Spatiotemporal Control of Viscoelasticity in Phototunable Hyaluronic Acid Hydrogels (original) (raw)
. Author manuscript; available in PMC: 2020 Jul 1.
Published in final edited form as: Biomacromolecules. 2019 Oct 22;20(11):4126–4134. doi: 10.1021/acs.biomac.9b00965
Abstract
Viscoelasticity has emerged as a critical regulator of cell behavior. However, there is an unmet need to develop biomaterials where viscoelasticity can be spatiotemporally controlled to mimic the dynamic and heterogeneous nature of tissue microenvironments. Toward this objective, we developed a modular hyaluronic acid hydrogel combining light-mediated covalent and supramolecular cross-linking to afford spatiotemporal control of network viscoelastic properties. Covalently cross-linked elastic hydrogels or viscoelastic hydrogels combining covalent and supramolecular interactions were fabricated to match healthy and fibrotic liver mechanics. LX-2 human hepatic stellate cells cultured on viscoelastic hydrogels displayed reductions in spreading, actin stress fiber organization, and myocardin-related transcription factor A (MRTF-A) nuclear localization compared to cells on elastic hydrogels. We further demonstrated the dynamic capabilities of our hydrogel system through photo-mediated secondary incorporation of either covalent or supramolecular cross-links to modulate viscoelastic properties. We used photopatterning to create hydrogels with well-controlled patterned regions of stiff elastic mechanics representing fibrotic tissue nodules surrounded by regions of soft viscoelastic hydrogel mimicking healthy tissue. Cells responded to the local mechanics of the patterned substrates with increased spreading in fibrosis-mimicking regions. Together, this work represents an important step forward toward the creation of hydrogel models with spatiotemporal control of both stiffness and viscoelastic cell-instructive cues.
Graphical Abstract
1. INTRODUCTION
The interplay between cells and their surrounding extracellular matrix (ECM) plays a critical role in regulating development, wound healing, and disease progression.1-3 Through mechanisms including mechanotransduction, a process in which mechanical forces are converted into biochemical signals, cells are constantly probing and responding to their evolving microenvironment.4 Cell–ECM interactions are especially important in pathologies such as fibrosis, a heterogeneous pathological scarring process that can lead to irreversible loss of tissue function and organ failure. During fibrosis progression, healthy tissue mechanics transition from softer and viscoelastic to stiffer and less viscous.5,6 Moreover, fibrosis progresses in a heterogeneous manner, leading to microscale spatial heterogeneity in the form of patchy, stiff fibrotic nodules surrounded by areas of softer, less-affected tissue where nodule size often directly correlates with the severity of fibrosis.7-9 The presence of a stiff microenvironment can guide mechanotransduction by providing necessary biophysical cues for the activation of resident cells into fibrosis-promoting myofibroblasts,10 and elevated stiffness alone has been shown to drive progression of both fibrosis11 and cancer.12
Hydrogels have become valuable model systems to better understand the complex roles that matrix biophysical properties play in regulating cell behaviors through their ability to mimic salient properties of natural tissue, including soft tissue mechanics and high water content,13,14 and numerous systems have already investigated the influence of hydrogel mechanics on cell behavior.3,9,15-21 In particular, many groups have shown a direct correlation between increasing hydrogel Young’s modulus (stiffness) and elevated cell spreading in twodimensional (2D) cultures.10,22-25 Although many studies have developed homogenous substrates to study cell–ECM interactions, healthy and especially diseased tissues are inherently heterogeneous. During pathologies such as fibrosis, changes in the physical environment have direct implications on cell mechanotransduction, where activated cell patches begin depositing excessive amounts of ECM proteins, resulting in nodules of nonfunctional scar tissue.7 Therefore, it is necessary to develop methods to recapitulate tissue heterogeneity in hydrogel models. Recent work using light-based chemistries to spatially pattern elastic substrates has shown that cells will exhibit behavior correlating to their local mechanics such as increased spreading on stiffer areas.9,26,27
Although these findings are informative, they typically involve covalently cross-linked hydrogels that primarily behave as elastic solids and do not display time-dependent tissue-relevant mechanical properties. The majority of native tissues exhibits viscoelastic behaviors including stress relaxation,6,28 which can occur through both external and cell-mediated forces exerted onto the matrix. For this reason, viscoelasticity has recently emerged as a critical parameter for probing cell behaviors and functions. Viscoelastic hydrogels have been developed using ionic,15,16 supramolecular,29 and dynamic covalent cross-linking30 mechanisms. Viscoelastic hydrogels with stress relaxation properties similar to native tissues have been shown to affect cell spreading, focal adhesion organization, proliferation, and differentiation in comparison with elastic hydrogels.15-18,31,32 This can be attributed in part to cell-mediated reorganization and/or relaxation of the energy-dissipative viscoelastic hydrogel network. Recent work from Charrier et al.18 showed changes in the behavior of hepatic stellate cells, the primary cellular source of hepatic myofibroblasts, when cultured on viscoelastic hydrogels. Stellate cells displayed lower spread area and reduced expression of _α_-smooth muscle actin (_α_-SMA), a hallmark of myofibroblast activation, with increasing hydrogel loss modulus.18 This study highlighted the importance of hydrogel viscoelasticity in regulating disease-relevant cellular behaviors.
Although the importance of incorporating viscoelasticity into hydrogel cellular microenvironments is clearly established, an approach to spatially control viscoelastic properties in a manner that mimics heterogeneous tissue has not been developed. The ability to pattern regions of hydrogel stiffness and/or viscoelasticity in a manner that captures both the dynamic stiffening that occurs during fibrosis progression and the overall heterogeneity of fibrotic tissue would help establish more robust disease models to study pathological cell behaviors. Here, we designed a phototunable viscoelastic hydrogel system where stiffness and viscoelasticity can be independently tuned through control of network covalent and supramolecular interactions. Using this modular approach, we developed photopatterned substrates where stiffness and viscoelasticity could be spatiotemporally controlled and investigated the role that matrix mechanical properties played in regulating cell behavior in an in vitro model of fibrosis.
2. MATERIALS AND METHODS
2.1. NorHA Synthesis.
Norbornene-modified HA was synthesized similar to previous methods.33 Briefly, sodium hyaluronate (Lifecore, 74 kDa) was reacted with Dowex 50W proton-exchange resin, filtered, titrated to pH 7.05, frozen, and lyophilized to yield hyaluronic acid _tert_-butyl ammonium salt (HA-TBA). HA-TBA was then reacted with 5-norbornene-2-methylamine and benzotriazole-1-yloxytris-(dimethylamino)phosphonium hexafluorophosphate (BOP) in dimethylsulfoxide (DMSO) for 2 h at 25 °C. The reaction was quenched with cold water, dialyzed (molecular weight cutoff: 6–8 kDa) for 5 days, filtered, dialyzed for 5 more days, frozen, and lyophilized. The degree of modification was 22% as determined by 1H NMR (500 MHz Varian Inova 500, Figure S1).
2.2. _β_-CD-HDA Synthesis.
