COVID-19 cardiac injury: Implications for long-term surveillance and outcomes in survivors (original) (raw)
Abstract
Up to 20%–30% of patients hospitalized with coronavirus disease 2019 (COVID-19) have evidence of myocardial involvement. Acute cardiac injury in patients hospitalized with COVID-19 is associated with higher morbidity and mortality. There are no data on how acute treatment of COVID-19 may affect the convalescent phase or long-term cardiac recovery and function. Myocarditis from other viral pathogens can evolve into overt or subclinical myocardial dysfunction, and sudden death has been described in the convalescent phase of viral myocarditis. This raises concerns for patients recovering from COVID-19. Some patients will have subclinical and possibly overt cardiovascular abnormalities. Patients with ostensibly recovered cardiac function may still be at risk of cardiomyopathy and cardiac arrhythmias. Screening for residual cardiac involvement in the convalescent phase for patients recovered from COVID-19–associated cardiac injury is needed. The type of testing and therapies for post COVID-19 myocardial dysfunction will need to be determined. Therefore, now is the time to plan for appropriate registries and clinical trials to properly assess these issues and prepare for long-term sequelae of “post–COVID-19 cardiac syndrome.”
Keywords: Arrhythmia, Cardiac injury, Cardiomyopathy, COVID-19, Myocarditis
A. Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has demonstrated a broad spectrum of presentations ranging from asymptomatic disease to severe respiratory failure, myocardial injury, and death. Up to 20%–30% of patients hospitalized with COVID-19 have evidence of myocardial involvement manifested by elevated troponin levels.1, 2, 3, 4, 5, 6 The prevalent cardiac expression of angiotensin I–converting enzyme 2 (ACE2),7 the target for SARS-CoV-2’s spike protein, is implicated in the pathophysiology of the associated myocardial injury. There are multiple pathways to myocardial injury (Figure 1), including type 1 or 2 myocardial infarction (MI), myocarditis, vasculitis, or other mechanisms related to inflammation, thrombosis, and/or stress. Depending on the type of myocardial injury, there may be important sequelae if residual inflammation or fibrosis exists. As SARS-CoV-2 is a new pathogen, there are no long-term data on cardiovascular abnormalities or dysrhythmias that may occur in the convalescent phase.
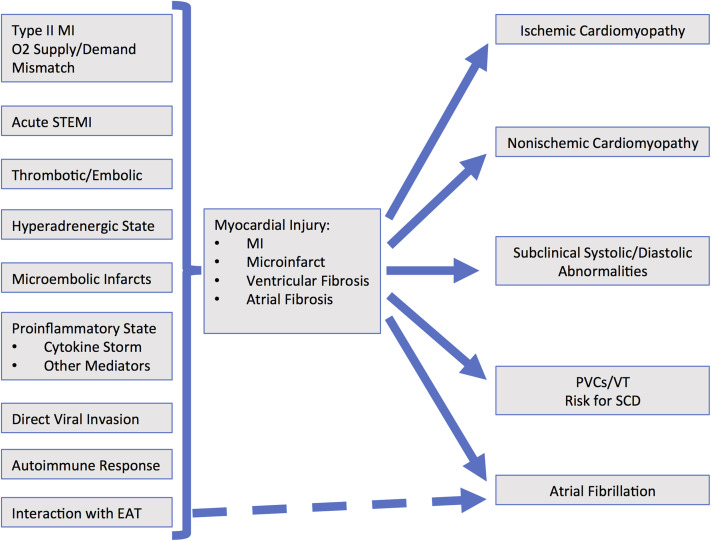
Figure 1.
Flowchart demonstrating the pathophysiology and various mechanisms of cardiac injury during acute coronavirus disease 2019 infection. Possible sequelae after recovery are then demonstrated. EAT = epicardial adipose tissue; MI = myocardial infarction; PVC = premature ventricular complex; SCD = sudden cardiac disease; STEMI = ST-segment elevation myocardial infarction; VT = ventricular tachycardia.
