Coronavirus Disease-2019 in Heart Transplant Recipients in Southeastern Michigan: A Case Series (original) (raw)
Abstract
Background
Since coronavirus disease 2019 (COVID-19) was first identified in Wuhan, China, in December 2019, the number of cases has risen exponentially. Clinical characteristics and outcomes among patients with orthotopic heart transplant (OHT) with COVID-19 remain poorly described.
Methods
We performed a retrospective case series of patients with OHT with COVID-19 admitted to 1 of 2 hospitals in Southeastern Michigan between March 21 and April 22, 2020. Clinical data were obtained through review of the electronic medical record. Final date of follow-up was May 7, 2020. Demographic, clinical, laboratory, radiologic, treatment, and mortality data were collected and analyzed.
Results
We identified 13 patients with OHT admitted with COVID-19. The mean age of patients was 61 ± 12 years, 100% were black males, and symptoms began 6 ± 4 days before admission. The most common symptoms included subjective fever (92%), shortness of breath (85%), and cough (77%). Six patients (46%) required admission to the intensive care unit. Two patients (15%) died during hospitalization.
Conclusions
Black men may be at increased risk for COVID-19 among patients with OHT. Presenting signs and symptoms in this cohort are similar to those in the general population. Elevated inflammatory markers on presentation appear to be associated with more severe illness.
Key Words: Advanced heart failure, heart transplant, immunosuppression, COVID
Coronavirus disease 2019 (COVID-19) is the illness caused by severe acute respiratory syndrome coronavirus-2, first detected in Wuhan, China. Although COVID-19 most commonly causes a mild respiratory illness, there are wide ranges of presenting symptoms and disease severity. Owing to the immunosuppression required to prevent allograft rejection, patients with orthotopic heart transplantation (OHT) represent a population who may have an atypical presentation or be at higher risk for adverse outcomes from COVID-19. Currently, COVID-19 in patients with OHT remains poorly characterized.1 We describe the presentation, course, and outcomes of patients with OHT hospitalized for COVID-19.
Material and Methods
Study Population and Setting
We included adult patients (≥18 years) with OHT with laboratory-confirmed COVID-19 admitted to 1 of 2 hospitals in Southeastern Michigan from March 21 to April 22, 2020. The final date of follow-up was May 7, 2020. A confirmed case of COVID-19 was defined as a positive result on a reverse-transcriptase polymerase chain reaction assay of a nasopharyngeal swab specimen. The institutional review boards at Michigan Medicine and Henry Ford Health System approved this study.
Data Collection
Data were collected through review of OHT databases and electronic medical records. Data included demographics, clinical history, laboratory and radiologic results, and therapies administered.
Statistical Methods
Descriptive statistics were used to summarize the data. Results are reported as medians and interquartile ranges or means and standard deviations as appropriate. Categorical variables were summarized as counts and percentages. Analysis was performed with Microsoft Excel (Microsoft, Redmond, WA).
Results
Demographics and Medical History
More than 375 heart transplantations have been performed at Michigan Medicine since 2006. Recipients from 2006 to 2018 had a median age of 55 years at time of transplantation. The majority were male (78%) and white (81%), with 16% black. At Henry Ford, more than 200 heart transplants have been performed since 2006. Recipients from 2006 to 2019 had a median age of 54 years at time of transplantation. The majority were male (79%) and white (56%), with 39% black.
From March 21 to April 22, 2020, 13 adult patients with OHT were hospitalized for COVID-19, 8 admitted to Michigan Medicine and 5 to Henry Ford Hospital. No patients were diagnosed via asymptomatic screening. The mean age was 61 ± 12 years (range, 34–77 years). All were black males. Comorbid conditions were common, including hypertension (85%), chronic kidney disease (85%), and diabetes mellitus (69%; Table 1). Two patients were heart-kidney transplant recipients and one was a heart–lung transplant recipient.
Table 1.
