Targeted Disruption of CDK4 Delays Cell Cycle Entry with Enhanced p27Kip1 Activity (original) (raw)
Abstract
The mechanism by which cyclin-dependent kinase 4 (CDK4) regulates cell cycle progression is not entirely clear. Cyclin D/CDK4 appears to initiate phosphorylation of retinoblastoma protein (Rb) leading to inactivation of the S-phase-inhibitory action of Rb. However, cyclin D/CDK4 has been postulated to act in a noncatalytic manner to regulate the cyclin E/CDK2-inhibitory activity of p27Kip1 by sequestration. In this study we investigated the roles of CDK4 in cell cycle regulation by targeted disruption of the mouse CDK4 gene. _CDK4_−/− mice survived embryogenesis and showed growth retardation and reproductive dysfunction associated with hypoplastic seminiferous tubules in the testis and perturbed corpus luteum formation in the ovary. These phenotypes appear to be opposite to those of p27-deficient mice such as gigantism and gonadal hyperplasia. A majority of _CDK4_−/− mice developed diabetes mellitus by 6 weeks, associated with degeneration of pancreatic islets. Fibroblasts from _CDK4_−/− mouse embryos proliferated similarly to wild-type embryonic fibroblasts under conditions that promote continuous growth. However, quiescent _CDK4_−/− fibroblasts exhibited a substantial (∼6-h) delay in S-phase entry after serum stimulation. This cell cycle perturbation by CDK4 disruption was associated with increased binding of p27 to cyclin E/CDK2 and diminished activation of CDK2 accompanied by impaired Rb phosphorylation. Importantly, fibroblasts from _CDK4_−/− p27−/− embryos displayed partially restored kinetics of the G0-S transition, indicating the significance of the sequestration of p27 by CDK4. These results suggest that at least part of CDK4’s participation in the rate-limiting mechanism for the G0-S transition consists of controlling p27 activity.
In mammalian cells, the balance of growth-stimulatory and -inhibitory signals regulates the transition between proliferation and quiescence (42). Cyclin-dependent kinases (CDKs) activated by the regulatory subunits, cyclins, control cell cycle progression in all eukaryotes (21, 48, 52). Among several cyclin-CDK complexes, cyclin D- and cyclin E-dependent kinases play critical roles in regulating G1 progression and entry into S phase. D-type cyclins bind to and activate CDK4 during early to mid-G1. This is followed by activation of CDK2 in complex with cyclin E during late G1. These two types of CDKs seem to collaborate to determine the rate of the G1 to S transition (1, 40, 45). After cells enter S phase, cyclin A binds to and activates CDK2, which is required for maintenance of DNA replication (41). Three D-type cyclins (D1, D2, and D3) are expressed in tissue-specific but overlapping manners (32), whereas CDK4 and CDK2 and cyclins E and A are ubiquitously expressed. D-type cyclins also activate CDK6, a kinase closely related to CDK4 (2, 35). Although CDK6 and CDK4 are coexpressed in many cell types, it is unclear whether these two CDKs have completely overlapping functions.
Cyclin D-CDK4 plays an important role in inactivating the S-phase-inhibitory action of the retinoblastoma protein (Rb) by phosphorylation (10, 11, 19, 22, 60). During early G1, Rb is in a hypophosphorylated form and sequesters the E2F family of transcription factors (39). As cells proceed through G1, Rb is progressively phosphorylated, resulting in release of E2F from the sequestration and activation of a number of S-phase-specific genes by E2F. Accumulating evidence suggests that the kinase activity of cyclin D-CDK4 is at least partly responsible for the initial phosphorylation of Rb at specific sites, which allows subsequent phosphorylation of other sites, presumably by cyclin E-CDK2 (24, 29). Other Rb-related proteins, such as p130 and p107, could also be regulated by cyclin D-CDK4 in a phosphorylation-dependent manner (3, 9, 61), although details are not completely understood.
Cyclin D- and cyclin E-dependent kinases are inhibited by association with CDK inhibitor proteins, including p27Kip1 (53). We previously demonstrated that p27 plays a rate-limiting role in the transition between proliferation and quiescence by creating and examining mice deficient in p27 (25). T lymphocytes, oligodendrocytes, keratinocytes and ovarian granulosa cells in p27-deficient mice were refractory to growth-inhibitory signals (6, 30, 36, 59). The gigantism-like phenotype of p27-deficient mice further suggested that p27 determines the replicative capacity of cells in response to growth-inhibitory signals, while it remained unclear how p27 plays this role by interacting with multiple cyclin-CDK complexes. Although p27 has been categorized as a general inhibitor of the G1-S CDKs, cyclin D-CDK4 and cyclin E (or A)-CDK2 show different susceptibilities to inhibition by p27 (57). In proliferating cells, the association of p27 does not necessarily inhibit cyclin D-CDK4, whereas it always inactivates cyclin E (or A)-CDK2. Thus, cyclin D-CDK4 may sequester p27, controlling the amount of p27 available for inhibition of CDK2 activity. Therefore, cyclin D-CDK4 may play both catalytic and noncatalytic roles in the regulation of G1 progression. In this study, we attempted to determine the indispensable role(s) of CDK4 in cell cycle regulation by targeted disruption of the mouse CDK4 gene. Our analyses of CDK4-null mice and fibroblasts from these mice suggested that at least part of CDK4’s rate-limiting role for the G0-S transition consists of negative control of the activity of p27 by sequestration.
MATERIALS AND METHODS
Cloning and vector construction.
The CDK4 gene with its 5′ and 3′ flanking regions was cloned from a mouse 129/sv genomic library (Stratagene), with murine CDK4 cDNA (31) used as a probe. Exons 2 to 5 and a 3′ portion of exon 1 were deleted and replaced with the functional neomycin resistance gene cassette prepared from the pMC1-neo-poly(A)+ plasmid. A herpes simplex virus (HSV) thymidine kinase gene was inserted adjacent to the 3′ end of the CDK4 genomic fragment for the negative selection (47).
Targeted disruption of the CDK4 gene in ES cells.
CJ-7 ES cells (25, 58) were electroporated with the targeting vector and selected with G418 and ganciclovir, as described previously (25). The probe for the Southern analysis was a 1.2-kb _Bam_HI-_Eco_RI genomic fragment 5′ upstream to the region used for the targeting vector. We injected four of these clones individually into C57BL/6 blastocyst embryos (day 3.5), and the resulting male chimeras were crossed with wild-type C57BL/6 females to generate CDK4+/− offspring. The genotype of each mouse was determined by a PCR-based assay or Southern blotting by using DNA from part of the tail, sampled 14 to 21 days after birth.
Analyses of fertility and diabetes.
We examined the mating ability and fertility of _CDK4_−/− mice by keeping each mutant and a wild-type fertile partner together in a cage and checking daily for the presence of a vaginal plug. Females with plugs were separated and monitored throughout pregnancy, delivery, and nursing. Glucosuria was monitored in the morning (9 to 11 a.m.) by using Chemstrip (Boehringer Mannheim). Each mouse was analyzed for glucosuria on at least three different days.
Histology.
Tissues were fixed with 10% buffered formalin, embedded in paraffin, and cut in 3-μm-thick sections with a microtome. Slides were stained with hematoxylin and eosin (20).
Culture of embryonic fibroblasts.
Fibroblasts were prepared from day 12.5 embryos according to standard protocols (20) and cultured in Dulbecco’s modified minimum essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Cells from one embryo were cultured in a 60-mm-diameter culture dish until confluent (passage 0) and then kept under subconfluent conditions by passages with a dilution of 1:3. Following three passages, cells were serum starved by 72-h culture in DMEM supplemented with 0.1% FBS and then stimulated by 10% FBS. At different times, cells were pulse-labeled with 100 μM bromodeoxyuridine (BrdU) for 30 min and then harvested. For flow cytometry, cells were fixed with 70% ethanol, stained with 10 μg of fluorescein-conjugated anti-BrdU antibody (Pharmingen)/ml and then stained with 50 μg of propidium iodide/ml in the presence of 200 μg of RNase A/ml for 30 min at 37°C according to the manufacturer’s protocols. The samples were analyzed with a FACScan (Becton Dickinson), and the data were processed with Cell Quest (Becton Dickinson) software.
