Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans (original) (raw)
. Author manuscript; available in PMC: 2020 Nov 6.
Summary
Rediscovery of cold-activated brown adipose tissue (BAT) in humans has boosted research interest into identifying BAT activators for metabolic benefits. Of particular interest are cytokines capable of fat browning. Irisin, derived from FNDC5, is an exercise-induced myokine that drives brown fat-like thermogenesis in murine white fat. Here we explored whether cold exposure is an afferent signal for irisin secretion in humans and compared it with FGF21, a brown adipokine in rodents. Cold exposure increased circulating irisin and FGF21. We found an induction of irisin secretion proportional to shivering intensity, in magnitude similar to exercise-stimulated secretion. FNDC5 and/or FGF21 treatment up-regulated human adipocyte brown fat gene/protein expression and thermogenesis in a depot-specific manner. These results suggest exercise-induced irisin secretion could have evolved from shivering-related muscle contraction, serving to augment brown fat thermogenesis in concert with FGF21. Irisin-mediated muscle-adipose crosstalk may represent a thermogenic, cold-activated endocrine axis, exploitable in obesity therapeutics development.
Keywords: irisin, FNDC5, FGF21, brown adipose tissue, beige adipose tissue, shivering thermogenesis, non-shivering thermogenesis
Introduction
Cold-induced thermogenesis (CIT) is the increase in heat production in response to acute ambient temperature reduction. It comprises non-shivering thermogenesis (NST) and shivering thermogenesis (ST). In rodents, the chief tissue mediating NST is brown adipose tissue (BAT), which releases heat through the action of uncoupling protein 1 (UCP1) (Cannon and Nedergaard, 2004). Heat demand not met by NST recruits ST, thereby generating heat from muscle contractions. Long-term cold exposure reduces shivering, conceivably a result of NST enhancement from cold acclimatization (Davis, 1961). In humans, the re-discovery of cold-activated BAT suggests a possible regulatory role of BAT in NST (Cypess et al., 2009; Saito et al., 2009; van Marken Lichtenbelt et al., 2009; Virtanen et al., 2009). However, the physiologic cues orchestrating NST and ST recruitment are unclear.
While adequate shelter and clothing in the modern society have minimized the hazards of cold temperatures, the obesity epidemic has re-ignited interest into exploring whether harnessing BAT may benefit weight control (Yoneshiro et al., 2013). Activated BAT may contribute up to 20% of CIT following mild cold exposure (Chen et al., 2013), representing a proportion of total energy expenditure (EE) sufficient to impact long-term energy balance. Identification of BAT endocrine activators may open new directions in obesity therapeutics development (Lee et al., 2013b).
Irisin is an exercise-induced myokine, which is secreted into the circulation following proteolytic cleavage from its cellular form, fibronectin-type III domain-containing 5 (FNDC5) (Bostrom et al., 2012). It reverses diet-induced obesity and diabetes by stimulating thermogenesis in rodents through increasing brown adipocyte-like cell abundance (brite (Petrovic et al., 2010)/beige (Wu et al., 2012) adipocytes) within white fat. As it appears paradoxical that exercise should increase secretion of a thermogenic hormone, it has been hypothesized that the mechanism evolved from shivering-related muscle contraction to augment NST through BAT expansion (Bostrom et al., 2012).
In this study, we tested this hypothesis by investigating the impact of cold exposure in healthy adults on irisin secretion and compared its excursion with the sympatho-thyroid-adrenal axes, principal regulators of CIT (Celi et al., 2010), as well as fibroblast growth factor 21 (FGF21), a recently identified brown adipokine that predicts NST response in humans (Lee et al., 2013a; Lee et al., 2013c). Finally, we examined in vitro the bioenergetic profiles of FNDC5- and FGF21-treated human adipocytes to determine their thermogenic significance.
Results and Discussion
Irisin detection in human serum
Circulating irisin, cleaved from FNDC5, is heavily glycosylated, and multiple bands are visible on serum immunoblot against anti-FNDC5 antibody (Bostrom et al., 2012). Because of the recent controversy over the circulating form of irisin (Erickson, 2013), we first determined the identity of FNDC5-immunoreactive bands detectable in human serum by mass spectrometry (MS).
