Retinoid X Receptor (RXR) Agonist-Induced Activation of Dominant-Negative RXR-Retinoic Acid Receptor α403 Heterodimers Is Developmentally Regulated during Myeloid Differentiation (original) (raw)
Abstract
The multiple biologic activities of retinoic acid (RA) are mediated through RAR and retinoid X receptor (RXR) nuclear receptors that interact with specific DNA target sequences as heterodimers (RXR-RAR) or homodimers (RXR-RXR). RA receptor activation appears critical to regulating important aspects of hematopoiesis, since transducing a COOH-terminally truncated RARα exhibiting dominant-negative activity (RARα403) into normal mouse bone marrow generates hematopoietic growth factor-dependent cell lines frozen at the multipotent progenitor (EML) or committed promyelocyte (MPRO) stages. Nevertheless, relatively high, pharmacological concentrations of RA (1 to 10 μM) overcome these differentiation blocks and induce terminal granulocytic differentiation of the MPRO promyelocytes while potentiating interleukin-3 (IL-3)-induced commitment of EML cells to the granulocyte/monocyte lineage. In the present study, we utilized RXR- and RAR-specific agonists and antagonists to determine how RA overcomes the dominant-negative activity of the truncated RARα in these different myeloid developmental stages. Unexpectedly, we observed that an RXR-specific, rather than an RAR-specific, agonist induces terminal granulocytic differentiation of MPRO promyelocytes, and this differentiation is associated with activation of DNA response elements corresponding to RAR-RXR heterodimers rather than RXR-RXR homodimers. This RXR agonist activity is blocked by RAR-specific antagonists, suggesting extensive cross-talk between the partners of the RXR-RARα403 heterodimer. In contrast, in the more immature, multipotent EML cells we observed that this RXR-specific agonist is inactive either in potentiating IL-3-mediated commitment of EML cells to the granulocyte lineage or in transactivating RAR-RXR response elements. RA-triggered GALdbd-RARα hybrid activity in these cells indicates that the multipotent EML cells harbor substantial nuclear hormone receptor coactivator activity. However, the histone deacetylase (HDAC) inhibitor trichostatin A readily activates an RXR-RAR reporter construct in the multipotent EML cells but not in the committed MPRO promyelocytes, indicating that differences in HDAC-containing repressor complexes in these two closely related but distinct hematopoietic lineages might account for the differential activation of the RXR-RARα403 heterodimers that we observed at these different stages of myeloid development.
The biologic effects of retinoic acid (RA) are critical in regulating development and differentiation of diverse cell types. RA exerts these effects through specific nuclear receptors possessing discrete DNA-binding and RA (ligand)-binding domains. Two general families of RA receptors include the RARs and retinoid X receptors (RXRs), both containing at least three members designated α, β, and γ. The effects of RA are thought to be mediated through either RXR-RAR heterodimers or RXR-RXR homodimers that regulate gene transcription by interacting with specific response elements in their respective target gene promoters (30, 41).
Hematopoietic cells preferentially express RARα (12, 36), and we have previously observed that the RA-mediated granulocytic differentiation of HL-60 leukemia cells is directly mediated through RARα (9). Moreover, the central role that RARα plays in granulocytic differentiation is further indicated by the observation that RARα is involved in the 15;17 translocation that characterizes most cases of acute promyelocytic leukemia (APL) (2, 13, 29), a subtype of human leukemia that is uniquely sensitive to RA-induced granulocytic differentiation (4, 5, 26, 59). However, the dramatic response of APL cells to RA appears confined to this particular subtype of human leukemia, and most other forms of human myelogenous leukemia exhibit little if any response to retinoids (4, 42).
One important approach in defining the biologic role of RA receptors in controlling the differentiation of specific cell lineages involves the use of RA receptor constructs exhibiting dominant-negative activity. Truncating or introducing specific point mutations into the COOH-terminal end of RARα results in an altered RA receptor that inhibits the function of normal RA receptors (10, 11, 15, 45, 47). Such dominant-negative constructs inhibit RA activity in a number of different cell types, including cultured CV1 cells (11, 45), transgenic mouse epidermis (27, 48), multipotent embryonal carcinoma cells (10, 11), and mammary epithelial cells (50). The truncated receptors lack the COOH-terminal activation domain (AF2) while retaining the DNA-binding domain as well as the ability to heterodimerize with RXR (11, 15). It is likely that the truncated RA receptor acts as a dominant negative by competing with the normal RA receptors in the formation of biologically active RXR-RAR heterodimers. In our own studies, we have observed that introducing a mutated RARα harboring a 59-amino-acid truncation at the COOH terminus (designated RARα403) into normal mouse bone marrow generates hematopoietic growth factor-dependent cells frozen at distinct stages of myeloid differentiation (53, 54). These include granulocyte-macrophage colony-stimulating factor (GM-CSF)-dependent MPRO cells, which are frozen at the promyelocyte stage of granulocyte differentiation (53), and the more primitive SCF (stem cell factor or kit ligand)-dependent EML cells, which are multipotent and exhibit erythroid, lymphoid, and myeloid potential (54). Curiously, in these hematopoietic cell lines the effect of the dominant-negative RARα403 construct does not appear to be absolute but can be overcome with the addition of relatively high, pharmacological concentrations of RA. Thus, RA (1 to 10 μM) induces terminal granulocytic differentiation of the GM-CSF-dependent MPRO cells (53), while in the pluripotent, SCF-dependent EML cells, RA potentiates the interleukin-3 (IL-3)-mediated commitment of these cells to the granulocyte/monocyte lineage (54).
We initiated the present studies to determine the mechanism by which such relatively high concentrations of RA overcome the dominant-negative activity of the RARα403 construct in the MPRO and EML hematopoietic cell lines and presumably trigger activation of the aberrant RXR-RARα403 heterodimer. Our approach involved assessing the effect of different synthetic retinoids with specific activity as RXR or RAR agonists and/or RXR or RAR antagonists on both the terminal differentiation of the MPRO promyelocytes and the granulocyte lineage commitment of the pluripotent EML cells. Surprisingly, we observed that granulocytic differentiation of the MPRO promyelocytes is induced by RXR- rather than RAR-specific agonists, and transient-transfection studies indicated that this differentiation is associated with activation of RXR-RAR rather than RXR-RXR response elements. However, this potent effect of the RXR agonist on RXR-RAR activation appears to be developmentally regulated during myeloid differentiation, because we could detect little if any effect of the same RXR agonist in the multipotent EML cells, in which the developmental block is at a more immature progenitor stage. Moreover, utilizing histone deacetylase (HDAC) inhibitors we observed significant differences in HDAC repressor complexes between the multipotent EML cells and the committed MPRO promyelocytes that might account for the differential activation of RXR-RAR response elements in these two related but distinct hematopoietic lineages.
MATERIALS AND METHODS
Cell cultures.
MPRO cells were cultured in Dulbecco minimal essential medium (DMEM) (GIBCO, Grand Island, N.Y.) supplemented with 10% fetal calf serum and 1% l-glutamine. These cells are absolutely dependent upon GM-CSF (recombinant murine GM-CSF at 5 to 10 ng/ml) (Peprotech, Rocky Hill, N.J.). EML cells were cultured in Iscove modified Dulbecco medium (IMDM) supplemented with 2% horse serum (GIBCO) and 1% l-glutamine. These cells are absolutely dependent upon SCF (100 ng/ml) (Peprotech). A Friend murine erythroleukemia cell line (MEL) was cultured in DMEM supplemented with 10% fetal calf serum. These cells were stably transduced with a retroviral vector harboring human RARα (LRARαSN) as previously detailed (9). Cells were cultured in a 37°C incubator with 5% CO2–95% air.
Receptor-specific synthetic retinoids.
Synthetic retinoids that were specific for either RXR or RAR were screened by both direct ligand binding assays and transactivation assays. The ligand binding screen involved determining the ability of a given retinoid to competitively inhibit specific binding of all-trans [3H]RA ([3H]ATRA) or 9-_cis_-[3H]RA to baculovirus-expressed RARs and RXRs (3, 22). Receptor-specific agonists were identified by their ability to transactivate a luciferase reporter construct driven by either an RAR-RXR (TRE pal-Luc) (55) or an RXR-RXR (pTK-CRBPII-Luc) (40) selective element in CV1 cells. RAR and RXR selective antagonists were identified by their ability to inhibit ATRA-mediated transactivation of RAR-RXR response elements (28) or 9-_cis_-RA-mediated activation of RXR-RXR response elements (35). The compounds utilized in the present study were the RAR(α,β,γ) selective panagonist (AGN 193695), the RXR(α,β,γ) selective panagonist (AGN 194204) (58), the RAR antagonist (AGN 193109) (28), and the RXR antagonist (LGN100849) (51). These compounds were dissolved at 5 mM concentrations in dimethyl sulfoxide and stored at −70°C in small aliquots until use. Trichostatin A (TSA) was obtained from Wako Chemicals USA (Richmond, Va.).
Construction of GALdbd-RAR hybrid.
We synthesized two oligonucleotides, one corresponding to codons 135 to 142 of human RARα (17) (5′ ACGTGAATTCGTGACCCGGAACCGCTGCCAGTAC 3′) and the other corresponding to nucleotides 1601 to 1625 in the 3′ untranslated region of human RARα (5′ ACGTGAATTCTTTTTCCCCAGGGAAGGTCCCCAGTACTG-3′). _Eco_RI sites were included at the 5′ ends of both these oligonucleotides (underlined). With the retroviral vector construct LRARαSN (9) as the template, these oligonucleotides were used as primers to PCR amplify a 1.1-kb RARα fragment harboring codons 135 to 462 of RARα. This fragment was digested with _Eco_RI and then cloned in the in-frame sense direction into the _Eco_RI-digested GALdbd(1–147) expression vector, pSG424 (46).