In patients with COVID-19, it is plausible that myocardial involvement can be the initiator of a pathway of inflammation and subsequent fibrosis.8 If the extent and distribution of fibrosis produces electrophysiological abnormalities that predispose to atrial fibrillation and ventricular arrhythmias, early detection and intervention could improve long-term outcomes. Patients with asymptomatic but overt clinical cardiac disease would benefit from standard therapy. Patients with subclinical disease may be at risk of cardiac arrhythmias. Therefore, the identification of survivors of COVID-19 infections with subclinical myocardial disease and/or arrhythmias creates a rationale to consider agents with demonstrated cardioprotective properties, such as mineralocorticoid antagonists, β-blockers, and statins.9 Given the large number of COVID-19 survivors, enhanced surveillance and treatment for those with significant electrophysiological abnormalities could substantially lower the burden of subsequent morbidity and mortality.
Treatment of COVID-19 infections has included standard, novel, and experimental therapies. Specific antiviral, anti-inflammatory, immunosuppressive, and cell-based therapies continue to evolve.5 , 10 Nevertheless, we hypothesize that a substantial number of patients who survive COVID-19 and have evidence for myocardial injury at the time of initial presentation may have long-term overt or subclinical myocardial and/or electrophysiological abnormalities. It is unknown whether the severity of cardiopulmonary injury, the type of treatment, or other host factors influences long-term sequelae. Heightened surveillance is therefore warranted in these patients with consideration of primary prevention approaches. Despite the novelty of COVID-19 infections, there have been numerous case reports, case series, editorials, and review articles on cardiovascular complications during acute COVID-19 infection.1 , 3, 4, 5 , 11, 12, 13, 14, 15 In this review, we highlight potential cardiovascular or arrhythmia complications that may occur during the convalescent phase in survivors of COVID-19 infection.
B. COVID-19 cardiovascular complications
SARS-CoV-2 has caused a global COVID-19 pandemic with serious infections, and major morbidity and mortality.1 , 2 , 4 , 5 , 11 , 16, 17, 18 This virus attaches to ACE2, which is found extensively in alveolar tissue and myocardial tissue.7 Numerous studies have shown that ACE2 is a cardioprotective transmembrane protein whose expression is downregulated by SARS-CoV-2 infection.5 , 6
A comparison of COVID-19 with other coronavirus infections shows similarity with SARS-CoV. Experimental data show that the original SARS-CoV can also cause ACE2-dependent myocardial injury.5 Moreover, autopsy samples from the Toronto SARS epidemic demonstrated SARS-CoV RNA in 35% of autopsy hearts of the expired patients, revealing that the virus has cardiotropism and can infect the myocardium.19 In that study by Oudit et al,19 the 7 of 20 patients who died of SARS-related coronavirus, had SARS-CoV genome detected in the heart. Although the presence of SARS-CoV RNA does not automatically mean there was myocardial injury, in this small case series by Oudit et al, all patients with detectable SARS COVID genome had increased myocardial inflammation, including macrophage infiltration. This suggests a direct causal effect from viral infiltration and the associated inflammation. It was also noted in this study that patients with SARS-CoV infection in the heart suffered from a significantly more aggressive illness and had earlier death compared with those with SARS-CoV without myocardial infiltration.19 A small long-term follow-up study of 25 patients (mean age 48 years) who recovered from SARS-CoV infection in 2003 demonstrated lipid abnormalities and increased cardiovascular abnormalities.1
Cardiac involvement in patients with COVID-19 (Table 1) has been attributed to multiple mechanisms and patterns of injury (Figure 1).4 , 15 Up to 20%–30% of patients hospitalized with COVID-19 have evidence of myocardial involvement manifested by elevated troponin levels, which is associated with worse short-term outcomes (Table 1).1, 2, 3 , 6 , 12 , 20 In a case series of 187 patients with COVID-19, elevated troponin levels, with or without a history of cardiovascular disease, were associated with malignant arrhythmias, acute respiratory failure, and higher mortality. Interestingly, patients with known cardiovascular disease but without elevated troponin levels had a more favorable outcome.12 Acute cardiac injury portends a worse prognosis, greater need for mechanical ventilation, and higher mortality. Shi et al3 reported that cardiac injury was associated with acute respiratory distress syndrome, acute kidney injury, and coagulation disorders. Coagulation disorders and metabolic derangements with acute kidney injury can also contribute to cardiac injury. Other mechanisms of cardiac injury (Figure 1) include acute ST-segment MI, type 2 MI due to oxygen supply/demand mismatch, vascular endothelial injury, microthrombi/emboli, and systemic hyperinflammatory state leading to the cytokine storm syndrome.15
Table 1.
