Association of Routine Infant Vaccinations With Antibody Levels Among Preterm Infants (original) (raw)
Key Points
Question
What is the association between receipt of a routine schedule of vaccinations and subsequent antibody levels among preterm infants over the first year of life?
Findings
In this prospective observational study that included 296 preterm infants and 66 historical control term-born infants, administration of a routine schedule of vaccinations was associated with 95% or more of preterm infants across all gestational ages achieving protective IgG levels for most vaccine antigens on completion of the primary series and the booster dose. However, after the primary series and booster, 40.6% and 88.1% of preterm infants, respectively, achieved protective levels against Haemophilus influenzae type b. In general, postimmunization antibody levels were significantly lower in preterm infants than in the historical control group, with the lowest values in extremely premature infants.
Meaning
Among preterm infants, administration of a routine schedule of vaccinations during the first year was associated with protective antibody levels against most antigens, except for Haemophilus influenzae type b.
Abstract
Importance
The standard schedule of national immunization programs for infants may not be sufficient to protect extremely and very preterm infants.
Objective
To evaluate the immunogenicity of routine vaccinations administered to preterm infants.
Design, Setting, and Participants
A multicenter, prospective, observational cohort study of preterm infants stratified according to gestational age recruited from 8 hospitals across the Netherlands between October 2015 and October 2017, with follow-up until 12 months of age (October 2018). In total, 296 premature infants were enrolled and compared with a control group of 66 healthy term infants from a 2011 study, immunized according to the same schedule with the same vaccines.
Exposures
Three primary doses of the diphtheria–tetanus toxoids–acellular pertussis–inactivated poliomyelitis–Haemophilus influenza type b–hepatitis B combination vaccine were given at 2, 3, and 4 months after birth followed by a booster at 11 months and a 10-valent pneumococcal conjugate vaccine at 2, 4, and 11 months after birth.
Main Outcomes and Measures
Primary end points were (1) proportion of preterm infants who achieved IgG antibody against vaccine antigens at concentrations above the internationally defined threshold for protection after the primary series and booster dose and (2) serum IgG geometric mean concentrations after the primary series and booster vaccination. Proportions and geometric mean concentrations were compared in preterm infants and the control group of term infants.
Results
Of 296 preterm infants (56.1% male; mean gestational age, 30 weeks), complete samples before vaccination, 1 month after the primary series, and 1 month after the booster were obtained from 220 preterm infants (74.3%). After the primary series, the proportion of preterm infants across all gestational age groups who achieved protective IgG antibody levels against pertussis toxin, diphtheria, tetanus and 6 of 10 pneumococcal serotypes varied between 83.0% and 100%, Haemophilus influenzae type b between 34.7% and 46.2% (40.6% among all preterm infants overall), and pneumococcal serotypes 4, 6B, 18C, and 23F between 45.8% and 75.1%. After the booster dose, protective antibody levels were achieved in more than 95% of all preterm groups, except for Haemophilus influenzae type b (88.1%). In general, geometric mean concentrations of all vaccine-induced antibodies were significantly lower in all preterm infants vs term infants, except for pertussis toxin and pneumococcal serotypes 4 and 19F after the primary series and booster vaccination.
Conclusions and Relevance
Among preterm infants, administration of routine vaccinations during the first year of life was associated with protective antibody levels against most antigens in the majority of infants after the primary series and booster, except for Haemophilus influenzae type b. However, antibody concentrations were generally lower among preterm infants compared with historical controls.
This cohort study evaluates IgG antibody concentrations in preterm infants after administration of combination diphtheria–tetanus toxoids–acellular pertussis–inactivated poliomyelitis–Haemophilus influenza type b–and hepatitis B (DTaP-IPV-Hib-HepB) and pneumococcal conjugate vaccines.
Introduction
National immunization programs for infants aim to protect individuals and the population at large against vaccine-preventable target diseases. Whereas in some countries primary vaccination schedules are adjusted in case of prematurity, most countries, including the Netherlands and the US, apply the same primary schedule and timing for all newborn infants.1,2 This universal dose and time schedule is usually based on clinical studies in healthy term-born infants, with limited data on preterm infants. However, the number of preterm infants (<36 weeks’ gestational age), and in particular extremely preterm infants (<28 weeks’ gestational age), is increasing. In the Netherlands in 2018, 8.5% of births, approximately 15 500 infants, were born preterm, with 0.7% born extremely preterm.3
Preterm infants have increased susceptibility to infections due to decreased transfer of protective maternal antibodies4 and possibly due to relative immaturity of the immune system. The limited available data on vaccine responses in preterm and especially extremely preterm infants indicate that antibody responses against protein vaccine antigens are generally adequate,5,6,7,8 but immune responses to polysaccharide antigens of Streptococcus pneumoniae and Haemophilus influenzae type b may be reduced.9,10,11 However, the heterogeneity between studies is substantial and the studied population often consists of preterm infants with a gestational age between 32 and 36 weeks. Especially for infants born before gestational age of 28 weeks, there is a paucity of data on vaccine responses.
A prospective, multicenter, cohort study was conducted to determine the immunogenicity of the Dutch routine national immunization program in the first year of life in preterm infants in 3 gestational age groups: less than 28, 28 to less than 32 weeks, and 32 to 36 weeks. Vaccine antigen responses also were compared with a historical control cohort of term infants.
Methods
Study Design and Participants
Written informed consent was obtained from either parents or guardians of all infants before enrollment. Ethical approval was obtained from the Medical Ethical Committee of the University Medical Centre Utrecht.