Plasmids.
Two luciferase reporter plasmids based on the plasmid designated ΔMTV-Luc (23), which contains the MTV long terminal repeat in which the glucocorticoid response elements have been deleted and replaced with DR5 (RAR-RXR) and DR1 (RXR-RXR) response elements, were used. The DR5 Luc plasmid (A5-Luc) harbors the sequence AGCTTTCAGGTCACCAGGAGGTCAGAA (5-bp spacer underlined). The DR1 plasmid (MTV-DR-1-Luc) is identical, with the exception of the response element, which is one copy of the cytosolic retinol-binding protein II promoter sequence (40), AGCTTACAGGTCACAGGTCACAGGTCACAGTTCATTT (single-base spacer underlined). The growth hormone expression plasmid pCMVGH, which serves as an internal control for transfection efficiency, has been previously described (1). As the reporter for assessing activity of the GALdbd-RARα hybrid, we utilized the p(UAS)5- GL3 construct, which harbors five Gal4 binding sites (17-mers) cloned into the polylinker of the pGL3-promoter vector (Promega, Madison, Wis.).
RARα antibodies.
Anti-human RARα amino-terminal monoclonal antibodies were generated against peptides corresponding to amino acids 10 to 25 near the amino terminus of human RARα. This peptide contained a glycine-cysteine linker for conjugation to keyhole limpet hemocyanin (KLH-CG-10TPGGGHLNGYPVPPYA25). Mice were immunized with the conjugated peptide in Freund’s complete adjuvant and boosted with peptide in Freund’s incomplete adjuvant. Fusions were performed, and resulting hybridomas were cloned and screened for activity by enzyme-linked immunosorbent assay against free peptide and by Western blotting with recombinantly expressed human RARα. The appropriate clonal producers were expanded, and antibodies were purified from cell culture supernatant by protein G-Sepharose. A rabbit polyclonal immunoglobulin G generated against amino acids 443 to 462 at the COOH terminus of human RARα and supplied as TransCruz Gel Supershift reagent was purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.).
Assay of MPRO granulocytic differentiation.
MPRO cells were cultured in their standard growth medium harboring GM-CSF in the presence of different concentrations of retinoids. After 5 days of culture, differential counts were performed on Wright-Giemsa-stained cytospin preparations of 0.2-ml samples of the cell suspension (approximately 104 cells). By morphology, mature granulocytes harboring lobulated or doughnut-shaped nuclei and pale cytoplasm are readily distinguished from immature MPRO promyelocytes (53).
Assay of EML CFU-GM generation.
EML cells were cultured at 104/ml in growth medium supplemented with SCF or SCF plus IL-3 plus different concentrations of retinoid agonists and antagonists. All experiments involving CFU-GM generation were performed on EML cells between passage 10 and passage 20. The sources of IL-3 included either 5% WEHI 3B-conditioned medium or murine recombinant IL-3 (10 ng/ml) (Peprotech) with equivalent results obtained with either source of IL-3. Following 1 to 3 days of this liquid suspension culture, the cells were harvested and washed and 5 × 103 cells were resuspended in 0.7 ml of IMDM supplemented with 0.75 ml of 2.2% methylcellulose (Methocult; Stem Cell Technologies, Vancouver, British Columbia, Canada), 5% horse serum, and 10 ng of murine recombinant GM-CSF (Peprotech) per ml. Cultures were incubated in 12-well plates (0.7 ml/plate), and GM-CSF-dependent colonies (>20 cells) were counted following 5 to 7 days of incubation in a humidified incubator.
Derivation of MPRO-like cells from EML cells.
EML cells were cultured in their standard SCF-containing growth medium for 2 days in the presence of IL-3 (10 ng/ml) and ATRA (10 μM). The cells were then washed, resuspended in medium containing GM-CSF alone (10 ng/ml), and plated in 96-well microtiter dishes. After 3 to 4 days of culture, GM-CSF-dependent cell growth was readily detected, and these cells were subsequently expanded to generate the MPRO-like cell lines.
Transactivation assays.
Both EML cells and MPRO cells were transiently transfected by electroporation. For EML cells, 107 cells were harvested, washed twice with phosphate-buffered saline, and resuspended in 0.8 ml of IMDM supplemented with 2 to 5% horse serum, SCF, and 10 mM HEPES (pH 7.9). Forty micrograms of the DR5 or DR1 luciferase reporter construct together with 20 μg of pCMVGH plasmid (1) was added, and the solution was placed in 0.4-cm electroporation cuvettes, kept at room temperature for 10 to 15 min, and then electroporated at 950 μF, 270 V, with a Bio-Rad (Hercules, Calif.) Gene Pulser II electroporator. The identical transfection procedure was utilized for MPRO cells except that the electroporation medium was DMEM supplemented with 20% fetal calf serum, 10 mM HEPES, and GM-CSF, and electroporation parameters were 700 μF and 450 V. Following electroporation, the cuvettes were immediately placed at 4°C for 10 min, and the cells were then cultured overnight at approximately 106/ml in 10 ml of their respective growth media supplemented with different concentrations of RAR and RXR agonists and antagonists.
Reporter gene assays.
The electroporated cells were cultured for 18 to 24 h under standard conditions, the cells were pelleted, and 1 ml of supernatant medium was harvested and subjected to the human growth hormone assay with the Allegro growth hormone assay kit (Nichols Institute, Capistrano, Calif.). The cell pellet was lysed, and following centrifugation at 13,200 rpm for 20 s, the supernatant was harvested and assayed for luciferase activity with the Promega luciferase assay kit. The cell lysate supernatant was also assayed for protein content with the Pierce Micro bicinchoninic acid kit (Rockford, Ill.) according to the manufacturer’s directions. Final luciferase activity was determined following correction for both transfection efficiency, with the growth hormone activity as an internal control, and cell number, with protein content as the internal control. All experiments were done a minimum of three times.
Nuclear extracts.
Cultured cells (5 × 108 to 1 × 109) were washed twice in ice-cold phosphate-buffered saline plus 2 mM EDTA, pH 7.4. The remainder of the extraction was performed on ice. The cell pellets were resuspended in 5 ml of hypotonic buffer (10 mM HEPES [pH 7.9], 750 μM spermidine, 150 μM spermine, 0.1 mM EDTA [pH 8.0], 0.1 mM EGTA, 1 mM dithiothreitol [DTT] [in NaAc], 10 mM KCl) supplemented with specific protease inhibitors. Since both EML and MPRO cells harbor significant protease activity, these protease inhibitors were critical and included 0.5 mg of AEBSF (Boehringer Mannheim) per ml, 2 μg of aprotinin (Calbiochem) per ml, 50 μg of antipain (Calbiochem) per ml, 40 μg of bestatin (Calbiochem) per ml, 10 μg of E-64 (Boehringer Mannheim) per ml, 0.5 μg of leupeptin (Sigma) per ml, and 0.7 μg of pepstatin A (Sigma) per ml. The cells were pelleted again, resuspended in 10 ml of hypotonic buffer with the above-listed protease inhibitors, allowed to swell on ice for 10 to 15 min, and then Dounce homogenized with 20 to 30 strokes of the tight (A) pestle. To the lysed cells was added 1 ml of sucrose restore buffer (50 mM HEPES [pH 7.9], 750 μM spermidine, 150 μM spermine, 0.2 mM EDTA [pH 8.0], 1 mM DTT [in NaAc], 10 mM KCl, 75% RNase-free sucrose), which was mixed with two strokes of the Dounce homogenizer. The nuclei were pelleted at 10,000 rpm in chilled Corex tubes at 4°C in a Sorvall rotor. The nuclear pellet was washed once with 5 ml of ice-cold hypotonic buffer plus the above-described protease inhibitors plus 250 μl of sucrose restore buffer. The nuclei were spun again for 30 s at 10,000 rpm at 4°C, washed in 10 ml of ice-cold sucrose buffer (0.32 M sucrose, 1.5 mM MgCl2, 0.1 mM EDTA [pH 8.0], 10 mM Tris [pH 8.0], 1 mM DTT plus protease inhibitors), pelleted for 20 s, and then resuspended in 400 μl of low-salt nuclear resuspension buffer (20 mM HEPES [pH 7.9], 25% glycerol, 1.5 mM MgCl2, 20 mM KCl, 0.2 mM EDTA, 0.5 mM DTT) plus protease inhibitors. Three 100-μl aliquots of high-salt nuclear resuspension buffer (same as low-salt buffer except with 800 mM KCl) were slowly added and gently mixed with the pipette tip, and the pellets were kept on ice for 20 min with gentle mixing every 5 min. Two milliliters of diluent buffer (20 mM HEPES [pH 7.9], 25% glycerol, 0.1 mM EDTA, 0.5 mM DTT) plus protease inhibitors was added, and the nuclei were spun at 10,000 rpm in a 4°C Sorvall rotor for 20 min. The nuclear extract (supernatant) was removed and stored in aliquots frozen at −80°C until use.
Electromobility shift assay (EMSA).