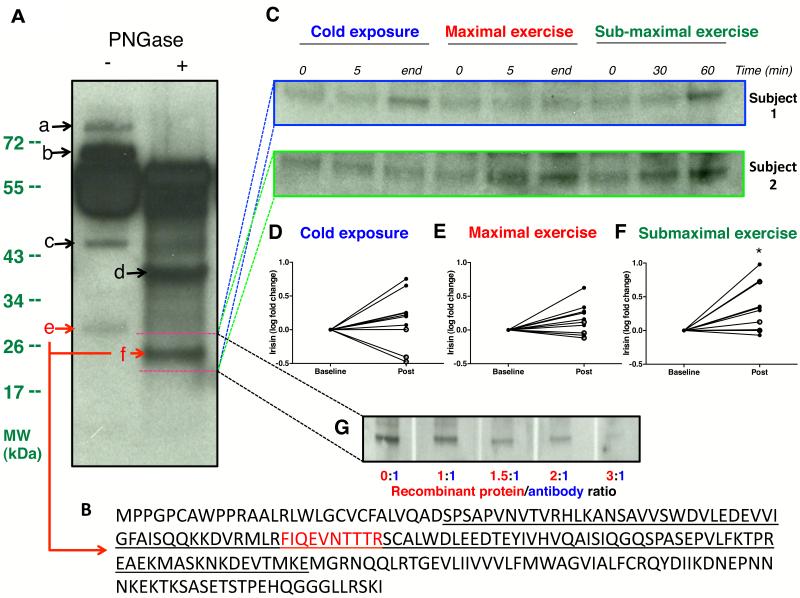
Consistent with previous reports, immunoblot of albumin/immunoglobulin depleted serum revealed multiple bands reactive to anti-FNDC5 antibody. Deglycosylation reduced the size of a 32 kDa band to 24 kDa, corresponding to reported molecular weights (MW) of glycosylated and deglycosylated irisin, respectively [Figure 1A] (Bostrom et al., 2012; Schumacher et al., 2013). MS analysis identified a unique peptide, mapped to the known sequence of irisin, only within the 32 kDa and 24 kDa bands [Figure 1B]. These results thus validated immunoblot identification of circulating irisin in humans. To further ascertain specificity of antibody used, we also demonstrated successful quenching of irisin signal by excess recombinant protein [Figure 1G].
Figure 1. Validation of immunoblot-detected irisin by mass spectrometry.
Immunoblot of paired serum samples following albumin/immunoglobulin depletion, against anti-FNDC5 antibody revealed multiple distinct bands (a-f) [Panel A]. PNGase treatment reduced size of band e (~32 kDa) to band f (~24 kDa). Panel B showed the amino acid sequence of full length FNDC5 with the secreted irisin segment underlined. Mass spectrometry analysis of all bands (a-f) identified a specific peptide (in red), unique to irisin, only in band e and band f, with molecular weights matching those of glycosylated and deglycosylated irisin, respectively. Panel C showed representative immunoblots of serum irisin for fold change quantification from 2 subjects during cold exposure, maximal exercise and sub-maximal exercise. Subject 1 shivered during cold exposure while subject 2 did not. Accordingly, deglycosylated irisin band (~24 kDa) was stronger at the end of cold exposure only in Subject 1. In contrast, irisin band was stronger after submaximal exercise in both subjects. Full sized blots are shown in Figure S1. Panels D-F are graphical representation of serum irisin fold changes during cold exposure, maximal and sub-maximal exercise tests, respectively, of all 10 subjects. “Post” indicates the average band intensity of irisin extracted from the mid- and final blood samples of each clinical test. Similar results were obtained when analysis was conducted comparing irisin band intensity between baseline and final sample alone. Irisin level rose significantly following sub-maximal exercise [Panel F], and trended higher (p=0.07) after maximal exercise [Panel E]. Irisin levels increased only in the 7 subjects who shivered [closed circles, Panel D], but not those who did not [open circles, Panel D]. Panel G demonstrates neutralization of anti-FNDC5 antibody by FNDC5 recombinant protein. FNDC5 antibody mixture in increasing ratio resulted in quenching of western signal in a dose-dependent manner by excess FNDC5 recombinant protein. *P<0.05. Data are presented as mean ± SD.
Exercise increases serum irisin levels in humans
To understand the interrelationships between exercise, cold exposure and irisin, we compared irisin secretion in 10 healthy adults (4 females, 27±5 years old, body mass index (BMI): 22±2 kg/m2, body fat (BF): 24±9%) following graded, step-wise cold exposure (27°C to 12°C, Figure S1) with two forms of standard exercise tests: exercise on cycloergometer to maximal capacity (VO2max) and sub-maximal exercise test at 40% VO2max for 1 hour.
Serum irisin levels trended higher (p=0.07) after maximal exercise [Figure 1C and E]. After 60 minutes of sub-maximal exercise, irisin levels rose by 3.1±2.8 fold (p<0.05) [Figure 1C and F]. These results thus replicated the known stimulatory effect of exercise on irisin secretion (Bostrom et al., 2012; Huh et al., 2012). The greater irisin increase during sub-maximal exercise compared to maximal exercise suggests endurance exercise maybe a more potent stimulus of irisin secretion, consistent with finding of higher FNDC5 expression in oxidative vs. glycolytic muscle fibres (Wrann et al., 2013).