This prospective, observational, multicenter, cohort study evaluated the immunological responses to routine national immunizations of preterm infants in the first year of life. Preterm infants eligible for the study (gestational age ≤36 weeks) were enrolled between October 2015 and November 2017 in 8 Dutch hospitals, including 5 centers hosting neonatal intensive care units and 3 general hospitals with a neonatal high care unit, where prematurely born infants receive additional monitoring because of clinical problems although not intensive care. Enrollment was stratified based on gestational age into 3 groups: less than 28 weeks, 28 to less than 32 weeks, and 32 to 36 weeks. Follow-up was until age 12 months and the end date of follow-up was through October 2018. A historical cohort of 66 term-born infants from a 2011 pneumococcal conjugate vaccine intervention study was used as reference control group.12,13
Infants received vaccinations as per normal practice. At the time of the study, a combination vaccine for diphtheria–tetanus toxoids–acellular pertussis–inactivated poliomyelitis–Haemophilus influenza type b–and hepatitis B (DTaP-IPV-Hib-HepB; Infanrix-hexa [GlaxoSmithKline]) was administered at 6 to 9 weeks, 3 months, 4 months, and 11 months of age according to the Dutch national immunization program. Pneumococcal conjugate vaccine (PCV10; Synflorix [GlaxoSmithKline]) was administered at 6 to 9 weeks, 4 months, and 11 months of age.1 Infants from the control group were vaccinated with the same vaccines according to the same vaccination schedule.12
Data Collection and Serological Analysis
Preterm infants hospitalized at the neonatal ward were recruited before the start of immunizations by research nurses and/or hospital staff. Parents were asked to fill in a questionnaire, including information on pregnancy, delivery, and duration of hospitalization. Capillary blood samples (heel-prick) were collected before the primary vaccinations (300 μL at approximately 6 weeks after birth) and 1 month after the last dose of the primary series (500 μL at approximately 5 months) during home visits. Venous blood samples up to 8 mL were obtained 1 month after the booster vaccination (at approximately 12 months). In the control group, blood samples also were collected at 5 and 12 months after birth.13
IgG antibody concentrations against pertussis antigens (pertussis toxin, filamentous hemagglutinin, and pertactin), diphtheria toxoid, tetanus toxin, the capsular polysaccharide polyribosylribitol phosphate of Haemophilus influenzae type b, and the PCV10 pneumococcal polysaccharides were measured with a fluorescent bead-based multiplex immunoassay as previously described.14,15,16 The lower limits of quantification (LLOQ) were 0.85 IU/mL for pertussis toxin, 0.41 IU/mL for filamentous hemagglutinin, 0.21 IU/mL for pertactin, 0.001 IU/mL for diphtheria toxoid, 0.001 IU/mL for tetanus toxin, 0.01 μg/mL for Haemophilus influenzae type b, and 0.01 μg/mL for the pneumococcal serotypes. IgG antibody concentrations below the LLOQ were set at 0.5 LLOQ for the 3 pertussis antigens. In the absence of an established protective level of antibodies for pertussis toxin, we used the arbitrary threshold of 20 IU/mL or greater.17 The antibody thresholds for protection for diphtheria toxoid and tetanus toxin are 0.01 IU/mL or greater for basic immunity and 0.10 IU/mL or greater for protection.18,19 For Haemophilus influenzae type b, the protective threshold is 0.15 μg/mL or greater.20 For all pneumococcal serotypes, an IgG level of 0.35 μg/mL or greater was considered protective.21 Antibody levels of the samples from the control group were determined in the same laboratory with the same methodology, and similar results, with a correlation varying between R = 0.91 and 0.97, were obtained in a retest of these samples in 2020, underlining the reproducibility of these assays.
Outcomes
Primary outcomes were the proportion of infants with protective IgG antibody concentrations against pertussis toxin, diphtheria toxoid, tetanus toxin, Haemophilus influenzae type b, and 10 pneumococcal serotypes and serum IgG geometric mean concentrations both 1 month after the primary series and the booster vaccination. Secondary outcomes included prevaccination IgG levels, which are maternally derived. In addition, the geometric mean concentrations of the absolute differences between postprimary series and prevaccination antibody levels were compared between the preterm groups and between preterm infants and the term control group.
Statistical Analysis
Sample size calculations estimated that a minimum of 60 infants per gestational age group would allow detection of a difference of at least 15% with 100% protection in the control group by vaccine component according to internationally defined thresholds, and a 17% difference with 99% protection in the control group, with 90% power and α = .05,22 using the method of Fleiss, Tytun, and Ury to estimate the sample size to achieve a given power of a 2-sided test for the difference in 2 proportions. All analyses were performed including all available samples, which ranged between 82% and 92% per time point in the 3 gestational age groups. No differences were found if only infants with samples at all 3 time points were included, and these results are not presented.
Proportions of infants with protective vaccine antibody concentrations at each sampling point were calculated. IgG antibody concentrations were log-transformed to calculate geometric mean concentrations and 95% CIs. Log-transformed data followed a normal distribution. Differences in proportions achieving the protective antibody level for each antigen were compared between all preterm vs term control infants and in case of significant differences further evaluated for differences between gestational age groups with χ2 test. Vaccine responses for each antigen at all time points were compared between all preterm vs term infants and between the 3 gestational age groups of preterm infants with independent t test. Absolute differences between postprimary series and prevaccination antibody levels were assessed with independent t test for each antigen between the preterm gestational age groups on log-transformed data. All statistical analyses were performed by comparing 2 groups each time; therefore, no correction for multiple comparison was made. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary outcomes should be interpreted as exploratory. Blood samples collected within 10 days after the last vaccination were excluded from all analyses. Two-sided P values less than .05 were considered significant. Statistical analysis was done using SPSS version 24 (IBM) and GraphPad Prism version 8.0.1.
Results
In total, 296 infants were enrolled (gestational age group <28 weeks: n = 87; ≥28-<32 weeks: n = 119; ≥32-≤36 weeks: n = 90) (Figure 1). The overall mean gestational age was 30.0 weeks (range, 24.0-36.0) with corresponding mean birth weight of 1415 g (range, 465-3355) (Table). The proportion of infants who were male was higher in the gestational age group less than 28 weeks compared with the 2 other groups (Table). Differences in the timing of the first vaccination were found between preterm groups, with a delayed first vaccination in the gestational age group less than 28 weeks with a mean age of 69.2 days relative to 59.4 days for the gestational age group 28 to less than 32 weeks and 61.0 days for the gestational age group 32 to 36 weeks.23 A difference in the time window between the booster vaccination and the postbooster blood sample was observed between gestational age groups 28 to less than 32 and 32 to 36 weeks. From the 296 enrolled preterm infants, 264 (89.2%) completed the prevaccination sampling and 220 preterm infants (74.3%; Figure 1) completed blood sampling at all 3 time points.
Figure 1. Study Flowchart.
