Comparative Effectiveness of the Sodium–Glucose Cotransporter 2 Inhibitor Empagliflozin Versus Other Antihyperglycemics on Risk of Major Adverse Kidney Events (original) (raw)
Abstract
OBJECTIVE
To examine the comparative effectiveness of the sodium–glucose cotransporter 2 inhibitor (SGLT2i) empagliflozin and other non-SGLT2i antihyperglycemics on the risk of major adverse kidney events (MAKE) of estimated glomerular filtration rate (eGFR) decline >50%, end-stage kidney disease, or all-cause mortality.
RESEARCH DESIGN AND METHODS
In a cohort study of 379,033 new users of empagliflozin or other non-SGLT2i antihyperglycemics, predefined variables and covariates identified by a high-dimensional variable selection algorithm were used to build propensity scores. Weighted survival analyses were then applied to estimate the risk of MAKE.
RESULTS
Compared with other antihyperglycemics, empagliflozin use was associated with 0.99 (95% CI 0.51, 1.55) mL/min/1.73 m2 less annual reduction in eGFR, 0.25 (95% CI 0.16, 0.33) kg/m2 more annual decrease in BMI, and reduced risk of MAKE (hazard ratio [HR] 0.68 [95% CI 0.64, 0.73]). Empagliflozin use was associated with reduced risk of MAKE in eGFR ≥90, ≥60 to <90, ≥45 to <60, and ≥30 to <45 mL/min/1.73 m2 (HR 0.70 [95% CI 0.60, 0.82], 0.66 [0.60, 0.73], 0.78 [0.69, 0.89]), and 0.71 [0.55, 0.92], respectively), in participants without albuminuria, with microalbuminuria and macroalbuminuria (HR 0.65 [95% CI 0.57, 0.75], 0.72 [0.66. 0.79], and 0.74 [0.62, 0.88], respectively), and in participants with and without cardiovascular disease (HR 0.67 [95% CI 0.61, 0.74] and 0.76 [0.69, 0.83], respectively). The association was evident in per-protocol analyses, which required continuation of the assigned antihyperglycemic medication (empagliflozin or other antihyperglycemics) during follow-up (HR 0.64 [95% CI 0.60, 0.70]), and in analyses requiring concurrent use of metformin in at least the first 90 days of follow-up (HR 0.63 [0.57–0.69]).
CONCLUSIONS
Among people with type 2 diabetes, empagliflozin use was associated with eGFR preservation, a greater decline in BMI, and a reduced risk of MAKE compared with other non-SGLT2i antihyperglycemics.
Introduction
The global prevalence of type 2 diabetes in 2017 was 462 million (uncertainty interval 423–509 million) (1). Nearly 40% of people with type 2 diabetes develop diabetic kidney disease, making it the leading driver of chronic kidney disease (CKD) burden in the U.S. and globally (1–3). Diabetic kidney disease is associated with substantial morbidity and reduced life expectancy (4,5). While intensive glucose control with conventional antihyperglycemic medications resulted in a reduction in the risk of major kidney outcomes in patients with type 2 diabetes (6), the burden of major adverse kidney events in these patients remains substantially high (7). In the past 5 years, four randomized clinical trials (RCTs) provided evidence that when compared with placebo, the newest class of antihyperglycemic agents, sodium–glucose cotransporter 2 inhibitor (SGLT2i), reduced the risk of end-stage kidney disease (ESKD) and death (8–11). However, these RCTs included mostly participants with established or at high risk of cardiovascular disease and enrolled a limited number of people with reduced kidney function. The only exception is Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE), which enrolled patients with albuminuria and an estimated glomerular filtration rate (eGFR) of 30 to <90 mL/min/1.73 m2 (8–22). Evaluating the comparative effectiveness of SGLT2i versus other active antihyperglycemics in a real-world setting, including people with and without cardiovascular disease and people with a broad spectrum of kidney function, including advanced kidney disease, would address an important knowledge gap to complement evidence from RCTs and help inform the choice of antihyperglycemic therapy among people with type 2 diabetes.
In this work, we used real-world data from the U.S. Department of Veterans Affairs (VA) to examine the comparative effectiveness of incident use of the SGLT2i empagliflozin versus incident use of other non-SGLT2i antihyperglycemics on the risk of major adverse kidney events (MAKE), defined as a composite end point of eGFR decline >50%, ESKD, or all-cause mortality.
Research Design and Methods
Cohort Design
Participants who received antihyperglycemic medications from the VA Health Care System between 1 October 2015 and 30 September 2019 were selected as an initial cohort (n = 1,406,662). A flowchart of cohort construction is included in Supplementary Fig. 1_A_. Within these participants, we selected as the empagliflozin group those who received an empagliflozin prescription between 1 October 2016 and 30 September 2019 (n = 66,012) and then limited this group to those with no prior history of SGLT2i exposure in past 1 year (n = 63,208). Time zero (T0) was defined as time of the first empagliflozin prescription during this period. From the rest of the initial cohort, we then selected those who switched to or added on an antihyperglycemic medication that was not an SGLT2i to build the control group (n = 438,127). Change in antihyperglycemic medication was defined by receipt of prescription of a new class of antihyperglycemics that had not been used in the past year. This approach constructs a control group that may be more similar in clinical characteristics to the empagliflozin group by requiring a change in treatment regimen. T0 in this group was defined as time of this first new antihyperglycemic medication prescription (time of switch or add-on). Participants in the control group were excluded if they received SGLT2i within 1 year before T0 (n = 435,312). To ensure that all participants had a 1-year covariate ascertainment period, we only included those enrolled in the health care system for >1 year at T0 (empagliflozin n = 62,083; control n = 419,979). Within them, 59,156 participants in the empagliflozin group and 401,388 participants in the control group did not experience ESKD or have type 1 diabetes. The groups were further selected to only include participants with an eGFR ≥30 mL/min/1.73 m2 within 1 year before T0 (empagliflozin n = 56,261; control n = 375,037). After excluding participants who did not have glycated hemoglobin (HbA1c), LDL, blood pressure, height, or weight measured within 1 year before T0, the final cohort included 52,535 participants in the empagliflozin group and 326,498 participants in the control group. Participants were monitored until the occurrence of an outcome or administrative end of follow-up (31 January 2020).
Data Sources
Data from VA Corporate Data Warehouse (CDW) were used in this study (23–26). CDW Outpatient and Inpatient Encounters domains were used to collect ICD-10 diagnosis codes, Current Procedural Terminology (CPT) codes, and ICD-10 procedure codes during an outpatient visit or hospitalization (27). The CDW Outpatient Pharmacy domain was used to collect prescription data and provider data. Inpatient and outpatient laboratory results were both obtained from the CDW Laboratory Results domain (28). Vital measurements were collected from the CDW Vital Signs domain, and demographic information, including date of death, was collected from the CDW Patient domain and VA Vital Status Databases (29).
