-mediated responses against MERS-CoV. A dose–effect relationship was demonstrated for reactogenicity, but not for vaccine-induced immune responses. The data presented here support further clinical testing of MVA-MERS-S in larger cohorts to advance MERS vaccine development.
Funding
German Center for Infection Research.
Research in context.
Evidence before this study
Middle East respiratory syndrome (MERS) is a zoonotic viral respiratory illness caused by the MERS coronavirus (MERS-CoV). MERS is under active surveillance scrutiny by WHO, and so far there is no licensed vaccine available to prevent disease and viral spread. MVA-MERS-S, a novel vaccine candidate based on the recombinant modified vaccinia virus Ankara (MVA) vaccine vector platform, has been reported to be safe and immunogenic in mice and dromedary camels. We searched ClinicalTrials.gov on Aug 23, 2019, with the keywords “MERS” and “vaccine”; five studies were listed. Except for one study that evaluated immunoglobulins as treatment against MERS, the other four tested MERS vaccine platforms. Three of these vaccine platforms were to be tested in phase 1 trials: ChAdox1, MVA, and a DNA vaccine, with the latter progressing to a phase 1/2a trials. The DNA vaccine phase 1 trial was done in the USA and a phase 1/2a study was recently initiated in Seoul (South Korea). The first human data on a MERS vaccine candidate were published in July, 2019, presenting the safety and immunogenicity of the DNA vaccine candidate GLS-5300. We aimed to assess the safety and immunogenicity data of the first MVA viral vector vaccine candidate against MERS proceeding to clinical trials.
Added value of this study
This study provides comprehensive results of a phase 1 trial testing an MVA vector-based vaccine expressing the MERS-CoV-S protein. The study reveals a benign safety profile of MVA-MERS-S and provides first insight into the immunogenicity of the vaccine candidate. Participants who received the high dose of 1 × 108 plaque-forming unit (PFU) experienced mild-to-moderate adverse events and showed seroconversion, and 91% of these patients showed T-cell responses. The low-dose group (1 × 107 PFU) showed a similar safety profile, seroconversion in 75% of participants, and T-cell immunity in 83% of participants.
Implications of all the available evidence
In the aftermath of the Ebola virus outbreak in west Africa in 2013–16, WHO has flagged the need for the timely development of vaccines for high-threat pathogens, including MERS-CoV. The data presented here support the further clinical development of the candidate vaccine MVA-MERS-S and add critical insight into the field of vaccine research against coronaviruses and other emerging pathogens.
Introduction
Middle East respiratory syndrome (MERS) is a viral respiratory illness under active surveillance by WHO, and, in 2015, it was placed on the list of priority diseases with high epidemic potential that warrant urgent research and development of countermeasures.1 As a disease of the lower respiratory tract in humans, MERS can progress rapidly from unspecific, influenza-like symptoms to severe pneumonia, multiple organ failure, and death.
The causative agent of MERS is MERS coronavirus (MERS-CoV), which was first identified in 2012 in Saudi Arabia.2 Dromedary camels serve as reservoir hosts, and the majority of primary human cases can be traced back to close or frequent contact with dromedaries. Additionally, human-to-human transmission is amplified in health-care settings, adding health-care workers to the populations at high risk.
As of Jan 31, 2020, 2519 cases have been reported, with 866 deaths in 27 countries, resulting in a case-fatality rate of 34·3%.3 Large MERS outbreaks occurred in Saudi Arabia in 2014 (255 cases and 93 deaths)4 and in South Korea in 2015 (186 cases and 38 deaths).5 Although transmission is predominantly reported from the Arabian Peninsula, there is a high risk of exporting cases to areas outside the Middle East as a result of travel. So far, supportive therapy, isolation of patients, and contact tracing remain the main pillars of MERS treatment and prevention, highlighting the urgent need to develop countermeasures such as vaccines to block transmission and combat potential future disease outbreaks.
The development of MERS vaccine candidates has been challenging because the determinants of protective immunity remain incompletely understood and the limited number of MERS cases has made it difficult to do efficacy trials. Although optimal animal models for the identification of protective immune correlates for MERS are still scarce, current mouse models suggest a crucial role for antibodies and T cells in the induction of protective immunity.6, 7, 8 In humans, one study showed a positive correlation of CD4 T-cell responses and neutralising antibodies with severe disease progression, and a positive correlation of strong virus-specific CD8 T-cell responses with reduced morbidity;9 conversely, another study reported a positive association between early CD8 T-cell responses and disease severity in acute MERS-CoV infection.10 A role of antibodies in protection was shown in mice that received convalescent sera from survivors.9 The animal and human data underline that a MERS vaccine candidate should preferentially induce humoral and cell-mediated immune responses and that the timing and dose of a vaccine could be crucial for protection.
In this study, we used the recombinant modified vaccinia virus Ankara (rMVA) vaccine vector platform, which has been shown to be safe and immunogenic in humans.11 MVA vaccine candidates have demonstrated a favourable safety profile in various populations and disease settings.11, 12, 13 The MERS-CoV spike glycoprotein consists of S1 and S2 subunits and mediates viral attachment to host cells, entry, and membrane fusion.14 It is a target for neutralising antibodies and, therefore, vaccine development. The MERS vaccine candidate MVA-MERS-S investigated in this study encodes the full MERS-CoV spike glycoprotein. Preclinical studies have shown that MVA-MERS-S induced neutralising antibodies6 and conferred protection against MERS-CoV in mice15 expressing the dipeptidyl peptidase 4 (DPP4) receptor. Furthermore, a reduction in viral replication in a MERS-CoV challenge after MVA-MERS-S vaccination was successfully demonstrated in dromedary camels.16 These preclinical data support the advancement of the vaccine candidate MVA-MERS-S to test the safety and immunogenicity in human clinical trials.
We aimed to assess the safety, tolerability, and immunogenicity data for a novel viral-vectored MERS candidate vaccine in healthy adults.
Methods
Study design and participants
This open-label, phase 1 study was done at the University Medical Center Hamburg-Eppendorf (Hamburg, Germany). Participants were recruited through public advertisement. We included men and women aged 18–55 years with no clinically significant health problems as determined during medical history and physical examination, a body-mass index of 18·5–30·0 kg/m2 and weight of more than 50 kg at screening, and a negative pregnancy test for women. A key exclusion criterion was previous MVA immunisation. The full list of inclusion and exclusion criteria are provided in the protocol (appendix pp 45–46).
All participants provided written, informed consent. The study design was reviewed and approved by the Competent National Authority (Paul-Ehrlich-Institut, Langen, Germany) and the Ethics Committee of the Hamburg Medical Association. The study was done in accordance with the Declaration of Helsinki (Fortaleza, Brazil, 2013) and International Conference on Harmonisation Good Clinical Practice. A local safety monitoring board provided clinical oversight.
Procedures
MVA-MERS-S is based on a rMVA vector encoding the full-length MERS-CoV spike glycoprotein, based on the sequence of EMC/2012 (GenBank accession number JX869059).6 The vaccine was manufactured by IDT Biologika (Dessau, Germany) in primary chicken embryo fibroblasts.