The synthesis of _β_-cyclodextrin hexamethylene diamine (_β_-CD-HDA) followed the procedure outlined previously.34 _p_-Toluenesulfonyl chloride (TosCl) was dissolved in acetonitrile and added dropwise to an aqueous _β_-cyclodextrin (CD) suspension (5:4 molar ratio of TosCl to CD) at 25 °C. After 2 h, the solution was cooled on ice and an aqueous NaOH solution was added dropwise (3.1:1 molar ratio of NaOH to CD). The solution was reacted for 30 min at 25 °C before adding ammonium chloride to reach a pH of 8.5. The solution was cooled on ice, precipitated using cold water and acetone, and dried overnight. The CD-Tos product was then charged with hexamethylene diamine (HDA) (4 g/g CD-Tos) and dimethylformamide (DMF) (5 mL/g CD-Tos), and the reaction was carried out under nitrogen at 80 °C for 12 h before being precipitated with cold acetone (5 × 50 mL/g CD-Tos), washed with cold diethyl ether (3 × 100 mL), and dried. The degree of modification was 61% as determined by 1H NMR (Figure S2).
2.3. CD-HA Synthesis.
_β_-Cyclodextrin-modified hyaluronic acid (CD-HA) was prepared through coupling of _β_-CD-HDA to HA-TBA. A reaction containing HA-TBA, 6-(6-aminohexyl)amino-6-deoxy-_β_-cyclodextrin (_β_-CD-HDA), and BOP in DMSO was carried out at 25 °C for 3 h. The reaction was quenched with cold water, dialyzed for 5 days, filtered, dialyzed for 5 more days, frozen, and lyophilized. The degree of modification was 27% as determined by 1H NMR (Figure S3).
2.4. Peptide Synthesis.
Solid-phase peptide synthesis was performed on a Gyros Protein Technologies Tribute peptide synthesizer. A thiolated adamantane (Ad) peptide (Ad-KKKCG) and a fluorescently-labeled thiolated peptide (Fluorescein-KKCG) were synthesized on either Rink Amide MBHA high-loaded (0.78 mmol/g) or Wang (1 mmol/g) resins using standard solid-supported Fmoc-protected peptide synthesis. The resin was swelled with 20% (v/v) piperidine in DMF, and the amino acids were activated using HBTU and 0.4 _N_-methyl morpholine in DMF (5:1 excess). Peptides were cleaved in a solution of 95% trifluoroacetic acid, 2.5% triisopropylsilane, and 2.5% H2O for 2–3 h, precipitated in cold ethyl ether, and dried overnight. The peptide products were resuspended in H2O, frozen, and lyophilized. Synthesis was confirmed by matrix-assisted laser desorption/ionization (MALDI) (Figures S4 and S5).
2.5. HA Hydrogel Fabrication.
2D hydrogel thin films were made between untreated and thiolated coverslips (50 _μ_L, 18 × 18 mm). Elastic NorHA hydrogels were fabricated using ultraviolet (UV) light-mediated thiol–ene addition. Soft (2 wt % NorHA) and stiff (6 wt % NorHA) hydrogel precursor solutions containing 1 mM thiolated RGD peptide (GCGYGRGDSPG, Genscript) and dithiothreitol (DTT, thiol–norbornene ratios of 0.35 for both groups) were photopolymerized (365 nm, 5 mW/cm2) in the presence of 1 mM lithium acylphosphinate (LAP) photoinitiator for 2 min. Soft (2 wt % NorHA-CD-HA) and stiff (6 wt % NorHA-CD-HA) viscoelastic NorHA-CD-HA hydrogels were fabricated by first mixing CD-HA (5 and 8 wt % stock solutions for soft and stiff viscoelastic groups, respectively) with the thiolated adamantane peptide (1:1 molar ratio of CD to Ad) to introduce Ad–CD guest–host interactions before mixing in RGD, DTT (thiol-norbornene ratios of 0.45 and 0.55 for soft and stiff viscoelastic groups, respectively), and 8 wt % NorHA stock solution. The 2 and 6 wt % NorHA-CD-HA precursor solutions were then photopolymerized using the same conditions as elastic hydrogels. Hydrogels were swelled in phosphate-buffered saline (PBS) overnight at 37 °C before subsequent cell-seeding procedures.
2.6. Rheological Characterization.
All rheological measurements were performed at 25 °C on an Anton Paar MCR 302 rheometer using a cone-plate geometry (25 mm diameter, 0.5°, 25 _μ_m gap). Rheological properties were tested using oscillatory time sweeps (1 Hz, 1% strain) with a 2 min UV irradiation (365 nm, 5 mW/cm2), oscillatory frequency sweeps (0.001–10 Hz, 1% strain), cyclic stress relaxation and recovery tests alternating between 0.1 and 5% strain (1 Hz), and creep tests where a constant stress of 100 Pa was applied to the sample for 50 S.18
2.7. Cell Culture.
Human hepatic stellate cells (LX-2s,35 Millipore Sigma) were used between passages 6–8 for all experiments. Culture media contained Dulbecco’s modified Eagle’s medium supplemented with 10 v/v% fetal bovine serum (Gibco) and 1 v/v% penicillin/streptomycin/amphotericin B (1000 U/mL, 1000 _μ_g/mL, and 0.25 _μ_g/mL final concentrations, respectively, Gibco). For cell seeding, swelled thin film hydrogels (18 × 18 mm) were sterilized using germicidal UV irradiation for 2 h and incubated in culture media for at least 30 min prior to cell seeding. Cultures were treated with 5–10 _μ_g/mL mitomycin C (Sigma-Aldrich) in serum-free media for 2 h, washed thrice with PBS, and incubated in complete culture media for at least 1 h prior to cell seeding. Cells were seeded atop hydrogels placed in untreated 6-well plates at a density of 2 × 104 cells per hydrogel. For all experiments, media was replaced every 2–3 days for 7 day cultures.
2.8. Immunocytochemistry, Imaging, and Analysis.
For immunostaining, cell-seeded hydrogels were fixed in 10% buffered formalin for 15 min, permeabilized in 0.1% Triton X-100 for 10 min, and blocked in 3% bovine serum albumin (BSA) in PBS for at least 1 h at room temperature. Hydrogels were then incubated overnight at 4 °C with primary antibodies against myocardin-related transcription factor A (MRTF-A, rabbit polyclonal anti-Mk11 antibody, 1:600, Abcam) and either _α_-SMA (mouse monoclonal anti-_α_-SMA clone 1A4, 1:400, Sigma-Aldrich) or rhodamine phalloidin to visualize F-actin (1:600, Invitrogen). The hydrogels were washed three times in PBS and incubated with secondary antibodies (AlexaFluor 488 goat anti-rabbit IgG, 1:800; AlexaFluor 555 goat anti-mouse, 1:800) for 2 h in the dark at room temperature. The hydrogels were then rinsed three times with PBS and stained with a DAPI nuclear stain (1:10 000) for 1 min before rinsing twice with 3% BSA. Stained hydrogels were stored in the dark at 4 °C until imaging. Microscopy was performed on a Zeiss AxioObserver 7 inverted microscope. 2D hydrogels were covered with an 18 × 18 mm glass coverslip and inverted for imaging. Exposure time and other image settings for each respective channel were held constant while imaging. Cell spread area, cell shape index (CSI), and MRTF-A nuclear localization were determined using a CellProfiler (Broad Institute, Harvard/MIT) pipeline modified to include adaptive thresholding. CSI determines the circularity of the cell, where a line and a circle have values of 0 and 1, respectively, and was calculated using the formula
where A is the cell area and P is the cell perimeter. MRTF-A nuclear/cytosolic ratio was determined using the formula
Nuclear MRTF−A=nuclear MRTF−a signal∕area of nuclescytosolic MRTF−a signal∕area of cytosol
where the signal intensities were taken and normalized to their respective areas.
