Association of sudden sensorineural hearing loss with dementia: a nationwide cohort study (original) (raw)
Abstract
Background
Impaired cochlear blood perfusion and microvascular damage can cause sudden sensorineural hearing loss (SSHL), which is a potential risk factor for dementia. This study explored the association between SSHL and dementia.
Methods
This retrospective cohort study used a random sample of 1000,000 individuals from Taiwan’s National Health Insurance Research Database. We identified 3725 patients newly diagnosed with SSHL between January 1, 2000, and December 31, 2009, and propensity score matching according to age, sex, index year, comorbidities, and medications was used to select the comparison group of 11,175 patients without SSHL. Participants were stratified by age (<65 and ≧65 years) and sex for the subgroup analyses. The outcome of interest was all cause dementia (ICD-9-CM codes 290.0, 290.4, 294.1, 331.0). Both groups were followed up until December 31, 2010, for diagnoses of dementia. Cox regression models were used to estimate the hazard ratio (HR) of dementia.
Results
During the average 5-year follow-up period, the incidence rate of dementia in the SSHL cohort was 6.5 per 1000 person-years compared with 5.09 per 10,000 person-years in the comparison group. After adjustment for potential confounders, patients with SSHL were 1.39 times more likely to develop dementia than those without SSHL (95% confidence interval = 1.13–1.71). When stratified by patients’ age and sex, the incidence of dementia was 1.34- and 1.64-fold higher in patients with SSHL aged ≥65 years (P = .013) and in women (P = .001), respectively, compared with the comparison group. Women with SSHL who were < 65 years old had the highest risk (2.14, 95% CI = 1.17–4.11, P = .022). In addition, a log-rank test revealed that patients with SSHL had significantly higher cumulative incidence of dementia than those without SSHL (P = .002).
Conclusions
Patients with SSHL, especially women aged < 65 years, were associated with higher risk of dementia than those without SSHL. Thus, clinicians managing patients with SSHL should be aware of the increased risk of dementia.
Keywords: Sudden sensorineural hearing loss, Dementia, Nationwide cohort study, Female, Older adults
Background
The proportion of older adults (≧65 years) in Taiwan has been gradually increasing—from 4.1% in 1980 to 14.05% in 2018 and is estimated to reach 20% in 2025 [1], which will make Taiwan a super-aged society [2]. The global population, in general, is rapidly aging, and the global prevalence of dementia will therefore have significantly increased by 2050, with an estimated prevalence of 59% in Asia [3], indicating a potential dementia epidemic. However, no measures exist for preventing cognitive decline in older adults [4, 5]. Therefore, it is crucial to determine which patients are at risk of dementia.
Any type of hearing loss (HL), especially when related to aging, is a risk factor for cognitive decline, cognitive impairment, and dementia [6]. Although the causal relation between HL and cognitive decline remains unclear [7], the two conditions may have a common etiology, as both are related to vascular dysfunction and extended physiological decline. Aged-related HL (ARHL) is also linked to multiple indicators of functional decline [8]. Another theory postulates that HL in older adults can lead to a lower quality of life, social isolation, depression, and disability, all of which increase the risk of dementia [9].
Sudden sensorineural HL (SSHL) is defined as a sensorineural HL of ≥30 dB in three sequential frequencies within 3 days [10]. It is an urgent neurotology disorder requiring immediate identification and treatment [11]. SSHL occurs at any age but usually affects people in their 40s and 50s [12], with an annual incidence of 5–20 per 100,000 people per year in developed countries and with an equal sex distribution [13]. It can occur in isolation or as part of a systemic disorder. Four potential causes of SSHL include vascular disorder, autoimmune diseases, inner ear membrane rupture, and viral or bacterial infections. Currently, the vascular basis is the favored explanation; the abrupt onset of SSHL, such as by cerebral stroke or acute myocardial infarction [14, 15], may be correlated with a vascular event also contributing to cognitive impairment. For example, Phillips et al. [16] described a case of SSHL coupled with cerebral autosomal dominant arteriopathy, subcortical infarcts, and leukoencephalopathy, including intermittent cerebrovascular events, migraines, and dementia.
Here, we used Taiwan’s national population-based database to examine whether SSHL contributes to the development of dementia. Our results have practical implications for clinicians and may guide the development of preventive measures for dementia in patients with SSHL.
Methods
Data source
In this cohort study, data were collected from the National Health Insurance (NHI) Research Database (NHIRD) [17], which includes data on inpatient, outpatient, ambulatory care, and previous medical conditions dating from January 1, 1997, to December 31, 2010. Taiwan’s NHI program, established in 1995, is a government-operated single-payer insurance system that covers the health care of the entire nation in hopes of eradicating social problems caused by poverty and disease. As of 2019, 99.9% of Taiwan’s population is participating in the NHI program.
