Real‐world evidence of the effectiveness on glycaemic control of early simultaneous versus later sequential initiation of basal insulin and glucagon‐like peptide‐1 receptor agonists (original) (raw)
Abstract
Aim
To assess the impact of the timing of initiating both basal insulin and glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs) on reaching glycaemic control targets over 6 and 12 months in people with type 2 diabetes (T2D) uncontrolled on oral antihyperglycaemic drugs with an HbA1c of 9% or higher.
Methods
This retrospective cohort study assessed the impact of the timing of initiating both basal insulin and GLP‐1 RA therapies on reaching glycaemic targets (HbA1c < 7% and <8%, and ≥1% and ≥2% HbA1c reduction) over 12 months in people with markedly uncontrolled T2D (HbA1c ≥ 9%) on oral antihyperglycaemic drugs identified on the Optum Humedica database (electronic medical records; 1 January 2011 to 30 June 2017). Study cohorts were defined by the days between initiating each injectable: cohort A, 30 days or less (simultaneous initiation) and cohorts B, 31‐90, C, 91‐180, D, 181‐270 and E, 271‐360 days (sequential initiation).
Results
Cohort A had the best glycaemic outcomes at 6 and 12 months for all four endpoints, followed by cohort B. The likelihood of achieving an HbA1c of less than 7% did not significantly differ between cohorts A and B (hazard ratio [95% confidence interval]: 0.87 [0.76‐1.01]); cohorts C, D and E were significantly less likely to achieve an HbA1c of less than 7% than cohort A (0.62 [0.53‐0.72]; 0.62 [0.53‐0.72]; 0.63 [0.54‐0.73]).
Conclusions
In people with uncontrolled T2D requiring treatment with a GLP‐1 RA and basal insulin, greater improvements in glycaemic control were observed when both therapies were initiated within close proximity of one another (≤90 days) compared with initiation 91‐360 days apart.
Keywords: basal insulin, cohort study, database research, GLP‐1 analogue, glycaemic control, type 2 diabetes
1. INTRODUCTION
Consensus guidelines from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) recommend that people with type 2 diabetes (T2D) who need a greater glucose‐lowering effect than is achieved by their current dual/triple oral diabetes therapy should add an injectable medication. 1 Glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs) are the preferred first‐choice injectable for most patients, however, insulin is recommended first for people with significant and symptomatic hyperglycaemia (HbA1c ≥ 10%). 1 Furthermore, guidelines currently recommend that if an individual requires further glucose‐lowering therapy despite treatment with a GLP‐1 RA or basal insulin, the other injectable can be promptly added as a treatment intensification. 1 , 2
In people with a baseline HbA1c of 9.0% or higher, the likelihood of reaching glycaemic control is even lower than in people with a baseline HbA1c of 7% to less than 8%, or of 8% to less than 9%. 3 In a retrospective, longitudinal, observational study, in those subjects with a baseline HbA1c of 9% or higher, the cumulative probability of achieving an HbA1c of less than 7.0% with either basal insulin or a GLP‐1 RA alone was 15% and 19% at 12 months, and 26% and 28% at 24 months, respectively. 3 By contrast, in those with a baseline HbA1c of 8% to less than 9%, the cumulative probability of achieving an HbA1c of less than 7.0% with either a basal insulin or GLP‐1 RA was 24% and 41% at 12 months, and 36% and 59% at 24 months, respectively, and in those with a baseline HbA1c of 7% to less than 8%, the probability was 34% and 75% at 12 months, and 50% and 91% at 24 months, respectively. 3 Thus, in people with an HbA1c of 9.0% or higher, combined treatment intensification with a GLP‐1 RA and basal insulin should be considered, administered either separately or together in the form of a fixed‐ratio combination. 1 , 3
Real‐world evidence from a large US electronic medical records (EMR) database shows that in people with T2D who initiated basal insulin after being insufficiently controlled on oral antihyperglycaemic drugs (OADs), the conditional probability of reaching glycaemic control (HbA1c < 7%) diminished progressively every 3 months (26.6% between 3 and 6 months, 17.6% between 6 and 9 months and 8.6% between 9 and 12 months) and was very low (<7%) after 12 months, leading to the conclusion that earlier additional treatment should be considered if glycaemic control is not reached in the first 6‐9 months, and ideally even earlier. 4
In addition, evidence is sparse as to whether patient outcomes are affected by the length of time between the initiation of these two injectable therapies. In this retrospective study using data collected from real‐world clinical practice, we assessed the impact of the timing of initiating both basal insulin and GLP‐1 RAs on reaching glycaemic control targets over 6 and 12 months in people with T2D uncontrolled on OADs with an HbA1c of 9% or higher.