Nuclear extracts (10 μg) were incubated with 4 μg of poly(dI-dC) (Boehringer Mannheim) in 10 mM Tris (pH 7.5)–50 mM NaCl–1 mM DTT–1 mM EDTA (pH 8.0)–5% glycerol followed by the addition of 4,000 cpm of 32P-labelled probe. The protease inhibitors described above for the nuclear extract procedure were also included. For the gel supershifts, the appropriate anti-RARα antibody was added to the nuclear extracts alone, and the mixture was incubated at 25°C for 10 to 15 min prior to the addition of the labelled probe. The reaction mixture was incubated at room temperature for an additional 10 to 15 min and then loaded onto a 5% polyacrylamide gel prerun at 100 V for 30 min in 250 mM Tris (pH 8.3)–1.9 M glycine–10 mM EDTA. The gel was run at 100 V for 5 to 7 h at 4°C with recirculation. The gel was dried and autoradiographed overnight. The probes included a 138-bp fragment from the actin promoter for use as a control for the nuclear extract integrity. The βRARE probe corresponded to the DR5 RA response element in the RARβ promoter, 5′ GAGGGTAGGGTTCACCGAAAGTTCACTCG 3′ (the 5-bp spacer is underlined). This oligonucleotide was annealed with its complementary oligonucleotide by heating to 70°C. Probes were end labelled with Klenow fragment.
RESULTS
MPRO and EML cells as models of myeloid differentiation.
The hematopoietic growth factor-dependent cell lines utilized in these studies were previously derived by transducing a dominant-negative human RARα construct that harbors a 59-amino-acid truncation at the COOH terminus (RARα403) into normal mouse bone marrow (53, 54). The GM-CSF-dependent MPRO cells express relatively high levels of the RARα403 mRNA and are predominantly promyelocytes, which can be induced to terminally differentiate to granulocytes by relatively high (1 to 10 μM) concentrations of ATRA (53). The SCF-dependent EML cells are multipotent (having erythroid, lymphoid, and myeloid potential), but with the addition of IL-3 and ATRA (again 1 to 10 μM), the EML cells commit to GM-CSF-dependent granulocyte/monocyte precursors as assessed by standard CFU-GM assays in semisolid medium (54).
EMSA complexes harboring the dominant-negative RARα403 predominate in EML and MPRO cells.
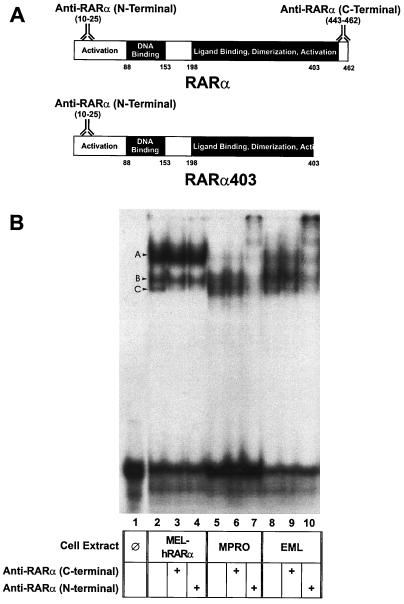
There is relatively high expression of the RARα403 mRNA in both the MPRO and EML cells compared with that of normal RARα mRNA (53, 54). Since this COOH-terminally truncated RARα403 retains the capacity to heterodimerize with RXRs and bind to RXR-RAR DNA response elements (11, 15), it is likely that RXR-RARα403 heterodimers are predominant in both the EML and the MPRO cells. To confirm this, we utilized the EMSA to compare complexes generated by nuclear extracts from both MPRO and EML cells on an oligonucleotide harboring an RXR-RAR consensus binding sequence (DR5) (56). As a control, we used nuclear extracts from Friend murine erythroleukemia cells (MEL) stably transduced with full-length human RARα (9). The nuclear extracts from these different cell lines generated two higher-mobility complexes (labelled B and C on Fig. 1B). Complex C in both EML and MPRO cells consistently migrated at a slightly higher mobility than the comparable complex from MEL extracts harboring the full-length RARα, suggesting that this complex may be generated by the truncated RARα403. To confirm this, we performed gel supershift assays with antibodies specific for the amino-terminal and COOH-terminal ends of RARα. The truncated RARα403 cannot react with the COOH-terminal antibody but will react with the amino-terminal antibody, while the full-length RARα reacts with both antibodies (Fig. 1A). If the truncated RARα403 predominates in the DR5 complexes generated from MPRO and EML extracts, then we would expect these complexes to be inhibited by the amino-terminal RARα antibody but not the COOH-terminal antibody. Indeed, the generation of both complex B and complex C is inhibited in the EML and MPRO nuclear extracts by the amino-terminal RARα antibody (Fig. 1B, lanes 7 and 10), while these same complexes are not inhibited by the COOH-terminal RARα antibody (Fig. 1B, lanes 6 and 9). In contrast, each of these antibodies inhibits formation of the higher-mobility complex C in the control MEL extracts harboring the full-length RARα (Fig. 1B, lanes 3 and 4). These studies indicate that in EML and MPRO cells the RARα403 predominates in complexes interacting with RXR-RAR (DR5) response elements.
FIG. 1.
EMSA on a DR5 (RXR-RAR) oligonucleotide. (A) Schematic of the molecular structure of both the full-length human RARα and the dominant-negative, truncated RARα403 illustrating the location of the RARα peptides utilized to generate both the N-terminal and the C-terminal RARα antibodies. (B) Nuclear extracts from the indicated cells were incubated with a radiolabelled DR5 oligonucleotide in the presence or absence of the indicated RARα antibodies and run on a Tris-glycine gel. MEL-hRARα cells are a mouse erythroleukemia cell line stably transduced with full-length human RARα. The composition of complex A, which is particularly predominant in the MEL-hRARα nuclear extracts, is unknown, but it does not appear to involve RARα, since this complex is not inhibited by either of the RARα antibodies.
RXR- and RAR-specific agonists and antagonists.
As noted above, relatively high concentrations of ATRA appear to overcome the effect of the dominant-negative RARα403 construct in both MPRO promyelocytes (where ATRA induces terminal granulocyte differentiation) (53) and the multipotent EML cells (where ATRA potentiates IL-3-induced commitment to the granulocyte/monocyte lineage) (54). Our experimental plan involved utilizing synthetic retinoids displaying specific RXR-RAR agonist and antagonist activity to determine how ATRA might be mediating such effects. Such synthetic retinoids were previously identified by both direct ligand binding assays and transactivation assays as described in Materials and Methods. The compounds utilized in the present study were the RAR selective agonist (AGN 193695) and the RXR selective agonist (AGN 194204), which bind to their respective receptors with high affinity (Table 1) and transactivate reporter constructs harboring RXR-RAR or RXR-RXR response elements, respectively. In contrast, the RAR antagonist (AGN 193109) and the RXR antagonist (LGN100849) also bind to their respective receptors with high affinity (Table 1), but these antagonists selectively inhibit ATRA- or 9-_cis_-RA-mediated activation of reporter constructs harboring RXR-RAR or RXR-RXR response elements, respectively.
TABLE 1.
Binding affinities and transcriptional activation characteristics of the RAR- and RXR-specific agonists and antagonistsa
| Receptor | AGN 193695 (RAR agonist) | AGN 194204 (RXR agonist) | AGN 193109b (RAR antagonist) K d | LGN100849c (RXR antagonist) K d | ||
|---|---|---|---|---|---|---|
| K d | EC50 | K d | EC50 | |||
| RARα | 44 | 31 | >30,000 | >1,000 | 2 | >10,000 |
| RARβ | 28 | 4 | >30,000 | >1,000 | 2 | 6,000 |
| RARγ | 99 | 3 | >30,000 | >1,000 | 3 | >10,000 |
| RXRα | >1,000 | >1,000 | <3 | 1.5 | >30,000 | 16 |
| RXRβ | >1,000 | >1,000 | <3 | 2.0 | >30,000 | 3 |
| RXRγ | >1,000 | >1,000 | <3 | 1.0 | >30,000 | 4 |
Granulocytic differentiation of MPRO cells is selectively induced by an RXR-specific agonist.
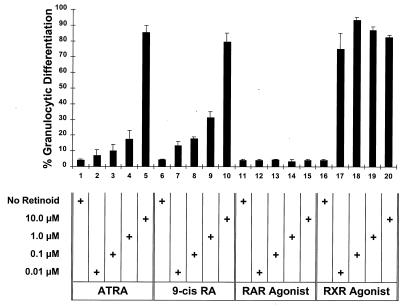
We determined the differentiative responses of the GM-CSF-dependent MPRO promyelocytes to these RXR and RAR selective agonists and antagonists. MPRO cells were grown in liquid suspension in these different receptor-selective retinoids, and granulocyte differentiation was assessed by morphological changes after 5 days of culture. As previously observed (53), relatively high concentrations of ATRA (1 to 10 μM) as well as of 9-_cis_-RA induce granulocytic differentiation of the MPRO cells (Fig. 2). Previous studies had indicated that ATRA binds the truncated RARα403 with approximately 10-fold-less affinity than it does the normal RARα (52). Thus, if RA binding to RARα403 is involved in MPRO differentiation, then this reduced binding affinity might account for the relatively high concentrations of ATRA required to trigger MPRO differentiation. However, contrary to this hypothesis, we found that the RXR-specific agonist (AGN 194204) is a potent inducer of MPRO granulocytic differentiation while the RAR-specific agonist (AGN 193695) had virtually no activity even at relatively high concentrations (10 μM) (Fig. 2). Comparing the relative concentrations of these different retinoids required to induce maximal MPRO differentiation indicated that the RXR agonist was approximately 100- to 1,000-fold more potent than either ATRA or 9-_cis_-RA (Fig. 2). As expected, neither the RXR antagonist nor the RAR antagonist induced any significant granulocytic differentiation of MPRO cells (data not shown).
FIG. 2.