Immunoprecipitation and immunoblotting.
Cells were lysed by sonication in NP-40 lysis buffer (50 mM HEPES-KOH [pH 7.5], 150 mM NaCl, 0.1% NP-40, 10% glycerol, 1 mM EDTA, 2.5 mM EGTA, 1 mM dithiothreitol, 10 mM β-glycerophosphate, 1 mM NaF, 0.1 mM sodium orthovanadate, 0.2 mM phenylmethylsulfonyl fluoride (PMSF), 20 μg of aprotinin/ml, 20 μg of leupeptin/ml, 1 μg of pepstatin/ml, 10 μg of soy bean trypsin inhibitor/ml). Lysates were then clarified by centrifugation, and protein contents were measured by using the Bradford reagent (Sigma). CDK2-associated kinase activity was measured in immunoprecipitates by using histone H1 as a substrate (57). Fifty micrograms of protein was loaded onto a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel for quantitative immunoblotting. For the analysis of CDKs in complex with p27, lysates with 300 μg of protein were immunoprecipitated with 1 μg of affinity-purified anti-p27 antibody (57) or normal rabbit immunoglobulin G (IgG) as a control. For measurement of p27 in complex with cyclin E, 600 μg of protein was immunoprecipitated with 2 μg of anti-cyclin E antibody (M-20; Santa Cruz Biotechnology). Immune complexes were collected by incubation with protein A-agarose (Pierce) for 1 h at 4°C. After an extensive washing of the protein A-agarose beads with lysis buffer, immune complexes were subjected to SDS-PAGE. Immunoblotting was performed by using the following primary antibodies purchased from Neomarkers and Pharmingen: CDK2 (clone 2B6 plus 8D4), CDK4 (DCS-31), CDK6 (K6.90 plus K6.83), cyclin D1 (DCS-6), cyclin D2 (DCS-5.2), cyclin D3 (DCS-28.1), p27 (DCS-72.F6), and Rb (G3-245). The antibody specific for Rb with Ser-780 phosphorylation was obtained from New England Biolabs. Antibody binding was detected by using peroxidase-conjugated anti-mouse IgG (Pierce) and the Supersignal chemiluminescence reagent (Pierce). Signals on X-ray films were quantified by using GS-700 Imaging Densitometer and Molecular Analyst software (Bio-Rad).
RESULTS
CDK4 gene targeting.
First, we generated a _CDK4_-null mouse strain, using a standard gene targeting technique with murine embryonic stem (ES) cells (47). We constructed a genomic homologous recombination vector in which 81% of the CDK4 coding sequence (from the middle of exon 1 through exon 5) and the intervening introns were replaced with a neomycin resistance gene (Fig. 1a). Of the 348 ES clones examined after transfection and selection, 6 had legitimate homologous recombination, exhibiting the CDK4+/− genotype, and 2 exhibited germ line transmission in the resulting chimeras (Fig. 1b). CDK4+/− mice were overtly normal and fertile. We then set up intercross mating and cultured fibroblasts from embryos on day 12.5. No CDK4 protein was detected in _CDK4_−/− fibroblasts (Fig. 1c and d) by immunoblotting, indicating that a CDK4-null mutation had been created.
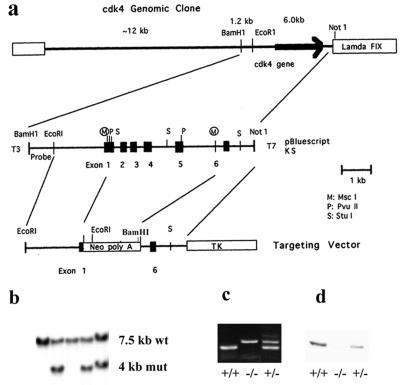
FIG. 1.
Gene targeting of the CDK4 gene in ES cells and generation of CDK4-null mice. (a) Targeting replacement vector. A genomic fragment containing the six exons encoding CDK4 was isolated from a 129/svj mouse genomic library, and a 6.0-kb _Eco_RI-_Not_I fragment was subcloned into the pBluescript plasmid vector. The homologous recombination was constructed by replacing a 2.2-kb _Msc_I fragment of this plasmid with a neomycin-phosphotransferase gene under the control of the thymidine kinase promoter for the positive selection. An HSV thymidine kinase gene under the control of the polyoma enhancer was then inserted at the 3′ side of the 1.2-kb homologous flanking region for the negative selection. A 1.2-kb _Bam_HI-_Eco_RI genomic fragment upstream of the 2.6-kb 5′ homologous region was used as a probe to distinguish the wild-type (wt) and mutant (mut) alleles. (b) Germ line transmission of the CDK4 mutation. The gene targeting event was identified by Southern blotting with _Bam_HI-digested genomic DNAs and the probe shown in panel a. Germ line transmission was confirmed by the Southern blot with genomic DNAs of the F1 offspring from crossbreeding of chimeras with wild-type C57BL/6 mice. An analysis of a representative F1 litter is shown. (c) PCR genotyping of day 12.5 embryos obtained from intercross between _CDK4_-heterozyous mice. Wild-type (+/+), homozygous (−/−), and heterozygous (+/−) mice were identified by the presence of PCR products specific for either the wt or mut allele. (d) Immunoblotting for CDK4 with CDK4 monoclonal antibody (DCS-31) with extracts of fibroblasts obtained from (+/+), (−/−), and (+/−) embryos.
Growth retardation in CDK4-deficient mice.
To characterize the impact of CDK4 deficiency on development and homeostasis in vivo, we examined _CDK4_−/− mice generated by intercross breeding of CDK4+/− mice (Fig. 2a). Of 98 mice born from the intercross, 22 (22%) were homozygotes, a figure which is approximately consistent with the Mendelian rule. However, six (27%) of the homozygotes died by 28 days after birth, while no wild type and only one heterozygote died during this period, indicating increased postnatal lethality of _CDK4_-null mice. Gross autopsy examination did not reveal major defects related to the lethality, while detailed pathological examinations are still ongoing. _CDK4_−/− mice were 20% smaller at birth than their littermates with the CDK4+/+ genotype (Fig. 2b). The growth retardation of the mutants became more obvious during prepuberty, and the adult homozygotes of both sexes were about 42 to 43% smaller than wild-type controls. Body weights of CDK4+/+, CDK4+/−, and _CDK4_−/− males at 7 weeks were 28.8 ± 3.5, 27.3 ± 1.2, and 17.2 ± 1.9 g, while those of CDK4+/+, CDK4+/−, and _CDK4_−/− females were 20.8 ± 0.9, 20.4 ± 1.8, and 11.9 ± 2.6 g, respectively (means ± standard deviations; n = 7). None of homozygous mice attained the size of the wild-type mice.
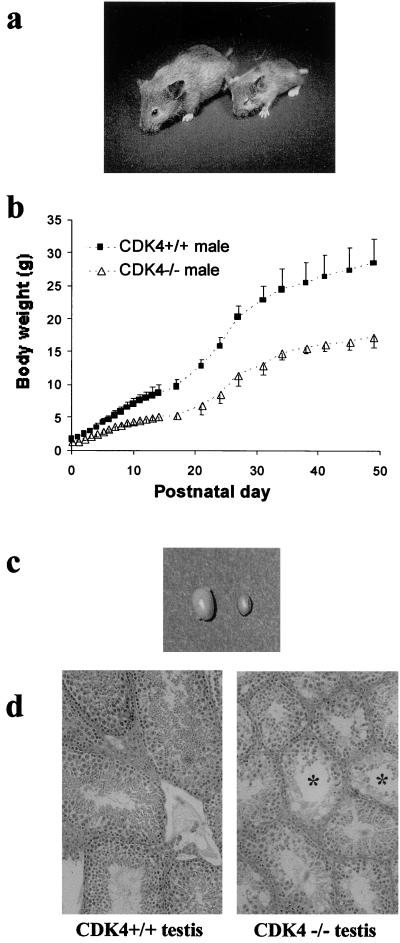
FIG. 2.