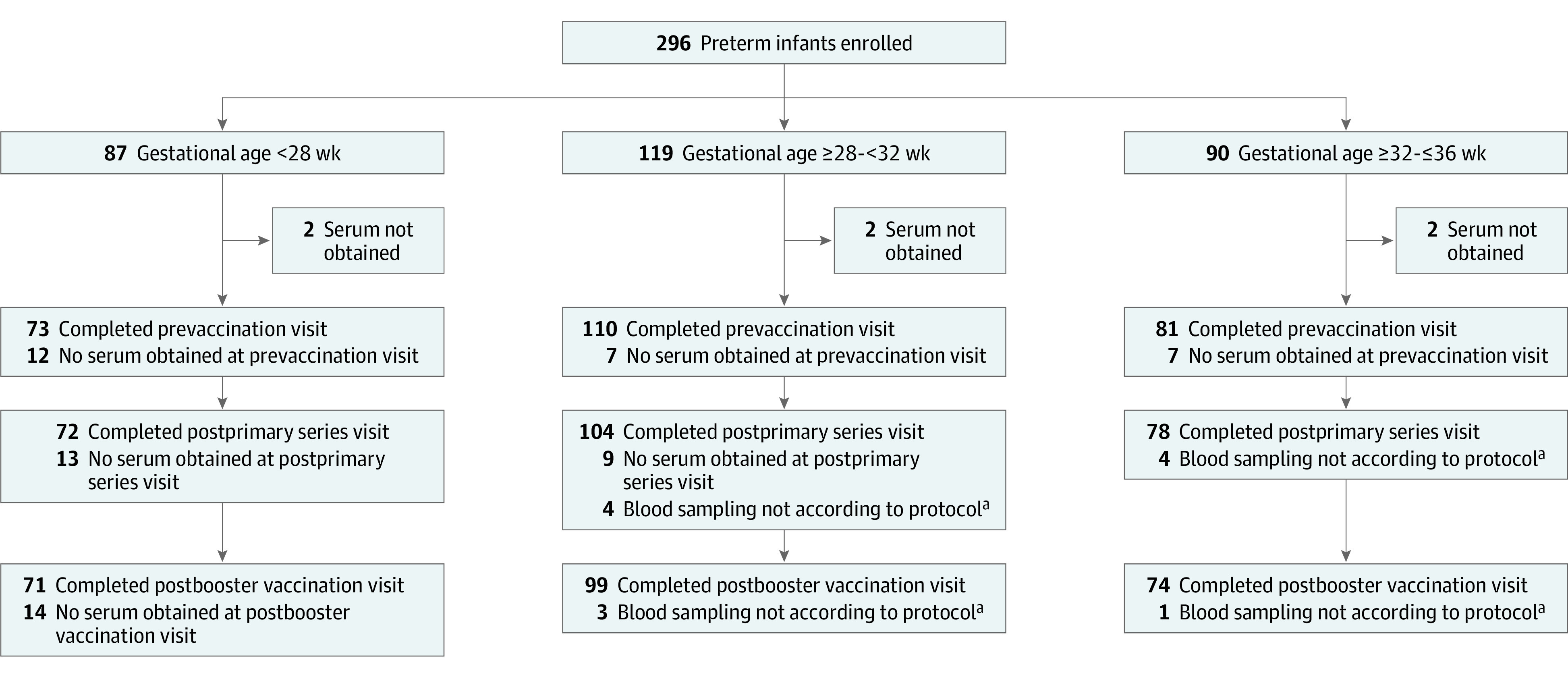
The prevaccination period was 42 to 56 days after birth; the postprimary series period, 28 to 56 days after the primary series; and postbooster vaccination, 28 to 56 days after the booster vaccination.
aNot according to protocol indicates sample was drawn less than 10 days after final vaccinations.
Table. Baseline Characteristicsa.
| Characteristic | Gestational age, wk | ||||
|---|---|---|---|---|---|
| <28 (n = 87) | ≥28-<32 (n = 119) | ≥32-≤36 (n = 90) | All preterm (N = 296) | Term (n = 66) | |
| Sex, No. (%) | |||||
| Male | 59 (68) | 62 (68) | 45 (50) | 166 (56.1) | 33 (50) |
| Female | 28 (32) | 57 (48) | 45 (50) | 130 (43.9) | 33 (50) |
| Gestational age, mean (range), wk | 26.3 (24.0-27.6) | 30.0 (28.0-31.6) | 33.6 (32.0-36.0) | 30.0 (3.1) | 39.8 (1.3) |
| Birth weight, mean (range), g | 914 (465-1300) | 1313 (530-2142) | 2034 (1160-3355) | 1415 (465-3355) | 3636 (471) |
| Age at, mean (SD), d | |||||
| Prevaccination sample | 53.7 (11.2) | 49.7 (15.2) | 44.9 (10.7) | 49.3 (13.3) | NA |
| First vaccination | 69.2 (14.3) | 59.4 (10.2) | 61.0 (8.3) | 62.5 (11.9) | 62 (3.0) |
| Postprimary sample | 179.1 (22.5) | 170.1 (19.1) | 169.5 (19.9) | 172.5 (20.7) | 164 (9.3) |
| Postbooster sample | 397.6 (27.9) | 383.2 (19.8) | 387.3 (19.1) | 388.6 (23.0) | 378 (9.3) |
| Window between, mean (SD), d | |||||
| Primary dose 3 and postprimary sample | 40.7 (12.4) | 43.5 (17.3) | 40.9 (21.3) | 41.9 (17.3) | 29 (6.0) |
| Booster dose and postbooster sample | 38.3 (14.7) | 37.9 (13.8) | 43.3 (20.0) | 39.7 (16.3) | 30 (6.0) |
Prevaccination Antibody Levels
Across the 3 preterm groups, mean IgG levels for the different vaccine antigens prior to vaccination were low, varying between 1.5% and 54.9% with IgG levels below the internationally defined threshold for protection (Figure 2, Figure 3, and Figure 4). Only for tetanus toxin, IgG levels above the protective level (>0.01 IU/mL) were observed in 99.2% of infants.
Figure 2. IgG Antibody Concentrations and Proportion of Infants With Protective Antibody Levels per Gestational Age Group by Study Time Points .
