Trends in thyroid cancer incidence and mortality in the United States, 1974–2013 (original) (raw)
. Author manuscript; available in PMC: 2021 Jun 21.
Published in final edited form as: JAMA. 2017 Apr 4;317(13):1338–1348. doi: 10.1001/jama.2017.2719
Abstract
Importance:
Thyroid cancer incidence has more than tripled in the United States over the last four decades, driven largely by increases in papillary thyroid cancer. It is unclear whether the rising incidence of papillary thyroid cancer has been related to thyroid cancer mortality trends.
Objective:
To compare trends in thyroid cancer incidence and mortality by tumor characteristics at diagnosis.
Design, setting, and participants:
Trends in thyroid cancer incidence and incidence-based mortality rates were evaluated using data from the Surveillance, Epidemiology, and End Results-9 cancer registry program.
Exposures:
Tumor characteristics.
Main outcomes and measures:
Log-linear regression was used to calculate annual percent changes in age-adjusted thyroid cancer incidence and incidence-based mortality rates by histologic type and Surveillance, Epidemiology, and End Results stage for cases diagnosed during 1974–2013.
Results:
Among 77,276 patients (mean age at diagnosis, 48 [standard deviation=16] years; 58,213 [75%] women) diagnosed with thyroid cancer from 1974–2013, papillary thyroid cancer was the most common histologic type (64,625 cases), and 2,371 deaths from thyroid cancer occurred during 1994–2013. Thyroid cancer incidence increased, on average, 3.6% per year (95% confidence interval 3.2–3.9%) during 1974–2013 (from 4.56 per 100,000 person-years in 1974–77 to 14.42 per 100,000 person-years in 2010–13), primarily driven by increases in papillary thyroid cancer (annual percent change=4.4%, 95% confidence interval 4.0–4.7%). Papillary thyroid cancer incidence increased for all stages at diagnosis (4.6% per year for localized, 4.3% per year for regional, 2.4% per year for distant, 1.8% per year for unknown). During 1994–2013, incidence-based mortality increased 1.1% per year (95% confidence interval 0.6–1.6%) (from 0.40 per 100,000 person-years in 1994–97 to 0.46 per 100,000 person-years in 2010–13) overall and 2.9% per year (95% confidence interval 1.1–4.7%) for distant papillary thyroid cancer.
Conclusions and Relevance:
Among patients in the United States diagnosed with thyroid cancer from 1974–2013, the overall incidence of thyroid cancer increased 3% annually, with increases in the incidence rate and thyroid cancer mortality rate for advanced-stage papillary thyroid cancer. These findings are consistent with a true increase in the occurrence of thyroid cancer in the United States.
Introduction
In the United States, thyroid cancer incidence rates have more than tripled between 1975 and 2013, with papillary thyroid cancer (PTC), the most common and least aggressive histologic type, accounting for most of the new cases1. Some investigators have suggested that over-diagnosis, or the increased ability to detect and diagnose small, indolent tumors that would never otherwise cause symptoms or require treatment, explains a substantial proportion of the increase2–5.
However, there is growing evidence in support of a true increase in the occurrence of thyroid cancer. An analysis of Surveillance, Epidemiology and End Results (SEER) cancer registry data from 1980–2005 revealed substantial increases in the incidence of advanced-stage PTCs and PTCs >5 cm in diameter; these tumors are generally large enough to be detected via palpation or to cause symptoms6. The rates of increase for the largest (>5 cm) and the smallest PTCs (≤1 cm) were nearly equal among white women, a group considered to be particularly susceptible to over-diagnosis6. While thyroid cancer mortality rates are much lower relative to incidence, providing the impression of stability over time2–5, thyroid cancer mortality rates have increased significantly since the late 1980s (0.7% per year, P<0.001)1. These trends are consistent with temporal changes in the prevalence of some risk factors, including obesity and non-current smoking7.
As advanced-stage PTC is less amenable to treatment than localized PTC, the rising mortality rates may be a direct consequence of the trends in advanced-stage PTC. To address this question, the current analysis used SEER data during 1974–2013 to systematically compare thyroid cancer incidence and mortality trends by demographic and tumor characteristics at the time of diagnosis.
Methods
Data sources
Thyroid cancer cases diagnosed during 1974–2013 were ascertained from the SEER cancer incidence file maintained by the National Cancer Institute8. The file includes information from nine high-quality, population-based registries (SEER-9: Connecticut, Iowa, New Mexico, Utah, Hawaii, Detroit, San Francisco-Oakland, Atlanta, and Seattle-Puget Sound) that include approximately 10% of the U.S. population. Demographic and cancer diagnosis information was available for each case. Data on nationwide and SEER-9 thyroid cancer deaths were derived from information recorded in death certificates and ascertained from the National Center for Health Statistics9.
The incidence-based mortality file uses cancer registry information from the SEER-9 cancer incidence file to link characteristics of the cancer at diagnosis with death certificate information10,11. Thus, unlike traditional mortality rates, incidence-based mortality rates can be examined according to variables recorded at diagnosis (e.g., histology, stage, tumor size). The incidence-based mortality analysis was restricted to deaths during 1994–2013 and diagnoses during 1974–2013 to ensure maximum data for those deaths and to prevent underestimation of the incidence-based mortality rates in the earliest years.
Demographic characteristics
Demographic characteristics of interest for this analysis included gender, race, and age at diagnosis. This information was originally abstracted from medical records and submitted to regional or state cancer registries. Information on age at thyroid cancer death was abstracted from death certificates.
Tumor characteristics
Thyroid cancer cases (International Classification of Diseases for Oncology version 3 topography code C73) were classified according to histologic type12: PTC (histologic codes 8050, 8260, 8340–8344, 8350, 8450–8460), follicular thyroid cancer (FTC, 8290, 8330–8335), medullary thyroid cancer (MTC, 8345,8510–8513), anaplastic thyroid cancer (ATC, 8020–8035), others (including unspecified, poorly specified [e.g., insular], and others).
SEER Historic Stage A was used to classify cases according to stage at diagnosis: localized (limited to the thyroid gland), regional (tumor extension beyond the limits of the thyroid gland or spread by more than one lymphatic or vascular supply route), distant (extracervical metastasis), and unknown stage13. Cases diagnosed since 2004 additionally were classified as Stage I-IV (or unknown) according to the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) Tumor-Node-Metastasis (TNM) staging system, 6th edition, which accounts for age at diagnosis (<45 and ≥45 years, for PTC and FTC), tumor size (T stage), extent of spread to the regional lymph nodes (N stage), and presence or absence of distant metastases (M stage)14. Although AJCC/TNM stage information was not available for the entire study period, it is the staging system most used for thyroid cancer in clinical practice and is more readily interpretable than SEER stage.
Tumor size has been recorded in SEER since 1983 using three different schemes: Extent of Disease-4 codes for 1983–1987, Extent of Disease-10 codes for 1988–2003, and Collaborative Staging codes for 2004–2013. These codes were combined to categorize cases diagnosed during 1983–2013 by tumor size15.