Comparison of early studies of acute coronary injury in patients hospitalized with coronavirus disease 2019
| Study | Huang et al16 | Guo et al12 | Chen et al11 | Shi et al3 | Zhou et al20 | Goyal et al17 | Bhatraju et al2 | Richardson et al18 |
|---|---|---|---|---|---|---|---|---|
| Location | Wuhan, China | Wuhan, China | Wuhan, China | Wuhan, China | Wuhan, China | New York City, NY | Seattle, WA | New York City, NY |
| Date | 12/16/19–1/2/20 | 1/23/20–2/23/20 | 1/13/20–2/12/20 | 1/20/20–2/10/20 | 12/29/19–1/31/20 | 3/5/20–3/27/20 | 2/24/20–3/9/20 | 3/1/20–4/4/20 |
| N | 41 | 187 | 274∗ | 416 | 191 | 393 | 24 | 5700 |
| Characteristics | ||||||||
| Age (median) | 49 | 58.5 | 62 | 64 | 56 | 62.2 | 64 | 63 |
| Male | 73 | 48.7 | 62 | 49.3 | 62 | 60.6 | 63 | 60.3 |
| HTN | 15 | 32.6 | 34 | 30.5 | 30 | 50.1 | – | 56.6 |
| CAD | 15 | 11.2 | 8 | 10.6 | 8 | 13.7 | – | 11.1 |
| CHF | – | 4.3 | <1 | 4.1 | – | 7.1 | – | 6.9 |
| DM | 20 | 15 | 17 | 14.4 | 19 | 25.2 | 58 | 33.8 |
| Laboratory indices | ||||||||
| Troponin I or T (>99th percentile) | 12 | 27.8 | 41† | 19.7 | 17 | 4.5 | 15 | 22.6 |
| Complications | ||||||||
| Cardiac injury | 12 | – | 44† | 19.7 | 17 | – | – | – |
| HF | – | – | 24† | – | 23 | 1.8 | – | – |
| ACS | – | – | – | – | – | 3.6 | – | – |
| Arrhythmia | – | – | – | – | – | 7.4 | – | – |
| Atrial | – | – | – | – | – | 7.1 | – | – |
| Ventricular | – | 5.9 | – | – | – | 0.3 | – | – |
| Outcomes | ||||||||
| ICU admission | 31.7 | – | – | – | 26 | 33.1‡ | 100 | 22.5 |
| Death | 15 | 23 | 41.2 | 13.7 | 28.3 | 10.2 | 50 | 9.7 |
Given the abundance of ACE2 receptors on myocardial and vascular tissue, direct viral myocardial infection with SARS-CoV-2 is one of the mechanisms of cardiac injury and myocarditis.6 , 21 Tavazzi et al22 described a case of myocarditis in a COVID-19–positive patient, where direct viral infection was seen by endomyocardial biopsy showing low-grade inflammation and SARS-CoV-2 particles in interstitial cells of the myocardium. The pathophysiology of acute myocarditis may be related to direct viral infection and/or immune-mediated hyperinflammation (Figure 1).21 , 22 The systemic hyperinflammatory response is thought to be the third stage of infection in a theoretical model proposed by Atri et al.15
Up to 20%–30% of patients hospitalized with COVID-19 have evidence of myocardial involvement manifested by elevated troponin levels, which is associated with worse short-term outcomes (Table 1).1, 2, 3 , 12 , 20 The arrhythmia risk in patients with COVID-19 is likely multifactorial related to the viral infection, severity of the illness, severity of cardiac injury, inflammation, and potentially drug treatment with QT-prolonging drugs. However, the presence of ventricular arrhythmias is higher in patients with elevated troponin levels, up to 17% in 1 series compared with 1.2% in patients without elevated troponin levels.12 Lazzerini and colleagues23 demonstrated the direct effect that inflammatory cytokines, including interleukin 6, have on hERG-K+ channels, which prolongs ventricular action potential duration, leading to lethal ventricular arrhythmias.