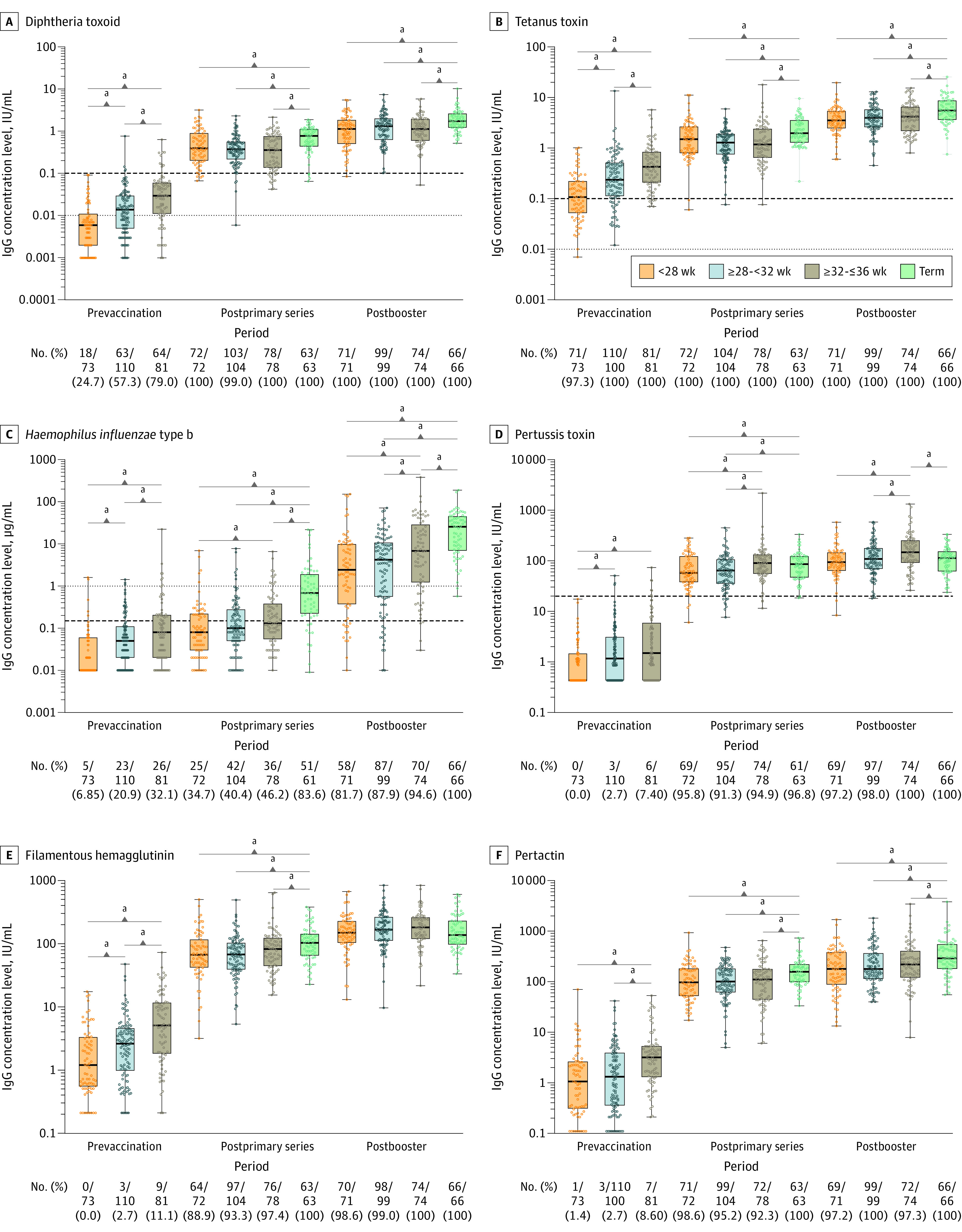
The dots represent individual children’s values. The box plots represent the 25th, 50th, and 75th percentiles. The horizontal bars in the box plots represent the geometric mean concentrations. In panels A and B, the thin dotted lines indicate the antibody threshold for basic immunity and the thick dashed lines indicate the antibody threshold for protection. In panel C, the thick dashed line indicates basic immunity and the thin dotted line indicates long-term protection. In panel D, the dashed line indicates the threshold for protection.
a_P_ < .05.
Figure 3. IgG Antibody Concentrations and Proportion of Infants With Protective Antibody Levels per Gestational Age Group for Pneumococcal Serotypes 1, 4, 5, 6B, 7F, and 9V at the Different Study Time Points .
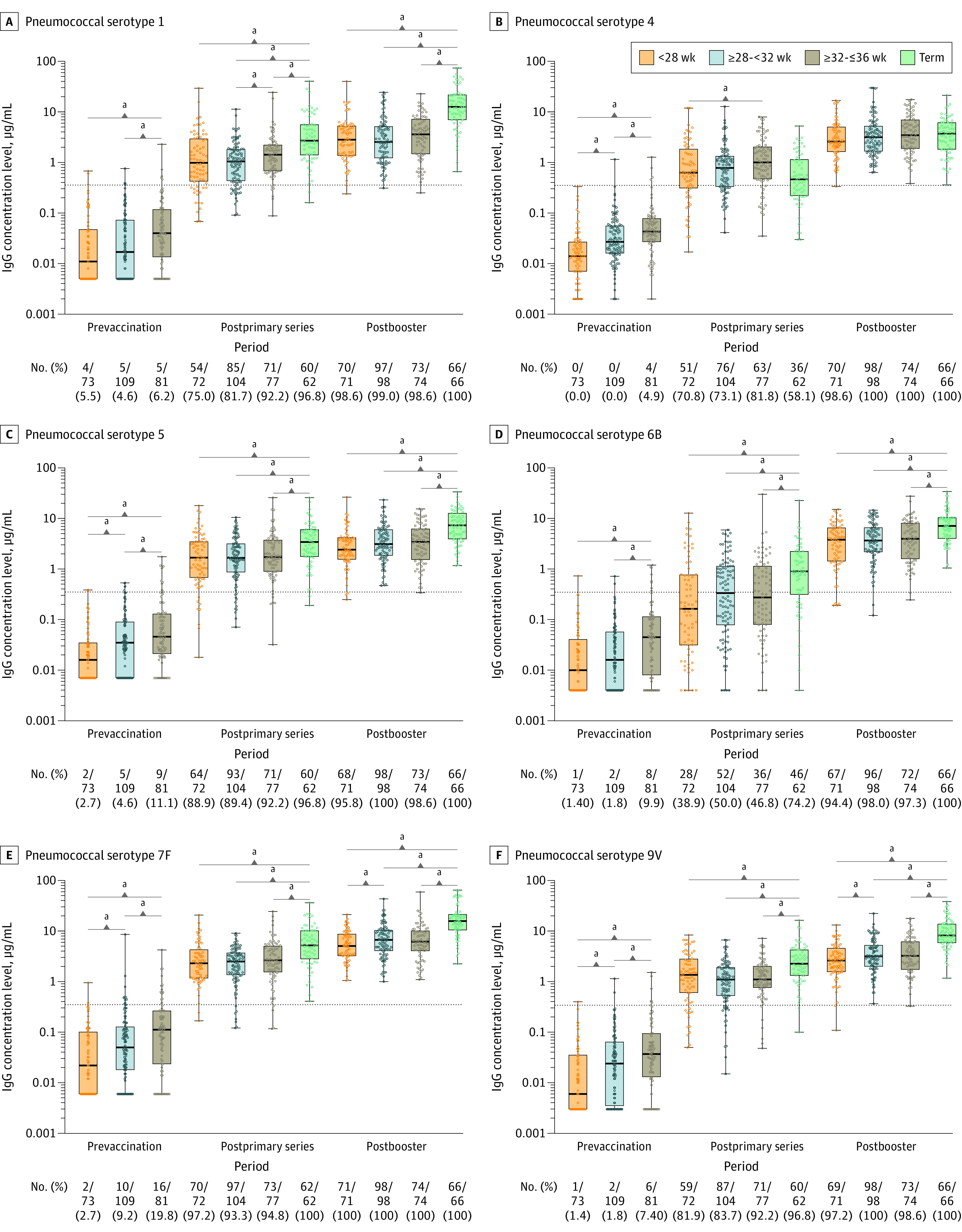
The dots represent individual children’s values. The box plots represent the 25th, 50, and 75th percentiles. The horizontal bars in the box plots represent the geometric mean concentrations. The dotted lines indicate the antibody thresholds for protection.