Granulocytic differentiation of MPRO in response to different retinoids. MPRO cells were cultured in liquid suspension for 5 days with the indicated concentration of retinoids. The percent granulocytic differentiation in the culture (myelocytes, metamyelocytes, banded, doughnut, and segmented neutrophils) was then determined on Wright-Giemsa-stained cytospin preparations.
In MPRO cells, the RXR agonist preferentially triggers activation of RXR-RAR heterodimers rather than RXR-RXR homodimers.
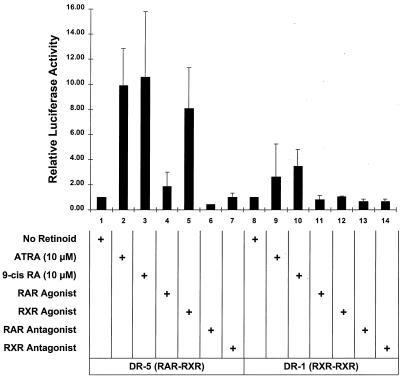
The truncated RARα403 retains the capacity to bind RXR and form RARα403-RXR heterodimers which can bind to RXR-RAR target sequences (11, 15). Since RXR-RARα403 heterodimers appear to predominate in MPRO cells (Fig. 1) (53), the RXR agonist likely mediates MPRO differentiation by triggering the activation of such RXR-RARα403 heterodimers. However, it is also possible that the RXR agonist may be mediating MPRO differentiation by activating any RXR-RXR homodimers that also might be present in these cells. To distinguish these possibilities, we utilized reporter constructs harboring DNA response elements that are selectively activated by either RAR-RXR or RXR-RXR complexes. The RXR-RXR homodimers preferentially interact with specific response elements consisting of a consensus binding sequence separated by a single base pair (DR1) (22, 40), while RXR-RAR heterodimers preferentially activate consensus sequences separated by 5 bp (DR5) (56). We performed transient-transfection assays of retinoid-treated MPRO cells with reporter constructs driven by promoters harboring either the DR1 or DR5 RA response element (Fig. 3). In the transfected MPRO cells, the RXR agonist (AGN 194204) consistently triggered an 8- to 10-fold activation of the DR5 (RXR-RAR) response elements while displaying little if any activation of the DR1 (RXR-RXR) reporter (Fig. 3, compare lane 5 with lane 12). Similar to its potency as a differentiation inducer of MPRO (Fig. 2), this RXR agonist triggered the DR5 reporter activation in MPRO cells at concentrations as low as 10 nM (data not shown). Similarly, ATRA- and 9-_cis_-RA-induced activation of the DR5 reporter was consistently greater than the activation of the DR1 reporter (Fig. 3, compare lanes 2 and 3 with lanes 9 and 10). In contrast, the RAR agonist, which is ineffective in activating MPRO differentiation (Fig. 2), mediated only a twofold activation of the DR5 (RXR-RAR) reporter and no activation of the DR1 (RXR-RXR) reporter (Fig. 3, lanes 4 and 11). As expected, the RXR and RAR antagonists activated neither reporter construct. The observation that the RXR agonist selectively activates the DR5 (RXR-RAR) rather than the DR1 (RXR-RXR) reporter construct suggests that this compound triggers MPRO granulocyte differentiation by activating the RXR-RARα403 heterodimers rather than RXR-RXR homodimers.
FIG. 3.
Relative activity of DR5 and DR1 response elements in retinoid-treated MPRO cells. Relative luciferase activity was determined in MPRO cells which were transfected with either the DR5 Luc (RXR-RAR responsive) or DR1 Luc (RXR-RXR responsive) reporter construct and then treated for 24 h with the indicated retinoid. The concentration of ATRA and 9-_cis_-RA utilized was 10 μM, while that of the other retinoids was 2.5 μM. Calculated luciferase activity was normalized for transfection efficiency with the cotransfected growth hormone reporter (pCMVGH) as an internal control. Solid bars represent the means of at least three independent experiments.
The activity of the RXR agonist in MPRO cells is inhibited by both RXR and RAR antagonists.
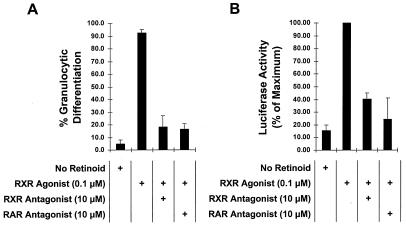
To further analyze the presumed RXR agonist-mediated activation of the RXR-RARα403 complex in MPRO cells, we assessed the MPRO response to this RXR agonist in the presence of specific RXR or RAR antagonists. As expected, the addition of the RXR antagonist (LGN100849) in a 100-fold excess inhibited both RXR agonist-induced MPRO differentiation (Fig. 4A) and RXR agonist-induced activation of the DR5 (RXR-RAR) reporter construct (Fig. 4B). This likely occurs because the RXR antagonist directly competes with the RXR agonist for binding to the ligand-binding domain of the RXR partner in the RXR-RARα403 heterodimer. Unexpectedly, we noted that the RAR antagonist also inhibits both RXR agonist-induced MPRO differentiation (Fig. 4A) and RXR agonist-induced activation of the DR5 (RXR-RAR) reporter (Fig. 4B). This was surprising because the RAR antagonist binds specifically to RARs, displaying virtually no binding affinity for RXRs (Table 1), and thus would not be expected to inhibit the RXR agonist activity. This observed ability of the RAR antagonist to inhibit the biological effects of the RXR agonist in MPRO cells suggests that the RXR and RAR heterodimeric partners do not behave independently after interaction with their respective ligands. Rather, there appears to be considerable cross-talk between the RXR and RAR components of the heterodimer after ligand stimulation (see Discussion).
FIG. 4.
Both RXR and RAR antagonists inhibit the biological effects of the RXR agonist in MPRO cells. (A) MPRO cells were cultured in liquid suspension with the indicated concentrations of retinoid agonist and/or antagonist. After 5 days, the percentage of morphologically mature granulocytes in the cultures was determined on Wright-Giemsa-stained cytospin preparations. (B) MPRO cells were transfected with the DR5 Luc reporter construct (responsive to RXR-RAR) and then cultured overnight in liquid suspension with the indicated concentrations of retinoid agonist and/or antagonist. Relative luciferase activity was then determined in cell lysates, and results were normalized for transfection efficiency with the cotransfected pCMVGH growth hormone expression plasmid as an internal control.
Response of EML cells to RXR and RAR selective retinoids.
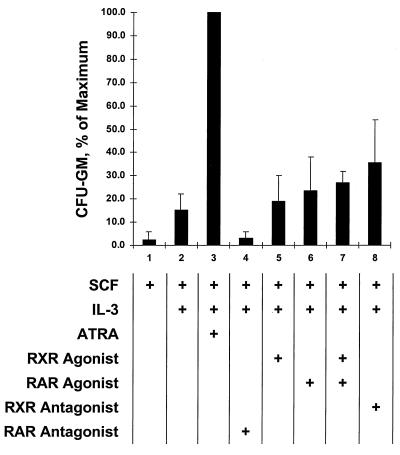
We next wished to characterize the response of the multipotent EML cells, in which the truncated RARα403 complexes are also predominant (Fig. 1), to different RAR- and RXR-specific agonists and antagonists. Unlike the GM-CSF-dependent MPRO cells, which are strictly committed to granulocyte differentiation, the SCF-dependent EML cells are multipotent, exhibiting erythroid, lymphoid, and myeloid potential (54). The addition of IL-3 to EML cultures will induce commitment of these cells to the monocyte/granulocyte lineage (as measured by CFU-GM generation), and this IL-3-induced commitment to the monocyte/granulocyte lineage is potentiated by relatively high concentrations of ATRA (Fig. 5, lanes 1 to 3) (54). As in the above-described studies of MPRO cells, we utilized the synthetic retinoids specific for RAR and RXR to determine whether the CFU-GM generation observed in EML cells induced by relatively high concentrations of ATRA was mediated through RXR or through RAR. We observed that, in marked contrast to MPRO cells, where the RXR agonist (AGN 194204) exhibited potent activity in inducing granulocyte differentiation (Fig. 2), this same RXR agonist had little effect in potentiating the IL-3-mediated CFU-GM production in the EML cells (Fig. 5, compare lanes 2 and 5). Moreover, the RAR agonist also exhibited no significant biological effect (Fig. 5, lane 6), and the combination of RAR and RXR agonists exhibited no significant increase compared with the effect of either agonist alone (Fig. 5, lane 7). Curiously, it was the RXR antagonist that exhibited slightly enhanced CFU-GM production in the EML cells (Fig. 5, lane 8), although this level of CFU-GM production was consistently lower than that observed with ATRA (Fig. 5, lane 3). In contrast, the RAR antagonist consistently diminished CFU-GM production in the IL-3-treated EML cells (Fig. 5, lane 4).
FIG. 5.
Generation of CFU-GM in EML cultures treated with IL-3 and different retinoids. EML cells were cultured in liquid suspension with the indicated concentration of IL-3 and/or retinoid agonist or antagonist. After 2 to 3 days of this liquid culture, the cells were harvested, and the numbers of CFU-GMs in the cultures were determined in colony assays as described in Materials and Methods.