Acute cardiac injury portends a worse prognosis, greater need for mechanical ventilation, and higher mortality. Shi et al3 reported that cardiac injury was associated with acute respiratory distress syndrome, acute kidney injury, and coagulation disorders. Coagulation disorders and metabolic derangements with acute kidney injury can also contribute to cardiac injury.
Acute ST-segment elevation MI has been well recognized in viral respiratory illnesses.24 A study by Kwong et al25 showed that the incidence of acute coronary syndrome is 5–10 times as high within the first 7 days of influenza diagnosis. Recommendations from experts suggest that these patients should be preferentially treated with fibrinolytic therapy.26 Therefore, patients with acute ST-segment MI and COVID-19 may be expected to have larger infarcts if fibrinolytic therapy does not result in timely and complete reperfusion.
Given the high prevalence of cardiac injury, it is reasonable to expect that a spectrum of heart disease is present with some residual postmyocarditis abnormalities. In fact, recent media reports have already suggested some patients suffer from new-onset cardiomyopathy during the convalescent phase after COVID-19 infection (https://www.nbcnewyork.com/news/coronavirus/coronavirus-after-effects-ny-doctor-develops-heart-disease-after-recovery/2397699/).
C. Long-term sequelae
As there are no current data on long-term COVID-19 cardiovascular complications, it would be instructive to review recovery from other types of myocardial injury. For patients with acute coronary syndrome or type 1 MI, standard follow-up and care are warranted. Chapman et al27 studied consecutive inpatients with elevated troponin levels. Patients with type 2 MI (supply/demand mismatch with evidence for ischemia) and cardiac injury (elevated troponin levels and no evidence for ischemia) were compared with patients with type 1 MI during 5 years of follow-up. Patients with type 2 MI and/or cardiac injury had significantly higher rates of major cardiovascular events (after adjustment for clinical covariates) and higher rates of noncardiovascular death.27 These data would be relevant for patients recovered from COVID-19–associated cardiac injury.
For patients with stress-induced myocardial injury, the long-term follow-up of patients with takotsubo cardiomyopathy may be relevant. In a recent small study, patients who recovered from takotsubo cardiomyopathy demonstrated long-term symptomatic and functional impairment despite normalized ejection fraction.28 However, the magnetic resonance imaging (MRI) study did not reveal late gadolinium enhancement. Although atrial and ventricular arrhythmias are common during the acute phase, there does not appear to be a significant risk of ventricular arrhythmias in the convalescent phase after recovery from takotsubo cardiomyopathy.29
Microemboli and/or microvascular coronary dysfunction has been posited to be one of the mechanisms of acute coronary injury during COVID-19. Preclinical studies on microemboli demonstrated vasoconstriction and inflammation, with increase in tumor necrosis factor α.30 Microemboli have been described during acute percutaneous coronary interventions and may lead to microvascular dysfunction and heart failure.30
Long-term studies in patients with viral myocarditis may be relevant for patients with COVID-19 myocarditis and/or fibrosis due to inflammation (regional or local) associated with their acute illness. In 1 study of 502 patients with biopsy-proven inflammatory carditis, up to 6.6% of patients had aborted or actual sudden cardiac death and/or appropriate implantable cardioverter-defibrillator shocks.31 In a recent study in inpatients with symptomatic active or prior myocarditis, there was an increased prevalence of atrial and ventricular arrhythmias.32 Moreover, the arrhythmic burden was not different among patients with active and previous myocarditis, although the type of arrhythmias differed. In the 62 patients with previous myocarditis with a mean ejection fraction of 47% ± 14%, atrial fibrillation was present in 34% and ventricular tachycardia was present in 47%. Late gadolinium enhancement in a nonischemic pattern was observed on cardiac MRI in all 62 patients with prior myocarditis. Ventricular arrhythmias occurred even in patients with a left ventricular ejection fraction of >50%.32