Growth retardation and testicular atrophy in _CDK4_−/− mice. (a) CDK4+/+ female (left) and the littermate _CDK4_−/− female (right) at 28 days after birth. (b) Growth curves of CDK4+/+ and _CDK4_−/− males. Body weights are indicated as means of mice with each genotype (n = 6), and bars represent standard deviations. (c) Testes from 12-week-old CDK4+/+ and _CDK4_−/− mice. Left, CDK4+/+; right, _CDK4_−/−. (d) Morphology of testes of 12-week-old mice. Sections (3 μm thick) were stained with hematoxylin-eosin. Asterisks indicate severely dysplastic seminiferous tubules. Magnification, ×200.
Testicular atrophy in CDK4-deficient mice.
Fertility depends on proliferation and differentiation in the gonads and other reproductive organs and could be decreased by perturbed cell cycle regulation (51, 59). We found that fertility was compromised in both _CDK4_−/− males and females. Only 10% of male homozygous mutants examined (n = 10) were fertile, whereas all female mutants (n = 10) were infertile. The testes of 12-week-old _CDK4_−/− mice were remarkably small and displayed dysplastic changes in histological examination (Fig. 2c and d). Cross sections of seminiferous epithelial tubules in _CDK4_−/− testes had smaller diameters than those in CDK4+/+ testes and showed reduced numbers of the spermatogonia/spermatocytes with perturbed layer formation. Mature spermatids were rarely found in CDK4+/+ testes. Thus, defective proliferation of gonocytes, together with hypoplastic supporting structure (i.e., Sertoli cells) of seminiferous epithelia (34), may contribute to impaired spermatogenesis in mutants.
Perturbed corpus luteum formation in CDK4−/− ovaries.
Granulosa cells in the ovary proliferate during follicular maturation and differentiate into luteal cells following ovulation (50). Ovaries of 8-week-old _CDK4_−/− mice exhibited some well-developed antral follicles and corpora luteum-like structures (Fig. 3b and d). However, the organization of granulosa/luteal cells in the corpora luteum-like structures was significantly disturbed, and aberrant luteinization was often observed with oocytes trapped in the middle (Fig. 3c). These observations suggest that female infertility in _CDK4_−/− mice may be associated with perturbed regulation of proliferation and differentiation of granulosa cells.
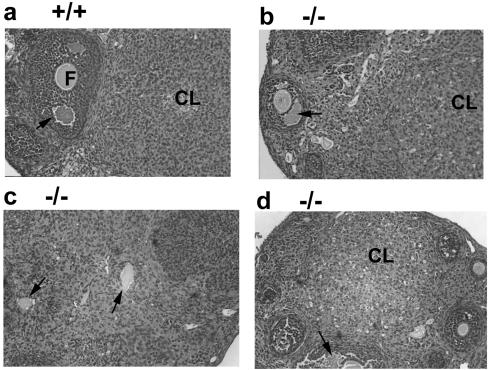
FIG. 3.
Perturbed differentiation of granulosa cells in _CDK4_−/− ovaries. (a) Ovary of 8-week-old CDK4+/+ wild-type mice. (b, c, d) Ovary of 8-week-old _CDK4_−/− mutant mice. Sections (3 μm thick) were stained with hematoxylin-eosin. Magnification, ×200. F, follicle; CL, corpus luteum. The arrows in panels a, b, and d indicate antral follicles, and those in panel c indicate oocytes trapped in structures showing abnormal luteinization.
Diabetes mellitus in _CDK4_−/− mice.
We found that a majority of adult _CDK4_−/− mice exhibit glucosuria (Table 1). While no _CDK4_−/− mice displayed glucosuria at 2 to 3 weeks of age, 60 to 80% of homozygous mutants started exhibiting glucosuria by 6 to 7 weeks. The rest of mutants did not develop this diabetic phenotype, at least not during our observations up to 40 weeks. Furthermore, _CDK4_−/− mice had degenerative changes in islets of Langerhans of the pancreas (Fig. 4). The pancreatic islets are an endocrine organ secreting insulin, glucagon, and other peptide hormones (56). Insufficient secretion of insulin from the islets is a major cause of clinical diabetes mellitus. At 3 weeks of age, _CDK4_−/− pancreas had fewer islets, many of which exhibited disorganized cellularity and dying cells with condensed nuclei (Fig. 4c). In 6-week-old _CDK4_−/− mice that showed glucosuria, most islets were markedly small, with only a few cells, as can be seen in Fig. 4d. No sign of inflammation, i.e., infiltration of lymphocytes or granulocytes, was observed in _CDK4_−/− pancreas. There was no significant change in exocrine acinar cells in pancreas of _CDK4_−/− mice. This diabetic phenotype with islet degeneration was observed in both males and females with no distortion. These data suggest that diabetes mellitus in _CDK4_−/− mice is associated with degeneration of islets of Langerhans.
TABLE 1.
Diabetes mellitus in _CDK4_−/− mice
| Genotype and age in weeks | No. of mice analyzed | No. of mice with glucosuria | % Glucosuria |
|---|---|---|---|
| _CDK4_−/− | |||
| 2 | 5 | 0 | 0 |
| 3 | 5 | 0 | 0 |
| 6–7 | 5 | 4 | 80 |
| 12 | 5 | 3 | 60 |
| 20 | 7 | 5 | 71 |
| 40 | 3 | 2 | 67 |
| CDK4+/+ | |||
| 6–7 | 6 | 0 | 0 |
| 40 | 6 | 0 | 0 |
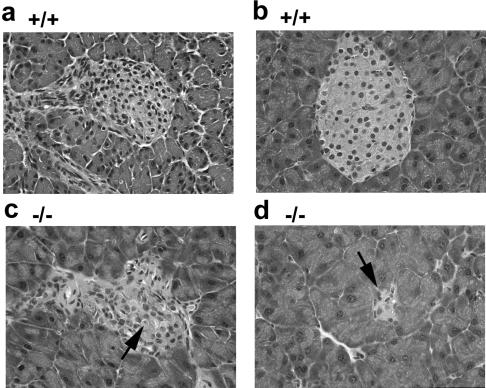
FIG. 4.
Degeneration of endocrine islets in the pancrease of _CDK4_−/− mice. (a) Pancreas of wild-type mice at 3 weeks of age. A well-developed islet is surrounded by exocrine pancreatic tissue. (b) Pancreas of wild-type mice at 6 weeks. (c) Pancreas of _CDK4_−/− mice at 3 weeks. The arrow indicates a representative condensed nucleus in the islet. (d) Pancreas of _CDK4_−/− mice at 6 weeks. The arrow indicates a severely degenerated islet. Magnification, ×400.
CDK6 expression in organs of _CDK4_−/− mice.
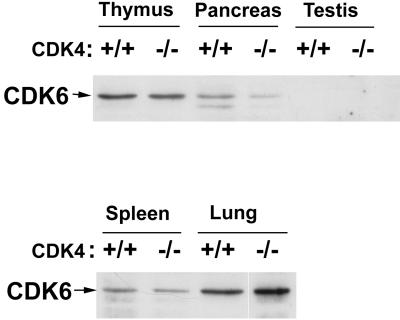
Since CDK4 and CDK6 possess common biochemical properties as Rb kinases (2, 35), we examined by immunoblotting whether expression of CDK6 was affected in organs of _CDK4_−/− mice (Fig. 5). In wild-type mice, CDK6 protein in the testis was almost undetectable, and that in the pancreas was less abundant than in the thymus and lung. In _CDK4_−/− mice, similar patterns of CDK6 expression were detected. Pancreatic expression of CDK6 appeared lower in _CDK4_−/− mice. Thus, there is apparently no compensatory increase in CDK6 expression, at least not in these organs of _CDK4_−/− mice.
