Spiking activity in the human hippocampus prior to encoding predicts subsequent memory (original) (raw)
Significance
Brain activity in the medial temporal lobe associated with the onset of a study item is thought to influence encoding and, therefore, subsequent memory for that item. However, our study found that spiking activity in the hippocampus prior to the onset of the to-be-studied item predicted both activity during encoding and subsequent memory. Thus, when the hippocampus is in a “ready-to-encode” mode before stimulus presentation, the stimulus is likely to be encoded and subsequently remembered. By contrast, if the hippocampus is not in a ready-to-encode mode before the presentation of a stimulus, the stimulus is likely to be poorly encoded and subsequently forgotten. We conclude that prestimulus “attention to encoding” predicts subsequent memory.
Keywords: human hippocampus, single-unit activity, multiunit activity, subsequent memory, encoding
Abstract
Encoding activity in the medial temporal lobe, presumably evoked by the presentation of stimuli (postonset activity), is known to predict subsequent memory. However, several independent lines of research suggest that preonset activity also affects subsequent memory. We investigated the role of preonset and postonset single-unit and multiunit activity recorded from epilepsy patients as they completed a continuous recognition task. In this task, words were presented in a continuous series and eventually began to repeat. For each word, the patient’s task was to decide whether it was novel or repeated. We found that preonset spiking activity in the hippocampus (when the word was novel) predicted subsequent memory (when the word was later repeated). Postonset activity during encoding also predicted subsequent memory, but was simply a continuation of preonset activity. The predictive effect of preonset spiking activity was much stronger in the hippocampus than in three other brain regions (amygdala, anterior cingulate, and prefrontal cortex). In addition, preonset and postonset activity around the encoding of novel words did not predict memory performance for novel words (i.e., correctly classifying the word as novel), and preonset and postonset activity around the time of retrieval did not predict memory performance for repeated words (i.e., correctly classifying the word as repeated). Thus, the only predictive effect was between preonset activity (along with its postonset continuation) at the time of encoding and subsequent memory. Taken together, these findings indicate that preonset hippocampal activity does not reflect general arousal/attention but instead reflects what we term “attention to encoding.”
When episodic memory is tested for a previously presented list of items, participants invariably remember some items and forget others. What accounts for this variation? Part of the explanation is that subsequently remembered items tend to be associated with higher encoding activity in the medial temporal lobe (MTL) than subsequently forgotten items (1–7). The level of encoding activity observed in such studies is presumed to be evoked by the presentation of the to-be-studied items: An image or word is presented, triggering a change in firing rates in MTL neurons, thereby increasing memory strength.
However, there is also research showing that conditions prior to stimulus presentation can affect encoding and improve subsequent memory. For example, in an electroencephalographic study, nonlocalized brain activity elicited by a cue presented just before a to-be-studied word predicted whether the word would be subsequently remembered (ref. 8; see also ref. 9). In a follow-up study using functional magnetic resonance imaging (fMRI), a similar effect was observed in the hippocampus (10). These effects of preonset and postonset activity have not been investigated in a study that measured single-unit and multiunit activity.
Do prestimulus and poststimulus activity during encoding both predict subsequent memory performance, and, if so, how are these effects related to each other? In addition, are these predictive effects, if they exist in single-unit and multiunit data, evident only in the hippocampus, or also in other brain regions? To address these questions, we tested recognition memory performance in epilepsy patients (n = 34) while clinical microwires measured single-unit activity (SUA) and multiunit activity (MUA) in four brain regions bilaterally: hippocampus, prefrontal cortex (PF), anterior cingulate cortex (AC), and amygdala (A) (11). We used a continuous recognition memory procedure in which words were presented consecutively and repeated once after varying lags. Patients judged each word as “novel” or “repeated.” The words were presented visually in some sessions and auditorily in other sessions. Collectively, the patients completed 55 sessions. As is typically done, we treated these 55 sessions as if they were independent, although some patients completed more than one session. Different sessions for any given patient were conducted on different days and it is typically assumed that different neurons were recorded during each session because the depth electrodes shift slightly as patients move around. (See SI Appendix for by-patient analyses, which were similar to the by-session analyses, and detailed methods.)
Results
On average, behavioral performance was well above chance and well below ceiling: 83.5 ± 2.0% correct for the first presentation of words (i.e., for novel words, correct rejection rate, 0.84; false alarm rate, 0.17) and 80.6 ± 2.8% correct for the second presentation of words (i.e., for repeated words, hit rate, 0.81; miss rate, 0.19).