In a long-term follow-up study of 1142 patients who recovered from acute myocarditis (mean age 40.2 years), the authors noted heart failure hospitalizations between 6% and 8%.33
Currently, there are no studies that have specifically evaluated the burden of arrhythmias postmyocarditis during follow-up, particularly in patients whose ventricular function has recovered. In 1 study of long-term survival of 112 patients with biopsy-proven myocarditis, an ejection fraction of ≤40% was only a borderline predictor of mortality (P = .052), suggesting that a substantial number of patients with preserved left ventricular function died during follow-up.34 Other series of patients with myocarditis also show diminished survival and sudden cardiac death.35 A case report describes a patient with myocarditis who had a monitored ventricular fibrillation event 2 months later when left ventricular function was normal.36 Autopsy series37 , 38 of patients with sudden cardiac death have identified myocarditis as a potential explanation in a significant number of cases, even with a grossly normal appearing heart. The finding of myocarditis as a significant cause of sudden death in the young is notable and relevant to survivors of COVID-19.39
While myocarditis is typically considered for its manifestations on the ventricle, the atrium is also involved.40 Endomyocardial biopsies in patients with “idiopathic” atrial fibrillation has shown a high incidence of myocarditis in both atrial septal and right ventricular biopsy specimens.41 A Taiwanese study found an adjusted odds ratio of 1.182 (P = .03) for developing atrial fibrillation in individuals who had had influenza vs those who did not.42
Obesity has been observed to be a risk factor for worse outcomes during COVID-19 infection. ACE2 is also expressed in adipose tissue. Recent publications point to a pathophysiological link between COVID-19 and obesity.43 Obesity modulates inflammatory response through secretion of pro- and anti-inflammatory adipokines, modulation of interleukin 6; therefore, COVID-19 infection and its interaction with adipocytes may contribute to deleterious outcomes.43 Inflammation involving the epicardial adipose tissue may be affected by myocarditis because of the contiguity of the myocardium and epicardial adipose tissue. Epicardial fat has been linked with atrial fibrillation and coronary artery disease.44 , 45 Hence, the COVID-19 interaction with epicardial fat may provide a plausible link to acute or long-term atrial fibrillation and coronary artery disease.
Conclusions and recommendations
There is much unknown about COVID-19 infection. Just like other entities with acute cardiac injury, there is likely to be a diverse response, depending on the mechanism of myocardial injury, severity of acute illness, therapy delivered, hemodynamic response, host factors, immune-mediated factors, and postrecovery care and follow-up. Based on other studies of patients with recovered myocarditis, type 2 MI, or other cardiac injury, it is expected that some patients will have subclinical and possibly overt cardiovascular abnormalities. Patients with ostensibly recovered cardiac function may still be at risk of coronary artery disease, atrial fibrillation, or ventricular arrhythmias.
While current paradigms for treatment appropriately focus on acute recovery, it is unknown whether the treatment given during the acute illness may affect future cardiovascular abnormalities. Given the size of the pandemic, it is important to determine whether acute delivery of antifibrotic therapy, anti-inflammatory therapy, cell-based therapy, or antiviral therapy affects long-term as well as short-term outcomes.