2. METHODS
2.1. Study design and population
This retrospective cohort study captured and analysed EMR data from the Optum Humedica database for people with T2D severely uncontrolled (HbA1c ≥ 9%) on OADs, and who initiated injectables (both a GLP‐1 RA and basal insulin in any order) from 1 January 2011 to 30 June 2017. National drug codes were used to identify basal insulin and GLP‐1 RAs in participants’ records in the EMR data.
Study index date was defined as the date upon which the second injectable treatment was initiated. The baseline period was defined as the 12‐month period immediately prior to and including the index date. The follow‐up period included the day after the index date and continued until the end of medication persistence, death, the end of EMR activity, or the end of the study period, whichever came first.
Eligible participants were aged from 18 to 80 years inclusive with a T2D diagnosis (International Classification of Diseases, 9th revision, clinical modification [ICD‐9‐CM] codes 250.x0 or 250.x2, or International Classification of Diseases, 10th revision, Clinical Modification [ICD‐10‐CM] code E11). 5 , 6 All participants initiated second‐line treatment with either basal insulin or a GLP‐1 RA, and then received a second prescription of a different injectable drug type (a GLP‐1 RA or basal insulin) within the next 12 months. Other eligibility criteria included: a baseline HbA1c of 9% or higher; one or more valid HbA1c record (4%‐20%) in the 90 days prior to and 14 days post‐index date (defined as the baseline HbA1c value) and one or more valid HbA1c record at any time post‐index; a prescription for one or more OAD prior to initiating injectable treatments; 12 months or more of continuous EMR data prior to initiating first second‐line therapy; no type 1 diabetes, secondary diabetes or gestational diabetes at any time during the study period.
Participants were classified into five different treatment cohorts based on the gap between the calendar prescription dates for initiating basal insulin and GLP‐1 RA therapies: cohort A included those who initiated their second injectable within 30 days of the first prescription; cohort B within 31‐90 days; cohort C within 91‐180 days; cohort D within 181‐270 days; and cohort E within 271‐360 days. Cohort A was considered to have initiated both treatments simultaneously, while cohorts B‐E were considered to have initiated the two treatments sequentially.
2.2. Outcomes
The primary outcomes of this analysis were glycaemic control defined as the first observed HbA1c value of less than 7.0% following the index date (the date when the second injectable treatment was initiated), the first observed reduction of 1% or higher in HbA1c from baseline following the index date, and the change in HbA1c and weight from baseline to month 6 or month 12 (with a window of ±90 days). Demographic and clinical characteristics were also assessed in the baseline period. The probability of reaching an HbA1c of less than 7.0% was assessed by baseline demographics and clinical characteristics.
Sensitivity analyses included the first observed HbA1c value of less than 8.0% following the index date and the first observed reduction of 2% or higher in HbA1c from baseline following the index date.
2.3. Data analysis
The study sample included all participants with complete data for all of the eligibility criteria within the observation period. Summary statistics included mean, standard deviation (SD), minimum, maximum and quartiles for continuous study measures, and counts and proportions for categorical measures. Demographics and baseline characteristics were captured and, if multiple values were present in the data during the baseline period for a given variable, the value closest to the index date was used. HbA1c and weight values had to occur within ±90 days of the 6‐month and 12‐month follow‐up dates. If there were multiple HbA1c values that fell within the ±90‐day window, the test value closest to the 6‐ or 12‐month date was selected. If there were values at equal intervals around the 6‐ and 12‐month follow‐up assessments, the lowest value was selected. If any participant had more than one HbA1c test on the same day, the test with the lowest value was selected for analysis. Missing data were not imputed. It was anticipated that missing data may not be random but may reflect participant characteristics, so the influence of missing values was reduced by ensuring that participants met acceptable quality standards and had complete data in the baseline and follow‐up periods, with one or more valid HbA1c result during both the baseline and 12‐month follow‐up periods.
Glycaemic control in terms of reaching an HbA1c of less than 7% was summarized by the number of patients, by treatment cohort, by those who achieved glycaemic control during the follow‐up period, and by the proportion of patients achieving glycaemic control (calculated as the number of patients with an outcome over the number of patients with one or more HbA1c value) at 6 and 12 months. Multivariate Cox proportional hazards models were used to estimate unadjusted and adjusted hazard ratios (HRs) for the associations between the baseline clinical and demographic characteristics and time to achieving glycaemic control in all patients. The characteristics assessed included treatment group, age, ethnicity, treatment sequence, type of health plan, index year, baseline HbA1c, Charlson co‐morbidity index (CCI) group, index basal insulin prescription, index GLP‐1 RA prescription and co‐morbidity.