Study design and population
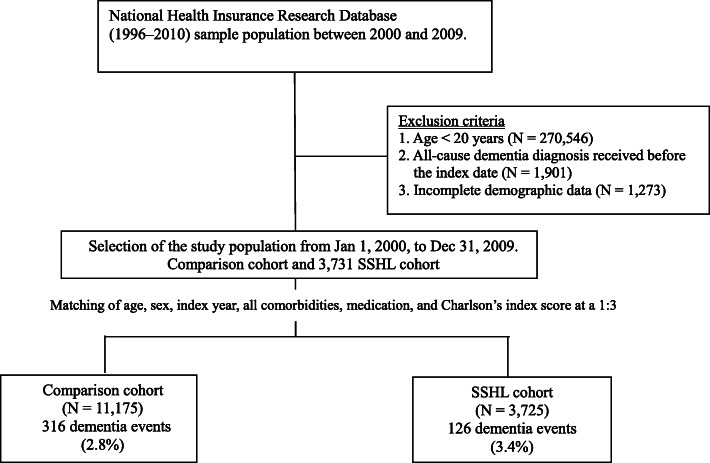
A population-based retrospective matched cohort study was conducted. Disease diagnosis followed the regulations of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). ICD-9-CM code 388.2 was used to define SSHL. Medical records that matched with these codes were obtained from the NHIRD between 2000 and 2009 for further analysis. Patients diagnosed with SSHL between January 1, 2000, and December 31, 2009, for > 1 year who had attended ≥2 outpatient visits or received any inpatient diagnosis were included as the SSHL group. Patients diagnosed before 2000, aged <20 years, or diagnosed with dementia before SSHL were excluded. A total of 3731 newly diagnosed patients with SSHL were included, and through propensity score matching at a 1:3 ratio according to age, sex, index year, comorbidities, and medications (Fig. 1), the comparison group was constructed. For patients without SSHL or dementia, the index date was designated as 365 days after the diagnosis date. At last, 3725 patients with SSHL and 11,175 without SSHL were selected. The study was approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB-EXEMPT (II)-20,160,028). All methods were carried out in accordance with relevant guidelines and regulations. Because the patient identifiers were scrambled to the public for research purposes to protect confidentiality, the requirement for written or verbal consent from patients for data linkage study was waived.
Fig. 1.
Flowchart of the study design
Covariates
Details of the patients’ sex, age, area of residence, insurance premium (≤NT$15,840, NT$15,841–25,000, and ≥ NT$25,001), comorbidities, and medications were obtained from the database. To evaluate the contribution of SSHL to the risk of dementia based on age, the participants were divided into three age groups (< 40, 40–59, and ≥ 60 years). We analyzed comorbidities that are well-known risk factors for dementia: hypertension, hyperlipidemia, diabetes, depression, ischemic heart disease, and cerebral vascular disease, and medications, including anticoagulant agents, antiplatelet agents, statins, antidiabetic agents, and antihypertension agents. Comorbidities were considered present if they were diagnosed between 2000 and 2009 prior to the diagnosis of dementia. Incidences of dementia and all-cause endpoints were defined as the study endpoints. Those who were alive and had no disease on December 31, 2010, were excluded after this time point. Person-year risk, the risk of dementia between the SSHL and comparison group, was deemed to be the time between the patient’s respective endpoint and the date of the diagnosis of SSHL or January 1, 2000 (for the non-SSHL group).
Outcome variable
Dementia (ICD-9-CM codes 290.1, 290.4, 294.1, 294.2, and 331.0) constituted the study outcome (Fig. 1). A diagnosis of dementia was based on at least two outpatient visits or at least one inpatient visit with a diagnosis of dementia listed on the claims data. Patients were followed from the index date to the earliest occurrence of dementia, death, disenrollment from the national health insurance, or the end of the study date (December 31, 2010), whichever came first.
Statistical analysis
Incidence rates for dementia per 1000 person-years were defined as the number of patients with dementia divided by person-years at risk. To identify whether SSHL increased the risk of dementia, we calculated the hazard ratios (HRs) and 95% confidence intervals (CIs; adjusted for other predictor variables) using Cox proportional hazards regression analysis. The proportional hazards assumption would be checked using statistical tests and graphical diagnostics based on the scaled Schoenfeld residuals. A plot that shows a non-random pattern against time is evidence of violation of the assumption. If the proportional hazard assumption is violated for some covariate, it is possible to stratify taking this variable into account and use the proportional hazards model in each stratum for the other covariates. With respect to the subgroup analysis, participants were stratified by age (<65 and ≧65 years), and sex to compare the outcome of interest in the specific subgroup. We used the Kaplan–Meier method to analyze the cumulative incidence of dementia between the SSHL cohort and the comparison cohort and investigated the difference with a log-rank test. SAS (version 9.3 for Windows; SAS Institute, Cary, NC, USA) was used for all statistical operations. All _P_-values were 2-sided, and P < .05 was considered significant.
Results
Patient characteristics
The two cohorts comprised 3725 newly diagnosed patients with SSHL and 11,175 age- and sex-matched controls; their data were extracted from the NHIRD 2000–2010. Table 1 summarizes the cohort characteristics. Most cases of SSHL occurred in the 40–59 age group (40.5%), followed by the ≥60 age group (35%). Most patients were men. No significant differences were observed in the distribution of comorbidities and medication between the SSHL and matched comparison cohorts (Table 1).
