Human Osteogenesis Involves Differentiation-Dependent Increases in the Morphogenically Active 3′ Alternative Splicing Variant of Acetylcholinesterase (original) (raw)
Abstract
The extended human acetylcholinesterase (AChE) promoter contains many binding sites for osteogenic factors, including 1,25-(OH)2 vitamin D3 and 17β-estradiol. In differentiating osteosarcoma Saos-2 cells, both of these factors enhanced transcription of the AChE mRNA variant 3′ terminated with exon 6 (E6-AChE mRNA), which encodes the catalytically and morphogenically active E6-AChE isoform. In contrast, antisense oligodeoxynucleotide suppression of E6-AChE mRNA expression increased Saos-2 proliferation in a dose- and sequence-dependent manner. The antisense mechanism of action was most likely mediated by mRNA destruction or translational arrest, as cytochemical staining revealed reduction in AChE gene expression. In vivo, we found that E6-AChE mRNA levels rose following midgestation in normally differentiating, postproliferative fetal chondrocytes but not in the osteogenically impaired chondrocytes of dwarf fetuses with thanatophoric dysplasia. Taken together, these findings suggest morphogenic involvement of E6-AChE in the proliferation-differentiation balance characteristic of human osteogenesis.
Osteogenesis in terrestrial vertebrates involves the progeny of common mesenchymal progenitors (3) that become committed to bone or cartilage lineages in parallel pathways controlled by osteogenic hormones and growth factors (9). These include 1,25-(OH)2 vitamin D3 [1,25-(OH)2D3], which has been found to be essential for endochondral ossification (6, 41). Another osteogenic agent, 17β-estradiol, interacts with high-affinity binding sites in normal human osteoblast-like and osteosarcoma cells (16, 24). This suggests osteoblast mediation for the action of estrogen on bone. The resultant skeletal tissue supports hematopoiesis through the function of bone marrow stromal cells, which share a common progenitor with the osteogenic lineages. These complex interrelationships predict the existence of common coordinator proteins with control functions in the proliferation-differentiation balance characteristic of both osteogenic and hematopoietic processes.
An intriguing candidate protein for such a role(s), with recently established morphogenic capacities, is the acetylcholine-hydrolyzing enzyme acetylcholinesterase (acetylcholine acetyl hydrolase [AChE]; EC 3.1.1.7). AChE is expressed in both mesenchyme (26) and hematopoietic cells (28, 29). Antisense AChE suppression attenuates erythropoiesis and induces stem cell expansion in primary murine bone marrow cultures (39), and the chondrogenic expression of AChE parallels the early development of rat lower limbs (43) and embryonic chick limbs (1). Thus, AChE fulfills at least some of the requirements for an osteogenic/hematopoietic coordinator. This provides an incentive for exploring genomic and transcriptional evidence to test whether it does indeed function as such a coordinator.
In transgenic Xenopus embryos (35), transfected rat gliomata (22), and cultured Xenopus motoneurons (40), the morphogenic capacities of AChE were limited to E6-AChE, translated from the alternative splicing E6-AChE mRNA variant, 3′ terminated with exon 6. Therefore, we examined E6-AChE mRNA expression in human osteosarcoma cells, which are amenable to controlled differentiation by 1,25-(OH)2D3 and 17β-estradiol, and tested the outcome of antisense AChE suppression on cell proliferation. In parallel, we searched for E6-AChE mRNA in vivo in developing chondrocytes from normal fetuses and from dwarf bones of proliferation-impaired fetuses with thanatophoric dysplasia (8). This inherited skeletal disorder is one of the most severe forms of chondrodysplasia, causing dwarfism and abnormal body proportions that lead to late prenatal or early postnatal death. While data on its frequency are not yet available, chondrodysplastic disorders are believed to occur with a frequency as high as 1:10,000.
Thanatophoric dysplasia, like other severe forms of chondrodysplasia, is frequently associated with point mutations in fibroblast growth factor receptor 3 (13). Its diagnosis is based on ultrasonography, X-ray findings, and pathologic changes in bone structure. The lack of definitive therapy calls for studying the molecular mechanisms which underlie the impaired chondrocyte proliferation in affected embryos. Here, we report findings which suggest involvement of AChE in osteo- and chondrogenesis, as well as impairments of AChE gene expression in thanatophoric dysplasia.
MATERIALS AND METHODS
Osteoblast cell culture.
Human osteosarcoma Saos-2 cells were maintained in a fully humidified atmosphere at 37°C and 5% CO2 in Ham’s F-10 growth medium containing 10% (vol/vol) fetal calf serum, 2 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 0.12% (wt/vol) bicarbonate and were passaged once a week. 17β-Estradiol and 1,25-(OH)2D3 (Sigma Chemical Co., St. Louis, Mo.) dissolved in ethanol were diluted into growth medium (1:5,000 [vol/vol]) before use. For antisense manipulations, 20,000 cells/well, plated in 96-well flat-bottomed plates, were washed twice with phosphate-buffered saline and maintained in RPMI 1640 medium without phenol red (which exerts an estrogenic effect on osteoblasts [17]) and with a serum substitute, Biogro-1 (Biological Industries Co., Beit-Haemek, Israel). Oligonucleotides were added without carrier at the noted doses for 12 h of incubation at 37°C and 5% CO2, followed by two washes in phosphate-buffered saline.
RT-PCR analyses.
Kinetic follow-up of reverse transcription-PCR (RT-PCR) analyses was performed by using selective primers for AChE mRNA, essentially as described previously (28), with actin and glyceraldehyde 3-phosphate dehydrogenase mRNA measurement (14) as a control. Plasmid DNAs containing exons E2 to E6 and the pseudointron 14 region (5) served as probes for blot hybridization. Densitometry calibration curves showing linearity and parallelism of intensity of product with cycle number over the range encompassing the percent differences between treated and untreated cells were as previously described (21, 23).
Antisense oligonucleotide experiments.
2-O-methylated or phosphorothioated 15- and 20-mer oligonucleotides, antisense (designated by the prefix “AS”) and inversely oriented sequences, were targeted against the common sequence domain in human AChE mRNA and were used as detailed elsewhere (15, 18, 29, 39), except for the addition of a terminal 5′-hexa-ethylene glycol (HEG) group where indicated. Oligonucleotides targeted against butyrylcholinesterase (BChE) mRNA served as a control (18). Cell proliferation was evaluated by 5-bromo-2′-deoxyuridine (BrdU) incorporation (19) or binding of methylene blue (33), both as previously described.
In situ hybridization.
The following 5′-biotinylated, 2-O-methylated AChE cRNA probes complementary to 3′ alternative human AChE exons (5) were used: E6 (morphogenic form), (5402) 5′-CCGGGGGACGUCGGGGUGGGGUGGGGAUGGGCAGAGUCUGGGGCUCGUCU-3′ (5352); E5 (hematopoietic form), (4457) 5′-AGGAAGAGGAGGAGAAGCUGGUGGAGGAGGAGGAGGGGCAGGGGGAGGCC-3′ (4506); and I4 (readthrough form), (4397) 5′-CUAGGGGGAGAAGAGAGGGGUUACACUGGCGGGCUCCCACUC CCCUCCUC-3′ (4349). (Numbers denote nucleotide positions in the GenBank entry [accession no. M55040].)
The project was approved by the Sourasky Medical Center Ethics Committee, and written informed consent was obtained from the parents. In each of the selected gestational stages, bone tissues were derived from two aborted human fetuses with normal histological morphology (hematoxylin and eosin staining). Samples of distal femur bone were also obtained from two 18-week fetuses aborted due to thanatophoric bone dysplasia. Diagnosis was confirmed histologically, with demonstration of drastically reduced chondrocyte proliferation and hypertrophy zones of the growth plate along with normal appearance of the resting cartilage (38). Following postmortem examination, tissues were fixed and cut sections were placed on slides pretreated with 3-aminopropyltriethoxysilane, dried at 37°C overnight, and kept at 4°C until use. In situ hybridization procedures were as detailed elsewhere (23). Photography was carried out at a magnification of ×1,000, and scanned images were evaluated for red staining efficiencies in cytoplasmic regions, using Adobe Photoshop 4.0 (Adobe Systems, Inc., San Jose, Calif.) at 255 output levels. Percentage of cytoplasmic red color pixels out of the entire image’s red color was normalized by subtraction of control (no-probe) values. Background values were lower than 10%. Findings were expressed as mean ± standard error, and analysis of variance (ANOVA) was performed with the superANOVA statistical package (Abacus Concepts, Inc., Berkeley, Calif.).
Immunohistochemistry, cytochemistry, and AChE catalytic activity measurements.
For immunohistochemistry, cryocut sections were incubated with human-specific anti-AChE monoclonal antibody 101-1 (primary antibody) (36) and biotinylated anti-mouse antibody (secondary antibody) (Vectastain; Vector Laboratories, Burlingame, Calif.); detection was carried out with 3,3′-diaminobenzidine and urea-H2O2 as instructed by the manufacturer (Sigma). Cytochemical staining for AChE activity on nonfixed cells grown on glass slides and on cryocut sections, as well as determination of hydrolysis rates of acetylthiocholine iodide in homogenates, was performed essentially as detailed elsewhere (28) for the noted time periods. Nuclear staining was done with 4′,6-diamidino-2-phenylindole (DAPI).
Confocal laser scanning microscopy.
An MRC-1024 Bio-Rad confocal microscope equipped with an inverted microscope and a 63×/1.4 oil immersion objective was used to scan the fast red precipitate used for detection during in situ hybridization of Saos-2 cells. The fast red was excited at 488 nm, and emission was measured with a 580df32 filter. A section was scanned every 0.54 μm, and a three-dimensional projection was created from all sections.
RESULTS
AChE gene expression in cultured Saos-2 cells.
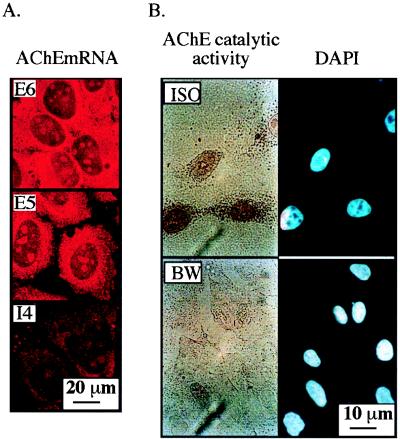
AChE mRNA transcripts were first detected in cultured Saos-2 cells by high-resolution in situ hybridization using alternative splicing-specific probes for the various AChE mRNA transcripts. An exon 6-derived probe revealed efficient production of the morphogenic E6-AChE mRNA, terminated with exon E6 (40). Smaller amounts of E5-AChE mRNA 3′ terminated with exon E5, which encodes the erythrocyte AChE, and negligible levels of the readthrough I4-AChE mRNA form including pseudointron I4 (22) were also detected (Fig. 1A). Similar to the situation in cells of hematopoietic origin (21, 28), the major detectable transcript was that encoding E6-AChE. Especially prolonged (72-h) cytochemical staining of nonfixed Saos-2 cells revealed accumulation of acetylthiocholine hydrolysis products that were suppressed by the AChE-specific inhibitor BW 284C51 but resistant to the BChE-specific inhibitor tetraisopropylpyrophosphoramide (iso-OMPA) (Fig. 1B). We conclude that the AChE gene is expressed in Saos-2 cells to yield its catalytically and morphogenically active E6-AChE form, with considerably smaller amounts of its other variants.
FIG. 1.
AChE gene expression in Saos-2 osteosarcoma cells. (A) AChE mRNA alternative splicing variants. Presented are three-dimensional projections created from confocal scanned sections of Saos-2 cells subjected to in situ hybridization with 5′-biotinylated AChE cRNA probes selective for the morphogenic E6-AChE mRNA variant (E6), the hematopoietic E5-AChE mRNA variant encoding glycophospholipid-anchored AChE (E5), and the readthrough form including pseudointron I4 (I4). Detection was carried out by forming alkaline phosphatase-streptavidin conjugates producing fast red precipitates, which were excited at 488 nm. Note that E6 is the predominant AChE mRNA transcript in Saos-2 cells. (B) Cytochemical staining. Saos-2 cells were subjected to cytochemical staining of AChE catalytic activity in the presence of 10−5 M iso-OMPA (ISO) or BW 284C51 (BW), selective inhibitors of BChE and AChE, respectively. Nuclear staining was done with DAPI. Note the selective depiction of brown precipitates of AChE but not BChE reaction products.
AChE transcription in Saos-2 cells is up-regulated by 17β-estradiol and 1,25-(OH)2D3.
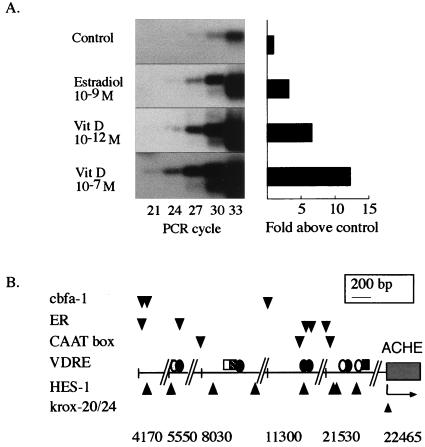
Kinetic follow-up of RT-PCR amplification (21, 23) using Saos-2 RNA yielded a logarithmic increase in a 482-bp fragment derived from E6-AChE mRNA, as expected, up to cycle 33. Calculations based on actin mRNA levels from the same preparations revealed 106 E6-AChE mRNA molecules per 200 ng of total Saos-2 RNA. For average yields of 20 ± 5 μg of total RNA from 3 × 106 Saos-2 cells, this indicates fewer than 50 AChE mRNA molecules per cell, at the same order of magnitude as hematopoietic DAMI cells (75 molecules/cell [28]) but at much lower concentrations than brain cells (104 molecules/cell [21]). Physiologically relevant concentrations of the osteogenic agent 17β-estradiol (10−9 M [32]) increased E6-AChE mRNA levels in Saos-2 cells 3.3-fold within 24 h (Fig. 2A). A more prominent effect was caused by 1,25-(OH)2D3, which increased E6-AChE mRNA levels in a dose-dependent manner. Increases of 6.7- and 12.3-fold for 10−12 and 10−7 M 1,25-(OH)2D3 (Fig. 2A), respectively, demonstrated an association between AChE transcription and 1,25-(OH)2D3-induced osteogenesis.
FIG. 2.
Osteogenic control of AChE expression. (A) 17β-Estradiol and 1,25-(OH)2D3 enhance expression of E6-AChE mRNA. Presented are RT-PCR products of the morphogenic E6-AChE mRNA variant (482 bp). Detection was done by DNA blot hybridization using a plasmid DNA probe containing exons E2 to E6 (22). 17β-Estradiol (10−9 M) and 1,25-(OH)2D3 (Vit D; 10−12 and 10−7 M) were added to Saos-2 cells for 24 h. Note the densitometric analysis of kinetic follow-up showing, at the phase of PCR product accumulation, logarithmic 24-cycle increases in E6-AChE mRNA levels with these osteogenic agents. (B) Upstream human ACHE sequence includes clusters of bone enhancer motifs. Depicted is the reverse sequence of the cosmid insert (accession no. AF002993) of the human AChE gene promoter. The arrow represents the position of a transcription start site. Five putative bone enhancer regions are shown with their first nucleotide positions designated; fully conserved consensus sequences known to bind osteogenic transcription factors are represented by circles and rectangles (for VDREs) or by wedges (for other transcription factors). Non-VDRE consensus sequences: Cbfa1 (cbfa-1), AACCAC; HES-1, CACNAG; CAAT box, GCCAAT; estrogen receptor (ER) half site, GGTCA; Krox-20/24, GCGGGGGCGGG. VDRE consensus sequences: AGGACA (light grey circles); AGGTCA (dark gray circles); ATGCCA (open rectangles); GGTTCA (hatched rectangles); GGGTGA (filled circles); AGGTGA (dark gray rectangles).
Upstream AChE sequences include a plethora of binding sites for osteogenic factors.
The MatInspector (34) and FindPatterns programs of the University of Wisconsin software package were used to search for consensus motifs known to bind transcription factors important for osteoblast differentiation and bone remodeling. The recently sequenced 30-kb region upstream from the human AChE gene (accession no. AF002993) was found to include five clusters of such sequences, bearing 100% identity to the core consensus motifs. Of these, the most distal are located ca. 18 and 17 kb upstream from the transcription start site (Fig. 2B), a domain demonstrated to be active in regulating AChE expression (37). The osteogenic binding motifs include vitamin D receptor binding elements (VDREs) arranged in pairs or, in one case, in a triplet as found in the vitamin D-regulated promoter of the c-fos gene (10). Several of these VDREs are positioned near CAAT boxes which may act synergistically with them, as was shown for factors of the CTF/NF-1 family (30). Other elements include half-palindromic sequences capable of activating transcription by binding the estrogen receptor (42), in line with the recently demonstrated glucocorticoid effect on AChE gene expression (7). Recognition motifs were also found for the vitamin D-regulated repressor HES-1 (31) and for the brain development and bone-remodeling factor Krox-20/24 (27). Six elements for the fetus-specific core binding factor A1 (Cbfa1), essential for osteoblast development and bone formation in the mouse (25) (three of which are depicted in Fig. 2A), are characteristic of bone-specific genes (e.g., osteocalcin and osteopontin genes). In contrast, no Cbfa1 motif was found in the human liver-specific promoters for aldolase B (accession no. D00175), liver-type phosphofructokinase (accession no. X80853), and serum albumin (accession no. M12523 and J04457). Together with our RT-PCR data, this finding suggests that the binding elements in the AChE upstream sequences do indeed reflect functionally significant osteogenic regulation of AChE gene expression.
Antisense suppression of AChE gene expression enhances proliferation of Saos-2 cells.
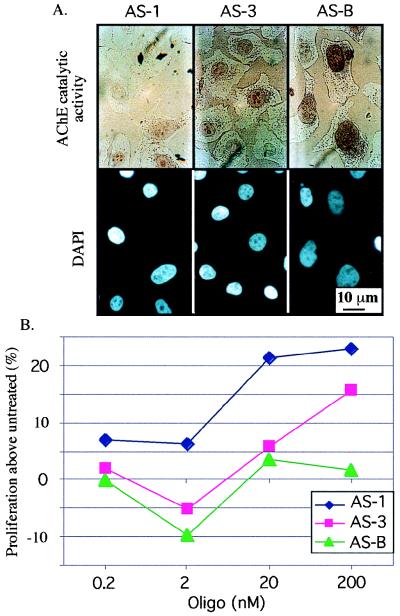
Since osteogenic differentiation is often associated with arrest of proliferation, we tested the effect of loss of AChE function on Saos-2 proliferation. To this end, two different 2-O-methylated AChE-targeted antisense oligonucleotides (AS1 and AS3 [18]) were added to Saos-2 cultures, and cell numbers were measured after a 12-h incubation in the presence of BrdU (see Materials and Methods). Both AS1 and AS3, at doses of 0.2 to 200 nM, suppressed the cytochemical staining capacity of treated cells under conditions where an irrelevant AS-BChE oligomer (18) had no effect on AChE activity (Fig. 3A). Dose-dependent increases in cell proliferation up to 25% above the level for untreated cells, reflecting enhanced BrdU incorporation, were observed for both AS1 and AS3 but not for AS-BChE. A phosphorothioated AS-AChE oligonucleotide, targeted against the initiator AUG domain in the AChE gene (39), was also used. This oligomer, with and without a HEG protecting group, was also found to be effective in enhancing proliferation (25 and 40% increases in methylene blue binding above control levels for untreated cells, respectively). In contrast, inverse and sense oligonucleotides were both ineffective in these tests (data not shown). The differences in proliferation that were induced by the three AS-AChE oligomers are considered significant in osteoblast cultures (17), indicating sequence dependence of the observed effects and suggesting functional significance for osteoblastic AChE in inhibiting proliferation.
FIG. 3.
Enhanced Saos-2 cell proliferation under antisense AChE suppression. (A) Sequence-dependent suppression of AChE activity. Saos-2 cells incubated for 12 h in the presence of the noted antisense oligonucleotides were stained for catalytically active AChE in the presence of 10−5 M iso-OMPA. Note reduction of enzyme activity staining in cells treated with AS1 and AS3 but not with the irrelevant AS-BChE oligomer (AS-B). (B) BrdU analysis. Cell proliferation was measured by BrdU incorporation in the presence of oligomers (Oligo) AS1, AS3, and AS-B in the noted concentrations. Presented are average results of three reproducible experiments with standard deviations less than 6%. Untreated cells served as controls. Note the dose-dependent increased proliferation associated with AS1 and AS3 treatment and the apparently higher efficiency of AS1 in enhancing proliferation, which matches its intense capacity to suppress AChE activity.
Developmental regulation of AChE mRNA expression in human fetal chondrocytes.
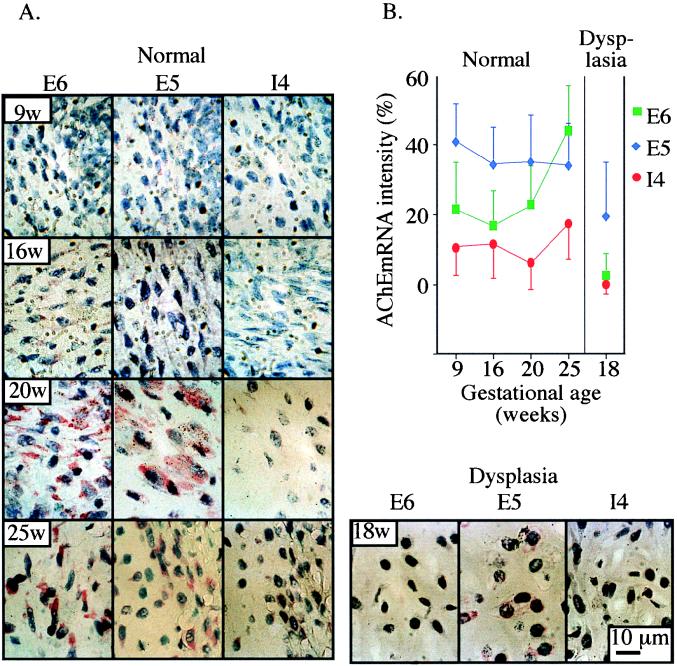
Cytochemical staining of AChE activity disclosed no catalytic activity of AChE in bones from aborted human fetuses at 18 weeks’ gestation, even under prolonged incubation conditions in which developing osteoblastic cells were positive. Also, immunohistochemistry staining for AChE revealed an exceedingly weak signal (data not shown). However, high-resolution in situ hybridization analyses demonstrated AChE mRNA transcripts in the cytoplasm of developing perichondria and mature chondrocytes within developing fetal axial bones. The three alternative forms of AChE mRNA, 3′ terminated with E5, E6, and I4, were all detected by fast red staining in bone cells throughout gestational weeks 9 to 25 (Fig. 4A), whereas control sections incubated with no probe revealed no staining. Image analysis of E5-AChE mRNA expression patterns showed relatively constant levels during the entire gestational period examined (Fig. 4B). In contrast, the level of E6-AChE mRNA labeling, which was lower than that of E5-AChE mRNA during the early gestational period, increased considerably (ANOVA, P ≤ 0.009) during advanced pregnancy, becoming significantly higher than that of E5-AChE mRNA by 25 weeks. In line with the analysis in Saos-2 cells, I4-AChE mRNA labeling was minimal in the bone tissues examined. This demonstrated developmentally regulated splicing choices for AChE mRNA in chondrocytes in vivo, with the morphogenic E6-AChE mRNA isoform appearing relatively late in fetal development.
FIG. 4.
AChE mRNA expression in human fetal bone. (A) Developmental regulation. Bone tissues from different gestational stages of normal human fetuses (left; 9, 16, 20, and 25 gestational weeks [9w, 16w, 20w, and 25w]) and from 18-gestational-week dwarf fetuses with thanatophoric dysplasia (bottom right) were subjected to high-resolution in situ hybridization using 5′-biotinylated AChE cRNA probes selective for the alternative human AChE mRNAs. Representative photographs of fast red staining at a magnification of ×200 are presented for the E5, E6, and I4 (synaptic, hematopoietic, and readthrough, respectively) forms of AChE mRNA. Note the considerably lower expression levels than in normal bone of E5-AChE mRNA transcripts in sections from fetuses with thanatophoric bone dysplasia. (B) Delayed increase of E6-AChE mRNA. Presented are image analysis data (mean ± standard error) for the zone of ossification and the area of the proliferative cells in five areas from each section. AChE mRNA labeling intensity is presented as the percentage of cytoplasmic red color pixels out of the entire image’s red color and normalized by subtraction of control values (without probe) from the in situ hybridization values (with specific probes). Note that in spite of the interregion variability, E5-AChE mRNA is stably expressed in fetal bone. In contrast, the level of E6-AChE mRNA is low at early gestation and increases later, and I4-AChE mRNA displays consistently lower levels than the other two forms. In thanatophoric bone dysplasia, note the general suppression of all AChE mRNA levels.
Attenuated E6-AChE mRNA production in thanatophoric bone dysplasia.
To study the association between normal osteogenic differentiation and AChE expression, we tested AChE mRNA labeling in femur bones from two unrelated fetuses with thanatophoric bone dysplasia. E5-AChE mRNA transcripts displayed expression levels much lower than those in normal bone, and E6-AChE mRNA was drastically suppressed (ANOVA, P ≤ 0.05), demonstrating consistently deficient E4-E6 splicing in addition to attenuated AChE transcription (Fig. 4). It is noteworthy that the hematopoietic organs expressed AChE mRNA with similar efficiencies in normal fetuses and fetuses with thanatophoric bone dysplasia (data not shown), reflecting bone-specific attenuation of AChE expression associated with bone dysplasia.
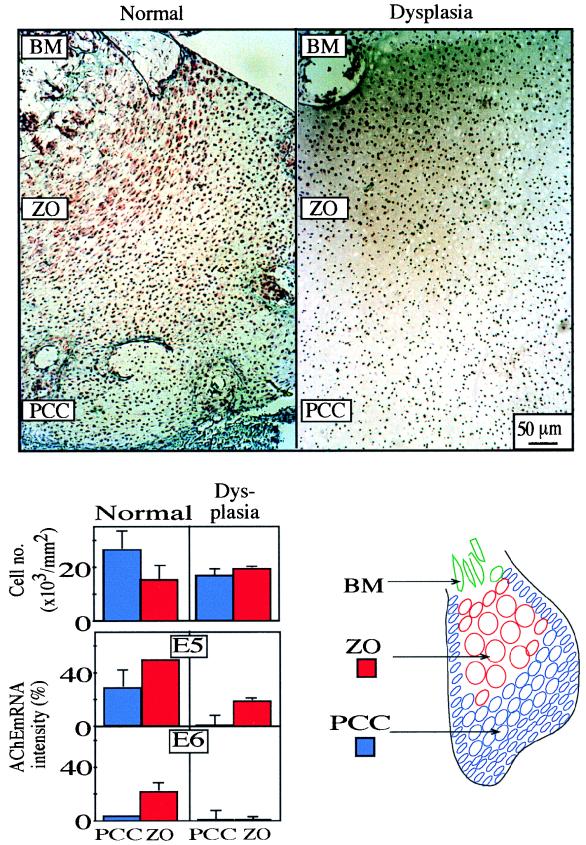
AChE mRNA labeling changes were also observed in the same bones between chondrocytes at various differentiation stages (Fig. 5). Early chondrocytes in peripheral proliferative bone areas expressed less AChE mRNA than cells in the zone of ossification located deeper in the bone. Digital imaging of four parallel regions at different distances from the periphery of bone sections revealed highly significant differentiation-associated increases in AChE mRNA levels (ANOVA, P ≤ 0.01), for both E5- and E6-AChE mRNAs. This difference was directly associated with the differentiation-related decrease in chondrocyte density (ANOVA, P ≤ 0.04). In bones from fetuses with thanatophoric bone dysplasia, considerably lower levels were observed for E5-AChE mRNA expression (ANOVA, P ≤ 0.01), although with parallel patterns of differentiation-related increases (Fig. 4). In contrast, E6-AChE mRNA remained absent in both zones of the dysplastic bone (Fig. 4 and 5). This absence of E6-AChE mRNA coincided with deficient chondrocyte condensation in the dysplastic bone (ANOVA, P ≤ 0.04). Taken together, these findings suggest a suppressive role for the E6-AChE protein in chondrocyte proliferation.
FIG. 5.
AChE mRNA levels increase inversely to chondrocyte density. (Top) In situ hybridization sections including early proliferative chondrocytic cells (PCC) and mature chondrocytes at the zone of ossification (ZO). E5-AChE mRNA labeling was performed at 20 and 18 weeks of gestation for normal and dysplastic bones. BM, bone marrow. (Bottom left) Cell density, expressed as 1,000 cells/mm2 units in the bone specimens (upper bar graph), decreases with ossification in normal bone but remains low and unchanged in thanatophoric dysplasia (mean ± standard deviation) for four proliferative (blue columns) and ossification (red columns) zones in each case. (Middle and lower bar graphs) E5- and E6-AChE mRNA labeling intensity in proliferative and ossification zones from normal bone and thanatophoric bone dysplasia. Note parallel increases in AChE mRNA expression levels and bone differentiation in normal bone. In dysplastic bone, differentiation-related increases in AChE mRNA expression were largely prevented. E5-AChE mRNA increases were limited, and E6-AChE mRNA was virtually absent when there was a lack of chondrocyte condensation. (Bottom right) Schematic of morphologic changes of chondrocyte locations within the ossifying bone.
DISCUSSION
By combining in vitro and in vivo approaches, we have demonstrated that the human AChE gene is expressed in osteoblasts and chondrocytes in a manner dependent both on their state of proliferation and differentiation and on the presence of binding sites for osteogenic factors such as 17β-estradiol and 1,25-(OH)2D3, which were found in the extended AChE promoter. In cultured tumor osteoblasts, E6-AChE mRNA expression increases with differentiation, whereas its suppression increases the proliferation rate. In the already committed, differentiated fetal chondrocytes, stable in vivo expression of E5-AChE mRNA persists through most of the gestation period. However, E6-AChE mRNA levels increase with differentiation, in parallel with decreases in cell density and after gestational week 20. In bones of fetuses with thanatophoric dysplasia, in which chondrocyte condensation is impaired, E5-AChE mRNA expression is drastically down-regulated. Its levels decrease further together with cell density and in a manner parallel to that observed in normal fetal bones. In contrast, E6-AChE mRNA levels did not increase in the dysplastic bones, suggesting that a threshold stage of development is required for such increases. The weak immunodetection and failure to observe catalytically active AChE in in vivo chondrocytes may be due to the low-level expression of the AChE gene in these cells, virtually at the limit of detection, as also seen in Saos-2 cells. That this protein nevertheless exerts a morphogenic effect further supports its in vivo function as a signaling factor, a role which could be fulfilled by a very small number of molecules.
Several arguments suggest that the osteogenic expression of E6-AChE mRNA is functionally significant: (i) the abundance of binding motifs for osteogenic factors in the extended AChE promoter; (ii) the increased expression under the influence of 17β-estradiol and 1,25-(OH)2D3; (iii) the direct correlation between E6-AChE mRNA expression and chondrocyte differentiation, itself inversely correlated with chondrogenic proliferation; and (iv) the proliferation-enhancing effect of antisense AChE suppression, which corroborates the inverse correlation with cell density. The deficient AChE mRNA expression in dysplastic bones demonstrates early induction during osteogenesis as well as dependence on those osteogenic agents missing in bone dysplasia and thus impairing chondrocyte condensation. The paucity of E6-AChE mRNA in dysplastic bones may, therefore, reflect deficiencies in transcription factors, splicing proteins such as SF2 and ASF, which were found to decrease in association with diminished splicing of E6-AChE mRNA in human megakaryocytes (28), or cell surface signals essential for E6-AChE production. Recently accumulated data on the cell-cell interaction capacities of AChE and its catalytically inactive homologs (4, 11, 12, 20) tentatively provide a mechanism for the morphogenic activities of cholinesterasic domains. Finally, the apparently normal hematopoietic expression of AChE in fetuses with thanatophoric dysplasia points to a clear distinction between the osteogenic and hematopoietic control processes governing AChE production.
Splicing choices of AChE mRNA transcripts were notably different in cultured osteoblasts, where E6-AChE mRNA predominated, from those in in vivo chondrocytes, where E6-AChE mRNA levels increased only late in gestation, following a long period when E5-AChE mRNA transcripts predominated. This difference may be related to the different growth conditions, cell type properties, or differentiation states involved. In addition, the predominance of E6-AChE may be attributed to its distinct morphogenic properties, so that E6 splicing deficiencies may reflect insufficient development of the dysplastic chondrocytes which are unprepared for the next phase in the cell-cell interaction activities particular to this AChE variant.
Antisense suppression of AChE production enhanced osteoblast proliferation in a dose- and sequence-dependent manner. The possibility of triple helix involvement had been excluded for all of the oligonucleotides used (15, 18). The suppressed cytochemical labeling points to mRNA destruction and/or interference with translation as the most likely mechanism(s) for functioning of these antisense oligonucleotides, which further suggests that interference with E6-AChE production impairs the proliferation-differentiation balance in osteoblasts. This argument is strengthened by the inverse in vivo relationship between AChE mRNA levels and chondrocyte density. The function implied by these studies for the AChE protein is, therefore, an association with the cell-cell signaling that arrests proliferation in committed, differentiating bone cells.
Having a developmental osteogenic function may imply involvement of AChE in the replacement of osteoblasts for maintenance of both fetal and adult bones. In this respect, it would be of interest to investigate the effect of antisense AChE agents on osteogenesis in fetuses of chondrodysplastic animal models. The association of bone defects (e.g., osteoporosis) with Alzheimer’s disease, in which peripheral AChE levels are reduced (2), further requires an examination of AChE mRNA levels following 17β-estradiol and 1,25-(OH)2D3 treatment in vivo. Finally, 17β-estradiol and 1,25-(OH)2D3 treatments should be examined for secondary effects on hematopoiesis through the predicted increase in bone marrow-supportive stromal cells. The expression of E6-AChE in bone therefore has both basic and applied significance, due to the morphogenic capacities of this intriguing protein.
ACKNOWLEDGMENTS
We are grateful to A. Cohen (Milford, Mass.) for a gift of 2-_O_-methyl antisense oligomers, to Letizia Shraiber for assistance with pathological analysis, to Avi Orr-Urteger and Joseph Weissman for genetic and scientific counseling, and to N. Melamed-Book for confocal imaging.
This research was supported by grants to H.S. from the German-Israeli Fund (grant I-0512-206.01/96) and Ester/Neuroscience, Ltd. D.G. was the recipient of a Sourasky Medical Center postdoctoral fellowship.
REFERENCES
- 1.Alber R, Sporns O, Weikert T, Willbold E, Layer P G. Cholinesterases and peanut agglutinin binding related to cell proliferation and axonal growth in embryonic chick limbs. Anat Embryol (Berlin) 1994;190:429–438. doi: 10.1007/BF00235489. [DOI] [PubMed] [Google Scholar]
- 2.Appleyard M E, McDonald B. Reduced adrenal gland acetylcholinesterase activity in Alzheimer’s disease. Lancet. 1991;338:1085–1086. doi: 10.1016/0140-6736(91)91947-s. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 3.Aubin J E, Liu F, Malaval L, Gupta K. Osteoblast and chondroblast differentiation. Bone. 1995;17:77S–83S. doi: 10.1016/8756-3282(95)00183-e. [DOI] [PubMed] [Google Scholar]
- 4.Auld V J, Fetter R D, Broadie K, Goodman C S. Gliotactin, a novel transmembrane protein on peripheral glia, is required to form the blood-nerve barrier in Drosophila. Cell. 1995;81:757–767. doi: 10.1016/0092-8674(95)90537-5. [DOI] [PubMed] [Google Scholar]
- 5.Ben Aziz-Aloya R, Seidman S, Timberg R, Sternfeld M, Zakut H, Soreq H. Expression of a human acetylcholinesterase promoter-reporter construct in developing neuromuscular junctions of Xenopus embryos. Proc Natl Acad Sci USA. 1993;90:2471–2475. doi: 10.1073/pnas.90.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boskey A L. Current concepts of the physiology and biochemistry of calcification. Clin Orthop. 1981;157:225–257. [PubMed] [Google Scholar]
- 7.Brank M, Zajc-Kreft K, Kreft S, Komel R, Grubic Z. Biogenesis of acetylcholinesterase is impaired, although its mRNA level remains normal, in the glucocorticoid-treated rat skeletal muscle. Eur J Biochem. 1998;251:374–381. doi: 10.1046/j.1432-1327.1998.2510374.x. [DOI] [PubMed] [Google Scholar]
- 8.Brenner R F, Nerlich A, Terinde R, Bartmann P. In vitro studies on clonal growth of chondrocytes in thanatophoric dysplasia. Am J Med Genet. 1996;63:401–405. doi: 10.1002/(SICI)1096-8628(19960517)63:2<401::AID-AJMG15>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 9.Bruder S P, Fink D J, Caplan A I. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J Cell Biochem. 1994;56:283–294. doi: 10.1002/jcb.240560809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Candeliere G A, Jurutka P W, Haussler M R, St-Arnaud R. A composite element binding the vitamin D receptor, retinoid X receptor α, and a member of the CTF/NF-1 family of transcription factors mediates the vitamin D responsiveness of the c-fos promoter. Mol Cell Biol. 1996;16:584–592. doi: 10.1128/mcb.16.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darboux I, Barthalay Y, Piovant M, Hipeau-Jacquotte R. The structure-function relationships in Drosophila neurotactin show that cholinesterasic domains may have adhesive properties. EMBO J. 1996;15:4835–4843. [PMC free article] [PubMed] [Google Scholar]
- 12.de la Escalera S, Bockamp E O, Moya F, Piovant M, Jimenez F. Characterization and gene cloning of neurotactin, a Drosophila transmembrane protein related to cholinesterases. EMBO J. 1990;9:3593–3601. doi: 10.1002/j.1460-2075.1990.tb07570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delezoide A L, Lasselin-Benoist C, Legeai-Mallet L, Brice P, Senee V, Yayon A, Munnich A, Vekemans M, Bonaventure J. Abnormal FGFR 3 expression in cartilage of thanatophoric dysplasia fetuses. Hum Mol Genet. 1997;6:1899–1906. doi: 10.1093/hmg/6.11.1899. [DOI] [PubMed] [Google Scholar]
- 14.Dukas K, Sarfati P, Vaysse N, Pradayrol L. Quantitation of changes in the expression of multiple genes by simultaneous polymerase chain reaction. Anal Biochem. 1993;215:66–72. doi: 10.1006/abio.1993.1555. [DOI] [PubMed] [Google Scholar]
- 15.Ehrlich G, Patinkin D, Ginzberg D, Zakut H, Eckstein F, Soreq H. Use of partially phosphorothioated “antisense” oligonucleotides for sequence-dependent modulation of hematopoiesis in culture. Antisense Res Dev. 1994;4:173–183. doi: 10.1089/ard.1994.4.173. [DOI] [PubMed] [Google Scholar]
- 16.Eriksen E F, Colvard D S, Berg N J, Graham M L, Mann K G, Spelsberg T C, Riggs B L. Evidence of estrogen receptors in normal human osteoblast-like cells. Science. 1988;241:84–86. doi: 10.1126/science.3388021. [DOI] [PubMed] [Google Scholar]
- 17.Ernst M, Schmid C, Froesch E R. Enhanced osteoblast proliferation and collagen gene expression by estradiol. Proc Natl Acad Sci USA. 1988;85:2307–2310. doi: 10.1073/pnas.85.7.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grifman M, Soreq H. Differentiation intensifies the susceptibility of pheochromocytoma cells to antisense oligodeoxynucleotide-dependent suppression of acetylcholinesterase activity. Antisense Nucleic Acid Drug Dev. 1997;7:351–359. doi: 10.1089/oli.1.1997.7.351. [DOI] [PubMed] [Google Scholar]
- 19.Huong P L, Kolk A H, Eggelte T A, Verstijnen C P, Gilis H, Hendriks J T. Measurement of antigen specific lymphocyte proliferation using 5-bromo-deoxyuridine incorporation. An easy and low cost alternative to radioactive thymidine incorporation. J Immunol Methods. 1991;140:243–248. doi: 10.1016/0022-1759(91)90377-r. [DOI] [PubMed] [Google Scholar]
- 20.Ichtchenko K, Nguyen T, Sudhof T C. Structures, alternative splicing, and neurexin binding of multiple neuroligins. J Biol Chem. 1996;271:2676–2682. doi: 10.1074/jbc.271.5.2676. [DOI] [PubMed] [Google Scholar]
- 21.Karpel R, Ben Aziz-Aloya R, Sternfeld M, Ehrlich G, Ginzberg D, Tarroni P, Clementi F, Zakut H, Soreq H. Expression of three alternative acetylcholinesterase messenger RNAs in human tumor cell lines of different tissue origins. Exp Cell Res. 1994;210:268–277. doi: 10.1006/excr.1994.1039. [DOI] [PubMed] [Google Scholar]
- 22.Karpel R, Sternfeld M, Ginzberg D, Guhl E, Graessmann A, Soreq H. Overexpression of alternative human acetylcholinesterase forms modulates process extensions in cultured glioma cells. J Neurochem. 1996;66:114–123. doi: 10.1046/j.1471-4159.1996.66010114.x. [DOI] [PubMed] [Google Scholar]
- 23.Kaufer D, Friedman A, Seidman S, Soreq H. Acute stress facilitates long-lasting changes in cholinergic gene expression. Nature. 1998;393:373–377. doi: 10.1038/30741. [DOI] [PubMed] [Google Scholar]
- 24.Komm B S, Terpening C M, Benz D J, Graeme K A, Gallegos A, Kore M, Greene G L, O’Malley B W, Hausler M R. Estrogen binding, receptor mRNA, and biologic response in osteoblast-like osteosarcoma cells. Science. 1988;241:81–84. doi: 10.1126/science.3164526. [DOI] [PubMed] [Google Scholar]
- 25.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson R T, Gao Y H, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturation arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 26.Layer P G, Willbold E. Novel functions of cholinesterases in development, physiology and disease. Prog Histochem Cytochem. 1995;29:1–94. doi: 10.1016/s0079-6336(11)80046-x. [DOI] [PubMed] [Google Scholar]
- 27.Lemaire P, Vesque C, Schmitt J, Stunnenberg H, Frank R, Charnay P. The serum-inducible mouse gene Krox-24 encodes a sequence-specific transcriptional activator. Mol Cell Biol. 1990;10:3456–3467. doi: 10.1128/mcb.10.7.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lev-Lehman E, Deutsch V, Eldor A, Soreq H. Immature human megakaryocytes produce nuclear-associated acetylcholinesterase. Blood. 1997;89:3644–3653. [PubMed] [Google Scholar]
- 29.Lev-Lehman E, Ginzberg D, Hornreich G, Ehrlich G, Meshorer A, Eckstein F, Soreq H, Zakut H. Antisense inhibition of acetylcholinesterase gene expression causes transient hematopoietic alterations in vivo. Gene Ther. 1994;1:127–135. [PubMed] [Google Scholar]
- 30.Liu M, Freedman L P. Transcriptional synergism between the vitamin D3 receptor and other nonreceptor transcription factors. Mol Endocrinol. 1994;8:1593–1604. doi: 10.1210/mend.8.12.7708050. [DOI] [PubMed] [Google Scholar]
- 31.Matsue M, Kageyama R, Denhardt D T, Noda M. Helix-loop-helix-type transcription factor (HES-1) is expressed in osteoblastic cells, suppressed by 1,25(OH)2 vitamin D3, and modulates 1,25(OH)2 vitamin D3 enhancement of osteopontin gene expression. Bone. 1997;20:329–334. doi: 10.1016/s8756-3282(97)00005-7. [DOI] [PubMed] [Google Scholar]
- 32.Moore J W, Clark G M G, Takatani O, Wakabayashi Y, Hayward J L, Bulbrook R D. Distribution of 17 beta-estradiol in the sera of normal British and Japanese women. J Natl Cancer Inst. 1983;71:749–755. [PubMed] [Google Scholar]
- 33.Oliver M H, Harrison N K, Bishop J E, Cole P J, Laurent G J. A rapid and convenient assay for counting cells cultured in microwell plates: application for assessment of growth factors. J Cell Sci. 1989;92:513–518. doi: 10.1242/jcs.92.3.513. [DOI] [PubMed] [Google Scholar]
- 34.Quandt K, Frech K, Karas H, Wingender E, Werner T. MatInd and MatInspector—new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res. 1995;23:4878–4884. doi: 10.1093/nar/23.23.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seidman S, Sternfeld M, Ben Aziz-Aloya R, Timberg R, Kaufer-Nachum D, Soreq H. Synaptic and epidermal accumulations of human acetylcholinesterase are encoded by alternative 3′-terminal exons. Mol Cell Biol. 1995;15:2993–3002. doi: 10.1128/mcb.15.6.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seidman S, Ben Aziz-Aloya R, Timberg R, Loewenstein Y, Velan B, Shafferman A, Liao J, Norgaard-Pedersen B, Brodbeck U, Soreq H. Overexpressed monomeric human acetylcholinesterase induces subtle ultrastructural modifications in developing neuromuscular junctions of Xenopus laevis embryos. J Neurochem. 1994;62:1670–1681. doi: 10.1046/j.1471-4159.1994.62051670.x. [DOI] [PubMed] [Google Scholar]
- 37.Shapira, M., M. Korner, L. Bosgraaf, I. Tur-Kaspa, and H. Soreq. The human ACHE locus includes a polymorphic enhancer domain 17 kb upstream from the transcription start site. In D. M. Quinn, B. P. Doctor, and P. Taylor (ed.), Cholinesterases and related proteins, in press. Plenum Press, New York, N.Y.
- 38.Sillence D O, Horton W A, Rimoin D L. Morphologic studies in skeletal dysplasias. Am J Pathol. 1979;96:813–859. [PMC free article] [PubMed] [Google Scholar]
- 39.Soreq H, Patinkin D, Lev-Lehman E, Grifman M, Ginzberg D, Eckstein F, Zakut H. Antisense oligonucleotide inhibition of acetylcholinesterase gene expression induces progenitor cell expansion and suppresses hematopoietic apoptosis ex vivo. Proc Natl Acad Sci USA. 1994;91:7907–7911. doi: 10.1073/pnas.91.17.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sternfeld M, Ming G, Song H, Sela K, Timberg R, Poo M, Soreq H. Acetylcholinesterase enhances neurite growth and synapse development through alternative contributions of its hydrolytic capacity, core protein, and variable C termini. J Neurosci. 1998;18:1240–1249. doi: 10.1523/JNEUROSCI.18-04-01240.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sylvia V L, Schwartz Z, Schuman L, Morgan R T, Mackey S, Gomez R, Boyan B D. Maturation-dependent regulation of protein kinase C activity by vitamin D3 metabolites in chondrocyte culture. J Cell Physiol. 1993;157:271–278. doi: 10.1002/jcp.1041570209. [DOI] [PubMed] [Google Scholar]
- 42.Tora L, Gaub M P, Madar S, Dierich A, Bellard M, Chambon P. Cell-specific activity of a GGTCA half-palindromic oestrogen-responsive element in the chicken ovalbumin gene promoter. EMBO J. 1988;7:3771–3778. doi: 10.1002/j.1460-2075.1988.tb03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Umezu Y, Nagata N, Doi Y, Furukawa H, Sagara T, Hayashida T, Ogata H, Fujimoto S. Cytochemical and immunocytochemical demonstration of acetylcholinesterase of the prenatal rat lower limb. Arch Histol Cytol. 1993;56:217–224. doi: 10.1679/aohc.56.217. [DOI] [PubMed] [Google Scholar]