Data analysis
Only microscopically-confirmed cases and the first matching record were selected, and cases identified only from autopsy records or death certificates were excluded. Incidence and mortality rates were calculated using SEER*Stat version 8.3.216. All rates were age-adjusted to the 2000 U.S. standard population and expressed per 100,000 person–years. Incidence-based mortality rates were calculated as number of thyroid cancer deaths among cases diagnosed in the SEER-9 registries over person-time at risk among individuals in the SEER areas. Standardized dimensions were used for the y- and x-axes, in which a slope of 10 degrees represents a change of 1% per year17. Rates for thyroid cancers with missing or unknown histology, stage, or size were calculated and plotted separately from the known values. Rate differences were calculated to evaluate the degree to which reductions or increases in rates for thyroid cancers with unknown stage or size may have affected trends for thyroid cancers with known values. The National Cancer Institute’s Joinpoint Regression Analysis program, version 4.2.018, calculated annual percentage changes (APCs) and 95% confidence intervals (CIs) to quantify trends in incidence and mortality, overall and by demographic and tumor characteristics, using T-tests to determine whether APCs were statistically significantly different from zero. The program also selected the best-fitting log-linear regression model to identify calendar years (i.e., the “joinpoints”) when APCs changed significantly, allowing for the minimum number of “joinpoints” necessary to fit the data.18 Statistical significance was assessed at the P<0.05 alpha level, and all hypotheses were two-sided.
Results
Of the 79,409 thyroid cancer cases diagnosed among residents of the SEER-9 areas during 1974–2013, 77,276 (97%) met the case definition and were included in the incidence analysis (Table 1). Women (75%) and whites (82%) comprised the majority of the cases. Mean age at diagnosis was 48 (standard deviation=16) years. The most common histologic types were PTC (84%) and FTC (11%). Of the eligible cases, 2,371 died of thyroid cancer during 1994–2013 and were included in the incidence-based mortality analysis. Of the deaths, 57% occurred in women and 81% in whites. Compared with all cases, patients who died of thyroid cancer were more likely to have been diagnosed at older ages, with non-PTC histologies, and advanced-stage and/or larger tumors. Among those who died of thyroid cancer, the median time between thyroid cancer diagnosis and death was 25 months; 19% survived >10 years after diagnosis. Among PTC patients who died of thyroid cancer, 27% survived >10 years after diagnosis.
Table 1.
Thyroid cancer incidence (1974–2013) and incidence-based mortality (1994–2013): the SEER-9 Registry Database
| Incidence | Incidence based mortality | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic at diagnosis | Thyroid cancer | Papillary thyroid cancer | Thyroid cancer | Papillary thyroid cancer | ||||||||||||
| No. of cases a | % | Rateb | 95% CI | No. of casesa | % | Rateb | 95% CI | No. of deathsc | % | Rateb | 95% CI | No. of deathsc | % | Rateb | 95% CI | |
| Overall | 77,276 | 100 | 7.98 | 7.93, 8.04 | 64,625 | 100.0 | 6.66 | 6.61, 6.71 | 2,371 | 100 | 0.44 | 0.42, 0.46 | 1063 | 100 | 0.20 | 0.19, 0.21 |
| Gender | ||||||||||||||||
| Male | 19,063 | 24.7 | 4.20 | 4.14, 4.26 | 15,074 | 23.3 | 3.28 | 3.23, 3.33 | 1,017 | 42.9 | 0.44 | 0.41, 0.47 | 464 | 43.7 | 0.20 | 0.18, 0.22 |
| Female | 58,213 | 75.3 | 11.63 | 11.53, 11.72 | 49,551 | 76.7 | 9.92 | 9.83, 10.01 | 1,354 | 57.1 | 0.44 | 0.41, 0.46 | 599 | 56.3 | 0.19 | 0.18, 0.21 |
| Race | ||||||||||||||||
| White | 63,479 | 82.1 | 8.18 | 8.12, 8.24 | 53,219 | 82.4 | 6.85 | 6.80, 6.91 | 1,914 | 80.7 | 0.43 | 0.41, 0.45 | 841 | 79.1 | 0.19 | 0.18, 0.20 |
| Black | 4,582 | 5.9 | 4.88 | 4.73, 5.03 | 3,473 | 5.4 | 3.64 | 3.52, 3.77 | 143 | 6 | 0.32 | 0.27, 0.38 | 48 | 4.5 | 0.11 | 0.08, 0.15 |
| Othersd | 8,442 | 10.9 | 9.14 | 8.95, 9.34 | 7,305 | 11.3 | 7.85 | 7.66, 8.03 | 312 | 13.2 | 0.59 | 0.52, 0.66 | 173 | 16.3 | 0.33 | 0.28, 0.38 |
| Age (years) | ||||||||||||||||
| <20 | 1,794 | 2.3 | 0.62 | 0.59, 0.65 | 1,512 | 2.30 | 0.52 | 0.49, 0.55 | - | - | - | - | - | - | - | - |
| 20–39 | 23,877 | 30.9 | 8.00 | 7.89, 8.09 | 21,106 | 32.7 | 7.06 | 6.97, 7.16 | 102 | 4.3 | 0.02 | 0.02, 0.02 | 47 | 4.4 | 0.01 | 0.01, 0.01 |
| 40–59 | 32,188 | 41.7 | 13.28 | 13.14, 13.43 | 27,727 | 42.9 | 11.45 | 11.32, 11.59 | 631 | 26.6 | 0.11 | 0.10, 0.12 | 314 | 29.5 | 0.06 | 0.05, 0.06 |
| 60–79 | 16,877 | 21.8 | 13.15 | 12.95, 13.35 | 12,825 | 19.8 | 9.92 | 9.75, 10.10 | 1,148 | 48.4 | 0.22 | 0.21, 0.24 | 515 | 48.4 | 0.10 | 0.09, 0.11 |
| ≥80 | 2,540 | 3.3 | 8.81 | 8.47, 9.16 | 1,455 | 2.3 | 5.05 | 4.79, 5.31 | 479 | 20.2 | 0.09 | 0.08, 0.10 | 184 | 17.3 | 0.03 | 0.03, 0.04 |
| Histologic type | ||||||||||||||||