The Optum Humedica EMR database is fully compliant with the Health Insurance Portability and Accountability Act (HIPAA) of 1996. All data were de‐identified prior to acquisition, negating the need for institutional review board approval. The study was conducted in accordance with ethical principles that are consistent with the Declaration of Helsinki, International Conference on Harmonisation, Good Clinical Practice, Good Pharmacy Practice, and the applicable legislation on non‐interventional studies and/or observational studies.
3. RESULTS
3.1. Baseline characteristics
Of the 5,842,115 people with T2D screened, 6339 met all eligibility criteria and were included in the analyses. Participants were 47% male with a mean ± SD age of 54 ± 10.7 years, weight of 108 ± 25.5 kg and baseline HbA1c of 10.7% ± 1.45% (93.4 ± 15.8 mmol/mol). The most common co‐morbidities were dyslipidaemia (82%), hypertension (80%), chronic kidney disease (26%) and neuropathy (23%). More than 1000 people were included in each cohort; cohort A was the largest cohort (n = 1420), followed by cohorts C (n = 1325), B (n = 1259), D (n = 1205) and E (n = 1130). Accounting for the distribution of CCI and specific co‐morbidities in the cohorts at baseline, the baseline demographics and clinical characteristics were basically similar between the cohorts (Table 1).
TABLE 1.
Baseline demographics and disease characteristics
| Baseline characteristics | All participants (N = 6339) | Cohort A ≤30 days (N = 1420) | Cohort B 31‐90 days (N = 1259) | Cohort C 91‐180 days (N = 1325) | Cohort D 181‐270 days (N = 1205) | Cohort E 271‐360 days (N = 1130) |
|---|---|---|---|---|---|---|
| Age group, years | ||||||
| 18‐29 | 108 (1.7) | 22 (1.5) | 28 (2.2) | 25 (1.9) | 16 (1.3) | 17 (1.5) |
| 30‐44 | 1079 (17.0) | 256 (18.0) | 226 (18.0) | 200 (15.1) | 202 (16.8) | 195 (17.3) |
| 45‐64 | 4084 (64.4) | 917 (64.6) | 813 (64.6) | 844 (63.7) | 788 (65.4) | 722 (63.9) |
| ≥65 | 1068 (16.8) | 225 (15.8) | 192 (15.3) | 256 (19.3) | 199 (16.5) | 196 (17.3) |
| Mean ± SD age at index, years | 54.1 ± 10.72 | 54.0 ± 10.66 | 53.3 ± 10.89 | 54.6 ± 10.86 | 54.4 ± 10.46 | 54.4 ± 10.66 |
| Mean ± SD length of follow‐up, years | 3.3 ± 1.62 | 3.2 ± 1.66 | 3.2 ± 1.59 | 3.4 ± 1.62 | 3.4 ± 1.67 | 3.2 ± 1.55 |
| Gender, male | 2977 (47.0) | 666 (46.9) | 567 (45.0) | 645 (48.7) | 553 (45.9) | 546 (48.3) |
| Race | ||||||
| White | 4786 (75.5) | 1048 (73.8) | 966 (76.7) | 1015 (76.6) | 897 (74.4) | 860 (76.1) |
| Black | 1072 (16.9) | 264 (18.6) | 198 (15.7) | 210 (15.8) | 203 (16.8) | 197 (17.4) |
| Asian | 92 (1.5) | 20 (1.4) | 18 (1.4) | 19 (1.4) | 20 (1.7) | 15 (1.3) |
| Other/missing | 389 (6.1) | 88 (6.2) | 77 (6.1) | 81 (6.1) | 85 (7.1) | 58 (5.1) |
| Ethnicity | ||||||
| Hispanic | 543 (8.6) | 103 (7.3) | 102 (8.1) | 134 (10.1) | 110 (9.1) | 94 (8.3) |
| Prescribing physician type | ||||||
| Primary care provider | 4698 (81.3) | 1003 (79.5) | 930 (81.2) | 986 (81.9) | 916 (81.7) | 863 (82.7) |
| Specialist | 1079 (18.7) | 259 (20.5) | 216 (18.8) | 218 (18.1) | 205 (18.3) | 181 (17.3) |