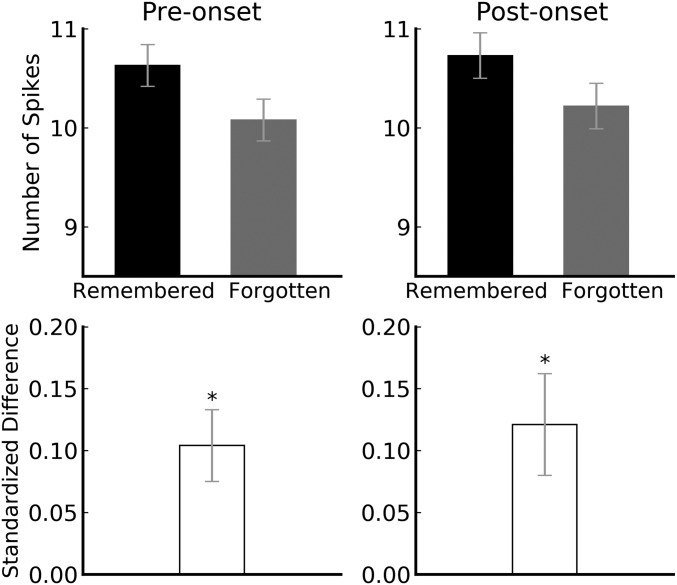
To examine how encoding activity around the first presentation of words (encoding) relates to performance at the second presentation (i.e., subsequent memory), we analyzed spikes from clusters in bilateral hippocampus (SUA and MUA) both before and after word onset. The preonset time window was 1,000 to 200 ms (i.e., before encoding), and the postonset time window was 200 to 1,000 (i.e., during encoding). Within each session, i, and for each hippocampal cluster, j, we computed the mean number of spikes for subsequently remembered words (X¯Rij) and subsequently forgotten words (X¯Fij), respectively. We then computed the mean of those cluster means for a given session, yielding two grand means (X¯Ri and X¯Fi) for each session. These measures were computed separately for preonset activity and postonset activity (Fig. 1, Top).
Fig. 1.
Bilateral spiking activity in the hippocampus around the first presentation of words. (Top) Preonset and postonset mean spike counts as a function of subsequently remembered vs. forgotten words. (Bottom) Standardized difference scores of preonset and postonset activity for subsequently remembered vs. forgotten words were both significantly larger than 0 (P = 0.0008 and 0.004). The two standardized difference scores did not differ from each other (P = 0.556). Error bars denote SEs. *P < 0.01.
The difference between X¯Ri and X¯Fi varied widely across sessions because some sessions were composed mostly of SUA whereas others were composed mostly of MUA (e.g., the difference for SUA was much smaller than that for MUA). We therefore standardized the data by dividing the difference score for each session (X¯Ri − X¯Fi) by the pooled SD of X¯Rij and X¯Fij across clusters in that session. That is, for each session, we computed a Cohen’s d effect-size score, one for preonset activity (dprei) and one for postonset activity (dposti). In the absence of a difference in activity around encoding for subsequently remembered vs. forgotten words, the expected value of both Cohen’s d scores (d¯pre and d¯post) is 0. A value significantly greater than 0 indicates that activity around encoding for subsequently remembered words exceeded that for subsequently forgotten words, whereas a value significantly less than 0 indicates the opposite.
In agreement with prior studies using more global measures, d¯post in bilateral hippocampus was significantly greater than 0 [d¯post=0.12, _t_(54) = 2.97, _P_ = 0.004; Fig. 1, _Bottom_]. Thus, postonset activity for novel words was significantly higher for subsequently remembered words, relative to subsequently forgotten words. Interestingly, d¯pre in bilateral hippocampus was also significantly greater than 0 [d¯pre=0.10, _t_(54) = 3.57, _P_ = 0.0008; Fig. 1, _Bottom_]. Thus, both before and after word onset, hippocampal activity was higher for subsequently remembered words compared to subsequently forgotten words. In addition, the difference between d¯pre and d¯post was negligible, indicating that the postonset difference was a continuation of the preonset difference [t(54) = 0.59, P = 0.556]. (Analysis of the raw spiking data also indicated that postonset spiking level was a continuation of the preonset spiking level for both the subsequently remembered and the subsequently forgotten words. See SI Appendix). Thus, the onset of novel stimuli did not trigger changes in hippocampal spiking activity (See SI Appendix for an illusion of a change in postonset spiking level if data were normalized without awareness of difference in preonset spiking level.)