The optimal screening for patients after recovery from COVID-19 is unknown. One paradigm (Figure 2) would be to define the population at highest risk by identifying patients with COVID-19 infection and elevated high-sensitivity troponin and/or brain natriuretic peptide levels, as this has already been shown to provide important short-term prognostic information. These patients should be followed to monitor and assess the long-term prognostic effect of COVID-19 myocardial involvement.
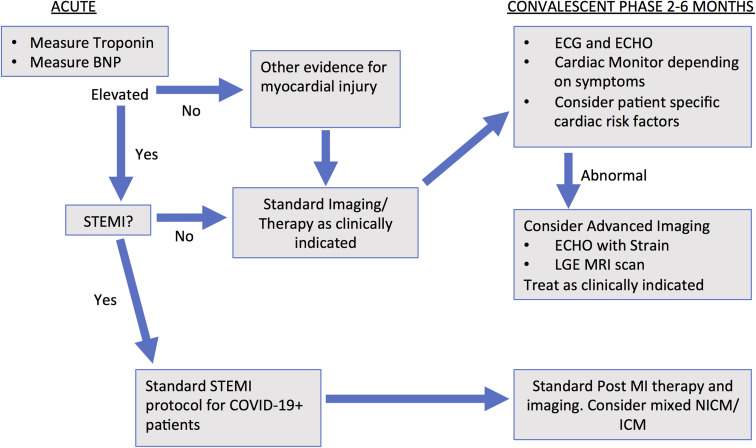
Figure 2.
Flowchart with recommendations to identify patients with cardiac injury during the acute phase- obtain troponin and brain natriuretic peptide (BNP). After the identification of patients with potential cardiac injury, the recommendation is to screen patients with an electrocardiogram (ECG) and echocardiogram (ECHO). Depending on symptoms, a cardiac monitor may be considered. Further testing and treatment would be guided by the patient’s symptoms, cardiac risk factors, and findings from initial testing. COVID-19 = coronavirus disease 2019; COVID-19+ = patients who test positive for COVID-19; ICM = ischemic cardiomyopathy; LGE = late gadolinium enhancement; MRI = magnetic resonance imaging; NICM = nonischemic cardiomyopathy; STEMI = ST-segment elevation myocardial infarction.
Screening for residual cardiac involvement in the convalescent phase is needed to establish the population burden of long-term cardiac disease contributed by COVID-19. If a significant burden of disease is identified, trials of prophylactic therapies to prevent long-term complications may be appropriate. The type of testing and cost-effectiveness of screening tests for post–COVID-19 myocardial dysfunction/arrhythmias will need to be determined. We recommend standard electrocardiogram and echocardiogram recordings and possibly cardiac monitoring 2–6 months postrecovery, with the recognition that even these tests may not detect subtle clinical abnormalities. Consideration should be given for advanced imaging (ie, MRI with gadolinium enhancement or echocardiographic strain) when initial testing reveals abnormalities or as clinically indicated. Future studies will clarify whether there will be “post–COVID-19 cardiac syndrome” and how best to manage patients recovering from COVID-19’s cardiac involvement.
We are currently facing a pandemic unseen in our lifetime with potential long-term cardiovascular complications. Now is the time for action to plan appropriate registries, such as the American Heart Association COVID-19 registry and clinical studies to assess the incidence and significance of potential mid- and long-term cardiac abnormalities and dysrhythmias, with the hope and promise to mitigate these long-term sequelae.
Footnotes
Funding sources: Drs Mitrani and Goldberger received funding from the Miami Heart Research Institute, Miami, Florida.
Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Zheng Y.-Y., Ma Y.-T., Zhang J.-Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatraju P.K., Ghassemieh B.J., Nichols M. Covid-19 in critically Ill patients in the Seattle region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China [published online ahead of print March 25, 2020]. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed]
- 4.Siripanthong B., Nazarian S., Muser D. Recognizing COVID-19-related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management [published online ahead of print May 5, 2020]. Heart Rhythm. https://doi.org/10.1016/j.hrthm.2020.05.001 [DOI] [PMC free article] [PubMed]
- 5.Akhmerov A., Marbán E. COVID-19 and the heart. Circ Res. 2020;126:1443–1455. doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu P.P., Blet A., Smyth D., Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020;142:68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 7.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116:1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suthahar N., Meijers W.C., Sillje H.H.W., de Boer R.A. From inflammation to fibrosis-molecular and cellular mechanisms of myocardial tissue remodelling and perspectives on differential treatment opportunities. Curr Heart Fail Rep. 2017;14:235–250. doi: 10.1007/s11897-017-0343-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitrani R.D., Ilkhanoff L., Goldberger J.J. Impact of nontraditional antiarrhythmic drugs on sudden cardiac death. In: Zipes D.P.J.J., Stevenson W., editors. Cardiac Electrophysiology: From Cell to Bedside. 7th ed. Elsevier; Philadelphia: 2018. pp. 1084–1091. [Google Scholar]
- 10.Grein J., Ohmagari N., Shin D. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) [published online ahead of print March 27, 2020]. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed]
- 13.Bonow RO, Fonarow GC, O’Gara PT, Yancy CW. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality [published online ahead of print March 27, 2020]. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2020.1105. [DOI] [PubMed]
- 14.Inciardi R.M., Lupi L., Zaccone G. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) [published online ahead of print March 27, 2020]. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2020.1096 [DOI] [PMC free article] [PubMed]
- 15.Atri D., Siddiqi H.K., Lang J., Nauffal V., Morrow D.A., Bohula E.A. COVID-19 for the cardiologist: a current review of the virology, clinical epidemiology, cardiac and other clinical manifestations and potential therapeutic strategies. JACC Basic Transl Sci. 2020;5:518–536. doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goyal P., Choi J.J., Pinheiro L.C. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oudit G.Y., Kassiri Z., Jiang C. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tavazzi G., Pellegrini C., Maurelli M. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capecchi P.L., Laghi-Pasini F., El-Sherif N., Qu Y., Boutjdir M., Lazzerini P.E. Autoimmune and inflammatory K+ channelopathies in cardiac arrhythmias: clinical evidence and molecular mechanisms. Heart Rhythm. 2019;16:1273–1280. doi: 10.1016/j.hrthm.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 24.Bangalore S., Sharma A., Slotwiner A. ST-segment elevation in patients with Covid-19—a case series. N Engl J Med. 2020;82:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwong J.C., Schwartz K.L., Campitelli M.A. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378:345–353. doi: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 26.Mahmud E., Dauerman H.L., Welt F.G. Management of acute myocardial infarction during the COVID-19 pandemic [published online ahead of print April 21, 2020]. J Am Coll Cardiol. https://doi.org/10.1016/j.jacc.2020.04.039 [DOI] [PMC free article] [PubMed]