Outcomes
The outcome of MAKE was defined as time until the first occurrence of eGFR decline >50% from baseline, ESKD, or all-cause mortality. Time of ESKD was identified by first occurrence of eGFR <15 mL/min/1.73 m2, chronic dialysis, or kidney transplant, identified in inpatient, outpatient, and laboratory data sets. The Chronic Kidney Disease Epidemiology Collaboration creatinine equation was used to compute eGFR based on serum creatinine, age, race, and sex (30). We also estimated the trajectory of eGFR and BMI during follow-up.
Exposure
Prescriptions of antihyperglycemic medications were identified from outpatient pharmacy records. Classes of antihyperglycemics included SGLT2i, biguanides (metformin), insulin, sulfonylureas, dipeptidyl peptidase 4 inhibitors (DPP4), glucagon-like peptide 1 receptor agonists (GLP1), thiazolidinediones, α-glucosidase inhibitors, meglitinides, and amylin analogs (26,31). A list of medications within each class is provided in Supplementary Table 1. A distribution of antihyperglycemic medications at T0 is presented in Supplementary Table 2.
Covariates
Predictors of antihyperglycemic prescription were selected as predefined covariates and ascertained in the year before T0. Predefined covariates included age, race (White, Black, and other), sex, HbA1c, eGFR, systolic blood pressure, diastolic blood pressure, LDL, and BMI computed from weight and height. We also included diseases that may affect the choice of antihyperglycemics, such as congestive heart failure, cardiovascular diseases, cancer, alcoholism, hypoglycemia, diabetic ketoacidosis, acute kidney injury, bladder and urinary tract infections, venous thromboembolism, pancreatitis, and bone fracture, and albuminuria (32). Acute kidney injury was defined as increased serum creatinine of 0.3 mg/dL or 50% within 30 days, and albuminuria status was categorized into no albuminuria (≤30 mg/g), microalbuminuria (>30 to ≤300 mg/g), and macroalbuminuria (>300 mg/g). Any prescription of GLP1, DPP4, sulfonylureas, thiazolidinediones, metformin, insulin, α-glucosidase inhibitors, meglitinides, amylin analogs, statins, ACE inhibitors, or angiotensin receptor blockers (ARBs), β-blockers, diuretics, and calcium channel blockers were also included as predefined covariates (32). Total number of diabetes medications, smoking status (never, former, current), hospital complexity (outpatient clinic or health care system), and the calendar year of T0 were also included as predefined covariates.
High-dimensional information from VA electronic health records were additionally used to reduce potential biases that were not adjusted for by the predefined covariates (33,34). A high-dimensional variable selection algorithm used all participant records within the 1 year before T0 from data dimensions, including outpatient ICD-10 diagnostic codes, outpatient CPT, inpatient ICD-10 diagnostic codes, inpatient CPT, and inpatient ICD-10 procedure codes for operations, pharmacy records, and laboratory results. The 300 most frequently occurring items (diagnosis, procedure, abnormal laboratory result, etc.) from each of the seven dimensions were categorized into three binary variables: ever, sometimes, and frequently occurring, which generated in total 300  7
7  3 = 6,300 variables. Variables were further selected based on their association with assignment to the empagliflozin or control group, where the 300 variables with the largest risk ratios were selected. High-dimensional variable selection was conducted independently in the overall cohort and within each subgroup for subgroup analyses.
3 = 6,300 variables. Variables were further selected based on their association with assignment to the empagliflozin or control group, where the 300 variables with the largest risk ratios were selected. High-dimensional variable selection was conducted independently in the overall cohort and within each subgroup for subgroup analyses.
For estimation of the per-protocol effect, predefined covariates were time updated. High-dimensional variables were selected based on association with adherence to the treatment protocol and were also time updated (35).
Ethical Approval
The VA St. Louis Health Care System Institutional Review Board (St. Louis, MO) reviewed and approved this research project.
Statistical Analyses
Characteristics of the overall cohort and within the SGLT2i empagliflozin and control groups are presented as mean and SD or number and percentage, as appropriate. Kaplan-Meier curves for MAKE in the empagliflozin and control groups are presented.
Supplementary Figure 1_B_ displays the analytic framework used in this study. We used an inverse weighting approach to adjust for confounding. The probability of initiating empagliflozin, in the form of a high-dimensional propensity score, was estimated from logistic regression, where independent variables included both predefined and high-dimensional variables (33). Continuous variables, including age, HbA1c, eGFR, LDL, systolic and diastolic blood pressure, and BMI, were treated as restricted cubic spline with knots at the 5th, 35th, 65th, and 95th percentiles. Based on the propensity score, we then weighted the control group based on probability of initiating empagliflozin/(1 − probability of initiating empagliflozin) (36). A weighted pseudocohort was then generated where the empagliflozin and control groups had similar baseline characteristics to facilitate comparing the two groups. Weights were truncated at the 0.1th and 99.9th percentile. To demonstrate the reduction of imbalance in baseline characteristics, characteristics in the weighted cohort and the absolute standardized difference between groups were computed. Weighted survival probability in the form of Kaplan-Meier curves for MAKE in the empagliflozin and the control groups are presented.
We applied a weighted linear mixed model to estimate the eGFR and BMI trajectories in the weighted empagliflozin and the control groups. A cubic spline of time was used to capture potential nonlinear trajectories during follow-up, where knots were placed at 0, 90, 365, 730, and 1,095 days. The difference in eGFR trajectory between treatment arms was estimated from the interaction between treatment group and the splined time. eGFR and BMI in the empagliflozin and control groups at 90 days and at 1, 2, and 3 years were plotted. eGFR and BMI changes from baseline (T0) were estimated. The difference between the two trajectories, where the control group served as the reference, represented the eGFR and BMI changes associated with empagliflozin at each time point.
Weighted Cox survival models were built to estimate the intention-to-treat hazard ratio (HR) and risk difference between groups in the weighted cohort. A robust sandwich estimator was applied to account for the influence of weighting on variance. To estimate the effect in different populations of interest to the clinical community, we conducted subgroup analyses according to eGFR category (≥90, ≥60 to <90, ≥45 to <60, and ≥30 to <45 mL/min/1.73 m2), albuminuria status (no albuminuria, microalbuminuria, and macroalbuminuria) at baseline, both albuminuria status and kidney disease status (eGFR ≥60 and <60 mL/min/1.73 m2), in those with and without cardiovascular disease at baseline, according to baseline use of ACE/ARB, metformin, statins, diuretics, and insulin, and according to BMI category (>30, >25 to ≤30, and ≤25 kg/m2). For each subgroup analysis, the propensity score and the related weighting was estimated independently. We also examined the association between empagliflozin and risk of each component of the composite outcome (MAKE): eGFR decline >50%, ESKD, and death, and a composite outcome of eGFR decline >50% or ESKD, separately. Event rate differences per 1,000 person-years between the empagliflozin and the control groups in the overall cohort and in subgroups were computed based on the difference in estimated survival probability at 3 years.
Multiple sensitivity analyses were conducted to test robustness of study results.
- Instead of weighting, variable ratio matching with a maximum of five controls per treatment was conducted to estimate the association in a matched cohort (37).
- To examine whether the association between empagliflozin and risk of MAKE was independent of change of BMI during follow-up, we controlled for time-dependent BMI in the form of restricted cubic splines.