For the prime immunisation, participants received doses of 1 × 107 plaque-forming unit (PFU) (low-dose group) or 1 × 108 PFU (high-dose group) MVA-MERS-S intramuscularly into the deltoid muscle of the non-dominant arm. A second identical dose was administered intramuscularly as a booster immunisation 28 days after first injection. For safety reasons, participants were vaccinated in a staggered manner (appendix p 49). 7 days after administration of prime and boost doses to the last study participant in the low-dose group and review of all safety data by the local safety board, the same staggered mode of vaccinations was applied in the high-dose group. As a control group for immunogenicity analyses, blood samples were drawn at identical study timepoints from six healthy adults, who did not receive any injections.
Each individual underwent physical examination and drug and pregnancy testing, and received an electrocardiogram the evening before each immunisation at the Clinical Trial Center North (Hamburg, Germany). Heart rate, blood pressure, and body temperature were recorded by study personnel 2 h, 4 h, 6 h, 8 h, 12 h, and 24 h after each vaccination. Blood was drawn for chemical and haematological safety analyses. Participants were monitored longitudinally for 180 days, with study visits the day before vaccination (day −1), the day of vaccination (day 0), and days 1, 3, 7, 14, 27, 28, 29, 35, 42, 56, 84, and 180 after vaccination, including two overnight stays with an approximate duration of 36 h (days −1 to 1 for the prime immunisation and days 27 to 29 for the booster immunisation). Clinical and laboratory evaluations were done during each study visit (appendix p 4). Laboratory analyses included measurements of complete blood counts, creatinine, C-reactive protein (CRP), troponin, and liver function markers.
Local and systemic reactogenicity as well as medication use were recorded by the participants for 14 days after each vaccination on a daily notification sheet and were further recorded on follow-up visits by study personnel. Adverse events were classified as mild (no interference with activities of daily living), moderate (interference with daily living activities, but pose no substantial or permanent risks to the participants), or severe (considerably affect daily living and clinical status). Adverse events were assessed and categorised on the basis of whether they were solicited (expected and therefore predefined in the protocol) or unsolicited events. Solicited events included both local (pain, induration, redness, haematoma, and swelling at injection site) and systemic adverse events (fever, chills, fatigue, malaise, myalgia, arthralgia, headaches, and gastrointestinal symptoms such as diarrhoea, loose stools, abdominal cramps, and nausea). Unsolicited adverse events were all other events not defined as solicited and all events that arose between days 14 and 28 as well as after day 42 (14 days after the booster vaccination). The occurrence of serious adverse events was monitored throughout the study. A serious adverse event was defined as any event that results in death, was life-threatening, required inpatient hospital admission or caused prolongation of an existing hospital stay, resulted in persistent disability, or required intervention to prevent permanent impairment or damage. Adverse events were listed and graded by study personnel according to the Common Terminology Criteria for Adverse Events (version 4.0) and the Medical Dictionary for Regulatory Activities (appendix pp 67–68).
Serum was analysed at days 0, 28, 35, 42, 56, 84, and 180 to evaluate humoral immune responses. We did an ELISA by coating 96-well microtitre plates with 1 μg/mL MERS-CoV S1 protein, as previously described.17 Absorbance was measured at 450 nm. A cutoff was set at an optical density of 0·5. An optimised commercial MERS-CoV S1 ELISA assay (EuroImmun, Lubeck, Germany) was done as a post-hoc additional analysis.
The presence of MERS-CoV-neutralising antibodies in sera of participants was investigated with the virus neutralisation test, which was used to measure the neutralising capacity of sera on HuH-7 cells infected with MERS-CoV (EMC/2012) in four replicates, with a reciprocal titre of 8 considered to be positive, as previously described.15 Neutralisation was defined as absence of cytopathic effects.
Additionally, sera were tested for neutralisation capacity with a plaque reduction neutralisation test (using EMC/2012).17 The plaque reduction neutralisation test (PRNT) titre of each serum sample is the reciprocal value of the highest dilution resulting in an infection reduction of 80% or more (PRNT80). A titre of 20 or more was considered to be positive. Details of all three methods are described in the appendix (pp 4–5).
Peripheral blood mononuclear cells (PBMCs) were isolated and cryopreserved from EDTA blood using Ficoll density gradient centrifugation. T-cell responses were assessed with interferon-γ (IFNγ)-linked enzyme-linked immune absorbent spot (ELISpot; ImmunoSpot, Cellular Technology, Cleveland, OH, USA; 384-well plate). Following thawing and resting, PBMCs were stimulated for 16 h in triplicates with five overlapping peptide pools spanning the entire MERS-CoV-S amino acid sequence. A phytohemagglutinin pool and a cytomegalovirus, Epstein-Barr virus, and influenza peptide pool (JPT Peptide Technologies, Berlin, Germany) served as positive controls, and serum-free medium (Cellular Technology) supplemented with dimethyl sulphoxide served as negative controls. A response was defined as positive when two criteria were met: first, more than 50 spot-forming cells (SFCs) per million PBMCs; and second, the number of SFCs per million PBMCs was more than four times higher than the baseline (day 0) value. For more details, see appendix (p 5).
Antigen-specific CD4 IFNγ-expressing and CD8 IFNγ-expressing T cells were analysed using flowcytometry. Following overnight resting, PBMCs were incubated for 6 h at 37°C with overlapping peptide pools. Cells were analysed on an LSRFortessa (BD Biosciences, Franklin Lakes, NJ, USA) and evaluated with FlowJo10 (version 10.6.1; FlowJo, Ashland, OR, USA).
Outcomes
The primary endpoints of frequency and severity of adverse events was measured as the occurrence of solicited local and systemic reactogenicity signs and symptoms for 14 days after vaccination; occurrence of unsolicited adverse events for 28 days after vaccination; change from baseline of safety laboratory measures; and occurrence of serious adverse events throughout the study period of 6 months. The secondary endpoint of immunogenicity was the magnitude of MERS-CoV spike-specific antibody responses as measured by ELISA. Preplanned exploratory analyses included evaluation of T-cell immunity by ELISpot.
Statistical analysis
This trial was designed as an exploratory trial and was not powered statistically to measure a specific outcome or determine dose finding. All participants who were vaccinated at least once were included in the safety analysis. Immunogenicity was analysed in the participants who completed 6 months of follow-up. Recorded adverse events are depicted in frequency (%) of participants in each dose group who experienced at least one adverse event in the indicated symptom group. In case a participant reported two adverse events with differing degrees in the same symptom group, the more severe event was recorded.
To correlate binding and neutralising antibodies against MERS-CoV, we did a Spearman correlation between ELISA optical density values and the reciprocal titre of neutralising antibodies.
We analysed data by Wilcoxon signed-rank testing for paired samples and Mann-Whitney U testing for unpaired samples. A p value of 0·05 or less was considered to be significant. p values were not corrected for multiple comparisons. We did statistical analyses using GraphPad Prism (version 8).
This trial is registered at ClinicalTrials.gov, NCT03615911, and EudraCT, 2014-003195-23.