Shivering is an afferent signal of irisin secretion
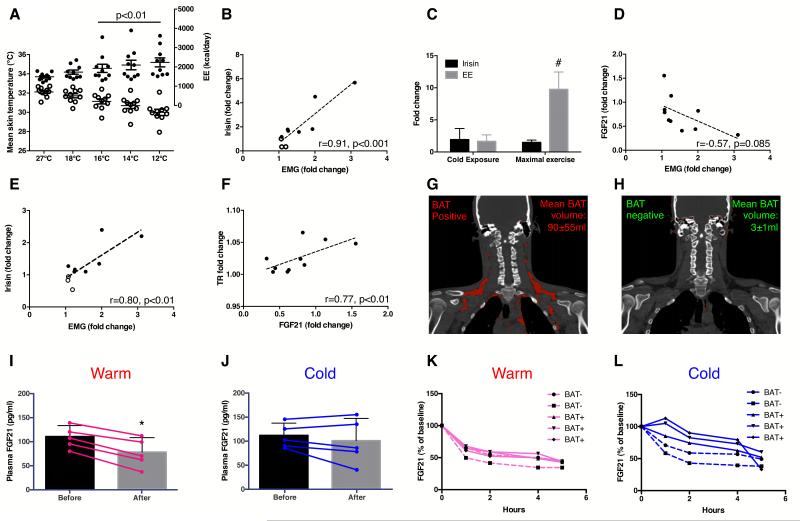
We next determined the impact of cold temperature on irisin changes. Upon cold exposure, skin temperature decreased in all subjects (p<0.0001) while core temperature was preserved [Table 1 and Figure 2A]. Arm to hand, skin to core and supraclavicular to chest temperature gradients increased by 13±18% (p=0.01), 45±15% (p<0.0001) and 4±3% (p<0.0001), signifying vasoconstrictive, insulative and thermogenic responses, respectively. EE rose by 48±37% (p<0.01), representing CIT response [Figure 2A]. Seven subjects reported shivering and shivering activity, quantified by surface electromyography (EMG), increased during cold exposure (p<0.01) [Table 1]. The increase in EMG activity was 88±80% in individuals who shivered, and 13±9%, among those who did not (p<0.05). Our cooling protocol thus elicited the full spectrum of CIT response, allowing the interrogation of irisin-CIT interrelationships. Irisin changes correlated the strongest with shivering among all CIT components [Table S1]. Circulating irisin rose in the 7 subjects who shivered [Figure 1C and D] and changes in irisin levels correlated positively with shivering activity (r=0.91, p<0.001) [Figure 2B]. To ensure accuracy of our quantification, we also demonstrated concordant irisin changes measured with a commercially available irisin enzyme-linked immunosorbant assay [Figure 2E and Figure S1D-F], which has been validated against immunoblotting (Wen et al., 2013).
Table 1.
Physiologic changes during cold exposure and hormonal profile during cold exposure and exercise tests in 10 subjects.
| Physiologic variables | ||||||
|---|---|---|---|---|---|---|
| Body temperature (°C) | 27°C | 18°C | 16°C | 14°C | 12°C | Trendp-value |
| Core | 36.9±0.2 | 36.9±0.2 | 36.9±0.2 | 36.9±0.2 | 36.9±0.2 | 0.65 |
| Skin | 32.1±0.5 | 31.7±0.7 | 31.1±0.8 | 30.7±0.9 | 30.0±1.0 | <0.0001 |
| Gradient, core-skin | 4.5±0.8 | 4.8±0.9 | 5.4±1.1 | 5.9±1.2 | 6.5±1.2 | <0.0001 |
| Gradient, arm-hand | −6.7±1.7 | −7.1±1.8 | −7.4±1.8 | −7.2±1.8 | −7.3±1.5 | 0.01 |
| Gradient, supraclavicular-chest | 1.04±0.03 | 1.05±0.03 | 1.06±0.04 | 1.07±0.05 | 1.09±0.06 | <0.0001 |
| Resting EE (kcal/day) | 1488±196 | 1731±337 | 1926±673 | 2100±785 | 2235±738 | 0.007 |
| Respiratory quotient | 0.78±0.03 | 0.85±0.07 | 0.79±0.07 | 0.77±0.07 | 0.79±0.08 | 0.001 |
| Surface electromyography (×10−6 RMS) | 2.5±5.7 | 3.1±2.1 | 4.0±4.0 | 4.7±3.6 | 4.9±3.3 | 0.044 |
| Hormonal variables | ||||||
| Cold exposure | Maximal exercise | Sub-maximal exercise | ||||
| Baseline | End | Baseline | End | Baseline | End | |
| Epinephrine (pg/ml) | 38±25 | 110±73* | 237±418 | 300±316 | 180±406 | 311±558* |
| Norepinephrine (pg/ml) | 658±306 | 1101±449* | 727±264 | 1568±541* | 714±173 | 974±209* |
| Glucose (mg/dL) | 84±3 | 86±2 | 91±6 | 117±22* | 87±4 | 90±10 |
| Insulin (U/L) | 4.2±1.1 | 5.3±1.1 | 6.8±3.6 | 21.4±22.6* | 6.6±3.5 | 7.9±5.2 |
| HOMA IR | 1.0±0.8 | 1.3±0.8 | 1.5±0.9 | 6.5±6.7* | 1.4±0.8 | 1.8±1.2 |
| NEFA (μEq/L) | 0.55±0.07 | 0.57±0.07 | 0.49±0.19 | 0.45±0.19 | 0.47±0.23 | 0.65±0.22* |
| TSH (mlU/L) | 1.9±0.3 | 2.1±0.3 | 2.1±0.7 | 2.5±0.9* | 1.9±0.6 | 1.9±0.7 |
| Free T4 (ng/dL) | 1.0±0.0 | 1.0±0.0 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 |
| Free T3 (pg/dL) | 320±17 | 346±13 | 299±43 | 296±47 | 312±45 | 311±41 |
| Total T3 (ng/dL) | 134±22 | 139±17 | 115±29 | 114±16 | 121±23 | 117±17 |
| ACTH (pg/ml) | 17±9 | 19±6 | 27±16 | 156±133* | 19±10 | 37±31* |
| Cortisol (μg/dL) | 12±3 | 15±5 | 16±5 | 18±6 | 14±4 | 16±5 |
| FGF21 (pg/ml) | 129±76 | 98±90 | 135±80 | 102±97 | 119±79 | 97±85 |
Figure 2. Relationship between irisin, FGF21, BAT and temperature.
