The Mre11-Rad50-Xrs2 Protein Complex Facilitates Homologous Recombination-Based Double-Strand Break Repair in Saccharomyces cerevisiae (original) (raw)
Abstract
_Saccharomyces cerevisiae mre11_Δ mutants are profoundly deficient in double-strand break (DSB) repair, indicating that the Mre11-Rad50-Xrs2 protein complex plays a central role in the cellular response to DNA DSBs. In this study, we examined the role of the complex in homologous recombination, the primary mode of DSB repair in yeast. We measured survival in synchronous cultures following irradiation and scored sister chromatid and interhomologue recombination genetically. _mre11_Δ strains were profoundly sensitive to ionizing radiation (IR) throughout the cell cycle. Mutant strains exhibited decreased frequencies of IR-induced sister chromatid and interhomologue recombination, indicating a general deficiency in homologous recombination-based DSB repair. Since a nuclease-deficient mre11 mutant was not impaired in these assays, it appears that the role of the S. cerevisiae Mre11-Rad50-Xrs2 protein complex in facilitating homologous recombination is independent of its nuclease activities.
Repair of DNA double-strand breaks (DSBs) by homologous recombination requires a sister chromatid or a homologous chromosome as a template. In Saccharomyces cerevisiae, the sister chromatid is the preferred template for the repair of damaged DNA (20). Consequently, ionizing radiation (IR) resistance of wild-type haploid and diploid cells is maximal in the G2 phase of the cell cycle when sister chromatids are present (8, 9). However, wild-type diploid strains are more resistant to IR-induced DNA damage than haploid strains, reflecting that chromosomal homologues can also serve as templates for repair (28, 33).
Genetic and biochemical analyses have implicated the Mre11-Rad50-Xrs2 protein complex in nonhomologous end joining (NHEJ) (14, 21, 29, 31). The rate of spontaneous heteroallelic recombination is increased in _mre11_Δ, _rad50_Δ, and _xrs2_Δ diploid strains relative to that of the wild type (1), indicating that deficiency in the complex does not abrogate homologous recombination. However, the extent to which DSB repair is impaired in _mre11_Δ mutants (7, 26) suggests that the impact of Mre11 deficiency extends beyond NHEJ. Therefore, we hypothesized that _mre11_Δ mutants are deficient in homologous recombination and that this defect results from a diminished ability to utilize the sister chromatid as a template for recombinational DNA repair.
We tested this hypothesis by measuring cell survival of synchronous cultures following irradiation and by scoring sister chromatid recombination (SCR) and interhomologue recombination genetically. We found that Mre11 deficiency leads to a decrease in homologous recombination-based DSB repair. Both SCR and interhomologue homologous recombination are affected by Mre11 deficiency, but the defect is most pronounced with respect to SCR. Since SCR and interhomologue recombination were normal in _hdf1_Δ mutants, the defects observed in _mre11_Δ strains are not a general feature of NHEJ mutants. Further, the data indicate that the nuclease activity of the S. cerevisiae Mre11-Rad50-Xrs2 protein complex is not required for homologous recombination, as SCR and interhomologue recombination were normal in a nuclease-deficient mre11 strain.
The phenotypic features of _mre11_Δ mutants described herein are consistent with the hypothesis that the S. cerevisiae Mre11-Rad50-Xrs2 protein complex stabilizes chromatid interactions, and thus plays a structural role in the homologous recombination process. The data also suggest that the complex may regulate resection of DSB ends to facilitate homologous recombination.
MATERIALS AND METHODS
Yeast strains.
The genotypes of yeast strains used in this study are listed in Table 1. MATa/− diploid strains JPY145, JPY146, JPY260, and JPY264, capable of arresting the cell cycle in response to the mating pheromone α-factor, were constructed by transformation of strains JPY41, JPY45, JPY84, and JPY259, respectively, with plasmid pFP18 (a gift of Jim Haber) linearized with _Pvu_II. Integrative transformation of this construct results in replacement of the HO-site-containing 138-bp _Bgl_II/_Bsa_AI fragment of the _MAT_α locus with the hisG-URA3-hisG cassette (2). Disruption was assessed morphologically by response to α-factor and was confirmed by Southern blotting. The _hdf1_Δ disruption was introduced into diploid strains JPY45, JPY67, and JPY115 using plasmid pHSX-YKuLEU2 as described (5). Double mutant haploids were obtained by sporulation and tetrad dissection of double heterozygotes. For XRS2 disruption in diploid strain JPY115 by one-step gene replacement, a hisG-URA3-hisG cassette (2) was inserted into the _Hin_cII/_Bgl_II site in XRS2, deleting all but 213 bp at the 5′ end and 383 bp at the 3′ end of the coding sequence.