| Mean ± SD HbA1c at baseline, % | 10.7 ± 1.45 | 10.9 ± 1.53 | 10.7 ± 1.51 | 10.5 ± 1.31 | 10.5 ± 1.41 | 10.6 ± 1.44 |
| Mean ± SD body weight at baseline, kg | 108.2 ± 25.49 | 108.3 ± 24.10 | 109.1 ± 25.86 | 107.6 ± 26.04 | 107.8 ± 26.36 | 108.3 ± 25.19 |
| CCI group | ||||||
| 0 | 4316 (68.1) | 999 (70.4) | 855 (67.9) | 879 (66.3) | 843 (70.0) | 740 (65.5) |
| 1 | 785 (12.4) | 158 (11.1) | 153 (12.2) | 174 (13.1) | 139 (11.5) | 161 (14.2) |
| 2 | 732 (11.5) | 164 (11.5) | 146 (11.6) | 163 (12.3) | 135 (11.2) | 124 (11.0) |
| ≥3 | 506 (8.0) | 99 (7.0) | 105 (8.3) | 109 (8.2) | 88 (7.3) | 105 (9.3) |
| Co‐morbidities | ||||||
| Hypertension | 5088 (80.3) | 1103 (77.7) | 976 (77.5) | 1090 (82.3) | 994 (82.5) | 925 (81.9) |
| Dyslipidaemia | 5206 (82.1) | 1097 (77.3) | 1011 (80.3) | 1108 (83.6) | 1034 (85.8) | 956 (84.6) |
| Chronic kidney disease | 1654 (26.1) | 313 (22.0) | 325 (25.8) | 363 (27.4) | 327 (27.1) | 326 (28.8) |
| Congestive heart failure | 412 (6.5) | 81 (5.7) | 85 (6.8) | 87 (6.6) | 73 (6.1) | 86 (7.6) |
| Stroke | 151 (2.4) | 29 (2.0) | 36 (2.9) | 30 (2.3) | 30 (2.5) | 26 (2.3) |
| Neuropathy | 1443 (22.8) | 257 (18.1) | 288 (22.9) | 327 (24.7) | 291 (24.1) | 280 (24.8) |
| Peripheral vascular disease | 318 (5.0) | 47 (3.3) | 68 (5.4) | 72 (5.4) | 57 (4.7) | 74 (6.5) |
| Retinopathy/blindness | 306 (4.8) | 63 (4.4) | 55 (4.4) | 70 (5.3) | 61 (5.1) | 57 (5.0) |
| Hypoglycaemia | 454 (7.2) | 98 (6.9) | 84 (6.7) | 87 (6.6) | 89 (7.4) | 96 (8.5) |
| Obesity | 2372 (37.4) | 513 (36.1) | 503 (40.0) | 483 (36.5) | 445 (36.9) | 428 (37.9) |
3.2. Glycaemic control outcomes
In each cohort, 22%‐26% of participants did not have one or more HbA1c value at 6 months and 10%‐16% did not have one or more HbA1c value at 12 months, and therefore were not included in the analysis. The proportion of participants reaching glycaemic control outcomes was highest in cohort A followed by cohort B for all four endpoints (Figure 1). The proportions of participants reaching glycaemic control outcomes in cohorts C‐E were significantly lower compared with cohorts A and B for all four endpoints.
FIGURE 1.
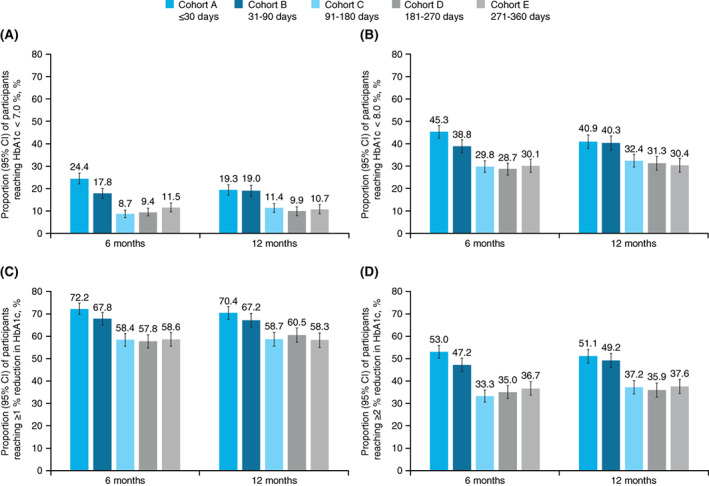
Proportion of participants reaching A, HbA1c < 7.0%, B, HbA1c < 8.0%, C, ≥1% reduction in HbA1c from baseline and D, ≥2% reduction in HbA1c from baseline at 6 and 12 months in each cohort. Proportion calculated as number of patients with outcome over the number of patients with ≥1 HbA1c value. CI, confidence interval
At 6 and 12 months follow‐up, an HbA1c of less than 7.0% was reached by less than 25% of participants in cohorts A (6 months: 24.4%; 12 months: 19.3%) and B (17.8%; 19.0%), but by less than 12% of those in cohorts C (8.7%; 11.4%), D (9.4%; 9.9%) and E (11.5%; 10.7%). Cox proportional hazards models showed that participants in cohorts C, D and E were significantly less likely to reach glycaemic control (HbA1c < 7.0%) than participants in cohort A (HR [95% CI] 0.62 [0.53, 0.72]; 0.62 [0.53, 0.72]; 0.63 [0.54, 0.73]). Cohort B was not significantly less likely than cohort A, despite having a numerically lower target achievement than cohort A (HR [95% CI] 0.87 [0.76, 1.01]; Figure 2).