Role of the funding source
The funder of this investigator-initiated study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
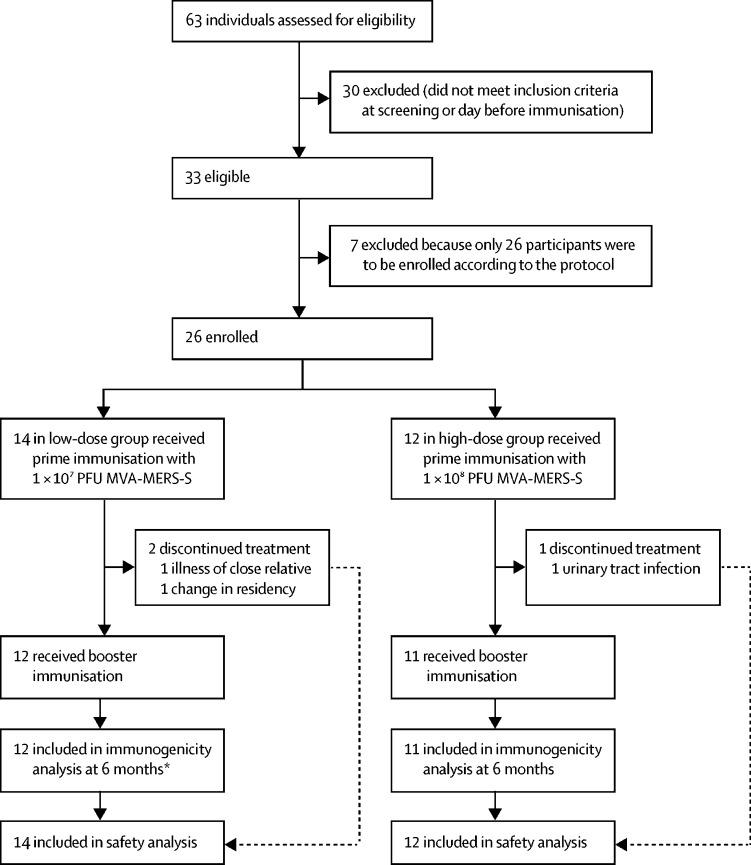
Between Dec 17, 2017, and June 5, 2018, 63 individuals were screened and 26 participants were enrolled (figure 1). Details of the participants' baseline characteristics are summarised in the table 1.
Figure 1.
Trial profile
Reasons for screening failures of the ineligible individuals are provided in the appendix (p 9). MERS=Middle East respiratory syndrome. MVA=modified vaccinia virus Ankara. PFU=plaque-forming unit. S=spike. *One participant had to move the day 180 visit to day 110; this data point was therefore removed from immunological analysis.
Table 1.
Baseline characteristics
| Low-dose group (n=14) | High-dose group (n=12) | All participants*(n=26) | Control group (n=6) | |
|---|---|---|---|---|
| Sex | ||||
| Female | 10 (71%) | 10 (83%) | 20 (77%) | 4 (67%) |
| Male | 4 (29%) | 2 (17%) | 6 (23%) | 2 (33%) |
| Age, years | 28·7 (8·4; 18–47) | 30·8 (8·7; 21–52) | 29·7 (8·4; 18–52) | 35·0 (5·1; 27–40) |
| Ethnicity | ||||
| White | 12 (86%) | 10 (83%) | 22 (85%) | 5 (83%) |
| Black or African American | 1 (7%) | 0 | 1 (4%) | 0 |
| Native Hawaiian or Pacific Islander | 0 | 1 (8%) | 1 (4%) | 0 |
| Hispanic or Latino | 1 (7%) | 1 (8%) | 2 (8%) | 0 |
| American Indian or Alaska Native | 0 | 0 | 0 | 0 |
| Asian | 0 | 0 | 0 | 1 (17%) |
| BMI, kg/m2 | 23·6 (2·5) | 23·5 (3·7) | 23·6 (3·0) | 22·9 (1·2) |
Three individuals (one in the low-dose group and two in the high-dose group) did not receive the booster immunisation and were excluded from the immunogenicity analysis; however, all data were included in the safety investigations. The two participants in the low-dose group discontinued because of personal reasons within the first 14 days of study and were replaced. Both replacements completed the follow-up visits. A third person (high-dose group) who dropped out received no booster immunisation because of an unrelated urinary tract infection on day 27. This person was not replaced at this stage of the trial.
The full prime–boost vaccination regimen was administered to 23 participants at an interval of 28 days. All 23 participants completed the scheduled follow-up visits up to 6 months. One participant from the low-dose group moved the day 180 follow-up visit to day 110 because of a change of residence. This timepoint was excluded from the immunogenicity analysis. There were no other protocol violations.
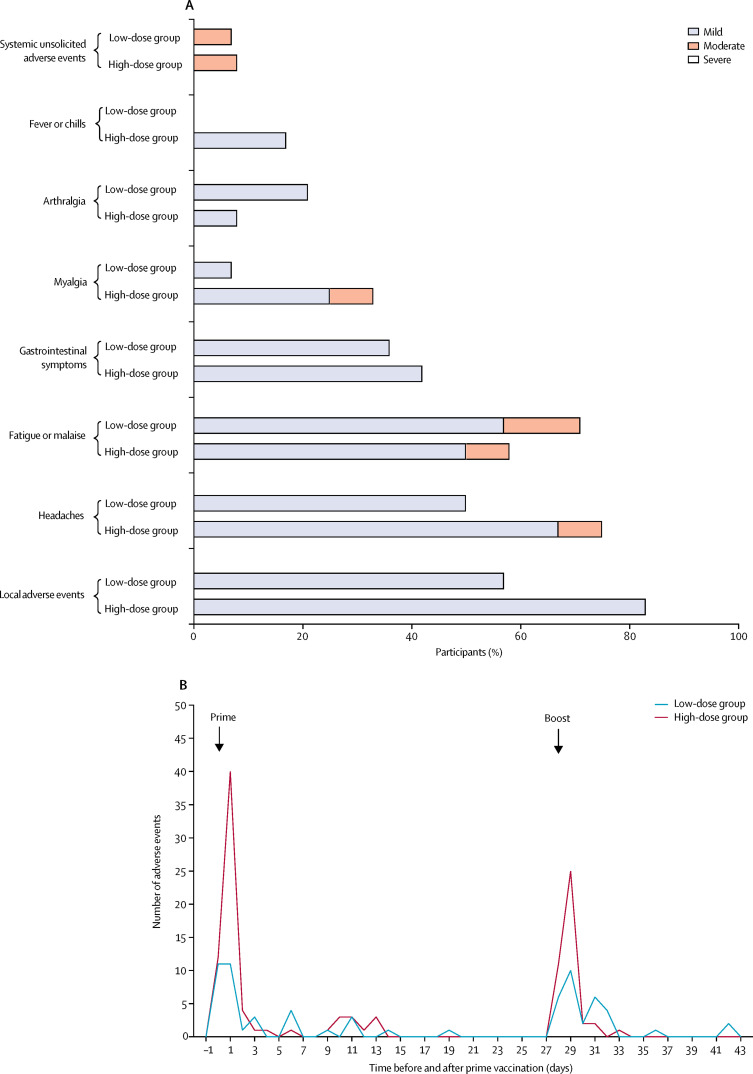
In the monitoring of acute reactogenicity following immunisations, no serious adverse events were reported. 67 vaccine-related adverse events were reported in ten (71%) of 14 participants in the low-dose group and 111 were reported in ten (83%) of 12 participants in the high-dose group. The majority was solicited and mild, with a few moderate and no severe adverse events. Most adverse events appeared early after administration of the vaccine (median 1 day [IQR 1–3]). The high-dose group had more adverse events after prime and boost immunisations than the low-dose group (figure 2A). All adverse events resolved quickly (median 1 day [IQR 0–3]) and generally required no treatment.
Figure 2.
Treatment-related local and systemic adverse events
(A) The proportion of participants in each dose group who experienced at least one adverse event in the indicated symptom group. In case a participant reported two adverse events with differing degrees in the same symptom group, the more severe event was recorded. (B) Absolute number of related adverse events over time up to 43 days after prime immunisation with MVA-MERS-S in each dose group. MERS=Middle East respiratory syndrome. MVA=modified vaccinia virus Ankara. S=spike.