Cold exposure resulted in reduction in skin temperature [open circles, Panel A], accompanied by a rise in energy expenditure (EE) [closed circles], both reaching significance from 16-12°C. Panels B (by immunoblot) and E (by ELISA) showed positive associations between irisin and EMG fold changes during cold exposure. Panel C compared irisin and EE fold changes during cold exposure with maximal exercise test. Changes in FGF21 levels correlated negatively with shivering [Panel D] but positively with thermogenic response (TR; difference between supraclavicular skin and chest T°C) [Panel F]. Panels G-H showed representative PET-CT images of BAT positive (N=3) and negative (N=2) individuals, respectively (BAT in red). FGF21 diurnal reduction was more markedly blunted in BAT positive (solid lines) compared to negative (dashed lines) individuals at 19°C [Panel L] vs. 24°C [Panel K]. Panels I-J compared FGF21 changes (N=5) measured between 8-10 am at either warm (27°C) or shivering (12°C) conditions. FGF21 reduction was significantly blunted in the cold. *P<0.05 compared to warm condition; #p<0.001 compared to cold exposure. Data are presented as mean ± SD.
As exercise is the only known activator of irisin secretion, we compared cold- and exercise-induced irisin changes. The increment in irisin was similar during the two tests [Figure 2C]. However, the increase in EE was significantly greater during maximal exercise, compared to cold exposure (9.8±2.4 vs. 1.5±0.4 fold, p<0.0001). The dissociation between irisin and EE responses suggests additional cold-specific signals, unrelated to muscle contraction, potentiating irisin secretion during shivering. As NST, mediated by BAT (Cannon and Nedergaard, 2004) and muscle (Bal et al., 2012), is activated with ST, we explored whether fibroblast growth factor-21 (FGF21), a brown adipokine in rodents (Chartoumpekis et al., 2011; Hondares et al., 2011) and humans (Lee et al., 2013c) relates to shivering-induced irisin secretion.
Distinct involvement of irisin and FGF21 during shivering and non-shivering thermogenesis
In agreement with known diurnal reduction in circulating FGF21 levels in humans (Yu et al., 2011), FGF21 concentration trended lower during all three tests (cold exposure and exercise tests) undertaken between 8:00 and 10:00 am [Table 1]. Greater FGF21 reduction was associated with more intense shivering, albeit not reaching significance (p=0.08) [Figure 2D]. We interpret a greater reduction in FGF21 levels as lesser FGF21 secretion, indicating a lower NST response (Lee et al., 2013a), leading to ST recruitment for additional heat generation. This is corroborated by a positive correlation observed between supraclavicular skin temperature (i.e. an index of BAT activity (Lee et al., 2011)) and FGF21 changes [Table S1, Figure 2F]. To further substantiate our interpretation of cold-induced FGF21 secretion, we undertook two additional experiments in separate groups of subjects to characterize i) relationships between BAT and FGF21 and ii) temperature-dependency of FGF21 diurnal rhythm.
First, to elucidate whether BAT is a significant source of cold-augmented FGF21 secretion, we profiled FGF21 excursions in 5 men (21±2 years old, BMI: 22±1 kg/m2, BF: 21±2%) stratified to BAT status during 5 hours of either mildly cold, non-shivering condition (19°) vs. thermoneutrality (24°) [Figure 2G-H]. FGF21 diurnal reduction was blunted at 19° in the group as a whole by 23±17% (p<0.05). However, the blunting effect was markedly greater in BAT positive, compared to BAT negative subjects [Figure 2K-L], translating to a total FGF21 output more than 6-fold higher in BAT positive individuals. Since the subjects were of similar age and leanness, and differed only by BAT status, these associative results support BAT as a source of FGF21 during cold exposure in humans.
Second, to verify FGF21 diurnal rhythm is indeed temperature-sensitive, we measured FGF21 changes in another 5 men (26±6 years old, BMI: 23±2 kg/m2, BF: 19±3%) under same shivering-inducing cold exposure employed in the main study, but on a separate day exposed the same subjects to a warm temperature (27°), during which FGF21 was measured at matching time points. Cold exposure blunted FGF21 diurnal reduction by 28±23% (p=0.02) [Figure 2I-J]. Pooling the results in these 5 subjects with the 10 subjects originally studied, we observed a significant positive association between FGF21 diurnal reduction and shivering intensity (r=0.53, p<0.05).
Collectively, our findings indicate concerted stimulated secretion of FGF21 and irisin during NST and ST, respectively. In other words, shivering stimulates irisin secretion in a FGF21-primed milieu, through a mechanism mimicked by muscle contraction during exercise, which offers a plausible reconciliation for the paradox of why exercise, an energy-dissipating process, should stimulate the release of a thermogenic hormone.