FIGURE 2.
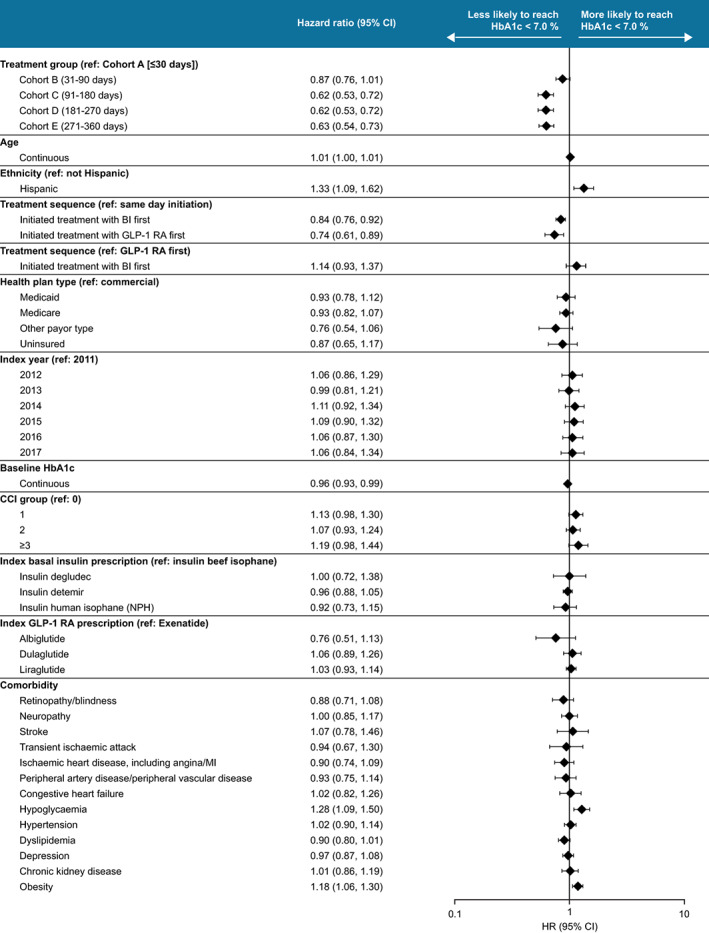
Characteristics affecting glycaemic control (HbA1c < 7.0%) achievement. Associations calculated by multivariate cox regression model. BI, basal insulin; CCI, Charlson co‐morbidity index; CI, confidence interval; GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist; HR, hazard ratio; MI, myocardial infarction; NPH, neutral protamine Hagedorn
Similarly, at 6 and 12 months, an HbA1c of less than 8.0% target achievement was higher in participants in cohorts A (45.3%; 40.9%) and B (38.8% and 40.3%) than in cohorts C (29.8%; 32.4%), D (28.7%; 31.3%) and E (30.1%; 30.4%).
More than 50% of participants in each cohort achieved a reduction of 1% or higher in HbA1c from baseline to 6 months (57.8%‐72.2%) and 12 months (58.3%‐70.4%), and at least one‐third achieved a reduction of 2% or higher in HbA1c from baseline to 6 months (33.3%‐53.0%) and 12 months (35.9%‐51.1%). The proportions of participants reaching glycaemic control were similar between 6 and 12 months in each cohort.
Mean ± SD change in HbA1c from baseline to month 6 or month 12 was greater in cohort A (−2.4% ± 2.39%; −2.2% ± 2.34%) and cohort B (−2.0% ± 2.21%; −2.0% ± 2.27%) compared with cohorts C‐E (Figure 3A).
FIGURE 3.