| Papillary | 64,625 | 83.6 | 6.66 | 6.61, 6.71 | 1,063 | 44.8 | 0.20 | 0.19, 0.21 | ||||||||
| Follicular | 8,359 | 10.8 | 0.87 | 0.85, 0.89 | 404 | 17 | 0.08 | 0.07, 0.08 | ||||||||
| Medullary | 1,685 | 2.2 | 0.18 | 0.17, 0.18 | 189 | 8 | 0.04 | 0.03, 0.04 | ||||||||
| Anaplastic | 975 | 1.3 | 0.11 | 0.10, 0.11 | 471 | 19.9 | 0.09 | 0.08, 0.10 | ||||||||
| Othere | 1,632 | 2.1 | 0.17 | 0.17, 0.18 | 244 | 10.3 | 0.05 | 0.04, 0.05 | ||||||||
| SEER Historic Stage A | ||||||||||||||||
| Localized | 45,919 | 59.4 | 4.75 | 4.71, 4.79 | 39,971 | 61.9 | 4.13 | 4.09, 4.17 | 280 | 11.8 | 0.05 | 0.05, 0.06 | 143 | 13.5 | 0.03 | 0.02, 0.03 |
| Regional | 25,835 | 33.4 | 2.66 | 2.62, 2.69 | 21,435 | 33.2 | 2.20 | 2.17, 2.23 | 1,045 | 44.1 | 0.19 | 0.18, 0.21 | 566 | 53.2 | 0.11 | 0.10, 0.11 |
| Distant | 3,658 | 4.7 | 0.39 | 0.37, 0.40 | 2,045 | 3.2 | 0.21 | 0.20, 0.22 | 922 | 38.9 | 0.17 | 0.16, 0.18 | 308 | 29.0 | 0.06 | 0.05, 0.06 |
| Unknown | 1,864 | 2.4 | 0.19 | 0.18, 0.20 | 1,174 | 1.8 | 0.12 | 0.11, 0.13 | 124 | 5.2 | 0.02 | 0.02, 0.03 | 46 | 4.3 | 0.01 | 0.01, 0.01 |
| AJCC/TNM stagef | ||||||||||||||||
| Stage I | 25,580 | 67.4 | 8.91 | 8.80, 9.02 | 23,974 | 71.5 | 8.34 | 8.24, 8.45 | 17 | 0.7 | 0.01 | 0.003, 0.01 | - | - | - | - |
| Stage II | 2,870 | 7.6 | 0.92 | 0.89, 0.96 | 2,091 | 6.20 | 0.67 | 0.64, 0.70 | - | - | - | - | - | - | - | - |
| Stage III | 4,562 | 12.0 | 1.46 | 1.42, 1.50 | 3,821 | 11.4 | 1.22 | 1.18, 1.25 | 45 | 1.9 | 0.02 | 0.01, 0.02 | 24 | 8.7 | 0.01 | 0.01, 0.01 |
| Stage IV | 3,045 | 8.0 | 1.01 | 0.97, 1.04 | 2,111 | 6.3 | 0.69 | 0.66, 0.72 | 680 | 28.7 | 0.23 | 0.21, 0.25 | 218 | 77.6 | 0.07 | 0.06, 0.08 |
| Unknown | 1,881 | 5.0 | 0.62 | 0.59, 0.65 | 1,541 | 4.6 | 0.51 | 0.48, 0.53 | 49 | 68.1 | 0.02 | 0.01, 0.02 | 19 | 6.9 | 0.01 | 0.00, 0.01 |
| Tumor size (cm)g | ||||||||||||||||
| ≤1 | 19,943 | 28.6 | 2.50 | 2.47, 2.54 | 19,257 | 32.5 | 2.42 | 2.38, 2.45 | 71 | 3.1 | 0.01 | 0.01, 0.02 | 53 | 5.2 | 0.01 | 0.01, 0.01 |
| >1-≤2 | 18,113 | 26.0 | 2.26 | 2.23, 2.29 | 16,477 | 27.8 | 2.05 | 2.02, 2.09 | 188 | 8.2 | 0.04 | 0.03, 0.04 | 130 | 12.9 | 0.02 | 0.02, 0.03 |
| >2-≤4 | 16,031 | 23.0 | 2.00 | 1.97, 2.03 | 12,772 | 21.6 | 1.59 | 1.56, 1.61 | 483 | 21.1 | 0.09 | 0.08, 0.10 | 274 | 27.2 | 0.05 | 0.05, 0.06 |
| >4 | 6,713 | 9.6 | 0.85 | 0.83, 0.87 | 3,973 | 6.7 | 0.50 | 0.48, 0.51 | 852 | 37.1 | 0.16 | 0.15, 0.17 | 278 | 27.5 | 0.05 | 0.05, 0.06 |
| Unknown | 8,879 | 13.0 | 1.12 | 1.10, 1.14 | 6,721 | 11.4 | 0.84 | 0.82, 0.86 | 700 | 30.5 | 0.13 | 0.12, 0.14 | 276 | 27.3 | 0.05 | 0.05, 0.06 |
Of the 77,276 cases occurring during 1974–2013, 10% were diagnosed in years prior to the availability of tumor size data starting in 1983, and 51% were diagnosed prior to the availability of AJCC/TNM stage information starting in 2004 (Table 1). Of the 2,371 incidence-based mortality deaths occurring during 1994–2013, 3% had been diagnosed in years prior to the availability of tumor size data and 66% were diagnosed prior to the availability of AJCC/TNM stage information. During the years in which specific tumor characteristics were reported to the registries, SEER stage (1974–2013) was unknown for 2% of cases and 5% of deaths, tumor size (1983–2013) was unknown for 13% of cases and 31% of deaths, and AJCNN/TNM stage (2004–2013) was unknown for 5% of cases and 6% of deaths.
A comparison of the agreement between SEER Historic Stage A, tumor size, and AJCC/TNM stage among PTC cases diagnosed between 2004 and 2013 by age (<45 and ≥45 years) is shown in eTable 1. In general, more advanced stage was associated with larger tumor size. There was greater agreement of SEER stage with size and AJCC/TNM stage for patients aged ≥45 years compared with younger patients, reflecting the differences in AJCC/TNM staging guidelines for PTC patients diagnosed before and after age 45 14.
Trends in thyroid cancer incidence by demographic and tumor characteristics are described in Table 2, with joinpoints denoted as Trends 1–5. Trends by selected tumor characteristics are shown graphically in Figures 1a-1c. Thyroid cancer incidence rates increased over the study period (from 4.56 [95% CI 4.40–4.73] per 100,000 in 1974–77 to 14.42 [95% CI 14.20–14.64] per 100,000 in 2010–13), rising 3.6% (95% CI 3.2–3.9%) per year, on average. Rates increased 6.7% (95% CI 6.1–7.2%) per year during 1997–2009, but did not increase during 2009–2013 (APC=1.8%, 95% CI −0.7–4.4%). Thyroid cancer incidence rates increased for all sex, race, and age groups. Significant increases were observed for PTC (APC=4.4%, 95% CI 4.0–4.7%), FTC (APC=0.6%, 95% CI 0.2–0.8%), and MTC (APC=0.7%, 95% CI 0.2–1.1%). PTC incidence increased significantly for every stage and tumor size category. During 2009–2013, incidence rates did not increase significantly for overall, localized, Stage I, or small (≤2 cm) PTCs, while there was no evidence of a reduction in the increase for regional, distant, or large PTCs.
Table 2.