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Referencea |
|---|---|---|
| JPY27 | MATa ura3-52 lys2-801 ade2-101 trp1_-Δ_63 his3_-Δ_200 leu2_-Δ_1 | |
| JPY35 | _MAT_α ura3-52 lys2-801 ade2-101 trp1-63 his3-200 leu2-1 mre11::hisG | 7 |
| JPY41 | MATa_/MAT_α his7/his7 leu2/leu2 ura3/ura3 trp1/trp1 hom3/+ can1/+ | 7 |
| JPY45 | MATa_/MAT_α his7/his7 leu2/leu2 ura3/ura3 trp1/trp1 hom3/+ can1/+ mre11::hisG/mre11::hisG | 7 |
| JPY67 | MATa_/MAT_α his7/his7 leu2/leu2 ura3/ura3 trp1/trp1 hom3/+ can1/+ mre11::hisG/+ | 7 |
| JPY69 | MATa his7 leu2 ura3 trp1 mre11::hisG (spore of JPY67) | |
| JPY70 | MATa his7 leu2 ura3 trp1 (spore of JPY67) | |
| JPY84 | MATa_/MAT_α his7/his7 leu2/+ ura3/ura3 trp1/trp1 hom3/+ can1/+ mre11::hisG/mre11::hisG | |
| JPY92 | _MAT_α ura3-52 lys2-801 ade2-101 trp1::SCE his3-Δ200 leu2-Δ1 (spore of JPY104) | |
| JPY94 | MATa ura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1 (spore of JPY104) | |
| JPY97 | _MAT_α ura3-52 lys2-801 ade2-101 trp1::SCE his3-Δ200 leu2-Δ1 mre11::hisG (spore of JPY104) | |
| JPY98 | MATa ura3-52 lys2-801 ade2-101 trp1::SCE his3-Δ200 leu2-Δ1 mre11::hisG (spore of JPY104) | |
| JPY102 | MATa_/MAT_α ura3-52/ura3-52 lys2-801/lys2-801 ade2-101/ade2-101 trp1-Δ63/trp1-Δ63 his3-Δ200/his3-Δ200 leu2-Δ1/leu2-Δ1 mre11::hisG/+ (JPY27 × JPY35) | |
| JPY104 | Same as JPY102 except trp1-Δ63/trp1::SCE | |
| JPY115 | MATa_/MAT_α ura3-52/ura3-52 lys2-801/lys2-801 ade2-101/ade2-101 trp1-Δ63/trp1::SCE his3-Δ200/his3-Δ200 leu2-Δ1/leu2-Δ1 (JPY92 × JPY94) | |
| JPY145 | Same as JPY45 except MATa_/mat_α::hisG | |
| JPY146 | Same as JPY41 except MATa_/mat_α::hisG | |
| JPY154 | Same as JPY115 except xrs2::hisG-URA3-hisG/+ | |
| JPY155 | _MAT_α ura3-52 lys2-801 ade2-101 trp1::SCE his3-Δ200 leu2-Δ1 xrs2::hisG-URA3-hisG (spore of JPY154) | |
| JPY156 | MATa ura3-52 lys2-801 ade2-101 trp1::SCE his3-Δ200 leu2-Δ1 xrs2::hisG-URA3-hisG (spore of JPY154) | |
| JPY169 | Same as JPY67 except hdf1::LEU2/+ | |
| JPY170 | Same as JPY115 except hdf1::LEU2/+ | |
| JPY174 | MATa ura3-52 lys2-801 ade2-101 trp1::SCE his3-Δ200 leu2-Δ1 hdf1::LEU2 (spore of JPY170) | |
| JPY176 | _MAT_α ura3-52 lys2-801 ade2-101 trp1::SCE his3-Δ200 leu2-Δ1 hdf1::LEU2 (spore of JPY170) | |
| JPY177 | MATa_/MAT_α ura3-52/ura3-52 lys2-801/lys2-801 ade2-101/ade2-101 trp1::SCE/trp1::SCE his3-Δ200/his3-Δ200 leu2-Δ1/leu2-Δ1 mre11::hisG/+ hdf1::LEU2/+ (JPY97 × JPY174) | |
| JPY181 | MATa his7 leu2 ura3 trp1 hdf1::LEU2 (spore of JPY169) | |
| JPY202 | MATa ura3-52 lys2-801 ade2-101 trp1::SCE his3-Δ200 leu2-Δ1 (spore of JPY177) | |
| JPY205 | _MAT_α ura3-52 lys2-801 ade2-101 trp1::SCE his3-Δ200 leu2-Δ1 mre11::hisG hdf1::LEU2 (spore of JPY177) | |
| JPY206 | MATa ura3-52 lys2-801 ade2-101 trp1::SCE his3-Δ200 leu2-Δ1 mre11::hisG hdf1::LEU2 (spore of JPY177) | |
| JPY247 | Same as JPY45 except hdf1::LEU2/+ | |
| JPY250 | MATa his7 leu2 ura3 trp1 mre11::hisG (spore of JPY247) | |
| JPY254 | MATa his7 leu2 ura3 trp1 mre11::hisG hdf1::LEU2 (spore of JPY247) | |
| JPY255 | _MAT_α his7 leu2 ura3 trp1 mre11::hisG hdf1::LEU2 (spore of JPY247) | |
| JPY259 | MATa_/MAT_α his7/his7 leu2/leu2 ura3/ura3 trp1/trp1 mre11::hisG/mre11::hisG hdf1::LEU2/hdf1::LEU2 (JPY254 × JPY255) | |
| JPY260 | Same as JPY259 except MATa_/mat_α::hisG-URA3-hisG | |
| JPY264 | Same as JPY84 except MATa_/mat_α::hisG-URA3-hisG |
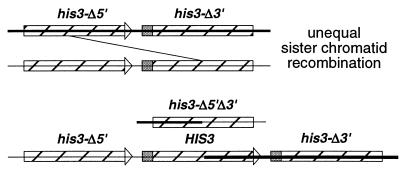
SCR was monitored in haploid spores derived from strain JPY104, obtained by the integration of MFp102 (11) into diploid strain JPY102, which resulted in the replacement of the TRP1 locus with the MFp102 SCR construct (see Fig. 3). The his3-Δ200 mutation in JPY102 deletes HIS3 sequences contained in the SCR construct so that histidine prototrophy can be generated only by an unequal SCR event. All strains were cultured at 30°C. Disruptions were confirmed by Southern blotting. Yeast media were prepared and strain manipulations were carried out according to standard procedures (4, 13).
FIG. 3.
Sister chromatid recombination assay. Integrative transformation of MFp102 (11) results in the replacement of the TRP1 locus with the SCR substrate. Striped bars indicate the location of his3 fragments (_his3_-Δ_5_′ and _his3_-Δ_3_′) in a head-to-tail arrangement along the sister chromatids (solid horizontal lines). The arrowhead and shaded box represent regions of HIS3 deleted in the opposite allele. A functional HIS3 gene can be generated only by an unequal sister chromatid recombination event as indicated by the diagonal line. An intrachromatid recombination event will produce a HIS3 circle (not shown).
Plasmids.
For construction of pScM11-314, a 2.9-kb _Bam_HI/_Kpn_I MRE11 fragment was subcloned from pSK-_MRE11_-BNX (pSK-ScMRE11 [7] digested with _Nru_I and _Xho_I, blunted, and reclosed, deleting sequences 3′ of the MRE11 stop codon) into the centromeric vector pRS314 (34). MRE11 expression from this construct is under the control of the native MRE11 promoter. The ADH1 promoter-driven MRE11 and mre11-3 expression constructs have previously been described (7).
Cell cycle arrest.
Cultures were grown to a density of approximately 5 × 106 cells/ml and arrested in G1 with α-factor (3 μM final concentration, incubated for 2 to 3 h) (U.S. Biologicals) or in G2 with carbendazim (150 μg/ml final concentration, incubated for 2 to 3 h) (Aldrich). Synchronization was assessed morphologically and by flow cytometry (see below).