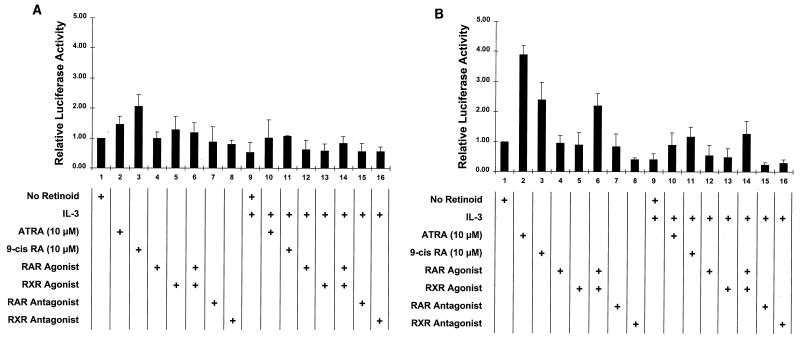
The relative inactivity of the RXR agonist in potentiating CFU-GM generation in the IL-3-treated EML cells was puzzling given the potent activity of this agonist in inducing granulocyte differentiation of MPRO cells (Fig. 2). To further explore the activity of the RXR agonist in EML cells, we assessed the activity of the DR1 (RXR-RXR) and DR5 (RXR-RAR) reporter constructs transfected into retinoid-stimulated EML cells. Little activation of the DR1 (RXR-RXR) reporter was noted in the EML cells treated with the different retinoids, with the greatest activation (approximately twofold) observed with 9-_cis_-RA induction (Fig. 6A, lanes 3 and 11). In addition, IL-3 had little if any effect in enhancing activation of the DR1 reporter in EML cells (Fig. 6A, lanes 9 to 16). Similarly, in the transfected EML cells the DR5 (RAR-RXR) reporter exhibited a markedly reduced retinoid-mediated activation compared with that in MPRO cells (compare Fig. 6B with Fig. 3). For example, the RXR agonist, which markedly stimulates the DR5 (RXR-RAR) reporter in MPRO cells (Fig. 3, lane 5), stimulated little if any activation of the same DR5 reporter in the transfected EML cells either in the absence or in the presence of IL-3 (Fig. 6B, lanes 5 and 13). Interestingly, while neither the RXR nor the RAR agonist induced any DR5 reporter activation in EML cells in the presence or absence of IL-3 (Fig. 6B, lanes 4 and 5 and lanes 12 and 13), both agonists together reproducibly induced a two- to threefold activation of this reporter (Fig. 6B, lanes 6 and 14). ATRA and 9-_cis_-RA, rather than any of the synthetic agonists or antagonists, consistently induced the greatest activation of the DR5 construct in EML cells, but this activation (three- to fourfold) was consistently less than the approximately 10-fold activation of the same construct induced by ATRA or 9-_cis_-RA in the transfected MPRO cells (Fig. 3, lanes 2 and 3). Thus, in comparison with the response in MPRO promyelocytes, in the more immature, multipotent EML cells the DR5 (RXR-RAR) reporter exhibits a markedly blunted response to retinoid-induced activation, and in these cells, the RXR agonist by itself exhibits virtually no activation of the DR5 reporter.
FIG. 6.
Relative activities of DR1 and DR5 response elements in IL-3- and/or retinoid-treated EML cells. Relative luciferase activity was determined in EML cells transfected with either the DR1 Luc (RXR-RXR responsive) (A) or the DR5 Luc (RXR-RAR responsive) (B) reporter construct and treated for 24 h with the indicated retinoid and/or IL-3. The concentration of ATRA and 9-_cis_-RA utilized was 10 μM, while that of the other retinoids was 2.5 μM. Calculated luciferase activity was normalized for transfection efficiency with the cotransfected growth hormone reporter (pCMVGH) as an internal control.
Response of MPRO cells directly derived from EML cells to the RXR selective retinoids.
The MPRO and EML cells utilized in the above experiments were derived at different times from different stocks of retroviral vectors, and it is possible that subtle mutations in the transduced construct or in the cultured hematopoietic target cells could account for the observed dramatic differences between the responses of MPRO and EML cells to the RXR selective compounds. To address this possibility, we exploited our previous observation that GM-CSF-dependent MPRO-like promyelocytes could be directly derived from the SCF-dependent EML cells by switching the hematopoietic growth factor in the EML culture from SCF to GM-CSF (54). As detailed in Materials and Methods, we derived EML subclones that were GM-CSF dependent and exhibited the morphological appearance of MPRO promyelocytes. These EML subclones exhibited responses to ATRA and the RXR selective agonists and antagonists that were virtually identical to those of the MPRO cells described above (data not shown). Thus, the marked differences in responses to the RXR agonist that we have observed between the MPRO and the EML cells appear to be developmentally regulated during myeloid differentiation, such that the RXR selective agonist is active in the committed MPRO promyelocytes but inactive in the more immature, uncommitted EML cells.
Nuclear hormone receptor coactivator activity in EML and MPRO cells.
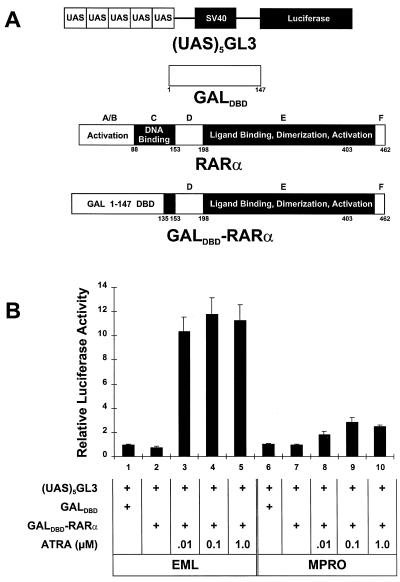
Our observations indicate that the RXR-RARα403 heterodimer is readily activated in one cell lineage (MPRO promyelocytes) but not in another closely related though distinct lineage (multipotent EML cells). Since nuclear hormone receptors regulate transcription by interacting with a complex array of coactivator (6, 7, 19, 24, 31, 44, 57) or corepressor (8, 25, 33, 38) proteins, our observations suggest that there may be significant differences in such coactivators or corepressors between the EML and MPRO cells. We first compared the functional activities of RAR transcriptional coactivators in these different cells by assessing the activity of an RA-responsive GALdbd-RARα hybrid on a luciferase reporter harboring five GAL binding sites [p(UAS)5- GL3] (Fig. 7A). In neither EML nor MPRO cells does this GALdbd-RARα hybrid significantly repress the (UAS)5 reporter compared with the GALdbd construct alone (Fig. 7B, compare lane 1 with lane 2 and lane 6 with lane 7). Thus, the effect of ligand on this hybrid provides a relative assessment of nuclear hormone receptor transcriptional coactivator activity in these different cell types. Surprisingly, we noted that the addition of RA triggered remarkably more activity of the luciferase reporter in the EML cells than in the MPRO cells (Fig. 7B). These observations, indicating that there is abundant functional nuclear hormone receptor coactivator activity in EML cells, suggest that the difference in RXR-RARα403 activation between EML and MPRO cells cannot be accounted for by a relative deficiency of coactivator activity in the pluripotent EML cells.
FIG. 7.
RAR coactivator activity in EML cells compared with that in MPRO cells. (A) Schematic illustrating the upstream activation sequence (UAS) luciferase reporter [p(UAS)5-GL3] and the construction of the GALdbd-RARα hybrid. (B) The EML and MPRO cells were transfected with 20 μg of each of the indicated plasmids and then cultured for 24 h in the presence or absence of different concentrations of ATRA. Relative luciferase activity was then determined in cell lysates.
Nuclear hormone receptor corepressor activity in EML and MPRO cells.
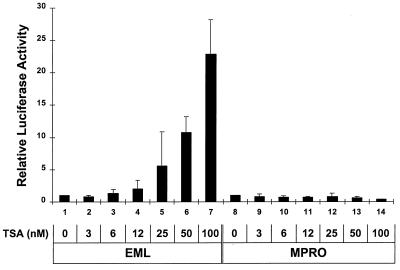
Transcriptional repression by RA receptors may in part be mediated by specific corepressors such as N-CoR and SMRT which interact with nuclear hormone receptors as multicomponent complexes, including mSin3A and HDACs (8, 21, 25, 43). To compare the activities of such HDAC-containing repressor complexes in EML cells with those in MPRO cells, we assessed the ability of the HDAC inhibitor TSA (62) to activate the DR5 (RAR-RXR) reporter in these different cell types. We observed that TSA readily activated this reporter in EML cells but had no effect on activating this same reporter in the MPRO cells even at higher concentrations (Fig. 8). Thus, repression of DR5 appears dependent on HDAC activity in the EML but not the MPRO cells, indicating that there are significant differences in transcriptional repressor complexes in these different cell types.
FIG. 8.
TSA activates the DR5 (RXR-RAR) reporter in EML but not MPRO cells. EML and MPRO cells were transfected with 40 μg of the DR5 Luc (RXR-RAR responsive) reporter construct and then cultured for 24 h with the indicated concentration of TSA. Relative luciferase activity was then determined in cell lysates.
DISCUSSION
Our present observations can be summarized as follows. (i) In MPRO promyelocytes, which overexpress the truncated, dominant-negative RARα403 construct, an RXR rather than an RAR agonist is a potent inducer of granulocytic differentiation. (ii) This RXR agonist-induced granulocytic differentiation is associated with activation of RXR-RAR rather than RXR-RXR response elements (Fig. 9A). (iii) The same RXR agonist exhibits virtually no activity in the more immature EML cells, which express the same dominant-negative RARα403 as the MPRO promyelocytes (Fig. 9C). (iv) There are functionally significant differences in HDAC-containing repressor complexes between the multipotent EML cells and the committed MPRO promyelocytes.