FNDC5/FGF21 induce expression of beige gene transcriptome in human neck adipocytes
These results led us to probe the biological significance underlying enhanced irisin and FGF21 secretion during cold exposure. We hypothesize the two hormones are cold-activated to boost whole body thermogenic capacity by switching on brown fat-like program in white fat. We thus examined in vitro the bioenergetic profiles of FNDC5- and FGF21-treated primary human adipocytes established from neck fat biopsies, a location known to be enriched with beige adipocytes.
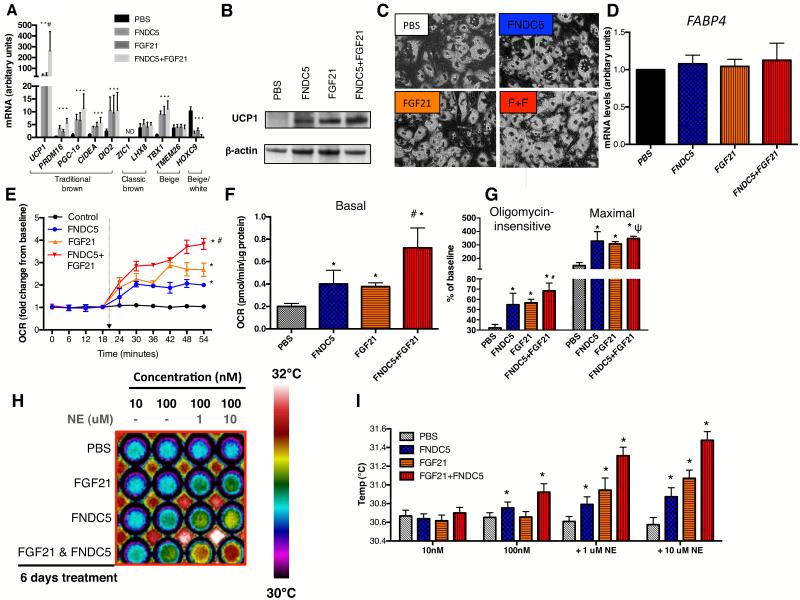
While stimulated beige adipocytes manifest similar thermogenic capacity as classic brown adipocytes, they are characterized by a distinct gene signature (Cypess et al., 2013; Jespersen et al., 2013; Sharp et al., 2012; Wu et al., 2012). We therefore first determined the impact of FNDC5 and/or FGF21 treatment on classic brown and beige gene expression in human neck adipocytes. FNDC5- and/or FGF21 treatment increased general BAT and beige gene expression without altering those belonging to the classic brown fat lineage [Figure 3A]. UCP1 protein, absent in untreated adipocytes, became strongly expressed following FNDC5 and/or FGF21 treatment [Figure 3B]. Pre- and post-treatment adipocytes displayed similar morphology, lipid-accumulation and general adipogenic gene expression [Figure 3C and D], indicating thermogenic gene up-regulation was not a result of more efficient differentiation.
Figure 3. Effects of FNDC5 and/or FGF21 treatment on gene/protein expression and bioenergenetics of neck adipocytes.
Panel A showed effects of FGF21 and/or FNDC5 treatment on BAT/beige/white gene markers in neck adipocytes (N=6). UCP1 protein was absent in PBS-treated adipocytes, but was detected following FGF21 and/or FNDC5 treatment [Panel B]. UCP1 protein was highest in adipocytes treated with dual FGF21/FNDC5. Neck adipocytes displayed multi-lobulated lipid droplets (40x), similar before and after treatment [Panel C, F+F=FNDC5+FGF21 treatment]. Expression of FABP4, a general adipogenic gene, was not different following treatment [Panel D]. Induction of UCP1 was accompanied by up-regulation of basal [Panel F], oligomycin-insensitive, maximal uncoupled [Panel G] and norepinephrine-induced [Panel E] oxygen consumption, most robust in dual FGF21/FNDC5-treated adipocytes (N=4). Panel H showed infrared thermographic images of adipocytes in microplates treated with PBS, FGF21 and/or FNDC5 (N=4). The temperature scale showed color representation of temperature variation. Heat production was increased in the basal state by FNDC5- but not FGF21. Addition of norepinephrine (NE) increased heat production in increasing magnitude in FGF21-, FNDC5- and dual FGF21/FNDC5-treated adipocytes. These results are displayed in graphical format in Panel I. *p<0.05 compared to PBS, #p<0.05 compared to FNDC5- or FGF21-treated adipocytes and ψp<0.05 compared to FGF21-treated adipocytes. Data are presented as mean ± SD.