Table 1.
Basic characteristics of the SSHL and comparison cohorts (N = 14,900)
| Variables | Comparison cohort (N = 11,175) | SSHL cohort (N = 3725) | P value |
|---|---|---|---|
| N (%) | N (%) | ||
| Age | 0.823 | ||
| < 40 | 2731 (24.4) | 918 (24.6) | |
| 40–59 | 4484 (40.1) | 1508 (40.5) | |
| ≧60 | 3960 (35.4) | 1299 (34.9) | |
| Mean (±SD) | |||
| Age (years) | 52.1 (±16.4) | 52.0 (±16.4) | 0.726 |
| Sex | 0.977 | ||
| Female | 5199 (46.5) | 1734 (46.6) | |
| Male | 5976 (53.5) | 1991 (53.4) | |
| Insurance range | 0.640 | ||
| < NT 15,840 | 3928 (35.1) | 1339 (35.9) | |
| NT 15,841-25,000 | 4193 (37.5) | 1371 (36.8) | |
| > NT 25,001 | 3054 (27.3) | 1015 (27.2) | |
| Comorbidities | |||
| Diabetes | 1613 (14.4) | 569 (15.3) | 0.209 |
| Hyperlipidemia | 2358 (21.1) | 801 (21.5) | 0.603 |
| hypertension | 3700 (33.1) | 1230 (33.0) | 0.920 |
| Depression | 1001 (9.0) | 323 (8.7) | 0.595 |
| Ischemic heart disease | 197 (1.8) | 74 (2.0) | 0.376 |
| Cerebral vascular disease | 360 (3.2) | 133 (3.6) | 0.302 |
| Charlson’s index score | 0.723 | ||
| ≦1 | 8395 (75.1) | 2793 (75.0) | |
| 2 | 1276 (11.4) | 414 (11.1) | |
| ≧3 | 1504 (13.5) | 518 (13.9) | |
| Medications | |||
| Anticoagulant agents | 64 (0.6) | 24 (0.6) | 0.621 |
| Antiplatete agents | 366 (3.3) | 137 (3.7) | 0.239 |
| Antidiabetic agents | 1088 (9.7) | 386 (10.4) | 0.267 |
| Antihypertension agents | 2730 (24.4) | 935 (25.1) | 0.410 |
| Statin | 523 (4.7) | 190 (5.1) | 0.298 |
Association of SSHL with risk of dementia
Of the 14,900 patients whose data were analyzed in this study, 442 developed dementia in the follow-up period: 126 (3.4%) in the SSHL cohort and 316 (2.8%) in the comparison cohort (Table 2). The mean (standard deviation) follow-up intervals were 5.20 (2.83) years for the SSHL cohort and 5.55 (2.79) years for the comparison cohort (Table 3). SSHL was associated with increased hazard of developing dementia (HR = 1.39; 95% CI = 1.13–1.71; P = .002) in the Cox regression model adjusted for age, sex, area of residence, insurance premium, all comorbidities, and medications (Table 2).
Table 2.
Risk of all-cause dementia in the comparison and SSHL cohorts
| No. cases | Per 1000 Person year | Crude HR (95%CI) | P value | Adjusted HR (95%CI) | P value | |
|---|---|---|---|---|---|---|
| Comparison cohort | 316 | 5.09 | Ref. | Ref. | ||
| SSHL cohort | 126 | 6.50 | 1.28 (1.04–1.57) | 0.019 | 1.39 (1.13–1.71) | 0.002 |
Table 3.
The average follow-up duration and new onset dementia duration of the two cohorts
| Follow-up duration | New onset dementia duration | |||
|---|---|---|---|---|
| Mean (SD) | p value | Mean (SD) | p value | |
| Comparison cohort | 5.55 (2.79) | < 0.001 | 3.68 (2.59) | 0.287 |
| SSHL cohort | 5.20 (2.83) | 3.39 (2.43) |
Stratification by age and sex
To determine whether SSHL is a sex- and age-dependent risk factor for dementia, patients were stratified into four groups by age (< 65 years vs. ≥65 years) and sex (female vs. male). Women and older adults (≥65 years) with SSHL had a higher risk for dementia than the comparison group in the Cox regression models after adjustment for the potential confounding factors (HR = 1.64; 95% CI = 1.22–2.22; P = .001 and HR = 1.34; 95% CI = 1.06–1.68; P = .013, respectively); women with SSHL who were < 65 years old had the highest risk (2.14, 95% CI = 1.17–4.11, P = .022; Table 4).
Table 4.