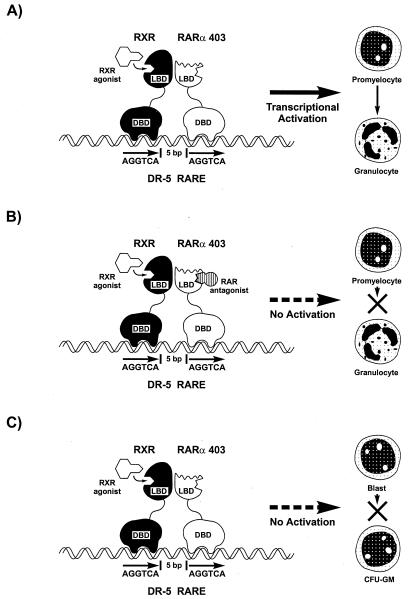
FIG. 9.
Summary of RXR-RARα403 activation in MPRO and EML cells. (A) The RXR agonist selectively activates DR5 response elements and induces granulocytic differentiation of MPRO cells. (B) In MPRO cells, this RXR agonist-induced DR5 activation and differentiation are inhibited by an RARα antagonist. (C) In EML cells, the RXR agonist is inactive in inducing DR5 activation. RARE, RA response element; DBD, DNA-binding domain; LBD, ligand-binding domain.
RA receptors and myeloid differentiation.
The important role that RA receptors play in hematopoietic differentiation is emphasized by our previous observation that overexpressing a truncated RA receptor exhibiting dominant-negative activity (RARα403) in normal mouse hematopoietic progenitors generates hematopoietic growth factor-dependent cell lines blocked at distinct stages of myeloid development (53, 54). The MPRO cell line consists of GM-CSF-dependent promyelocytes and is firmly committed to granulocyte differentiation (53), while the SCF-dependent EML cells are multipotent, exhibiting erythroid, lymphoid, and myeloid potential (54). Both of these cell lines overexpress the C-terminally truncated dominant-negative RARα403, which lacks the C-terminal activation domain (AF2) but retains the capacity to heterodimerize with RXRs and bind to RXR-RAR response elements (11, 15). Utilizing gel shift assays, we observed that EML and MPRO complex formation on a DR5 (RXR-RAR) oligonucleotide shifted with an antibody directed against the amino terminus of RARα but not with an antibody against the RARα COOH terminus, indicating that the COOH-terminally truncated RARα complexes are predominant in these cells (Fig. 1). These predominant RXR-RARα403 heterodimers present in EML and MPRO cells likely interfere with normal RXR-RAR function, which somehow leads to the block in hematopoietic differentiation that characterizes these cells. However, this block is not absolute because relatively high, pharmacological concentrations (1 to 10 μM) of ATRA induce terminal granulocyte differentiation of MPRO cells while similar ATRA concentrations potentiate IL-3-mediated CFU-GM generation in the EML cells (53, 54). The question addressed in the present study was how such pharmacological concentrations of ATRA might activate these aberrant RXR-RARα403 heterodimers.
RXR agonist-mediated activation of RXR-RARα403 heterodimers in MPRO cells.
In the course of these experiments, we made a number of unexpected observations. First, we noted that an RXR agonist (AGN 194204), known to specifically bind to RXRs rather than to RARs, exhibited potent induction of MPRO granulocyte terminal differentiation while the RAR agonist (AGN 193695) exhibited virtually no such activity (Fig. 2). The effect of the RXR agonist was likely mediated through activation of aberrant RXR-RARα403 heterodimers rather than any residual RXR-RXR homodimers, because in MPRO cells the RXR-specific agonist consistently activated a reporter construct harboring DR5 (RXR-RAR) response elements while inducing little if any activation of a similar reporter harboring DR1 (RXR-RXR) elements (Fig. 3). Moreover, it is very unlikely that this RXR agonist-mediated DR5 activation is triggered through normal residual wild-type RXR-RAR heterodimers, because previous studies have indicated that there is an allosteric block that inhibits binding of RXR-specific agonists to the RXR partner of normal RXR-RAR heterodimers (32, 37). Consistent with this, we have noted in our screening assays (see Materials and Methods) that the RXR agonist utilized in our studies (AGN 194204) specifically activated RXR-RXR response elements and exhibited no activation of normal RXR-RAR heterodimers. Therefore, the RXR agonist-mediated activation of the RXR-RARα403 heterodimers in MPRO cells suggests that the conformation of the RXR-RARα403 heterodimer must differ significantly from that of the normal RXR-RAR heterodimer to allow binding of the RXR-specific retinoid to the RXR partner with consequent activation of the RXR-RARα403 heterodimer. Indeed, recent in vitro binding studies indicate that RXR-specific ligands preferentially trigger recruitment of coactivators to RXR-RARα403 heterodimers compared with wild-type RXR-RAR heterodimers, indicating an important role for the RARα C-terminal AF2 domain (the domain that has been truncated in the RARα403 construct) in inhibiting RXR ligand-specific activation of RXR-RAR complexes (60). In this respect, the behavior of the aberrant RXR-RARα403 heterodimer mimics that of other RXR heterodimers which are activated by RXR-specific agonists, including RXR-Nurr1 (16), RXR-LXR (61), and RXR-PPAR (14) complexes.
Cross-talk between partners of the RXR-RARα403 heterodimer.
Curiously, we observed that in MPRO cells the RARα antagonist inhibited the RXR agonist-induced differentiation of MPRO as well as the RXR agonist-induced transactivation of the DR5 element (Fig. 4). This was unexpected because the RARα antagonist has virtually no affinity for RXRs (Table 1) and thus cannot interfere with the RXR agonist-induced activation by competitively binding to the RXR ligand-binding domain. Instead, this observation suggests that there is considerable cross-talk between the RXR and RARα403 partners such that RXR agonist-induced conformational changes might trigger activation of the complex through the RAR partner. Such communication between heterodimeric partners has been previously observed for RXR-LXR heterodimers, where 9-_cis_-RA binding to the RXR partner triggers transcriptional activation that is mediated through the LXR AF2 domain (61). Similarly, binding of the RXR antagonist LG10074 (35) to the RXR subunit of RXR-RAR heterodimers triggers activation of the complex by inducing conformational changes in the RAR partner, a phenomenon termed the “phantom ligand” effect (49). Our observation for MPRO cells that an RARα antagonist blocks RXR agonist-induced granulocyte differentiation and DR5 (RXR-RARα) activation suggests that similar cross-talk mechanisms are also involved in activation of the RXR-RARα403 complex in MPRO cells. It is possible that occupation of the RARα403 ligand-binding domain by the RARα antagonist might interfere with RXR agonist-induced conformational changes that trigger activation of the complex through the RAR partner. Alternatively, conformational changes in the RARα403 partner resulting from binding to the RARα antagonist might interfere with RXR agonist binding to the RXR partner. In either case, the inhibition of RXR agonist activity by the RARα antagonist suggests considerable cross-talk between the individual partners of the RXR-RARα403 heterodimer (Fig. 9B).
Blunted activation of the RXR-RARα403 heterodimer in the more immature EML cells.
Another unexpected observation was the relative lack of activity of the RXR agonist in activating the DR5 (RXR-RAR) reporter in the multipotent EML cells, which harbor the same RXR-RARα403 complex as the MPRO promyelocytes. Nevertheless, from these multipotent EML cells we have repeatedly established cultures of MPRO-like promyelocytes which readily exhibit, unlike the parental EML cells, RXR agonist-induced activation of the DR5 (RXR-RAR) reporter. Why would the RXR-RARα403 complex be readily activated in one cell lineage (MPRO promyelocytes) but not in another closely related though distinct lineage (multipotent EML cells)? To address this question, we assessed nuclear hormone receptor coactivator and corepressor function in these two cell types. Utilizing an RA-inducible GALdbd-RARα hybrid, we observed no functional deficiency of hormone receptor coactivator activity in EML cells compared with activity in MPRO cells. Indeed, in EML cells the (UAS)5 reporter unexpectedly exhibited an enhanced response to ligand-induced activation of the GALdbd-RAR hybrid (Fig. 7). In contrast, our observations suggest that differences in corepressor activity more likely account for the differential activation of DR5 between EML and MPRO cells. Transcriptional repression by RA receptors involves interaction with multiprotein complexes that include N-CoR, SMRT, mSin3A, and HDACs (8, 21, 25, 43). Our observation that the HDAC inhibitor TSA readily activates (derepresses) the DR5 reporter in EML but not in MPRO cells (Fig. 8) suggests that there are functionally significant differences in repressor complexes harboring HDAC activity in these different hematopoietic lineages. Indeed, recent biochemical evidence suggests that multiple, functionally distinct HDAC complexes may indeed exist in vivo (34, 63). The specific compositions of such repressor complexes in EML compared with those in MPRO cells is presently unknown, but determining the nature of such complexes in these different cell types will likely be critical in determining the molecular basis for the difference in RA receptor activity that we have observed in these distinct stages of myeloid development.
RA receptor activity in normal and malignant hematopoiesis.