Solicited local reactions were the most common adverse events: pain was observed in 17 (65%; seven participants in the low-dose group vs ten in the high-dose group) participants, swelling in ten (38%; two vs eight) participants, and induration in ten (38%; one vs nine) participants. Headaches (observed in seven participants in the low-dose group vs nine participants in the high-dose group) and fatigue or malaise (ten vs seven participants) were the most common solicited systemic adverse events (figure 2B). Details on adverse events are listed in the appendix (pp 10–16).
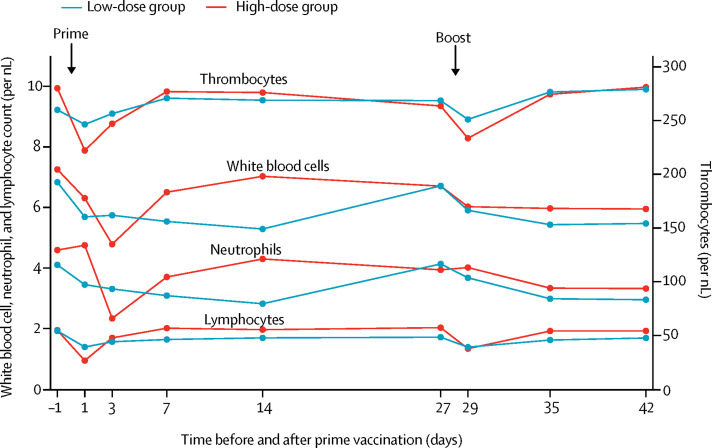
Two participants in the high-dose group had an elevated temperature and fever about 16 h after prime immunisation (temperatures of 37·8°C and 38·8°C, respectively; appendix pp 7, 8, 18). Significant, but transient, asymptomatic decreases in white blood cell and neutrophil counts from day −1 to day 3, as well as lymphocyte and thrombocyte counts from day −1 to day 1, were observed in the high-dose group (figure 3; table 2). The low-dose group also had similar differences, but to a lower extent. CRP concentration at day 3 was significantly higher in the high-dose group than in the low-dose group (appendix p 7). Other laboratory parameters and vital signs did not show significant alterations after vaccinations (data not shown).
Figure 3.
Biological monitoring
Haematological changes after prime–boost immunisation with MVA-MERS-S. Lines represent the median value of measured counts of white blood cells, neutrophils, lymphocytes, and thrombocytes in the low-dose and high-dose groups. MERS=Middle East respiratory syndrome. MVA=modified vaccinia virus Ankara. S=spike.
Table 2.
Haematological changes
| Low-dose group (n=14) | High-dose group (n=12) | |||
|---|---|---|---|---|
| Geometric mean per nL (95% CI) | p value | Geometric mean per nL (95% CI) | p value | |
| Thrombocytes | .. | 0·048 | .. | 0·0005 |
| Day −1 | 257 (236–280) | .. | 273 (237–315) | .. |
| Day 1 | 241 (214–272) | .. | 216 (186–251) | .. |
| White blood cells | .. | 0·041 | .. | 0·0010 |
| Day −1 | 7·0 (5·9–7·6) | .. | 7·1 (6·2–8·1) | .. |
| Day 3 | 5·6 (4·9–6·4) | .. | 4·6 (4·0–5·4) | .. |
| Neutrophils | .. | 0·080 | .. | 0·0010 |
| Day −1 | 4·0 (3·5–4·6) | .. | 4·5 (3·8–5·3) | .. |
| Day 3 | 3·2 (2·8–3·7) | .. | 2·2 (1·8–2·7) | .. |
| Lymphocytes | .. | 0·0052 | .. | 0·0005 |
| Day −1 | 1·8 (1·4–2·3) | .. | 1·9 (1·5–2·3) | .. |
| Day 1 | 1·3 (1·1–1·6) | .. | 0·9 (0·8–1·1) | .. |
Seroconversion was detected in 20 (87%) of 23 participants (nine [75%] of 12 participants in the low-dose group and 11 [100%] participants in the high-dose group) at any timepoint throughout the study (table 3).
Table 3.
Antibody responses of study participants as measured by ELISA MERS-CoV S1 assay
| Low-dose group (n=12) | High-dose group (n=11) | All participants*(n=23) | Control group (n=6) | |||||
|---|---|---|---|---|---|---|---|---|
| Geometric mean (95% CI) | Seropositivity | Geometric mean (95% CI) | Seropositivity | Geometric mean (95% CI) | Seropositivity | Geometric mean (95% CI) | Seropositivity | |
| Day 0 | 0·20 (0·16–0·24) | 0 | 0·21 (0·18–0·24) | 0 | 0·20 (0·18–0·23) | 0 | 0·24 (0·16–0·38) | 1 (16%) |
| Day 28 | 0·23 (0·16–0·32) | 1 (8%) | 0·34 (0·26–0·43) | 1 (9%) | 0·28 (0·22–0·34) | 2 (9%) | 0·21 (0·14–0·33) | 0 |
| Day 35 | 0·52 (0·28–0·94) | 6 (50%) | 0·87 (0·65–1·16) | 9 (81%) | 0·66 (0·47–0·93) | 15 (65%) | 0·23 (0·15–0·35) | 0 |
| Day 42 | 0·88 (0·48–1·57) | 8 (67%) | 0·90 (0·70–1·20) | 11 (100%) | 0·89 (0·66–1·21) | 19 (83%) | 0·25 (0·19–0·34) | 0 |
| Day 56 | 0·80 (0·47–1·34) | 8 (67%) | 0·74 (0·57–0·98) | 11 (100%) | 0·77 (0·59–1·02) | 19 (83%) | 0·20 (0·17–1·23) | 0 |
| Day 84 | 0·64 (0·39–1·05) | 8 (67%) | 0·54 (0·41–0·70) | 8 (73%) | 0·56 (0·46–0·76) | 16 (70%) | 0·13 (0·07–0·22) | 0 |
| Day 180† | 0·27 (0·14–0·51) | 3/11 (27%) | 0·18 (0·13–0·26) | 0 | 0·22 (0·15–0·33) | 3/22 (14%) | 0·14 (0·10–0·21) | 0 |
| Day 28–180† | 0·55 (0·44–0·70) | 9 (75%) | 0·63 (0·54–0·73) | 11 (100%) | 0·59 (0·51–0·68) | 20 (87%) | 0·18 (0·16–0·22) | 0 |
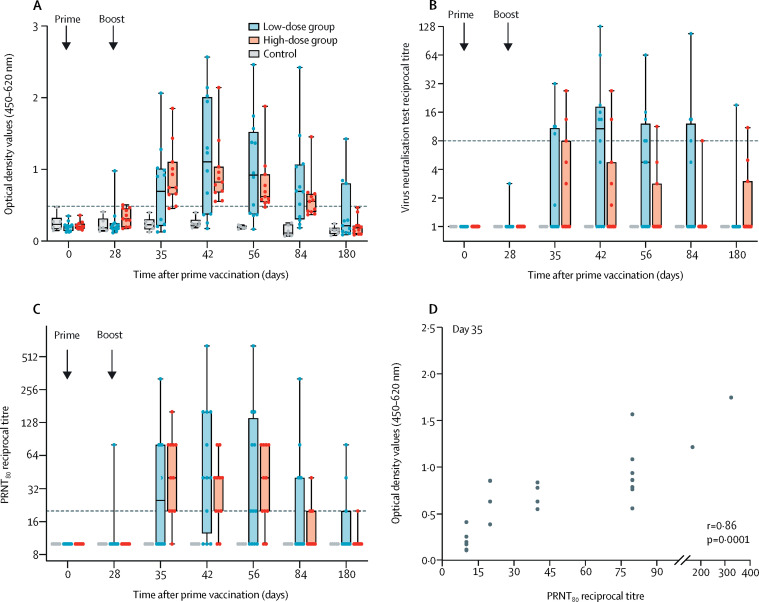
Antibody responses were predominantly detected after the second vaccine dose of either the low or the high dose of MVA-MERS-S. Two vaccinated participants (one from the low-dose group and one from the high-dose group) showed a positive ELISA response on day 28 after a single MVA-MERS-S administration. 7 days after boost immunisation (day 35), MERS-CoV S1-specific antibodies were detectable in both dose groups (table 3). The majority of vaccinated participants showed positive ELISA responses on days 42 and 56 (table 3). In the control group, all participants were seronegative except for one participant, who showed a borderline optical density value of 0·5 on day 0, the exact cutoff value for seropositivity (figure 4A; table 3).