Bioenergetic activation of human neck adipocytes by FNDC5/FGF21
We next investigated the functional impact of FNDC5 and FGF21 on adipocyte thermogenic function. We chose a treatment duration of 6 days, as guided by previous studies reporting a reduction of shivering in cold acclimatized humans after 1 week (Davis, 1961). FNDC5 and/or FGF21 enhanced adipocyte basal oxygen consumption rate (OCR) [Figure 3F], measured using an extracelluar fluid bioanalyzer. Pharmacological interrogation of mitochondrial respiration revealed augmentation of both forms of respiratory uncoupling (oligomycin-insensitive and maximal) by FNDC5 and FGF21 treatment [Figure 3G]. To mimic cold exposure in vitro, we measured norepinephrine-induced thermogenesis. While untreated adipocytes did not respond to norepinephrine, FNDC5 and FGF21 both induced a robust increase in OCR upon norepinephrine exposure [Figure 3E], thus recapitulated the observed cold-induced hormonal response in vivo. Combined FNDC5/FGF21 treatment produced greater responses than either hormone alone [Figure 3]. Treating adipocytes with irisin instead of FNDC5 resulted in similar extent of fat browning on gene, protein and functional levels [Figure S2A, C, D and E], consistent with previous findings in murine fat cells (Wu et al., 2012), and suggests both FNDC5 and irisin in the circulation could be biologically active at adipose tissue.
FNDC5/FGF21 increases human neck adipocyte heat production
As the primary function of BAT is to generate heat, we next quantified heat production from adipocytes directly by infrared thermography (IRT) [Figure S2G]. FNDC5 treatment enhanced adipocyte heat production dose-dependently [Figure 3H-I], which was further augmented by norepinephrine. In contrast, FGF21 increased heat production only after norepinephrine exposure. Additive effects were again observed following combined FNDC5/FGF21 treatment.
Taken together, these in vitro experiments provide mechanistic insight into our in vivo observations. It is conceivable that shivering-stimulated irisin, in concert with FGF21, phenotypically transform white adipocytes to BAT-like cells to expand overall thermogenic capacity. This heat-generating hormonal response may confer an evolutionary advantage in the defense against environmental hypothermic challenges by boosting the more energy-efficient NST response over shivering.
Fat depot-specific effects of FNDC5/FGF21
From a clinical perspective, a 2-3 fold increase in adipocyte OCR following FNDC5/FGF21 treatment, if extrapolated to the whole body EE level, could be substantial. However, the in vivo relevance is dependent on the generalizability of our findings to other fat depots. We therefore repeated FNDC5/FGF21 experiments in primary human subcutaneous and visceral adipocytes. FNDC5/FGF21 enhanced BAT-like thermogenic program in subcutaneous but not omental adipocytes, and the magnitude of thermogenic activation was less compared to those observed in neck adipocytes [Figure S3 and S2F]. Beige gene expression was either low (subcutaneous adipocytes) or absent (omental adipocytes). In rodents, irisin treatment only increased UCP1 in beige fat gene-expressing adipocytes (Wu et al., 2012). Lower/absent beige gene expression in subcutaneous and omental adipocytes may account for the modest or lack of response of these adipocytes to FNDC5/FGF21.
Metabolic significance and clinical implications
Although our sample size is relatively small, our results are physiologically and clinically relevant. First, they uncover an intriguing evolutionary interconnection between exercise and shivering, juxtaposing at the muscle-fat interface through cold-induced endocrine BAT activators. As irisin levels were higher in shivering subjects, we hypothesize muscle to be the main contributor to the observed irisin rise, although adipose-derived irisin cannot be excluded (Roca-Rivada et al., 2013). Second, while the sympathetic nervous system (SNS) is the best-known mediator of CIT, recent evidence points to the existence of specific cold-induced neuroendocrine signals in animals, whose actions are highly fat-specific, without undesirable global SNS activation (Villarroya and Vidal-Puig, 2013). Our study provided evidence supporting similar fat browning capacity of two of these novel cytokines, irisin and FGF21. Third, our irisin detection validation clarifies recent concerns over specificity of irisin immunoblotting (Erickson, 2013). Although the antibody used recognizes a peptide present in FNDC5, which is theoretically lost during irisin-specific proteolytic cleavage, it is possible that shivering induces release of FNDC5 fragments that harbor the antibody-reactive peptide. While the dynamics of irisin secretion remains to be clarified in future studies, identification of the same peptide sequence shared by circulating irisin (shown in our clinical study) and recombinant FNDC5/irisin proteins used in our in vitro experiments offers renewed perspective over controversy on the relevance of irisin in human biology (Atherton and Phillips, 2013). Fourth, our finding of fat depot-specific browning by FNDC5 suggests adipocyte browning potential may impact response to FNDC5/irisin, and may account for negative results utilizing solely subcutaneous adipocytes (Raschke et al., 2013). Finally, long-term maintenance of regular exercise is challenging, and natural human tendency for thermal comfort limits cold exposure in contemporary society. Irisin and FGF21 may represent endocrine mimics of these thermogenic stimuli, and are therefore potential therapeutic targets to attain weight control and to improve overall metabolic profile.
EXPERIMENTAL PROCEDURES
Clinical Studies
Healthy volunteers provided written informed consent. NIDDK-NIAMS institutional review board approved the studies (ClinicalTrials.gov: NCT00521729 and NCT01730105). We conducted 3 sets of studies: i) Main Study consisted of 3 experiments: cold exposure, maximal and sub-maximal exercise tests, ii) PET-CT Study involved FGF21 profiling in volunteers stratified to BAT status and iii) Temperature Study to determine temperature dependence of FGF21 diurnal rhythm.