- To examine whether the association was independent of change of HbA1c, we controlled for time-dependent HbA1c in form of restricted cubic splines.
- We also controlled for both time-dependent BMI and HbA1c spline terms in the same model.
- Because antihyperglycemic prescribing preferences may have changed over time, we examined the association in two cohort enrollment periods (2016 and 2017) and separately (2018 and 2019).
- To remove events that are less likely related to long-term effects of antihyperglycemics, we excluded participants who experienced events in the first 180 days of the follow-up.
- We tested the association with an alternatively defined outcome of eGFR decline >50% based on two eGFR measurements separated by at least 30 days.
- We examined the association with ESKD, defined as receipt of chronic dialysis, kidney transplantation, or eGFR <15 mL/min/1.73 m2 on two separate occasions at least 30 days apart.
As a means of testing for possible spurious associations, we used the approach outlined by Lipsitch et al. (38) to test the association between empagliflozin and risk of traffic-related injury as a negative outcome control where a priori evidence suggests that an association is not expected. A Cox survival model was used to estimate the association between traffic-related injury and empagliflozin in the weighted cohort.
Per-protocol, analyses were conducted to estimate the effect of empagliflozin when following a defined treatment protocol (39,40). In the weighted cohort, we additionally inverse weighted by the time dependent probability of not following the treatment protocol. Two treatment protocols were specified and examined separately. The first treatment protocol required the continuation of the antihyperglycemic medication initiated at T0 throughout duration of follow-up. The second treatment protocol required the concurrent use of metformin with empagliflozin or the other antihyperglycemics in the first 90 days of follow-up. For every time interval k with length of 90 days, the stabilized weight for adherence to treatment protocol was estimated as: 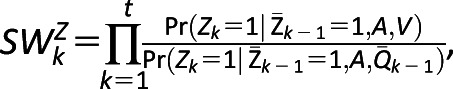 where Z is an indicator of adherence, A is the treatment group, V is a vector of time-independent predictors, including age, race, sex, complexity of hospital where initial prescription occurred, and year of T0, and
where Z is an indicator of adherence, A is the treatment group, V is a vector of time-independent predictors, including age, race, sex, complexity of hospital where initial prescription occurred, and year of T0, and 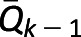 is the history of predefined variables and high-dimensional variables through time k−1. Stabilized weights were truncated at the 0.1th and 99.9th percentile, and a pooled logistic regression was then used to estimate the per-protocol effect (41,42).
is the history of predefined variables and high-dimensional variables through time k−1. Stabilized weights were truncated at the 0.1th and 99.9th percentile, and a pooled logistic regression was then used to estimate the per-protocol effect (41,42).
Reproducible Research Statement
The study protocol and statistical code are available from Z.A.-A. (email: zalaly@gmail.com). The data in the data set are available through the U.S. Department of Veterans Affairs.
Results
There were 379,033 participants in the unweighted cohort corresponding to 644,311.59 person-years of follow-up (details provided in Supplementary Table 3). Survival probabilities in the SGLT2i empagliflozin and the control groups in the unweighted cohort are provided in Supplementary Fig. 2. A weighted cohort was generated based on the probability of initiating empagliflozin, resulting in 52,535 users in the empagliflozin group and 52,850 in the control group. Demographic and health characteristics in the weighted cohort are reported in Table 1.
Table 1.
Demographic and health characteristics of the weighted cohort
| Baseline characteristics | Weighted cohort (n = 105,385) | Empagliflozin (n = 52,535 [49.85%]) | Other non-SGLT2i antihyperglycemics (n = 52,850 [50.15%]) | Absolute standardized difference |
|---|---|---|---|---|
| Age, years | 65.63 (9.12) | 65.35 (9.23) | 65.92 (9.00) | 0.06 |
| Race, n (%) | ||||
| White | 77,869 (73.89) | 38,697 (73.66) | 39,172 (74.12) | 0.01 |
| Black | 15,534 (14.74) | 7,943 (15.12) | 7,591 (14.36) | 0.02 |
| Other | 11,982 (11.37) | 5,895 (11.22) | 6,087 (11.52) | <0.01 |
| Sex, n (%) | ||||
| Male | 100,725 (95.58) | 50,172 (95.50) | 50,553 (95.65) | <0.01 |
| Female | 4,660 (4.42) | 2,363 (4.50) | 2,297 (4.35) | <0.01 |