Figure 4.
MERS-CoV-specific antibody responses
Dots represent data from individual participants, and the median is depicted by horizontal line within the boxes. Error bars represent minimum to maximum values. (A) Binding antibodies were measured against the MERS-CoV S1 protein using an in-house ELISA. The horizontal dashed line depicts the positive threshold. The optical density value of 0·5 was considered to be the threshold for seropositivity. (B) MERS-CoV-specific neutralising antibody responses were measured by the virus neutralisation test assay after prime and boost immunisation. Data are represented as reciprocal neutralisation titre. The geometric mean of four replicates per participant per timepoint is shown. The signal from samples with no neutralising capacity was set to 1. The horizontal dashed line represents the positive threshold. (C) PRNT titre was calculated on the basis of 80% or greater reduction of infected cells. Data are shown as reciprocal titre. The horizontal dashed line represents the positive threshold. (D) Correlation analysis between binding antibodies (optical density value) and reciprocal PRNT80 titres at day 35 after vaccination. MERS-CoV=Middle East respiratory syndrome coronavirus. PRNT80=plaque reduction neutralisation test titre calculated on the basis of 80% or greater reduction of infected cells.
The geometric mean of optical density values for antibody concentrations on day 42 were similar for both cohorts (table 3). Antibody concentrations for both groups decreased over time. Positive ELISA responses were still observed in 16 (70%) of 23 participants at day 84. At day 180, three (27%) of 11 participants in the low-dose group and none in the high-dose group remained positive for MERS-CoV spike-specific antibodies. We observed no significant dose dependency in immunogenicity readouts between the low-dose and high-dose groups (day 35 p=0·2944, day 42 p=0·8463, and day 56 p=0·8801, with the Mann-Whitney U test; data not shown). A post-hoc additional immunogenicity investigation with optimised commercial ELISA assay (EuroImmun) showed similar confirmatory results (appendix pp 4–5).
At any timepoint throughout the study, sera from 12 (58%) of 23 participants who were vaccinated neutralised MERS-CoV using virus neutralisation test (figure 4B). A single injection of MVA-MERS-S showed no induction of neutralising antibodies. The virus neutralisation test revealed detectable neutralising capacity only after boosting, but mainly in participants in the low-dose group, with a peak response on day 42, when seven (58%) of 12 participants reacted positively, compared with two (18%) of 11 participants in the high-dose group. At the end of the study (day 180), two (9%; one in each dose group) still had detectable neutralising capacity by the virus neutralisation test.
In the more sensitive PRNT80 assay (figure 4C), antibody responses mirrored a similar pattern as observed using the in-house ELISA (figure 4A). A single vaccine administration induced neutralising antibodies in one participant (in the low-dose group). After boost immunisation, nine (75%) participants in the low-dose group and nine (82%) participants in the high-dose group showed responses. Boosting elicited a significant increase in neutralising antibody responses at day 42 compared with baseline in both groups (day 42 p<0·0010 in both groups; Wilcoxon signed-rank test; appendix p 17).
A linear correlation between ELISA optical density values and the reciprocal titre of neutralising antibodies (PNRT80) was observed for day 35 (r=0·86 [95% CI 0·6960–0·9427], p=0·0001; figure 4D), suggesting a high proportion of functional antibodies among the vaccine-induced MERS-CoV spike-specific binding antibodies. The highest number of positive responses in both the in-house MERS-CoV S1 ELISA and the PRNT80 assays were detected on days 42 and 56 in 16 (70%) of 23 vaccinated participants (seven in the low-dose group and nine in the high-dose group; data not shown). Antibodies directed against the vaccines vector were also assessed. After boost vaccination, MVA-specific neutralising antibodies were detectable in 19 (83%) of 23 vaccine recipients (eight in the low-dose group and 11 in the high-dose group). No correlation with MERS-CoV-specific binding or neutralising antibody responses was observed (data not shown).
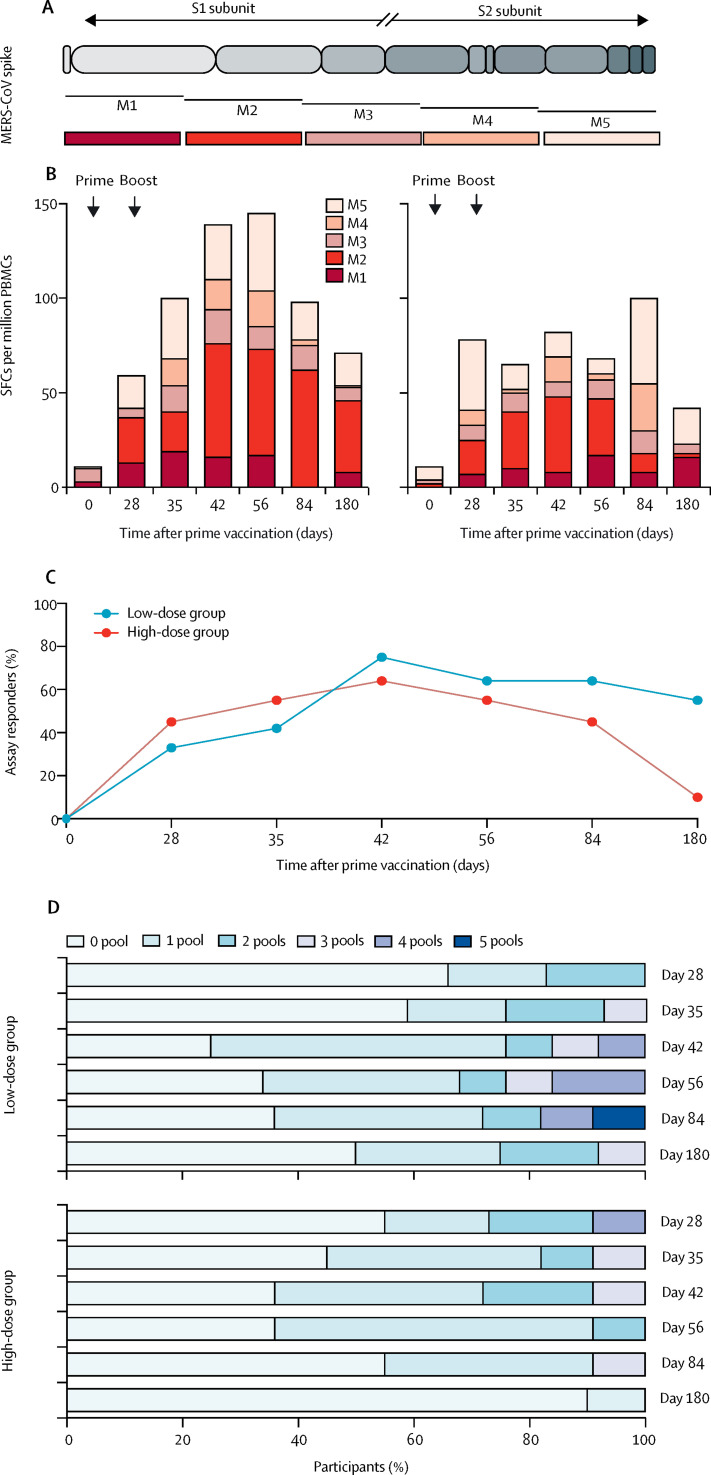
MERS-CoV spike-specific T-cell responses were evaluated by ELISpot, using five overlapping peptide pools (figure 5A). Representative ELISpot wells are shown in the appendix (p 21). T-cell responses against MERS-CoV spike emerged after a single vaccination with MVA-MERS-S in some of the participants and were enhanced after boost immunisation. Overall, IFNγ secretion was detected in ten (83%) of 12 participants in the low-dose group and ten (91%) of 11 participants in the high-dose group at one or more timepoints throughout the study.