Clinical History of Patients
| Characteristic | All Patients (N = 13) | Acute Care (N = 7/13 [54%]) | Intensive Care (N = 6/13 [46%]) |
|---|---|---|---|
| Enrollment site – no. (%) | |||
| Michigan Medicine, Ann Arbor, MI | 8/13 (62) | 2/7 (29) | 6/6 (100) |
| Henry Ford Hospital, Detroit, MI | 5/13 (38) | 5/7 (71) | 0/6 (0) |
| Demographics | |||
| Mean age (range), years | 61 (34–77) | 65 (52–77) | 57 (34–73) |
| Male sex, no./total no. (%) | 13/13 (100) | 7/7 (100) | 6/6 (100) |
| Body-mass index,* mean ± SD | 29 ± 8 | 27 ± 5 | 32 ± 9 |
| Black race, no./total no. (%) | 13/13 (100) | 7/7 (100) | 6/6 (100) |
| Baseline transplant and immunosuppression information | |||
| Time since heart transplant, years, median (IQR) | 8 (5–14) | 8 (6–13) | 9 (4–14) |
| Use of calcineurin inhibitor before diagnosis, no./total no. (%) | 12/13 (92) | 7/7 (100) | 5/6 (83) |
| Use of prednisone before admission, no./total no. (%) | 7/13 (54) | 3/7 (43) | 4/6 (67) |
| Use of mycophenolate mofetil before admission, no./total no. (%) | 7/13 (54) | 5/7 (71) | 2/6 (33) |
| Coexisting disorder, no./total no. (%) | |||
| Chronic anemia | 8/13 (62) | 5/7 (71) | 3/6 (50) |
| Chronic heart failure† | 5/13 (38) | 1/7 (14) | 4/6 (67) |
| Chronic kidney disease‡ | 11/13 (85) | 7/7 (100) | 4/6 (67) |
| Coronary artery disease§ | 6/13 (46) | 2/7 (29) | 4/6 (67) |
| Former tobacco smoker | 7/13 (54) | 6/7 (86) | 1/6 (17) |
| Diabetes mellitus | 9/13 (69) | 5/7 (71) | 4/6 (67) |
| Hypertension | 11/13 (85) | 5/7 (71) | 6/6 (100) |
| Obesity | 5/13 (38) | 2/7 (29) | 3/6 (50) |
| Obstructive sleep apnea | 6/13 (46) | 3/7 (43) | 3/6 (50) |
| Baseline laboratory values | |||
| Mean tacrolimus trough over prior 3 months (ng/mL), median (IQR) | 5.4 (4.8–6.0) | 5.2 (4.8–5.9) | 5.5 (5.2–6.5) |
| Most recent lymphocyte count (per mm3), median (IQR) | 1,600 (1,100–2,300) | 1,100 (1,000–1,950) | 1,885 (1,406–2,443) |
| Most recent platelet count (per mm3), median (IQR) | 202,000 (159,000–224,000) | 203,000 (141,500–219,000) | 194,500 (166,000–236,500) |
| Most recent high-sensitivity troponin ≥19 pg/mL, no./total no. (%) | 6/10 (60) | 2/4 (50) | 4/6 (66) |
| History | |||
| Duration of symptoms before diagnosis (days), mean ± SD | 6 ± 4 | 6 ± 5 | 6 ± 2 |
| Symptom, no./total no. (%) | |||
| Fever | 12/13 (92) | 7/7 (100) | 5/6 (83) |
| Cough | 10/13 (77) | 6/7 (86) | 4/6 (67) |
| Shortness of breath | 11/13 (85) | 5/7 (71) | 6/6 (100) |
| Diarrhea | 6/13 (46) | 4/7 (57) | 2/6 (33) |
| Contact with health care system within 14 days before admission‖ | 4/13 (31) | 1/7 (14) | 3/6 (50) |
| Reported sick contact without confirmed COVID-19 diagnosis | 7/13 (54) | 5/7 (71) | 2/6 (33) |
The average time from OHT to presentation was 9.6 ± 6.3 years (range, 1.5–22.7). Heart failure or left ventricular systolic dysfunction was present in 5 patients (38%) and cardiac allograft vasculopathy in 6 (46%). Before COVID-19, a calcineurin inhibitor was prescribed in 12 patients (83% tacrolimus), prednisone in 7 (54%), and mycophenolate in 7 (54%). The median of the 3-month individual average tacrolimus trough among patients prescribed tacrolimus was 5.4 ng/mL (range, 4.8–6.0 ng/mL; Table 1).