Main Study
These tests allowed comparison of hormone/substrate profiles during active muscle contractions with cold-induced shivering. Volunteers were admitted after an overnight fast (September 2012-March 2013) for each test, performed at least 3 days apart.
Cold exposure test
Ten volunteers wearing hospital scrubs rested in a bed in a room at 24°C. Two water-infused thermo-blankets (Gaymar Medi Therm, Louisville KY) were used to adjust rapidly temperature exposure [Figure S1A]. Thirty minutes of resting energy expenditure (EE) measurement was obtained by indirect calorimetry when water temperature was at 27°C, after which it was cooled to 18°C, then further lowered by 2°C every 3 minutes until 12°C was reached. EE measurement continued throughout this period and the test concluded after 5 minutes at 12°C. Shivering intensity was measured by surface electromyography (EMG) (Trigno, DelSys Inc, Boston, MA).
Exercise tests
Maximal exercise test was performed on a mechanically braked cycle ergometer (Ergomedic 839E, Monark Exercise, Vansbro, Sweden). A stepwise incremental exercise test was performed to assess maximal aerobic capacity (VO2max) using breath-by-breath analysis. For the sub-maximal exercise test, volunteers cycled for 1 h at an intensity of 40% of VO2max.
Laboratory measurements
Three blood samples were obtained during each test for hormone/substrate measurements: baseline, 5 minutes after the start of cooling or maximal exercise, and at the conclusion of the test. For the sub-maximal exercise test, samples were obtained at baseline, 30 minutes into, and at the end of the test. Serum irisin was measured by western blotting (Bostrom et al., 2012) and plasma FGF21 by enzyme immunoabsorbant assays.
PET-CT study
Five subjects were studied after an overnight fast. They were exposed to either 24 hour of mild cold (19°C) or thermoneutral temperature (24°C). Blood samples were obtained at 0800, 0900, 1000, 1200 and 1300, corresponding to 0, 1, 2, 4, 5 hours after exposure to testing temperature. FGF21 levels were measured by same ELISA as Main Study. At the end of 24-hour exposure to 19°C, each subject received a 5mCi dose of 18Fluro-deoxyglucose (FDG) at 08:00 and underwent PET-CT scanning.
Temperature study
Five subjects underwent same cold exposure study as those in the Main Study. Blood samples were obtained at baseline and at the end of the cold exposure study for FGF21 measurements and correlation with shivering activity. On a separate day, subjects returned and were exposed to a constant warm temperature at 27°C for the same duration as the cold exposure testing period. Blood samples were obtained at matching time points for FGF21 measurements to directly compare with levels obtained during cold exposure.
In vitro studies
Adipocyte culture
Thermogenic effects of FNDC5/FGF21 were tested on primary adipocytes established from human cervical, subcutaneous and omental fat, as previously described (Lee et al., 2013c).
Gene/protein expression and thermogenesis
Standard techniques were used for RNA/protein extraction and analysis by semi-quantitative real-time PCR and immunoblotting. Cellular respiration was measured by XF24-3 extracellular flux analyzer (Seahorse Bioscience). Heat production was measured by infrared thermography (FLIR systems), as previously described (Lee et al., 2013c).
Additional clinical/laboratory experimental details and PET-CT scanning analytical methods are available in Supplemental File.
Statistical analysis
Statistical analysis was performed using SPSS 20.0 (SPSS, Inc., Chicago, IL, USA). Data are expressed as mean±standard deviation (SD). Comparisons between results during graded cold exposure (Main Study) and FGF21 time course (PET-CT study) were performed using repeated measure ANOVA with Bonferroni’s correction. Paired t test was used for comparison of measurements at 24 and 19°C. Data not normally distributed were log-transformed before analysis but are presented in the text non-transformed. Pearson correlation coefficients were used to examine linear relations between variables. Areas under the curve were calculated using the trapezoidal rule. An α error of 0.05 was considered the threshold for statistical significance.