Figure 5.
MERS-CoV spike-specific T-cell responses after prime–boost vaccination with MVA-MERS-S
(A) Schematic represents the five MERS-CoV spike-specific overlapping peptide pools (M1–M5) that span the entire MERS-CoV spike glycoprotein. (B) Magnitude of T-cell responses targeting MERS-CoV spike glycoprotein. ELISpot responses to the MERS-CoV spike glycoprotein are shown for the five peptide pools by the median values. The sum of the medians of SFCs per million PBMCs is depicted per cohort, per pool, and per timepoint. (C) Proportion of assay responders. A positive response was defined as such if more than 50 SFCs per million PBMCs and the number of SFCs per million PBMCs was more than four times higher than the baseline (day 0) value. (D) Graph showing the breadth of the response to peptide pools. The darker the blue, the more peptide pool responses were observed. ELISpot=interferon-γ-linked enzyme-linked immune absorbent spot assay. MERS-CoV=Middle East respiratory syndrome coronavirus. MVA=modified vaccinia virus Ankara. RBD=receptor binding domain. S=spike. SFCs=spot-forming cells. PBMCs=peripheral blood mononuclear cells.
T-cell responses were observed to all peptide pools; however, responses to pool M2 were more frequently detected (seven [58%] of 12 participants in the low-dose group vs seven [64%] of 11 participants in the high-dose group) than to other pools (four [33%] vs five [45%] for M1, four [33%] vs one [9%] for M3, three [25%] vs three [27%] for M4, and five [42%] vs five [45%] for M5; figure 5B). Both groups showed an increase in IFNγ secretion compared with day 0, which was detected in the low-dose group on day 28 and in the high-dose group on day 35 at the earliest. A peak response was observed for M2 at day 42 (appendix p 21).
The highest magnitudes of IFNγ responses were observed for two participants in the low-dose group upon M1-pool stimulation (622 and 522 SFCs per million PBMCs, respectively). An additional post-hoc analysis of a subgroup of six participants (three in each group) demonstrated MERS-CoV spike-specific secretion of IFNγ predominantly from CD8 T cells rather than from CD4 T cells when using flowcytometry (appendix p 22).
Taking into account the number of assay responders over time, both dose cohorts showed a similar pattern: the earliest assay responders were identified at day 28 (figure 5C). At day 180, in the low-dose group, six (55%) of 11 participants (one participant was removed from day 180 analysis, as outlined previously) still showed positive T-cell responses, and in the high-dose group, one participant showed a response. To evaluate the breadth of T-cell responses, we assessed the number of pools to which the participants responded. The high-dose group revealed a response to more than one pool already at day 28, which reduced in breadth over time. By comparison, the low-dose group showed an increase in breadth only after the boost immunisation (figure 5D).
Taken together, these data show that immunisation with MVA-MERS-S induced T-cell responses, which decreased during the study period but remained detectable.
Discussion
Following homologous prime and boost immunisations with MVA-MERS-S, no single severe or serious adverse events were observed in participants who received either the low dose or the high dose of the vaccine. All participants experienced transient, self-limiting, mild-to-moderate adverse events, mostly dose-dependent local reactions as well as unspecific systemic adverse events. These data demonstrate a favourable safety profile for both doses and two administrations. Safety signals were similar to those observed in a phase 1 trial testing the influenza vaccine MVA-H5-sfMR,18 in which participants received the same doses with the identical MVA backbone. Similar safety profiles have been observed in other trials using the MVA platform with inserted antigens derived from pathogens.11, 12, 19 Comparison of MVA-MERS-S vaccine-induced safety profiles suggests vector-specific rather than insert-specific safety signals. No MERS-CoV-specific adverse events as outlined by the Brighton Collaboration were observed.
Vaccination with MVA-MERS-S elicited both humoral and cellular immune responses to MERS-CoV spike, which were mostly detectable following boost rather than prime immunisation. The humoral immune responses, measured by ELISA and two different viral neutralisation assays, revealed a peak of antibody responses at days 42 and 56, were maintained through day 84, and declined to baseline levels in the majority of study participants by end of study (6 months after vaccination). Although the exact contribution of MERS-CoV-specific antibodies to immune protection against MERS remains to be further elucidated, preclinical data support their role in protection against virus challenge in animal models.6, 9, 20, 21 A significant reduction of excreted infectious virus and viral RNA transcripts in MVA-MERS-S-vaccinated dromedary camels was observed following MERS-CoV challenge that was associated with MERS-CoV-specific neutralising antibodies.16
Albeit not an optimal animal model, mouse studies can support the investigation into protective mechanisms. Mice are not susceptible to MERS-CoV, but with expression of human DPP4 (transgenic mice or adenoviral-mediated delivery), MERS-CoV can infect mouse cells and challenge experiments can be supported. The impact of antibodies on immune protection through passive immunotherapy with subsequent challenge experiments was evaluated. Passive immunotherapy before MERS-CoV challenge using MERS-CoV-specific monoclonal antibodies or sera obtained from MERS-CoV-positive immune dromedary camels showed protection in mice expressing human DPP4.20, 21, 22 Although safety and tolerability of anti-MERS-CoV polyclonal antibody treatment in humans has been demonstrated,23 protection against MERS in humans has not yet been documented.