FIG. 5.
Expression of CDK6 protein in tissues of 3-week-old CDK4+/+ and _CDK4_−/− mice. Extracts from the tissues indicated were analyzed by immunoblotting by using monoclonal CDK6 antibodies (K6.90 plus K6.83).
Growth of _CDK4_−/− embryonic fibroblasts.
As growth retardation and reproductive dysfunction of _CDK4_−/− mice suggested that cell cycle progression might be perturbed in CDK4-null cells, we studied growth properties of fibroblasts from _CDK4_−/− mouse embryos. Cultures of _CDK4_−/− fibroblasts were morphologically indistinguishable from those of CDK4+/+ fibroblasts. The growth curves of passage 4 _CDK4_−/− fibroblasts in the presence of 10% FBS were similar to those of CDK4+/+ fibroblasts from the same litters (Fig. 6a), and the distribution of _CDK4_−/− cells in different phases of the cell cycle during exponential growth was comparable to that of CDK4+/+ cells (Fig. 6b). Cells with these two genotypes displayed contact inhibition at similar cell densities. These observations were confirmed in experiments by using embryos from three different litters. Growth of _CDK4_−/− fibroblasts at earlier passages also appeared to be similar to that of wild-type cells (data not shown). These data suggest that CDK4 disruption has a minimal effect on the rate of continuous proliferation of embryonic fibroblasts.
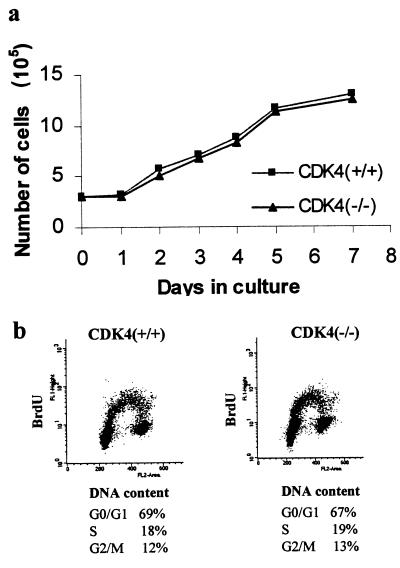
FIG. 6.
Continuous proliferation is not affected by targeted disruption of CDK4. (a) Growth curves of CDK4+/+ and _CDK4_−/− embryonic fibroblasts at passage 4. Embryonic fibroblasts were harvested from CDK4+/+ and _CDK4_−/− embryos (day 12.5 postcoitus) and cultured in the presence of 10% FBS. (b) Cell cycle analysis by flow cytometry after staining with BrdU for DNA replication and propidium iodide for DNA content. Cells at day 3 of passage 4 were pulse-labeled with 100 μM BrdU for 30 min, stained with propidium iodide and anti-BrdU antibody, and subjected to flow cytometry. These data represent three separate experiments with different clones of embryonic fibroblasts.
Delayed cell cycle entry of _CDK4_−/− fibroblasts.
To further determine whether CDK4 has a rate-limiting role in the transition between quiescence and proliferation, we examined cell cycle entry of _CDK4_−/− and CDK4+/+ fibroblasts from quiescence. Passage 4 fibroblasts were starved by 72-h culture in medium supplemented with 0.1% FBS and then stimulated by the addition of 10% FBS. Flow cytometric analysis of cells stained with anti-BrdU antibody and propidium iodide demonstrated that by 18 h, wild-type fibroblasts started to enter the S phase, while most of _CDK4_−/− cells did not enter S phase until 21 to 24 h (Fig. 7a and b). These results indicate that the absence of CDK4 significantly delays the G0-S transition but does not prevent it.
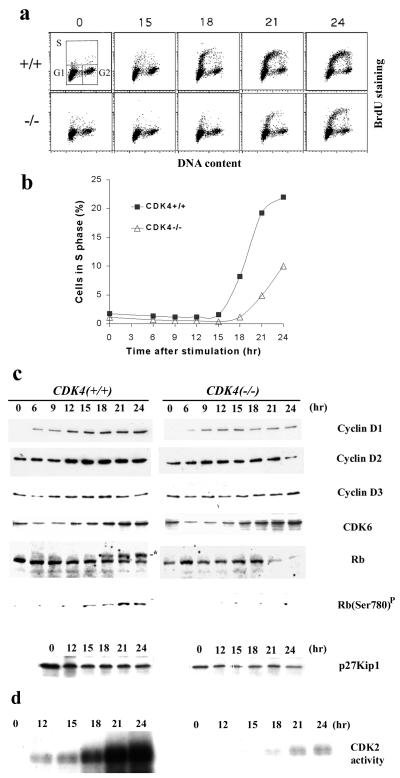
FIG. 7.
Delayed entry into the S phase of _CDK4_−/− fibroblasts after serum stimulation. (a) Cell cycle analysis by staining with BrdU and propidium iodide. Embryonic fibroblasts were harvested from CDK4+/+ and _CDK4_−/− embryos (day 12.5 postcoitus) and cultured in the presence of 10% FBS for three passages. Quiescence was then induced by culturing cells in 0.1% serum for 72 h (time zero), followed by stimulation of cells with medium containing 10% serum. Cells were pulse-labeled with 100 μM BrdU for 30 min and harvested at the times indicated and labeled hours after stimulation. Cells were stained with anti-BrdU antibody and propidium iodide and subjected to flow cytometry. The x axis represents the DNA content of each cell by the intensity of propidium iodide staining, and the y axis demonstrates the amount of BrdU incorporated into newly synthesized DNA in each cell by the intensity of BrdU staining. The upper left panel shows the gate settings for cells in G1, S, and G2/M. (b) Kinetics of S-phase entry. The percentage of cells in S phase from each time point was figured and plotted according to the flow cytometric analysis (a). (c) Expression of cell cycle-regulatory proteins and Rb phosphorylation. Cells were harvested at the times indicated, and lysates were prepared. Lysates with 50 μg of proteins were analyzed by SDS-PAGE, followed by immunoblotting for cell cycle-regulatory proteins as indicated. The asterisk in the lane for Rb indicates the hyperphosphorylated form of Rb. Rb(Ser780)P indicates immunoblotting with an antibody specific for Rb phosphorylated on the Ser-780 residue (New England Biolabs). (d) CDK2-associated kinase activity. CDK2-associated protein complexes were immunoprecipitated from cell lysates prepared at the times indicated, and histone H1-kinase activity was measured in vitro as described in Materials and Methods. These data represent three separate experiments with different clones of embryonic fibroblasts.