Supplementary Material
01
Acknowledgements
Paul Lee was supported by an Australian National Health Medical Research Council (NHMRC) Early Career Fellowship, the Royal Australasian College of Physicians (RACP) Foundations Diabetes Australia Fellowship and Bushell Travelling Fellowship, and the School of Medicine, University of Queensland, Australia and. This study was supported by the Intramural Research Program of NIDDK: programs Z01-DK047057-02, Z01-DK071044, Z01-DK071013 and Z01-DK071014.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Atherton PJ, Phillips BE. Greek goddess or Greek myth: the effects of exercise on irisin/FNDC5 in humans. J Physiol. 2013;591:5267–5268. doi: 10.1113/jphysiol.2013.265371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal NC, Maurya SK, Sopariwala DH, Sahoo SK, Gupta SC, Shaikh SA, Pant M, Rowland LA, Bombardier E, Goonasekera SA, et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat Med. 2012;18:1575–1579. doi: 10.1038/nm.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Celi FS, Brychta RJ, Linderman JD, Butler PW, Alberobello AT, Smith S, Courville AB, Lai EW, Costello R, Skarulis MC, et al. Minimal changes in environmental temperature result in a significant increase in energy expenditure and changes in the hormonal homeostasis in healthy adults. Eur J Endocrinol. 2010;163:863–872. doi: 10.1530/EJE-10-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoumpekis DV, Habeos IG, Ziros PG, Psyrogiannis AI, Kyriazopoulou VE, Papavassiliou AG. Brown adipose tissue responds to cold and adrenergic stimulation by induction of FGF21. Mol Med. 2011;17:736–740. doi: 10.2119/molmed.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KY, Brychta RJ, Linderman JD, Smith S, Courville A, Dieckmann W, Herscovitch P, Millo CM, Remaley A, Lee P, et al. Brown fat activation mediates cold-induced thermogenesis in adult humans in response to a mild decrease in ambient temperature. J Clin Endocrinol Metab. 2013;98:E1218–1223. doi: 10.1210/jc.2012-4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, Sass CA, Huang TL, Roberts-Toler C, Weiner LS, Sze C, et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med. 2013;19:635–639. doi: 10.1038/nm.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TR. Chamber cold acclimatization in man. J Appl Physiol. 1961;16:1011–1015. doi: 10.1152/jappl.1961.16.6.1011. [DOI] [PubMed] [Google Scholar]
- Erickson HP. Irisin and FNDC5 in retrospect: An exercise hormone or a transmembrane receptor? Adipocyte. 2013;2:289–293. doi: 10.4161/adip.26082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T, Villarroya F. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem. 2011;286:12983–12990. doi: 10.1074/jbc.M110.215889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, Mantzoros CS. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61:1725–1738. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen NZ, Larsen TJ, Peijs L, Daugaard S, Homoe P, Loft A, de Jong J, Mathur N, Cannon B, Nedergaard J, et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 2013;17:798–805. doi: 10.1016/j.cmet.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Lee P, Brychta RJ, Linderman J, Smith S, Chen KY, Celi FS. Mild cold exposure modulates fibroblast growth factor 21 (FGF21) diurnal rhythm in humans: relationship between FGF21 levels, lipolysis, and cold-induced thermogenesis. J Clin Endocrinol Metab. 2013a;98:E98–102. doi: 10.1210/jc.2012-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Ho KK, Greenfield JR. Hot fat in a cool man: infrared thermography and brown adipose tissue. Diabetes Obes Metab. 2011;13:92–93. doi: 10.1111/j.1463-1326.2010.01318.x. [DOI] [PubMed] [Google Scholar]
- Lee P, Swarbrick MM, Ho KK. Brown adipose tissue in adult humans: a metabolic renaissance. Endocr Rev. 2013b;34:413–438. doi: 10.1210/er.2012-1081. [DOI] [PubMed] [Google Scholar]
- Lee P, Werner CD, Kebebew E, Celi FS. Functional thermogenic beige adipogenesis is inducible in human neck fat. Int J Obes (Lond) 2013c doi: 10.1038/ijo.2013.82. doi: 10.1038/ijo.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschke S, Elsen M, Gassenhuber H, Sommerfeld M, Schwahn U, Brockmann B, Jung R, Wisloff U, Tjonna AE, Raastad T, et al. Evidence against a Beneficial Effect of Irisin in Humans. PLoS One. 2013;8:e73680. doi: 10.1371/journal.pone.0073680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca-Rivada A, Castelao C, Senin LL, Landrove MO, Baltar J, Belen Crujeiras A, Seoane LM, Casanueva FF, Pardo M. FNDC5/irisin is not only a myokine but also an adipokine. PLoS One. 2013;8:e60563. doi: 10.1371/journal.pone.0060563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher MA, Chinnam N, Ohashi T, Shah RS, Erickson HP. The Structure of Irisin Reveals a Novel Intersubunit beta-Sheet Fibronectin Type III (FNIII) Dimer: IMPLICATIONS FOR RECEPTOR ACTIVATION. J Biol Chem. 2013;288:33738–33744. doi: 10.1074/jbc.M113.516641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, Hu H, Wang L, Pavlova Z, Gilsanz V, et al. Human BAT Possesses Molecular Signatures That Resemble Beige/Brite Cells. PLoS One. 2012;7:e49452. doi: 10.1371/journal.pone.0049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- Villarroya F, Vidal-Puig A. Beyond the sympathetic tone: the new brown fat activators. Cell Metab. 2013;17:638–643. doi: 10.1016/j.cmet.2013.02.020. [DOI] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- Wen MS, Wang CY, Lin SL, Hung KC. Decrease in irisin in patients with chronic kidney disease. PLoS One. 2013;8:e64025. doi: 10.1371/journal.pone.0064025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM. Exercise Induces Hippocampal BDNF through a PGC-1alpha/FNDC5 Pathway. Cell Metab. 2013;18:649–659. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, Iwanaga T, Saito M. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest. 2013;123:3404–3408. doi: 10.1172/JCI67803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Xia F, Lam KS, Wang Y, Bao Y, Zhang J, Gu Y, Zhou P, Lu J, Jia W, et al. Circadian rhythm of circulating fibroblast growth factor 21 is related to diurnal changes in fatty acids in humans. Clin Chem. 2011;57:691–700. doi: 10.1373/clinchem.2010.155184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01