Trends in thyroid cancer incidence rates a (1974–2013): the SEER-9 Registry Database
| Overall | Trend 1 | Trend 2 | Trend 3 | Trend 4 | Trend 5 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic at diagnosis | APC (95% CI) | _P_value | Year | APC (95% CI) | _P_value | Year | APC (95% CI) | _P_value | Year | APC (95% CI) | _P_value | Year | APC (95% CI) | _P_value | Year | APC (95% CI) | _P_value |
| Total | 3.6 (3.2, 3.9) | <0.001 | 1974–77 | 5.5 (1.4, 9.8) | 0.01 | 1977–80 | −5.9 (−13.1, 1.9) | 0.10 | 1980–97 | 2.6 (2.3, 2.9) | <0.001 | 1997–09 | 6.7 (6.1, 7.2) | <0.001 | 2009–13 | 1.8 (−0.7, 4.4) | 0.20 |
| Gender | |||||||||||||||||
| Male | 3.1 (2.7, 3.5) | <0.001 | 1974–80 | −2.9 (−5.9, 0.2) | 0.07 | 1980–98 | 2.5 (1.8, 3.2) | <0.001 | 1998–13 | 5.6 (4.7, 6.4) | <0.001 | ||||||
| Female | 3.7 (3.3, 4.1) | <0.001 | 1974–77 | 5.8 (0.7, 11.2) | 0.03 | 1977–80 | −5.3 (−14.2, 4.6) | 0.30 | 1980–96 | 2.6 (2.2, 3.0) | <0.001 | 1996–09 | 6.7 (6.1, 7.4) | <0.001 | 2009–13 | 1.6 (−1.5, 4.8) | 0.30 |
| Race | |||||||||||||||||
| White | 3.8 (3.4, 4.2) | <0.001 | 1974–77 | 6.2 (2.2, 10.4) | <0.001 | 1977–80 | −5.9 (−12.9, 1.6) | 0.10 | 1980–97 | 2.9 (2.6, 3.2) | <0.001 | 1997–09 | 7.0 (6.5, 7.6) | <0.001 | 2009–13 | 1.4 (−1.1, 3.9) | 0.30 |
| Black | 3.4 (2.8, 4.0) | <0.001 | 1974–88 | −1.3 (−2.8, 0.3) | 0.10 | 1988–13 | 5.4 (4.7, 6.1) | <0.001 | |||||||||
| Othersb | 1.5 (1.1, 1.9) | <0.001 | 1974–96 | −0.2 (−0.9, 0.4) | 0.50 | 1996–13 | 3.9 (2.9, 5.0) | <0.001 | |||||||||
| Age (years) | |||||||||||||||||
| <20 | 1.9 (1.3, 2.4) | <0.001 | 1974–06 | 1.1 (0.5, 1.7) | <0.001 | 2006–13 | 9.8 (3.4, 16.7) | 0.003 | |||||||||
| 20–39 | 3.0 (2.6, 3.3) | <0.001 | 1974–82 | −1.4 (−3.1, 0.3) | 0.10 | 1982–93 | 1.8 (0.6, 3.1) | 0.005 | 1993–13 | 4.7 (4.3, 5.2) | <0.001 | ||||||
| 40–59 | 3.9 (3.5, 4.3) | <0.001 | 1974–81 | −1.2 (−3.9, 1.5) | 0.40 | 1981–95 | 2.8 (1.7, 3.9) | <0.001 | 1995–13 | 6.2 (5.5, 6.9) | <0.001 | ||||||
| 60–79 | 4.2 (3.8, 4.6) | <0.001 | 1974–96 | 2.2 (1.8, 2.7) | <0.001 | 1996–09 | 7.7 (6.5, 8.9) | <0.001 | 2009–13 | 0.8 (−5.2, 7.2) | 0.80 | ||||||
| ≥80 | 2.3 (1.8, 2.7) | 0.002 | 1974–96 | 1.0 (0.1, 2.0) | 0.03 | 1996–13 | 4.1 (2.7, 5.5) | <0.001 | |||||||||
| Histologic type | |||||||||||||||||
| Papillary | 4.4 (4.0, 4.7) | <0.001 | 1974–77 | 6.9 (1.5, 12.6) | 0.01 | 1977–80 | −5.9 (−15.2, 4.4) | 0.20 | 1980–97 | 3.6 (3.2, 4.0) | <0.001 | 1997–09 | 7.3 (6.6, 8.1) | <0.001 | 2009–13 | 2.6 (−0.7, 6.1) | 0.10 |
| Follicular | 0.6 (0.2, 0.8) | <0.001 | 1974–00 | −0.2 (−0.7, 0.2) | 0.30 | 2000–06 | 6.0 (0.8, 11.4) | 0.03 | 2006–13 | −3.0 (−5.9, −0.1) | 0.05 | ||||||
| Medullary | 0.7 (0.2, 1.1) | 0.005 | |||||||||||||||
| Anaplastic | −0.1 (−0.7, 0.6) | 0.80 | |||||||||||||||
| Otherc | −0.6 (−1.3, 0.0) | 0.06 | 1974–94 | −3.0 (−4.6, −1.7) | <0.001 | 1994–13 | 1.9 (0.2, 3.7) | 0.03 | |||||||||
| Papillary | |||||||||||||||||
| SEER Historic Stage A | |||||||||||||||||
| Localized | 4.6 (4.1, 5.1) | <0.001 | 1974–77 | 8.2 (0.0, 17.2) | 0.05 | 1977–80 | −7.3 (−20.9, 8.7) | 0.30 | 1980–93 | 2.6 (1.6, 3.5) | <0.001 | 1993–09 | 7.8 (7.1, 8.5) | <0.001 | 2009–13 | 1.1 (−3.8, 6.3) | 0.70 |
| Regional | 4.3 (4.0, 4.6) | <0.001 | 1974–77 | 8.4 (1.4, 15.9) | 0.02 | 1977–81 | −3.3 (−9.5, 3.4) | 0.30 | 1981–84 | 11.7 (−2.2, 27.5) | 0.10 | 1984–99 | 2.8 (2.1, 3.4) | <0.001 | 1999–13 | 6.7 (6.0, 7.4) | <0.001 |
| Distant | 2.4 (1.7, 3.2) | <0.001 | 1974–81 | −7.7 (−15.0, 0.3) | 0.06 | 1981–13 | 3.5 (2.6, 4.4) | <0.001 | |||||||||
| Unknown | 1.8 (0.9, 2.7) | <0.001 | 1974–78 | −18.2 (−33.2, 0.1) | 0.05 | 1978–95 | 7.7 (5.1, 10.4) | <0.001 | 1995–01 | −11.8 (−23.6, 1.7) | 0.08 | 2001–13 | 5.6 (1.7, 9.6) | <0.001 | |||
| AJCC/TNM staged | |||||||||||||||||
| Stage I | 5.8 (4.5, 7.1) | <0.001 | 2004–09 | 8.2 (5.7, 10.7) | <0.001 | 2009–13 | 2.6 (−0.7, 6.1) | 0.10 | |||||||||
| Stage II | 3.9 (0.6, 7.3) | 0.03 | |||||||||||||||
| Stage III | 9.2 (6.6, 11.9) | <0.001 | 2004–06 | 26.0 (7.8, 47.3) | 0.01 | 2006–13 | 6.5 (4.3, 8.7) | <0.001 | |||||||||
| Stage IV | 5.2 (3.3, 7.2) | <0.001 | |||||||||||||||
| Unknown | −3.9 (−6.4, −1.3) | 0.009 | |||||||||||||||
| Tumor size (cm)e | |||||||||||||||||
| ≤1 | 9.3 (8.8, 9.8) | <0.001 | 1983–88 | 2.6 (−1.0, 6.3) | 0.15 | 1988–98 | 9.0 (7.4, 10.6) | <0.001 | 1998–08 | 12.2 (10.6, 13.8) | <0.001 | 2008–13 | 3.1 (−0.5, 6.9) | 0.09 | |||
| >1-≤2 | 5.4 (4.9, 5.9) | <0.001 | 1983–99 | 3.1 (2.6, 3.7) | <0.001 | 1999–09 | 9.1 (7.6, 10.6) | <0.001 | 2009–13 | 2.2 (−2.5, 7.2) | 0.40 | ||||||
| >2-≤4 | 4.5 (4.2, 4.9) | <0.001 | 1983–95 | 2.5 (1.4, 3.6) | <0.001 | 1995–13 | 5.7 (5.1, 6.3) | <0.001 | |||||||||
| >4 | 6.1 (5.4, 6.7) | <0.001 | 1983–88 | −2.8 (−9.7, 4.6) | 0.40 | 1988–13 | 6.9 (6.2, 7.6) | <0.001 | |||||||||
| Unknown | −1.8 (−2.6, −1.0) | <0.001 | 1983–00 | 1.4 (0.4, 2.4) | 0.005 | 2000–13 | −6.4 (−7.7, −5.1) | <0.001 |
Figure 1.