Risk of all-cause dementia stratified by sex and age between comparison and SSHL cohorts
| Comparison cohort | SSHL cohort | SSHL cohort Verse Comparison cohort | ||||
|---|---|---|---|---|---|---|
| No. cases | Per 1000 PY | No. cases | Per 1000 PY | Adjusted HR (95% CI) | p value | |
| Sex | ||||||
| Female (N = 6933) | 143 | 4.93 | 62 | 6.90 | 1.64 (1.22–2.22) | 0.001 |
| Male (N = 7967) | 173 | 5.24 | 64 | 6.16 | 1.24 (0.93–1.66) | 0.138 |
| Age | ||||||
| < 65 year old (N = 11,046) | 46 | 0.98 | 24 | 1.60 | 1.62 (0.98–2.65) | 0.058 |
| ≧65 year old (N = 3854) | 270 | 18.03 | 102 | 23.17 | 1.34 (1.06–1.68) | 0.013 |
| Age & Gender | ||||||
| Age < 65 & Female (N = 5291) | 23 | 1.02 | 15 | 2.08 | 2.14 (1.17–4.11) | 0.022 |
| Age < 65 & Male (N = 5755) | 23 | 0.94 | 9 | 1.16 | 1.20 (0.55–2.59) | 0.647 |
| Age≧65 & Female (N = 1642) | 120 | 18.73 | 47 | 26.28 | 1.55 (1.10–2.17) | 0.012 |
| Age≧65 & Male (N = 2212) | 150 | 17.40 | 55 | 21.03 | 1.23 (0.91–1.68) | 0.184 |
The Kaplan–Meier analysis revealed that patients with SSHL had a significantly higher cumulative incidence of dementia than the comparison group (log-rank P = .019; Fig. 2).
Fig. 2.
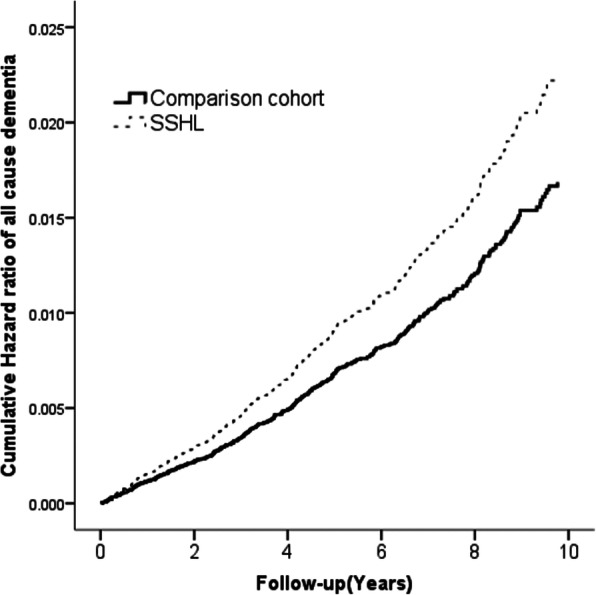
Cumulative hazard ratio of dementia by study groups (SSHL group [dashed line] vs comparison group [solid line]). Modified log-rank test P value = .019
Discussion
This population-based cohort study demonstrated SSHL was associated with increased hazard of developing dementia. Even after adjustment for multiple confounding factors, patients with SSHL had a greater risk of developing dementia than those without SSHL. Subgroup analyses revealed that women or older adults (≥65 years) with SSHL had a greater risk of dementia and that the risk was the highest in women with SSHL who were < 65 years old.
No large-scale study has addressed the correlation between SSHL and dementia using propensity score matching. Studies have focused on the relationship between ARHL, such as sensory HL or presbycusis, and the development of Alzheimer disease and the incidence of dementia [6, 7, 18–21]. Patients with HL, based on observation of hearing difficulties during testing or interview, have a faster rate of cognitive decline and are at greater risk of cognitive impairment [22]. This finding corroborates the notion that midlife HL may be responsible for on average 9.1% of dementia diagnoses worldwide [23]; thus, efforts should continue to reduce its impact.
The potential mechanisms underlying the association between ARHL and cognitive decline, particularly increased risk of dementia [19], remain unclear. Although Füllgrabe et al. suggested that audibility and processing effort can bias the cognitive test performance toward cognitive decline [24], Griffiths et al. proposed a mechanism linking auditory cognitive processing in the medial temporal lobe and dementia pathology [25]. Previously, Wayne and Johnsrude summarized the four directional hypotheses for the relationship between ARHL and cognitive decline, namely the cognitive load on perception hypothesis, sensory deprivation hypothesis, information degradation hypothesis, and common cause hypothesis [26]. Of them, the information degradation hypothesis is well supported by cognitive literature [27, 28], and the common cause hypothesis is another plausible mechanism, as it considers multiple sensory modalities (e.g., increased social isolation) and cognition decline concurrently [29]. However, we cannot exclude other physiological processes, such as factors related to the patient’s lineage (e.g., apolipoprotein E [ApoE] status) or a vascular disease causing both HL and dementia. However, we adjusted for vascular disease risk factors, such as hypertension, smoking, and diabetes, in our models, and a preliminary study did not reveal a positive correlation between ApoE status and HL [19]. Alternative variables such as leisure and mental activities were excluded because they do not cause HL and would thus not be relevant confounders in our models. Other specific associations may also exist between dementia and HL, such as different types of HL. SSHL is a unique disorder that often affects only one ear, and 70% of cases are idiopathic. Although many patients with SSHL recover spontaneously (32–65%), the condition’s underlying causes and pathogenesis remain unclear [10]. Moreover, the onset of SSHL is generally in the fifth or sixth decade of life, which is earlier than that of patients with ARHL [12].