Flow cytometry.
Cells were fixed in 70% ethyl alcohol at 4°C for at least 12 h, were pelleted, were resuspended in 1 ml of 50 mM sodium citrate (pH 7.5), and were sonicated for 15 s. Cells were resuspended in sodium citrate containing 0.25 mg of RNase A per ml, were incubated at 50°C for 1 h or at 37°C overnight, and were resuspended in sodium citrate containing 1 μM Sytox Green (1:5,000 dilution) (Molecular Probes). Samples were kept in the dark at room temperature for at least 1 h prior to flow cytometric analysis.
Irradiation studies.
Strains were irradiated in mid-log phase (approximately 107 cells/ml) or following cell cycle arrest (see above) as previously described (7).
Sister chromatid recombination.
To monitor spontaneous SCR, approximately 100 cells from an overnight culture were used to inoculate fresh 50-ml yeast extract-peptone-dextrose (YEPD) cultures, and the cultures were then grown to a density of approximately 5 × 107 cells/ml. Approximately 5 × 107 cells per synthetic complete (SC) medium plate lacking His (SC-His) and 500 cells per nonselective plate were plated in triplicate. The rate of spontaneous SCR was determined from at least nine independent cultures per strain by fluctuation analysis (23) with modifications as previously described (7).
To measure IR-induced SCR, cultures were grown to mid-log phase (approximately 107 cells/ml) in YEPD. Approximately 3 × 108 cells were harvested for each strain and were resuspended in 900 μl of double-distilled water (ddH2O). Each cell suspension was split into two aliquots, one of which was irradiated on ice with 50 Gy while the other served as the unirradiated control. Cells were then diluted 10-fold into fresh YEPD, were allowed to recover for 30 min at 30°C, and were plated as described above. The number of IR-induced SCR events was determined by subtracting the ratio of histidine prototrophs to total viable cells in the unirradiated sample from the same value in the irradiated sample.
Interhomologue recombination.
To measure IR-induced interhomologue recombination, cultures of JPY264 transformants were grown to early log phase (approximately 5 × 106 cells/ml) in SC-Trp-Met media. Cultures were then split into three aliquots for asynchronous and G1- and G2-synchronized samples as described above. Each was then resuspended in ddH2O and split into three aliquots, two of which were irradiated on ice with 50 Gy and 150 Gy while the third served as the unirradiated control. Cells were then diluted 10-fold into fresh SC-Trp media, were allowed to recover for 30 min at 30°C, and were plated onto SC-Trp and SC-Trp-Met canavanine plates. The number of IR-induced interhomologue recombination events was determined by subtracting the ratio of canavanine-resistant methionine prototrophs to total viable cells in the unirradiated sample from the same value in the irradiated sample.
RESULTS
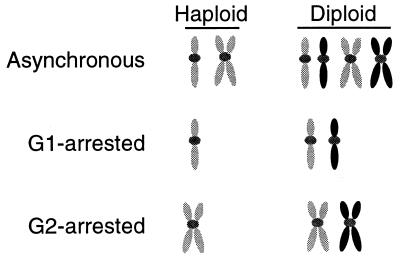
Synchronous cultures of haploid and diploid strains provide a means to examine the ability of cells to utilize different homologous templates for recombinational DSB repair. In G1-synchronous cultures, cells must rely on NHEJ to repair DSBs in the absence of homology (Fig. 1). Haploid cells contain a homologous template, in the form of a sister chromatid, only during the G2 phase. In contrast, diploid cells contain homologous templates, in the form of homologous chromosomes, throughout the cell cycle. Additionally, sister chromatids are present in the G2 phase and are the preferred template for repair (20). We monitored the contribution of each of these templates to the survival of _mre11_Δ cells following irradiation using synchronous cultures of haploid and diploid strains.
FIG. 1.
Schematic representation of asynchronous and synchronous cultures. The genotype shows that cells in G1 rely on NHEJ (haploids) or interhomologue recombination (diploids) for recombinational DNA repair. Cells in G2 can also undergo sister chromatid recombination (haploids and diploids). The petal-shaped figures represent chromosome arms; the gray dots represent the centromeres.
Radiation sensitivity of haploid _mre11_Δ strains.
Given the DSB repair deficiency observed in _mre11_Δ strains (7, 26), we asked whether homologous recombination was impaired by Mre11 deficiency, as suggested by previous work using _rad50_Δ and _xrs2_Δ strains (18). In order to determine whether _mre11_Δ strains were defective in SCR, we examined the IR sensitivity of synchronous cultures of wild-type (JPY70) and _mre11_Δ (JPY69) haploid strains. Cells were grown to early log phase and arrested in G1 or G2 by treatment with α-factor or carbendazim, respectively. Synchronization of cultures was confirmed morphologically and by flow cytometry. Cells were irradiated in suspension at 150 Gy and were plated onto rich media to score cell survival relative to that of unirradiated cultures.
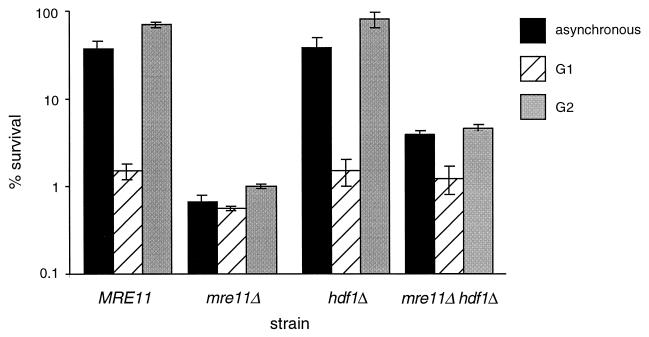
The wild-type haploid strain JPY70 exhibited 37% survival following irradiation during asynchronous growth, and the _mre11_Δ haploid strain JPY69 exhibited 0.7% survival (Fig. 2). Whereas wild-type cells arrested in G1 exhibited 1.5% survival, cells in G2 exhibited 70% survival, indicating that the presence of a sister chromatid in G2-synchronous cultures increases the survival of wild-type cells.
FIG. 2.