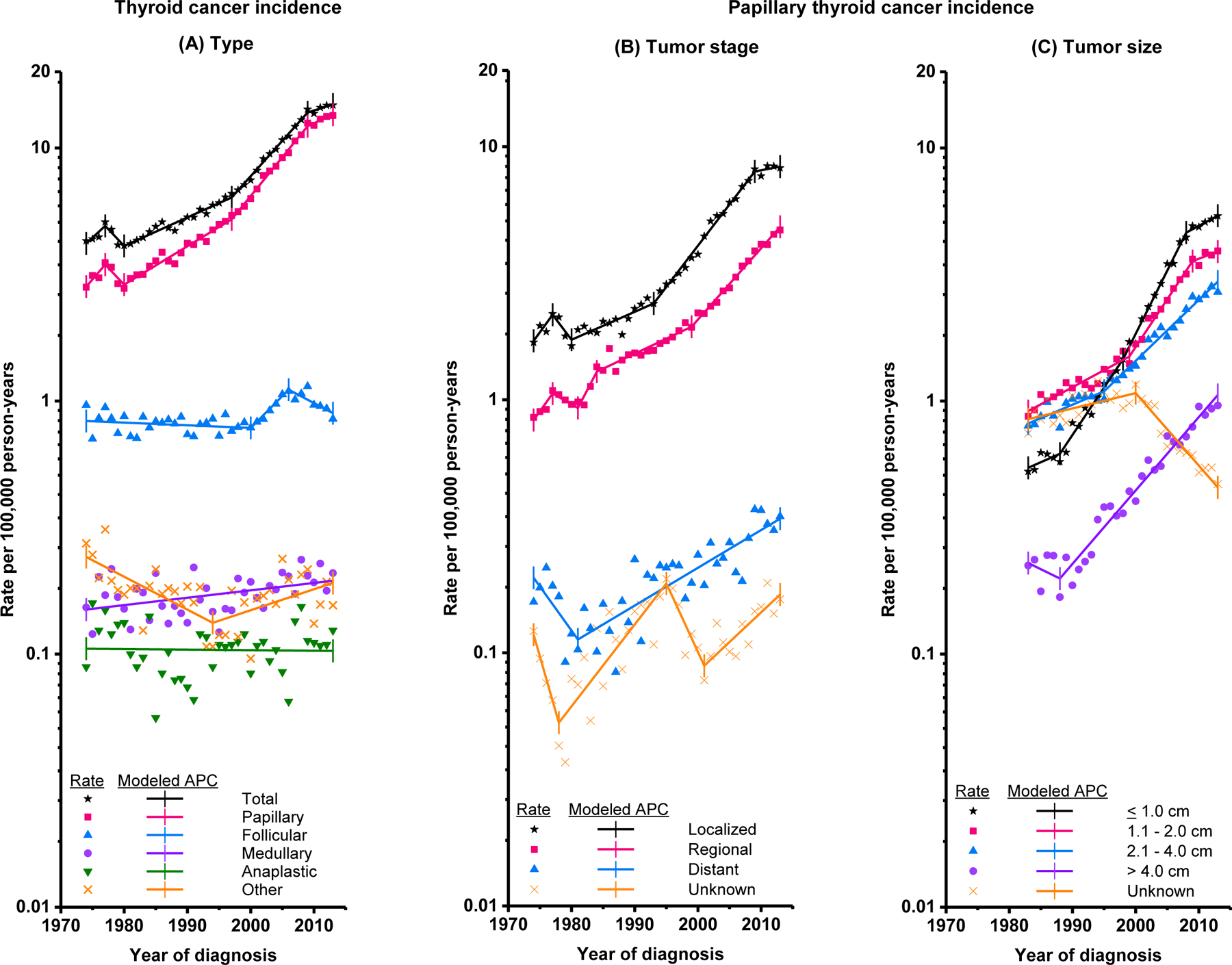
Trends in annual thyroid cancer incidence rates (cases per 100,000 person-years). (A) Thyroid cancer incidence, overall and by histologic type (1974–2013) (B) Papillary thyroid cancer incidence by SEER Historic Stage A at diagnosis (1974–2013) (C) Papillary thyroid cancer incidence by tumor size at diagnosis (1983–2013). Tumor size was not recorded for cases diagnosed 1974–1982. Rates are age-adjusted to the 2000 U.S. standard population. Each line represents the annual percent change (APC).
Thyroid cancer incidence-based mortality rates were underestimated in the earliest calendar years, but consistent with observed nationwide and SEER-9 thyroid cancer mortality rates during 1994–2013 (Figure 2a, eTable 2). Thyroid cancer incidence-based mortality increased, on average, 1.1% (95% CI 0.6–1.6%) annually during 1994–2013 (Table 3; Figure 2a), from 0.40 (95% CI 0.36–0.44) per 100,000 in 1994–97 to 0.46 (95% CI 0.43–0.50) per 100,000 in 2010–13. Positive APCs were observed for most demographic subgroups and statistically significant for females, whites, blacks, and patients diagnosed after age 79. By histologic type, the annual increase in incidence-based mortality rates was restricted to patients diagnosed with PTC (1.7%, 95% CI 0.6–2.9%). Positive APCs were observed for PTCs of all known stages at diagnosis, but were statistically significant only for patients with distant (2.9%, 95% CI 1.1–4.7%) and/or Stage IV (APC=12.9%, 95% CI 7.2–19.0%) disease (Table 3; Figure 2b). Positive APCs occurred for PTCs of all known sizes, with significant increases for tumors ≤2 and >2-≤4 cm (Table 3; Figure 2c).
Figure 2.