2.9. Photopatterning HA Hydrogels.
NorHA hydrogels (6 wt %) with low amounts of DTT (thiol–norbornene ratio = 0.12 for initially soft viscoelastic, 0.2 for initially soft elastic, 0.45 for initially stiff elastic) were fabricated and swelled overnight in PBS at 37 °C. The hydrogels were first swelled in a 2 wt % BSA in PBS solution for 2 h before being swelled in a 500 _μ_L PBS solution containing 1 wt % BSA, LAP, DTT, and fluorescent-thiolated peptide for 1 h at 37 °C before irradiation with a patterned photomask transparency (CAD/Art Services, Inc) for 2 min (5 mW/cm2). The resulting patterned hydrogels were washed with PBS several times prior to cell seeding and imaging.
2.10. Atomic Force Microscopy Characterization and Analysis.
Atomic force microscopy (AFM) force spectroscopy was performed using an Asylum Research MFP 3D AFM. A silicon nitride cantilever (MLCT-O10/Tipless/Ti–Au, cantilever C, Bruker) with a nominal spring constant of 0.1 N/m was functionalized with a 25 _μ_m diameter polystyrene bead at the tip. The spring constant of the cantilever was calibrated via thermal resonance curves prior to data collection. Nanoindentation tests (indentation rate, ν = 5–10 _μ_m/s) were performed on photopatterned hydrogels in PBS to determine the mechanics of the patterned and nonpatterned regions. Force versus distance curves were generated, and the instantaneous Young’s modulus (E(0)) at each indentation was calculated using the initial loading portion of the indentation curve by applying the Hertzian contact mechanics model and assuming a Poisson’s ratio of 0.5. Force relaxation tests were performed to study viscoelasticity of patterned substrates. Following indentation, the tip was held at a constant indentation depth for 10–30 s at a 500 Hz sampling rate. Indentation force and depth were recorded as a function of time.36,37 Temporal relaxation tests measuring time-dependent Young’s modulus (E(t)) normalized to the instantaneous modulus (E(0)) were used to assess viscoelasticity.
2.11. Statistical Analysis.
Student’s _t_-tests (two experimental groups) or one-way ANOVA with Tukey’s HSD post hoc tests (more than two experimental groups) were performed for all quantitative data sets. All experiments included at least 3 hydrogels and/or 20 individual cells quantified per experimental group. Box plots of single cell data had error bars that were the lower value of either 1.5 × interquartile range or the maximum/minimum value, with data points between 1.5 × interquartile range and the maximum/minimum indicated as open circles. Significance was indicated by *, **,or *** corresponding to P < 0.05, 0.01, or 0.001, respectively.
3. RESULTS AND DISCUSSION
3.1. Viscoelastic Hydrogels Were Synthesized with a Combination of Covalent and Supramolecular Cross-links.
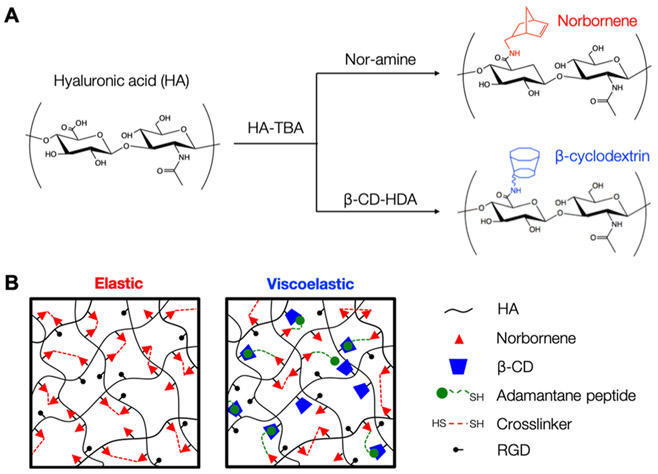
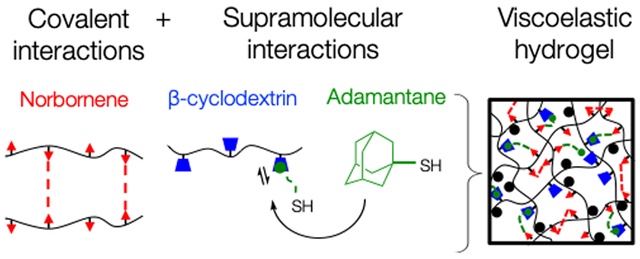
Hyaluronic acid was functionalized with norbornene groups (NorHA) to produce hydrogels containing a high degree of reactive sites (~20% of repeat units). Compared to common functional groups such as (meth)acrylates, which can react with each other to form kinetic chains, norbornene groups have high reactivity to thiyl radicals and low reactivity to themselves, allowing rapid and controllable thiol–ene click addition of both pendant and multifunctional thiolated groups.33 This bio-rthogonal system was also chosen for its ability to easily synthesize hydrogels with a wide range of tissue-relevant mechanics by a simple tuning of parameters such as cross-linker concentration or light intensity. In this study, soft (G′ ≈ 0.5 kPa) and stiff (G′ ≈ 5 kPa) hydrogels were fabricated to represent healthy and fibrotic liver tissue, respectively.10,11 Elastic NorHA hydrogels were fabricated via ultraviolet (UV) light-mediated thiol–ene addition between norbornenes on HA and thiols on DTT to create stable covalently cross-linked networks.
Viscoelasticity was introduced to the system by incorporating reversible guest–host interactions between adamantane (guest) and _β_-cyclodextrin (host) groups. The adamantane (Ad) guest moiety has a high affinity to the hydrophobic cavity of _β_-cyclodextrin (_K_a ≈ 105 M−2), and has previously been exploited to make viscoelastic, shear-thinning hydrogels.34,38,39 For viscoelastic hydrogel groups, _β_-cyclodextrin HA (CD-HA) and thiolated Ad peptide were mixed in solution (1:1 molar ratio of CD to Ad) to introduce supramolecular guest–host interactions, followed by the addition of NorHA and DTT, where the ratio of DTT to norbornene groups provided control over hydrogel modulus (Figure 1). This particular methodology involving Ad peptides allowed for a more modular approach to fabricate hydrogels because of its detachment from the HA backbone prior to the thiol–ene addition, making the hydrogel precursors less viscous and easier to pipet and mix. Following mixing of the Ad peptide, CD-HA, NorHA, and DTT, the thiols on the cysteine residues of the CD-associated Ad peptide reacted with the norbornenes to form stable supramolecular connections between HA chains, whereas the DTT formed covalent cross-links, creating a viscoelastic hydrogel network with both covalent and supramolecular cross-links.