Radiation sensitivity of haploid _mre11_Δ and _hdf1_Δ strains. Asynchronous cultures and cells synchronized with α-factor (G1 synchronous) or carbendazim (G2 synchronous) were irradiated at a dose of 150 Gy as described in Materials and Methods. Cell survival was scored for 5 days following irradiation. Values plotted represent the average of triplicate platings from at least three experiments. Error bars represent standard deviations. Haploid strains were JPY70 (MRE11), JPY69 (_mre11_Δ), JPY181 (_hdf1_Δ), and JPY254 (_mre11_Δ _hdf1_Δ).
In contrast to the wild-type strain, the presence of the sister chromatid in the G2-arrested _mre11_Δ cells did not increase survival following IR. G2-synchronous _mre11_Δ cells exhibited less than a twofold increase in survival upon irradiation relative to that of G1-synchronous cells (1 and 0.6% survival, respectively) (Fig. 2). Hence, the IR sensitivity of asynchronous haploid _mre11_Δ strains can primarily be attributed to the increased sensitivity of the G2 population. These data suggest that use of the sister chromatid for recombinational repair is substantially reduced in _mre11_Δ mutants.
The IR sensitivity of wild-type haploid cells in G1 demonstrates that NHEJ does not contribute significantly to cell survival following IR. Indeed, a substantial fraction of the surviving cells are unlikely to have received any DSBs at the IR dose used. A dose of 150 Gy is predicted to impart four to six DSBs per cell (32). Since IR-induced breaks are distributed stochastically, 0.25 to 2% of cells were predicted to sustain less than one DSB at the level of irradiation employed in this experiment—a dose at which we observed approximately 1.5% survival. Hence, most of the surviving cells in the G1-synchronous populations may not have sustained DSBs.
Radiation-induced sister chromatid recombination.
To assess SCR genetically, we used a chromosomal substrate consisting of a tandemly repeated HIS3 gene in which the first repeat is truncated at the 5′ end and the second is truncated at the 3′ end (11). In this configuration, a functional HIS3 gene can be generated only by an unequal SCR event (Fig. 3). The wild-type (JPY92 and JPY202) and _mre11_Δ (JPY97 and JPY98) haploid strains exhibited similar rates of spontaneous SCR ([1.4 to 2.0] × 10−6 per generation).
We next assessed radiation-induced unequal SCR following irradiation at 50 Gy. The wild-type strains exhibited an IR-induced increase in SCR frequency of 13.3 × 10−6 (Table 2). We consistently observed significantly smaller increases in SCR frequency following irradiation of the _mre11_Δ strains JPY97 and JPY98 (4.8 × 10−6). Similar data were obtained with a mutant of another member of the complex. The _xrs2_Δ strains JPY155 and JPY156 exhibited an IR-induced increase in SCR frequency of 5.4 × 10−6 (Table 2). At this IR dose, the decrease in survival of these mutant strains relative to the survival of the wild type (1.5-fold) is of similar magnitude to the decrease in the number of recombinants scored (2.6-fold).
TABLE 2.
Effect of mre11 and hdf1 mutations on radiation-induced sister chromatid recombination
| Genotype | Strain | IR-induced frequency (n)a | % Survival |
|---|---|---|---|
| MRE11 | JPY92 and JPY202 | 13.3 ± 5.9 (13) | 48 |
| _mre11_Δ | JPY97 and JPY98 | 4.8 ± 2.8 (8) | 31 |
| _xrs2_Δ | JPY155 and JPY156 | 5.4 ± 2.2 (8) | 35 |
| _hdf1_Δ | JPY174 and JPY176 | 16.3 ± 5.3 (12) | 46 |
| _mre11_Δ _hdf1_Δ | JPY205 and JPY206 | 6.1 ± 4.7 (15) | 52 |
| _mre11_Δ transformantsb | _mre11_Δ:DB-MRE11-TRP | 13.1 ± 5.2 (7) | 58 |
| _mre11_Δ:DB-mre11-3-TRP | 14.6 ± 6.5 (7) | 51 | |
| _mre11_Δ:DB-P-TRP | 3.7 ± 2.6 (7) | 43 |
Previous studies have shown that _mre11_Δ strains are deficient in NHEJ (14, 21, 31). We asked whether the decrease in SCR in _mre11_Δ cells was a general, and presumably indirect, outcome of NHEJ deficiency. We analyzed the IR sensitivity of synchronous cultures and the IR-induced frequency of SCR in the haploid _hdf1_Δ strains JPY181, JPY174, and JPY176, which are also impaired in NHEJ (6, 25). Haploid _hdf1_Δ cells were no more IR sensitive than wild-type cells, exhibiting survival of 38% in asynchronous cultures, 1.5% in G1-synchronous cultures, and 81% in G2-synchronous cultures (Fig. 2). The rate of spontaneous SCR in the haploid _hdf1_Δ strains JPY174 and JPY176 was 1.5 × 10−6 per generation, not significantly different from those of the other mutant and wild-type strains examined in this assay (data not shown). However, in contrast to the _mre11_Δ and _xrs2_Δ strains, the _hdf1_Δ strains showed an IR-dependent increase in SCR frequency equivalent to that of the wild type (16.3 × 10−6) (Table 2).
The IR sensitivity of haploid _mre11_Δ cells was partially suppressed by the _hdf1_Δ mutation. Survival of the _mre11Δ hdf1_Δ strain JPY254 was 5.7-fold greater than that of the _mre11_Δ strain JPY69 following irradiation of asynchronous cultures, with G1- and G2-synchronous cultures displaying 2.2- and 4.6-fold increases in survival, respectively (Fig. 2).
Radiation sensitivity of diploid _mre11_Δ strains.
We previously showed that diploid _mre11_Δ strains exhibit higher radiation resistance than their haploid counterparts (7), consistent with the behavior of _rad50_Δ and xrs2_Δ strains (18). As in wild-type cells, this effect is presumably due to the presence of homologous chromosomes for repair of DSBs (33). To assess the ability of Mre11-deficient diploid cells to use interhomologue recombination for DSB repair, MATa/mat_α::hisG disruptions were established in wild-type (JPY41) and _mre11_Δ (JPY45) diploid strains to allow G1 synchronization with α-factor. The IR sensitivity of synchronous populations of these diploid strains was examined as described above.
