Reply to Lebeaux D, Revest M. No evidence of clinical benefits of early treatment of COVID-19 patients with hydroxychloroquine and azithromycin (original) (raw)
Dear editor,
We read with interest the comment by Lebeaux et al. [1] questioning the evidence of clinical benefits of early testing and treatment of COVID-19 patients with hydroxychloroquine (HCQ) and azithromycin (AZ) reported in our study.
The authors of the commentary suggest that, due to our strategy of early and massive non-selective screening, the good evolution (low mortality of 0.75%) was close to that observed in the untreated general population and was influenced by the selection of young healthy patients with mild infections. However, the strategy of massive and early screening is the most effective non-pharmaceutical intervention according to several studies. Lai et al. [2] compared three major groups of non-pharmaceutical interventions: the restriction of intercity population movement, the identification and isolation of cases, and the reduction of travel and contact within cities to increase social distance. The early detection and isolation of cases quickly and substantially prevented more infections than did the introduction of contact reduction and social distancing measures across the country [2]. On the other hand, a French microbiologist, Vincent Jarlier, showed very clearly the negative correlation between the death attack rate (per 100,000 inhabitants) and the number of diagnostic tests, whereas there were weak correlations between the death attack rate and population density, the degree of urbanization or the proportion of the population over 65 years of age (https://infogram.com/graphiques-covid_new-1hd12ymxl5gx2km). As mentioned by the authors, countries that have applied an interventionist strategy of massive and early screening reported very low mortality rates of 2.1% (South Korea) and 0.5% (Iceland) respectively, to be compared to 18% case fatality rate in France (July 15, 2020 - www.who.int & https://ourworldindata.org/coronavirus-data?country=~FRA), where this strategy was not applied at this time while it was <1% in our series. From March 19th to June 2nd, 2020, the COVID-19 mortality was evaluated to be 798 per million inhabitants in Paris where no screening strategy was set up to be compared to 149 in our city, Marseille, where early and massive screening was already implemented with a similar prevalence of infection (https://www.data.gouv.fr/fr/datasets/donnees-hospitalieres-relatives-a-lepidemie-de-covid-19/#_).
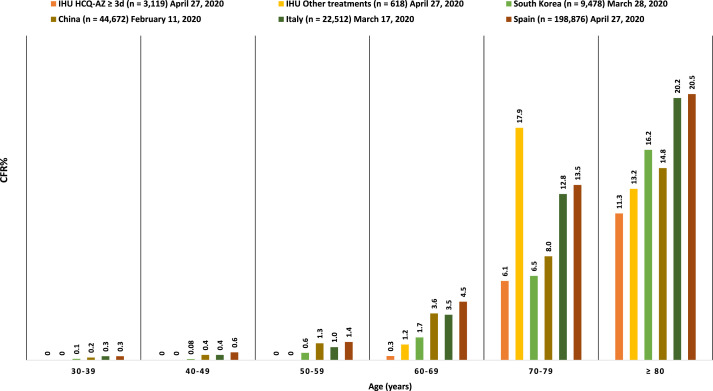
In addition, the authors commented that without a control group it is difficult to attribute the evolution of patients to the therapeutic intervention. There are descriptive and comparative studies. Before any comparison, description is an indispensable preliminary step. After the description of this cohort of 1061 patients, we have therefore reported a larger cohort of 3737 non-selected COVID 19 patients, including a control group of 618 patients, showing that receiving HCQ-AZ ≥ 3 days halved the risk of death, divided by 5 the risk of transfer to intensive care unit, and very significantly reduced the duration of viral shedding [3]. The risk of confounding associated with age, comorbidities and severity of the disease was controlled by multivariable analyses and propensity score matching approaches. This low mortality was also observed when our results were compared to the case fatality rates reported in South Korea, China, Italy and Spain for each age group and during the same period (see Fig. 1). In addition, we confirmed in another study the beneficial role of HCQ-AZ in the dependent elderly residents living in retirement homes [4], also confirmed by another team [5], allowing us to control for selection bias.
Fig. 1.
Case fatality rate in our center in patients treated or not treated with HCQ -AZ for at least 3 days compared to mortality in South Korea, China, Italy and Spain by age group for the same time period.
IHU:Institut Hospitalo-Universitaire Méditerranée Infection (our center),HCQ:hydroxychloroquine,AZ:azithromycin,CFR%:case fatality rate (%). This data is based on the number of confirmed cases and deaths in each age group as reported by our center as of 27th April 2020 [3] and national agencies: Chinese Center for Disease Control and Prevention (CDC) as of 11th February; Spanish Ministry of Health as of 27th April; Korea Centers for Disease Control and Prevention (KCDC) as of 24th March; and the Italian National Institute of Health, as presented in the paper by Onder et al. [6] as of 17th March. The relatively high CFR% of patients 70–79 years with other treatments and not receiving HCQ AZ in our center remain unexplained but may be related to the low sample size in this group (39 patients).
Altogether, these different facts strongly suggest that early massive screening and treatment with hydroxychloroquine and azithromycin are an effective global strategy to reduce mortality associated with COVID-19.
Funding source
None.
CRediT authorship contribution statement
Matthieu Million: Writing - original draft. Audrey Giraud-Gatineau: Formal analysis. Jean-Christophe Lagier: Writing - review & editing. Philippe Parola: Writing - review & editing. Philippe Gautret: Writing - review & editing. Didier Raoult: Supervision, Validation.
Declaration of competing interest
None.
References
- 1.Lebeaux D., Revest M. No evidence of clinical benefits of early treatment of COVID-19 patients with hydroxychloroquine and azithromycine. Trav Med Infect Dis. 2020;36:101819. doi: 10.1016/j.tmaid.2020.101819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai S., Ruktanonchai N.W., Zhou L. Effect of non-pharmaceutical interventions to contain COVID-19 in China. Nature. 2020;585(7825):410–413. doi: 10.1038/s41586-020-2293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lagier J.C., Million M., Gautret P. Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: a retrospective analysis [published online ahead of print, 2020 Jun 25] Trav Med Infect Dis. 2020:101791. doi: 10.1016/j.tmaid.2020.101791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ly T.D.A., Zanini D., Laforge V. Pattern of SARS-CoV-2 infection among dependant elderly residents living in long-term care facilities in Marseille, France, March-June 2020. Int J Antimicrob Agents. 2020;56(6):106219. doi: 10.1016/j.ijantimicag.2020.106219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heras E., Garibaldi P., Boix M. COVID-19 mortality risk factors in older people in a long-term care center [published online ahead of print, 2020 Nov 27] Eur Geriatr Med. 2020:1–7. doi: 10.1007/s41999-020-00432-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy [published correction appears in JAMA. 2020 Apr 28;323(16):1619] J Am Med Assoc. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]