The delay in S-phase entry of _CDK4_−/− fibroblasts was associated with diminished and delayed expression of cyclin D1 protein (Fig. 7c), suggesting that growth factor-dependent signals that regulate the induction of cyclin D1 (7, 27, 28) are also influenced by CDK4 deficiency. The retinoblastoma protein (Rb), a well-characterized substrate for cyclin D- and E-dependent kinases, became hyperphosphorylated just before CDK4+/+ cells entered S phase, consistent with previous studies (60). In contrast, Rb remained mostly hypophosphorylated in _CDK4_−/− cells up to 24 h after serum stimulation, and the total amount of Rb was lower than that in wild-type cells (Fig. 7c). We further measured phosphorylation of the serine-780 residue of Rb, which is catalyzed specifically by cyclin D-dependent kinases (24). While Ser-780 phosphorylation accumulated significantly during cell cycle entry of wild-type cells, it was almost undetectable in serum-stimulated _CDK4_−/− cells up to 24 h, suggesting that the cyclin D-dependent phosphorylation of Rb was defective. We found that even during exponential growth, the amount of Rb in _CDK4_−/− cells was about 30 to 50% of that in CDK4+/+ cells, and phosphorylation was diminished (data not shown). The expression of CDK6 was increased by serum stimulation in _CDK4_−/− and CDK4+/+ cells in similar manners. The expression of cyclins D2 and D3 did not differ significantly for _CDK4_−/− and CDK4+/+ cells. In addition, CDK4 was constantly expressed in CDK4+/+ cells during serum starvation and restimulation (data not shown). The cellular amount of p27 decreased somewhat when cells entered the cell cycle for both _CDK4_−/− and CDK4+/+ cells. We also found that levels of p27 in _CDK4_−/− cells tended to be 30 to 40% lower than those in CDK4+/+ cells. CDK2-associated kinase activity accumulated as wild-type cells entered S phase, whereas the induction of the kinase activity was substantially diminished and delayed in _CDK4_−/− cells (Fig. 7d). The cellular levels of CDK2 protein in _CDK4_−/− and CDK4+/+ cells were comparable and not affected by serum starvation and restimulation (data not shown). These data suggest that CDK4 is part of the rate-limiting mechanism for the G0-S transition.
Increased binding of p27 to other CDKs in _CDK4_−/− fibroblasts.
To determine whether alterations in p27-CDK interactions are involved in the delayed cell cycle entry of _CDK4_−/− cells, we measured the amounts of CDKs bound to p27 in cells 18 h after serum stimulation by immunoprecipitation, followed by immunoblotting (Fig. 8a). Wild-type cells showed a substantial amount of CDK4 in complex with p27, while no CDK4 was detected in complex with p27 in _CDK4_−/− cells. We detected increases in the amounts of CDK2 and CDK6 bound to p27 in serum-stimulated _CDK4_−/− cells, by 2.0- and 3.4-fold, respectively, compared with those in wild-type cells. In particular, two forms of CDK2 with different electrophoretic migrations were detected in the p27-associated complex from CDK4-null cells, whereas only the faster-migrating form of CDK2 bound to p27 in wild-type cells. Previous studies suggested that the faster-migrating form of CDK2 is phosphorylated on the Thr-160 residue by CDK-activating kinase (CAK) (17). These results suggest that p27 in CDK4-null cells may bind not only CAK-phosphorylated cyclin E-CDK2 complexes but also newly associated complexes that have not been phosphorylated by CAK. To further determine whether a larger amount of p27 is associated with cyclin E-CDK2 in serum-stimulated _CDK4_−/− fibroblasts than in wild-type cells, we measured the amounts of p27 in the cyclin E immunoprecipitates from cell lysates prepared 18 h after serum stimulation. The amount of p27 associated with cyclin E was 2.4-fold greater in _CDK4_−/− cells than in CDK4+/+ cells, although similar amounts of cyclin E are expressed (Fig. 8b and c). These results suggest that the elimination of CDK4 results in the association of more p27 protein with cyclin E-CDK2 and cyclin D-CDK6 complexes.
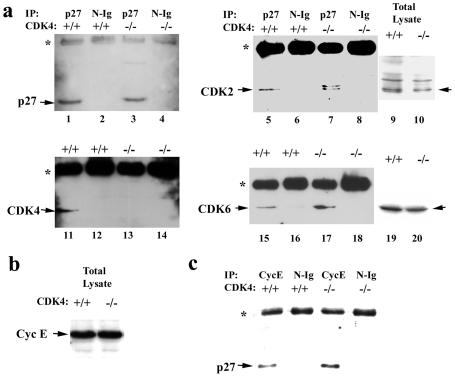
FIG. 8.
Increased binding of p27Kip1 to cyclin E-CDK2 in serum-stimulated fibroblasts with targeted disruption of CDK4. (a) CDK proteins in complex with p27. Fibroblasts from _CDK4_−/− and CDK4+/+ mouse embryos were serum starved for 72 h (time zero) and then restimulated with 10% FBS serum for 18 h, as described in Materials and Methods and the legend to Fig. 7. Cell lysates were immunoprecipitated with affinity-purified anti-p27 polyclonal antibody or normal IgG (N-Ig) as a control. The immune complexes were then analyzed by immunoblotting by using anti-p27 (lanes 1 to 4), -CDK2 (lanes 5 to 8), -CDK4 (lanes 11 to 14), and -CDK6 (lanes 15 to 18) monoclonal antibodies. Total lysates without immunoprecipitation were also analyzed by immunoblotting with anti-CDK2 and -CDK6 antibodies (lanes 9, 10, 19, and 20). The asterisks indicate heavy-chain IgG cross-reactive with secondary antibody used for immunoblotting. (b) Expression of cyclin E in _CDK4_−/− and CDK4+/+ fibroblasts at 18 h after serum stimulation measured by immunoblotting. (c) p27 protein in complex with cyclin E in in _CDK4_−/− and CDK4+/+ fibroblasts at 18 h after serum stimulation. These data represent three separate experiments with different clones of embryonic fibroblasts.
Restored kinetics of the G0-S transition in _CDK4_−/− _p27_−/− fibroblasts.
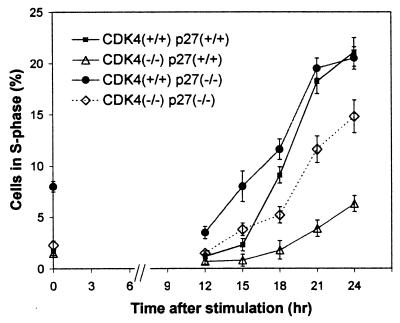
To further determine whether enhanced activity of p27 to bind cyclin E-CDK2 plays a significant role in the delayed cell cycle entry of CDK4-null fibroblasts, we examined fibroblasts from mouse embryos lacking both CDK4 and p27, which we generated by crossbreeding between the CDK4- and p27-deficient mouse strains (25). In the presence of 10% FBS, all cell types examined, such as _CDK4_−/− p27+/+, CDK4+/+ _p27_−/−, _CDK4_−/− _p27_−/−, and CDK4+/+ _p27_−/−, displayed similar patterns of cell cycle distribution, according to the double staining with BrdU and propidium iodide (data not shown). After serum starvation and restimulation, _CDK4_−/− _p27_−/− cells, which lacked both CDK4 and p27, entered S phase with only a modest delay compared to the time to entry of wild-type cells (Fig. 9). This contrasted sharply with the marked delay of _CDK4_−/− p27+/+ cells. In addition, CDK4+/+ _p27_−/− cells entered S phase with similar or slightly accelerated kinetics compared with those of wild-type cells. These observations that the elimination of p27 substantially restored the kinetics of cell cycle entry in CDK4-null cells suggest that the rate-limiting function of CDK4 for the G0-S transition depends at least partly on its activity in sequestering p27.
FIG. 9.
Restoration of cell cycle delay in CDK4-null fibroblasts by elimination of p27Kip1. Fibroblasts were harvested from embryos with the following genotypes: _CDK4_−/− p27+/+, CDK4+/+ _p27_−/−, _CDK4_−/− _p27_−/−, and CDK4+/+ p27+/+. Cells were serum starved for 72 h and then restimulated with 10% FBS. Cells were pulse-labeled with BrdU for 30 min before being harvested at the times indicated. Cells were stained with anti-BrdU antibody and subjected to flow cytometry, as described in Fig. 7. The x axis represents time (in hours) after the readdition of 10% FBS, and the y axis represents the percentages of cells in S phase determined by flow cytometry with propidium iodide and BrdU staining, as described in Materials and Methods and Fig. 7. Data are indicated as means ± standard deviations (n = 3), from experiments with different clones of embryonic fibroblasts.