Notably, mouse experiments demonstrated the generation of neutralising antibodies upon MVA-MERS-S vaccination, which only partially mediated neutralisation by the receptor-binding domain (RBD),6 indicating that other parts of the MERS-CoV spike glycoprotein might also induce neutralising antibodies. In this context, a notable outcome of the mouse experiments was the protective capacity of S1-specific non-RBD and S2-specific neutralising mouse antibodies against MERS-CoV.6, 7, 24 Furthermore, it was demonstrated that S2 monoclonal antibodies showed only moderate neutralising capacity in vitro but were highly protective from challenge in vivo, suggesting that strong neutralising activity is not conditional for protection.7 Future analyses should therefore include not only MERS-CoV S1-specific but also S2-specific antibody assays, and other functional antibody read-outs beyond neutralisation capacity, which are currently understudied. Systems-serology approaches including non-neutralising functions such as antibody-dependent cellular cytotoxicity or antibody-dependent cellular phagocytosis might provide insight into MVA-MERS-S-induced immunity and dissect additional mechanisms of antibody-mediated protection. However, it should be noted that although animal studies indicate a crucial role for antibodies in protection against MERS, the human data available from MERS-CoV-infected individuals so far did not reveal a clear and strong correlation between MERS-CoV-specific antibody responses and MERS-CoV viral load.25, 26
Reports on individuals with MERS-CoV infection revealed predominantly T-cell responses as opposed to antibody responses in survivors as well as exposed dromedary camel workers.9, 27 Although it has not yet been determined that T-cell responses are crucial for MERS-CoV clearance in humans, animal models have provided evidence that T cells support viral clearance in mice.8, 15 In this study, we detected vaccine-induced T-cell responses against MERS-CoV spike, which were present even before the second immunisation. Responses persisted until day 180 in a third of study participants (seven [32%] of 22 participants), suggesting that vaccine-induced T-cell responses were maintained slightly longer than humoral immune responses (three [14%] of 22 participants). We observed stronger and more robust T-cell responses in the low-dose group than in the high-dose group. Although this finding was not significant, it might be considered in future studies that focus on dose finding when including a larger number of participants. However, the exact contribution of T-cell immunity to vaccine-induced or natural protection against the disease and viral clearance remains to be determined and needs further investigation.
To complement our analyses on vaccine-induced immunity to the MERS-CoV spike antigenic insert of the vector vaccine, we also investigated vector immunity to the MVA backbone. MVA-specific humoral responses against the MVA vector were assessed post-hoc using a virus neutralisation test assay against wild-type MVA to address their potential effect on MERS-CoV spike-specific antibodies. Although MVA-specific neutralising antibodies were induced, no correlation with MERS-CoV-specific binding or neutralising antibody responses was observed. Currently, there is no strong evidence that induced or pre-existing MVA-specific immunity interferes with vaccine-induced immune responses when using MVA as a vector. Our study and the trial using MVA-H5-sfMR18 show that MVA-based vaccines can elicit antibody responses against the antigenic insert following the second and third immunisation, despite the induction of anti-vector immunity after the prime injection. The exact impact of pre-existing immune responses against MVA requires further investigation in future studies.
Rapid induction of vaccine-induced immunity following single-shot vaccination schemes is considered to be particularly useful in outbreak scenarios; however, MVA-MERS-S showed no induction of strong MVA-MERS-S-specific immune responses following a single prime vaccination, which might reduce its direct applicability in acute outbreak scenarios. In the context of limited human data on correlates of protection, it is currently challenging to predict whether MVA-MERS-S is suitable for rapidly evolving epidemic situations, because it remains unclear which specific parameters are relevant for the defence against MERS-CoV. It is conceivable that homologous MVA prime–boost vaccination might not be the final and optimised vaccination schedule for MVA-MERS-S. Different strategies including heterologous prime–boost regimens should be evaluated in future trials. Several MERS vaccine candidates are currently being tested in phase 1 and 2 clinical trials. The Coalition for Epidemic Preparedness Innovations is funding five different vaccine platforms, with MVA-MERS-S, ChAdOx1, and DNA being the most advanced. In the first MERS vaccine trial in humans reported to date, which investigated a DNA-based vaccine (GLS-5300),28 a homologous prime–boost–boost regime was used. Similar to our study, the authors observed a favourable safety profile and induction of both humoral and cellular immune responses. Although seroreactivity and cellular responses were largely maintained over the study period of 60 weeks, neutralising antibodies were detectable in only 48% of vaccine recipients, peaked about 2 weeks after the second boost, and rapidly declined.
Additional clinical trials are needed to evaluate whether heterologous prime–boost vaccination schedules with a MVA-MERS-S, ChAdOx1, or DNA prime and a boost with MVA-MERS-S can induce immune responses protective against MERS-CoV infection or can at least favourably modulate the MERS disease course.
Further studies are warranted to improve our detailed understanding of the role of antibody titres, neutralising and non-neutralising antibodies, and cellular immune responses in both natural and vaccine-induced protective MERS-CoV-specific immunity, to further enhance and accelerate strategic vaccine development against MERS. The ultimate assessment of vaccine efficacy will be challenging because outbreaks are unpredictable and the number of MERS cases has been relatively low.
Although the data presented here make a valuable contribution to the MERS vaccine agenda and enterprise, the study also has limitations. The restricted number of study participants in this phase 1 trial limits the generalisability of results and necessitates follow-up studies in larger cohorts. The study did also not include an additional late boost, which induced strong and increased antibody responses in a previous MVA-H5-sfMR trial.18 This early study format also did not allow for data generation on antibody dependent enhancement in the context of MERS-CoV infections, which has been previously discussed for severe acute respiratory syndrome (SARS) coronavirus.29 Future studies in animal models, possibly including adoptive transfer experiments with sera derived from vaccinated individuals, as well as clinical trials will need to address this crucial aspect.
In conclusion, the phase 1 trial investigating the candidate vaccine MVA-MERS-S showed a benign safety profile and provides the first evidence of humoral and cellular immunogenicity induced by this candidate vaccine in humans. The data generated so far support its further development as a MERS vaccine candidate for homologous or heterologous vaccination schemes, possibly involving other viral vector vaccines. In 2018, the Coalition for Epidemic Preparedness Innovations included MVA-MERS-S in its funding portfolio to support scalable manufacturing and further clinical development to evaluate safety and immunogenicity in larger cohorts and endemic areas. The favourable profile of MVA-MERS-S might also make useful contributions for the development of future vaccine strategies against other coronavirus pathogens, such as the recently emerged SARS coronavirus 2. However, crucial knowledge gaps remain to be addressed.
Data sharing
Individual participant data will be shared, including data dictionaries. All of the individual participant data collected during the trial will be shared after de-identification. Other documents that will be made available include the study protocol and informed consent forms. The data will be available immediately following publication with no end date. The data will be shared with anyone who wishes to access the data. The data will be available for any purpose of analyses. For data, please contact the corresponding author.
Acknowledgments
Acknowledgments
We are grateful for generous funding of this study through the German Center of Infectious Disease Research (DZIF; number FKZ8009801908, DZIF Thematic Translational Unit [TTU] 01.908, and TTU 01.702). TK and AF received DZIF Clinical Leave Scholarships. JSHP received a DZIF medical doctor stipend. We specifically thank the members of the DZIF TTU Emerging Infections for the excellent collaborative work that made this study possible. We thank all participants for their participation in this phase 1 vaccine trial for MVA-MERS-S and for their dedicated commitment to combating MERS-CoV outbreaks. We also express our sincere gratitude to all trial centre members for their extraordinary work (Clinical Trial Center North, Hamburg, Germany). We also thank the Hamburg local safety board members and Anneke Novak-Funk for input and critical review of the manuscript.