| eGFR, mL/min/1.73 m2 | 77.41 (18.38) | 77.91 (18.40) | 76.92 (18.35) | 0.05 |
| eGFR category, n (%) | ||||
| ≥90 mL/min/1.73 m2 | 28,222 (26.78) | 14,468 (27.54) | 13,794 (26.10) | 0.03 |
| ≥60 to <90 mL/min/1.73 m2 | 57,077 (54.16) | 28,206 (53.69) | 28,846 (54.58) | 0.02 |
| ≥45 to <60 mL/min/1.73 m2 | 17,294 (16.41) | 8,515 (16.21) | 8,768 (16.59) | 0.01 |
| ≥30 to <45 mL/min/1.73 m2 | 2,793 (2.65) | 1,346 (2.56) | 1,443 (2.73) | 0.01 |
| HbA1c, % | 8.70 (1.40) | 8.69 (1.40) | 8.70 (1.41) | <0.01 |
| HbA1c, mmol/mol | 72 (11.59) | 71 (11.44) | 72 (11.59) | <0.01 |
| BMI, kg/m2 | 34.12 (6.43) | 34.06 (6.41) | 34.17 (6.45) | 0.02 |
| LDL, mg/dL | 81.04 (34.12) | 81.50 (34.37) | 80.59 (33.86) | 0.03 |
| Blood pressure, mmHg | ||||
| Systolic | 132.19 (16.33) | 132.19 (16.25) | 132.19 (16.40) | <0.01 |
| Diastolic | 74.84 (9.83) | 75.08 (9.83) | 74.60 (9.82) | 0.05 |
| Congestive heart failure, n (%) | 10,951 (10.39) | 5,320 (10.13) | 5,630 (10.65) | 0.01 |
| Alcoholism, n (%) | 4,220 (4.00) | 2,211 (4.21) | 2,009 (3.80) | 0.02 |
| Bone fracture, n (%) | 1,273 (1.21) | 613 (1.17) | 660 (1.25) | <0.01 |
| Cancer, n (%) | 21,413 (20.32) | 10,627 (20.23) | 10,786 (20.41) | <0.01 |
| Cardiovascular disease, n (%) | 43,395 (41.18) | 21,294 (40.53) | 22,101 (41.82) | 0.03 |
| Diabetic ketoacidosis, n (%) | 212 (0.20) | 108 (0.21) | 104 (0.20) | 0.002 |
| Hypoglycemia, n (%) | 3,626 (3.44) | 1,730 (3.29) | 1,896 (3.59) | 0.02 |
| Pancreatitis, n (%) | 1,192 (1.13) | 625 (1.19) | 567 (1.07) | 0.01 |
| Bladder and urinary tract infections, n (%) | 2,233 (2.12) | 1,077 (2.05) | 1,156 (2.19) | 0.01 |
| Venous thromboembolism, n (%) | 608 (0.58) | 325 (0.62) | 283 (0.53) | 0.01 |
| Acute kidney injury, n (%) | 9,705 (9.01) | 4,705 (8.96) | 4,789 (9.06) | <0.01 |
| Albuminuria, n (%) | ||||
| None (≤30 mg/g) | 41,314 (39.20) | 20,827 (39.64) | 20,487 (38.76) | 0.02 |
| Microalbuminuria (>30 to ≤300 mg/g) | 52,999 (50.29) | 26,371 (50.20) | 26,628 (50.38) | <0.01 |
| Macroalbuminuria (>300 mg/g) | 11,072 (10.51) | 5,337 (10.16) | 5,735 (10.85) | 0.02 |
| Metformin, n (%)* | 84,393 (80.08) | 42,156 (80.24) | 42,237 (79.92) | <0.01 |
| Insulin, n (%)* | 59,402 (56.37) | 29,011 (55.22) | 30,391 (57.50) | 0.05 |
| Sulfonylureas, n (%)* | 50,157 (47.59) | 24,901 (47.40) | 25,256 (47.79) | 0.03 |
| DPP4, n (%)* | 25,272 (23.98) | 12,817 (24.40) | 12,455 (23.57) | 0.03 |
| GLP1, n (%)* | 13,608 (12.91) | 6,557 (12.48) | 7,051 (13.34) | 0.02 |
| Thiazolidinediones, n (%)* | 8,150 (7.73) | 4,277 (8.14) | 3,873 (7.33) | 0.03 |
| α-Glucosidase inhibitors, n (%)* | 1,841 (1.75) | 878 (1.67) | 963 (1.82) | 0.06 |
| Meglitinides, n (%)* | 271 (0.26) | 165 (0.31) | 106 (0.20) | 0.05 |
| Amylin analogs, n (%)* | 50 (0.05) | 28 (0.05) | 22 (0.04) | 0.02 |
| Total number of diabetes medications used | 2.31 (0.96) | 2.30 (0.94) | 2.32 (0.99) | 0.02 |
| ACE/ARB, n (%)* | 73,851 (70.08) | 36,566 (69.60) | 37,285 (70.55) | 0.02 |
| Calcium channel blockers, n (%)* | 32,218 (30.57) | 15,918 (30.30) | 16,300 (30.84) | 0.01 |
| β-Blockers, n (%)* | 55,122 (52.31) | 26,989 (51.37) | 28,133 (53.23) | 0.04 |
| Diuretics, n (%)* | 46,648 (44.26) | 23,003 (43.79) | 23,645 (44.74) | 0.02 |
| Statins, n (%)* | 89,615 (85.04) | 44,515 (84.73) | 45,100 (85.34) | 0.02 |
| Hospital complexity, n (%) | ||||
| Outpatient clinic | 55,543 (52.70) | 28,123 (53.53) | 27,420 (51.88) | 0.03 |
| Health care system | 49,842 (47.30) | 24,412 (46.47) | 25,430 (48.12) | 0.03 |
| Year of treatment initial, n (%) | ||||
| 2016 | 2,025 (1.92) | 956 (1.82) | 1,067 (2.02) | 0.01 |
| 2017 | 17,874 (16.96) | 8,439 (16.06) | 9,435 (17.85) | 0.05 |
| 2018 | 35,078 (33.29) | 16,957 (32.28) | 18,121 (34.29) | 0.04 |
| 2019 | 50,408 (47.83) | 26,183 (49.84) | 24,225 (45.84) | 0.08 |
| Smoking status, n (%) | ||||
| Never | 48,845 (46.35) | 24,389 (46.42) | 24,456 (46.27) | <0.01 |
| Former | 35,238 (33.44) | 17,260 (32.85) | 17,978 (34.02) | 0.02 |
| Current | 21,302 (20.21) | 10,886 (20.72) | 10,416 (19.71) | 0.03 |
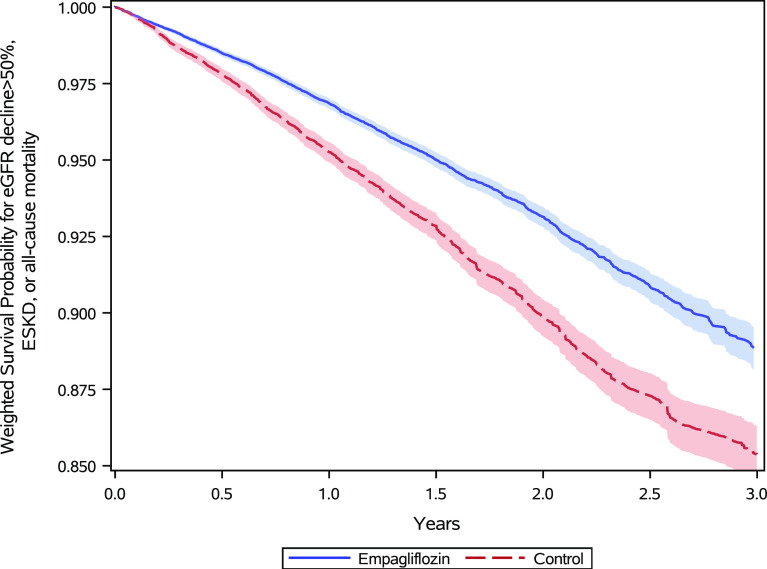
In the weighted cohort, during 3 years of follow-up, there were 2,305 (4.39%) composite outcomes (MAKE) in the empagliflozin group (34.80 per 1,000 person-years) and 3,642.17 (6.89%) in the control group (51.18 per 1,000 person-years). Weighted survival probabilities in the empagliflozin and the control groups are provided in Fig. 1.
Figure 1.
Survival probability for MAKE of eGFR decline >50%, ESKD, or all-cause mortality in the empagliflozin group (blue) and the control group of other non-SGLT2i antihyperglycemics (red) in the weighted cohort.