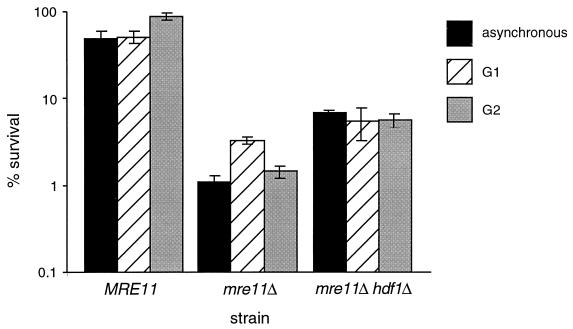
The wild-type diploid strain JPY146 was more resistant to IR than its haploid counterpart JPY70, exhibiting survival of 51% following irradiation of asynchronous cultures (Fig. 4). As with the wild-type haploid strain, G2-synchronous diploid cultures exhibited increased IR resistance relative to the resistance of G1-arrested cells (90 and 59%, respectively). Although asynchronous cultures of the _mre11_Δ JPY145 strain did exhibit an increase in IR resistance relative to its haploid counterpart JPY69 (compare Fig. 2 and Fig. 4) (7), the G2-synchronous _mre11_Δ diploid culture did not show increased survival relative to that of the G1-synchronous culture (Fig. 4). Instead, we reproducibly observed a two- to threefold decrease in survival of mre11_Δ diploid cells in G2 (1.5% survival) relative to that of G1-synchronous cultures (3.3% survival) (Fig. 4). This effect was not due to mating-type hemizygosity, as MATa/MAT_α strains synchronized in G1 by growth to saturation exhibited an even more dramatic increase in survival following IR (data not shown).
FIG. 4.
Radiation sensitivity of diploid _mre11_Δ and _mre11_Δ _hdf1_Δ strains. Methods of synchronization and irradiation are as described in the legend to Fig. 3 and in Materials and Methods. IR dose = 150 Gy. Values plotted represent the averages of triplicate platings from at least three experiments. Error bars represent standard deviations. Diploid strains were JPY146 (MRE11), JPY145 (_mre11_Δ), and JPY260 (_mre11_Δ _hdf1_Δ).
As in haploid strains, the _hdf1_Δ mutation partially suppressed the IR sensitivity of the diploid _mre11_Δ strain JPY145. Survival of the homozygous _mre11Δ hdf1_Δ diploid strain JPY260 was 6.3-fold greater than that of the diploid _mre11_Δ strain following irradiation of asynchronous cultures (Fig. 4). _mre11Δ hdf1_Δ diploid cells arrested in G1 and G2 exhibited 1.7- and 4-fold increases in survival, respectively, relative to that of _mre11_Δ cells.
The IR sensitivity of _mre11_Δ diploid cells suggested that _mre11_Δ strains are defective in IR-induced interhomologue recombination, as shown previously for rad50_Δ strains (33). We measured the induction of interhomologue recombination by IR in the diploid strain JPY264 transformed with either a wild-type MRE11 expression construct (pScM11-314) or an empty vector. JPY264 is an Mre11-deficient MATa/mat_α::hisG diploid strain heterozygous for can1 and hom3 on opposite arms of chromosome V. In this strain, the frequency of interhomologue recombination can be determined by scoring the frequency of canavanine-resistant methionine prototrophs (7, 16). Heteroallelic (intragenic) recombination cannot be distinguished from intergenic recombination by this assay.
As in previous studies, we found that spontaneous interhomologue recombination was increased in _mre11_Δ diploid cells relative to that of the wild type, with frequencies of 4.0 × 10−3 and 3.7 × 10−4 recombinants per viable cell, respectively (1). We observed greater IR induction of interhomologue recombination in both wild-type and _mre11_Δ G1-synchronous cells relative to that of asynchronous and G2-synchronous cultures (Table 3), consistent with the observation that sister chromatids are preferred over homologous chromosomes as templates for recombinational repair (20). However, the IR-induced frequency of interhomologue recombination in the _mre11_Δ strain was indistinguishable from that of the wild type at a dose of 50 Gy (Table 3).
TABLE 3.
Radiation-induced interhomologue recombination in _mre11_Δ/_mre11_Δ transformants
| Plasmida | IR-induced frequency (n)b | |||||
|---|---|---|---|---|---|---|
| 50 Gy | 150 Gy | |||||
| Asynchronous | G1 arrested | G2 arrested | Asynchronous | G1 arrested | G2 arrested | |
| pScM11-314 | 7.7 ± 2.5 (14) | 33.5 ± 16.7 (16) | 7.1 ± 3.5 (16) | 7.3 ± 1.4 (5) | 35.0 ± 8.4 (6) | 4.8 ± 2.2 (6) |
| pRS314 | 11.1 ± 12.1 (10) | 49.0 ± 15.6 (8) | 8.5 ± 15.9 (8) | −4.7 ± 1.9 (6) | 31.5 ± 6.9 (5) | −3.2 ± 6.9 (6) |
We found that IR induction of interhomologue recombination following irradiation at 150 Gy was reduced in asynchronous cultures of the _mre11_Δ strain relative to that of the wild-type (pScM11-314) transformants (Table 3), with frequencies of −4.7 × 10−4 and 7.3 × 10−4 recombinants per viable cell, respectively. In contrast, the frequency of IR-induced interhomologue recombination observed in G1-synchronous _mre11_Δ cultures (31.5 × 10−4) was not different from that of the wild type (35.0 × 10−4) following irradiation at 150 Gy (Table 3). The extent to which IR-induced interhomologue recombination was reduced in the asynchronous and G2-synchronous _mre11_Δ cells is consistent with the decrease in cell survival at this dose. The negative IR induction of recombinants in the _mre11_Δ strain reflects that the degree of cell killing at this dose exceeds the frequency of viable recombinants.
Mre11 nuclease activity in homologous recombination.
The data described above indicates that Mre11 deficiency profoundly impairs homologous recombination. Since nucleolytic processing is required for homologous recombination (15, 35), we asked whether the role of the Mre11-Rad50-Xrs2 complex in homologous recombination was dependent upon the nuclease activity of Mre11 (12, 27, 29, 36, 37). For this analysis, we used the mre11-3 mutant, in which the conserved histidine residue at position 125 is altered (7). Alteration of this residue in the Scmre11 H125N allele inactivates the nuclease function of Mre11 and disrupts the early stages of meiotic recombination (27). Like Scmre11 H125N, mre11-3 mutants are unable to produce viable spores (data not shown).
