Colorectal Cancer Screening and COVID-19 (original) (raw)
As the COVID-19 pandemic spread throughout the United States, the San Francisco Health Network, an urban integrated safety-net health system, rapidly altered how it delivered care. Routine outpatient visits were converted to telehealth appointments, and nonurgent endoscopic procedures were cancelled, resulting in dramatic declines in colorectal cancer (CRC) screening. With ongoing surges of COVID-19, prolonged social distancing and limitations on procedures will likely be necessary. Without prompt action, the impact of missed diagnoses and resultant excess mortality could be substantial.
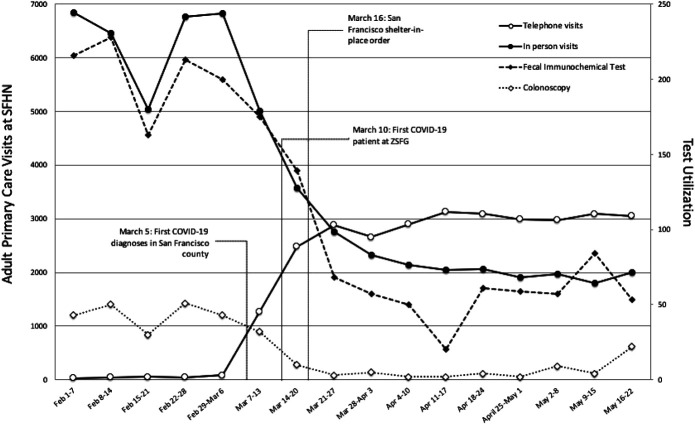
San Francisco Health Network serves ∼90,000 individuals through multisite clinics and a specialty referral center, Zuckerberg San Francisco General Hospital. Between February and May 2020, total adult primary care in-person visits decreased by 70% (from 6,800 to 2,000 visits/wk). Telehealth visits increased from 1.2% of adult primary care visits in the first week of March to 60% by April (from 40 to 3,000 visits/wk) (Figure 1).
Figure 1.
Changes in healthcare delivery at San Francisco Health Network (SFHN).
Coinciding with the decrease of in-person visits was a drastic reduction in CRC screening tests. Fecal immunochemical test (FIT) volume decreased by ∼85% (from 370 tests ordered per week in February to 60 tests per week by April) and colonoscopy volume declined by ∼90% (from 150 to 200 cases per month to less than 15). Reduced screening and delays in colonoscopies, especially for those with positive FIT, will lead to increased incident and later-stage CRC diagnoses (1). Estimates suggest that if decreased screening persisted through early June, there could be nearly 19,000 fewer CRC diagnoses and over 4,000 excess deaths from CRC nationally because of the effects of the COVID-19 pandemic (2,3).
A concerted effort is needed to mitigate the decline in CRC screening by shifting to organized outreach programs. Most cancer screening in the United States operates on an opportunistic visit-based model; however, there is a growing need for organized cancer screening independent of in-person clinic visits. Organized outreach is more effective than provider-initiated screening with CRC screening rates improving from ∼40% to over 80% after implementation in 1 large healthcare system (4).
To achieve organized outreach, health systems must embrace the shift to telehealth. Electronic medical records and registries can identify patients not up to date with screening, use of at-home FIT kits mailed directly to patients can be expanded, and positive tests can be followed up with automated calls and text prompts from direct patient messaging platforms—all strategies that have proven effective at increasing screening rates and follow-up. Increased utilization of FIT would triage higher-risk patients to colonoscopy, allowing endoscopy units to better manage procedure backlog volume and limit unnecessary patient exposures (5).
The duration of the COVID-19 pandemic seems to be long lasting. Black, American Indian/Alaska Natives, Hispanic communities, and low socioeconomic individuals disproportionately affected by COVID-19 are more likely to die from CRC and now face even greater challenges to timely CRC screening and follow-up colonoscopy completion. Accelerating and expanding use of telehealth and organized outreach will be a positive outcome of this crisis and has the potential to reach vulnerable patients in increased numbers. Collectively, we have an opportunity to proactively improve patient-centered preventive care, address worsening health disparities, and reduce the excess burden of disease from COVID-19.
CONFLICTS OF INTEREST
Guarantor of the article: Shreya Patel, MD, MPH.
Specific author contributions: S.P.: study concept and design, acquisition of data, analysis and interpretation of the data, drafting of the manuscript, and critical revision of the manuscript. R.B.I.: critical revision of the manuscript. E.C.: critical revision of the manuscript. M.S.: study concept and design, analysis and interpretation of the data, critical revision of the manuscript, and study supervisor.
Financial support: R.B.I. receives funding from National Institutes of Health/National Cancer Institute award number K08 CA241296. M.S. was supported in part by the San Francisco Cancer Initiative.
Potential competing interests: None to report.
REFERENCES
- 1.Corley DA, Jensen CD, Quinn VP, et al. Association between time to colonoscopy after a positive fecal test and risk of colorectal cancer stage at diagnosis. JAMA 2017;317(16):1631–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aitken M, Kleinrock M, IQVIA Institute for Human Data Science. Shifts in Healthcare Demand, Delivery and Care during the COVID-19 Era: Tracking the Impact in the United States. IQVIA Institute for Human Data Science: Parsippany, NJ, 2020. [Google Scholar]
- 3.Sharpless NE. COVID-19 and cancer. Science 2020;368(6497):1290. [DOI] [PubMed] [Google Scholar]
- 4.Levin TR, Corley DA, Jensen CD, et al. Effects of organized colorectal cancer screening on cancer incidence and mortality in a large community-based population. Gastroenterology 2018;155(5):1383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somsouk M, Rachocki C, Mannalithara A, et al. Effectiveness and cost of organized outreach for colorectal cancer screening: A randomized, controlled trial. J Natl Cancer Inst 2020;112(3):305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]