Contributors
TH, SBo, SS, AWL, GS, and MMA designed the study. TK, CD, AK, VK, NMAO, SBo, BLH, GS, and MMA planned the experiments. TK, CD, AF, AK, VK, NMAO, SH, CR, ME, MLL, MEZ, EB, JSHP, and RN ran the assays. TK, CD, AF, AK, VK, NMAO, AJ, SBo, BLH, SBe, and MMA gathered the data. TK, CD, AF, AK, VK, NMAO, AV, AJ, SBo, RF, BLH, GS, SBe, and MMA analysed the data. TK, CD, AF, and MMA wrote the manuscript. TK, CD, AF, AV, SS, AWL, BLH, GS, SBe, and MMA reviewed the manuscript.
Declaration of interests
BLH is listed as inventor on a Middle East respiratory syndrome coronavirus patent application. All other authors declare no competing interests.
Supplementary Material
Supplementary appendix
References
- 1.Modjarrad K, Moorthy VS, Ben Embarek P, Van Kerkhove M, Kim J, Kieny MP. A roadmap for MERS-CoV research and product development: report from a World Health Organization consultation. Nat Med. 2016;22:701–705. doi: 10.1038/nm.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 3.WHO MERS monthly summary. January 2020. http://www.emro.who.int/health-topics/mers-cov/mers-outbreaks.html
- 4.Oboho IK, Tomczyk SM, Al-Asmari AM. 2014 MERS-CoV outbreak in Jeddah—a link to health care facilities. N Engl J Med. 2015;372:846–854. doi: 10.1056/NEJMoa1408636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO 2015 MERS outbreak in Republic of Korea. https://www.who.int/westernpacific/emergencies/2015-mers-outbreak
- 6.Song F, Fux R, Provacia LB. Middle East respiratory syndrome coronavirus spike protein delivered by modified vaccinia virus Ankara efficiently induces virus-neutralizing antibodies. J Virol. 2013;87:11950–11954. doi: 10.1128/JVI.01672-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Widjaja I, Wang C, van Haperen R. Towards a solution to MERS: protective human monoclonal antibodies targeting different domains and functions of the MERS-coronavirus spike glycoprotein. Emerg Microbes Infect. 2019;8:516–530. doi: 10.1080/22221751.2019.1597644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao J, Li K, Wohlford-Lenane C. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci USA. 2014;111:4970–4975. doi: 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao J, Alshukairi AN, Baharoon SA. Recovery from the Middle East respiratory syndrome is associated with antibody and T-cell responses. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aan5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin H-S, Kim Y, Kim G. Immune responses to Middle East respiratory syndrome coronavirus during the acute and convalescent phases of human infection. Clin Infect Dis. 2019;68:984–992. doi: 10.1093/cid/ciy595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbert SC. Clinical development of modified vaccinia virus Ankara vaccines. Vaccine. 2013;31:4241–4246. doi: 10.1016/j.vaccine.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Volz A, Sutter G. Modified vaccinia virus Ankara: history, value in basic research, and current perspectives for vaccine development. Adv Virus Res. 2017;97:187–243. doi: 10.1016/bs.aivir.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gómez CE, Perdiguero B, García-Arriaza J, Esteban M. Clinical applications of attenuated MVA poxvirus strain. Expert Rev Vaccines. 2013;12:1395–1416. doi: 10.1586/14760584.2013.845531. [DOI] [PubMed] [Google Scholar]
- 14.Zhang N, Jiang S, Du L. Current advancements and potential strategies in the development of MERS-CoV vaccines. Expert Rev Vaccines. 2014;13:761–774. doi: 10.1586/14760584.2014.912134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volz A, Kupke A, Song F. Protective efficacy of recombinant modified vaccinia virus Ankara delivering Middle East respiratory syndrome coronavirus spike glycoprotein. J Virol. 2015;89:8651–8656. doi: 10.1128/JVI.00614-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haagmans BL, van den Brand JMA, Raj VS. An orthopoxvirus-based vaccine reduces virus excretion after MERS-CoV infection in dromedary camels. Science. 2016;351:77–81. doi: 10.1126/science.aad1283. [DOI] [PubMed] [Google Scholar]
- 17.Okba NMA, Raj VS, Widjaja I. Sensitive and specific detection of low-level antibody responses in mild Middle East respiratory syndrome coronavirus infections. Emerg Infect Dis. 2019;25:1868–1877. doi: 10.3201/eid2510.190051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreijtz JHCM, Goeijenbier M, Moesker FM. Safety and immunogenicity of a modified-vaccinia-virus-Ankara-based influenza A H5N1 vaccine: a randomised, double-blind phase 1/2a clinical trial. Lancet Infect Dis. 2014;14:1196–1207. doi: 10.1016/S1473-3099(14)70963-6. [DOI] [PubMed] [Google Scholar]
- 19.La Rosa C, Longmate J, Martinez J. MVA vaccine encoding CMV antigens safely induces durable expansion of CMV-specific T cells in healthy adults. Blood. 2017;129:114–125. doi: 10.1182/blood-2016-07-729756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao J, Perera RAPM, Kayali G, Meyerholz D, Perlman S, Peiris M. Passive immunotherapy with dromedary immune serum in an experimental animal model for Middle East respiratory syndrome coronavirus infection. J Virol. 2015;89:6117–6120. doi: 10.1128/JVI.00446-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stalin Raj V, Okba NMA, Gutierrez-Alvarez J. Chimeric camel/human heavy-chain antibodies protect against MERS-CoV infection. Sci Adv. 2018;4 doi: 10.1126/sciadv.aas9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pascal KE, Coleman CM, Mujica AO. Pre- and postexposure efficacy of fully human antibodies against Spike protein in a novel humanized mouse model of MERS-CoV infection. Proc Natl Acad Sci USA. 2015;112:8738–8743. doi: 10.1073/pnas.1510830112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beigel JH, Voell J, Kumar P. Safety and tolerability of a novel, polyclonal human anti-MERS coronavirus antibody produced from transchromosomic cattle: a phase 1 randomised, double-blind, single-dose-escalation study. Lancet Infect Dis. 2018;18:410–418. doi: 10.1016/S1473-3099(18)30002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Shi W, Chappell JD. Importance of neutralizing monoclonal antibodies targeting multiple antigenic sites on the Middle East respiratory syndrome coronavirus spike glycoprotein to avoid neutralization escape. J Virol. 2018;92:e02002–e02017. doi: 10.1128/JVI.02002-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corman VM, Albarrak AM, Omrani AS. Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clin Infect Dis. 2016;62:477–483. doi: 10.1093/cid/civ951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Min C-K, Cheon S, Ha N-Y. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep. 2016;6 doi: 10.1038/srep25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alshukairi AN, Zheng J, Zhao J. High prevalence of MERS-CoV infection in camel workers in Saudi Arabia. MBio. 2018;9:e01985–e02018. doi: 10.1128/mBio.01985-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Modjarrad K, Roberts CC, Mills KT. Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: a phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect Dis. 2019;19:1013–1022. doi: 10.1016/S1473-3099(19)30266-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yip MS, Cheung CY, Li PH, Bruzzone R, Peiris JSM, Jaume M. Investigation of antibody-dependent enhancement (ADE) of SARS coronavirus infection and its role in pathogenesis of SARS. BMC Proc. 2011;5(suppl 1):80. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary appendix
Data Availability Statement
Individual participant data will be shared, including data dictionaries. All of the individual participant data collected during the trial will be shared after de-identification. Other documents that will be made available include the study protocol and informed consent forms. The data will be available immediately following publication with no end date. The data will be shared with anyone who wishes to access the data. The data will be available for any purpose of analyses. For data, please contact the corresponding author.