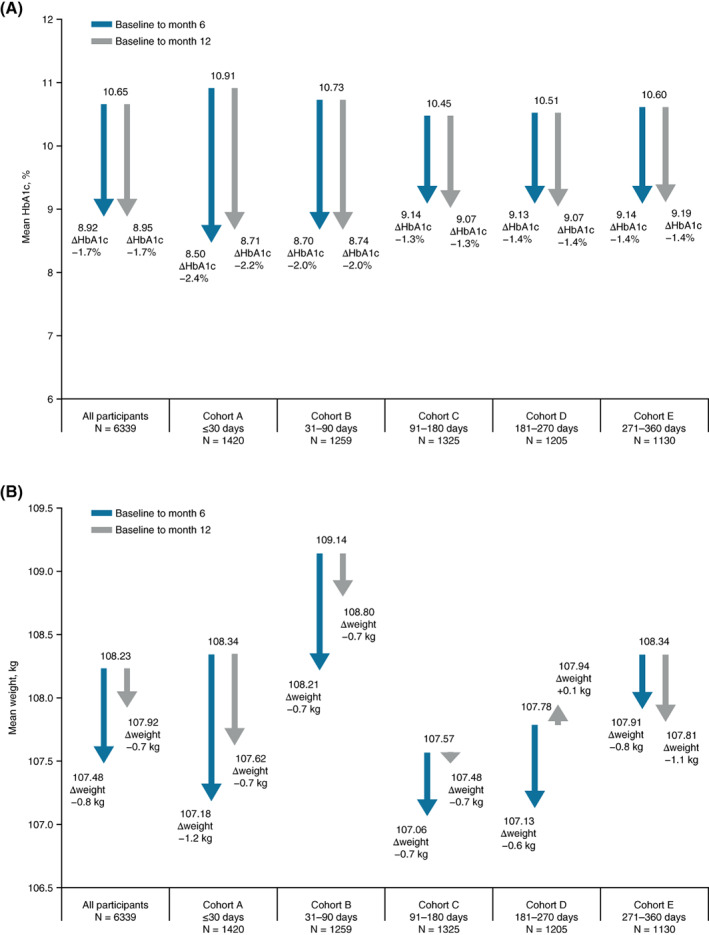
Change in A, HbA1c, and B, weight from baseline to months 6 and 12 by cohort. ΔHbA1c is mean change from baseline to month 6 or 12. Δweight is mean change from baseline to month 6 or 12. Baseline weight data include every participant with a baseline weight value. Change from baseline weight data include every participant with a baseline and a month 6 or month 12 weight value, as appropriate
3.3. Factors associated with reaching glycaemic control (HbA1c < 7.0%)
In addition to differing by cohort, participants were less likely to reach glycaemic control if they initiated sequentially either basal insulin treatment first (n = 3408; HR [95% CI] relative to same day initiation 0.84 [0.76, 0.92]), or GLP‐1RA treatment first (n = 2931; HR [95% CI] relative to same day initiation 0.74 [0.61, 0.89]), than if they initiated both therapies on the same day (n = 577) (Figure 2). Analyses showed that there were no differences in reaching an HbA1c of less than 7.0% in those who initiated basal insulin first versus those who initiated a GLP‐1 RA first (HR [95% CI] 1.14 [0.94‐1.37]). Participants were also more likely to reach glycaemic control if they were Hispanic (HR [95% CI] 1.33 [1.09, 1.62]). As expected, participants were also less likely to reach glycaemic control if they had a higher baseline HbA1c (HR [95% CI] 0.96 [0.93, 0.99]). No significant associations were observed between participants reaching glycaemic control and index year, type of basal insulin, type of GLP‐1 RA, healthcare plan, CCI group, or most comorbidities.
3.4. Change in weight
Weight was reduced in all cohorts at 6 and 12 months with the exception of cohort D at 12 months (Figure 3B).
4. DISCUSSION
In this retrospective cohort study, participants who initiated basal insulin and GLP‐1 RA therapies close together in time (within 30 days or up to 90 days of one another) had a significantly greater probability of reaching glycaemic control compared with those initiating therapies 91‐360 days apart. Furthermore, analysis showed that when both therapies were initiated on the same day there was an increased likelihood of a participant reaching glycaemic control, compared with when basal insulin and a GLP‐1 RA were initiated separately. The complementary mechanisms of action make both therapies more effective in combination than either drug alone. For patients with highly uncontrolled hyperglycaemia, it is suboptimal to add one drug after the other with an extended period in between. Our study provides further evidence on the benefits of early combination therapy being given almost simultaneously rather than sequentially before glucotoxicity, which could compromise the response of a late addition of a second agent.
A US retrospective cohort study by Tong et al., using the IMPACT (Impact National Managed Care Benchmark) claims database, studied 1552 adults with T2D uncontrolled (HbA1c ≥ 7%) on basal insulin who intensified their therapy to include a GLP‐1 RA early (≤6 months after basal insulin initiation), delayed (>6‐24 months after basal insulin initiation), or not at all. 7 Participants who initiated a GLP‐1 RA early had better clinical outcomes (measured as changes in HbA1c) compared with those with delayed initiation. Our results confirm these findings using a different methodology in a larger, more diverse dataset, one which is more representative of the whole US population. This study also expands on these previous findings by assessing time to second initiation irrespective of initiation order, as opposed to only assessing adults who initiated basal insulin followed by a GLP‐1 RA, and includes a greater number of narrower time periods, with five periods ranging from 30 to 90 days in length as opposed to two periods of 6 and 18 months. The shorter timeframes in our study enabled us to provide a more detailed picture of how each delay in time may affect outcomes compared with the Tong et al. study.