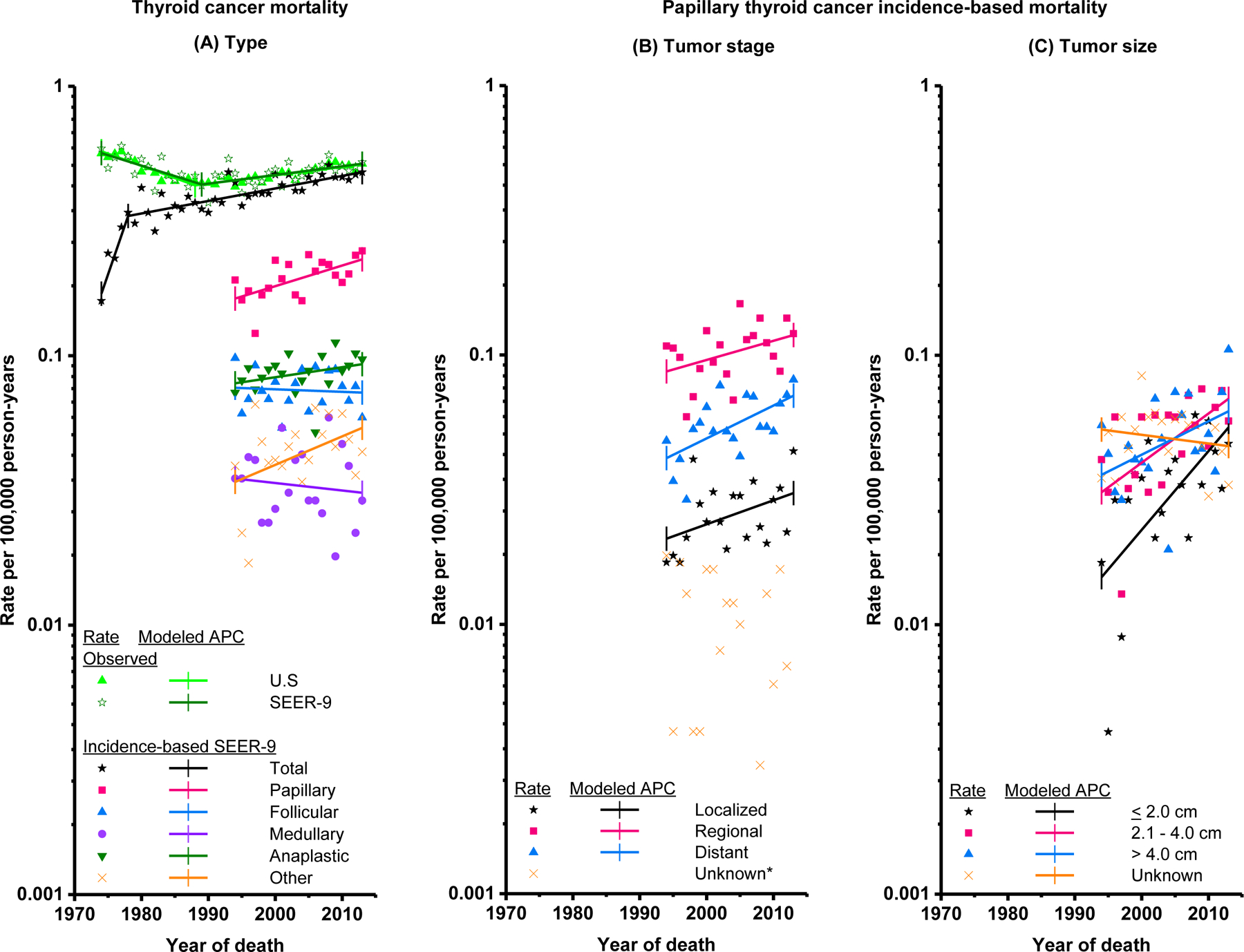
Trends in annual thyroid cancer mortality rates (deaths per 100,000 person-years). (A) Observed total U.S. thyroid cancer mortality (1974–2013), observed SEER-9 thyroid cancer mortality (1974–2013), thyroid cancer incidence–based mortality, overall (1974–2013) and by histologic type (1994–2013), based on cases diagnosed during 1974–2013 (B) Thyroid cancer incidence-based mortality by SEER Historic Stage A at diagnosis (1994–2013), based on papillary thyroid cancer cases diagnosed during 1974–2013 C) Thyroid cancer incidence-based mortality by tumor size at diagnosis (1994–2013), based on papillary thyroid cancer cases diagnosed during 1983–2013. Tumor size was not recorded for cases diagnosed 1974–1982. Rates are age-adjusted to the 2000 U.S. standard population. Each line represents the annual percent change (APC). *APCs were not calculated if 0 deaths occurred in one or more years.
Table 3.
Trends in observed thyroid cancer mortality rates a (total U.S. and SEER-9) and thyroid cancer incidence-based mortality rates a (1994–2013): the SEER-9 Registry Database
| Characteristic at diagnosis | Overall | |
|---|---|---|
| APC (95% CI) | P value | |
| Observed | ||
| Total U.S | 0.9 (0.7, 1.5) | <0.001 |
| SEER-9 | 1.0 (0.4, 1.5) | <0.001 |
| Incidence-based b | ||
| Total | 1.1 (0.6, 1.6) | <0.001 |
| Gender | ||
| Male | 1.0 (−0.1, 2.1) | 0.10 |
| Female | 1.2 (0.4, 2.0) | 0.01 |
| Race | ||
| White | 0.9 (0.3, 1.6) | 0.01 |
| Black | 3.8 (0.2, 7.6) | 0.04 |
| Others c | −0.2 (−2.4, 2.2) | 0.90 |
| Age (years) | ||
| <20 | - | - |
| 20–39 | - | - |
| 40–59 | 1.4 (0.0, 2.8) | 0.05 |
| 60–79 | 0.8 (−0.2, 1.8) | 0.10 |
| ≥80 | 1.3 (0.5, 2.1) | 0.002 |
| Histologic type | ||
| Papillary | 1.7 (0.6, 2.9) | 0.01 |
| Follicular | −0.2 (−1.6, 1.2) | 0.80 |
| Medullary | −0.7 (−3.2, 1.9) | 0.60 |
| Anaplastic | 0.9 (−0.4, 2.2) | 0.20 |
| Other d | 2.4 (−0.1, 5.1) | 0.06 |
| Papillary (incidence-based) | ||
| SEER Historic Stage A b | ||
| Localized | 2.1 (−0.1, 4.2) | 0.06 |
| Regional | 1.7 (−0.3, 3.6) | 0.09 |
| Distant | 2.9 (1.1, 4.7) | 0.003 |
| Unknown | - | - |
| AJCC/TNM stage e | ||
| Stage I | - | - |
| Stage II | - | - |
| Stage III f | 14.5 (−6.1, 39.7) | 0.20 |
| Stage IV f | 12.9 (7.2, 19.0) | <0.001 |
| Unknown f | 16.5 (−3.4, 40.4) | 0.09 |
| Tumor size (cm) g | ||
| ≤2 | 6.8 (2.4, 11.4) | 0.004 |
| >2-≤4 | 4.3 (1.3, 7.3) | 0.01 |
| >4 | 2.8 (−0.1, 5.9) | 0.06 |
| Unknown | −0.6 (−2.7, 1.5) | 0.50 |
Rate differences were calculated for PTCs by known/unknown values of SEER stage and tumor size (eTables 3 and 4). The incidence rate for unstaged PTC increased by 0.07 (95% CI 0.04–0.10) from 1974–77 to 2010–13, indicating that the observed increase in PTC with known SEER stage was underestimated by this amount. PTC with unknown tumor size declined by 0.57 (95% CI 0.49–0.65) from 1994–97 to 2010–13, which may have overestimated the observed increase in PTC with known tumor size by this amount; however, apart from PTCs >4 cm, the observed increases for PTCs with known tumor sizes were much larger. The unstaged PTC mortality rate declined by −0.01 (95% CI [−0.01–0.01]) from 1994–97 to 2010–13, indicating that the increase in mortality for PTCs with known SEER stage was overestimated by this amount. The mortality rate for PTCs with unknown tumor size decreased by 0.02 (95% CI 0.00–0.04) since 1998–2001, which may have overestimated mortality rates for PTC with known tumor size by this amount.