SSHL can result from various identifiable causes (e.g., neoplastic, infectious, autoimmune, neurologic, otologic, metabolic, or vascular diseases; ototoxic drugs; and trauma) with many proposed etiologies and risk factors [30]. Postulated causes of idiopathic SSHL include viral cochleitis [31], microvascular events due to a hypercoagulable state [32], and autoimmune disorders [33, 34]. Furthermore, several disease susceptibility genes, such as ITGB3, MTHFR, HSP70, and PRKCH, have been studied in association with SSHL. Most of these genes are related to thrombosis, inflammatory response, and free-radical processes or oxidative stress [35]. Accordingly, factors such as metabolism, inflammation, thrombosis, immunology, and oxidative stress may play key pathogenic roles in the onset and development of SSHL [30]. However, these genes may also be related to dementia or other cerebrovascular diseases [14, 36, 37]. Previous studies have consistently reported that patients with SSHL have significantly higher plasma fibrinogen and cholesterol levels than the general population [38, 39]. Elevated plasma fibrinogen and cholesterol levels also contribute to dementia [40, 41]. Moreover, inflammation and immune responses play critical roles in both SSHL and dementia [42, 43]. Elevated serum levels of proinflammatory cytokines, including interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-α), and C-reactive protein, can cause cognitive impairment [44, 45]. These associations between systemic inflammation and cognitive impairment have been found in all age groups—young [46], middle-aged [47], and older [48] adults. TNF-α and IL-6 concentrations are higher in patients with SSHL than in controls [49, 50]. Taken together, these findings lead us to speculate that SSHL shares a common etiology with dementia through these underlying mechanisms. Additional investigations are required to verify this.
Notably, women with SSHL, especially those < 65 years of age, had a higher risk of dementia than women without SSHL. The epidemiologic data revealed that more women than men had dementia. This agrees with the worldwide data, which indicate dementia is twice as prevalent in women as in men [51]. Although the risk of dementia is related to age, dementia is ultimately deterioration of the brain and not caused by aging alone. Using data from the NHIRD, Lee et al. [52] reported that HL was positively correlated with the risk of dementia, especially in patients aged 45–64 years. However, unlike in our study, they did not stratify the participants by age and sex, which may have confounded the results. Women have higher estrogen levels, which affects their physiology throughout life, including mental health, brain, and cardiovascular health. Estrogen may protect brain cells [53, 54], and higher estrogen levels in aging women may decrease their susceptibility to dementia [55–57]. In our findings, women aged < 65 years with SSHL were most prone to dementia. Assessing the risk of dementia is complicated; understanding the differences in the relationship between dementia and sex and its association with various diseases may help elucidate its causes and the development preventive strategies.
Strengths and limitations
Our study used a representative nation-based sample and included physicians’ diagnoses of SSHL, a longitudinal analysis, and a wide array of covariates, including general health status and sociodemographic factors. Nonetheless, our study has several limitations. In particular, the NHIRD does not include information on other potential confounders, such as education level, level of cognitive function at baseline, ApoE ε4, stressful life events, body mass index, smoking habits, air pollution exposure, or family history. Furthermore, the follow-up time was short compared with the preclinical phase of Alzheimer disease. Participants who developed dementia were likely already on a trajectory of cognitive decline. Without knowing the participants’ cognitive status, even if we matched SSHL cases to non-SSHL controls, it would be unclear whether SSHL is a consequence of a common underlying pathology that is also related to dementia risk or whether SSHL may aggravate an underlying condition, precipitating dementia. Moreover, although our study revealed that women with SSHL aged < 65 years had the highest risk of dementia, subgroup analysis revealed that there were only 38 dementia cases (23 in the comparison cohort and 15 in the SSHL cohort), indicating the possibility of overfitting. Further studies should explore the association between dementia and sex in the middle-aged population. Finally, this study included only Taiwanese citizens, so our findings may not be generalizable to other countries.
Conclusion
The present study investigated a possible link between SSHL and dementia development. We observed that patients with SSHL, especially women aged < 65 years, were associated with higher risk of dementia than those without SSHL. Given the rapidly aging population in Taiwan and worldwide, clinicians should be aware of the risk of dementia in patients with SSHL. Further research is required to explore the mechanisms underlying this association.
Acknowledgments
The authors thank the Statistical Analysis Laboratory, Department of Management, Kaohsiung Municipal Ta-Tung Hospital.