Physicians often may delay intensifying therapy, and wait to determine the effectiveness of an existing treatment. However, current data suggest this may not be the most effective approach as it leads to clinical inertia. Previous EMR data show that people with uncontrolled T2D who initiated basal insulin after OADs had a reduced likelihood of reaching glycaemic control (<7%) over time, with the highest likelihood occurring at the earliest time‐bracket observed (3‐6 months) and a low likelihood beyond 12 months. 4 More recently, a study of people uncontrolled (HbA1c ≥ 7.0%) on OADs from the REACHnet database initiating basal insulin and GLP‐1 RA treatment at the same time, resulted in greater HbA1c reductions and greater glycaemic target achievement than those who initiated sequentially a basal insulin followed by a GLP‐1 RA between 1 and 90 days, or more than 90 days later. 8 In line with these results, the current study shows that people with an HbA1c of 9.0% or higher who initiated basal insulin and GLP‐1 RAs within 90 days of one another have a significantly greater probability of reaching glycaemic control than those who initiated both therapies between 91 and 360 days apart. In addition, there was no perceived increase in glycaemic target achievement in any group between months 6 and 12.
The aforementioned studies show superior glycaemic control outcomes when GLP‐1 RAs and basal insulin are initiated closer together in time. However, it could be suggested that clinicians are only prescribing both injectable therapies within close time proximity for patients whom they perceive to be able to benefit from and tolerate such a regimen, for example, patients who are younger, less frail, and those who have fewer co‐morbidities. Nevertheless, our cohorts did not differ by age or CCI group, which negates the argument that the difference in outcomes observed is attributable to a difference in prescribing patterns.
Furthermore, diabetes guidelines may have changed between 2011 and 2017, which could have impacted changes in diabetes care in clinical practice. The use of basal insulin and GLP‐1 RAs did not have significant changes during this period, however, the latest consensus guidelines from ADA and EASD recommend that a GLP‐1 RA is given as the first injectable in most patients with T2D, with basal insulin considered in special clinical circumstances. 1 Regardless of which is chosen first, adding the other injectable is recommended if it is necessary to further improve glucose control, and our results show that there is no difference in glycaemic outcome achievement between those who initiated basal insulin first and those who initiated a GLP‐1 RA first.
This analysis only included people with T2D with an HbA1c of 9.0% or higher. While many guidelines recommend an HbA1c target of less than 7.0%, less stringent targets (such as less than 8.0%) are often recommended, depending on participant characteristics, medical history, and the risk of adverse events or hypoglycaemia. 2 , 9 In this study, the mean baseline HbA1c was 10.7%, which some physicians would consider as too high to target an HbA1c of less than 7.0%. Therefore, we included a post hoc analysis with a glycaemic target of an HbA1c of less than 8.0%. This target was reached by 6 months in almost 50% of participants who initiated basal insulin and a GLP‐1RA simultaneously.
Despite the observed differences in target achievement, glycaemic control was still unsatisfactory across all cohorts. This could be a result of the participant population having a high baseline HbA1c and a high prevalence of co‐morbidities such as chronic kidney disease, which can make target achievement less attainable, and also focuses on those people who had to intensify their treatment regimen because it was uncontrolled on OADs.
One limitation of this study is that it includes wide cohort brackets that do not allow for a more precise picture of the benefits of simultaneous co‐administration of basal insulin and a GLP‐1 RA. There was also a large variation in those initiating on different days (1‐360 days apart), therefore, further investigation powered to focus on participants who initiated both therapies 90 days or less apart, or preferably 30 days or less apart, might provide a clearer indication of the time cut‐off point for the perceived benefit of initiating both therapies together. Another limitation is that HbA1c was not measured on a set schedule, so each participant may have had a different testing interval, leading to differences in follow‐up times between participants. OAD use was not reliably captured beyond baseline and was assumed to be randomly distributed during the follow‐up; however, OAD use may have influenced outcomes. In addition, the dataset was limited by the available data on the Optum Humedica database, for example, socioeconomic status and insurance status were not captured on the database, so the effect of these characteristics on our results is not known. The requirements designed to mitigate for missing data only partially did so. The strengths of the study were that the analysed data were real‐world evidence, which showed trends in participants by how they were treated by physicians, and also that EMR data include a rich collection of clinical and laboratory information.