The haploid _mre11_Δ strains JPY69, JPY97, and JPY98 were each transformed with a centromeric plasmid containing no insert (empty vector) or MRE11 or mre11-3 (both expressed from the ADH1 promoter) (7). Transformants were examined with respect to cell survival of synchronous cultures following irradiation and IR-induced SCR as described above.
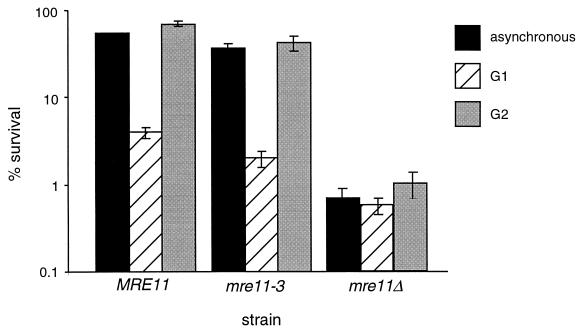
As with the wild-type JPY69 transformants, the presence of the sister chromatid in the G2-synchronous mre11-3 transformants increased cell survival following IR relative to that of G1-synchronous cultures. Asynchronous and G2-synchronous cultures of mre11-3 transformants exhibited 37 and 42% survival, respectively, whereas G1-synchronous cells exhibited 2% survival (Fig. 5). Consistent with cell survival, we found that the frequency of IR-induced SCR in the haploid JPY69 mre11-3 transformants was 14.6 × 10−6 (Table 2), indistinguishable from that of the wild type (13.1 × 10−6).
FIG. 5.
Radiation sensitivity of synchronous mre11-3 cultures. Methods of synchronization and irradiation are as described in the legend to Fig. 3 and Materials and Methods. IR dose = 150 Gy. Haploid _mre11_Δ strain JPY69 was transformed with an ADH1 promoter-driven MRE11 or mre11-3 expression construct (DB-MRE11-TRP or DB-mre11-3-TRP) or an empty vector (DB-P-TRP) (7). Asynchronous, G1-synchronous, and G2-synchronous cultures of JPY69 transformants were plated onto SC-Trp media and scored for cell survival for 5 days following irradiation. Values plotted represent the averages of triplicate platings. Error bars represent standard deviations. The standard deviation for the asynchronous MRE11 culture (1.2%) is not visible at the scale of this plot.
DISCUSSION
We examined the response of _mre11_Δ mutants to DSBs using cell survival, SCR, and interhomologue recombination assays. We found that Mre11 deficiency leads to a dramatic reduction in homologous recombination, as evidenced by decreased survival following irradiation of synchronous cultures and by decreased frequencies of IR-induced sister chromatid and interhomologue recombination. Although previous studies have implicated the S. cerevisiae Mre11-Rad50-Xrs2 protein complex in NHEJ, this study clearly indicates that the primary role of the complex in the cellular DNA damage response is in facilitating homologous recombination. This role in the damage response does not appear to depend upon the nuclease activities exhibited by the complex, as a nuclease-deficient allele of mre11 did not affect homologous recombination in our assays.
It is likely that deficiency in the S. cerevisiae Mre11-Rad50-Xrs2 protein complex destabilizes the association of homologous chromatids during recombinational DNA repair, thereby impairing homologous recombination as well as NHEJ (26). In homologous recombination, the effect is more severe for SCR than for interhomologue recombination. For example, we observed that G1-synchronous _mre11_Δ diploid cultures were more IR resistant than G2-synchronous diploid cultures (Fig. 4).
The mechanistic basis for the putative reduction in chromatid interactions in S. cerevisiae Mre11-Rad50-Xrs2 protein complex-deficient cells is not clear. In _mre11_Δ cells, the induction of interhomologue recombination by IR treatment is reduced in G2-synchronous cultures relative to that of G1-synchronous cultures (Table 3), as shown previously for wild-type cells (10). This suggests that the bias toward the use of sister chromatids for homologous recombination is intact in _mre11_Δ cells. The increased survival of G1-synchronous cultures relative to that of G2-synchronous diploid cultures (Fig. 4) is consistent with this interpretation. The increased sensitivity of G2 cultures suggests that the completion, rather than the initiation, of SCR events is affected by Mre11 deficiency and that reduced survival relative to that of G1 cultures may be caused by abortive SCR events.
The spontaneous hyperrecombination phenotype observed in _mre11_Δ, _rad50_Δ, and _xrs2_Δ strains is seemingly paradoxical in light of the homologous recombination defects in these mutant strains (1, 3, 18). Two factors may interact to influence the frequency of spontaneous interhomologue recombination. First, Mre11 deficiency may result in an increased steady-state level of recombinogenic lesions and thereby lead to an elevated frequency of interhomologue recombination. Second, the slow resection of DSB ends in _mre11_Δ strains may lead to shorter heteroduplex tracts during gene conversion. The result would be an apparent increase in gene conversion between heteroalleles in a diploid strain (14, 30). This scenario can also explain the observation that at low doses of IR (50 Gy), both wild-type and _mre11_Δ mutants exhibited an induction of can1 recombinants (Table 3), despite a relative decrease in survival of the _mre11_Δ strain (data not shown). That is, although IR-induced DNA damage is repaired inefficiently in the _mre11_Δ strain, the decreased number of successful recombination events that do occur do so with DNA ends that are minimally resected and are thus more likely to result in a conversion of the heteroallele. At higher doses of IR (150 Gy), the frequency of cell death in _mre11_Δ cells exceeds the frequency of successfully completed interhomologue recombination events. Consequently, we observed a negative induction of interhomologue recombination in _mre11_Δ cells at this dose (Table 3).
In contrast, the spontaneous rates of SCR among null mutants of the S. cerevisiae Mre11-Rad50-Xrs2 protein complex are indistinguishable from each other and from rates in wild-type cells. However, the frequency of IR-induced SCR events is reduced in _mre11_Δ cells relative to that in the wild type (Table 2). Although the magnitude of the observed effects is subtle, these data offer evidence that the repair of IR-induced DNA damage may be mechanistically distinct from the repair of DNA lesions that lead to spontaneous SCR. In this regard, it is important to consider the possibility that spontaneous SCR events occur in close proximity to the replication fork to repair spontaneously occurring DSBs (22), whereas IR-induced events occur at essentially random locations.