Abbreviations
SSHL
Sudden sensorineural hearing loss
HL
Hearing loss
ARHL
Aged-Related Hearing Loss
NHIRD
National Health Insurance Research Database
NHI
National Health Insurance
(ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
HRs
Hazard ratios
CIs
Confidence intervals
ApoE
Apolipoprotein E
Authors’ contributions
S.Y.T and C.Y.C designed the study. C.T.S and C.Y.C had full access to all the data in the study and take responsibility for data integrity and the accuracy of the data analysis. S.Y.T, L.F.W., C.T.S, and C.Y.C contributed substantially to the study design, data analysis, and data interpretation. S.Y.T and C.Y.C contributed substantially to the writing of the manuscript. All authors have read and approved the final manuscript.
Funding
This research received funding from the Taiwan Ministry of Science and Technology (MOST 109–2635-B-037-005) and Kaohsiung Municipal Ta-Tung Hospital (kmtth-106-046 and kmtth-109-R007). The sponsors or funding sources had no role in study design, data collection and analysis, decision to publish, or manuscript preparation.
Availability of data and materials
The data that support our findings this study are available from the NHIRD, but restrictions apply to the availability of these data, which were used under license for the current study; thus, they are not publicly available. Data are, however, available from the authors upon reasonable request and with the permission of the NHIRD.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB-EXEMPT (II)-20160028). All methods were carried out in accordance with relevant guidelines and regulations. As study participants were collected from the NHIRD, the requirement of patient consent was waived due to the anonymity of the database.
Consent for publication
Not applicable.
Competing interests
None of the authors have any conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Department of Civil Affair, Ministry of the Interior, Taiwan. http://www.moi.gov.tw/english/cl.aspx?n=7662. Accessed 12 Feb 2021.
- 2.staff TCPn . Taiwan must address the challenges of an aging society. 2013. [Google Scholar]
- 3.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3(3):186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 4.Drugs for Alzheimer's disease: best avoided. No therapeutic advantage. Prescrire Int. 2012;21(128):150. [PubMed]
- 5.Birks J, Flicker L. Donepezil for mild cognitive impairment. Cochrane Database Syst Rev. 2006;(3):CD006104. 10.1002/14651858.CD006104. [DOI] [PubMed]
- 6.Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2018;144(2):115–126. doi: 10.1001/jamaoto.2017.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomson RS, Auduong P, Miller AT, Gurgel RK. Hearing loss as a risk factor for dementia: a systematic review. Laryngoscope Investig Otolaryngol. 2017;2(2):69–79. doi: 10.1002/lio2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panza F, Solfrizzi V, Logroscino G. Age-related hearing impairment—a risk factor and frailty marker for dementia and AD. Nat Rev Neurol. 2015;11(3):166–175. doi: 10.1038/nrneurol.2015.12. [DOI] [PubMed] [Google Scholar]
- 9.Amieva H, Ouvrard C, Meillon C, Rullier L, Dartigues J-F. Death, depression, disability, and dementia associated with self-reported hearing problems: a 25-year study. J Gerontol A. 2018;73(10):1383–1389. doi: 10.1093/gerona/glx250. [DOI] [PubMed] [Google Scholar]
- 10.Chandrasekhar SS, Tsai Do BS, Schwartz SR, et al. Clinical practice guideline: sudden hearing loss (update) Otolaryngol Head Neck Surg. 2019;161(1_suppl):S1–S45. doi: 10.1177/0194599819859885. [DOI] [PubMed] [Google Scholar]
- 11.Rauch SD. Clinical practice. Idiopathic sudden sensorineural hearing loss. N Engl J Med. 2008;359(8):833–840. doi: 10.1056/NEJMcp0802129. [DOI] [PubMed] [Google Scholar]
- 12.Xie W, Dai Q, Liu J, Liu Y, Hellström S, Duan M. Analysis of clinical and laboratory findings of idiopathic sudden sensorineural hearing loss. Sci Rep. 2020;10(1):6057. doi: 10.1038/s41598-020-63046-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh A, Kumar Irugu DV. Sudden sensorineural hearing loss – a contemporary review of management issues. J Otol. 2020;15(2):67–73. doi: 10.1016/j.joto.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J-Y, Hong JY, Kim D-K. Association of sudden sensorineural hearing loss with risk of cardiocerebrovascular disease: a study using data from the Korea National Health Insurance Service. JAMA Otolaryngol Head Neck Surg. 2018;144(2):129–135. doi: 10.1001/jamaoto.2017.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SY, Lim JS, Sim S, Choi HG. Sudden sensorineural hearing loss predicts ischemic stroke: a longitudinal follow-up study. Otol Neurotol. 2018;39(8):964–969. doi: 10.1097/MAO.0000000000001902. [DOI] [PubMed] [Google Scholar]
- 16.Phillips JS, King JA, Chandran S, Prinsley PR, Dick D. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) presenting with sudden sensorineural hearing loss. J Laryngol Otol. 2005;119(2):148–151. doi: 10.1258/0022215053419880. [DOI] [PubMed] [Google Scholar]
- 17.Institues NHR. National health insurance research database. http://nhird.nhri.org.tw/date_01_en.html. Accessed 12 June 2020.