Clinical Presentation
The mean duration of symptoms before admission was 6 ± 4 days (range, 1–14 days). The most common symptoms included subjective fever (92%), shortness of breath (85%), and cough (85%). Diarrhea occurred in 6 patients (46%; Table 1). No patients were hospitalized within 30 days before admission, had traveled outside the United States in the 90 days before admission, or had known contact with an individual who tested positive for COVID-19.
Objective Findings
Documented fever was present in 10 patients (77%) on admission. Lymphopenia occurred in all patients and thrombocytopenia in 8 (62%). Acute kidney injury occurred in the majority (85%). High-sensitivity troponin was elevated in 7 patients (54%) and lactate was elevated in 5 patients (38%). Elevated laboratory markers of inflammation were common, with higher median values observed in patients requiring intensive care. No patients had laboratory-confirmed coinfection on presentation. The median tacrolimus trough on admission was 5.1 ng/mL (range, 2.8–6.0 ng/mL). Chest radiographs were obtained in all patients and almost all (92%) showed bilateral pulmonary infiltrates (Table 2).
Table 2.
Objective Findings and Outcomes of Patients
| Characteristics | All Patients (N = 13) | Acute Care (N = 7/13 [54%]) | Intensive Care (N = 6/13 [46%]) |
|---|---|---|---|
| Vital signs* | |||
| Temperature >100.4°F or 38°C | 10/13 (77) | 4/7 (57) | 6/6 (100) |
| Temperature <96.8°F or 36°C | 1/13 (8) | 0/7 (0) | 1/6 (17) |
| Heart rate >100 beats per minute | 9/13 (69) | 5/7 (71) | 4/6 (67) |
| Respiratory rate ≥20 breaths per minute | 11/13 (85) | 5/7 (71) | 6/6 (100) |
| Cell counts† | |||
| White cell count/mm3 | 4800 (3600–6600) | 3600 (3000–4800) | 6550 (5000–11,550) |
| Lowest lymphocyte count | |||
| Per mm3 | 700 (400–900) | 700 (450–850) | 600 (425–850) |
| <1500/mm3 | 13/13 (100) | 7/7 (100) | 6/6 (100) |
| Lowest platelet count | |||
| Per mm3 | 134,000 (120,000–156,000) | 133,000 (115–166,000) | 138,500 (123,500–150,500) |
| <150,000/mm3 | 8/13 (62) | 4/7 (57) | 4/6 (67) |
| Chemistries† | |||
| Acute kidney injury‡ | 11/13 (85) | 6/7 (86) | 5/6 (83) |
| High-sensitivity troponin ≥19 pg/mL | 7/13 (54) | 3/7 (43) | 4/6 (67) |
| Lactate ≥2.0 mmol/L | 5/13 (38) | 1/7 (14) | 4/6 (67) |
| Tacrolimus trough (ng/mL) | 5.1 (2.8–6.0) | 5.75 (4.7–6.5) | 3.5 (1.7–5.0) |
| Inflammatory markers† | |||
| Highest d-dimer (μg/mL) | 0.9 (0.5–1.8) | 0.4 (0.3–0.7) | 1.9 (1.2–6.0) |
| Highest ferritin (ng/mL) | 1163 (540–1828) | 540 (381–1030) | 1502 (1166–2258) |
| Highest lactate dehydrogenase (U/L) | 315 (215–627) | 215 (186–285) | 644 (626–729) |
| Highest procalcitonin (ng/mL) | 1.2 (0.4–7.5) | 0.4 (0.3–10.2) | 3.0 (0.6–6.8) |
| Chest radiograph with bilateral infiltrates, no./total no. (%) | 12/13 (92) | 6/7 (86) | 6/6 (100) |
| Medical therapies | |||
| Tocilizumab | 3/13 (23) | 0/7 (0) | 3/6 (50) |
| Hydroxychloroquine | 8/13 (62) | 6/7 (86) | 2/6 (33) |
| High-dose corticosteroids§ | 8/13 (62) | 3/7 (43) | 5/6 (83) |
| Intensive care therapies, no/total no. (%) | |||