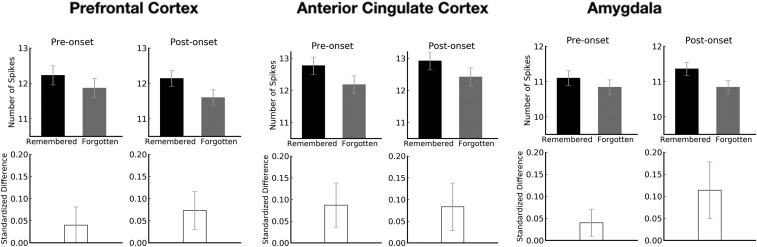
We next analyzed the data from PF, AC, and A in the manner described above for the hippocampus. All three regions exhibited trends of higher preonset and postonset activity for subsequently remembered vs. forgotten words, but none of the trends was significant [PF: d¯pre=0.04, _t_(45) = 0.97, _P_ = 0.339; d¯post=0.07, _t_(45) = 1.68, _P_ = 0.100; AC: d¯pre= 0.09, _t_(46) = 1.72, _P_ = 0.092; d¯post=0.08, _t_(46) = 1.49, _P_ = 0.142; A: d¯pre=0.04, _t_(49) = 1.28, _P_ = 0.208; d¯post=0.11, _t_(49) = 1.78, _P_ = 0.081; Fig. 2]. The number of sessions analyzed varied by brain area because recordings were not available from every area for every session.
Fig. 2.
Bilateral spiking activity in prefrontal cortex, anterior cingulate cortex, and amygdala around the first presentation of words. (Top) Preonset and postonset raw spike counts as a function of subsequently remembered vs. forgotten words for each brain area. (Bottom) Preonset and postonset standardized differences in spiking activity between subsequently remembered vs. forgotten words in each brain area. Although trends were evident for each brain area, the standardized difference score of both preonset and postonset spiking activity for subsequently remembered vs. forgotten words was numerically larger than 0 for each brain area (prefrontal: P = 0.339 and 0.100; anterior cingulate: P = 0.092 and 0.142; amygdala: P = 0.208 and 0.081). Error bars denote SEs.
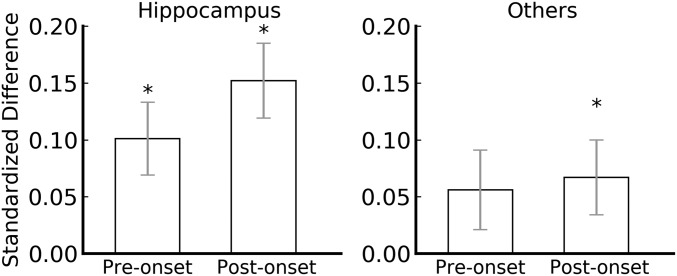
To compare the subsequent memory effect in the hippocampus with that in the other three brain areas, we next analyzed the subset of sessions for which recordings were available from all four brain areas (40 sessions in all). In the hippocampus, both preonset and postonset subsequent memory effects remained significant [d¯pre=0.10, _t_(39) = 2.87, _P_ = 0.007; d¯post=0.15, _t_(39) = 2.81, _P_ = 0.008; Fig. 3]. Because the trends in PF, AC, and A were very similar, we combined them to increase statistical power. For the combined areas, the preonset subsequent memory effect was marginal and the postonset subsequent memory effect was significant [d¯pre=0.06, _t_(39) = 1.81, _P_ = 0.078; d¯post=0.07, _t_(39) = 2.08, _P_ = 0.044; Fig. 3]. An ANOVA comparing hippocampus vs. the combined three areas yielded a main effect of brain area [F(1) = 7.11, P = 0.011], with no main effect of preonset vs. postonset [F(1) = 1.87, P = 0.179] and no interaction [F(1) = 1.87, P = 0.179]. Thus, the effect was significantly larger in the hippocampus compared to the other three areas combined.
Fig. 3.
Preonset and postonset subsequent memory effects in the hippocampus and in the three other areas combined for the 40 sessions that had recordings from all four brain areas. (Left) Preonset and postonset subsequent memory effect remained significant for the hippocampus (P = 0.007 and 0.008). (Right) Preonset and postonset subsequent effects were marginally significant and significant for the three other areas combined, respectively (P = 0.078 and 0.044). An ANOVA comparing hippocampus vs. the combined three areas yielded a main effect of brain area (P = 0.011), with no main effect of preonset vs. postonset (P = 0.179) and no interaction (P = 0.179). Error bars denote SEs. *P < 0.05.