a_P_ < .05.
Figure 4. IgG Antibody Concentrations and Proportion of Infants With Protective Antibody Levels per Gestational Age Group for Pneumococcal Serotypes 14, 18C, 19F, and 23F at the Different Study Time Points .
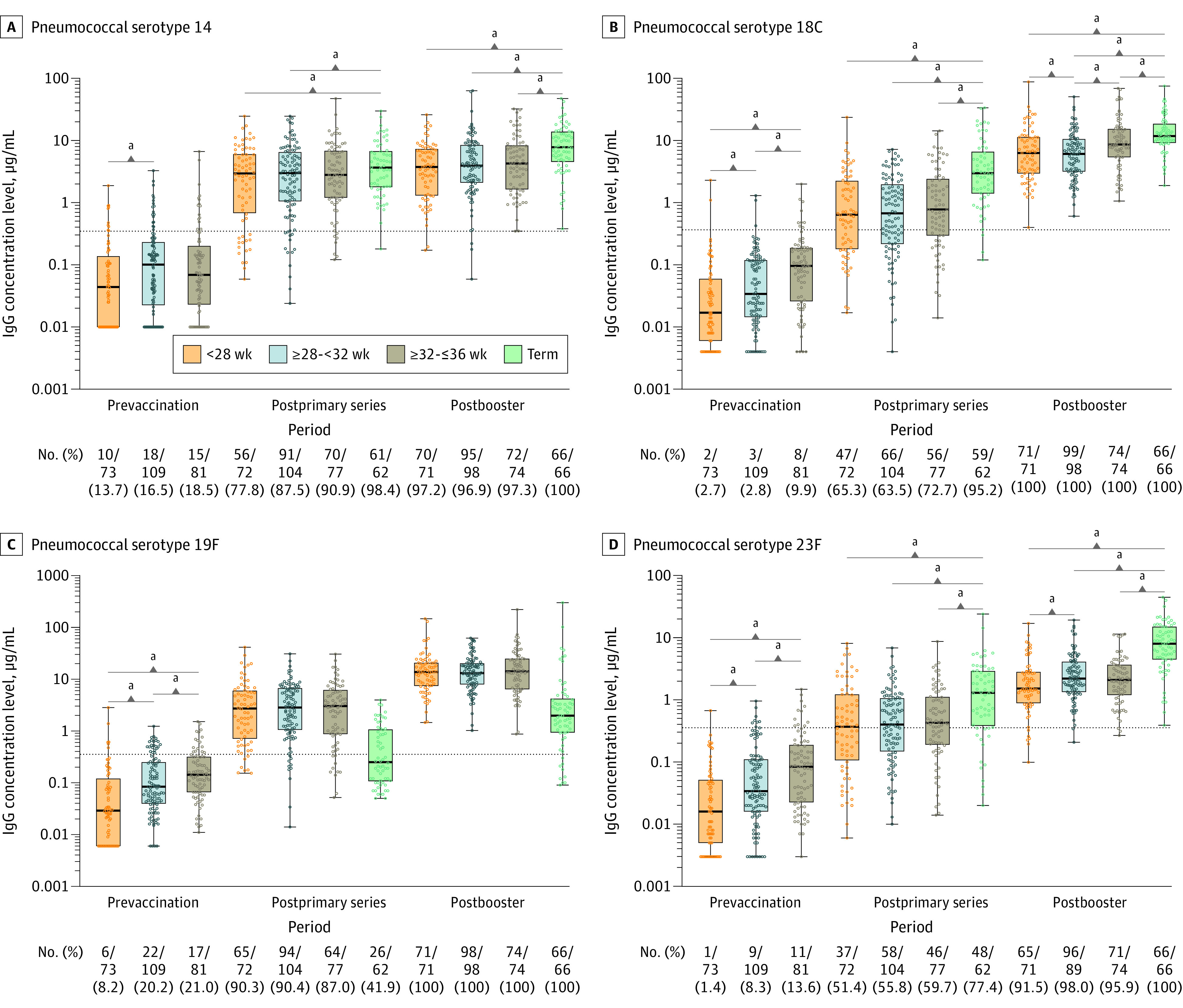
The dots represent individual children’s values. The box plots represent the 25th, 50, and 75th percentiles. The horizontal bars in the box plots represent the geometric mean concentrations. The dotted lines indicate the antibody thresholds for protection.
a_P_ < .05.
For all vaccine antigens, the prevaccination geometric mean concentrations were lowest in the group with gestational age less than 28 weeks and significantly lower for almost all antigens compared with the other 2 groups. Only for the pertussis antigen pertactin and pneumococcal serotypes 1 and 6B was there no significant difference between gestational age less than 28 weeks and 28 to less than 32 weeks. Additional significant differences in geometric mean concentrations were observed between gestational age groups 28 to less than 32 weeks and 32 to 36 weeks for all antigens except pertussis toxin.