| Vasopressor use | 5/13 (38) | – | 5/6 (83) |
| Mechanical ventilation | 5/13 (38) | – | 5/6 (83) |
| Continuous renal replacement therapy | 5/13 (38) | – | 5/6 (83) |
| Neuromuscular blockade | 5/13 (38) | – | 5/6 (83) |
| Outcomes | |||
| Died in hospital | 2/13 (15) | 0/7 (0) | 2/6 (33) |
| Discharged from hospital | 9/13 (69) | 7/7 (100) | 2/6 (33) |
| Remain hospitalized | 2/13 (15) | 0/7 (0) | 2/6 (33) |
| Length of hospital stay among those discharged (days) | 6 (3–11) | 5 (3–9) | 19 (8–29) |
Interventions
Among COVID-19–specific experimental/empiric therapies delivered, 3 patients (23%) received tocilizumab, 8 (62%) received hydroxychloroquine, and 8 (62%) received high-dose corticosteroids. Vasopressors, mechanical ventilation, neuromuscular blockade, and continuous renal replacement therapy were required in 5 of the 6 patients (83%) requiring intensive care (Table 2). Only 1 patient (8%) received inhaled pulmonary vasodilators and prone positioning. No patients received extracorporeal membrane oxygenation.
Among the 7 patients prescribed mycophenolate before presentation, it was discontinued in 6 on presentation. Although tacrolimus trough goals remained unchanged, dose reduction was required in 6 patients (60% of all patients on tacrolimus) to achieve the target range.
Outcomes
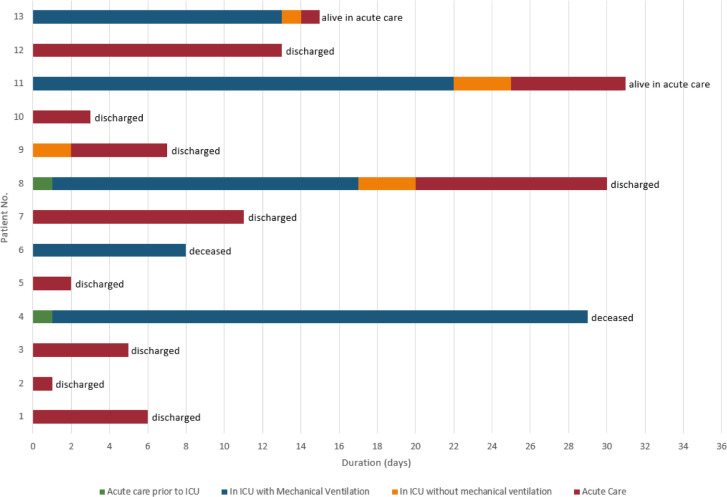
All patients had at least 15 days of follow-up. As of May 7, 2020, of the 13 admitted patients, 2 (15%) had died, 2 (15%) remain hospitalized in acute care, and 9 (69%) had been discharged from the hospital (Fig. 1). The median length of hospital stay among those discharged was 6 days (range, 3–11 days; Table 2).
Fig. 1.
Outcomes of individual patients. The current condition of patients with orthotopic heart transplantation with coronavirus disease-2019.
Discussion
To our knowledge, this is the first case series of a cohort of patients with OHT hospitalized with COVID-19. There are several notable observations.
All patients in this cohort were black males. Racial disparities in COVID-19 outcomes have been observed in several states.2 In Michigan, 14% of the population is black, and 33% of COVID-19 cases and 41% of deaths occurred in the black community.3 Although this imbalance is potentially due to the prevalence of comorbid conditions or health care disparities, genetic polymorphisms in the renin-angiotensin system may also contribute to this risk. Previous reports have identified alterations in the renin-angiotensin system activity profile of blacks.4 Severe acute respiratory syndrome coronavirus-2 enters human cells through angiotensin-converting enzyme 2 receptors and angiotensin II activity potentially contributes to disease severity and organ damage.5 Owing to the small sample size of this case series, no definitive conclusions can be drawn regarding COVID-19 susceptibility in black males with OHT. As such, further research is needed to determine if disease severity and COVID-19 risk is greater in black males with OHT and whether patient characteristics or treatments specific to OHT can be modified to decrease this risk.