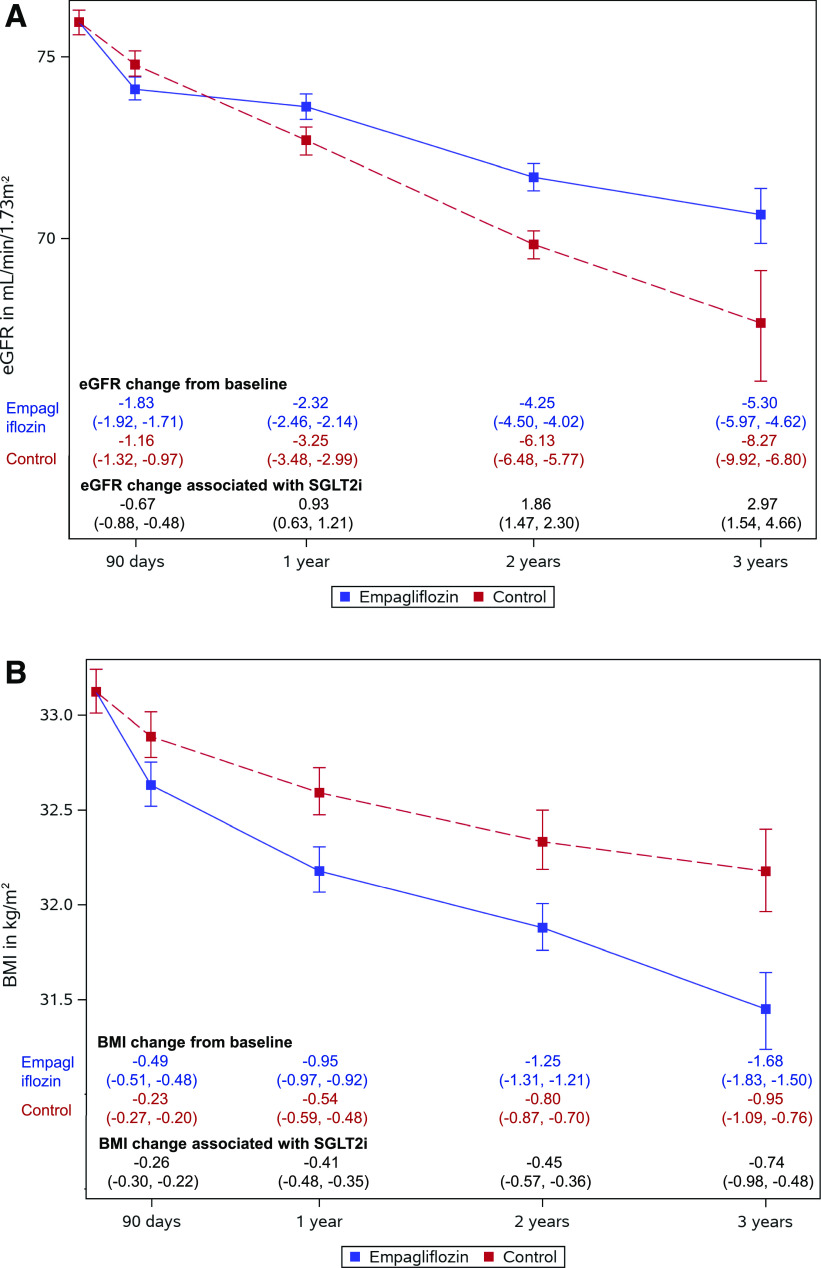
Estimated eGFR trajectories in the empagliflozin and control groups showed decline in eGFR (Fig. 2_A_). Although the empagliflozin group experienced a rapid drop of eGFR in the first 90 days after treatment initiation, over the span of 3 years, the SGLT2i and control groups exhibited a 5.30 (95% CI 4.62, 5.97) and 8.27 (6.80, 9.92) mL/min/1.73 m2 decline in eGFR, respectively. Compared with the control group, empagliflozin use was associated with an annual eGFR preservation of 0.99 (0.51, 1.55) mL/min/1.73 m2/year and a net 2.97 (1.54, 4.66) mL/min/1.73 m2 of eGFR preserved in 3 years (P < 0.001).
Figure 2.
A: eGFR trajectory during follow-up. Estimated eGFR values and 95% CIs at 90 days and at 1, 2, and 3 years were plotted for the empagliflozin group (blue) and the control group of other non-SGLT2i antihyperglycemics (red). eGFR change associated with empagliflozin at each time point represents the difference between the two trajectories where the control group served as the reference. B: BMI trajectory during follow-up. Estimated BMI values and 95% CIs at 90 days and at 1, 2, and 3 years were plotted for the empagliflozin group (blue) and the control group of other non-SGLT2i antihyperglycemics (red). BMI change associated with empagliflozin at each time point represents the difference between the two trajectories where the control group served as the reference.
The empagliflozin and control groups both experienced decline in BMI during the follow up (Fig. 2_B_). The empagliflozin group was associated with a greater reduction in BMI than the control group. Over the span of 3 years, the empagliflozin and the control groups exhibited 1.68 (95% CI 1.50, 1.83) and 0.95 (0.76, 1.09) kg/m2 decline in BMI, respectively. Compared with the control group, empagliflozin use was associated with an additional annual decrease in BMI of 0.25 (0.16, 0.33) kg/m2 in 1 year, and 0.74 (0.480, 0.98) kg/m2 in 3 years (P < 0.001) (Fig. 2_B_).
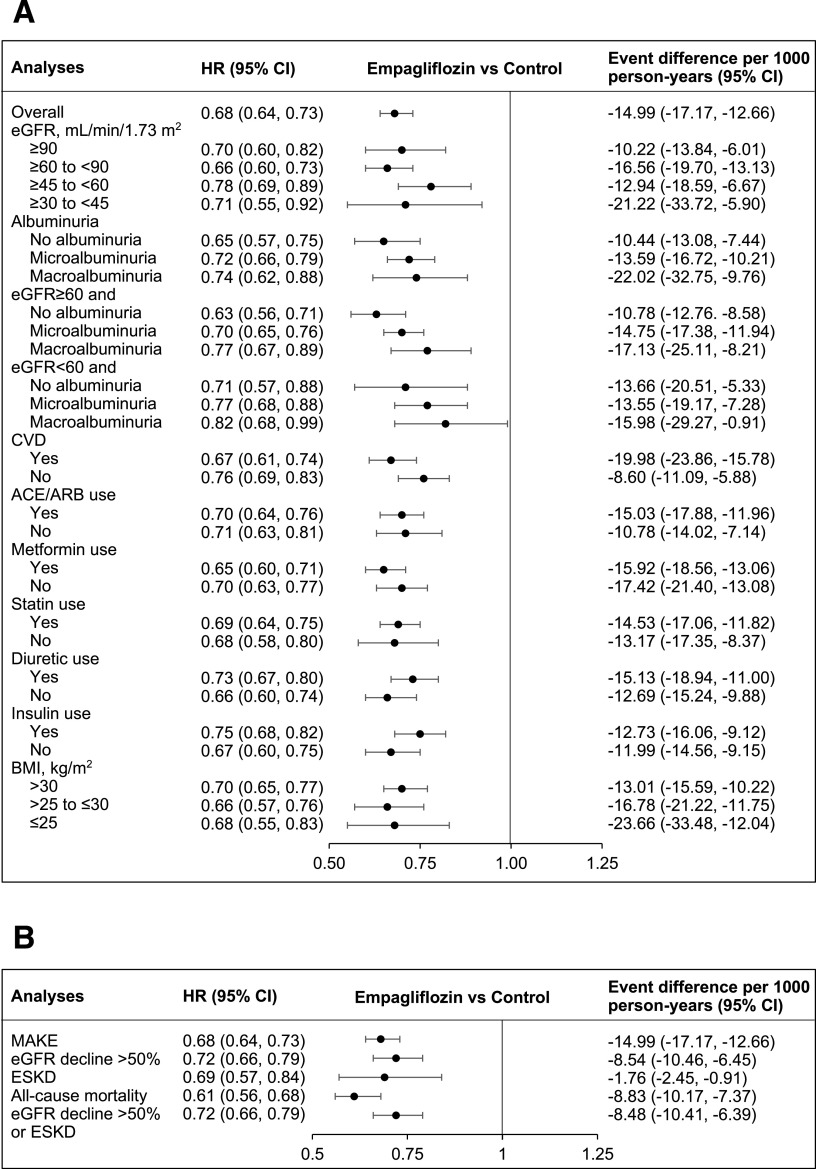
Empagliflozin use was associated with reduced risk of MAKE (HR 0.68 [95% CI 0.64, 0.73]), yielding an adjusted rate difference of −14.99 (−17.17, −12.66) fewer events per 1,000 person-years of empagliflozin use compared with other non-SGLT2i antihyperglycemics (Fig. 3). Analyses by eGFR category suggested that empagliflozin use was associated with reduced risk of MAKE in each of the eGFR categories examined, including eGFR ≥90, ≥60 to <90, ≥45 to <60, and ≥30 to <45 mL/min/1.73 m2 and in people with no albuminuria, microalbuminuria, and macroalbuminuria. In addition, empagliflozin use was associated with reduced risk of MAKE in participants with eGFR above and below 60 mL/min/1.73 m2, regardless of albuminuria status. Empagliflozin use was associated with reduced risk of MAKE in cohort participants with and without cardiovascular disease, and regardless of receipt of ACE/ARB, metformin, statins, diuretics, or insulin, and regardless of BMI category. Compared with other non-SGLT2i antihyperglycemics, empagliflozin use was associated with fewer events per 1,000 person-years in all subgroups examined (Fig. 3_A_ and Supplementary Table 4_A_).