Figure 1.
Overview of hydrogel synthesis and cross-linking. (A) Hyaluronic acid was first converted to HA-TBA salt before modification with norbornene or _β_-cyclodextrin groups using BOP coupling chemistry to synthesize NorHA and CD-HA. (B) For the elastic hydrogel system, covalent cross-links between the norbornene groups were introduced using di-thiol cross-linkers via light-mediated thiol–ene addition. For the viscoelastic hydrogel system, thiol–ene photochemistry was used to introduce supramolecular interactions between CD-HA and Ad groups on thiolated peptides in addition to dithiol-mediated covalent cross-links between the norbornenes.
3.2. Viscoelastic Hydrogels Display Stress Relaxation and Frequency-Dependent Behavior.
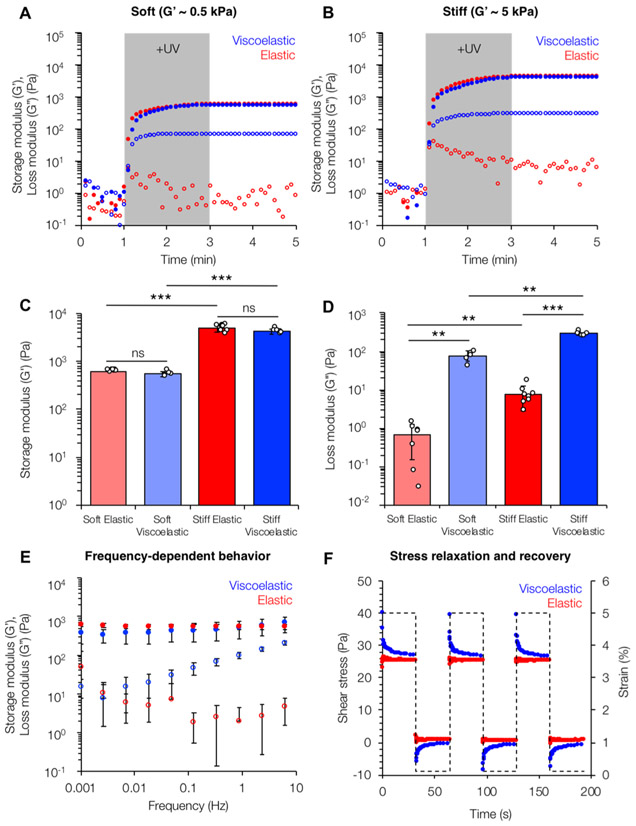
Hydrogel mechanical properties were characterized through shear oscillatory rheology (Figure 2). In situ gelation of hydrogel precursor solutions demonstrated rapid gelation kinetics controlled by light exposure, resulting in a nearly immediate plateau in storage and loss moduli once light irradiation was stopped (Figure 2A,B). Similar storage moduli at 1 Hz were observed for the soft (elastic: G′ = 0.51 ± 0.08 kPa, viscoelastic: G′ = 0.46 ± 0.07 kPa) and stiff (elastic: 4.59 ± 0.24 kPa, viscoelastic: G′ = 4.93 ± 0.77 kPa) hydrogel groups corresponding to healthy and fibrotic liver tissues, respectively. However, as expected, the viscoelastic hydrogels had significantly higher loss moduli at 1 Hz (soft viscoelastic: G″ = 67.2 ± 2.44 Pa, stiff viscoelastic: G″ = 330 ± 45.5 Pa) compared to elastic groups (soft elastic: G″ = 0.99 ± 0.89 Pa, stiff elastic: G″ = 2.78 ± 2.21 Pa). Notably, the G″ values for the viscoelastic hydrogels were within an order of magnitude of the G′ similar to the ratios observed in native viscoelastic tissue.18,40 Hydrogel frequency sweeps revealed relatively constant storage and loss moduli for the elastic groups (Figure 2C). However, the viscoelastic hydrogels showed frequency-dependent behavior; at higher frequencies, the loss modulus increased, demonstrating that guest–host interactions were being disrupted with less time to reassociate. Although there were no statistically significant differences in G′ between the elastic and viscoelastic groups over the range of frequencies tested (0.001–10 Hz), the strong frequency-dependent behavior shown by the viscoelastic hydrogels for G″ is similar to trends seen in other comparable viscoelastic systems.18,34 Stress relaxation and recovery tests showed that at a constant applied strain of 5%, the elastic hydrogels showed no stress relaxation over time because of their stable covalently cross-linked network (Figure 2D). In contrast, the viscoelastic hydrogel groups showed cyclic stress relaxation in which high stress was observed, followed by a plateau to a final stress value equal to the corresponding elastic groups. The ability of the hydrogels to fully recover their mechanical properties upon repeated bouts of applied strain highlighted their viscoelasticity as opposed to viscoplasticity.
Figure 2.
Rheological characterization of viscoelastic hydrogels. Viscoelastic hydrogels (blue) of equivalent storage moduli (closed circles) to their elastic counterparts (red) showed loss moduli (open circles) within an order of magnitude for both (A) “soft” (G′ ≈ 0.5 kPa) and (B) “stiff” (G′ ≈ 5 kPa) hydrogel formulations corresponding to healthy and fibrotic tissue, respectively. (C) Average values of storage moduli (G′) measured at a constant frequency (1 Hz) and strain (1%). (D) Average values of loss moduli (G″) measured at a constant frequency (1 Hz) and strain (1%). (E) Viscoelastic hydrogels also showed frequency-dependent behavior with increasing loss moduli as frequency was increased, whereas the elastic hydrogel properties remained relatively constant. (F) Stress relaxation and recovery tests showed full recovery of the mechanical properties of the viscoelastic hydrogels. For the frequency and stress relaxation tests, the soft groups are shown; similar trends were seen for the stiff groups (Figure S6). **: P < 0.01, ***: P < 0.001.