Patients with OHT seem to present with COVID-19 similarly to patients without OHT, exhibiting a wide range of symptoms, but sharing the most common symptoms of fever, cough, and shortness of breath. However, diarrhea occurred more commonly than previously observed.6 , 7 In addition, particularly among patients requiring intensive care, comorbid conditions such as hypertension, diabetes mellitus, heart failure, and chronic kidney disease were common, as was cardiac allograft vasculopathy, a condition unique to patients with OHT.
Patients with OHT requiring intensive care in this series had significant elevations in markers of inflammation. This occurred despite these patients requiring immunosuppression to preserve allograft function. It seems that, similar to the general population,8 these laboratory findings denote a greater illness severity in patients with OHT with COVID-19 and may be used to risk stratify patients. Of note, although elevated high-sensitivity troponin levels are seen in approximately 20% of COVID-19 cases,9 levels were elevated in more than one-half of our cohort, although these levels were also elevated before admission among the 10 patients for whom these data were available. Lymphopenia and thrombocytopenia rates were higher in this study compared with previous reports in the nontransplanted and kidney transplant populations.7 , 10 This finding may be explained by a lower baseline lymphocyte and thrombocyte count, yet median values of these cell counts were lower on admission. Although myocardial injury has been associated with worse outcomes,11 no signal of an association was evident in this small series and no patients experienced cardiac complications.
Our study has several limitations. First, our sample size is small and it may lack generalizability. Owing to sample size, we were unable to detect significant differences between critically ill patients and those not requiring intensive care. We also cannot make any conclusions regarding COVID-19–specific treatments or the management of immunosuppression in this setting, although it should be noted that one-half of our cohort required dose reductions of tacrolimus despite similar tacrolimus levels before and on admission. Second, 2 patients (15%) remained hospitalized at the conclusion of data collection on May 7, 2020. Outcomes for these patients are unknown. Last, this study did not capture outcomes for patients treated in the outpatient setting. This factor limits our understanding of the spectrum of symptoms and disease severity among patients with OHT with COVID-19.
Conclusions
In this case series of patients with OHT hospitalized at 2 centers in Southeastern Michigan, all patients were black males, most of whom had comorbid conditions. Despite immunosuppression, the clinical presentation and laboratory markers of disease severity showed similarities to what has been observed in the general population. Further research should focus on racial and gender disparities in COVID-19 and on the identification of prognostic markers, treatments, and appropriate immunosuppression modifications for patients with OHT with COVID-19.
Acknowledgments
Competing Interests
The authors have no competing interests to declare.
Disclosures
The authors have no conflicts of interest to report.
Footnotes
Appendix. Supplementary materials
References
- 1.Li F., Cai J., Dong N. First cases of COVID-19 in heart transplantation from China. J Heart Lung Transplant. 2020;39:496–497. doi: 10.1016/j.healun.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Why black Americans are at higher risk for coronavirus - CNN [Internet]. Available at: https://www.cnn.com/2020/04/07/us/coronavirus-black-americans-race/index.html. Accessed April 12, 2020.
- 3.Black people are dying of COVID-19 at alarming rates. Here's why. | HuffPost [Internet]. Available at: https://www.huffpost.com/entry/black-people-are-dying-of-covid-19-at-alarming-rates-heres-why_n_5e8cdb76c5b62459a930512a. Accessed April 12, 2020.
- 4.Williams S.F. African Americans, hypertension and the renin angiotensin system. World J Cardiol. 2014;6:878. doi: 10.4330/wjc.v6.i9.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaduganathan M., Vardeny O., Michel T., McMurray JJ V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin X., Lian J.-S., Hu J.-H., Gao J., Zheng L., Zhang Y.-M. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 Apr 14 doi: 10.1001/jamacardio.2020.0950. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akalin E., Azzi Y., Bartash R., Seethamraju H., Parides M., Hemmige V. Covid-19 and kidney transplantation. N Engl J Med. 2020 Apr 24 doi: 10.1056/NEJMc2011117. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruan Q., Yang K., Wang W., Jiang L., Song J. Vol. 46. 2020. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China; pp. 846–848. (Intensive Care Med). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.