Figure 3.
A: HRs and event rate differences for the MAKE of eGFR decline >50%, ESKD, or all-cause mortality in the overall cohort and in subgroups. B: HRs and event rate differences for individual components of the MAKE of eGFR decline >50%, ESKD, or all-cause mortality. Albuminuria status defined as no albuminuria (albumin-to-creatinine ratio ≤30 mg/g), microalbuminuria (>30 to ≤300 mg/g), and macroalbuminuria (>300 mg/g).
We also examined the association of empagliflozin with each component of the composite outcome of MAKE. Empagliflozin was associated with reduced risk of each of the three components, including eGFR decline >50% (HR 0.72 [95% CI 0.66, 0.79]), ESKD (HR 0.69 [0.57, 0.84]) and death (HR 0.61 [0.56, 0.68]). Analyses that considered the outcome of eGFR decline >50% or ESKD yielded consistent results (Fig. 3_B_ and Supplementary Table 4_B_).
Sensitivity Analyses
To examine the robustness of our findings, we conducted multiple sensitivity analyses (Supplementary Table 5).
- We conducted a variable ratio matching analysis, which allows each treatment to be matched with up to five controls, and the results suggested that empagliflozin use was associated with reduced risk of the composite outcome (HR 0.73 [95% CI 0.69, 0.77]).
- To examine whether the observed association was independent of change in BMI, we additionally controlled for time-dependent BMI as a restricted cubic spline, and the result was consistent (HR 0.71 [95% CI 0.66, 0.76]).
- We additionally controlled for time-dependent HbA1c as a spline, resulting in a HR of 0.73 (95% CI 0.68, 0.78).
- Results were consistent after controlling for both time-dependent BMI and HbA1c (HR 0.72 [95% CI 0.67, 0.77]).
- To examine whether the observed association of empagliflozin with the outcome varied depending on temporal differences in the availability of the medication and prescription criteria, we examined the effect of empagliflozin in years 2016 and 2017 when SGLT2i was less accessible and in years 2018 and 2019 when SGLT2i use became relatively more popular. Compared with the control group, empagliflozin use exhibited a reduced risk of the composite outcome in both time periods examined, HR 0.70 (95% CI 0.64, 0.77) in years 2016 and 2017 and HR 0.65 (0.59, 0.72) in years 2018 and 2019.
- We also conducted analyses where we removed users with an event in the first 180 days of follow-up, because these events may be unlikely related to exposure to the antihyperglycemic medication. Results showed that compared with the control group, empagliflozin use was associated with reduced risk of the composite kidney outcome (HR 0.67 [95% CI 0.62, 0.73]).
- We alternatively defined eGFR decline >50% based on two eGFR measurements separated by at least 30 days, and the results were consistent (HR 0.61 [95% CI 0.51, 0.73]).
- We also alternatively defined ESKD as receipt of chronic dialysis, kidney transplantation, or eGFR <15 mL/min/1.73 m2 on two separate occasions at least 30 days apart, and the results were consistent (HR 0.53 [95% CI 0.36, 0.80]) (Supplementary Table 5).
To further test for possible spurious biases, we examined the association between empagliflozin and traffic-related injury as a negative control, where no prior knowledge suggests a causal association exists. No significant association between SGLT2i and traffic-related injury was found (HR 1.10 [95% CI 0.88, 1.38]) (Supplementary Table 5).
Per-Protocol Analyses
In a prespecified protocol, which required the continuation of the antihyperglycemic medication initiated at T0 throughout duration in cohort, 64.12% of the empagliflozin group and 61.66% of the control group adhered to the protocol in the weighted cohort. Empagliflozin use was associated with reduced risk of MAKE compared with the control group of other non-SGLT2i antihyperglycemics (HR 0.64 [95% CI 0.60, 0.70]).
In analyses of an additional prespecified protocol, which required participants to have concurrent use of metformin with empagliflozin or other non-SGLT2i antihyperglycemics in the first 90 days of follow-up, 46.54% of the empagliflozin group and 56.11% of the control group adhered to this protocol in the weighted cohort. Empagliflozin use was associated with reduced risk of MAKE compared with the control group (HR 0.63 [95% CI 60.57, 0.69]).
Conclusions
In this real-world study of people with type 2 diabetes and eGFR ≥30 mL/min/1.73 m2 corresponding to 644,311.59 person-years, incident use of the SGLT2i empagliflozin versus other non-SGLT2i antihyperglycemics was associated with eGFR preservation, a greater decline in BMI, and a reduced risk of MAKE, a composite of eGFR decline >50%, ESKD, or death. The salutary association was observed regardless of baseline eGFR, regardless of albuminuria status, in people with and without cardiovascular disease, and in several other prespecified subgroups. The association was also observed in two prespecified per-protocol analyses 1) requiring adherence to empagliflozin or the other non-SGLT2i antihyperglycemic medication throughout duration of follow-up, and 2) requiring concomitant use of metformin for the first 90 days. The results were robust to challenge in multiple sensitivity analyses.
Our results suggest that empagliflozin use is associated with eGFR preservation of 0.99 (95% CI 0.51, 1.55) mL/min/1.73 m2/year and BMI reduction of 0.25 (95% CI 0.16, 0.33) kg/m2/year, an observation that is consistent with results from RCTs and prior real-world evidence (8–11,17,43,44).