3.3. Cell Spreading is Modulated by Both Hydrogel Stiffness and Viscoelasticity.
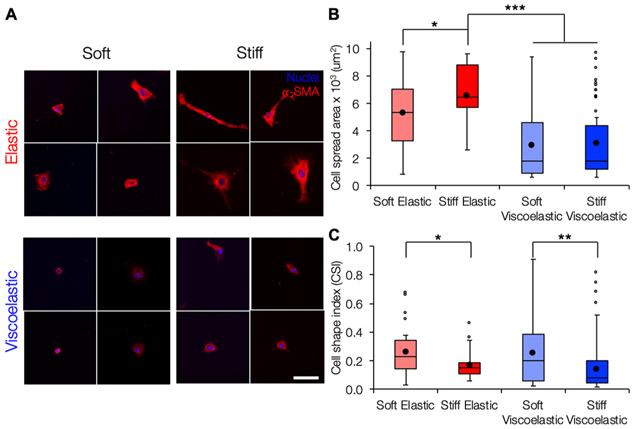
After rheological characterization highlighted the tunable viscoelastic nature of our hydrogel design, we investigated the behavior of LX-2s, a human hepatic stellate cell line, when cultured on four hydrogel groups: soft elastic, stiff elastic, soft viscoelastic, and stiff viscoelastic. Cells on stiff elastic substrates showed increased spreading compared to cells on soft elastic substrates, similar to what has previously been reported for elastic substrates of increasing stiffness (Figure 3). In comparison the viscoelastic hydrogels, which had the same storage moduli as the corresponding elastic groups but higher loss moduli, supported decreased cell spreading and more rounded morphologies as measured by CSI compared to the corresponding elastic substrates for both the soft and stiff groups. The differences in cell spreading and circularity were the greatest between cells cultured on the stiff elastic hydrogels, which became more elongated and extended protrusions (average spread area: 6600 _μ_m2, CSI: 0.17), and cells cultured on the soft viscoelastic hydrogels, which showed smaller, more rounded morphologies (average spread area: 3000 _μ_m2, CSI: 0.26). The reduction in stellate cell spreading is similar to observations from a recent study where stellate cells showed reduced spreading and reduced expression of _α_-SMA, a marker of myofibroblast activation, when cultured on polyacrylamide substrates with higher loss moduli.18 Similarly, although cells on our viscoelastic hydrogels showed positive _α_-SMA staining, we also observed reduction in the organization of _α_-SMA stress fibers that is typical of activated myofibroblasts.18,41,42 Although around 85% of cells on stiff elastic hydrogels displayed at least some organized _α_-SMA stress fibers, only 6 and 22% of cells on soft and stiff viscoelastic hydrogels, respectively, displayed well-organized _α_-SMA stress fibers (Figure S7).
Figure 3.
Cell spreading is modulated by both stiffness and viscoelasticity. (A) Representative images of LX-2 hepatic stellate cells stained for _α_-SMA (red) and nuclei (blue) after 7 days of culture. Scale bar 100 _μ_m. (B) Although cell spreading was increased on stiff elastic compared to soft elastic hydrogels, spreading was significantly reduced on both soft and stiff viscoelastic hydrogels compared to the stiff elastic group. (C) CSI was significantly higher for cells on soft hydrogels compared to their respective stiff counterparts, indicating that the cells displayed more rounded morphologies. *: P < 0.05, **: P < 0.01, and ***: P < 0.001.
Because _α_-SMA expression is a relatively late marker of myofibroblast activation, we also investigated earlier markers of fibrogenic mechanotransduction. MRTF-A, a transcriptional coactivator implicated in the regulation and progression of fibrosis, which has been shown to drive _α_-SMA expression and subsequent myofibroblast activation.43-45 Specifically, activation of mechanotransduction pathways through cell–matrix interactions can promote RhoA/ROCK signaling, actin polymerization, and subsequent MRTF-A nuclear translocation. MRTF-A then interacts with serum response factor, the transcription factor that promotes upregulation of the Acta2 gene encoding for _α_-SMA.44,46-48 We measured the ratio of MRTF-A nuclear to cytosolic signaling intensity and found elevated MRTF-A nuclear localization for cells on stiff compared to soft elastic hydrogels (Figure S8). However, cells cultured on the viscoelastic hydrogel groups showed reduced MRTF-A nuclear localization compared to the stiff elastic group. Overall, both soft and stiff viscoelastic hydrogels promoted reduced stellate cell spreading, _α_-SMA stress fiber organization, and MRTF-A nuclear localization. A possible explanation for these results could be that the higher loss moduli and rapid viscous dissipation of cell-generated traction forces into the matrix prevented spreading and the activation of the mechanores-ponsive signaling pathways was investigated here.49 Differences in cell spreading and MRTF-A nuclear localization between elastic and viscoelastic groups could also be attributed to differences in relaxation timescales and initial moduli.
3.4. Light-Mediated Thiol–Ene Addition Enables Secondary Incorporation of Covalent or Supramolecular Cross-links.
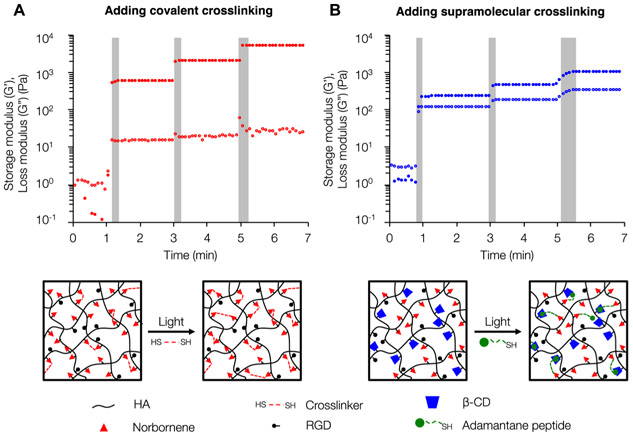
Following the evaluation of cell behavior on static elastic and viscoelastic hydrogels, we next demonstrated the dynamic capabilities of our viscoelastic hydrogel system through specific secondary introduction of either covalent or supramolecular interactions. First, we fabricated elastic hydrogels with increasing covalent cross-linking density controlled by sequential bouts of light exposure, permitting further thiol–ene cross-linking. Rheological analysis indicated that each additional irradiation corresponded with increasing storage modulus but relatively little change in loss modulus as expected for an elastic network (Figure 4A). Next, we made initially soft viscoelastic hydrogels containing unreacted norbornene and _β_-cyclodextrin groups and introduced additional supramolecular cross-links through sequential thiol–ene addition of thiolated adamantane peptide. Each additional light irradiation led to increases in both the storage and loss moduli as the hydrogel maintained its viscoelastic nature (Figure 4B). We observed that incorporating additional supramolecular cross-linking required progressively longer irradiation times, which is likely because of the difficulty of new incorporated supramolecular cross-links (via the Ad peptide) in finding both a free cyclodextrin group and neighboring norbornene group to associate with in the already cross-linked network. However, this change in irradiation time does not significantly impact the tunability of the system. Overall, the unique amenability of our system to the light-mediated introduction of either new covalent or supramolecular cross-links sets the stage for the creation of dynamic, heterogeneous viscoelastic hydrogels.
Figure 4.
Secondary introduction of covalent or supramolecular cross-links to modulate viscoelastic properties. (A) When incorporating new covalent cross-links through DTT addition, each subsequent UV light exposure (gray bars) results in an increased storage modulus but minor changes in loss modulus. (B) Following initial formation of a viscoelastic hydrogel, incorporating new supramolecular guest–host cross-links leads to increases in both the storage and loss moduli with each UV light exposure.