So far, we have examined the relationship between activity around the first presentation of words and memory performance at the second presentation. We also examined whether activity around a word’s presentation predicted memory performance on that trial (i.e., correct novel responses during the first presentation and correct repeated responses during the second presentation). During the first presentation, preonset and postonset hippocampal activity did not differ for words that were correctly judged as novel vs. incorrectly judged as repeated [d¯pre=0.05, t(51) = −1.03, P = 0.306; d¯post=0.03, t(51) = −0.86, P = 0.396]. Similarly, during the second presentation, no significant effect was observed [d¯pre=0.04, t(54) = 0.92, P = 0.359; d¯post=0.03, t(54) = −0.90, P = 0.371].
These last two analyses indicate that spiking activity in the hippocampus around item presentation did not predict whether memory performance would be correct on that trial, regardless of whether the correct response was novel or repeated. The only predictive relationship between spiking activity and memory was the positive relationship observed between preonset and postonset encoding activity and subsequent memory. Moreover, this effect was stronger in the hippocampus than in other areas, similar to an earlier study reporting that theta oscillations in the hippocampus (but not in temporal or frontal regions) predict subsequent memory (12). Thus, the findings do not simply reflect variations in general arousal/attention but instead reflect a more specific phenomenon that we term “attention to encoding.”
Discussion
In this study, 34 epilepsy patients carried out 55 sessions of a continuous recognition task by judging whether each word was novel or repeated. Clinical microwires recorded the spiking activity in the hippocampus and three other brain areas (i.e., PF, A, and AC) during the task. We found that preonset spiking activity in the hippocampus predicted subsequent memory: Subsequently remembered words were associated with higher preonset spike counts, and subsequently forgotten words were associated with lower preonset spike counts. Postonset activity also predicted subsequent memory, but the activity level was merely a continuation of preonset activity level. Moreover, the preonset and postonset predicting effects were larger in the hippocampus than in the other three areas combined. We also found that spiking activity around the presentation of novel words did not predict memory performance on the novel words, and that spiking activity around the presentation of repeated words did not predict memory performance on the repeated words. Thus, the only predictive relationship was between spiking activity (both preonset and postonset) around the encoding of novel words and, subsequently, correct recognition when the words were repeated.
A previous fMRI study found that intentionally suppressing memory retrieval reduces hippocampal encoding activity for experiences that occur during the suppression via control mechanisms mediated by the lateral prefrontal cortex (13). Our findings may also reflect trial-to-trial variability in a similar prefrontal control mechanism that influences the efficacy of encoding. Although prefrontal activity in our study did not strongly predict subsequent memory (we recorded from ventromedial, not lateral, prefrontal cortex), prefrontally mediated fluctuations in attention to encoding may govern preonset neural activity in the hippocampus, perhaps by elevating neural activity when attention to encoding is high.
How might preonset hippocampal activity determine encoding efficiency? Prior work has shown that increasing excitability in neurons results in those neurons being biased to represent a new memory (e.g., ref. 14). More excitable neurons preferentially fire in response to the presentation of a stimulus, and synaptic changes in these neurons constitute the memory trace (15). Conceivably, in our study, the more excitable neurons were already active at the time of stimulus presentation and created stronger memory traces (16–24).
The preonset and postonset effects observed in the hippocampus must reflect the activity of a relatively large fraction of hippocampal neurons (i.e., activity that is easily detected despite recording from relatively few clusters per patient). It might seem surprising that memory performance at the time of the second presentation could be predicted by broadly distributed hippocampal activity only at the time of the first presentation and not by broadly distributed hippocampal activity at the time of the second presentation. However, longstanding neurocomputational models hold that initial encoding involves recruiting a small fraction of hippocampal neurons. In that case, although activity in a large fraction of hippocampal neurons is predictive of later performance, relatively few neurons would represent the successfully encoded memory trace and later be reactivated at the second presentation of the word. Moreover, on the basis of earlier work, we expect that the few neurons that are active at retrieval will differ for different repeated words (25, 26). Although speculative, we suggest that a small subset of active hippocampal neurons is recruited to encode a specific episodic memory (27, 28) and that such recruitment is more likely to succeed when preonset activity in the hippocampus is relatively high compared to when it is relatively low.