RA as a differentiating agent induces complete remissions in human APL (5, 26, 59) but is generally inactive in other subtypes of acute myelogenous leukemia (4, 42). Such clinical observations are indeed paradoxical because the RA-responsive leukemias harbor the aberrant PML-RARα fusion gene (2, 13, 29) while the RA-resistant leukemias express normal RA receptors (42). The PML-RARα fusion protein appears to inhibit normal RA receptor function by selectively recruiting corepressors to RAR target gene promoters (18, 20, 39) and thus likely mediates transformation, at least in part, by inhibiting normal RA receptors. The specific presence of this aberrant fusion protein in malignant promyelocytes rather than in leukemias of a more immature lineage suggests that inhibition of RA receptor activity is an important event in the transformation of normal promyelocytes but that it has little effect in mediating transformation of other more immature hematopoietic cells. Our observation that DR5 (RXR-RAR) is readily activated by certain retinoids in committed promyelocytes (MPRO) but not in more immature multipotent hematopoietic cells (EML) may be related to this differential response of human leukemia cells to RA. The murine MPRO cells closely resemble human promyelocytic leukemia cells in their block to differentiation at the promyelocytic stage and the terminal granulocytic differentiation that they display in response to RA. In contrast, the multipotent, more primitive EML cells resemble cells from those other cases of myelogenous leukemia that are generally less well differentiated than promyelocytic leukemia cells and which exhibit little if any therapeutic response to RA. Thus, determining the molecular basis for the marked difference in retinoid-mediated activation of the RXR-RARα403 heterodimer between the MPRO promyelocytes and the more undifferentiated EML cells may have direct relevance to the question of why RA is an effective differentiating agent in one form of human myelogenous leukemia but not in others. Our observations suggest that such a molecular analysis should emphasize potential differences in HDAC-containing repressor complexes that might exist among leukemias of different hematopoietic lineages.
ACKNOWLEDGMENTS
We thank Grant McArthur and Bob Eisenman for a critical reading of the manuscript.
This work was supported by NIH grant CA58292 to S.J.C.
REFERENCES
- 1.Agura E, Howard M, Collins S J. Identification and sequence analysis of the promoter for the leukocyte integrin beta subunit (CD18): a retinoic acid-inducible gene. Blood. 1992;79:602–609. [PubMed] [Google Scholar]
- 2.Alcalay M, Zangrilli D, Pandolfi P, Longo L, Mencarelli A, Giacomucci A, Rocchi M, Biondi A, Rambaldi A, LoCoco F, Diverioi D, Donti E, Grignani F, Pelicci P. Translocation breakpoint of acute promyelocytic leukemia lies within the retinoic acid receptor α locus. Proc Natl Acad Sci USA. 1991;88:1977–1981. doi: 10.1073/pnas.88.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allegretto E, McClurg M R, Lazarchik S B, Clemm D L, Kerner S A, Elgort M G, Boehm M F, White S K, Pike J W, Heyman R A. Transactivation properties of retinoic acid and retinoid X receptors in mammalian cells and yeast: correlation with hormone binding and effects of metabolism. J Biol Chem. 1993;268:26625–26633. [PubMed] [Google Scholar]
- 4.Breitman T, Collins S J, Keene B. Terminal differentiation of human promyelocytic leukemia cells in primary culture in response to retinoic acid. Blood. 1981;57:1000–1004. [PubMed] [Google Scholar]
- 5.Castaigne S, Chomienne C, Daniel M, Berger N, Fenaux P, Degos L. All-trans retinoic acid as a differentiation therapy for acute promyelocytic leukemia. I. Clinical results. Blood. 1990;76:1704–1712. [PubMed] [Google Scholar]
- 6.Cavailles V, Dauvois S, L’Horset F, Lopez G, Hoare S, Kushner P, Parker M. Nuclear factor RIP140 modulates transcriptional activation by the estrogen receptor. EMBO J. 1995;14:3741–3751. doi: 10.1002/j.1460-2075.1995.tb00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H, Lin R J, Schiltz R L, Chakravarti D, Nash A, Nagy L, Privalsky M L, Nakatani Y, Evans R. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell. 1997;90:569–580. doi: 10.1016/s0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- 8.Chen J D, Evans R. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 9.Collins S J, Robertson K A, Mueller L. Retinoic acid-induced granulocytic differentiation of HL-60 myeloid leukemia cells is mediated directly through the retinoic acid receptor (RAR-α) Mol Cell Biol. 1990;10:2154–2163. doi: 10.1128/mcb.10.5.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costa S L, McBurney M W. Dominant negative mutant of retinoic acid receptor-α inhibits retinoic acid-induced P19 cell differentiation by binding to DNA. Exp Cell Res. 1996;225:35–43. doi: 10.1006/excr.1996.0154. [DOI] [PubMed] [Google Scholar]
- 11.Damm K, Heyman R A, Umesono K, Evans R M. Functional inhibition of retinoic acid response by dominant negative retinoic acid receptor mutants. Proc Natl Acad Sci USA. 1993;90:2989–2993. doi: 10.1073/pnas.90.7.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de The H, Marchio A, Tiollais P, Dejean A. Differential expression and ligand regulation of the retinoic acid receptor alpha and beta genes. EMBO J. 1989;8:429–439. doi: 10.1002/j.1460-2075.1989.tb03394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de The H, Lavfau C, Marchio A, Chomienne C, Degos L, Dejean A. The PML-RARα fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell. 1991;66:675–684. doi: 10.1016/0092-8674(91)90113-d. [DOI] [PubMed] [Google Scholar]
- 14.DiRenzo J, Soderstrom M, Kurokawa R, Ogliastro M-H, Ricote M, Ingrey S, Horlein A, Rosenfeld M, Glass C. Peroxisome proliferator-activated receptors and retinoic acid receptors differentially control the interactions of retinoid X receptor heterodimers with ligands, coactivators, and corepressors. Mol Cell Biol. 1997;17:2166–2176. doi: 10.1128/mcb.17.4.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durand B, Saunders M, Gaudon C, Roy B, Losson R, Chambon P. Activation function 2 (AF-2) of retinoic acid receptor and 9-cis retinoic acid receptor: presence of a conserved autonomous constitutive activating domain and influence of the nature of the response element on AF-2 activity. EMBO J. 1994;13:5370–5382. doi: 10.1002/j.1460-2075.1994.tb06872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forman B, Umesono K, Chen J, Evans R. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell. 1995;81:541–550. doi: 10.1016/0092-8674(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 17.Giguere V, Ong E, Segui P, Evans R. Identification of a receptor for the morphogen retinoic acid. Nature. 1987;330:624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- 18.Grignani F, De Matteis S, Nervi C, Tomassoni L, Gelmetti V, Cioce M, Fanelli M, Ruthardt M, Ferrara F, Zamir I, Seiser C, Grignani F, Lazar M, Minucci S, Pelicci P G. Fusion proteins of the retinoic acid receptor-α recruit histone deacetylase in promyelocytic leukemia. Nature. 1998;319:815–818. doi: 10.1038/35901. [DOI] [PubMed] [Google Scholar]
- 19.Halachmi S, Marden E, Martin G, MacKay H, Abbondanza C, Brown M. Estrogen receptor-associated proteins: possible mediators of hormone-induced transcription. Science. 1994;264:1455–1458. doi: 10.1126/science.8197458. [DOI] [PubMed] [Google Scholar]
- 20.He L-Z, Guidez F, Tribioli C, Peruzzi D, Ruthardt M, Zelent A, Pandolfi P P. Distinct interactions of PML-RARα and PLZF-RARα with co-repressors determine differential responses to RA in APL. Nat Genet. 1998;18:126–135. doi: 10.1038/ng0298-126. [DOI] [PubMed] [Google Scholar]
- 21.Heinzel T, Lavinsky R, Mullen T M, Soderstrom M, Laherty C, Torchia J, Yang W-M, Brard G, Ngo S, Davie J, Seto E, Eisenman R, Rose D, Glass C, Rosenfeld M. A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature. 1997;387:43–48. doi: 10.1038/387043a0. [DOI] [PubMed] [Google Scholar]
- 22.Heyman R A, Mangelsdorf D J, Dyck J A, Stein R B, Eichele G, Evans R M, Thaller C. 9-Cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992;68:397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- 23.Hollenberg S, Evans R. Multiple and cooperative transactivation domains of the human glucocorticoid receptor. Cell. 1988;55:899–906. doi: 10.1016/0092-8674(88)90145-6. [DOI] [PubMed] [Google Scholar]
- 24.Hong H, Kohli K, Garabedian M, Stallcup M. Grip1, a transcriptional coactivator for the AF-2 transactivation domain of steroid, thyroid, retinoid, and vitamin D receptors. Mol Cell Biol. 1997;17:2735–2744. doi: 10.1128/mcb.17.5.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horlein A, Naar A, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Soderstrom M, Glass C, Rosenfeld M. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature. 1995;377:397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- 26.Huang M, Ye Y C, Chen S R, Chai J R, Lu J X, Zhoa L, Gu L J, Wang Z Y. Use of all trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72:567–574. [PubMed] [Google Scholar]