Immunogenicity After the Primary Series
After the primary series, protective antibody levels against pertussis toxin, diphtheria toxoid, and tetanus toxin were present in 93.7% to 100% of all preterm infants (Figure 2; eTable 4 in the Supplement). The proportion of protective antibody levels against diphtheria toxoid and tetanus toxin was greater than 99% across all preterm groups and did not significantly differ from the term control group. For Haemophilus influenzae type b, the proportion of all preterm infants with protective antibody levels was significantly lower (40.6%) compared with the term control group (83.6%) and levels were lowest in the group aged less than 28 weeks (34.7%) (Figure 2; eTable 4 in the Supplement). For pneumococcal serotypes, proportions of protective levels in all preterm infants varied substantially per serotype after the primary series. All preterm infants achieved high proportions of protective antibody levels, ranging from 83.0% to 94.9%, against pneumococcal vaccine serotypes 1, 5, 7F, 9V, 14, and 19F. However, for pneumococcal serotypes 4, 6B, 18C, and 23F, proportions were 75.1%, 45.8%, 66.8%, and 55.7% in all preterm groups together, respectively, with the lowest proportion in the gestational age group less than 28 weeks, except for serotype 18C. Compared with the term control group, the proportion of preterm infants protected against PCV10 serotypes after the primary series was significantly lower, except for pneumococcal serotypes 4 and 19F (Figure 3 and Figure 4; eTable 5 in the Supplement).
Overall, there were no significant differences in geometric mean concentrations between the 3 preterm groups after the primary series for most vaccine antigens. For pertussis toxin, Haemophilus influenzae type b, and pneumococcal serotype 4, geometric mean concentrations differed significantly between the group aged less than 28 weeks and the group aged 32 to 36 weeks. For pertussis toxin and pneumococcal serotype 1, geometric mean concentrations significantly differed between the group aged 28 to less than 32 weeks and the group aged 32 to 36 weeks (eTable 1 in the Supplement). For all vaccine antigens, geometric mean concentrations were significantly lower in all preterm infants compared with term-born control infants except for pertussis toxin and pneumococcal serotypes 4 and 19F (eTable 2 in the Supplement; Figure 2, Figure 3, and Figure 4).
eTable 3 in the Supplement describes the absolute antibody response after the primary series per preterm gestational age group. Only for diphtheria toxoid, tetanus toxin, and pneumococcal serotypes 7F and 9V, a significant difference between gestational age group less than 28 weeks and 28 to less than 32 weeks was observed, with the higher absolute response in the youngest gestational age group.
Immunogenicity After the Booster Vaccination
Following the booster immunization, 99% to 100% of all preterm infants achieved protective antibody levels for all antigens, except for Haemophilus influenzae type b. For Haemophilus influenzae type b, protective antibody levels were achieved in 88.1% of preterm infants overall; 81.7% in the preterm group aged less than 28 weeks and 87.9% in the group aged 28 to less than 32 weeks achieved protective antibody levels, which was significantly lower than the group aged 32 to 36 weeks and term infants (Figure 2; eTable 4 in the Supplement). Although most preterm infants achieved the protective antibody threshold after the booster vaccination, significant differences in geometric mean concentrations persisted, with lower levels for pertussis toxin; Haemophilus influenzae type b; and pneumococcal serotypes 7F, 9V, 18C, and 23F in the gestational age group less than 28 weeks. Other vaccine antigens did not significantly differ between preterm groups (eTable 1 in the Supplement). Compared with term control infants, geometric mean concentrations in preterm infants remained significantly lower after the booster vaccination, in particular for Haemophilus influenzae type b (eTable 2 in the Supplement; Figure 2). The pertussis antigens pertussis toxin and filamentous hemagglutinin and pneumococcal serotypes 4 and 19F did not significantly differ between preterm and term-born controls.
Discussion
In this study of 296 preterm infants, with 74.3% completing all samples at all 3 time points, most preterm infants—including infants born before 28 weeks’ gestational age—achieved protective IgG levels against most antigens following 3 primary doses of DTaP-IPV-Hib-HepB vaccinations at 2, 3, and 4 months. However, the protective IgG threshold for Haemophilus influenzae b was achieved in only 40.6% of preterm infants and for pneumococcal vaccine serotypes 4, 6B, 18C, and 23F in 45.8% to 75.1% of infants, which is significantly lower than in term infants. Preterm infants born before 28 weeks’ gestational age had the highest risk for lack of protective antibody levels after polysaccharide conjugate vaccinations.
Between the 3 preterm groups, geometric mean concentrations were not significantly different except for levels against pertussis toxin, Haemophilus influenzae type b, and pneumococcal serotypes 1 and 4. After the booster vaccination, more than 95% of all preterm infants achieved protective IgG levels for all vaccine antigens, except for Haemophilus influenzae type b, with 18.3% and 12.1% of preterm infants in the groups less than 28 weeks and 28 to less than 32 weeks, respectively, without protective IgG antibody levels. Overall, both after the primary series and the booster vaccination, geometric antibody concentration levels in preterm infants remained lower than in term infants for all vaccine antigens, except pertussis toxin and pneumococcal serotypes 4 and 19F. The high proportion of unprotected preterm infants against Haemophilus influenzae type b until the booster dose may have clinical implications if herd protection is low and should be considered when advising on national immunization schedules for preterm infants. An additional booster vaccination after the primary series might be needed, but further research to evaluate this is necessary.
Other studies reported similar proportions of protective antibody levels after the primary series and booster vaccination in preterm infants for the protein vaccine antigens diphtheria toxoid and tetanus toxin, and pertussis antigens filamentous hemagglutinin and pertactin.5,6,7,8 However, in line with this study, significantly lower geometric mean concentrations for these antigens were observed after the primary series in preterm infants compared with term infants. On the other hand, no difference between term and preterm infants for pertussis toxin was observed after the primary series in this study, while others found differences for this antigen.6,8 For Haemophilus influenzae type b antibody levels after the primary series, results of earlier studies were more diverse. The low proportion of protective antibody levels and the significantly lower geometric mean concentrations for Haemophilus influenzae type b compared with term infants in this study are in line with the results from Slack et al9 and Heath et al.10 In contrast, Omañaca et al7 found high proportions of Haemophilus influenzae type b protective antibody levels after the primary series.