In summary, spiking activity in the hippocampus prior to stimulus onset, as well as spiking activity during encoding (after stimulus onset), predicted subsequent memory. Thus, the onset of a stimulus did not itself trigger changes in hippocampal spiking activity. Just as Tulving (29) once suggested there is a retrieval mode, we suggest that the attention to encoding phenomenon described here indicates that there is an encoding mode as well. When the brain, especially the hippocampus, is ready to encode into long-term memory, spiking activity is high before the onset of the stimulus, and encoding is likely to succeed.
Methods
Participants.
The participants were 34 patients (mean age, 41 ± 2.02 y; 21 females and 14 males; all but 2 were right-handed) who had temporal lobe, drug-resistant epilepsy that required implantation of depth electrodes (Ad-Tech Medical) for clinical evaluation and consideration of possible surgical resection of their seizure foci. The patients participated in a total of 55 sessions, with each patient completing one, two, three, or four sessions. An additional 10 sessions from 6 of the 34 patients and all of the data from 1 additional patient were excluded from analysis because of low recognition memory performance (d′ no greater than 0). Another five sessions were excluded due to the lack of subsequent miss trials (i.e., there was no trials where a repeated word was judged as novel, and thus a comparison of brain activity between subsequent hit and subsequent miss trials could not be carried out for these sessions).
All patients provided informed consent to participate in the research, using a protocol approved by the Institutional Review Board of St. Joseph’s Hospital and Medical Center.
Materials and Procedure.
The patients were tested using a continuous recognition task with words as stimuli. Words were presented one after another, and most of the words were presented a second time after a certain interval. The patients’ task was to judge whether each word was novel (i.e., presented the first time) or repeated (i.e., presented a second time). The words were presented visually on a computer screen (i.e., visual sessions) or auditorily through headphones (i.e., auditory sessions).
For the visual sessions, 360 words (120 one-syllable, 120 two-syllable, and 120 three-syllable words) were used, each of which was repeated once. The words were taken from the Medical Research Council Psycholinguistic database (30). Another set of 45 one-syllable words were used as fillers that were never repeated. There were three separate sets of words that could be presented, and these were used for patients who volunteered for multiple sessions. Each session consisted of a sequence of 255 trials (i.e., 240 trials where each of the 120 words were presented twice and 15 filler trials where words were never repeated). Repeated words were presented after 0, 1, 3, 7, 15, or 31 intervening words. One patient completed more than three sessions (four sessions) and thus saw one repeated set of words, but the repetition of the stimuli set did not have an effect on the memory performance. In each trial, a word was displayed for 1,500 ms, followed by a question mark as a prompt for response. Patients had up to 2,000 ms to press a key to indicate whether a word was novel or repeated. The trial ended when a response was made. There was a jittered intertrial interval that lasted for an average of 888 ms with a SD of 552 ms. (On some trials of the visual task only, the prestimulus time period included the time when patients made response on the previous trial. However, excluding those trials from the analysis had a negligible effect on the results.)
The procedure for the auditory sessions was similar to that of the visual sessions except for the following aspects. Each auditory session consisted of a sequence of 615 trials. In these sessions, 300 different words were repeated once and 15 filler words were never repeated. The prompt for response appeared at the end of the sound file for the trial. There was a jittered intertrial interval that lasted for an average of 1,055 ms with a SD of 53 ms.
In total, we administered 55 sessions, including 35 visual sessions and 20 auditory sessions. Data are available at the Open Science Foundation repository at https://osf.io/9tgmx/.
Supplementary Material
Supplementary File
Acknowledgments
This is work is supported by Neurtex Brain Research Institute Grant 19-02, National Institute of Child Health and Human Development Grant HD075800-05, National Institute of Neurological and Communicative Disorders and Stroke Grant DC009781, Medical Research Service of the Department of Veterans Affairs (51K6CX001644), Award CX000359, and National Institute of Mental Health Grant 24600.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The neural and behavioral data reported in this paper have been deposited in the Open Science Foundation repository, https://osf.io/9tgmx/.