- 18.Deal JA, Betz J, Yaffe K, et al. Hearing impairment and incident dementia and cognitive decline in older adults: the health ABC study. J Gerontol A. 2016;72(5):703–709. doi: 10.1093/gerona/glw069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin FR, Metter EJ, O'Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Arch Neurol. 2011;68(2):214–220. doi: 10.1001/archneurol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su P, Hsu C-C, Lin H-C, et al. Age-related hearing loss and dementia: a 10-year national population-based study. Eur Arch Otorhinolaryngol. 2017;274(5):2327–2334. doi: 10.1007/s00405-017-4471-5. [DOI] [PubMed] [Google Scholar]
- 21.Ford AH, Hankey GJ, Yeap BB, Golledge J, Flicker L, Almeida OP. Hearing loss and the risk of dementia in later life. Maturitas. 2018;112:1–11. doi: 10.1016/j.maturitas.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Gurgel RK, Ward PD, Schwartz S, Norton MC, Foster NL, Tschanz JT. Relationship of hearing loss and dementia: a prospective, population-based study. Otol Neurotol. 2014;35(5):775–781. doi: 10.1097/MAO.0000000000000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Füllgrabe C. When hearing loss masquerades as cognitive decline. J Neurol Neurosurg Psychiatry. 2020;91(12):1248. doi: 10.1136/jnnp-2020-324707. [DOI] [PubMed] [Google Scholar]
- 25.Griffiths TD, Lad M, Kumar S, et al. How can hearing loss cause dementia? Neuron. 2020;108(3):401–412. doi: 10.1016/j.neuron.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wayne RV, Johnsrude IS. A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Res Rev. 2015;23(Pt B):154–166. doi: 10.1016/j.arr.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Rabbitt P. Mild hearing loss can cause apparent memory failures which increase with age and reduce with IQ. Acta Otolaryngol. 1991;111(sup476):167–176. doi: 10.3109/00016489109127274. [DOI] [PubMed] [Google Scholar]
- 28.Füllgrabe C. On the possible overestimation of cognitive decline: the impact of age-related hearing loss on cognitive-test performance. Front Neurosci. 2020;14:454. doi: 10.3389/fnins.2020.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3(6):343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 30.Chau JK, Lin JR, Atashband S, Irvine RA, Westerberg BD. Systematic review of the evidence for the etiology of adult sudden sensorineural hearing loss. Laryngoscope. 2010;120(5):1011–1021. doi: 10.1002/lary.20873. [DOI] [PubMed] [Google Scholar]
- 31.Schuknecht HF, Donovan ED. The pathology of idiopathic sudden sensorineural hearing loss. Arch Otorhinolaryngol. 1986;243(1):1–15. doi: 10.1007/BF00457899. [DOI] [PubMed] [Google Scholar]
- 32.Capaccio P, Ottaviani F, Cuccarini V, et al. Genetic and acquired prothrombotic risk factors and sudden hearing loss. Laryngoscope. 2007;117(3):547–551. doi: 10.1097/MLG.0b013e31802f3c6a. [DOI] [PubMed] [Google Scholar]
- 33.Toubi E, Ben-David J, Kessel A, Halas K, Sabo E, Luntz M. Immune-mediated disorders associated with idiopathic sudden sensorineural hearing loss. Ann Otol Rhinol Laryngol. 2004;113(6):445–449. doi: 10.1177/000348940411300605. [DOI] [PubMed] [Google Scholar]
- 34.Baek MJ, Park HM, Johnson JM, et al. Increased frequencies of cochlin-specific T cells in patients with autoimmune sensorineural hearing loss. J Immunol (Baltimore, Md: 1950) 2006;177(6):4203–4210. doi: 10.4049/jimmunol.177.6.4203. [DOI] [PubMed] [Google Scholar]
- 35.Cao Z, Gao J, Huang S, et al. Genetic polymorphisms and susceptibility to sudden sensorineural hearing loss: a systematic review. Audiol Neurootol. 2019;24(1):8–19. doi: 10.1159/000497032. [DOI] [PubMed] [Google Scholar]
- 36.Lin HC, Chao PZ, Lee HC. Sudden sensorineural hearing loss increases the risk of stroke: a 5-year follow-up study. Stroke. 2008;39(10):2744–2748. doi: 10.1161/STROKEAHA.108.519090. [DOI] [PubMed] [Google Scholar]
- 37.Lin C, Lin SW, Lin YS, Weng SF, Lee TM. Sudden sensorineural hearing loss is correlated with an increased risk of acute myocardial infarction: a population-based cohort study. Laryngoscope. 2013;123(9):2254–2258. doi: 10.1002/lary.23837. [DOI] [PubMed] [Google Scholar]
- 38.Suckfüll M, Wimmer C, Reichel O, Mees K, Schorn K. Hyperfibrinogenemia as a risk factor for sudden hearing loss. Otol Neurotol. 2002;23(3):309–311. doi: 10.1097/00129492-200205000-00013. [DOI] [PubMed] [Google Scholar]