The homologous recombination defects we observed may not be fully explained by the hypothesized reduction in chromatid association. Rather, data presented here and elsewhere suggest that the S. cerevisiae Mre11-Rad50-Xrs2 protein complex also plays a role in facilitating end resection at DSB sites. Resection in the 5′ to 3′ direction to create a protruding 3′ end is a requisite first step in the homologous recombination process (15, 35). A number of studies have shown that the rate of 5′ to 3′ end resection at HO-induced DSBs is reduced in mre11, rad50, and xrs2 deletion mutants (14). The magnitude of IR sensitivity we observed is disproportionate to the relatively modest end resection defects at HO-induced DSBs in these mutants (19, 24). However, IR-induced DSBs exhibit a significant degree of chemical and structural heterogeneity (17); therefore the end resection defects associated with Mre11 deficiency may be much more pronounced at IR- than at HO-induced DSBs.
In contrast to Mre11 deficiency, Hdf1 deficiency is associated with a sharp increase in the rate of end resection at HO-induced DSBs. Mre11 deficiency is apparently epistatic to Hdf1 deficiency in this regard, since resection rates are similar in _mre11_Δ and _mre11_Δ _hdf1_Δ mutants (24). However, we observed partial suppression of _mre11_Δ IR sensitivity in _mre11_Δ _hdf1_Δ mutants. We have hypothesized that homologous recombination defects in _mre11_Δ mutants result from impaired DSB end resection. We infer that the partial suppression of IR sensitivity in _mre11_Δ _hdf1_Δ double mutants may indicate that Hdf1 deficiency does indeed lead to a subtle increase in the rate of end resection at IR-induced DSBs. Given the relatively subtle degree of suppression observed, the increased rate of end resection imparted by Hdf1 deficiency in the _mre11_Δ background would not necessarily have been detectable by the physical methods employed by Lee et al. (24).
In this context, it is noteworthy that _mre11-3_-expressing strains, in which Mre11 nuclease activity is presumably abolished, are not grossly deficient in the resection of HO-induced DSBs (23a), do not exhibit mitotic homologous recombination defects, and do not show markedly increased IR sensitivity (references 7 and 27 and this study). Hence, although deficiency in the S. cerevisiae Mre11-Rad50-Xrs2 protein complex reduces the rate of DSB end resection in vivo, the complex may not be directly responsible for resection activity. The complex does specify nuclease activity, although Mre11 homologues from S. cerevisiae and humans exhibit 3′ to 5′ rather than 5′ to 3′ exonuclease activity in vitro. The reduction of 5′ to 3′ exonuclease activity in _mre11_Δ, _rad50_Δ, and _xrs2_Δ mutants suggests the possibility that the complex regulates or otherwise facilitates the activity of a bona fide 5′ to 3′ exonuclease in vivo (12, 27, 29, 36, 37).
In summary, these data illustrate the central importance of the S. cerevisiae Mre11-Rad50-Xrs2 protein complex in homologous recombination. The phenotypic features of mutations affecting the complex suggest that the complex is required to establish chromatid interactions and suggest a structural, rather than enzymatic, role in the recombinational DNA repair process. The data are also compatible with a substantial role for the complex in DSB end resection to facilitate homologous recombination.
ACKNOWLEDGMENTS
We are grateful to the members of our lab for insights throughout the course of this study, Michael Fasullo and Jim Haber for providing reagents, Barbara Garvik for advice and for providing yeast strains, and Mark Kaplan, Doug Bishop, and Michael Lichten for critical reading of the manuscript.
This work was supported by the Milwaukee Foundation, the Howard Hughes Medical Institute, the National Cancer Institute, NIH grant GM56888 (to J.H.J.P.), NIH predoctoral training grant 5T32GM07133 (to D.A.B.), and cancer biology postdoctoral training grant NCI T32-CA09471 to the University of Wisconsin Comprehensive Cancer Center (B.K.B.).
Footnotes
†
Manuscript 3538 from the University of Wisconsin—Madison Laboratory of Genetics.
REFERENCES
- 1.Ajimura M, Leem S-H, Ogawa H. Identification of new genes required for meiotic recombination in Saccharomyces cerevisiae. Genetics. 1993;133:51–66. doi: 10.1093/genetics/133.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alani E, Cao L, Kleckner N. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics. 1987;116:541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alani E, Subbiah S, Kleckner N. The yeast RAD50 gene encodes a predicted 153 kD protein containing a purine nucleotide-binding domain and two large heptad repeat regions. Genetics. 1989;122:47–57. doi: 10.1093/genetics/122.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. 1–3. New York, N.Y: John Wiley & Sons; 1989. [Google Scholar]
- 5.Barnes G, Rio D. DNA double-strand-break sensitivity, DNA replication, and cell cycle arrest phenotypes of Ku-deficient Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1997;94:867–872. doi: 10.1073/pnas.94.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulton S J, Jackson S P. Saccharomyces cerevisiae Ku70 potentiates illegitimate DNA double-strand break repair and serves as a barrier to error-prone DNA repair pathways. EMBO J. 1996;15:5093–5103. [PMC free article] [PubMed] [Google Scholar]
- 7.Bressan D A, Olivares H A, Nelms B E, Petrini J H J. Alteration of N-terminal phosphoesterase motifs inactivates Saccharomyces cerevisiae Mre11. Genetics. 1998;150:591–600. doi: 10.1093/genetics/150.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunborg G, Resnick M A, Williamson D H. Cell-cycle-specific repair of DNA double strand breaks in Saccharomyces cerevisiae. Radiat Res. 1980;82:547–558. [PubMed] [Google Scholar]
- 9.Brunborg G, Williamson D H. The relevance of the nuclear division cycle to radiosensitivity in yeast. Mol Gen Genet. 1978;162:277–286. doi: 10.1007/BF00268853. [DOI] [PubMed] [Google Scholar]