Until recently, SGLT2i use has been limited to people with eGFR >45 mL/min/1.73 m2. Except for the CREDENCE trial, which enrolled people with albuminuria and eGFR 30 to <90 mL/min/1.73 m2, all of the other RCTs of SGLT2i and kidney outcomes included a minority of patients with reduced kidney function or albuminuria. One real-world study included people with eGFR <60 mL/min/1.73 m2 (n = 2,747 [7.7%]), but did not report outcomes in those with eGFR <45 mL/min/1.73 m2 (43). Our data capture the recent increase in empagliflozin use in people with low eGFR and provide evidence of effectiveness that empagliflozin use was associated with a reduced risk of MAKE in people with eGFR 45 to <60 and ≥30 to <45 mL/min/1.73 m2—extending evidence of efficacy of canagliflozin in eGFR 45 to <60 and ≥30 to <45 mL/min/1.73 m2 from the CREDENCE trial and also providing plausibility to the notion of a salutary class effect of SGLT2i on kidney outcomes. Furthermore, the risk reduction associated with empagliflozin was observed regardless of albuminuria status at baseline, an observation congruent with analyses of the four major RCTs (Dapagliflozin Effect on Cardiovascular Events trial [DECLARE-TIMI 58], Canagliflozin Cardiovascular Assessment Study [CANVAS] Program, BI 10773 [Empagliflozin] Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients [EMPA-REG OUTCOME], and CREDENCE) (8–11,17). In addition, the reduced risk observed in patients with eGFR <60 mL/min/1.73 m2 and without albuminuria extends observations from CREDENCE and suggest that the beneficial effect of SGLT2i in patients with reduced kidney function is present regardless of albuminuria status. Taken together, the results from this analysis complement observations from prior RCTs by providing real-world evidence of effectiveness in several eGFR groups (including lower eGFR categories) and regardless of albuminuria status.
The observation that the SGLT2i empagliflozin use was associated with a reduced risk of MAKE in people with and without cardiovascular disease adds to known evidence from RCTs that mostly included people with established cardiovascular disease or at high risk of cardiovascular disease. In other subgroup analyses, empagliflozin use was associated with a reduced risk of MAKE regardless of baseline metformin, insulin, statin, or diuretic use and regardless of baseline BMI category. Overall, the results suggest that the salutary association of empagliflozin with MAKE is evident in clinically relevant subgroups and support the assessment that results from RCTs extend to broader populations with different characteristics.
Our analyses also provide supportive evidence that the risk reduction seen with the SGLT2i empagliflozin is evident regardless of baseline use of ACE/ARB. Most participants in EMPA-REG OUTCOME (80.7%), CANVAS Program (80%), DECLARE-TIMI 58 (81.3%), and CREDENCE (99.9%) received ACE/ARB (8–11,17). A meta-analysis of the effects of SGLT2i on substantial loss of kidney function, ESKD, and death due to kidney disease suggested a protective effect in participants who were on ACE/ARB at baseline and a trend toward a protective effect that was not statistically significant—likely due to the low number of trial participants—in those who were not on ACE/ARB at baseline (17). Our results complement and extend these observations by providing evidence that the salutary association of SGLT2i on the risk of kidney outcomes is present regardless of ACE/ARB use.
The association of empagliflozin with kidney outcomes was independent of glycemic control (HbA1c) and BMI, an observation consistent with prior studies that also suggests a glucose-independent mechanism likely involving reduced glomerular hyperfiltration, which may also explain the acute early reduction in eGFR in the SGLT2i group, which then stabilized to yield less annual reduction in eGFR (eGFR preservation) compared with the control arm (13,45,46). The results were robust to challenge in other sensitivity analyses, including variable ratio propensity score matching analysis, evaluation of the association when SGLT2i was starting to be used (2016 and 2017) and the most recent years (2018 and 2019), and evaluation of the individual components of the composite end point; the latter analysis also suggests that the association observed in the overall cohort was driven by the reduction in risk in each of the components of the composite outcome.
This study has several limitations. We relied on observational real-world data from the VA to build our cohort, which was mostly composed of older, White, and male participants, and therefore this may limit the generalizability of study findings. Although our analytic approach evaluated empagliflozin versus other active non-SGLT2i antihyperglycemics, considered known confounders, and applied a high-dimensional variable selection algorithm to more comprehensively capture potential confounding, we cannot completely rule out the possibility of residual confounding. We estimated the intention-to-treat effect, which may be limited by variable nonadherence among study participants; however, we also evaluated the study question in two prespecified per-protocol analyses that accounted for nonadherence. Our analyses did not examine risk of incident albuminuria or its progression, and we did not examine risk of adverse events.
The study has several strengths. We used large scale real-world data from the VA, which operates the largest integrated health care system in the U.S.; VA data are captured during routine clinical care, which might more closely recapitulate real-world experiences. We developed our research aim, study design, and execution to specifically address a knowledge gap of the comparative effectiveness of the SGLT2i empagliflozin versus an active comparator control on risk of kidney outcomes in people with type 2 diabetes who switched or added on an antihyperglycemic medication. In addition to reporting relative risk, we reported absolute risk differences in the overall cohort and in subgroups with different baseline risks, which may be clinically meaningful in informing choice of antihyperglycemic medication. We used a new user design with an active comparator, applied advanced statistical methodologies, including high-dimensional variable selection algorithms and inverse probability of treatment weighting, and reported both an intention-to-treat effect, which estimates the effectiveness of empagliflozin at the level of observed adherence in our cohort, and the per-protocol effect, which accounts for nonadherence and offers estimates of effectiveness that may be more generalizable across different settings (40). We examined the comparative effectiveness in prespecified subgroups, including those with low eGFR (≥45 to <60, and ≥30 to <45 mL/min/1.73 m2) and in subgroups based on albuminuria and cardiovascular disease. We tested robustness of results in multiple sensitivity analyses and applied a negative control to detect spurious associations.
In sum, real-world data suggest that compared with other antihyperglycemics, the SGLT2i empagliflozin was associated with eGFR preservation, a greater decline in BMI, and a significant reduction in the risk of MAKE of eGFR decline >50%, ESKD, or all-cause mortality among people with type 2 diabetes. The risk reduction was evident regardless of baseline eGFR, regardless of albuminuria status, and in several other prespecified clinically relevant subgroups. Overall, our results suggest that the salutary kidney effect of SGLT2i observed in RCTs likely extends to broader populations in real-world settings.
Article Information
Funding. This research was funded by the U.S. Department of Veterans Affairs (VA HSR&D I21-HX002995-01A1) and the Institute for Public Health at Washington University in St. Louis, St. Louis, MO (for Z.A.-A.), and American Society of Nephrology grants to Y.X. and B.B.
The funders of this study had no role in study design; collection, analysis, and interpretation of data; writing of the report; and the decision to submit the report for publication.
Duality of Interest. All authors have completed the International Committee of Medical Journal Editors uniform disclosure form at www.icmje.org/coi\_disclosure.pdf and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years, and no other relationships or activities that could appear to have influenced the submitted work. No potential conflicts of interest relevant to this article were reported.
Author Contributions. Y.X. performed statistical analysis. Y.X., B.B., A.K.G., J.B.M., Y.Y., G.M., and Z.A.-A. contributed to critical revision of the manuscript. Y.X., B.B., A.K.G., Y.Y., and Z.A.-A. contributed to data analysis and interpretation. Y.X., B.B., and Z.A.-A. contributed to research area and study design. Y.X. and A.K.G. acquired data. Y.X. and Z.A.-A. drafted the manuscript. Z.A.-A. provided administrative, technical, or material support. Z.A.-A. provided supervision and mentorship. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. Z.A.-A. takes responsibility that this study has been reported honestly, accurately, and transparently, that no important aspects of the study have been omitted, and that any discrepancies from the study as planned have been explained. Z.A.-A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government.