- 27.Chapman A.R., Shah A.S.V., Lee K.K. Long-term outcomes in patients with type 2 myocardial infarction and myocardial injury. Circulation. 2018;137:1236–1245. doi: 10.1161/CIRCULATIONAHA.117.031806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scally C., Rudd A., Mezincescu A. Persistent long-term structural, functional, and metabolic changes after stress-induced (takotsubo) cardiomyopathy. Circulation. 2018;137:1039–1048. doi: 10.1161/CIRCULATIONAHA.117.031841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jesel L., Berthon C., Messas N. Ventricular arrhythmias and sudden cardiac arrest in Takotsubo cardiomyopathy: incidence, predictive factors, and clinical implications. Heart Rhythm. 2018;15:1171–1178. doi: 10.1016/j.hrthm.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Heusch G., Skyschally A., Kleinbongard P. Coronary microembolization and microvascular dysfunction. Int J Cardiol. 2018;258:17–23. doi: 10.1016/j.ijcard.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Marc-Alexander O., Christoph M., Chen T.-H. Predictors of long-term outcome in patients with biopsy proven inflammatory cardiomyopathy. J Geriatr Cardiol. 2018;15:363–371. doi: 10.11909/j.issn.1671-5411.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peretto G., Sala S., Rizzo S. Ventricular arrhythmias in myocarditis: characterization and relationships with myocardial inflammation. J Am Coll Cardiol. 2020;75:1046–1057. doi: 10.1016/j.jacc.2020.01.036. [DOI] [PubMed] [Google Scholar]
- 33.Chang J.-J., Lin M.-S., Chen T.-H. Heart failure and mortality of adult survivors from acute myocarditis requiring intensive care treatment—a nationwide cohort study. Int J Med Sci. 2017;14:1241–1250. doi: 10.7150/ijms.20618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magnani J.W., Danik H.J., Dec G.W., Jr., DiSalvo T.G. Survival in biopsy-proven myocarditis: a long-term retrospective analysis of the histopathologic, clinical, and hemodynamic predictors. Am Heart J. 2006;151:463–470. doi: 10.1016/j.ahj.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 35.Anzini M., Merlo M., Sabbadini G. Long-term evolution and prognostic stratification of biopsy-proven active myocarditis. Circulation. 2013;128:2384–2394. doi: 10.1161/CIRCULATIONAHA.113.003092. [DOI] [PubMed] [Google Scholar]
- 36.Prochnau D., Surber R., Kuehnert H. Successful use of a wearable cardioverter-defibrillator in myocarditis with normal ejection fraction. Clin Res Cardiol. 2010;99:129–131. doi: 10.1007/s00392-009-0093-2. [DOI] [PubMed] [Google Scholar]
- 37.Junttila M.J., Hookana E., Kaikkonen K.S., Kortelainen M.L., Myerburg R.J., Huikuri H.V. Temporal trends in the clinical and pathological characteristics of victims of sudden cardiac death in the absence of previously identified heart disease. Circ Arrhythm Electrophysiol. 2016;9 doi: 10.1161/CIRCEP.115.003723. [DOI] [PubMed] [Google Scholar]
- 38.Tseng Z.H., Olgin J.E., Vittinghoff E. Prospective countywide surveillance and autopsy characterization of sudden cardiac death: POST SCD study. Circulation. 2018;137:2689–2700. doi: 10.1161/CIRCULATIONAHA.117.033427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maron B.J., Doerer J.J., Haas T.S., Tierney D.M., Mueller F.O. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980-2006. Circulation. 2009;119:1085–1092. doi: 10.1161/CIRCULATIONAHA.108.804617. [DOI] [PubMed] [Google Scholar]
- 40.Begieneman M.P., Emmens R.W., Rijvers L. Ventricular myocarditis coincides with atrial myocarditis in patients. Cardiovasc Pathol. 2016;25:141–148. doi: 10.1016/j.carpath.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Blagova O.V., Nedostup A.V., Kogan E.A. Myocardial biopsy in “idiopathic” atrial fibrillation and other arrhythmias: nosological diagnosis, clinical and morphological parallels, and treatment. J Atr Fibrillation. 2016;9:1414. doi: 10.4022/jafib.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang T.-Y., Chao T.-F., Liu C.-J. The association between influenza infection, vaccination, and atrial fibrillation: a nationwide case-control study. Heart Rhythm. 2016;13:1189–1194. doi: 10.1016/j.hrthm.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 43.Malavazos A.E., Marco Corsi Romanelli M., Bandera F., Iacobellis G. Targeting the adipose tissue in COVID-19. Obesity. 2020;28:1178–1179. doi: 10.1002/oby.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol. 2018;71:2360–2372. doi: 10.1016/j.jacc.2018.03.509. [DOI] [PubMed] [Google Scholar]
- 45.Goeller M., Achenbach S., Marwan M. Epicardial adipose tissue density and volume are related to subclinical atherosclerosis, inflammation and major adverse cardiac events in asymptomatic subjects. J Cardiovasc Comput Tomogr. 2018;12:67–73. doi: 10.1016/j.jcct.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]