3.5. Photopatterning Enables Presentation of Dynamic, Heterogeneous, and Cell-Instructive Viscoelastic Hydrogel Cues.
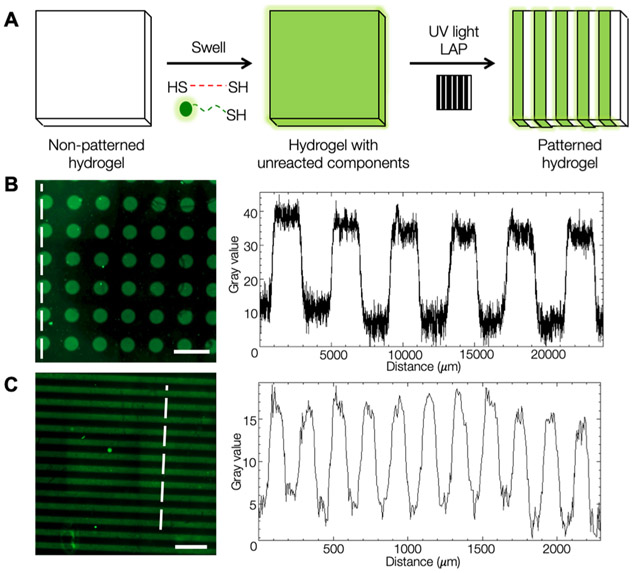
After developing our viscoelastic hydrogel system and demonstrating its amenability to secondary cross-linking reactions, we explored the use of photopatterning to recapitulate the heterogeneity of matrix mechanical properties during fibrogenesis in a well-defined manner (Figure 5). Using photomasks to control light penetration into the hydrogel during secondary cross-linking enabled spatial control over the thiol–ene addition reactions. Soft NorHA hydrogels were swelled in a solution containing 1 wt % BSA, LAP photoinitiator, DTT cross-linker or adamantane peptide, and thiolated fluorescent peptide for pattern visualization. Fluorescence microscopy confirmed pattern fidelity, with alternating fluorescent and nonfluorescent regions present in the hydrogel (Figure 5B).
Figure 5.
Photopatterning of hydrogels to introduce heterogeneous properties. (A) Schematic of the photopatterning process. NorHA hydrogels were swollen with thiolated molecules, covered with a photomask, and exposed to UV light, resulting in regions that underwent secondary cross-linking via light-mediated thiol–ene addition. A model of thiolated fluorescent peptide was used to demonstrate patterning capabilities. Color intensity profiles showed high pattern fidelity across pattern features for (B) 200 _μ_m diameter circles and (C) 200 _μ_m stripe patterns; signal intensity profiles were quantified along the white-dotted lines. Scale bars: 500 _μ_m.
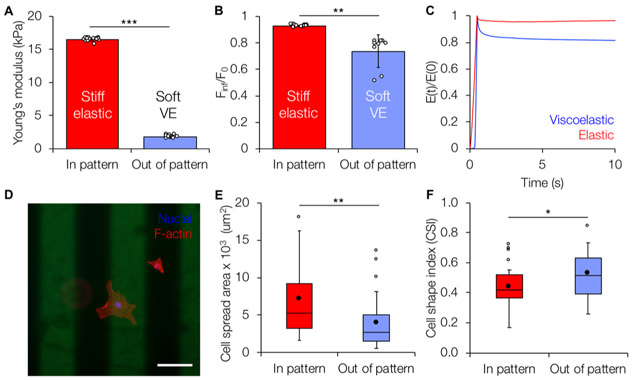
After establishing the photopatterning approach, we wanted to develop a patterned hydrogel model of fibrotic tissue. During the heterogeneous progression of fibrosis, the aberrant shift in healthy tissue mechanics from soft and viscoelastic to stiff and more elastic highlights the need for in vitro models enabling independent spatiotemporal control of both stiffness and viscoelasticity. Given the ability for multiple light-mediated thiol–ene click reactions to occur in series, our hydrogel system can model both the heterogeneity of fibrosis through photopatterning and the induction of fibrosis progression through the introduction of new cross-links to stiffen the hydrogel. Starting from an initial soft viscoelastic hydrogel, we photopatterned additional covalent cross-links to create stiff, less viscoelastic hydrogel regions mimicking fibrotic nodules. AFM was used to characterize the elastic and viscoelastic properties of the patterned substrates. AFM nanoindentation tests demonstrated that patterned hydrogel regions undergoing secondary covalent cross-linking displayed higher Young’s moduli (16.5 ± 0.23 kPa) compared to nonpatterned regions (1.78 ± 0.22 kPa) (Figure 6A). The viscoelasticity of the patterned hydrogel regions was characterized using a nanoindentation test, followed by a dwell period in which the AFM tip was held at a constant indentation depth to measure force as a function of time. The nonpatterned soft viscoelastic regions showed ~20% force relaxation over a period of 10 s, whereas the patterned stiff elastic regions showed negligible relaxation (Figure 6B,C), similar to bulk rheological measurements for homogeneous hydrogels. Importantly, this novel method for patterning viscoelasticity can be decoupled from changing the stiffness. As a demonstration of this, viscoelasticity can be patterned into a stiff elastic substrate through the introduction of supramolecular cross-links to produce regions of patterned, stiff viscoelasticity, as shown by force relaxation data without changing the overall Young’s modulus under the measuring conditions of the AFM (initial stiff elastic = 10.6 ± 0.38 kPa, patterned stiff viscoelastic = 10.7 ± 0.49 kPa) (Figure S9).
Figure 6.
Mechanical characterization and cell response on patterned viscoelastic hydrogels. (A) Patterned (stiff elastic) and nonpatterned (soft viscoelastic) regions showed differences in Young’s moduli similar to homogeneous substrates. (B) Quantification of the ratio of equilibrium force (_F_inf) to initial indentation force (_F_0) of patterned hydrogels indicates significantly greater levels of stress relaxation in the nonpatterned (soft viscoelastic) regions. (C) Time-dependent Young’s modulus E(t) normalized to the initial instantaneous modulus E(0) showed ~20% relaxation in the nonpatterned soft viscoelastic regions compared to negligible relaxation for the stiff elastic patterned regions. (D) Representative fluorescent image of LX-2 stellate cells (red: F-actin, blue: nuclei) cultured for 7 days on a patterned hydrogel (200 _μ_m wide stripes, green). Scale bar: 200 _μ_m. (E) Cells on the patterned (stiff elastic) region showed significantly increased spread area compared to those in the nonpatterned (soft viscoelastic) regions. (F) Cells in the patterned regions also showed significantly lower CSI, indicating a more elongated morphology, compared to more rounded cells in the nonpatterned regions. *: P < 0.05, **: P < 0.01, and ***: P < 0.001.
Next, we seeded LX-2 stellate cells onto soft viscoelastic hydrogels with patterned regions of stiff elastic mechanics (Figure 6D). Cells responded to the local mechanics of the patterned substrate and showed significantly increased spreading (Figure 6E) and significantly lower CSI (Figure 6F) on stiffer patterned regions. Although photopatterning was performed prior to cell seeding in these experiments, the reagents used are cytocompatible; therefore, we anticipate that these results will inform the future development of hydrogel models of heterogeneous tissue mechanics via in situ photopatterning in the presence of cells.