References
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017 [published correction appears in Lancet 2019;393:e44]. Lancet 2018;392:1789–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowe B, Xie Y, Li T, et al. Changes in the us burden of chronic kidney disease from 2002 to 2016: an analysis of the global burden of disease study. JAMA Netw Open 2018;1:e184412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie Y, Bowe B, Mokdad AH, et al. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int 2018;94:567–581 [DOI] [PubMed] [Google Scholar]
- 4.GBD 2017 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1859–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foreman KJ, Marquez N, Dolgert A, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018;392:2052–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perkovic V, Heerspink HL, Chalmers J, et al.; ADVANCE Collaborative Group . Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int 2013;83:517–523 [DOI] [PubMed] [Google Scholar]
- 7.Tuttle KR, Cherney DZ; Diabetic Kidney Disease Task Force of the American Society of Nephrology . Sodium glucose cotransporter 2 inhibition heralds a call-to-action for diabetic kidney disease. Clin J Am Soc Nephrol 2020;15:285–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosenzon O, Wiviott SD, Cahn A, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol 2019;7:606–617 [DOI] [PubMed] [Google Scholar]
- 9.Wanner C, Inzucchi SE, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–334 [DOI] [PubMed] [Google Scholar]
- 10.Neal B, Perkovic V, Mahaffey KW, et al.; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657 [DOI] [PubMed] [Google Scholar]
- 11.Perkovic V, Jardine MJ, Neal B, et al.; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306 [DOI] [PubMed] [Google Scholar]
- 12.Cefalu WT, Kaul S, Gerstein HC, et al. Cardiovascular outcomes trials in type 2 diabetes: where do we go from here? Reflections from a Diabetes Care Editors’ Expert Forum. Diabetes Care 2018;41:14–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heerspink HJ, Desai M, Jardine M, Balis D, Meininger G, Perkovic V. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol 2017;28:368–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scirica BM, Bhatt DL, Braunwald E, et al.; SAVOR-TIMI 53 Steering Committee and Investigators . Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–1326 [DOI] [PubMed] [Google Scholar]
- 15.Prischl FC, Wanner C. Renal outcomes of antidiabetic treatment options for type 2 diabetes-a proposed MARE definition. Kidney Int Rep 2018;3:1030–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 17.Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2019;7:845–854 [DOI] [PubMed] [Google Scholar]
- 18.Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus: systematic review and meta-analysis of cardiovascular outcomes trials. Circulation 2019;139:2022–2031 [DOI] [PubMed] [Google Scholar]
- 19.Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol 2019;7:776–785 [DOI] [PubMed] [Google Scholar]
- 20.Rosenstock J, Perkovic V, Johansen OE, et al.; CARMELINA Investigators . Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA 2019;321:69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkinson SV, Tomlinson LA, Iwagami M, Stirnadel-Farrant HA, Smeeth L, Douglas I. A systematic review comparing the evidence for kidney function outcomes between oral antidiabetic drugs for type 2 diabetes. Wellcome Open Res 2018;3:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019;393:31–39 [DOI] [PubMed] [Google Scholar]
- 23.Xie Y, Bowe B, Li T, Xian H, Balasubramanian S, Al-Aly Z. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J Am Soc Nephrol 2016;27:3153–3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z. Risk of death among users of Proton Pump Inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open 2017;7:e015735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int 2017;91:1482–1494 [DOI] [PubMed] [Google Scholar]
- 26.Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z. Higher blood urea nitrogen is associated with increased risk of incident diabetes mellitus. Kidney Int 2018;93:741–752 [DOI] [PubMed] [Google Scholar]
- 27.Vincent BM, Wiitala WL, Burns JA, Iwashyna TJ, Prescott HC. Using Veterans Affairs Corporate Data Warehouse to identify 30-day hospital readmissions. Health Serv Outcomes Res Methodol 2018;18:143–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.VA Information Resource Center VIReC Research User Guide: Veterans Health Administration Decision Support System Clinical National Data Extracts. Hines, IL, U.S. Department of Veterans Affairs, Health Servive Resource and Development Service, VA Information Resource Center, 2009
- 29.Maynard C. Ascertaining Veterans’ Vital Status: VA data sources for mortality ascertainment and cause of death. Database & Methods Cyberseminar Series, Washington, DC, U.S. Department of Veterans Affairs, 2017 [Google Scholar]
- 30.Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie Y, Bowe B, Li T, Xian H, Al-Aly Z. Blood urea nitrogen and risk of insulin use among people with diabetes. Diab Vasc Dis Res 2018;15:409–416 [DOI] [PubMed] [Google Scholar]
- 32.Hernán M. Antihyperglycemic Therapy and Cardiovascular Risk: Design and Emulation of a Target Trial Using Healthcare Databases, Washington, DC, Patient-Centered Outcomes Research Institute, 2019 [Google Scholar]
- 33.Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 2009;20:512–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie Y, Bowe B, Yan Y, Xian H, Li T, Al-Aly Z. Estimates of all cause mortality and cause specific mortality associated with proton pump inhibitors among US veterans: cohort study. BMJ 2019;365:l1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neugebauer R, Schmittdiel JA, Zhu Z, Rassen JA, Seeger JD, Schneeweiss S. High-dimensional propensity score algorithm in comparative effectiveness research with time-varying interventions. Stat Med 2015;34:753–781 [DOI] [PubMed] [Google Scholar]
- 36.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cepeda MS, Boston R, Farrar JT, Strom BL. Optimal matching with a variable number of controls vs. a fixed number of controls for a cohort study. trade-offs. J Clin Epidemiol 2003;56:230–237 [DOI] [PubMed] [Google Scholar]
- 38.Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology 2010;21:383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hernán MA, Hernández-Díaz S. Beyond the intention-to-treat in comparative effectiveness research. Clin Trials 2012;9:48–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernán MA, Robins JM. Per-protocol analyses of pragmatic trials. N Engl J Med 2017;377:1391–1398 [DOI] [PubMed] [Google Scholar]
- 41.Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000;11:561–570 [DOI] [PubMed] [Google Scholar]
- 42.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008;168:656–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heerspink HJL, Karasik A, Thuresson M, et al. Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): a multinational observational cohort study. Lancet Diabetes Endocrinol 2020;8:27–35 [DOI] [PubMed] [Google Scholar]
- 44.Wilkinson S, Williamson E, Pokrajac A, et al. Comparative effects of sulphonylureas, dipeptidyl peptidase-4 inhibitors and sodium-glucose co-transporter-2 inhibitors added to metformin monotherapy: a propensity-score matched cohort study in UK primary care. Diabetes Obes Metab 2020;22:847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heerspink HJL, Kosiborod M, Inzucchi SE, Cherney DZI. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int 2018;94:26–39 [DOI] [PubMed] [Google Scholar]
- 46.Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 2016;134:752–772 [DOI] [PubMed] [Google Scholar]