References
- 1.Brewer J. B., Zhao Z., Desmond J. E., Glover G. H., Gabrieli J. D., Making memories: Brain activity that predicts how well visual experience will be remembered. Science 281, 1185–1187 (1998). [DOI] [PubMed] [Google Scholar]
- 2.Long N. M., Kahana M. J., Successful memory formation is driven by contextual encoding in the core memory network. Neuroimage 119, 332–337 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Paller K. A., Wagner A. D., Observing the transformation of experience into memory. Trends Cogn. Sci. 6, 93–102 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Sederberg P. B., Kahana M. J., Howard M. W., Donner E. J., Madsen J. R., Theta and gamma oscillations during encoding predict subsequent recall. J. Neurosci. 23, 10809–10814 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staresina B. P., Duncan K. D., Davachi L., Perirhinal and parahippocampal cortices differentially contribute to later recollection of object- and scene-related event details. J. Neurosci. 31, 8739–8747 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thakral P. P., Benoit R. G., Schacter D. L., Characterizing the role of the hippocampus during episodic simulation and encoding. Hippocampus 27, 1275–1284 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner A. D. et al., Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science 281, 1188–1191 (1998). [DOI] [PubMed] [Google Scholar]
- 8.Otten L. J., Quayle A. H., Akram S., Ditewig T. A., Rugg M. D., Brain activity before an event predicts later recollection. Nat. Neurosci. 9, 489–491 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Sadeh T., Chen J., Goshen-Gottstein Y., Moscovitch M., Overlap between hippocampal pre-encoding and encoding patterns supports episodic memory. Hippocampus 29, 836–847 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Park H., Rugg M. D., Prestimulus hippocampal activity predicts later recollection. Hippocampus 20, 24–28 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valdez A. B., Hickman E. N., Treiman D. M., Smith K. A., Steinmetz P. N., A statistical method for predicting seizure onset zones from human single-neuron recordings. J. Neural Eng. 10, 16001 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Merkow M. B., Burke J. F., Stein J. M., Kahana M. J., Prestimulus theta in the human hippocampus predicts subsequent recognition but not recall. Hippocampus 24, 1562–1569 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hulbert J. C., Henson R. N., Anderson M. C., Inducing amnesia through systemic suppression. Nat. Commun. 7, 11003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai D. J. et al., A shared neural ensemble links distinct contextual memories encoded close in time. Nature 534, 115–118 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogerson T. et al., Synaptic tagging during memory allocation. Nat. Rev. Neurosci. 15, 157–169 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han J. H. et al., Neuronal competition and selection during memory formation. Science 316, 457–460 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Yin J. C., Del Vecchio M., Zhou H., Tully T., CREB as a memory modulator: Induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell 81, 107–115 (1995). [DOI] [PubMed] [Google Scholar]
- 18.Bartsch D., Casadio A., Karl K. A., Serodio P., Kandel E. R., CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell 95, 211–223 (1998). [DOI] [PubMed] [Google Scholar]
- 19.Josselyn S. A. et al., Long-term memory is facilitated by cAMP response element-binding protein overexpression in the amygdala. J. Neurosci. 21, 2404–2412 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park S. et al., Neuronal allocation to a hippocampal engram. Neuropsychopharmacology 41, 2987–2993 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sekeres M. J. et al., Increasing CRTC1 function in the dentate gyrus during memory formation or reactivation increases memory strength without compromising memory quality. J. Neurosci. 32, 17857–17868 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekeres M. J., Neve R. L., Frankland P. W., Josselyn S. A., Dorsal hippocampal CREB is both necessary and sufficient for spatial memory. Learn. Mem. 17, 280–283 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Wallace T. L., Stellitano K. E., Neve R. L., Duman R. S., Effects of cyclic adenosine monophosphate response element binding protein overexpression in the basolateral amygdala on behavioral models of depression and anxiety. Biol. Psychiatry 56, 151–160 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Josselyn S. A., Frankland P. W., Fear extinction requires reward. Cell 175, 639–640 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Wixted J. T. et al., Sparse and distributed coding of episodic memory in neurons of the human hippocampus. Proc. Natl. Acad. Sci. U.S.A. 111, 9621–9626 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wixted J. T. et al., Coding of episodic memory in the human hippocampus. Proc. Natl. Acad. Sci. U.S.A. 115, 1093–1098 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marr D., Simple memory: A theory for archicortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 262, 23–81 (1971). [DOI] [PubMed] [Google Scholar]
- 28.McClelland J. L., McNaughton B. L., O’Reilly R. C., Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 102, 419–457 (1995). [DOI] [PubMed] [Google Scholar]
- 29.Tulving E., Elements of Episodic Memory, (Oxford University Press, 1983). [Google Scholar]
- 30.Coltheart M., The MRC psycholinguistic database. Q. J. Exp. Psychol. A 33, 497–505 (1981). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File