The annual numbers of cases or deaths and incidence and incidence-based mortality rates used in this analysis are provided in eTables 5-16.
Discussion
To our knowledge, the current study is the first to describe U.S. trends in thyroid cancer mortality by demographic and tumor characteristics at diagnosis and to systematically compare trends in thyroid cancer incidence and mortality rates by these characteristics. The main finding from this study was the significant increase in thyroid cancer incidence-based mortality from 1994 to 2013 (about 1.1% per year, on average) for thyroid cancer patients overall and those who were diagnosed with advanced-stage PTC (2.9% per year). This finding appears to be associated with the increasing incidence of advanced-stage PTC (3.5% per year since 1981).
The results of this study challenge the prevailing notion that all of the increase in PTC incidence in the United States is related to over-diagnosis2,4,19, resulting from the introduction and increasing widespread use of diagnostic ultrasound and other imaging modalities and fine-needle aspiration biopsies that have allowed for incidental detection and diagnosis of localized and/or small (<2 cm) cancers that are mostly indolent20. Such changes could account for the rapid increases in the incidence rates for localized and/or small PTCs, which have been previously observed2,4,6,19,21. Likewise, the deceleration in rates for localized, but not advanced, PTC incidence rates since 2009, as observed previously19,21, may be explained by less aggressive diagnostic work-up of small thyroid tumors in recent years due to a rising awareness of the problems associated with over-treatment of low-risk thyroid cancers15,19,22. However, the significant, albeit less rapid, increase in advanced-stage and larger PTC incidence rates and increasing thyroid cancer mortality rates among patients diagnosed with advanced-stage PTC is incompatible with the notion that over-diagnosis is solely responsible for the changing trends in PTC incidence. Thus, trends in PTC incidence may be explained by two underlying processes, the dominant one being over-diagnosis, and the other being a small but actual increase in PTC incidence, possibly resulting from changes in exposure to environmental risk factors7.
Additional epidemiologic research is needed to identify the specific environmental factors that have contributed to increasing rates of PTC, namely those with greater aggressive potential. Ionizing radiation exposure in childhood is the most established risk factor for PTC23, and exposure has increased in the U.S. general population in recent decades, due primarily to more widespread use of diagnostic medical exams24. However, studies of changes in radiation-related somatic mutations have shown declines in the proportion of PTCs with radiation signatures, such as RET/PTC rearrangements, over time, whereas point mutations, such as BRAF or RAS that are more likely to have a non-radiation etiology, have increased25–28. There is growing evidence suggesting that changes in obesity and smoking prevalence have contributed to rising thyroid cancer rates7. Paralleling trends in thyroid cancer incidence, obesity prevalence has increased threefold among U.S. adults between 1960 and 2012, with the fastest rate of increase between 1980 and 201029. In contrast, the prevalence of daily cigarette smoking has significantly decreased in the United States since 198030. Epidemiologic studies have consistently found positive associations between excess adiposity in childhood and adulthood and subsequent risk of thyroid cancer, including PTC31,32, while current smoking consistently has been associated with a 30–40% reduction in thyroid cancer risk, independent of obesity and other risk factors33. Obesity and smoking could influence thyroid cancer development via insulin resistance, thyroid hormone, and estrogen-related pathways34,35. Together, these factors have been estimated to be related to >40% of all new cases of thyroid cancer annually in the United States 7. Endocrine-disrupting chemicals (e.g., pesticides, bisphenol A) also have been suspected to contribute to thyroid cancer incidence trends through their effects on thyroid hormone metabolism36. However, evidence in support of a causal association between environmental chemicals and thyroid cancer risk is currently lacking, largely due to the challenges in studying exposures that are ubiquitous and for which long-term exposure is extremely difficult to measure accurately.
The increasing mortality rates among patients with advanced-stage PTC suggest that for patients with these high-risk tumors, there should be renewed focus on aggressive transdisciplinary management that includes surgery, adjuvant radioactive iodine, and, when indicated for the 5–10% of patients who develop progressive disease, systemic therapy. While there is continued debate about the appropriate extent of surgery for low-risk tumors, total thyroidectomy and adjuvant radioactive iodine are indicated for high-risk disease15. Thorough preoperative imaging should be used to evaluate the neck for nodal metastases to inform whether simultaneous lymphadenectomy is necessary, and prophylactic central neck dissection should be considered for large and advanced tumors. Sorafenib and lenvatinib are approved for the management of advanced, iodine-resistant differentiated thyroid cancer including PTC, but unfortunately neither has been shown yet to afford a survival advantage, likely due to the indolent natural history of the disease37,38. Clinical trials to optimize the development and use of novel systemic therapies are necessary.
This study has several important limitations. Due to the descriptive nature of this study, it is only possible to speculate about potential explanations for the observed thyroid cancer trends. Individual-level environmental exposures and lifestyle-related factors were not captured by registries, nor were methods of thyroid cancer detection. While changes in mortality and incidence-based mortality rates capture secular trends in detection, diagnosis, and case ascertainment, as well as treatment and associated survival, the current study did not evaluate the influence of treatment on these trends. Analyses relying on tumor size and AJCC/TNM stage data were restricted to the years in which the information was reported to the registries. APC estimates for incidence-based mortality by tumor size and AJCC/TNM stage may be artificially inflated as this information was only available for cases diagnosed during 1983–2013 and 2004–2013, respectively, leaving a shorter latency period between diagnosis and death; however, the results generally agree with estimates by SEER stage. The results of an analysis evaluating the effect of unknown stage and size data during the study period suggest that the increasing incidence rates of PTC with unknown SEER stage information underestimated, rather than overestimated, the true increase in PTC incidence for known SEER stages. Unknown SEER stage data could only explain, at most, about one-third of the observed increase in mortality due to advanced-stage PTC. As specimens have been cut more finely and smaller tumors that are incidental findings have become better documented, the reduction in unknown size data over time most likely overestimated the observed trends for the smallest, rather than largest, PTCs. Finally, some results were based on small numbers of cases or deaths. It will be important to continue monitoring thyroid cancer incidence and mortality rates over time to see if the observed trends persist.
Conclusion
Among U.S. patients diagnosed with thyroid cancer from 1974–2013, the overall incidence of thyroid cancer increased 3% annually, with increases in the incidence rate and thyroid cancer mortality rate for advanced-stage PTC. These findings are consistent with a true increase in the occurrence of thyroid cancer in the United States.
Supplementary Material
eTables
KEY POINTS SECTION.
Question:
What have been the trends in U.S. thyroid cancer incidence and mortality, and have they differed by tumor characteristics at diagnosis?
Findings:
In this analysis of 77,276 thyroid cancer patients diagnosed during 1974–2013 and 2,371 thyroid cancer deaths during 1994–2013, average annual increases in incidence and mortality rates, respectively, were 3.6% and 1.1% overall and 2.4% and 2.9% for patients diagnosed with advanced-stage papillary thyroid cancer.
Meaning:
Thyroid cancer incidence and mortality rates have increased for patients diagnosed with advanced-stage papillary thyroid cancer in the United States since 1974, suggesting a true increase in the occurrence of thyroid cancer.
Acknowledgments
Funding/Support: This research was supported in part by the Intramural Research Program of the National Cancer Institute. The funders of the study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript or the decision to submit for publication.