DISCUSSION
In this study, we demonstrated that _CDK4_−/− mice survive embryogenesis, suggesting that CDK4 is not essential for mouse development. Furthermore, examination of _CDK4_−/− fibroblasts suggested that CDK4 is dispensable during continuous proliferation. However, our analysis with quiescent _CDK4_−/− fibroblasts indicated that CDK4 plays a rate-limiting role when quiescent cells enter the cell cycle. Growth retardation, reproductive dysfunction, and degeneration of pancreatic islets in _CDK4_−/− mice further suggest that CDK4 plays a critical role in in vivo regulation of cell cycle progression, the lack of which other CDKs cannot completely compensate for.
The dwarfism-like phenotype of _CDK4_-null mice is reminiscent of those in cyclin D1-deficient mice (13, 55) and transgenic mice with high copy numbers of the Rb gene (4). It also presents an image opposite to that of the gigantism-like phenotypes of mice deficient in the CDK inhibitor p27 (25) and mice lacking p18INK4c, an inhibitor of CDK4 and CDK6 (14, 16, 38). These observations suggest that the genetic pathway involving p27, p18, cyclin D1, CDK4, and Rb may be important for regulation of animal growth (46). Perturbed G1 progression in serum-stimulated _CDK4_−/− embryonic fibroblasts further implies that growth retardation of _CDK4_−/− mice is associated with defective cell cycle regulation during development. However, it is also possible that the dwarfism in _CDK4_−/− mice is due to another problem, e.g., a neurological or an endocrine disorder.
The gonads are highly proliferative organs. In the adult testis, germ cells continuously proliferate and differentiate into spermatids in response to hormonal signals. Disturbed control of proliferation in the gonads could lead to reduced or abrogated fertility. We have shown that CDK4 deficiency leads to reproductive dysfunction in both males and females. The testicular atrophy in _CDK4_−/− males showed some similarity to that in mice lacking E2F-1 (15, 62). Phosphorylation of Rb by cyclin D-CDK4 activates the transcriptional activity of E2F-1 by releasing E2F-1 from sequestration (37). Thus, this genetic pathway seems to be critical for proper regulation of spermatogenesis.
In contrast with proliferating spermatogonia in the testis, oocytes in the adult ovary are arrested in meiosis, while somatic granulosa cells in the follicle proliferate and differentiate in response to gonadotropins (50). Maintenance of the oocyte function depends on interactions between oocytes and granulosa cells via cell-cell contact and paracrine factors. Furthermore, upon ovulation, granulosa cells differentiate into luteal cells, which secrete steroid and peptide hormones required for the maintenance of pregnancy. _CDK4_−/− females were infertile, and their ovaries exhibited disturbed cellularity in corpora luteum and apparently abnormal luteinization. These morphological changes in _CDK4_−/− ovaries looked different from those in infertile _cyclin D2_−/− mice (51, 54). _cyclin D2_−/− ovaries had only small follicles and are defective in FSH-dependent proliferation of granulosa cells. In contrast, the absence of CDK4 seems to allow granulosa cells to proliferate to form large follicles, although the kinetics of differentiation may be disturbed. This difference between _cyclin D2_−/− and _CDK4_−/− ovaries implies that in the absence of CDK4, cyclin D2 may activate other CDKs to somehow promote granulosa cell proliferation. Female mice lacking p27 are also infertile in association with hyperproliferation of granulosa cells during luteal differentiation (25, 59). These studies recapitulate the importance of the pathway involving cyclin D2, CDK4, and p27 in the homeostasis of the proliferation and differentiation of granulosa cells.
We have found that a majority of _CDK4_−/− mice develop diabetes mellitus, associated with degenerative changes in pancreatic islets. This spontaneous development of nonobese diabetes resembles that in NOD mice or BB rats, which are animal models of autoimmune type I diabetes (8, 33). However, _CDK4_−/− pancreas displayed no sign of insulitis, the characteristic inflammation of islets due to autoimmunity. The marked decrease in cell numbers of _CDK4_−/− pancreatic islets and the presence of apoptotic cells suggest that CDK4 plays a critical role for the postnatal proliferation (and possibly for the maintenance) of these endocrine islet cells. The loss of islets, and especially of insulin-secreting B cells, leads to the development of diabetes (56). It is presently unclear why approximately 30% of CDK4-null mice do not develop diabetes. Although diabetes could affect postnatal growth, all _CDK4_−/− mice display similar extents of growth retardation whether or not they develop symptomatic diabetes. In addition, postnatal death often observed by 4 weeks of age does not seem to be related to diabetes, since no _CDK4_−/− mice developed glucosuria until 5 to 6 weeks. We are currently investigating the mechanism of the incomplete penetrance of diabetes and its relationship to other phenotypes of these mutant animals.
The delayed cell cycle entry of _CDK4_−/− fibroblasts with impaired Rb phosphorylation indicates that CDK4 participates in the rate-limiting mechanism for the G0-S transition. Diminished phosphorylation of Ser-780 of Rb, a specific target of cyclin D-dependent kinases (24), further implies that in fibroblasts, CDK4 plays a central role in the initiation of Rb phosphorylation. Although CDK6 is bound to D-type cyclins, Rb kinase activity detected in CDK6 immunoprecipitates is very weak in both wild-type and _CDK4_−/− fibroblasts (18a). The question of whether CDK6 is essential for proliferation without CDK4 awaits future experiments to inactivate CDK6 in _CDK4_−/− cells by gene targeting or other methods.
Our data also recapitulate the significance of CDK4-p27 interaction for the control of cyclin E-CDK2 activation. A larger amount of p27 that is free from sequestration could set a higher inhibitory threshold for the cyclin E-CDK2 activity. Therefore, p27 may act as an intermediate coordinator of CDK4 and CDK2 activation. The partial restoration of the kinetics of the G0-S transition in _CDK4_−/− _p27_−/− fibroblasts supports the hypothesis that CDK4 functions as an upstream regulator of p27, although the enhanced proliferation of _CDK4_−/− _p27_−/− cells may involve other regulatory mechanism(s) independent of the CDK4-p27 interaction, such as redistribution of other CDK inhibitors and modifications of CDK proteins. The mechanism of this enhanced S-phase entry is complex, since elevated CDK2 activity by p27 elimination might also compensate for the absence of CDK4, especially in Rb phosphorylation. We have observed that in serum-stimulated _CDK4_−/− _p27_−/− cells, the amounts of hyperphosphorylated Rb are slightly increased compared with those in _CDK4_−/− p27+/+ cells (data not shown).
In proliferating cells, a majority of p27 molecules are in complex with cyclin D-dependent kinases (44), which does not necessarily inhibit the kinase activity (5, 57). In contrast, cyclin E-CDK2 and cyclin A-CDK2 appear to be inhibited whenever they bind to p27 (5, 57). Moreover, studies with inducible expression of p27 or in vitro-purified proteins have demonstrated that the amount of p27 required for inhibition of cyclin D-CDK4 is much larger than that required for the inhibition of cyclin A-CDK2 (5, 43). Our study together with these other studies suggests the significance of the p27-regulatory action of CDK4 in the G0-S transition. The p27 reservoir function of CDK4 may allow multiple modes of control of G1-CDK activity in response to various extracellular signals. For instance, transforming growth factor β decreases the expression of CDK4 (12) and induces the CDK4-specific inhibitor p15INK4b (18, 49). These changes will disrupt the cyclin D-CDK4-p27 complex, leading to the inhibition of cyclin E-CDK2 and cyclin A-CDK2. Growth factor deprivation, differentiation, contact inhibition, or loss of anchorage will also decrease the expression of cyclin D and/or CDK4 (23, 26, 32, 44, 63), resulting in the mobilization of p27 to the CDK2 complexes. In contrast, when cells enter the cell cycle from quiescence, mitogenic signals facilitate complex formation of cyclin D-CDK4 (7), which will sequester p27 and allow the timely activation of cyclin E-CDK2.