4. CONCLUSIONS
This work developed an approach to make viscoelastic hydrogels via light-mediated thiol–ene addition of both covalent and supramolecular cross-links. The use of light as a trigger for cross-linking enabled secondary modification of the hydrogel network to increase stiffness (mimicking initiation of fibrosis) and/or modulate viscoelasticity (through the introduction of covalent and/or supramolecular cross-links). We showed that LX-2 human hepatic stellate cells responded to the viscoelastic hydrogels by displaying reductions in spread area, MRTF-A nuclear translocation, and organization of actin stress fibers. We also used photopatterning to create hydrogels with stiff, elastic areas surrounded by soft, viscoelastic regions to mimic a heterogeneous fibrotic environment and showed that cells spread more in the stiffer patterned regions. Moving forward, we expect that this hydrogel system affording spatiotemporal control of stiffness and viscoelasticity will be useful to model a range of healthy and diseased cellular microenvironments.
Supplementary Material
Supplementary Information
■ ACKNOWLEDGMENTS
The authors would like to thank Dr. Tom Barker and Dr. Wei Li for providing training and use of AFM, and Dr. Lin Han and Biao Han for providing the initial MATLAB code for AFM analysis. This work was supported by the University of Virginia and the Biotechnology Training Program NIGMS 5T32 GM008715.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.biomac.9b00965.
1H NMR spectra for NorHA, CD-HDA, and CD-HA; MALDI spectra for peptides; and additional hydrogel mechanical characterization and cell analysis (PDF)
The authors declare no competing financial interest.
■ REFERENCES
- (1).Wang N; Tytell JD; Ingber DE Mechanotransduction at a Distance: Mechanically Coupling the Extracellular Matrix with the Nucleus. Nat. Rev. Mol. Cell Biol 2009, 10, 75–82. [DOI] [PubMed] [Google Scholar]
- (2).Dupont S; Morsut L; Aragona M; Enzo E; Giulitti S; Cordenonsi M; Zanconato F; Le Digabel J; Forcato M; Bicciato S; Elvassore N; Piccolo S Role of YAP/TAZ in Mechanotransduction. Nature 2011, 474, 179–183. [DOI] [PubMed] [Google Scholar]
- (3).Engler AJ; Sen S; Sweeney HL; Discher DE Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [DOI] [PubMed] [Google Scholar]
- (4).Ingber DE Cellular Mechanotransduction: Putting All the Pieces Together Again. FASEB J. 2006, 20, 811–827. [DOI] [PubMed] [Google Scholar]
- (5).Wells RG Tissue Mechanics and Fibrosis. Biochim. Biophys. Acta 2014, 1832, 884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Fiore VF; Wong SS; Tran C; Tan C; Xu W; Sulchek T; White ES; Hagood JS; Barker TH Av_β_3 Integrin Drives Fibroblast Contraction and Strain Stiffening of Soft Provisional Matrix during Progressive Fibrosis. JCI Insight 2018, 3 (). 10.1172/jci.insight.97597. DOI: 10.1172/jci.insight.97597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Gurtner GC; Werner S; Barrandon Y; Longaker MT Wound Repair and Regeneration. Nature 2008, 453, 314–321. [DOI] [PubMed] [Google Scholar]
- (8).Wynn TA Integrating Mechanisms of Pulmonary Fibrosis. J. Exp. Med 2011, 208, 1339–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Guvendiren M; Perepelyuk M; Wells RG; Burdick JA Hydrogels with Differential and Patterned Mechanics to Study Stiffness-Mediated Myofibroblastic Differentiation of Hepatic Stellate Cells. J. Mech. Behav. Biomed. Mater 2014, 38, 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Olsen AL; Bloomer SA; Chan EP; Gaça MDA; Georges PC; Sackey B; Uemura M; Janmey PA; Wells RG Hepatic Stellate Cells Require a Stiff Environment for Myofibroblastic Differentiation. Am. J. Physiol. Gastrointest. Liver Physiol 2011, 301, G110–G118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Georges PC; Hui J-J; Gombos Z; McCormick ME; Wang AY; Uemura M; Mick R; Janmey PA; Furth EE; Wells RG Increased Stiffness of the Rat Liver Precedes Matrix Deposition: Implications for Fibrosis. Am.J. Physiol. Gastrointest. Liver Physiol 2007, 293, G1147–G1154. [DOI] [PubMed] [Google Scholar]
- (12).Levental KR; Yu H; Kass L; Lakins JN; Egeblad M; Erler JT; Fong SFT; Csiszar K; Giaccia A; Weninger W; Yamauchi M; Gasser DL; Weaver VM Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell 2009, 139, 891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Caliari SR; Burdick JA A Practical Guide to Hydrogels for Cell Culture. Nat. Methods 2016, 13, 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Baker BM; Chen CS Deconstructing the Third Dimension – How 3D Culture Microenvironments Alter Cellular Cues. J. Cell Sci 2012, 125, 3015–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Chaudhuri O; Gu L; Klumpers D; Darnell M; Bencherif SA; Weaver JC; Huebsch N; Lee H.-p.; Lippens E; Duda GN; Mooney DJ Hydrogels with Tunable Stress Relaxation Regulate Stem Cell Fate and Activity. Nat. Mater 2016, 15, 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Chaudhuri O; Gu L; Darnell M; Klumpers D; Bencherif SA; Weaver JC; Huebsch N; Mooney DJ Substrate Stress Relaxation Regulates Cell Spreading. Nat. Commun 2015, 6, 6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Cameron AR; Frith JE; Cooper-White JJ The Influence of Substrate Creep on Mesenchymal Stem Cell Behaviour and Phenotype. Biomaterials 2011, 32, 5979–5993. [DOI] [PubMed] [Google Scholar]
- (18).Charrier EE; Pogoda K; Wells RG; Janmey PA Control of Cell Morphology and Differentiation by Substrates with Independently Tunable Elasticity and Viscous Dissipation. Nat. Commun 2018, 9, 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Pelham RJ; Wang Y.-l. Cell Locomotion and Focal Adhesions Are Regulated by Substrate Flexibility. Proc. Natl.Acad. Sci. U.S.A 1997, 94, 13661–13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Mcbeath R; Pirone DM; Nelson CM; Bhadriraju K; Chen CS Cell Shape, Cytoskeletal Tension, and RhoA Regulate Stem Cell Lineage Commitment. Dev. Cell 2004, 6, 483–495. [DOI] [PubMed] [Google Scholar]