With respect to the conjugated pneumococcal polysaccharide vaccine, other studies also described considerable variation in proportions of protective antibody levels between serotypes after the primary series, both in term and preterm infants, in line with this study.11,24 After the booster dose in the other studies, all preterm and term infants achieved antibody levels above the internationally defined threshold for protection for all serotypes without significant differences, again in line with this study, although significantly lower IgG responses were found in preterm infants than in term infants both after the primary series and the booster vaccination.11,25
At birth, the immune system of neonates is adapted to pregnancy status and rapid exposure to many stimuli early in life, although the immune system of preterm infants differs from term infants.26 Preterm infants have fewer absolute numbers of lymphocytes, T cells, B cells, and T-helper cells in particular at the age at first immunizations (6-9 weeks in this study). However, a recent study described that the immune system of preterm infants rapidly converges and adapts after birth and follows a stereotypic pattern early in life.27 In addition, perinatal conditions and postnatal exposures influence the adaptive changes in the immune system of premature infants.27 The age of the infants when receiving their first vaccinations is important for antibody responses, with older infants responding with higher antibody levels.28
The start of vaccinations is often delayed in preterm infants, as in this study, with the longest delay in extremely premature infants, which might partly explain the good responses in this group.28 However, delaying first immunizations in preterm infants also prolongs the window of susceptibility to vaccine-preventable diseases, with waning protection by maternally derived antibodies.4 On the other hand, the low levels of maternal antibodies may contribute to the adequate responses to primary vaccinations in preterm infants because higher levels may blunt antibody responses upon vaccination.28,29 Except for tetanus toxin, the prevaccination IgG antibody levels in this study in all preterm infants were low, which implies that maternal antibodies were unlikely to affect the vaccine response in any preterm group. Quick adaption of the immune system after birth in preterm infants, including extremely premature infants, is the most likely explanation for the adequate antibody responses upon vaccinations.
One of the strengths of this study is that the cohort size and stratified enrollment by gestational age group resulted in at least 87 infants per group, including the extremely preterm group. For extremely preterm infants, good data on vaccine responses have been lacking due to, among other reasons, difficult enrollment of this group. This study was adequately powered to evaluate vaccine responses in preterm infants by gestational age and is, to our knowledge, the most comprehensive analysis of vaccine immunity in preterm infants to date. The availability of a historical control group of term infants who received the same vaccines in a similar schedule for comparison is another strength of this study. As current clinical practice of immunizing preterm infants was followed, results should be generalizable to preterm infants in similar communities.
Limitations
This study has several limitations. First, the control group of term infants dated from 5 years earlier, although the same vaccines and schedule were applied and analyses were performed in the same laboratory with the same procedures. Second, the timing of blood sampling varied between infants, although this variation was unlikely to affect the vaccine-induced antibody levels. Third, findings might not be generalizable to countries with different vaccines and vaccination schedules for preterm infants in the first year of life.
Conclusions
Among preterm infants, administration of routine vaccinations during the first year of life was associated with protective antibody levels against most antigens in the majority of infants after the primary series and booster, except for Haemophilus influenzae type b. However, antibody concentrations were generally lower among preterm infants compared with historical controls.
Supplement.
eTable 1. IgG Geometric Mean Concentrations With 95% CI Against the Vaccine Antigens for the Preterm Infants
eTable 2. IgG Geometric Mean Concentrations With 95% Confidence Intervals Against the Vaccine Antigens for all GA Groups Together and Term Infants
eTable 3. Mean of the Absolute Response (Difference Between T2-T1) for the Different Vaccine Components for all GA Groups Together and for GA Groups Separately
eTable 4. Proportion of Infants With Protective Antibody Levels per Antigen per GA Group
eTable 5. Proportion of Infants With Protective Antibody Levels per Antigen per GA Group
References
- 1.Rijksinstituut voor Volksgezondheid en Milieu RVP-richtlijn uitvoering. Accessed June 28, 2019. https://rijksvaccinatieprogramma.nl/professionals/richtlijnen/rvp-richtlijn-uitvoering-2019
- 2.European Center for Disease Prevention and Control Vaccine scheduler: Germany: recommended vaccinations. Accessed June 28, 2019. https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByCountry?SelectedCountryId=6&IncludeChildAgeGroup=true&IncludeChildAgeGroup=false&IncludeAdultAgeGroup=false
- 3.Volksgezondheid.info Vroeggeboortes en laag geboortegewicht. Accessed May 1, 2019. https://www.volksgezondheidenzorg.info/onderwerp/vroeggeboorte-en-laag-geboortegewicht/cijfers-context/trends
- 4.van den Berg JP, Westerbeek EA, Berbers GA, van Gageldonk PG, van der Klis FR, van Elburg RM. Transplacental transport of IgG antibodies specific for pertussis, diphtheria, tetanus, haemophilus influenzae type b, and Neisseria meningitidis serogroup C is lower in preterm compared with term infants. Pediatr Infect Dis J. 2010;29(9):801-805. doi: 10.1097/INF.0b013e3181dc4f77 [DOI] [PubMed] [Google Scholar]
- 5.D’Angio CT, Maniscalco WM, Pichichero ME. Immunologic response of extremely premature infants to tetanus, Haemophilus influenzae, and polio immunizations. Pediatrics. 1995;96(1, pt 1):18-22. [PubMed] [Google Scholar]
- 6.Slack MH, Schapira D, Thwaites RJ, et al. Acellular pertussis vaccine given by accelerated schedule: response of preterm infants. Arch Dis Child Fetal Neonatal Ed. 2004;89(1):F57-F60. doi: 10.1136/fn.89.1.F57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Omeñaca F, Garcia-Sicilia J, García-Corbeira P, et al. Response of preterm newborns to immunization with a hexavalent diphtheria-tetanus-acellular pertussis-hepatitis B virus-inactivated polio and Haemophilus influenzae type b vaccine: first experiences and solutions to a serious and sensitive issue. Pediatrics. 2005;116(6):1292-1298. doi: 10.1542/peds.2004-2336 [DOI] [PubMed] [Google Scholar]