Footnotes
Conflict of Interest Disclosures: Hyeyeun Lim, Susan S. Devesa, David Check and Cari M. Kitahara declare no competing interests. Julie A. Sosa is on the Data Monitoring Committee of the Medullary Thyroid Cancer Consortium Registry, which is sponsored by Astra Zeneca, Eli Lilly, GlaxoSmithKline and Novo Nordisk.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2013, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016. Accessed June 1, 2016. [Google Scholar]
- 2.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 2006;295(18):2164–2167. [DOI] [PubMed] [Google Scholar]
- 3.Kent WD, Hall SF, Isotalo PA, Houlden RL, George RL, Groome PA. Increased incidence of differentiated thyroid carcinoma and detection of subclinical disease. CMAJ 2007;177(11):1357–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 2014;140(4):317–322. [DOI] [PubMed] [Google Scholar]
- 5.Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide Thyroid-Cancer Epidemic? The Increasing Impact of Overdiagnosis. N Engl J Med 2016;375(7):614–617. [DOI] [PubMed] [Google Scholar]
- 6.Enewold L, Zhu K, Ron E, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev 2009;18(3):784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol 2016;12(11):646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Surveillance Epidemiology and End Results (SEER) Program (www.seer.cancer.gov). SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2015 Sub (1973–2013) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2014 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2016, based on the November 2015 submission. [Google Scholar]
- 9.Surveillance Epidemiology and End Results (SEER) Program (www.seer.cancer.gov). SEER*Stat Database: Mortality - All COD, Aggregated With State, Total U.S. (1969–2013 <Katrina/Rita Population Adjustment>, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2016. Underlying mortality data provided by NCHS (www.cdc.gov/nchs). [Google Scholar]
- 10.Chu KC, Miller BA, Feuer EJ, Hankey BF. A method for partitioning cancer mortality trends by factors associated with diagnosis: An application to female breat cancer. J Clin Epidemiol 1994;47(12):1451–1461. [DOI] [PubMed] [Google Scholar]
- 11.Surveillance Epidemiology and End Results (SEER) Program (www.seer.cancer.gov). SEER*Stat Database: Incidence-Based Mortality - SEER 9 Regs Research Data, Nov 2015 Sub (1973–2013) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2014 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2016, based on the November 2015 submission. [Google Scholar]
- 12.Forman D, Bray F, Brewster DH, et al. , eds. Cancer incidence in five contintents, volume X [electronic version]. Lyon, France: International Agency for Research on Cancer;2013. http://ci5.iarc.fr. Accessed June 1, 2016. [Google Scholar]
- 13.Young JL RS Jr, Ries LAG, Fritz AG, Hurlbut AA SEER Summary Staging Manual - 2000: Codes and Coding Instructions, National Cancer Institute, NIH Pub. No. 01–4969, Bethesda, MD, 2001. [Google Scholar]
- 14.Adjusted AJCC stage 6th edition. Surveillance Epidemiology and End Results Program; http://seer.cancer.gov/seerstat/variables/seer/ajcc-stage/6th/. Accessed August 1, 2016. [Google Scholar]
- 15.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016;26(1):1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Software 2016; https://seer.cancer.gov/seerstat/. Accessed April 30, 2016.
- 17.Devesa SS, Donaldson J, Fears T. Graphical presentation of trends in rates. Am J Epidemiol 1995;141(4):300–304. [DOI] [PubMed] [Google Scholar]
- 18.Surveillance, Epidemiology, and End Results (SEER) Program. Joinpoint Trend Analysis Software 2016; https://surveillance.cancer.gov/joinpoint/, 2016. Accessed August 1, 2016.
- 19.Morris LG, Tuttle RM, Davies L. Changing Trends in the Incidence of Thyroid Cancer in the United States. JAMA Otolaryngol Head Neck Surg 2016;142(7):709–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sosa JA, Hanna JW, Robinson KA, Lanman RB. Increases in thyroid nodule fine-needle aspirations, operations, and diagnoses of thyroid cancer in the United States. Surgery 2013;154(6):1420–1427. [DOI] [PubMed] [Google Scholar]
- 21.Mao Y, Xing M. Recent incidences and differential trends of thyroid cancer in the United States. Endocr Relat Cancer 2016;23(4):313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim BW, Yousman W, Wong WX, Cheng C, McAninch EA. Less is More: Comparing the 2015 and 2009 American Thyroid Association Guidelines for Thyroid Nodules and Cancer. Thyroid 2016;26(6):759–764. [DOI] [PubMed] [Google Scholar]
- 23.Dal Maso L, Bosetti C, La Vecchia C, Franceschi S. Risk factors for thyroid cancer: an epidemiological review focused on nutritional factors. Cancer Causes Control 2009;20(1):75–86. [DOI] [PubMed] [Google Scholar]
- 24.Fazel R, Krumholz HM, Wang Y, et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med 2009;361(9):849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romei C, Fugazzola L, Puxeddu E, et al. Modifications in the papillary thyroid cancer gene profile over the last 15 years. J Clin Endocrinol Metab 2012;97(9):E1758–E1765. [DOI] [PubMed] [Google Scholar]
- 26.Mathur A, Moses W, Rahbari R, et al. Higher rate of BRAF mutation in papillary thyroid cancer over time. Cancer 2011;117(19):4390–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung CK, Little MP, Lubin JH, et al. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab 2013;99(2):E276–E285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kowalska A, Walczyk A, Kowalik A, et al. Increase in Papillary Thyroid Cancer Incidence Is Accompanied by Changes in the Frequency of the BRAFV600E Mutation: A Single-Institution Study. Thyroid 2016;26(4):543–551. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Prevalence of Overweight, Obesity, and Extreme Obesity Among Adults: United States, 1960–1962 Through 2011–2012http://www.cdc.gov/nchs/data/hestat/obesity_adult_11_12/obesity_adult_11_12.htm. Accessed August 1, 2016.
- 30.Ng M, Freeman MK, Fleming TD, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA 2014;311(2):183–192. [DOI] [PubMed] [Google Scholar]
- 31.Kitahara CM, McCullough ML, Franceschi S, et al. Anthropometric Factors and Thyroid Cancer Risk by Histological Subtype: Pooled Analysis of 22 Prospective Studies. Thyroid 2016;26(2):306–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitahara CM, Gamborg M, de González AB, Sørensen TI, Baker JL. Childhood height and body mass index were associated with risk of adult thyroid cancer in a large cohort study. Cancer Res 2014;74(1):235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitahara CM, Linet MS, Freeman LEB, et al. Cigarette smoking, alcohol intake, and thyroid cancer risk: a pooled analysis of five prospective studies in the United States. Cancer Causes Control 2012;23(10):1615–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almquist M, Johansen D, Björge T, et al. Metabolic factors and risk of thyroid cancer in the Metabolic syndrome and Cancer project (Me-Can). Cancer Causes Control 2011;22(5):743–751. [DOI] [PubMed] [Google Scholar]
- 35.Wiersinga WM. Smoking and thyroid. Clin Endocrinol (Oxf) 2013;79(2):145–151. [DOI] [PubMed] [Google Scholar]
- 36.Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 2015;372(7):621–630. [DOI] [PubMed] [Google Scholar]
- 38.Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 2014;384(9940):319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTables