The Gab1 PH Domain Is Required for Localization of Gab1 at Sites of Cell-Cell Contact and Epithelial Morphogenesis Downstream from the Met Receptor Tyrosine Kinase (original) (raw)
Abstract
Stimulation of the hepatocyte growth factor (HGF) receptor tyrosine kinase, Met, induces mitogenesis, motility, invasion, and branching tubulogenesis of epithelial and endothelial cell lines in culture. We have previously shown that Gab1 is the major phosphorylated protein following stimulation of the Met receptor in epithelial cells that undergo a morphogenic program in response to HGF. Gab1 is a member of the family of IRS-1-like multisubstrate docking proteins and, like IRS-1, contains an amino-terminal pleckstrin homology domain, in addition to multiple tyrosine residues that are potential binding sites for proteins that contain SH2 or PTB domains. Following stimulation of epithelial cells with HGF, Gab1 associates with phosphatidylinositol 3-kinase and the tyrosine phosphatase SHP2. Met receptor mutants that are impaired in their association with Gab1 fail to induce branching tubulogenesis. Overexpression of Gab1 rescues the Met-dependent tubulogenic response in these cell lines. The ability of Gab1 to promote tubulogenesis is dependent on its pleckstrin homology domain. Whereas the wild-type Gab1 protein is localized to areas of cell-cell contact, a Gab1 protein lacking the pleckstrin homology domain is localized predominantly in the cytoplasm. Localization of Gab1 to areas of cell-cell contact is inhibited by LY294002, demonstrating that phosphatidylinositol 3-kinase activity is required. These data show that Gab1 is an important mediator of branching tubulogenesis downstream from the Met receptor and identify phosphatidylinositol 3-kinase and the Gab1 pleckstrin homology domain as crucial for subcellular localization of Gab1 and biological responses.
Hepatocyte growth factor/scatter factor (HGF) is a mesenchymally derived factor that stimulates a wide variety of cellular responses through activation of the Met receptor tyrosine kinase. HGF is a potent mitogen for primary hepatocytes and renal tubule cells (29, 43, 77), stimulates epithelial cell dissociation and invasion, and acts as an initiating signal for an intrinsic cellular morphogenic program of kidney, breast, and lung epithelium grown in matrix cultures (41, 59). In vivo, HGF is a potent angiogenic factor (21) and is involved in organ regeneration (39) as well as tumorigenesis (4, 53, 58, 64). Moreover, recent studies have demonstrated a role for Met and HGF in the development of the liver and placenta, the development and innervation of skeletal muscle, and in directing of the growth of axonal cones (9, 37, 57, 65, 74).
Using receptor chimeras, we and others have demonstrated that the Met cytoplasmic domain is sufficient to mediate the pleiotropic biological responses attributed to HGF in epithelial cells (32, 70, 80) and that these events require Met protein tyrosine kinase activity (70, 79). Phosphorylated tyrosine residues in the noncatalytic cytoplasmic domains of receptor tyrosine kinases act as specific binding sites for Src homology 2 (SH2) and phosphotyrosine binding domain-containing proteins, and these in turn transduce intracellular signals (reviewed in reference 47). While signaling pathways downstream from receptor tyrosine kinases involved in a mitogenic response have been characterized in detail, until recently little was known about the signaling pathways involved in cell dissociation, motility, and morphogenesis. Toward this end, the characterization of signaling pathways downstream from the Met receptor has been essential.
Upon stimulation with HGF, the Met receptor cytoplasmic domain becomes highly phosphorylated on tyrosine residues (52, 79). Structure-function analyses have shown that two tyrosine residues within the carboxyl terminus (Y1349 and Y1356), which are highly conserved between other members of the Met receptor tyrosine kinase gene family, Sea and Ron (54), are crucial for cell scatter and branching morphogenesis in Madin-Darby canine kidney (MDCK) epithelial cells (32, 70, 79, 80). Tyrosine 1356 forms a multisubstrate binding site, coupling the Met receptor with the Grb2 and Shc adapter proteins, the p85 subunit of phosphatidylinositol 3-kinase (PI3K), phospholipase Cγ1 (PLCγ1), and the phosphatase SHP2 (11, 13, 15, 16, 48, 79).
From a search for Met-specific substrates that could be implicated in branching morphogenesis, we have recently identified the Grb2-associated binder 1 (Gab1) as the major phosphorylated protein in epithelial cells that undergo a morphogenic program in response to HGF (44). Gab1 was initially identified in a library screen as a Grb2 binding protein and is phosphorylated downstream from the epidermal growth factor receptor, the insulin receptor, and the TrkA receptor (24, 25). More recently, it has been shown that interleukin-3 (IL-3), IL-6, and alpha and gamma interferons also induce the tyrosine phosphorylation of Gab1 (62). Gab1 is a member of the IRS-1 family of multisubstrate binding proteins, which includes IRS-1, IRS-2, p62_dok_, and DOS (6, 23, 49, 73, 75). While these proteins lack enzymatic activities, they are thought to function as multisubstrate docking proteins by virtue of their ability to associate with multiple signaling molecules.
Murine Gab1 contains eighteen tyrosine residues, some of which, if phosphorylated, provide potential binding sites for SH2 or PTB domain-containing proteins (24, 25, 62). In addition, Gab1 contains several proline rich regions which could interact with SH3 domain-containing proteins. The greatest homology observed with IRS-1 family members lies within the N terminus of Gab1, which contains a pleckstrin homology (PH) domain, suggesting a conserved role for the PH domain within these proteins (5, 24). While IRS-1 contains a phosphotyrosine binding domain involved in its recruitment to the insulin receptor, Gab1 lacks such a domain. In vivo, Gab1 is thought to be recruited to the Met receptor predominantly indirectly, via the Grb2 adapter protein, through the interaction with the carboxy-terminal SH3 domain of Grb2 (3, 14, 44) and association of the Grb2 SH2 domain with Y1356 of the multisubstrate binding site in the Met receptor. In addition, a direct interaction between Gab1 and Y1349 in the Met receptor was observed in the yeast two-hybrid system (69) and occurs to a lesser extent in vivo (44). This requires a proline-rich domain in Gab1, defined as the Met binding domain (69).
The observations that overexpression of Gab1 in neuronal cells promotes cell survival downstream from the TrkA receptor and that overexpression of Gab1 in epithelial cells promotes a morphogenesis program support a role for Gab1 as an important signaling molecule in multiple cell types (25, 69). Interestingly, MDCK cells expressing Met receptor mutants that fail to associate with Grb2 are unable to form branching tubules upon Met activation, suggesting that Grb2-dependent signaling pathways are involved in the morphogenic activities of HGF (16). We show here that overexpression of Gab1 rescues the tubulogenesis defect in cells expressing these Met mutants, consistent with the function of Gab1 lying downstream from Grb2. Importantly, this provides an assay system to investigate the structural domains of Gab1 required for Met-dependent branching tubulogenesis. From structure-function analyses, we have established that the PH domain of Gab1 is essential for the ability of Gab1 to support branching morphogenesis downstream from the Met receptor tyrosine kinase. We demonstrate that the PH domain is required to target Gab1 to the proximity of the cellular membrane at sites of cell-cell contact and that this localization is also dependent on the activity of PI3K. This study provides evidence for a key role for PI3K and the Gab1 PH domain in determining Gab1 cellular localization and biological responses downstream from the Met receptor.
MATERIALS AND METHODS
Cell culture and DNA transfections.
MDCK cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS). The generation of MDCK cell lines expressing wild-type colony-stimulating factor 1 (CSF)-Met receptor and mutants thereof by retroviral infection has been described previously (16, 80). For the generation of stable cell lines expressing wild-type and mutant HA-tagged Gab1, the Gab1 cDNA was cloned into the pCDNA1.1 vector and cotransfected with a PLX SH vector, which confers resistance to hygromycin, by the calcium phosphate method as described elsewhere (72). Cell lines were selected in hygromycin (300 μg/ml). For transient-transfection assays, 293T cells were seeded at 106/100-mm petri dish and transfected 24 h later with 2 μg of plasmid DNA encoding wild-type Gab1 without or with CSF-Met cDNA by the calcium phosphate precipitation method (72). At 16 h later, the cells were washed twice in DMEM medium lacking FBS and then cultured for another 48 h in medium containing 10% FBS; they were then harvested.
Antibodies and reagents.
Antibodies raised in rabbit against a C-terminal peptide of human Met were used (51). Anti-p85 was kindly provided by T. Pawson, Mount Sinai Hospital, University of Toronto. Antiphosphotyrosine (4G10) was obtained from Upstate Biotechnology Inc., Lake Placid, N.Y. Anti-HA (HA.11) was purchased from BABCO, Richmond, Calif. Anti-py PY20 was purchased from Transduction Laboratories. Anti-SHP2 was kindly provided by G.-S. Feng, Indiana University School of Medicine, and anti-E-cadherin (3G8) was provided by M. Pasdar, University of Alberta. The PI3K inhibitor LY294002 and the PLC inhibitor U73122 were purchased from Biomol, Plymouth Meeting, Pa. The p85 and p110 constructs were obtained from A. Klippel (31). CY3-conjugated goat anti-mouse immunoglobulin h (IgG) was purchased from Jackson ImmunoResearch Laboratories, Inc. The generation of Gab1ΔPI3K mutant protein was described elsewhere (25). The Gab1ΔPH domain mutant encompasses amino acids 116 to 695 of the murine Gab1 cDNA (24). It was constructed by performing PCR on full-length Gab1 cDNA by using a 5′ primer, GTGGGATCCTCGGATTCAATCCCACAGAAGAA, and a 3′ primer, CGAATTCACTTCACATTCTTGG. The PCR product was cloned into the _Bam_HI and _Eco_RI sites of pcDNA1.1 downstream from an in-frame HA tag.
HGF stimulation of MDCK cell lines expressing wild-type and mutant Gab1.
Cells were seeded at 106 per 100-mm dish. At 24 h later, they were washed once with DMEM and then starved for 24 h in 10 ml of DMEM containing 0.02% FBS. HGF was added at 100 U/ml in 2 ml for the indicated times. The cells were immediately lysed in 1 ml of lysis buffer (50 mM HEPES [pH 7.4], 150 mM NaCl, 10% glycerol, 0.1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 1 μg each of leupeptin and aprotinin per ml, 1 mM Na3VO4).
Immunoprecipitations and Western blotting.
MDCK cell lysates (2 mg of total protein) or 293T cell lysates (50 μg) were incubated with antibodies as indicated in the figures for 1 h at 4°C with gentle rotation. A 20-μl volume of a 50% slurry of either protein A or protein G-Sepharose was added for an additional 1 h to collect immune complexes. Following three washes in lysis buffer, proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane. The membranes were blocked for 1 h with 3% bovine serum albumin in TBST (10 mM Tris-HCl [pH 7.4], 2.5 mM EDTA, 150 mM NaCl, 0.1% Tween 20) and then for 1 h with primary antibody (1:1,000). Following five washes in TBST, the proteins were revealed with secondary anti-mouse (Jackson ImmunoResearch Laboratories, Inc.) or protein A (Gibco) conjugated to horseradish peroxidase. The proteins were visualized with an enhanced chemiluminescence detection system (Amersham).
PI3K assay.
The PI3K assay was performed as previously described (15). Briefly, 3 mg of 293T cell lysates was subjected to immunoprecipitation with either PY20, anti-Met, or anti-HA for 1 h at 4°C. Immune complexes were collected with protein A-Sepharose for the first two antibodies and protein G-Sepharose for the last antibody. Immunoprecipitates were washed three times with lysis buffer, once with phosphate-buffered saline (PBS), once with 100 mM Tris-HCl (pH 7.5)–0.5 M LiCl, once with distilled H2O, once with 20 mM Tris-HCl (pH 7.5)–100 mM NaCl–1 mM EDTA, and once with kinase buffer (20 mM Tris-HCl [pH 7.5], 100 mM NaCl, 0.5 mM EGTA). The beads were incubated for 10 min at room temperature with 50 μl of kinase buffer containing phosphatidylinositol (0.2 mg/ml). Then 20 μCi of [γ-32P]ATP and 20 mM MgCl2 were added for 10 min at room temperature. The reactions were stopped upon the addition of 150 μl of chloroform–methanol–11.6 M HCl (50💯1). The lipids were extracted with 100 μl of chloroform, and the organic phase was washed as described elsewhere (55), resuspended in 15 μl of chloroform, spotted on a silica gel 60 thin-layer chromatography plate (Merck), and resolved in chloroform–methanol–28% ammonium hydroxide–water (86:76:10:14) for 1 h. Phosphorylated phospholipids were visualized following autoradiography and quantitated with a Fuji Bas 1000 phosphorimage analyzer.
Collagen assays.
The ability of MDCK cells to form branching tubules was assayed as previously described (79). Briefly, 5 × 103 cells were resuspended in 500 μl of collagen solution (Vitrogen 100 [Celtrix]) prepared as specified by the manufacturer and layered over 350 μl of the collagen solution in a 24-well plate. The cells were maintained in Liebowitz medium containing 5% FBS and allowed to form cysts for 5 to 7 days. For stimulations, HGF or recombinant human CSF (rhCSF-1) (5 U/ml) (kindly provided by Genetics Institute, Boston, Mass.) was added to the Liebowitz medium containing 5% FBS. Tubules were apparent by light microscopy 5 to 10 days after the addition of stimuli. The medium was changed every 4 days, and photographs were taken 14 to 20 days later on Kodak TMY400 films at a magnification of ×10. For the quantitation of the morphogenic response, 60 colonies in each of six independent cultures (wells) were scored for their ability to form branching tubules (structures whose length is five times their diameter), and the results were plotted as the average number of cysts able to undergo tubulogenesis per culture per 100.
Immunofluorescence.
MDCK cells overexpressing wild-type Gab1 or the Gab1 mutants were plated for the indicated times on glass coverslips (Bellco Glass Inc.) in a 24-well dish (Nunc) in DMEM containing 10% FBS. For stimulations, 104 MDCK cells overexpressing wild-type Gab1 or Gab1ΔPH mutant protein were plated overnight in 10% serum-containing medium and 50 U of HGF per ml was added for 15 min at 37°C. The cells were fixed in 2% paraformaldehyde in PBS for 30 min at room temperature, washed twice in PBS, and incubated for 10 min in PBS containing 50 mM ammonium chloride. Following one additional wash in PBS, the cells were treated for 10 min at room temperature with PBS containing 0.1% Triton X-100 and 5% FBS (buffer A). Anti-HA (1:300 in buffer A) was then added to the cells, and after three washes in the same buffer, CY3-conjugated goat anti-mouse IgG (1:2,000) was added for 10 min, and the cells were given three washes in buffer A. For CSK treatments, cells were incubated for 10 min at room temperature in a buffer containing 10 mM piperazine-N,_N_′-bis(2-ethanesulfonic acid) (PIPES, pH 7.0), 300 mM sucrose, 50 mM NaCl, 3 mM MgCl2, 0.5% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 1 μg each of leupeptin and aprotinin per ml, and 1 mM Na3VO4. Following two washes in PBS, the cells were fixed and labeled with anti-HA in buffer A by the method described above. To induce the disruption of cell-cell adhesion, cells were cultured for 3 days in medium containing 10% FBS, washed once in PBS, incubated for 2 h in medium containing 5 mM EGTA as described by Takaishi et al. (63), and then fixed and labeled as described above. For the treatments with LY294002 and U73122, cells were incubated for 2 h at 37°C in 50 μM LY294002 or 2 μM U73122, washed twice, fixed in 2% paraformaldehyde in PBS, and labeled as described above. The glass coverslips were mounted onto slides in Immunofluore medium (ICN) and visualized with a Nikon Labophot-2 epifluorescence microscope. Photographs were taken with Kodak TMZ3200 film.
pEGFP Gab1 and microinjections.
Wild-type Gab1, the Gab1ΔPH mutant, and the Gab1PH domain (amino acids 1 to 116) cDNAs were subcloned as _Bam_HI-_Eco_RI fragments into the _Bgl_II-_Eco_RI sites in the multiple-cloning site of pEGFP-C2 (Clontech), downstream of GFP. Plasmids expressing these fusion proteins were microinjected at 50 μg of DNA per ml into nuclei of MDCK cells by using an Eppendorf microinjector. At 2 h after injection, the cells were fixed in 2% paraformaldehyde in PBS and visualized as described above. For the injection of constructs expressing the p110 and p85 subunits of PI3K (31), MDCK cells were serum starved in medium containing 0.02% FBS for 24 h prior to the microinjections. These constructs were microinjected into the nuclei of cells at 100 μg/ml. The pEGFP-Gab1 construct was comicroinjected where indicated in the figures, and cells were visualized 4 h after the injection.
RESULTS
Gab1 associates with multiple substrates downstream from the Met receptor tyrosine kinase.
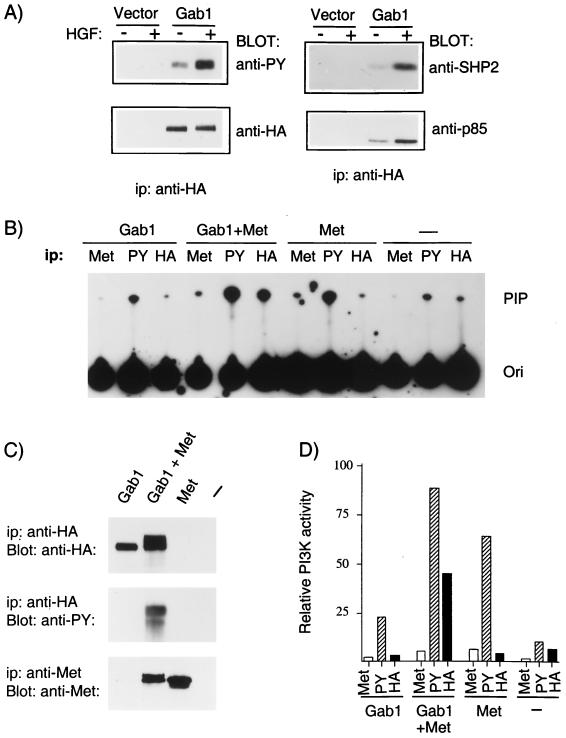
We have previously demonstrated that Gab1 is the predominant protein phosphorylated following stimulation of the Met receptor in epithelial cells (44). Gab1 associates with the p85 subunit of PI3K and the phosphatase SHP2 downstream from the insulin, EGF, and TrkA receptors (24, 25). To assess whether these signaling proteins associate with Gab1 following Met activation, we have generated MDCK epithelial cell lines stably expressing HA epitope-tagged wild-type Gab1 and assayed the ability of HA-Gab1 to associate with p85 and SHP2 in a Met-dependent fashion. Stimulation of HA-Gab1-expressing cell lines with HGF resulted in an increase in the phosphorylation of HA-Gab1 (Fig. 1A). Importantly, immunoprecipitation of HA-Gab1 followed by Western blotting with antibodies specific for SHP2 or the p85 subunit of PI3K revealed that HGF induced an increase in the association of these proteins with Gab1 (Fig. 1A). Therefore, in a similar manner to the insulin, EGF, and TrkA receptors, tyrosine phosphorylation of Gab1 downstream from the Met receptor was accompanied by an increase in the ability of Gab1 to associate with the p85 subunit of PI3K and with SHP2. These results indicate that Gab1 functions as a multisubstrate docking protein coupling the Met receptor with multiple signaling pathways in epithelial cells.
FIG. 1.
Association of Gab1 with cellular substrates is dependent on Met-mediated tyrosine phosphorylation of Gab1. (A) Stable MDCK cell lines expressing HA-Gab1 or vector control were serum starved in 0.02% FBS for 24 h and subsequently stimulated with HGF (100 U/ml) for 15 min. Cell lysates were subjected to immunoprecipitation (ip) with anti-HA followed by blotting with anti-PY. Products of parallel precipitations were blotted with anti-p85, anti-SHP2, or anti-HA. (B) 293T cells were transiently transfected with plasmids encoding epitope-tagged wild-type Gab1, with or without a Met chimera composed of the extracellular domain from CSF receptor fused to the Met transmembrane and intracellular domains. Cells were serum starved 24 h prior to harvest. Lysates were immunoprecipitated with anti-Met, anti-PY (PY20), or anti-HA. PI3K activity was determined as described in Materials and Methods. Phosphatidylinositol phosphates were separated by thin-layer chromatography on silica gel plates and revealed by autoradiography. The position of phosphatidylinositol 3-phosphate is shown (PIP), as is the origin (Ori). (C) Lysates from 293T cells transfected as described in panel B were subjected to immunoprecipitation with anti-HA or anti-Met and blotted with anti-HA, anti-Met, or anti-PY as indicated. (D) The incorporated radioactivity in the phosphatidylinositol 3-phosphate was quantitated with a Fujix BAS 1000 image analyzer, and the results are plotted on the bar graph as relative PI3K activity.
Binding of the p85 subunit of PI3K with IRS-1 correlated with activation of PI3K downstream from the insulin receptor (2, 42). Similarly, Gab1 was associated with activation of PI3K downstream from the TrkA receptor in PC12 cells (25). PI3K is activated downstream from the Met receptor (15, 55). However, although the Met receptor and oncoprotein can bind p85 directly, low levels of PI3K activity are coimmunoprecipitated with anti-Met compared to the levels in coimmunoprecipitations with antiphosphotyrosine (anti-PY) (15a). To establish if Met-stimulated PI3K activity is associated with Gab1, 293T cells were transiently transfected with HA-Gab1 alone, Met alone, or HA-Gab1 and Met together (Fig. 1B). Five independent transfections were performed, and the results of a representative experiment are shown in Fig. 1B through D. Overexpression of Met results in the induction of Met activation and phosphorylation in the absence of ligand (44), and Western blot analysis of anti-HA-Gab1 immunoprecipitates with anti-PY revealed robust tyrosine phosphorylation of Gab1 only in cells coexpressing Met (Fig. 1C). Significantly, in cells coexpressing HA-Gab1 and Met, anti-HA-Gab1 immunoprecipitates contained 50% of the PI3K activity observed in anti-PY immunoprecipitates whereas low levels of PI3K activity (5%) were observed in anti-Met immunoprecipitates (Fig. 1C). Thus, a significant portion of PI3K activated downstream from the Met receptor is associated with Gab1 (Fig. 1D).
Overexpression of Gab1 rescues the branching tubulogenesis defect of MDCK cells expressing mutant Met receptors.
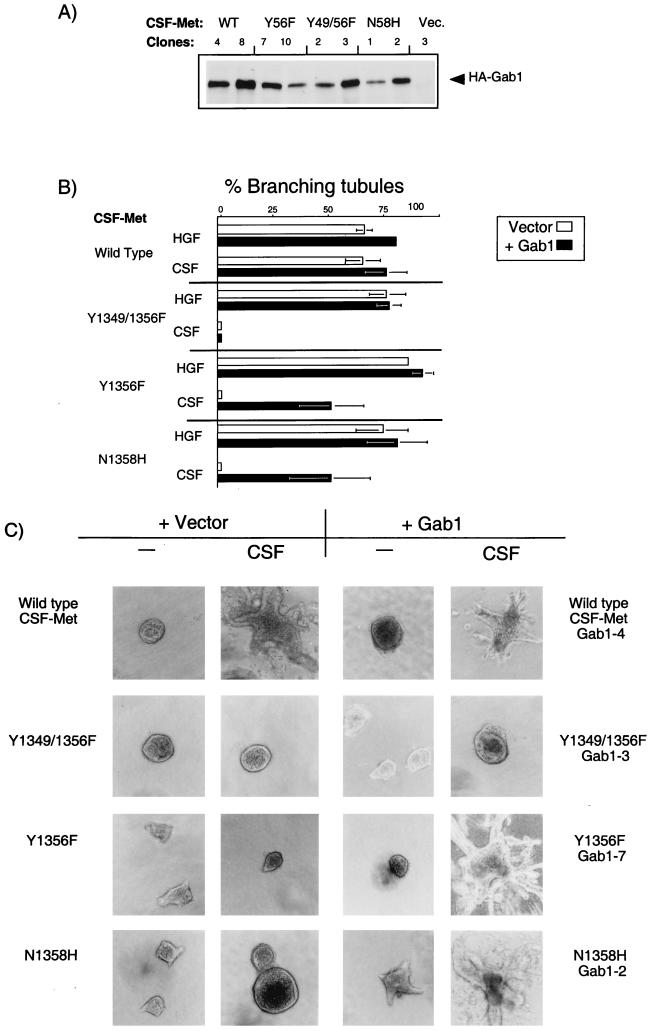
Structure-function studies with chimeric Met receptors containing the extracellular domain of the CSF-1 receptor fused to the transmembrane and cytoplasmic domains of Met revealed that Met receptor mutants with impaired Gab1 association fail to induce branching tubules following stimulation of the Met receptor (16, 79). To examine the biological function of Gab1, we have overexpressed HA-tagged Gab1 in cell lines expressing wild-type CSF-Met receptors that scatter and form branching tubules in response to CSF (80) and in cells expressing CSF-Met receptor mutants that fail to form branching tubules in response to CSF, including CSF-Met receptor multisubstrate binding-site mutants (Y1356F and Y1349/1356F) that have lost their ability to bind to multiple cellular substrates (16, 79) and a CSF-Met receptor mutant (N1358H), that is specifically unable to bind Grb2 (16).
Twenty independent clones of each HA-Gab1-transfected cell line were selected, and five showing similar levels of Gab1 expression were characterized in detail and assayed for branching tubulogenesis. The level of expression of Gab1 is shown for two representative clones of each cell line (Fig. 2A), and the tubulogenic response is shown for one clone (Fig. 2C). The tubulogenic response was quantitated in Fig. 2B, where the response to HGF is an indication of the total number of cysts capable of undergoing tubulogenesis. In cells expressing wild-type CSF-Met and Gab1, no branching tubules were observed in the absence of Met stimulation (Fig. 2C, clone 4). These cells formed cysts, as did parental cells, when grown in a collagen matrix, and they formed branching tubules (structures whose length is five times their width) only when the endogenous Met or the chimeric CSF-Met protein was stimulated with either HGF or CSF-1, respectively (Fig. 2B and C, clone 4). Importantly, in cells expressing the CSF-Met multisubstrate binding-site mutant (Y1356F), which fail to form tubules in response to CSF-1 (Fig. 2B and C, Y1356F + vector), overexpression of Gab1 rescued the tubulogenesis defect in the presence of CSF-1 in the five clones analyzed (Fig. 2B and C, clone 7). Moreover, overexpression of Gab1 rescued the branching tubulogenesis defect in 10 clones from two independent MDCK cell lines, expressing a CSF-Met receptor mutant that fails to bind only Grb2 (Fig. 2B and C, N1358H). In each case, overexpression of Gab1 rescued tubule formation in 75% of the cysts that were able to undergo tubulogenesis (50 to 60% of preformed cysts responded to CSF-1 by forming tubules, compared with 70 to 80% in response to HGF). However, cells expressing vector alone were unable to form tubules in response to CSF-1 (Fig. 2B and C, N1358H + vector). These results demonstrate that overexpression of Gab1 was sufficient to complement the branching tubulogenesis defect of cells expressing Met receptor mutants that fail to bind Grb2 and, as a consequence, show reduced ability to recruit Gab1 (44). In contrast, overexpression of Gab1 failed to rescue the branching tubulogenesis defect of cells expressing a CSF-Met Y1349/1356F mutant that fails to bind Gab1 (Fig. 2B and C, compare Y1349/1356F Vector with clone 3) demonstrating that Y1349 contributed to the ability of Gab1 to rescue tubulogenesis in the Y1356F or N1358H CSF-Met mutants.
FIG. 2.
Gab1 rescues branching tubulogenesis in MDCK cells expressing mutant CSF-Met receptors that fail to bind Grb2. MDCK cell lines expressing either wild-type (WT) CSF-Met or the CSF-Met mutants Y1349/1356F, Y1356F, or N1358H were stably transfected with vector or wild-type HA-tagged Gab1. (A) Lysates from two representative lines of each experimental group were subjected to immunoprecipitation with anti-HA, and proteins resolved by SDS-PAGE were transferred to a nitrocellulose membrane and immunoblotted with anti-HA. (B) Quantitation of the tubulogenic response following stimulation with HGF and CSF in cell lines expressing either CSF-Met or mutants thereof alone (open bars) or together with Gab1 (solid bars) was undertaken as described in Materials and Methods. The responses are plotted as the percentage of cysts that have undergone branching tubulogenesis. The values were derived from three independent experiments done in duplicate. None of the cysts formed tubules in the absence of stimulation. (C) Cell lines were grown in collagen for 5 days, during which they formed cysts. rhCSF-1 (5 U/ml) was added, and 14 days later branching tubules were visualized at a magnification of ×10 and photographs taken with Kodak TMY400 film. A representative cell line for each group is shown.
Gab1 is a substrate for the EGF receptor but fails to promote EGF-dependent epithelial branching tubulogenesis.
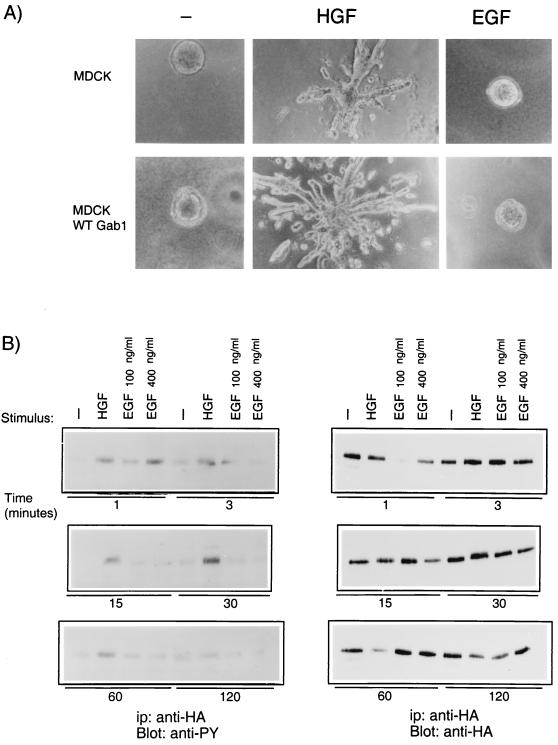
Overexpression of Gab1 promoted branching tubulogenesis induced through the HGF receptor, Met (Fig. 2). MDCK cells have abundant EGF receptors, and EGF induces tyrosine phosphorylation of Gab1 and its association with PI3K and SHP2 (24) but does not induce morphogenesis in MDCK cells. Therefore, we investigated whether EGF stimulation could induce branching tubulogenesis in MDCK cells overexpressing Gab1. As shown in Fig. 3A, while HGF stimulated the formation of branching tubules, EGF did not. To investigate whether this could be related to qualitative or quantitative differences in the phosphorylation of Gab1, in response to HGF or EGF, MDCK cells overexpressing Gab1 were stimulated with either growth factor for the time indicated (Fig. 3B). Gab1 phosphorylation in response to HGF was observed as early as 1 min following stimulation, reached a maximum at 15 to 30 min, was sustained for 1 h, and then returned to baseline within 2 h. In contrast, EGF-mediated Gab1 phosphorylation increased and then declined rapidly, reaching baseline within 15 min of stimulation (Fig. 3B). The distinct pattern of phosphorylation of Gab1 downstream from HGF and EGF was not due to different levels of expression of HA-Gab1 in the different experimental groups (Fig. 3B). Therefore, a sustained phosphorylation of Gab1 is observed downstream from HGF whereas only transient phosphorylation of Gab1 is observed downstream from EGF.
FIG. 3.
Overexpression of Gab1 does not mediate branching tubulogenesis downstream from EGF. (A) Control MDCK cells or MDCK cells overexpressing Gab1 were plated in a collagen matrix for 5 days. HGF (5 U/ml) or EGF (20 ng/ml) was added to the cultures, and photographs were taken 14 days later at a magnification of ×10. (B) MDCK cells overexpressing Gab1 were serum starved for 24 h prior to stimulation with either 100 U of HGF per ml or 100 400 ng of EGF per ml for the indicated time. Gab1 was immunoprecipitated (ip) with anti-HA. Proteins were resolved by SDS-PAGE, transferred to nitrocellulose, and probed with anti-PY or anti-HA. WT, wild type.
The PH domain of Gab1 is required for Met-dependent branching tubulogenesis.
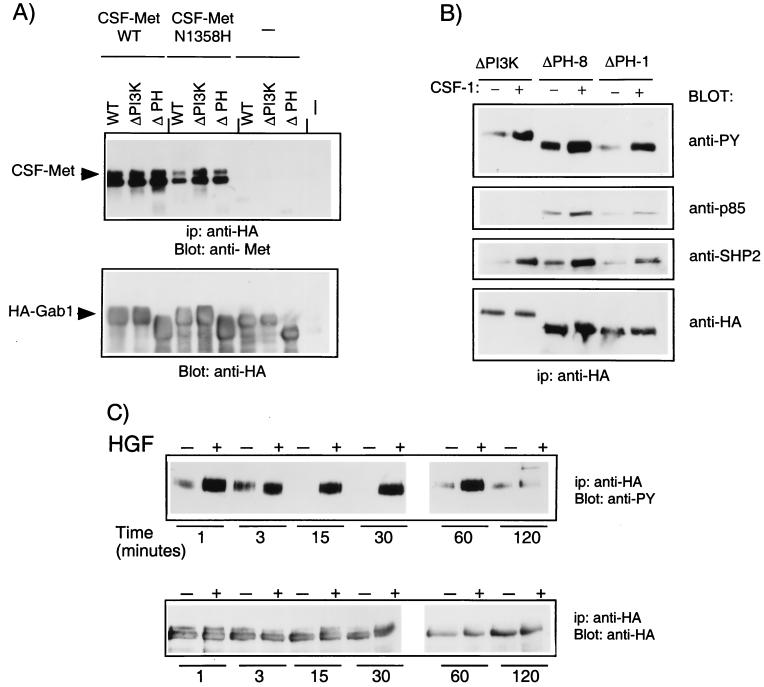
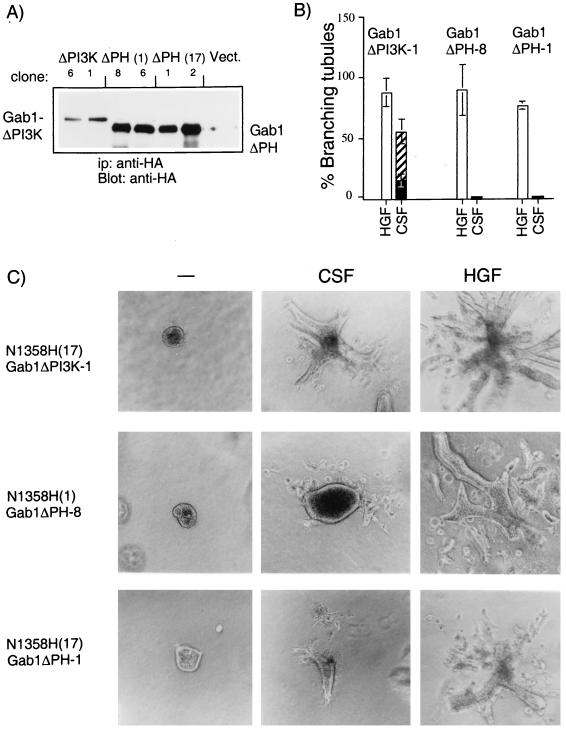
To identify domains in Gab1 that are critical for rescue of the tubulogenic response, mutant Gab1 proteins were expressed in the cell lines expressing the CSF-Met N1358H mutant, which fails to bind Grb2 and fails to induce tubule formation. Since a significant portion of the Met-induced PI3K activity was associated with Gab1 (Fig. 1B) and since PI3K is essential for cell dissociation and tubulogenesis (8, 55), we first examined a Gab1 mutant unable to bind PI3K. Sequences downstream from tyrosines 447, 472, and 589 in the carboxy-terminal portion of Gab1 contain putative binding sites for SH2 domains of the p85 subunit of PI3K, and substitution of these residues with phenylalanine residues results in the loss of p85 binding to Gab1 (25) (see Fig. 5B). To investigate whether the ability of Gab1 to bind p85 is essential for its ability to rescue tubulogenesis, MDCK cell lines expressing the CSF-Met N1358H Grb2-mutant were stably transfected with the Gab1ΔPI3K mutant (Fig. 4). Five of seven independent clones tested formed branching tubules in response to CSF-1. A representative clone is shown in Fig. 4C (ΔPI3K-1). Quantitation of the response in this clone revealed that while 60% of the preformed cysts were able to undergo morphogenic changes, an extensive branching tubulogenic response, where the individual tubule length was at least five times its width, was observed with 20% of the cysts. Thus, while wild-type Gab1 rescued tubulogenesis in all 10 cell lines tested, expression of the Gab1ΔPI3K mutant rescued to an intermediate level, suggesting that although the ability of Gab1 to associate with PI3K was not essential for the ability of Gab1 to rescue tubulogenesis, it was required for efficient rescue.
FIG. 5.
Gab1 mutant proteins associate with Met and are phosphorylated following Met activation. (A) 293T cells were transiently transfected with wild-type (WT) CSF-Met or the N1358H CSF-Met mutant, together with wild-type Gab1, Gab1ΔPI3K, or Gab1ΔPH. Lysates were subjected to immunoprecipitation (ip) with anti-HA, and proteins were resolved by SDS-PAGE (8% polyacrylamide), transferred to a nitrocellulose membrane, and blotted with anti-Met. The blot was stripped and reprobed with anti-HA. (B) MDCK cells expressing N1358H CSF-Met and either Gab1ΔPI3K or Gab1ΔPH were stimulated with 2 μg of CSF-1 per ml for 15 min at 37°C. Lysates were subjected to immunoprecipitation with anti-HA and blotting with anti-PY. The blots were stripped and reprobed with anti-p85 and then with anti-SHP2. A parallel blot was probed with anti-HA. (C) MDCK cells expressing Gab1ΔPH protein were stimulated for the indicated time, and lysates were subjected to immunoprecipitation with anti-HA. Proteins were resolved by SDS-PAGE and, following transfer to nitrocellulose, were blotted with either anti-PY or anti-HA as indicated.
FIG. 4.
The PH domain of Gab1 is essential for Met-mediated branching tubulogenesis. (A) MDCK cells expressing the N1358H CSF-Met mutant protein [N1358H(17)] were transfected with plasmids encoding for Gab1 ΔPI3K (ΔPI3K, clones 6 and 1). Two independent N1358H CSF-Met mutant expressing lines [N1358H(17) and N1358H(1)] were transfected with Gab1 ΔPH-encoding plasmids [ΔPH(1) clones 8 and 6, ΔPH(17) clones 1 and 2]. Lysates were subjected to immunoprecipitation (ip) and blotting with anti-HA. (B) The tubulogenic response was quantitated in cells expressing Gab1ΔPI3K and ΔPH, and results from representative clones are plotted as the percentage of cysts that have formed branching tubules in response to HGF or CSF-1. Solid bars represent results from cysts that have undergone a complete tubulogenic response; i.e., the tubule length is at least five times the size of the width; the hatched bar represents a partial response. (C) Cells expressing Gab1 mutants were plated in a collagen matrix and allowed to form cysts for 5 days. rh-CSF or HGF (5 U/ml) was added, and 14 days later branching tubules were visualized by light microscopy at a magnification of ×10. Representative lines are shown.
PH domains are found in many proteins with a broad spectrum of activities (reviewed in references 20, 22, and 34). Despite differences in the amino acid sequences of various PH domains, they adopt a common fold composed of seven β sheets and one α helix (34, 50). Evidence for in vitro binding to membrane phospholipids has been presented for a number of PH domains (17–19, 22, 35, 50, 56). This interaction has provided a possible mechanism through which these proteins could be recruited to the membrane and/or activated. To study the role of the PH domain in Gab1, a truncated Gab1 protein that lacks this domain (Gab1ΔPH) was assayed for its ability to rescue branching tubulogenesis in cell lines expressing the CSF-Met N1358H Grb2-mutant receptor. Two independent CSF-Met N1358H Grb2 mutant receptor-expressing cell lines, N1358H(1) and N1358H(17) were transfected with the Gab1ΔPH mutant protein. As shown in Fig. 4A, stable cell lines expressed the Gab1ΔPH protein to high levels. Although some change in the morphology of the cysts was occasionally observed, in all 10 independent clones derived from each of the two CSF-Met N1358H Grb2-mutant cell lines, the Gab1ΔPH mutant was unable to promote branching tubulogenesis following stimulation of CSF-Met (representative clones, Gab1ΔPH-8 and Gab1ΔPH-1, are shown in Fig. 4B and C). Furthermore, expression of Gab1ΔPH did not interfere with the intrinsic ability of these cells to form branching tubules, since stimulation of the endogenous, wild-type Met receptor with HGF induced branching tubules (Fig. 4B and C). This suggested that overexpression of the Gab1ΔPH protein did not inhibit the tubulogenic response. Importantly, these results indicate that Gab1 requires its PH domain to rescue branching tubulogenesis downstream from the Met receptor in MDCK cells.
Since the Gab1 mutant that lacks the PH domain failed to rescue tubulogenesis in cell lines expressing the N1358H CSF-Met mutant, we determined whether this reflected the inability of the Gab1ΔPH mutant protein to be phosphorylated by and/or be recruited to the CSF-Met receptor. The ability of Gab1 to coimmunoprecipitate with CSF-Met or the CSF-N1358H Met mutant was investigated following transient-transfection assays in 293T cells. Wild-type CSF-Met coimmunoprecipitated with the Gab1ΔPH and Gab1ΔPI3K mutant proteins as efficiently as with wild-type Gab1 (Fig. 5A). The N1358H CSF-Met receptor mutant, as described previously, associated less efficiently with Gab1 than did wild-type CSF-Met (44). Importantly, both the Gab1ΔPI3K and Gab1ΔPH mutants were comparable to wild-type Gab1 in their efficiency to coimmunoprecipitate with N1358H CSF-Met (Fig. 5A). Stripping of the blots and reblotting with anti-HA showed that similar levels of Gab1 were expressed in the different experimental groups (Fig. 5B).
To test the ability of the Gab1ΔPH mutant protein to be phosphorylated, the CSF-Met N1358H cell lines expressing the Gab1ΔPH mutant protein (Gab1ΔPH-8 and Gab1ΔPH-1 [Fig. 5B]) were stimulated with CSF-1 and the extent of tyrosine phosphorylation of HA-Gab1ΔPH was determined by Western blotting with anti-PY. Activation of CSF-Met resulted in an increase in the level of phosphorylation of Gab1ΔPH protein, and this increase was comparable to that observed for the Gab1ΔPI3K mutant (Fig. 5B). Further, the phosphorylation kinetics of Gab1ΔPH following Met activation (Fig. 5C) paralleled that observed for wild-type Gab1 (Fig. 3B). Phosphorylation of Gab1ΔPH was observed within 1 min of stimulation with HGF, and was maintained for 60 min, reaching baseline 2 h poststimulation (Fig. 5C and 3B). Moreover, as expected from its phosphorylation, Gab1ΔPH coimmunoprecipitated with the p85 subunit of PI3K and SHP2 (Fig. 5B). Taken together, these results indicate that the association and the phosphorylation of Gab1 with the Met receptor was not dependent on the Gab1 PH domain. Further, the Gab1 PH domain is not required for its ability to associate with signaling proteins (p85 and SHP2) following activation of the Met receptor.
Localization of Gab1 to sites of cell-cell contact requires its PH domain.
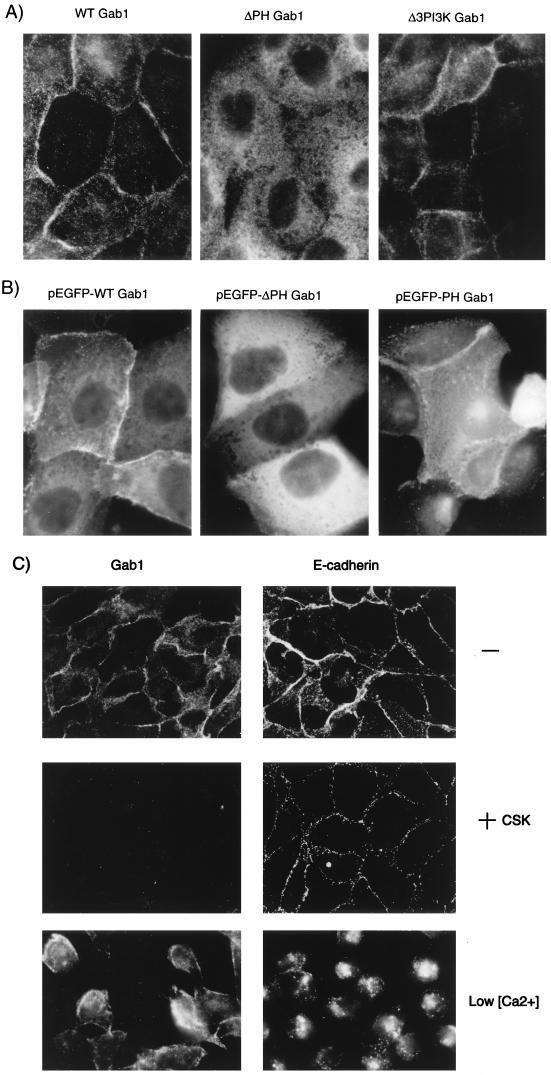
PH domains have been implicated in the recruitment of multiple proteins to the plasma membrane (10, 40, 46, 68). Gab1 was previously shown to localize to sites of cell-cell contact in epithelial cells (69). To investigate the cellular localization of Gab1 and Gab1 mutants, MDCK cells expressing wild-type Gab1, or mutants thereof, were grown on glass coverslips and subjected to indirect immunofluorescence with anti-HA. Fluorescence microscopy revealed that in tight colonies of MDCK cells, the majority of Gab1 was localized at the cell periphery, more specifically at sites of cell-cell contacts, although some Gab1 protein was detected in the cytoplasm (Fig. 6A). This is in accord with the results of Weidner et al. (69). Importantly, the Gab1ΔPH mutant did not localize to areas of cell-cell contact but instead localized in the cytoplasm (Fig. 6A). In contrast, the Gab1ΔPI3K mutant protein localized at sites of cell-cell contact in a similar manner to wild-type Gab1 protein (Fig. 6A).
FIG. 6.
The PH domain of Gab1 is required for the localization of Gab1 to sites of cell-cell contact. (A) MDCK cells stably transfected with wild-type Gab1, Gab1ΔPH, or Gab1ΔPI3K were grown on glass coverslips in DMEM containing 10% FBS. The cells were fixed in 2% paraformaldehyde and then labeled with anti-HA followed by CY3-conjugated anti-mouse antiserum. (B) Plasmids encoding pEGFP-Gab1, pEGFP-Gab1ΔPH, or pEGFP-Gab1PH were microinjected into nuclei of MDCK cells grown in DMEM plus 10% FBS. At 2 h following the microinjections, cells were visualized. (C) MDCK cells overexpressing Gab1 were either fixed in 2% paraformaldehyde (−), treated for 10 min in CSK buffer prior to fixation (+CSK), or treated for 2 h in 5 mM EGTA-containing media (Low [Ca2+]). The cells were subsequently labeled with anti-HA or anti-E-cadherin as indicated on the figure. Photographs were taken at a magnification of ×100.
The PH domains of SOS, the guanine nucleotide exchange factor for Ras, and PLCγ are sufficient for membrane targeting (7, 10), whereas deletion of the PH domain from PLCδ1 resulted in the localization of this protein to the cytoplasm (46). To determine if the Gab1PH domain is sufficient for subcellular localization, we have generated vectors expressing the pEGFP-Gab1PH domain, pEGFP-Gab1ΔPH, and pEGFP-Gab1ΔPI3K fusion proteins. Microinjection of these vectors into the nuclei of MDCK cells in colonies indicated that the PH domain of Gab1 was sufficient for localization to cell-cell contact sites (Fig. 6B). Taken together, these results indicate that the PH domain of Gab1 directs the subcellular localization of this protein to the cell periphery.
The localization of Gab1 to areas of cell-cell contact was similar to that of cell-cell adhesion proteins such as E-cadherin. However, unlike E-cadherin, Gab1 protein at the MDCK cell periphery was localized in a compartment that is Triton X-100 soluble. Treatment of cell lines expressing wild-type Gab1 prior to fixation with CSK buffer, which solubilizes proteins not stably associated with the cytoskeleton, revealed that E-cadherin remained insoluble and associated with the cytoskeleton whereas Gab1 was not stably associated with the cytoskeleton or a detergent insoluble compartment (Fig. 6C, +CSK). Furthermore, when cells were grown in media containing low Ca2+ concentrations, a condition that causes cell dissociation (45), E-cadherin relocalized to the cytoplasm whereas the membrane-proximal localization of Gab1 was not significantly altered, suggesting that at low extracellular Ca2+ concentrations, Gab1 was not coupled to E-cadherin (Fig. 6C, low [Ca2+]).
Localization of Gab1 to sites of cell-cell contacts requires PI3K activity.
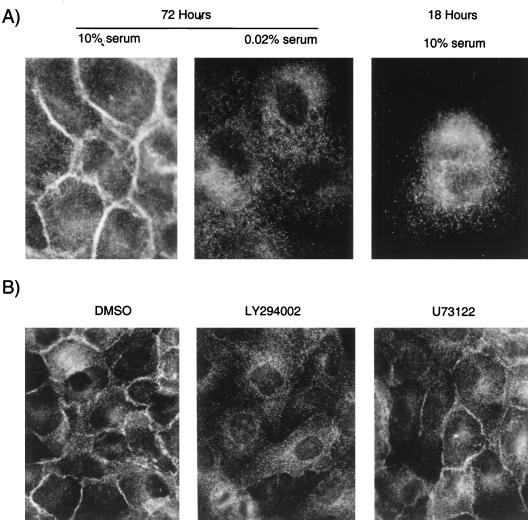
Increasing evidence suggests that PH domains may bind membrane phospholipids in vitro (17–19, 22, 35, 50, 56). This is supported by the crystal structure of several PH domains (12, 28, 78) and provides a molecular basis through which PH domain-containing proteins could be targeted to membranes. The recruitment of the PH domain of the Ras exchange factor SOS to the cell periphery is regulated by serum (7). Since Gab1 was expressed at the periphery when cells were grown in 10% serum, we determined whether its localization was serum dependent. MDCK cell colonies plated on glass coverslips for 48 h were starved in 0.02% FBS for 24 h and subsequently subjected to indirect immunofluorescence followed by microscopy. While Gab1 was located in a membrane-proximal compartment in the presence of 10% serum, serum depletion resulted in redistribution of Gab1 to the cytoplasm (Fig. 7A). Moreover, in cells cultured for 18 h, where cells remained as single cells or small colonies, Gab1 was localized in the cytoplasm even in the presence of high serum concentrations (Fig. 7A).
FIG. 7.
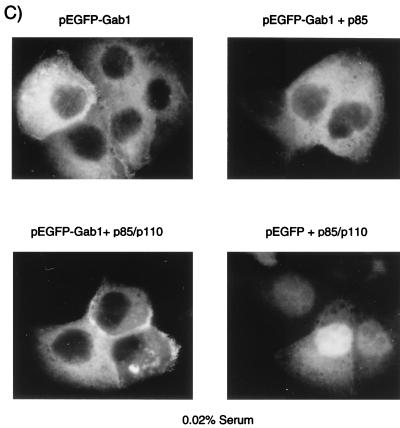
The localization of Gab1 is serum and PI3K dependent. (A) MDCK cells (104) stably expressing HA-Gab1 were grown in DMEM plus 10% FBS for 72 or 18 h as indicated. For serum starvation experiments, cells were first grown for 48 h in 10% FBS and then transferred for 24 h to medium containing 0.02% FBS. The cells were fixed in 2% paraformaldehyde, and the localization of Gab1 was determined following indirect immunofluorescence labeling with anti-HA followed by CY3-conjugated anti-mouse antiserum. (B) HA-Gab1-expressing MDCK cells were treated for 2 h at 37°C either with 50 μM LY294002 or with 2 μM U73122. The cells were subsequently fixed in 2% paraformaldehyde and labeled with anti-HA followed by CY3 anti-mouse antiserum. (C) MDCK cells were serum starved for 24 h prior to microinjection with plasmids encoding the p85 and p110 subunits of PI3K, together with pEGFP-Gab1 or control pEGFP plasmid. Photographs were taken 4 h following the injections. DMSO, dimethyl sulfoxide.
Several PH domain-containing proteins can bind the products of PI3K or PLCγ (17–22, 34, 50, 56). Therefore, it is possible that serum-stimulated PI3K activity is required for Gab1 cellular localization. To test this possibility, we have used pharmacological inhibitors that inhibit PI3K or phospholipase activity and the generation of membrane phospholipids. Treatment of MDCK cells expressing wild-type Gab1 with the PI3K inhibitor LY294002 (67) for 2 h resulted in redistribution of Gab1 to the cytoplasm, whereas in cells treated with vehicle alone (dimethyl sulfoxide) Gab1 remained localized at cell-cell contact sites (Fig. 7B). In contrast, treatment of these cells with an inhibitor of phospholipases, U73122, had no detectable effect on Gab1 distribution (Fig. 7B). This demonstrates that either a lipid(s) generated by PI3K or another effector downstream from PI3K was responsible for Gab1 recruitment and stabilization at cell-cell junctions. To establish whether PI3K or its downstream effector(s) is involved in the recruitment of Gab1 to the membrane, vectors encoding the p110 or the p85 subunits of PI3K (31) were comicroinjected with pEGFP-Gab1 into nuclei of MDCK cells in established colonies. Overexpression of p85 and p110 has been shown to correlate with enhanced PI3K activity in the absence of stimulation (31). Under serum-starved conditions, pEGFP-Gab1 was expressed in the cytoplasm (Fig. 7C). Importantly, comicroinjection of pEGFP-Gab1 with p110 and p85 resulted in localization of a fraction of pEGFP-Gab1 to areas of cell-cell contact, supporting a role for PI3K in the recruitment of Gab1 to these sites (Fig. 7C, pEGFP-Gab1 + p85/p110).
Met-mediated recruitment of Gab1 to the membrane is not dependent on the Gab1 PH domain.
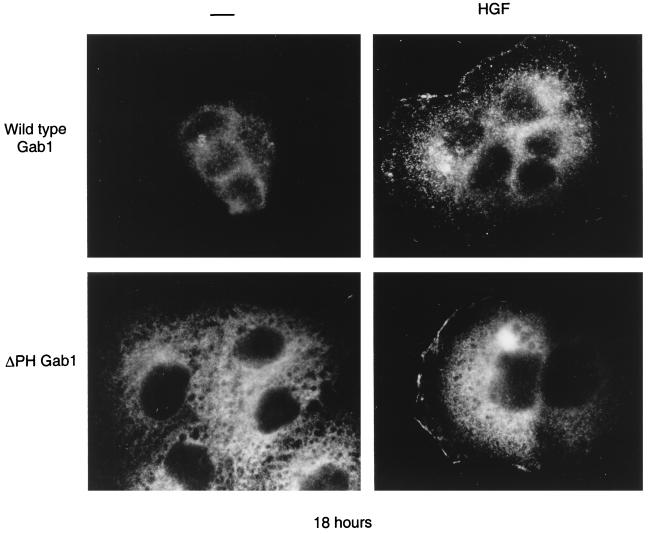
Activation of the Met receptor resulted in the association of Gab1 (directly or indirectly) with Met (Fig. 5); therefore, the prediction followed that Met activation recruits Gab1 to the vicinity of the cell membrane. To address if Gab1 is recruited to the cell periphery following Met activation, we have examined Gab1 localization following HGF stimulation of 18-h cultures of MDCK cells, a condition where Gab1 is localized in the cytoplasm (Fig. 7). Gab1 was translocated from the cytoplasm to a membrane-proximal localization within 15 min of stimulation with HGF. Moreover, the Gab1ΔPH mutant protein translocated to the membrane vicinity in a similar manner to wild-type Gab1 (Fig. 8). This was consistent with the ability of both wild-type Gab1 and the Gab1ΔPH mutant to associate with Met, as determined biochemically (Fig. 5B). Taken together, these results suggest that there are two mechanisms through which Gab1 could be recruited to the membrane; the first is dependent on the Gab1 PH domain and PI3K (Fig. 7), and the second is based on the recruitment of Gab1 to Met and is independent of the Gab1 PH domain (Fig. 8).
FIG. 8.
Gab1 is recruited to the membrane folowing Met activation. MDCK cells (104) expressing wild-type HA-Gab1 or HA-Gab1ΔPH mutant proteins were grown overnight in medium containing 10% FBS. They were then stimulated with 50 U of HGF per ml at 37°C for 15 min. Following fixation, the cells were labeled with anti-HA and photographs were taken at a magnification of ×100.
DISCUSSION
HGF stimulates a wide variety of cellular processes through activation of the Met receptor tyrosine kinase in epithelial cells. In some cells HGF is a mitogenic factor, whereas in others it stimulates cell dissociation and invasion and acts as an initiating signal for an intrinsic cellular morphogenic program. How the Met receptor orchestrates these distinct events was unclear. Here we show that Gab1 acts as a multisubstrate docking protein that is required for branching tubulogenesis in epithelial cells downstream from the Met receptor. From structure-function studies, we found that the Gab1 PH domain was essential for the subcellular localization of Gab1 to areas of cell-cell contact and for branching tubulogenesis.
Gab1 is the major phosphorylated protein in MDCK cells following activation of Met and acts to couple the Met receptor with the p85 subunit of PI3K and associated PI3K activity as well as with SHP2 and PLCγ1 (Fig. 1 and data not shown). Gab1 associates with similar substrates following stimulation of cells with EGF, insulin, nerve growth factor (NGF), and IL-3, indicating that Gab1 acts to target multiple receptors to common downstream signaling pathways (24, 25, 62). Gab1 is important for NGF-dependent PC12 cell survival (25), and in a similar manner, it functions as a critical signal transducer for branching tubulogenesis downstream from the Met receptor. Met receptor mutants that are impaired in their ability to associate with Gab1 fail to induce branching tubulogenesis in epithelial cells (16, 70, 79). Gab1 is recruited predominantly to the Met receptor in vivo to a Grb2 binding site at Y1356 (YVNV), at least in part through the Grb2 adapter protein (3, 14, 44), and to a lesser extent to Y1349 through a direct interaction (44, 69). Overexpression of Gab1 rescued the branching tubulogenesis defect of cells expressing mutant Met receptors with reduced Gab1 binding capacity (Y1356F and N1358H [Fig. 2B and C]) but not cells expressing mutant Met receptors that fail to bind Gab1 (Y1349/1356F [Fig. 2B and C]). This indicates that the recruitment and phosphorylation of Gab1 by Met is essential and that when Gab1 is overexpressed, Y1349 is sufficient for Gab1 recruitment and signaling (Fig. 2B and C and 5A and B).
These data support those of Weidner et al. that Gab1 is an important mediator of morphogenesis in epithelial cells (69). However, while these authors have demonstrated that overexpression of Gab1 promoted branching tubulogenesis in the absence of stimulation of the Met receptor, in our experience, overexpression of Gab1 in 40 independent cell lines examined was not sufficient to induce branching tubulogenesis in the absence of Met activation (Fig. 2). This discrepancy may simply reflect a difference in MDCK cell lines, expression levels of Gab1, or the criteria used to score positive tubulogenesis. In our assays, the ability to induce tubulogenesis was scored positive only when more than 50% of cystlike structures formed tubules (Fig. 2 and 4). Moreover, although Gab1 is phosphorylated downstream from the EGF receptor in MDCK cells (Fig. 3), overexpression of Gab1 did not promote branching tubulogenesis in response to EGF (Fig. 3). Thus, Gab1 phosphorylation per se is not sufficient to induce branching tubulogenesis in these cells.
The selectivity of the Gab1-dependent tubulogenic response downstream from Met and not the EGF receptor may suggest that a Met-specific substrate, in addition to Gab1, is required for branching tubulogenesis. Alternatively, we show that although the amplitude of phosphorylation of Gab1 is similar in response to HGF and EGF, HGF induces a prolonged tyrosine phosphorylation of Gab1 (60 min [Fig. 3B]) whereas Gab1 phosphorylation downstream from EGF is transient (15 min [Fig. 3B]). Similarly, a sustained activation of the MAPK pathway correlated with differentiation of MDCK cells in response to HGF (30) and of PC12 cells in response to NGF (38), whereas a transient activation was observed in either cell line in response to EGF and correlated with proliferation. Thus, the differentiation and tubulogenic responses in MDCK cells may require the sustained phosphorylation of Gab1 induced downstream of the Met receptor, indicating that the duration of Gab1 phosphorylation downstream from multiple extracellular signals may be an important factor in the Gab1 dependent response.
The ability of Gab1 to rescue branching tubulogenesis in cell lines expressing Met receptor mutants provided a means by which we could undertake a structure-function analysis of Gab1. An intact Gab1 PH domain, but not Gab1-associated PI3K activity, was essential for rescue of branching tubulogenesis downstream from Met receptor mutants (Fig. 4). The observation that PI3K activity was required for cell dissociation and branching tubulogenesis downstream from the Met receptor (8, 55), had suggested that Gab1-associated PI3K activity may be required for rescue of tubulogenesis. However, since the Gab1ΔPI3K mutant only partially rescued tubulogenesis (Fig. 4B and C), this may indicate a requirement for Gab1-associated PI3K activity for efficient rescue. Alternatively, the Met-associated or high basal cellular levels of PI3K observed in 5% serum used for these assays compensate for the lack of association of Gab1 with PI3K.
Gab1 is most homologous to the IRS-1 family of proteins in the PH domain (5, 25). The PH domain of IRS-1, in addition to its PTB domain, is required for efficient phosphorylation of IRS-1 by the insulin receptor (5, 76). Moreover, the Gab1 PH domain, but not those of spectrin, PLCγ, and βARK, can functionally substitute for the IRS-1 PH domain, suggesting a conserved modulatory function for the PH domains of IRS1 family proteins (5). The inability of the Gab1ΔPH mutant to rescue the tubulogenesis defect downstream from Met receptor mutants may reflect the possibility that the Gab1ΔPH mutant was not phosphorylated by the Met receptor. However, in MDCK cell lines, Met activation resulted in tyrosine phosphorylation of the Gab1ΔPH mutant, and this mutant associated with cellular substrates to a similar extent to wild-type Gab1 (Fig. 5). Thus, in agreement with previous data implicating Grb2 and the proline-rich Gab1 Met binding domain in recruitment of Gab1 to the Met receptor (Fig. 8) (44, 69), the Gab1 PH domain is not essential for phosphorylation of Gab1 by Met, suggesting a distinct role for the Gab1 PH domain in Met-regulated signal transduction.
An intact Gab1 PH domain was required for subcellular localization of Gab1 to areas of cell-cell contact in colonies of MDCK cells (Fig. 6). Increasing evidence supports a role for PH domains in the regulated targeting of proteins to cell membranes through their interactions with inositol phospholipids and/or additional interactions with proteins (17–19, 22, 28, 33, 35, 50, 56). The amino acids implicated in phospholipid binding are highly conserved among PH domains (12, 28, 36), including that of Gab1, supporting the possibility that the Gab1 PH domain also interacts with membrane phospholipids. Consistent with this, Gab1 is present at sites of cell-cell contact only in the presence of high serum concentrations (Fig. 7A). Moreover, the inhibition of PI3K by LY294002 resulted in the relocalization of Gab1 to the cytoplasm, even in the presence of high serum concentrations (Fig. 7B). The PH domains of the Rac exchange factor Tiam1, the ARF exchange factor family, and PLCγ bind preferentially to products of PI3K (phosphatidylinositol 3,4-bisphosphate or phosphatidylinositol 3,4,5-triphosphate [1, 61]), implicating PI3K-dependent phospholipids in the targeting of these proteins to the plasma membrane (10, 60, 66). Similarly, in in vitro binding studies, the Gab1 PH domain shows greatest affinity for phosphatidylinositol 3,4,5-triphosphate (PI3P) (25a), suggesting that the PI3K-dependent localization of Gab1 to sites of cell-cell contact involves the interaction of the Gab1 PH domain with PIP3 in the membrane. In support of this, overexpression of the p110 and p85 subunits of PI3K, which show elevated PI3K activity in the absence of stimulation (31), induces the translocation of Gab1 from the cytosol to the membrane in cells maintained at low serum concentrations (Fig. 7C).
The localization of Gab1 at sites of cell-cell contact requires extensive cell-cell interactions established after 3 days of culture, rather than initial cell-cell contacts observed after 18 h of culture (Fig. 7A). Unlike Tiam1, which is localized to E-cadherin-containing adherens junctions at sites of cell-cell contact in MDCK cells (26, 60), Gab1 is not associated with a detergent-insoluble compartment. Moreover, once localized to the proximity of the plasma membrane, the disruption of cell-cell interactions in media containing a low concentration of Ca2+ does not cause the redistribution of Gab1 to the cytoplasm as is the case for E-cadherin (Fig. 6C), suggesting that once recruited to the membrane, Gab1 can be retained at the membrane at high serum concentrations. However, it is possible that like Tiam1, the localization of Gab1 to areas of cell-cell contact requires both a lipid binding and protein-protein interaction, and further structure-function studies are required to distinguish these requirements (60).
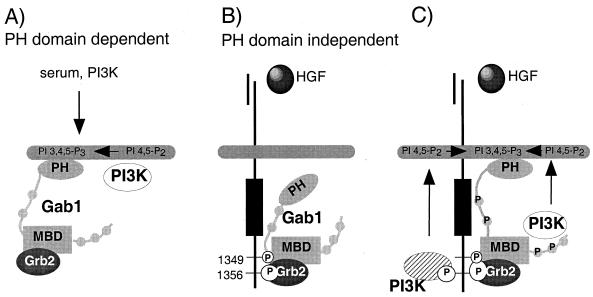
Our data indicate that Gab1 is recruited to the cell membrane by two distinct mechanisms. One, where Gab1 is localized to areas of cell-cell contact at high serum concentrations, is dependent on the formation of extensive cell-cell interactions in aged cultures, the Gab1 PH domain, and requires PI3K activity (Fig. 9A). The second, via recruitment of Gab1 to the Met receptor, is independent of the Gab1 PH domain (Fig. 8 and 9B) and PI3K activity (results not shown). However, recruitment of Gab1 to the Met receptor and its phosphorylation by the receptor are insufficient for tubulogenesis in the absence of the Gab1 PH domain. Therefore, while Met activation can result in Gab1 localization to the membrane, the Gab1 PH domain may act to stabilize its interaction with membrane bound phospholipids and enable Gab1 to potentiate and/or compartmentalize the signal downstream from Met (Fig. 9C), although we cannot rule out additional functions for the Gab1 PH domain. The elucidation of the mechanism(s) by which the Gab1 PH domain targets Gab1 to sites of cell-cell contact and the role of the PH domain in epithelial morphogenesis mediated by Gab1 will provide important insights into how these processes are normally regulated and how the organized epithelial architecture is altered in human cancers.
FIG. 9.
Gab1 is recruited to the cell surface by two distinct mechanisms. (A) One mechanism depends on the Gab1 PH domain and serum and requires PI3K activity. (B) The second mechanism is based on Met activation and is independent of the Gab1 PH domain (see Discussion for details). (C) Once recruited to and phosphorylated by Met, Met-associated or Gab1-associated PI3K activity is predicted to stabilize Gab1 association with the membrane.
ACKNOWLEDGMENTS
This research was supported by an operating grant from the National Cancer Institute of Canada with funds from the Canadian Cancer Society (to M.P.), an American Cancer Society Grant, and National Institutes of Health grants NS 34514 and CA69495 (to A.J.W.). Financial support was provided by the Medical Research Council as Fellowships (to C.R.M. and I.R.), a fellowship from the Ministerio de Educacion y Ciencia of Spain (to M.H.-M.), and the Royal Victoria Hospital Research Institute as a studentship (to T.M.F.). M.P. is a Scientist of the Medical Research Council of Canada.
We are grateful to Genetics Institute for recombinant CSF-1, T. Pawson for anti-p85, G. S. Feng for anti-SHP2, M. Pasdar for anti-E-cadherin, and members of the Park laboratory and A. Nepveu for helpful comments.
REFERENCES
- 1.Auger K R, Serunian L A, Soltoff S P, Libby P, Cantley L C. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell. 1989;57:167–175. doi: 10.1016/0092-8674(89)90182-7. [DOI] [PubMed] [Google Scholar]
- 2.Backer J M, Myers M J, Jr, Shoelson S E, Chin D J, Sun X J, Miralpeix M, Hu P, Margolis B, Skolnik E Y, Schlessinger J. Phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992;11:3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardelli A, Longati P, Gramaglia D, Stella M C, Comoglio P M. Gab1 coupling to the HGF/Met receptor multifunctional docking site requires binding of Grb2 and correlates with the transforming potential. Oncogene. 1997;15:3103–3111. doi: 10.1038/sj.onc.1201561. [DOI] [PubMed] [Google Scholar]
- 4.Bellusci S, Moens G, Gaudino G, Comoglio P, Nakamura T, Thiery J P, Jouanneau J. Creation of an hepatocyte growth factor/scatter factor autocrine loop in carcinoma cells induces invasive properties associated with increased tumorigenicity. Oncogene. 1994;9:1091–1099. [PubMed] [Google Scholar]
- 5.Burks D J, Pons S, Towery H, Smith-Hall J, Myers M G, Jr, Yenush L, White M F. Heterologous pleckstrin homology domains do not couple IRS-1 to the insulin receptor. J Biol Chem. 1997;272:27716–27721. doi: 10.1074/jbc.272.44.27716. [DOI] [PubMed] [Google Scholar]
- 6.Carpino N, Wisniewski D, Strife A, Marshak D, Kobayashi R, Stillman B, Clarkson B. p62dok: A constitutively tyrosine-phosphorylated, GAP-associated protein in chronic myelogenous leukemia progenitor cells. Cell. 1997;88:197–204. doi: 10.1016/s0092-8674(00)81840-1. [DOI] [PubMed] [Google Scholar]
- 7.Chen R H, Corbalan-Garcia S, Bar-Sagi D. The role of the PH domain in the signal-dependent membrane targeting of Sos. EMBO J. 1997;16:1351–1359. doi: 10.1093/emboj/16.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derman M P, Cunha M J, Barros E J, Nigam S K, Cantley L G. HGF-mediated chemotaxis and tubulogenesis require activation of the phosphatidylinositol 3-kinase. Am J Physiol. 1995;268:1211–1217. doi: 10.1152/ajprenal.1995.268.6.F1211. [DOI] [PubMed] [Google Scholar]
- 9.Ebens A, Brose K, Leonardo E D, Hanson M G Jr, Bladt F, Birchmeier C, Barres B A, Tessier-Lavigne M. Hepatocyte growth factor/scatter factor is an axonal chemoattractant and a neurotrophic factor for spinal motor neurons. Neuron. 1996;17:1157–1172. doi: 10.1016/s0896-6273(00)80247-0. [DOI] [PubMed] [Google Scholar]
- 10.Falasca M, Logan S K, Lehto V P, Baccante G, Lemmon M A, Schlessinger J. Activation of phospholipase Cγ by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 1988;17:414–422. doi: 10.1093/emboj/17.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faletto D L, Kaplan D R, Halverson D O, Rosen E M, Vande Woude G F. Signal transduction in c-met mediated motogenesis. Hepatocyte growth factor: scatter factor. EXS. 1993;65:107–130. [PubMed] [Google Scholar]
- 12.Ferguson K M, Lemmon M A, Schlessinger J, Sigler P B. Structure of the high affinity complex of inositol triphosphate with a phospholipase C pleckstrin homology domain. Cell. 1995;83:1037–1046. doi: 10.1016/0092-8674(95)90219-8. [DOI] [PubMed] [Google Scholar]
- 13.Fixman E D, Fournier T M, Kamikura D M, Naujokas M A, Park M. Pathways downstream of Shc and Grb2 are required for cell transformation by the Tpr-Met oncoprotein. J Biol Chem. 1996;271:13116–13122. doi: 10.1074/jbc.271.22.13116. [DOI] [PubMed] [Google Scholar]
- 14.Fixman E D, Holgado-Madruga M, Nguyen L, Kamikura D M, Fournier T M, Wong A J, Park M. Efficient cellular transformation by the Met oncoprotein requires a functional Grb2 binding site and correlates with phosphorylation of the Grb2-associated proteins Cbl and Gab1. J Biol Chem. 1997;272:20167–20172. doi: 10.1074/jbc.272.32.20167. [DOI] [PubMed] [Google Scholar]
- 15.Fixman E D, Naujokas M A, Rodrigues G A, Moran M F, Park M. Efficient cell transformation by the Tpr-Met oncoprotein is dependent upon tyrosine 489 in the carboxy-terminus. Oncogene. 1995;10:237–249. [PubMed] [Google Scholar]
- 15a.Fixman, E. D., I. Royal, and M. Park. Unpublished observations.
- 16.Fournier T, Kamikura D, Teng K, Park M. Branching tubulogenesis, but not scatter of Madin-Darby canine kidney cells requires a functional Grb2 binding site in the Met receptor tyrosine kinase. J Biol Chem. 1996;271:22211–22217. doi: 10.1074/jbc.271.36.22211. [DOI] [PubMed] [Google Scholar]
- 17.Franke T F, Kaplan D R, Cantley L C, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 18.Frech M, Andjelkovic M, Ingley E, Reddy K K, Falck J R, Hemmings B A. High affinity binding of inositol phosphates and phosphoinositides to the pleckstrin homology domain of RAC/protein kinase B and their influence on kinase activity. J Biol Chem. 1997;272:84474–84481. doi: 10.1074/jbc.272.13.8474. [DOI] [PubMed] [Google Scholar]
- 19.Garcia P, Gupta R, Shah S, Morris A J, Rudge S A, Scarlata S, Petrova V, McLaughlin S, Rebecchi M J. The pleckstrin homology domain of phospholipase C-δ1 binds with high affinity to phosphatidylinositol 4,5-biphosphate in bilayer membranes. Biochemistry. 1995;34:16228–16234. doi: 10.1021/bi00049a039. [DOI] [PubMed] [Google Scholar]
- 20.Gibson T J, Hyvönen M, Musacchio A, Saraste M, Birney E. PH domain: the first anniversary. Trends Biochem Sci. 1994;19:349–353. doi: 10.1016/0968-0004(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 21.Grant D S, Kleinman H K, Goldberg I D, Bhargava M M, Nickoloff B J, Kinsella J L, Polverini P, Rosen E M. Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci USA. 1993;90:1937–1941. doi: 10.1073/pnas.90.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harlan J E, Hajduk P J, Yoon H S, Fesik S W. Pleckstrin homology domains bind to phosphatidylinositol 4,5-bisphosphate. Nature. 1994;371:168–170. doi: 10.1038/371168a0. [DOI] [PubMed] [Google Scholar]
- 23.Herbst R, Caroll P M, Allard J D, Schilling J, Raabe T, Simon M A. Daughter of sevenless is a substrate of the phosphotyrosine phosphatase Corkscrew and functions during sevenless signaling. Cell. 1996;85:899–909. doi: 10.1016/s0092-8674(00)81273-8. [DOI] [PubMed] [Google Scholar]
- 24.Holgado-Madruga M, Emlet D R, Moscatello D K, Godwin A K, Wong A J. A Grb2-associated docking protein in EGF- and insulin-receptor signaling. Nature. 1996;379:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- 25.Holgado-Madruga M, Moscatello D K, Emlet D R, Dieterich R, Wong A J. Grb2-associated binder-1 mediates phosphatidylinositol 3-kinase activation and the promotion of cell survival by nerve growth factor. Proc Natl Acad Sci USA. 1997;94:12419–12424. doi: 10.1073/pnas.94.23.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25a.Holgado-Madruga, M., and A. J. Wong. Submitted for publication.
- 26.Hordijk P L, ten Klooster J P, van der Kammen R A, Michiels F, Oomen L C, Collard J G. Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science. 1997;278:1464–1466. doi: 10.1126/science.278.5342.1464. [DOI] [PubMed] [Google Scholar]
- 27.Hu Q, Klippel A, Muslin A J, Fantl W J, Williams L T. Ras-dependent induction of cellular responses by constitutively active phosphatidylinositol-3 kinase. Science. 1995;268:100–102. doi: 10.1126/science.7701328. [DOI] [PubMed] [Google Scholar]
- 28.Hyvönen M, Macias M J, Nilges M, Oschkinat H, Saraste M, Wilmanns M. Structure of the binding site for inositol phosphates in a PH domain. EMBO J. 1995;14:4676–4685. doi: 10.1002/j.1460-2075.1995.tb00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kan M, Zhang G, Zarnegar R, Michalopoulos G, Myoken Y, McKeehan W L, Stevens J I. Hepatocyte growth factor/hepatopoietin A stimulates the growth of rat kidney proximal tubule epithelial cells (RPTE), rat nonparenchymal liver cells, human melanoma cells, mouse keratinocytes and stimulates anchorage-independent growth of SV-40 transformed RPTE. Biochem Biophys Res Commun. 1991;174:331–337. doi: 10.1016/0006-291x(91)90524-b. [DOI] [PubMed] [Google Scholar]
- 30.Khwaja A, Lehmann K, Marte B M, Downward J. Phosphoinositide 3-kinase induces scattering and tubulogenesis in epithelial cells through a novel pathway. J Biol Chem. 1998;273:18793–18801. doi: 10.1074/jbc.273.30.18793. [DOI] [PubMed] [Google Scholar]
- 31.Klippel A, Escobedo J A, Hirano M, Williams L T. The interaction of small domains between the subunits of phosphatidylinositol 3-kinase determines enzyme activity. Mol Cell Biol. 1994;14:2675–2685. doi: 10.1128/mcb.14.4.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komada M, Kitamura N. The cell dissociation and motility triggered by scatter factor/hepatocyte growth factor are mediated through the cytoplasmic domain of the c-Met receptor. Oncogene. 1993;8:2381–2390. [PubMed] [Google Scholar]
- 33.Konishi H, Kuroda S, Kikkawa U. The pleckstrin homology domain of RAC protein kinase associates with the regulatory domain of protein kinase Cζ. Biochem Biophys Res Commun. 1994;205:1770–1775. doi: 10.1006/bbrc.1994.2874. [DOI] [PubMed] [Google Scholar]
- 34.Lemmon M A, Falasca M, Ferguson K M, Schlessinger J. Regulatory recruitment of signaling molecules to the cell membrane by pleckstrin homology domains. Trends Cell Biol. 1997;7:237–242. doi: 10.1016/S0962-8924(97)01065-9. [DOI] [PubMed] [Google Scholar]
- 35.Lemmon M A, Ferguson K M, O’Brien R, Sigler P B, Schlessinger J. Specific and high-affinity binding of inositol phosphates to an isolated pleckstrin homology domain. Proc Natl Acad Sci USA. 1995;92:10472–10476. doi: 10.1073/pnas.92.23.10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lemmon M A, Ferguson K M, Schlessinger J. PH domains: diverse sequences with a common fold recruit signaling molecules to the cell surface. Cell. 1996;85:621–624. doi: 10.1016/s0092-8674(00)81022-3. [DOI] [PubMed] [Google Scholar]
- 37.Maina F, Hilton M C, Ponzetto C, Davies A M, Klein R. Met receptor signaling is required for sensory nerve development and HGF promotes axonal growth and survival of sensory neurones. Genes Dev. 1997;11:3341–3350. doi: 10.1101/gad.11.24.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marshall C J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto K, Nakamura T S. Roles of HGF as a pleiotropic factor in organ regeneration. EXS. 1993;65:225–249. [PubMed] [Google Scholar]
- 40.Michiels F, Stam J C, Hordijk P L, van der Kammen R A, Ruuls-vanStalle L, Feltkamp C A, Collard J G. Regulated membrane localization of Tiam1, mediated by the NH2-terminal pleckstrin homology domain, is required for Rac-dependent membrane ruffling and C-Jun NH2-terminal kinase activation. J Cell Biol. 1997;137:387–398. doi: 10.1083/jcb.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montesano R, Schaller G, Orci L. Induction of epithelial tubular morphogenesis in vitro by fibroblast-derived soluble factors. Cell. 1991;66:697–711. doi: 10.1016/0092-8674(91)90115-f. [DOI] [PubMed] [Google Scholar]
- 42.Myers M G, Jr, Backer J M, Sun X J, Shoelson S, Hu P, Schlessinger J, Yoakim M, Schaffhausen B, White M F. IRS-1 activates phosphatidylinositol 3′-kinase by associating with src homology domains of p85. Proc Natl Acad Sci USA. 1992;89:10350–10354. doi: 10.1073/pnas.89.21.10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakamura T. Structure and function of hepatocyte growth factor. Prog Growth Factor Res. 1991;3:67–85. doi: 10.1016/0955-2235(91)90014-u. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen L, Holgado-Madruga M, Maroun C, Fixman E D, Kamikura D, Fournier T, Charest A, Tremblay M L, Wong A J, Park M. Association of the multisubstrate docking protein Gab1 with the hepatocyte growth factor receptor requires a functional Grb2 binding site involving tyrosine 1356. J Biol Chem. 1997;272:20811–20819. doi: 10.1074/jbc.272.33.20811. [DOI] [PubMed] [Google Scholar]
- 45.Pasdar M, Nelson W J. Regulation of desmosome assembly in epithelial cells: kinetics of synthesis, transport, and stabilization of desmoglein I, a major protein of the membrane core domain. J Cell Biol. 1989;109:163–177. doi: 10.1083/jcb.109.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paterson H F, Savopoulos J W, Perisic O, Cheung R, Ellis M V, Williams R L, Katan M. Phospholipase C δ1 requires a pleckstrin homology domain for interaction with the plasma membrane. Biochem J. 1995;312:661–666. doi: 10.1042/bj3120661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pawson T. Protein modules and signaling networks. Nature. 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- 48.Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla Zonca P, Giordano S, Graziani A, Panayotou G, Comoglio P M. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77:261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 49.Raabe T, Riesgo-Escovar J, Liu X, Bausenwein B S, Deak P, Maroy P, Hafen E. DOS, a novel pleckstrin homology domain-containing protein required for signal transduction between sevenless and RAS1 in Drosophila. Cell. 1996;85:911–920. doi: 10.1016/s0092-8674(00)81274-x. [DOI] [PubMed] [Google Scholar]
- 50.Rameh L E, Arvidsson A-K, Carraway III K L, Couvillon A D, Rathbun G, Crompton A, VanRenterghem B, Czech M P, Ravichandran K S, Burakoff S J, Wang D S, Chen C S, Cantley L C. A comparative analysis of the phosphoinositide binding specificity of pleckstrin homology domains. J Biol Chem. 1997;272:22059–22066. doi: 10.1074/jbc.272.35.22059. [DOI] [PubMed] [Google Scholar]
- 51.Rodrigues G A, Naujokas M A, Park M. Alternative splicing generates isoforms of the met receptor tyrosine kinase which undergo differential processing. Mol Cell Biol. 1991;11:2962–2970. doi: 10.1128/mcb.11.6.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodrigues G A, Park M. Autophosphorylation modulates the kinase activity and oncogenic potential of the Met receptor tyrosine kinase. Oncogene. 1994;9:2019–2027. [PubMed] [Google Scholar]
- 53.Rong S, Bodescot M, Blair D, Dunn J, Nakamura T, Mizuno K, Park M, Chan A, Aaronson S, Vande Woude G F. Tumorigenicity of the met proto-oncogene and the gene for hepatocyte growth factor. Mol Cell Biol. 1992;12:5152–5158. doi: 10.1128/mcb.12.11.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ronsin C, Muscatelli F, Mattei M G, Breathnach R. A novel putative receptor protein tyrosine kinase of the met family. Oncogene. 1993;8:1195–1202. [PubMed] [Google Scholar]
- 55.Royal I, Park M. Hepatocyte growth factor induced scatter of Madin-Darby canine kidney cells requires phosphatidylinositol 3-kinase. J Biol Chem. 1995;270:27780–27787. doi: 10.1074/jbc.270.46.27780. [DOI] [PubMed] [Google Scholar]
- 56.Salim K, Bottomly M J, Querfurth E, Zvelebil M J, Gout I, Scaife R, Margolis R L, Gigg R, Smith C I, Driscoll P C, Waterfield M D, Panayotou G. Distinct specificity in the recognition of phosphoinositides by the pleckstrin homology domains of dynamin and Bruton’s tyrosine kinase. EMBO J. 1996;15:6241–6250. [PMC free article] [PubMed] [Google Scholar]
- 57.Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 58.Schmidt L, Duh F M, Chen F, Kishida T, Glenn G, Choyke P, Scherer S W, Zhuang Z, Lubensky I, Dean M, Allikmets R, Chidambaram A, Bergerheim U R, Feltis J T, Casadevall C, Zamarron A, Bernues M, Richard S, Lips C J, Walther M M, Tsui L C, Geil L, Orcutt M L, Stackhouse T, Lipan J, Slife L, Brauch H, Decker J, Niehans G, Hughson M D, Moch H, Storkel S, Lerman M I, Linehan W M, Zbar B. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet. 1997;16:68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- 59.Soriano J V, Pepper M S, Nakamura T, Orci L, Montesano R. Hepatocyte growth factor stimulates extensive development of branching duct-like structures by cloned mammary gland epithelial cells. J Cell Sci. 1995;108:413–430. doi: 10.1242/jcs.108.2.413. [DOI] [PubMed] [Google Scholar]
- 60.Stam J C, Sander E E, Michiels F, van Leeuwen F N, Kain H E, van der Kammen R A, Collard J G. Targeting Tiam1 to the plasma membrane requires the cooperative function of the N-terminal pleckstrin homology domain and an adjacent protein interaction domain. J Biol Chem. 1997;272:28447–28454. doi: 10.1074/jbc.272.45.28447. [DOI] [PubMed] [Google Scholar]
- 61.Stephens L R, Hughes K T, Irvine R F. Pathway of phophatidylinositol (3,4,5)-trisphosphate synthesis in activated neutrophils. Nature. 1991;351:33–39. doi: 10.1038/351033a0. [DOI] [PubMed] [Google Scholar]
- 62.Takahashi-Tezuka M, Yoshida Y, Fukada T, Ohtany T, Yamanaka Y, Nishida K, Hibi M, Hirano T. Gab1 acts as an adapter molecule linking the cytokine receptor gp130 to ERK mitogen-activated protein kinase. Mol Cell Biol. 1998;18:4109–4117. doi: 10.1128/mcb.18.7.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell-cell adhesion by Rac and Rho small G proteins in MDCK cells. J Biol Chem. 1997;139:1047–1059. doi: 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takayama H, LaRochelle W J, Sharp R, Otsuka T, Kriebel P, Anver M, Aaronson S A, Merlino G. Diverse tumorigenesis associated with aberrant development in mice overexpressing hepatocyte growth factor/scatter factor. Proc Natl Acad Sci USA. 1997;94:701–706. doi: 10.1073/pnas.94.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702–705. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- 66.Venkateswarlu K, Oaty P B, Tavaré J M, Cullen P J. Insulin-dependent translocation of ARNO to the plasma membrane of adipocytes requires phosphatidylinositol 3-kinase. Curr Biol. 1998;8:463–466. doi: 10.1016/s0960-9822(98)70181-2. [DOI] [PubMed] [Google Scholar]
- 67.Vlahos C J, Matter W F, Hui K Y, Brown R F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 68.Wang D-S, Miller R, Shaw R, Shaw G. The pleckstrin homology domain of human ßIΣII spectrin is targeted to the plasma membrane in vivo. Biochem Biophys Res Commun. 1996;225:420–426. doi: 10.1006/bbrc.1996.1189. [DOI] [PubMed] [Google Scholar]
- 69.Weidner K M, Di Cesare S, Sachs M, Brinkmann V, Behrens J, Birchmeier W. Interaction between Gab1 and c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature. 1996;384:173–176. doi: 10.1038/384173a0. [DOI] [PubMed] [Google Scholar]
- 70.Weidner K M, Sachs M, Birchmeier W. The Met receptor tyrosine kinase transduces motility, proliferation, and morphogenic signals of scatter factor/hepatocyte growth factor in epithelial cells. J Cell Biol. 1993;121:145–154. doi: 10.1083/jcb.121.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weidner K M, Sachs M, Riethmacher D, Birchmeier W. Mutation of juxtamembrane tyrosine residue 1001 suppresses loss-of-function mutations of the met receptor in epithelial cells. Proc Natl Acad Sci USA. 1995;92:2597–2601. doi: 10.1073/pnas.92.7.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wigler M, Pellicer A, Silverstein S, Axel R, Urlaub G, Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci USA. 1979;76:1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamanashi Y, Baltimore D. Identification of the Abl- and rasGAP-associated 62 kDa protein as a docking protein, Dok. Cell. 1997;88:205–211. doi: 10.1016/s0092-8674(00)81841-3. [DOI] [PubMed] [Google Scholar]
- 74.Yang X M, Park M. Expression of the hepatocyte growth factor/scatter factor receptor tyrosine kinase is localized to epithelia in the adult mouse. Lab Investig. 1995;73:483–491. [PubMed] [Google Scholar]
- 75.Yenush L, White M F. The IRS-signaling system during insulin and cytokine action. Bioessays. 1997;19:491–500. doi: 10.1002/bies.950190608. [DOI] [PubMed] [Google Scholar]
- 76.Yenush L, Makati K J, Smith-Hall J, Ishibashi O, Myers M G, Jr, White M F. The pleckstrin homology domain is the principle link between the insulin receptor and IRS-1. J Biol Chem. 1996;271:24300–24306. doi: 10.1074/jbc.271.39.24300. [DOI] [PubMed] [Google Scholar]
- 77.Zarnegar R, Michalopoulos G. Purification and biological characterization of human hepatopoietin A, a polypeptide growth factor for hepatocytes. Cancer Res. 1989;49:3314–3320. [PubMed] [Google Scholar]
- 78.Zheng J, Chen R-H, Corblan-Garcia S, Cahill S M, Bar-Sagi D, Cowburn D. The solution structure of the pleckstrin homology domain of human SOS1. J Biol Chem. 1997;272:30340–30344. doi: 10.1074/jbc.272.48.30340. [DOI] [PubMed] [Google Scholar]
- 79.Zhu H, Naujokas M A, Fixman E D, Torossian K, Park M. Tyrosine 1356 in the carboxyl-terminal tail of the HGF/SF receptor is essential for the transduction of signals for cell motility and morphogenesis. J Biol Chem. 1994;269:29943–29948. [PubMed] [Google Scholar]
- 80.Zhu H, Naujokas M A, Park M. Receptor chimeras indicate that the Met tyrosine kinase mediates the motility and morphogenic responses of hepatocyte growth/scatter factor. Cell Growth Differ. 1994;5:359–366. [PubMed] [Google Scholar]