- 39.Doo JG, Kim D, Kim Y, et al. Biomarkers suggesting favorable prognostic outcomes in sudden sensorineural hearing loss. Int J Mol Sci. 2020;21(19):7248. doi: 10.3390/ijms21197248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panza F, Solfrizzi V, Colacicco AM, et al. Cerebrovascular disease in the elderly: lipoprotein metabolism and cognitive decline. Aging Clin Exp Res. 2006;18(2):144–148. doi: 10.1007/BF03327430. [DOI] [PubMed] [Google Scholar]
- 41.Xu G, Zhang H, Zhang S, Fan X, Liu X. Plasma fibrinogen is associated with cognitive decline and risk for dementia in patients with mild cognitive impairment. Int J Clin Pract. 2008;62(7):1070–1075. doi: 10.1111/j.1742-1241.2007.01268.x. [DOI] [PubMed] [Google Scholar]
- 42.Lin T, Liu GA, Perez E, et al. Systemic inflammation mediates age-related cognitive deficits. Front Aging Neurosci. 2018;10:236. doi: 10.3389/fnagi.2018.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li G, You D, Ma J, Li W, Li H, Sun S. The role of autoimmunity in the pathogenesis of sudden sensorineural hearing loss. Neural Plast. 2018;2018:7691473. doi: 10.1155/2018/7691473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59(3):371–378. doi: 10.1212/WNL.59.3.371. [DOI] [PubMed] [Google Scholar]
- 45.Schram MT, Euser SM, de Craen AJ, et al. Systemic markers of inflammation and cognitive decline in old age. J Am Geriatr Soc. 2007;55(5):708–716. doi: 10.1111/j.1532-5415.2007.01159.x. [DOI] [PubMed] [Google Scholar]
- 46.Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatry. 2008;63(11):1022–1029. doi: 10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marsland AL, Gianaros PJ, Kuan DC-H, Sheu LK, Krajina K, Manuck SB. Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav Immun. 2015;48:195–204. doi: 10.1016/j.bbi.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tegeler C, O'Sullivan JL, Bucholtz N, et al. The inflammatory markers CRP, IL-6, and IL-10 are associated with cognitive function—data from the Berlin Aging Study II. Neurobiol Aging. 2016;38:112–117. doi: 10.1016/j.neurobiolaging.2015.10.039. [DOI] [PubMed] [Google Scholar]
- 49.Yoon SH, Kim ME, Kim HY, Lee JS, Jang CH. Inflammatory cytokines and mononuclear cells in sudden sensorineural hearing loss. J Laryngol Otol. 2019;133(2):95–101. doi: 10.1017/S0022215119000100. [DOI] [PubMed] [Google Scholar]
- 50.Masuda M, Kanzaki S, Minami S, et al. Correlations of inflammatory biomarkers with the onset and prognosis of idiopathic sudden sensorineural hearing loss. Otol Neurotol. 2012;33(7):1142–1150. doi: 10.1097/MAO.0b013e3182635417. [DOI] [PubMed] [Google Scholar]
- 51.Beam CR, Kaneshiro C, Jang JY, Reynolds CA, Pedersen NL, Gatz M. Differences between women and men in incidence rates of dementia and Alzheimer’s disease. J Alzheimers Dis. 2018;64(4):1077–1083. doi: 10.3233/JAD-180141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu CM, Lee CT. Association of hearing loss with dementia. JAMA Netw Open. 2019;2(7):e198112. doi: 10.1001/jamanetworkopen.2019.8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu JH. Does estrogen provide “neuroprotection” for postmenopausal women? Menopause. 2019;26(12):1361–1362. doi: 10.1097/GME.0000000000001459. [DOI] [PubMed] [Google Scholar]
- 54.Matyi JM, Rattinger GB, Schwartz S, Buhusi M, Tschanz JT. Lifetime estrogen exposure and cognition in late life: the Cache County Study. Menopause. 2019;26(12):1366–1374. doi: 10.1097/GME.0000000000001405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waring SC, Rocca WA, Petersen RC, O'Brien PC, Tangalos EG, Kokmen E. Postmenopausal estrogen replacement therapy and risk of AD: a population-based study. Neurology. 1999;52(5):965–970. doi: 10.1212/WNL.52.5.965. [DOI] [PubMed] [Google Scholar]
- 56.Ratnakumar A, Zimmerman SE, Jordan BA, Mar JC. Estrogen activates Alzheimer’s disease genes. Alzheimers Dement (N Y) 2019;5:906–917. doi: 10.1016/j.trci.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song YJ, Li SR, Li XW, et al. The effect of estrogen replacement therapy on Alzheimer’s disease and Parkinson’s disease in postmenopausal women: a meta-analysis. Front Neurosci. 2020;14:157. doi: 10.3389/fnins.2020.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support our findings this study are available from the NHIRD, but restrictions apply to the availability of these data, which were used under license for the current study; thus, they are not publicly available. Data are, however, available from the authors upon reasonable request and with the permission of the NHIRD.