- (21).Wen JH; Vincent LG; Fuhrmann A; Choi YS; Hribar KC; Taylor-Weiner H; Chen S; Engler AJ Interplay of Matrix Stiffness and Protein Tethering in Stem Cell Differentiation. Nat. Mater 2014, 13, 979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Yeh Y-C; Corbin EA; Caliari SR; Ouyang L; Vega SL; Truitt R; Han L; Margulies KB; Burdick JA Mechanically Dynamic PDMS Substrates to Investigate Changing Cell Environments. Biomaterials 2017, 145, 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Mih JD; Marinkovic A; Liu F; Sharif AS; Tschumperlin DJ Matrix Stiffness Reverses the Effect of Actomyosin Tension on Cell Proliferation. J. Cell Sci 2012, 125, 5974–5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Guvendiren M; Burdick JA Stiffening Hydrogels to Probe Short- and Long-Term Cellular Responses to Dynamic Mechanics. Nat. Commun 2012, 3, 792–799. [DOI] [PubMed] [Google Scholar]
- (25).Wells RG The Role of Matrix Stiffness in Regulating Cell Behavior. Hepatology 2008, 47, 1394–1400. [DOI] [PubMed] [Google Scholar]
- (26).Yang C; DelRio FW; Ma H; Killaars AR; Basta LP; Kyburz KA; Anseth KS Spatially Patterned Matrix Elasticity Directs Stem Cell Fate. Proc. Natl. Acad. Sci U.S.A 2016, 113, E4439–E4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Marklein RA; Burdick JA Spatially Controlled Hydrogel Mechanics to Modulate Stem Cell Interactions. Soft Matter 2010, 6, 136–143. [Google Scholar]
- (28).Verdier C; Etienne J; Duperray A; Preziosi L Review: Rheological Properties of Biological Materials. C. R. Phys 2009, 10, 790–811. [Google Scholar]
- (29).Rodell CB; Dusaj NN; Highley CB; Burdick JA Injectable and Cytocompatible Tough Double-Network Hydrogels through Tandem Supramolecular and Covalent Crosslinking. Adv. Mater 2016, 28, 8419–8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).McKinnon DD; Domaille DW; Cha JN; Anseth KS Biophysically Defined and Cytocompatible Covalently Adaptable Networks as Viscoelastic 3d Cell Culture Systems. Adv. Mater 2014, 26, 865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Gong Z; Szczesny SE; Caliari SR; Charrier EE; Chaudhuri O; Cao X; Lin Y; Mauck RL; Janmey PA; Burdick JA; Shenoy VB Matching Material and Cellular Timescales Maximizes Cell Spreading on Viscoelastic Substrates. Proc. Natl. Acad. Sci. U.S.A 2018, 115, E2686–E2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Tang S; Ma H; Tu H-C; Wang H-R; Lin P-C; Anseth KS Adaptable Fast Relaxing Boronate-Based Hydrogels for Probing Cell–Matrix Interactions. Adv. Sci 2018, 5, 1800638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Gramlich WM; Kim IL; Burdick JA Synthesis and Orthogonal Photopatterning of Hyaluronic Acid Hydrogels with Thiol-Norbornene Chemistry. Biomaterials 2013, 34, 9803–9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Rodell CB; Kaminski AL; Burdick JA Rational Design of Network Properties in Guest-Host Assembled and Shear-Thinning Hyaluronic Acid Hydrogels. Biomacromolecules 2013, 14, 4125–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Xu L; Hui AY; Albanis E; Arthur MJ; Blaner WS; Mukherjee P; Friedman SL; Eng FJ Human Hepatic Stellate Cell Lines, LX-1 and LX-2: New Tools for Analysis of Hepatic Fibrosis. Gut 2005, 54, 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Han B; Chery DR; Yin J; Lu XL; Lee D; Han L Nanomechanics of Layer-by-Layer Polyelectrolyte Complexes: A Manifestation of Ionic Cross-Links and Fixed Charges. Soft Matter 2016, 12, 1158–1169. [DOI] [PubMed] [Google Scholar]
- (37).Li Q; Qu F; Han B; Wang C; Li H; Mauck RL; Han L Micromechanical Anisotropy and Heterogeneity of the Meniscus Extracellular Matrix. Acta Biomater. 2017, 54, 356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Rodell CB; MacArthur JW; Dorsey SM; Wade RJ; Wang LL; Woo YJ; Burdick JA Shear-Thinning Supramolecular Hydrogels with Secondary Autonomous Covalent Crosslinking to Modulate Viscoelastic Properties In Vivo. Adv. Funct. Mater 2015, 25, 636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Hörning M; Nakahata M; Linke P; Yamamoto A; Veschgini M; Kaufmann S; Takashima Y; Harada A; Tanaka M Dynamic Mechano-Regulation of Myoblast Cells on Supramolecular Hydrogels Cross-Linked by Reversible Host-Guest Interactions. Sci. Rep 2017, 7, 7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Perepelyuk M; Chin L; Cao X; Van Oosten A; Shenoy VB; Janmey PA; Wells RG Normal and Fibrotic Rat Livers Demonstrate Shear Strain Softening and Compression Stiffening: A Model for Soft Tissue Mechanics. PLoS One 2016, 11, e0146588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Balestrini JL; Chaudhry S; Sarrazy V; Koehler A; Hinz B The Mechanical Memory of Lung Myofibroblasts. Integr. Biol 2012, 4, 410. [DOI] [PubMed] [Google Scholar]
- (42).Caliari SR; Perepelyuk M; Cosgrove BD; Tsai SJ; Lee GY; Mauck RL; Wells RG; Burdick JA Stiffening Hydrogels for Investigating the Dynamics of Hepatic Stellate Cell Mechanotransduction during Myofibroblast Activation. Sci. Rep 2016, 6, 21387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Parmacek MS Myocardin-Related Transcription Factors. Circ. Res 2007, 100, 633–644. [DOI] [PubMed] [Google Scholar]
- (44).Zhao X-H; Laschinger C; Arora P; Szaszi K; Kapus A; McCulloch CA Force Activates Smooth Muscle -Actin Promoter Activity through the Rho Signaling Pathway. J. Cell Sci 2007, 120, 1801–1809. [DOI] [PubMed] [Google Scholar]
- (45).O’Connor JW; Gomez EW Cell Adhesion and Shape Regulate TGF-Beta1-Induced Epithelial-Myofibroblast Transition via MRTF-A Signaling. PLoS One 2013, 8, e83188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Hinson JS; Medlin MD; Lockman K; Taylor JM; Mack CP Smooth Muscle Cell-Specific Transcription Is Regulated by Nuclear Localization of the Myocardin-Related Transcription Factors. Am. J. Physiol. Gastrointest. Liver Physiol 2006, 292, H1170–H1180. [DOI] [PubMed] [Google Scholar]
- (47).Gupta M; Korol A; West-Mays JA Nuclear Translocation of Myocardin-Related Transcription Factor-A during Transforming Growth Factor Beta-Induced Epithelial to Mesenchymal Transition of Lens Epithelial Cells. Mol. Vis 2013, 19, 1017–1028. [PMC free article] [PubMed] [Google Scholar]
- (48).Marjoram RJ; Lessey EC; Burridge K Regulation of RhoA Activity by Adhesion Molecules and Mechanotransduction. Curr. Mol. Med 2014, 14, 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Goffin JM; Pittet P; Csucs G; Lussi JW; Meister J-J; Hinz B Focal Adhesion Size Controls Tension-Dependent Recruitment of _α_-Smooth Muscle Actin to Stress Fibers. J. Cell Biol 2006, 172, 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information