- 10.Esposito R E. Genetic recombination in synchronized cultures of Saccharomyces cerevisiae. Genetics. 1968;59:191–210. doi: 10.1093/genetics/59.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fasullo M T, Bennett T, AhChing P, Koudelik J. The Saccharomyces cerevisiae RAD9 checkpoint reduces the DNA damage-associated stimulation of directed translations. Mol Cell Biol. 1998;18:1190–1200. doi: 10.1128/mcb.18.3.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furuse M, Nagase Y, Tsubouchi H, Murakami-Murofushi K, Shibata T, Ohta K. Distinct roles of two separable in vitro activities of yeast Mre11 in mitotic and meiotic recombination. EMBO J. 1998;17:6412–6425. doi: 10.1093/emboj/17.21.6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guthrie C, Fink G R. Methods in enzymology. Vol. 194. San Diego, Calif: Academic Press, Inc.; 1991. [Google Scholar]
- 14.Haber J E. The many interfaces of Mre11. Cell. 1998;95:583–586. doi: 10.1016/s0092-8674(00)81626-8. [DOI] [PubMed] [Google Scholar]
- 15.Haber J E. Mating-type gene switching in Saccharomyces cerevisiae. Annu Rev Genet. 1998;32:561–599. doi: 10.1146/annurev.genet.32.1.561. [DOI] [PubMed] [Google Scholar]
- 16.Hartwell L H, Smith D. Altered fidelity of mitotic chromosome transmission in cell cycle mutants of S. cerevisiae. Genetics. 1985;110:381–395. doi: 10.1093/genetics/110.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henner W D, Grunberg S M, Haseltine W A. Sites and structures of γ radiation-induced DNA strand breaks. J Biol Chem. 1982;257:11750–11754. [PubMed] [Google Scholar]
- 18.Ivanov E L, Korolev V G, Fabre F. XRS2, a DNA repair gene of Saccharomyces cerevisiae, is needed for meiotic recombination. Genetics. 1992;132:651–664. doi: 10.1093/genetics/132.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivanov E L, Sugawara N, White C I, Fabre F, Haber J E. Mutations in XRS2 and RAD50 delay but do not prevent mating-type switching in Saccharomyces cerevisiae. Mol Cell Biol. 1994;14:3414–3425. doi: 10.1128/mcb.14.5.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kadyk L C, Hartwell L H. Sister chromatids are preferred over homologs as substrates for recombinational repair in Saccharomyces cerevisiae. Genetics. 1992;132:387–402. doi: 10.1093/genetics/132.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanaar R, Hoeijmakers J H J. Recombination and joining: different means to the same ends. Genes Funct. 1997;1:165–174. doi: 10.1046/j.1365-4624.1997.00016.x. [DOI] [PubMed] [Google Scholar]
- 22.Kuzminov A. Collapse and repair of replication forks in Escherichia coli. Mol Microbiol. 1995;16:373–384. doi: 10.1111/j.1365-2958.1995.tb02403.x. [DOI] [PubMed] [Google Scholar]
- 23.Lea D E, Coulson C A. The distribution of the numbers of mutants in bacterial populations. J Genet. 1947;49:264–285. doi: 10.1007/BF02986080. [DOI] [PubMed] [Google Scholar]
- 23a.Lee, S., D. Bressan, J. Petrini, and J. Haber. Unpublished data.
- 24.Lee S E, Moore J K, Holmes A, Umezu K, Kolodner R, Haber J E. Saccharomyces Ku70, Mre11/Rad50, and RPA proteins regulate adaptation to G2/M arrest after DNA damage. Cell. 1998;94:399–409. doi: 10.1016/s0092-8674(00)81482-8. [DOI] [PubMed] [Google Scholar]
- 25.Milne G T, Jin S, Shannon K B, Weaver D T. Mutations in two Ku homologs define a DNA end-joining repair pathway in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:4189–4198. doi: 10.1128/mcb.16.8.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore J K, Haber J E. Cell cycle and genetic requirements of two pathways of nonhomologous end-joining repair of double-strand breaks in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:2164–2173. doi: 10.1128/mcb.16.5.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreau S, Ferguson J R, Symington L S. The nuclease activity of mre11 is required for meiosis but not for mating-type switching, end joining, or telomere maintenance. Mol Cell Biol. 1999;19:556–566. doi: 10.1128/mcb.19.1.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mortimer R K. Radiobiological and genetic studies on a polyploid series (haploid to hexaploid) of Saccharomyces cerevisiae. Radiat Res. 1958;9:312–326. [PubMed] [Google Scholar]
- 29.Paull T T, Gellert M. The 3′ to 5′ exonuclease activity of Mre11 facilitates repair of DNA double-strand breaks. Mol Cell. 1998;1:969–979. doi: 10.1016/s1097-2765(00)80097-0. [DOI] [PubMed] [Google Scholar]
- 30.Petes T D, Malone R E, Symington L E. Recombination in yeast. In: Broach J R, Pringle J, Jones E, editors. The molecular and cellular biology of the yeast Saccharomyces: genome dynamics, protein synthesis, and energetics. I. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1991. pp. 407–521. [Google Scholar]
- 31.Petrini J H J, Bressan D A, Yao M S. The RAD52 epistasis group in mammalian double strand break repair. Semin Immunol. 1997;9:181–188. doi: 10.1006/smim.1997.0067. [DOI] [PubMed] [Google Scholar]
- 32.Resnick M A, Martin P. The repair of double-strand breaks in the nuclear DNA of Saccharomyces cerevisiae and its genetic control. Mol Gen Genet. 1976;143:119–129. doi: 10.1007/BF00266917. [DOI] [PubMed] [Google Scholar]
- 33.Saeki T, Machida I, Nakai S. Genetic control of diploid recovery after γ-irradiation in the yeast Saccharomyces cerevisiae. Mutat Res. 1980;73:251–265. doi: 10.1016/0027-5107(80)90192-x. [DOI] [PubMed] [Google Scholar]
- 34.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szostak J W, Orr-Weaver T L, Rothstein R J, Stahl F W. The double-strand break repair model for recombination. Cell. 1983;33:25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- 36.Trujillo K M, Yuan S S, Lee E Y, Sung P. Nuclease activities in a complex of human recombination and DNA repair factors Rad50, Mre11, and p95. J Biol Chem. 1998;273:21447–21450. doi: 10.1074/jbc.273.34.21447. [DOI] [PubMed] [Google Scholar]
- 37.Usui T, Ohta T, Oshiumi H, Tomizawa J, Ogawa H, Ogawa T. Complex formation and functional versatility of Mre11 of budding yeast in recombination. Cell. 1998;95:705–716. doi: 10.1016/s0092-8674(00)81640-2. [DOI] [PubMed] [Google Scholar]