In conclusion, in patients with T2D inadequately controlled on OADs requiring treatment with GLP‐1RA and basal insulin therapies, greater improvements in glycaemic control were observed when both therapies were initiated within close time proximity of one another (≤90 days) compared with when they were initiated 91‐360 days apart.
CONFLICT OF INTEREST
JR has served on advisory panels for Applied Therapeutics, Boehringer Ingelheim, Eli Lilly, Intarcia, Janssen, Lexicon, Novo Nordisk, Oramed and Sanofi; and has received research support from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Genentech, GlaxoSmithKline, Intarcia, Janssen, Lexicon, Merck, Novo Nordisk, Oramed, Pfizer and Sanofi. FJAB has served on advisory panels for Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, LifeScan, Medtronic, Merck, Novartis, Novo Nordisk, Pfizer, Roche and Sanofi; and has received research support from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, LifeScan Merck, Novo Nordisk, Pfizer, Sanofi and Servier. RL and AB are employees of Sanofi. XVP was employed by Sanofi at the time the study was conducted. LS has received research grants from Chiasma, Genentech and Sanofi; and has stock options with BRAVO4Health. VF has received research grants from Bayer, Boehringer Ingelheim and Gilead; has received honoraria for consulting and lectures from Abbott, Asahi, AstraZeneca, Eli, Intarcia, Novo Nordisk and Takeda; has stock options with BRAVO4Health, Insulin Algorithms and Microbiome Technologies; and has stock in Amgen.
AUTHOR CONTRIBUTIONS
All the named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article and had full access to all the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. Design: RL, XVP and AB designed and conceptualized the study. Conduct/data collection: RL contributed to the acquisition of data. Analysis: RL contributed to the analysis of data. Writing the manuscript: all of the authors participated in the interpretation of the data, the writing, reviewing and editing of the manuscript, and had final responsibility for approving the published version.
ACKNOWLEDGMENTS
Editorial assistance was provided by Tamsin Brown, MSc, and Jo Bentley, PhD, of Fishawack Communications Ltd, a Fishawack Health Company, and was funded by Sanofi, Paris, France.
Rosenstock J, Ampudia‐Blasco FJ, Lubwama R, et al. Real‐world evidence of the effectiveness on glycaemic control of early simultaneous versus later sequential initiation of basal insulin and glucagon‐like peptide‐1 receptor agonists. Diabetes Obes Metab. 2020;22:2295–2304. 10.1111/dom.14154
Funding information This study was funded by Sanofi, Paris, France
The copyright line for this article was changed on 17 December 2020 after original online publication.
REFERENCES
- 1.Davies M, D'Alessio D, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;61:2461‐2498. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association . Standards of medical care in diabetes ‐ 2020. Diabetes Care. 2020;43(Suppl 1):S1‐S213. [DOI] [PubMed] [Google Scholar]
- 3.Peng X, Blonde L, Shepherd L, Lubwama R, Ji L, McCrimmon R. A real‐world retrospective study evaluating glycaemic control with glucagon‐like peptide‐1 receptor agonists or basal insulin in type 2 diabetes in the UK. Diabetologia. 2019;62:S420‐S421. [Google Scholar]
- 4.Blonde L, Meneghini L, Peng X, et al. Probability of achieving glycemic control with basal insulin in patients with type 2 diabetes in real‐world practice in the USA. Diabetes Ther. 2018;9:1347‐1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dugan J, Shubrook J. International classification of diabetes, 10th revision, coding for diabetes. Clin Diabetes. 2017;35(4):232‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klompas M, Eggleston E, McVetta J, Lazarus R, Li L, Platt R. Automated detection and classification of type 1 versus type 2 diabetes using electronic health record data. Diabetes Care. 2013;36(4):914‐921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong L, Pan C, Wang H, Bertolini M, Lew E, Meneghini L. Impact of delaying treatment intensification with a glucagon‐like peptide‐1 receptor agonist in patients with type 2 diabetes uncontrolled on basal insulin: a longitudinal study of a US administrative claims database. Diabetes Obes Metab. 2018;20:831‐839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng X, Ayyagari R, Lubwama R, et al. Impact of simultaneous versus sequential initiation of basal insulin and glucagon‐like Peptide‐1 receptor agonists on HbA1c in type 2 diabetes: a retrospective observational study. Diabetes Ther. 2020;11:995‐1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Institute for Health and Care Excellence . NICE guideline [NG28]. Type 2 diabetes in adults: management. https://www.nice.org.uk/guidance/ng28. Updated August 2019. Accessed January 2020.