- 8.Vázquez L, Garcia F, Rüttimann R, Coconier G, Jacquet JM, Schuerman L. Immunogenicity and reactogenicity of DTPa-HBV-IPV/Hib vaccine as primary and booster vaccination in low-birth-weight premature infants. Acta Paediatr. 2008;97(9):1243-1249. doi: 10.1111/j.1651-2227.2008.00884.x [DOI] [PubMed] [Google Scholar]
- 9.Slack MH, Schapira D, Thwaites RJ, et al. Immune response of premature infants to meningococcal serogroup C and combined diphtheria-tetanus toxoids-acellular pertussis-Haemophilus influenzae type b conjugate vaccines. J Infect Dis. 2001;184(12):1617-1620. doi: 10.1086/324666 [DOI] [PubMed] [Google Scholar]
- 10.Heath PT, Booy R, McVernon J, et al. Hib vaccination in infants born prematurely. Arch Dis Child. 2003;88(3):206-210. doi: 10.1136/adc.88.3.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruggeberg JU, Collins C, Clarke P, et al. Immunogenicity and induction of immunological memory of the heptavalent pneumococcal conjugate vaccine in preterm UK infants. Vaccine. 2007;25(2):264-271. doi: 10.1016/j.vaccine.2006.07.036 [DOI] [PubMed] [Google Scholar]
- 12.Wijmenga-Monsuur AJ, van Westen E, Knol MJ, et al. Direct comparison of immunogenicity induced by 10- or 13-valent pneumococcal conjugate vaccine around the 11-month booster in Dutch infants. PLoS One. 2015;10(12):e0144739. doi: 10.1371/journal.pone.0144739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Westen E, Knol MJ, Wijmenga-Monsuur AJ, et al. Serotype-specific IgG antibody waning after pneumococcal conjugate primary series vaccinations with either the 10-valent or the 13-valent vaccine. Vaccines (Basel). 2018;6(4):82. doi: 10.3390/vaccines6040082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Gageldonk PG, van Schaijk FG, van der Klis FR, Berbers GA. Development and validation of a multiplex immunoassay for the simultaneous determination of serum antibodies to Bordetella pertussis, diphtheria and tetanus. J Immunol Methods. 2008;335(1-2):79-89. doi: 10.1016/j.jim.2008.02.018 [DOI] [PubMed] [Google Scholar]
- 15.de Voer RM, Schepp RM, Versteegh FG, van der Klis FR, Berbers GA. Simultaneous detection of Haemophilus influenzae type b polysaccharide-specific antibodies and Neisseria meningitidis serogroup A, C, Y, and W-135 polysaccharide-specific antibodies in a fluorescent-bead-based multiplex immunoassay. Clin Vaccine Immunol. 2009;16(3):433-436. doi: 10.1128/CVI.00364-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elberse KE, Tcherniaeva I, Berbers GA, Schouls LM. Optimization and application of a multiplex bead-based assay to quantify serotype-specific IgG against Streptococcus pneumoniae polysaccharides: response to the booster vaccine after immunization with the pneumococcal 7-valent conjugate vaccine. Clin Vaccine Immunol. 2010;17(4):674-682. doi: 10.1128/CVI.00408-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long SS, Welkon CJ, Clark JL. Widespread silent transmission of pertussis in families: antibody correlates of infection and symptomatology. J Infect Dis. 1990;161(3):480-486. doi: 10.1093/infdis/161.3.480 [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization Immunological basis for immunization: module 2: diphtheria—update 2009. Accessed June 28, 2019. https://apps.who.int/iris/handle/10665/44094
- 19.World Health Organization WHO immunological basis for immunization series module 3: tetanus update 2018. Accessed June 28, 2019. https://www.who.int/immunization/documents/ISBN9789241513616/en/
- 20.World Health Organization Immunological basis for immunization: Haemophilus influenzae type b vaccines. Published 2007. Accessed June 28, 2019. https://apps.who.int/iris/handle/10665/43799
- 21.Siber GR, Chang I, Baker S, et al. Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies. Vaccine. 2007;25(19):3816-3826. doi: 10.1016/j.vaccine.2007.01.119 [DOI] [PubMed] [Google Scholar]
- 22.Fleiss JL, Tytun A, Ury HK. A simple approximation for calculating sample sizes for comparing independent proportions. Biometrics. 1980;36(2):343-346. doi: 10.2307/2529990 [DOI] [PubMed] [Google Scholar]
- 23.Rouers EDM, Berbers GAM, van Dongen JAP, Sanders EAM, Bruijning-Verhagen P; PRIEMA Study Group . Timeliness of immunisations in preterm infants in the Netherlands. Vaccine. 2019;37(39):5862-5867. doi: 10.1016/j.vaccine.2019.08.006 [DOI] [PubMed] [Google Scholar]
- 24.Kent A, Ladhani SN, Andrews NJ, et al. ; PUNS Study Group . Schedules for pneumococcal vaccination of preterm infants: an RCT. Pediatrics. 2016;138(3):e20153945. doi: 10.1542/peds.2015-3945 [DOI] [PubMed] [Google Scholar]
- 25.Martinón-Torres F, Czajka H, Center KJ, et al. 13-Valent pneumococcal conjugate vaccine (PCV13) in preterm versus term infants. Pediatrics. 2015;135(4):e876-e886. doi: 10.1542/peds.2014-2941 [DOI] [PubMed] [Google Scholar]
- 26.Melville JM, Moss TJ. The immune consequences of preterm birth. Front Neurosci. 2013;7:79. doi: 10.3389/fnins.2013.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olin A, Henckel E, Chen Y, et al. Stereotypic immune system development in newborn children. Cell. 2018;174(5):1277-1292.e14. doi: 10.1016/j.cell.2018.06.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voysey M, Kelly DF, Fanshawe TR, et al. The influence of maternally derived antibody and infant age at vaccination on infant vaccine responses: an individual participant meta-analysis. JAMA Pediatr. 2017;171(7):637-646. doi: 10.1001/jamapediatrics.2017.0638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barug D, Pronk I, van Houten MA, et al. Maternal pertussis vaccination and its effects on the immune response of infants aged up to 12 months in the Netherlands: an open-label, parallel, randomised controlled trial. Lancet Infect Dis. 2019;19(4):392-401. doi: 10.1016/S1473-3099(18)30717-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement.
eTable 1. IgG Geometric Mean Concentrations With 95% CI Against the Vaccine Antigens for the Preterm Infants
eTable 2. IgG Geometric Mean Concentrations With 95% Confidence Intervals Against the Vaccine Antigens for all GA Groups Together and Term Infants
eTable 3. Mean of the Absolute Response (Difference Between T2-T1) for the Different Vaccine Components for all GA Groups Together and for GA Groups Separately
eTable 4. Proportion of Infants With Protective Antibody Levels per Antigen per GA Group
eTable 5. Proportion of Infants With Protective Antibody Levels per Antigen per GA Group