- 27.Imakado S, Bickenbach J, Bundman D, Rothnagel J, Attar P, Wang X-J, Walczak V, Wisniewski S, Pote J, Gordon J, Heyman R, Evans R, Roop D. Targeting expression of a dominant-negative retinoic acid receptor mutant in the epidermis of transgenic mice results in loss of barrier function. Genes Dev. 1995;9:317–329. doi: 10.1101/gad.9.3.317. [DOI] [PubMed] [Google Scholar]
- 28.Johnson A T, Klein E S, Gillet S J, Wang L, Song T K, Pino M E, Chandraratna R A S. Synthesis and characterization of a highly potent and effective antagonist of retinoic acid receptors. J Med Chem. 1995;38:4764–4767. doi: 10.1021/jm00024a003. [DOI] [PubMed] [Google Scholar]
- 29.Kakizuka A, Miller W, Umesono K, Warrell R, Frankel S, Murty V, Dmitrovsky E, Evans R. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RARα with a novel putative transcription factor, PML. Cell. 1991;66:663–674. doi: 10.1016/0092-8674(91)90112-c. [DOI] [PubMed] [Google Scholar]
- 30.Kastner P, Mark M, Chambon P. Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell. 1995;83:859–869. doi: 10.1016/0092-8674(95)90202-3. [DOI] [PubMed] [Google Scholar]
- 31.Kawasaki H, Eckner R, Yao T-P, Taira K, Chiu R, Livingston D, Yokoyama K. Distinct roles of the co-activators p300 and CBP in retinoic acid-induced F9 cell differentiation. Nature. 1998;393:284–288. doi: 10.1038/30538. [DOI] [PubMed] [Google Scholar]
- 32.Kurokawa R, DiRenzo J, Boehm M, Sugarman J, Gloss B, Rosenfeld M, Heyman R, Glass C. Regulation of retinoid signalling by receptor polarity and allosteric control of ligand binding. Nature. 1994;371:528–531. doi: 10.1038/371528a0. [DOI] [PubMed] [Google Scholar]
- 33.Kurokawa R, Soderstrom M, Horlein A, Halachmi S, Brown M, Rosenfeld M, Glass C. Polarity-specific activities of retinoic acid receptors determined by a co-repressor. Nature. 1995;377:451–454. doi: 10.1038/377451a0. [DOI] [PubMed] [Google Scholar]
- 34.Laherty C, Billin A, Lavinsky R, Yochum G, Bush A, Sun J-M, Mullen T-M, Davie J, Rose D, Glass C, Rosenfeld M, Ayer D, Eisenman R. SAP30, a component of the mSin3 corepressor complex involved in N-CoR-mediated repression by specific transcription factors. Mol Cell. 1998;2:33–42. doi: 10.1016/s1097-2765(00)80111-2. [DOI] [PubMed] [Google Scholar]
- 35.Lala D, Mukherjee R, Schulman I, Canan Koch S, Dardashti L, Nadzan A, Croston G, Evans R, Heyman R. Activation of specific RXR heterodimers by an antagonist of RXR homodimers. Nature. 1996;383:450–453. doi: 10.1038/383450a0. [DOI] [PubMed] [Google Scholar]
- 36.Largman C, Detmer K, Corral J, Hack F, Lawrence H J. Expression of retinoic acid receptor alpha in mRNA in human leukemia cells. Blood. 1989;74:99–102. [PubMed] [Google Scholar]
- 37.Leblanc B, Stunnenberg H. 9-Cis retinoic acid signaling: changing partners causes some excitement. Genes Dev. 1995;9:1811–1816. doi: 10.1101/gad.9.15.1811. [DOI] [PubMed] [Google Scholar]
- 38.Li H, Leo C, Schroen D, Chen J D. Characterization of receptor interaction and transcriptional repression by the corepressor SMRT. Mol Endocrinol. 1997;11:2025–2037. doi: 10.1210/mend.11.13.0028. [DOI] [PubMed] [Google Scholar]
- 39.Lin R, Nagy L, Inoue S, Shao W, Miller W, Evans R. Role of the histone deacetylase complex in acute promyelocytic leukemia. Nature. 1998;391:811–814. doi: 10.1038/35895. [DOI] [PubMed] [Google Scholar]
- 40.Mangelsdorf D, Umesono K, Kliewer S, Borgmeyer U, Ong E, Evans R. A direct repeat in the cellular retinol-binding protein type II gene confers differential regulation by RXR and RAR. Cell. 1991;66:555–561. doi: 10.1016/0092-8674(81)90018-0. [DOI] [PubMed] [Google Scholar]
- 41.Mangelsdorf D, Evans R. The RXR and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 42.Morosetti R, Grignani F, Liberatore C, Pelicci P, Schiller G, Kizaki M, Bartram C, Miller C, Koeffler H P. Infrequent alterations of the RAR alpha gene in acute myelogenous leukemias, retinoic acid-resistant acute promyelocytic leukemias, myelodysplastic syndromes and cell lines. Blood. 1996;87:4399–4403. [PubMed] [Google Scholar]
- 43.Nagy L, Kao H-Y, Chakravarti D, Lin R, Hassig C, Ayer D, Schreiber S, Evans R. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell. 1997;89:373–380. doi: 10.1016/s0092-8674(00)80218-4. [DOI] [PubMed] [Google Scholar]
- 44.Onate S, Tsai S, Tsai M-J, O’Malley B W. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 45.Robertson K, Emami B, Collins S J. Retinoic acid-resistant HL-60R cells harbor a point mutation in the RA receptor ligand binding domain that confers dominant negative activity. Blood. 1992;80:1885–1889. [PubMed] [Google Scholar]
- 46.Sadowski I, Ptashne M. A vector for expressing GAL4 (1-147) fusions in mammalian cells. Nucleic Acids Res. 1989;17:753. doi: 10.1093/nar/17.18.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saitou M, Narumiya S, Kakizuka A. Alteration of a single amino acid residue in retinoic acid receptor causes dominant-negative phenotype. J Biol Chem. 1994;269:19101–19107. [PubMed] [Google Scholar]
- 48.Saitou M, Sugai S, Tanaka T, Shimouchi K, Fuchs E, Narumiya S, Kakizuka A. Inhibition of skin development by targeted expression of a dominant-negative retinoic acid receptor. Nature. 1995;374:159–162. doi: 10.1038/374159a0. [DOI] [PubMed] [Google Scholar]
- 49.Schulman I, Li C, Schwabe J W R, Evans R. The phantom ligand effect: allosteric control of transcription by the retinoid X receptor. Genes Dev. 1997;11:299–308. doi: 10.1101/gad.11.3.299. [DOI] [PubMed] [Google Scholar]
- 50.Seewaldt V L, Caldwell L E, Johnson B S, Swisshelm K, Collins S J, Tsai S. Inhibition of retinoic acid receptor function in normal human mammary epithelial cells results in increased cellular proliferation and inhibits the formation of a polarized epithelium in vitro. Exp Cell Res. 1997;236:16–28. doi: 10.1006/excr.1997.3694. [DOI] [PubMed] [Google Scholar]
- 51.Solomin L, Johansson C, Zetterstrom R, Bissonnette R, Heyman R, Olson L, Lendahl U, Frisen J, Perlmann T. Retinoid-X receptor signalling in the developing spinal cord. Nature. 1998;395:398–401. doi: 10.1038/26515. [DOI] [PubMed] [Google Scholar]
- 52.Tate B, Allenby G, Janocha R, Kazmer S, Speck J, Sturzenbecker L, Abarzua P, Levin A, Grippo J. Distinct binding determinants for 9-cis retinoic acid are located within AF-2 of retinoic acid receptor α. Mol Cell Biol. 1994;14:2323–2330. doi: 10.1128/mcb.14.4.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsai S, Collins S. A dominant negative retinoic acid receptor blocks neutrophil differentiation at the promyelocyte stage. Proc Natl Acad Sci USA. 1993;90:7153–7157. doi: 10.1073/pnas.90.15.7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsai S, Bartelmez S, Sitnicka E, Collins S. Lymphohematopoietic progenitors immortalized by a retroviral vector harboring a dominant negative retinoic acid receptor can recapitulate lymphoid, myeloid and erythroid development. Genes Dev. 1994;8:2831–2842. doi: 10.1101/gad.8.23.2831. [DOI] [PubMed] [Google Scholar]
- 55.Umesono K, Giguere V, Glass C, Rosenfeld M, Evans R M. Retinoic acid and thyroid hormone induce gene expression through a common responsive element. Nature. 1988;336:262–265. doi: 10.1038/336262a0. [DOI] [PubMed] [Google Scholar]
- 56.Umesono K, Murakami K, Thompson C, Evans R M. Direct repeats as selective response elements for the thyroid hormone, retinoic acid and vitamin D3 receptors. Cell. 1991;65:1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voegel J, Heine M J S, Zechel C, Chambon P, Gronemeyer H. TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J. 1996;15:3667–3675. [PMC free article] [PubMed] [Google Scholar]
- 58.Vuligonda V, Lin Y, Chandraratna R A S. Synthesis of highly potent RXR-specific retinoids: the use of a cyclopropyl group as a double bond isostere. Biorg Med Chem Lett. 1996;6:213–218. [Google Scholar]
- 59.Warrell R, Frankel S, Miller W, Itri L, Andreef M, Jabukowski A, Gabrilove J, Gordon M, Dmitrovsky E. Differentiation therapy of acute promyelocytic leukemia with tretinoin (all-trans retinoic acid) N Engl J Med. 1991;324:1385–1390. doi: 10.1056/NEJM199105163242002. [DOI] [PubMed] [Google Scholar]
- 60.Westin S, Kurokawa R, Nolte R, Wisely G, McInerney E, Rose D, Milburn M, Rosenfeld M, Glass C. Interactions controlling the assembly of nuclear-receptor heterodimers and co-activators. Nature. 1998;395:199–202. doi: 10.1038/26040. [DOI] [PubMed] [Google Scholar]
- 61.Willy P, Mangelsdorf D J. Unique requirements for retinoid-dependent transcriptional activation by the orphan receptor LXR. Genes Dev. 1997;11:289–298. doi: 10.1101/gad.11.3.289. [DOI] [PubMed] [Google Scholar]
- 62.Yoshida M, Kijima M, Akita M, Beppu T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem. 1990;265:17174–17179. [PubMed] [Google Scholar]
- 63.Zhang Y, Sun Z-W, Iratni R, Erdjument-Bromage H, Tempst P, Hampsey M, Reinberg D. SAP30, a novel protein conserved between human and yeast, is a component of a histone deacetylase complex. Mol Cell. 1998;1:1021–1031. doi: 10.1016/s1097-2765(00)80102-1. [DOI] [PubMed] [Google Scholar]