Growth retardation and reproductive dysfunction in CDK4-null mice present an opposite image to the phenotype of mice deficient in p27, such as gigantism and gonadal hyperplasia (14, 25, 38). These observations imply that p27 and CDK4 genetically counteract to regulate animal growth and reproductive function, which may depend on the stoichiometric interaction between these two molecules. Interestingly, 7-week-old mice deficient in both p27 and CDK4 (n = 3) are 18 to 33% smaller than wild-type litter mice, whereas CDK4-null mice with intact p27 are 35 to 57% smaller than wild-type controls (24a). CDK4- and p27-null mice provide unique models in which we can further investigate interactions of cell cycle-regulatory proteins and their roles in development and oncogenesis.
ACKNOWLEDGMENTS
We thank Lida Aris and Angel Alvarez for technical assistance, Vera C. Soares for blastocyst injections, Rex Hess and Geula Gibori for comments on morphology of testes and ovaries, respectively, and William T. Beck for helpful suggestions.
This work was supported by funds provided to H.K. by the American Cancer Society, Illinois Division (no. 98-41), the Campus Research Board of the University of Illinois, and the Cancer Center of University of Illinois at Chicago and by funds provided to A.K. from the National Institutes of Health (GM52597) and the Pew Foundation. T.T. was supported by Osaka University.
REFERENCES
- 1.Ando K, Ajchenbaum-Cymbalista F, Griffin J D. Regulation of G1/S transition by cyclins D2 and D3 in hematopoietic cells. Proc Natl Acad Sci USA. 1993;90:9571–9575. doi: 10.1073/pnas.90.20.9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bates S, Bonetta L, MacAllan D, Parry D, Holder A, Dickson C, Peters G. CDK6 (PLSTIRE) and CDK4 (PSK-J3) are a distinct subset of the cyclin-dependent kinases that associate with cyclin D1. Oncogene. 1994;9:71–79. [PubMed] [Google Scholar]
- 3.Beijersbergen R L, Carlee L, Kerkhoven R M, Bernards R. Regulation of the retinoblastoma protein-related p107 by G1 cyclin complexes. Genes Dev. 1995;9:1340–1353. doi: 10.1101/gad.9.11.1340. [DOI] [PubMed] [Google Scholar]
- 4.Bignon Y J, Chen Y, Chang C Y, Riley D J, Windle J J, Mellon P L, Lee W H. Expression of a retinoblastoma transgene results in dwarf mice. Genes Dev. 1993;7:1654–1662. doi: 10.1101/gad.7.9.1654. [DOI] [PubMed] [Google Scholar]
- 5.Blain S W, Montalvo E, Massague J. Differential interaction of the cyclin-dependent kinase (Cdk) inhibitor p27Kip1 with cyclin A-Cdk2 and cyclin D2-Cdk4. J Biol Chem. 1997;272:25863–25872. doi: 10.1074/jbc.272.41.25863. [DOI] [PubMed] [Google Scholar]
- 6.Casaccia-Bonnefil P, Tikoo R, Kiyokawa H, Friedrich V J, Chao M V, Koff A. Oligodendrocyte precursor differentiation is perturbed in the absence of the cyclin-dependent kinase inhibitor p27Kip1. Genes Dev. 1997;11:2335–2346. doi: 10.1101/gad.11.18.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng M, Sexl V, Sherr C J, Roussel M F. Assembly of cyclin D-dependent kinase and titration of p27Kip1 regulated by mitogen-activated protein kinase kinase (MEK1) Proc Natl Acad Sci USA. 1998;95:1091–1096. doi: 10.1073/pnas.95.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheta D. Animal models of type I (insulin-dependent) diabetes mellitus. J Pediatr Endocrinol Metab. 1998;11:11–19. doi: 10.1515/jpem.1998.11.1.11. [DOI] [PubMed] [Google Scholar]
- 9.Dong F, Cress W D J, Agrawal D, Pledger W J. The role of cyclin D3-dependent kinase in the phosphorylation of p130 in mouse BALB/c 3T3 fibroblasts. J Biol Chem. 1998;273:6190–6195. doi: 10.1074/jbc.273.11.6190. [DOI] [PubMed] [Google Scholar]
- 10.Dowdy S F, Hinds P W, Louie K, Reed S I, Arnold A, Weinberg R A. Physical interaction of the retinoblastoma protein with human D cyclins. Cell. 1993;73:499–511. doi: 10.1016/0092-8674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- 11.Ewen M E, Sluss H K, Sherr C J, Matsushime H, Kato J, Livingston D M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993;73:487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- 12.Ewen M E, Sluss H K, Whitehouse L L, Livingston D M. TGF beta inhibition of Cdk4 synthesis is linked to cell cycle arrest. Cell. 1993;74:1009–1020. doi: 10.1016/0092-8674(93)90723-4. [DOI] [PubMed] [Google Scholar]
- 13.Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 1995;9:2364–2372. doi: 10.1101/gad.9.19.2364. [DOI] [PubMed] [Google Scholar]
- 14.Fero M L, Rivkin M, Tasch M, Porter P, Carow C E, Firpo E, Polyak K, Tsai L H, Broudy V, Perlmutter R M, Kaushansky K, Roberts J M. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell. 1996;85:733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 15.Field S J, Tsai F Y, Kuo F, Zubiaga A M, Kaelin W G J, Livingston D M, Orkin S H, Greenberg M E. E2F-1 functions in mice to promote apoptosis and suppress proliferation. Cell. 1996;85:549–561. doi: 10.1016/s0092-8674(00)81255-6. [DOI] [PubMed] [Google Scholar]
- 16.Franklin D S, Godfrey V L, Lee H, Kovalev G I, Schoonhoven R, Chen-Kiang S, Su L, Xiong Y. CDK inhibitors p18(INK4c) and p27(Kip1) mediate two separate pathways to collaboratively suppress pituitary tumorigenesis. Genes Dev. 1998;12:2899–2911. doi: 10.1101/gad.12.18.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu Y, Rosenblatt J, Morgan D O. Cell cycle regulation of CDK2 activity by phosphorylation of Thr160 and Tyr15. EMBO J. 1992;11:3995–4005. doi: 10.1002/j.1460-2075.1992.tb05493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hannon G J, Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994;371:257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 18a.Hesabi, B., T. Tsutsui, and H. Kiyokawa. Unpublished observations.
- 19.Hinds P W, Mittnacht S, Dulic V, Arnold A, Reed S I, Weinberg R A. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992;70:993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- 20.Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the mouse embryo. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 21.Hunter T, Pines J. Cyclins and cancer. II. Cyclin D and CDK inhibitors come of age. Cell. 1994;79:573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 22.Kato J, Matsushime H, Hiebert S W, Ewen M E, Sherr C J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993;7:331–342. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- 23.Kato J Y, Sherr C J. Inhibition of granulocyte differentiation by G1 cyclins D2 and D3 but not D1. Proc Natl Acad Sci USA. 1993;90:11513–11517. doi: 10.1073/pnas.90.24.11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitagawa M, Higashi H, Jung H K, Suzuki-Takahashi I, Ikeda M, Tamai K, Kato J, Segawa K, Yoshida E, Nishimura S, Taya Y. The consensus motif for phosphorylation by cyclin D1-Cdk4 is different from that for phosphorylation by cyclin A/E-Cdk2. EMBO J. 1996;15:7060–7069. [PMC free article] [PubMed] [Google Scholar]
- 24a.Kiyokawa, H. Unpublished observations.
- 25.Kiyokawa H, Kineman R D, Manova-Todorova K O, Soares V C, Hoffman E S, Ono M, Khanam D, Hayday A C, Frohman L A, Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1) Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 26.Kiyokawa H, Richon V M, Rifkind R A, Marks P A. Suppression of cyclin-dependent kinase 4 during induced differentiation of erythroleukemia cells. Mol Cell Biol. 1994;14:7195–7203. doi: 10.1128/mcb.14.11.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ladha M H, Lee K Y, Upton T M, Reed M F, Ewen M E. Regulation of exit from quiescence by p27 and cyclin D1-CDK4. Mol Cell Biol. 1998;18:6605–6615. doi: 10.1128/mcb.18.11.6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lavoie J N, L’Allemain G, Brunet A, Muller R, Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 29.Lundberg A S, Weinberg R A. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18:753–761. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo Y, Marx S O, Kiyokawa H, Koff A, Massague J, Marks A R. Rapamycin resistance tied to defective regulation of p27Kip1. Mol Cell Biol. 1996;16:6744–6751. doi: 10.1128/mcb.16.12.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsushime H, Ewen M E, Strom D K, Kato J Y, Hanks S K, Roussel M F, Sherr C J. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell. 1992;71:323–334. doi: 10.1016/0092-8674(92)90360-o. [DOI] [PubMed] [Google Scholar]
- 32.Matsushime H, Roussel M F, Ashmun R A, Sherr C J. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991;65:701–713. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- 33.McDuffie M. Genetics of autoimmune diabetes in animal models. Curr Opin Immunol. 1998;10:704–709. doi: 10.1016/s0952-7915(98)80092-7. [DOI] [PubMed] [Google Scholar]
- 34.McLaren A. Gonad development: assembling the mammalian testis. Curr Biol. 1998;8:R175–R177. doi: 10.1016/s0960-9822(98)70104-6. [DOI] [PubMed] [Google Scholar]
- 35.Meyerson M, Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol. 1994;14:2077–2086. doi: 10.1128/mcb.14.3.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Missero C, Di Cunto F, Kiyokawa H, Koff A, Dotto G P. The absence of p21Cip1/WAF1 alters keratinocyte growth and differentiation and promotes ras-tumor progression. Genes Dev. 1996;10:3065–3075. doi: 10.1101/gad.10.23.3065. [DOI] [PubMed] [Google Scholar]
- 37.Mulligan G, Jacks T. The retinoblastoma gene family: cousins with overlapping interests. Trends Genet. 1998;14:223–229. doi: 10.1016/s0168-9525(98)01470-x. [DOI] [PubMed] [Google Scholar]
- 38.Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh D Y, Nakayama K. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 39.Nevins J R, Leone G, DeGregori J, Jakoi L. Role of the Rb/E2F pathway in cell growth control. J Cell Physiol. 1997;173:233–236. doi: 10.1002/(SICI)1097-4652(199711)173:2<233::AID-JCP27>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 40.Ohtsubo M, Roberts J M. Cyclin-dependent regulation of G1 in mammalian fibroblasts. Science. 1993;259:1908–1912. doi: 10.1126/science.8384376. [DOI] [PubMed] [Google Scholar]
- 41.Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992;11:961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pardee A B. G1 events and regulation of cell proliferation. Science. 1989;246:603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- 43.Polyak K, Lee M H, Erdjument-Bromage H, Koff A, Roberts J M, Tempst P, Massague J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 44.Poon R Y, Toyoshima H, Hunter T. Redistribution of the CDK inhibitor p27 between different cyclin. CDK complexes in the mouse fibroblast cell cycle and in cells arrested with lovastatin or ultraviolet irradiation. Mol Biol Cell. 1995;6:1197–1213. doi: 10.1091/mbc.6.9.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quelle D E, Ashmun R A, Shurtleff S A, Kato J Y, Bar-Sagi D, Roussel M F, Sherr C J. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- 46.Raff M C. Size control: the regulation of cell numbers in animal development. Cell. 1996;86:173–175. doi: 10.1016/s0092-8674(00)80087-2. [DOI] [PubMed] [Google Scholar]
- 47.Ramirez-Solis R, Bradley A. Advances in the use of embryonic stem cell technology. Curr Opin Biotechnol. 1994;5:528–533. doi: 10.1016/0958-1669(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 48.Reed S I. Control of the G1/S transition. Cancer Surv. 1997;29:7–23. [PubMed] [Google Scholar]
- 49.Reynisdottir I, Polyak K, Iavarone A, Massague J. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev. 1995;9:1831–1845. doi: 10.1101/gad.9.15.1831. [DOI] [PubMed] [Google Scholar]
- 50.Richards J S, Fitzpatrick S L, Clemens J W, Morris J K, Alliston T, Sirois J. Ovarian cell differentiation: a cascade of multiple hormones, cellular signals, and regulated genes. Recent Prog Horm Res. 1995;50:223–254. doi: 10.1016/b978-0-12-571150-0.50014-7. [DOI] [PubMed] [Google Scholar]
- 51.Robker R L, Richards J S. Hormone-induced proliferation and differentiation of granulosa cells: a coordinated balance of the cell cycle regulators cyclin D2 and p27Kip1. Mol Endocrinol. 1998;12:924–940. doi: 10.1210/mend.12.7.0138. [DOI] [PubMed] [Google Scholar]
- 52.Sherr C J. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 53.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 54.Sicinski P, Donaher J L, Geng Y, Parker S B, Gardner H, Park M Y, Robker R L, Richards J S, McGinnis L K, Biggers J D, Eppig J J, Bronson R T, Elledge S J, Weinberg R A. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature. 1996;384:470–474. doi: 10.1038/384470a0. [DOI] [PubMed] [Google Scholar]
- 55.Sicinski P, Donaher J L, Parker S B, Li T, Fazeli A, Gardner H, Haslam S Z, Bronson R T, Elledge S J, Weinberg R A. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82:621–630. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 56.Slack J M. Developmental biology of the pancreas. Development. 1995;121:1569–1580. doi: 10.1242/dev.121.6.1569. [DOI] [PubMed] [Google Scholar]
- 57.Soos T J, Kiyokawa H, Yan J S, Rubin M S, Giordano A, DeBlasio A, Bottega S, Wong B, Mendelsohn J, Koff A. Formation of p27-CDK complexes during the human mitotic cell cycle. Cell Growth Differ. 1996;7:135–146. [PubMed] [Google Scholar]
- 58.Swiatek P J, Gridley T. Perinatal lethality and defects in hindbrain development in mice homozygous for a targeted mutation of the zinc finger gene Krox20. Genes Dev. 1993;7:2071–2084. doi: 10.1101/gad.7.11.2071. [DOI] [PubMed] [Google Scholar]
- 59.Tong W, Kiyokawa H, Soos T J, Park M, Soares V C, Manova-Todorova K O, Pollard J W, Koff A. The absence of p27Kip1, an inhibitor of G1 cyclin-dependent kinases, uncouples differentiation and growth arrest during the granulosa→luteal transition. Cell Growth Differ. 1998;9:787–794. [PubMed] [Google Scholar]
- 60.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 61.Xiao Z X, Ginsberg D, Ewen M, Livingston D M. Regulation of the retinoblastoma protein-related protein p107 by G1 cyclin-associated kinases. Proc Natl Acad Sci USA. 1996;93:4633–4637. doi: 10.1073/pnas.93.10.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamasaki L, Jacks T, Bronson R, Goillot E, Harlow E, Dyson N J. Tumor induction and tissue atrophy in mice lacking E2F-1. Cell. 1996;85:537–548. doi: 10.1016/s0092-8674(00)81254-4. [DOI] [PubMed] [Google Scholar]
- 63.Zhu X, Ohtsubo M, Bohmer R M, Roberts J M, Assoian R K. Adhesion-dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E-cdk2, and phosphorylation of the retinoblastoma protein. J Cell Biol. 1996;133:391–403. doi: 10.1083/jcb.133.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]